Abstract
Context: Following spinal cord injury (SCI), early prediction of future walking ability is difficult, due to factors such as spinal shock, sedation, impending surgery, and secondary long bone fracture. Accurate, objective biomarkers used in the acute stage of SCI would inform individualized patient management and enhance both patient/family expectations and treatment outcomes. Using magnetic resonance imaging (MRI) and specifically a midsagittal T2-weighted image, the amount of tissue bridging (measured as spared spinal cord tissue) shows potential to serve as such a biomarker. Ten participants with incomplete SCI received MRI of the spinal cord. Using the midsagittal T2-weighted image, anterior and posterior tissue bridges were calculated as the distance from cerebrospinal fluid to the damage. Then, the midsagittal tissue bridge ratio was calculated as the sum of anterior and posterior tissue bridges divided by the spinal cord diameter. Each participant also performed a 6-minute walk test, where the total distance walked was measured within six minutes.
Findings: The midsagittal tissue bridge ratio measure demonstrated a high level of inter-rater reliability (ICC = 0.90). Midsagittal tissue bridge ratios were significantly related to distance walked in six minutes (R = 0.68, P = 0.03).
Conclusion/clinical relevance: We uniquely demonstrated that midsagittal tissue bridge ratios were correlated walking ability. These preliminary findings suggest potential for this measure to be considered a prognostic biomarker of residual walking ability following SCI.
Keywords: Spinal cord injury, SCI, Magnetic resonance imaging, Tissue bridge, Walking
Introduction (context/objective)
One of the most pressing questions for patients following a spinal cord injury (SCI) is, “will I ever will walk again?1” Indeed, walking has been frequently identified by individuals post-SCI as one of their most important health priorities.2 Early prediction of walking recovery is difficult due to sedation, impending surgery, and secondary long bone fracture.3 Currently, clinicians rely mostly on physical examination.
The time-frame to establish a prognosis of residual motor function based on physical examination varies from 72 h up to 1-month post injury.3 Spinal shock, a condition characterized by complete loss of responses to external or internal stimuli of the body due to the sudden removal of descending inputs,4 could very well contribute to the limited prognostic value of physical examination findings alone. Furthermore, restoration of normal nerve function after SCI can take up to 2 months or even longer.4 Beyond isolated physical examination findings exists a need for early objective methods to effectively and reliably predict walking recovery following SCI on a patient-by-patient basis.
A hallmark of SCI is spinal cord edema, presenting as an increased signal intensity on T2-weighted magnetic resonance imaging (MRI).5 This edema develops within 72 h after SCI6 and impedes axonal re-growth through the injury site. The formation of a fluid-filled cavity occurs within 2 weeks and, on average, requires 4 weeks for completed edema formation, as evidenced by canine and murine weight-drop contusion models of SCI.7 Additionally, human work demonstrates that spinal cord edema can be detected using sagittal T2-weighted MRI within approximately 3–4 days post injury.5
Imaging of the acutely injured spinal cord is traditionally used to define the dimensions that reflect the extent of the resulting edema1,5,6,8–15 along the superior-inferior axis. Such an approach has been used to separate patients into different prognostic categories reflecting potential for functional recovery (i.e. fair or poor)5,10,11 and to demonstrate a relationship with residual lower extremity motor function.16,17
A recent investigation demonstrated the amount of spared spinal cord tissue bridging, measured using sagittal T2-weighted MRI as non-edema tissue, was predictive of neurological recovery after SCI.18 This preliminary study was based on a previous manuscript involving both rodent and human SCI, demonstrating significant relationships between midsagittal tissue bridges and the clinical scores of lower extremity motor testing and ordinal measures of walking ability.19 Participants were assigned to one of four categories based on current walking ability, with “0” equating to no voluntary ambulation and “4” as “few if any deficits” in walking ability.19
While lower extremity motor testing and categorical data of walking ability represent a reductionist measure of residual motor function, a walking test using ratio-level data such as the Six-Minute Walk Test (6MW)20 may provide a more distinguishing metric. Given the vast heterogeneity of residual motor function in persons with incomplete SCI,21 the 6MW test has been demonstrated to be a valid and reliable measure of walking ability in this population,20 with ratio data collected as distance walked in a 6-minute time-frame.
The purpose of this case series was to establish the relationship between midsagittal tissue bridges and distance walked in 6-minutes, in 10 individuals with incomplete SCI. We hypothesized that a significant positive linear relationship would exist between tissue bridges and the 6MW distance.
Methods
This study utilized a cross-sectional case series research design, completed in a university research setting. The study was approved by Northwestern University and Regis University Institutional Review Boards. Ten individuals with incomplete SCI agreed to participate, provided written and verbal informed consent, and completed the study (1 female, average age = 42 years old ± 13).
MRI measures: midsagittal tissue bridge ratio
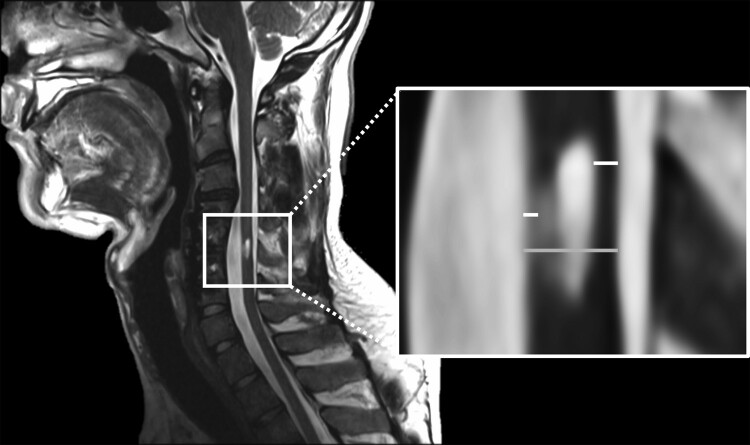
Sagittal T2-weighted imaging of the cervical spinal cord was performed using a 3.0 Tesla Prisma magnetic resonance (MR) scanner equipped with a 64-channel head/neck coil (Siemens, Erlangen, Germany). Sagittal T2-weighted structural imaging of the cervical spine and spinal cord damage site was acquired using a 2D turbo spin echo sequence using 24 consecutive slices (TR = 2300 ms, TE = 106 ms, flip angle = 88°, phase encoding direction: right to left, slice thickness = 2.0 mm, acquisition matrix = 512 × 512). Spinal cord sagittal T2-weighted MRI data were analyzed offline using OsiriX image processing software (Pixmeo, Geneva, Switzerland). Using the midsagittal T2-weighted image, anterior and posterior tissue bridges were calculated as the distance from cerebrospinal fluid to the edema (see Fig. 1). Then, the midsagittal tissue bridge ratio was calculated as the sum of anterior and posterior tissue bridges divided by the spinal cord diameter (see Fig. 1).
Figure 1.
(Left) One representative participant’s sagittal T2-weighted image (left). (Right) Midsagittal tissue bridge ratios were calculated as the sum of the tissue bridges (white) over the cord diameter (grey).
Six-minute walk test
Participants performed overground 6-minute walk tests, where the total distance walked was measured within six timed minutes.20 Each participant was permitted the use of assistive devices or braces as necessary to walk at a normal, self-selected pace.
Statistical analyses
All statistical analyses of the data were performed using IBM SPSS (Version 23, Armonk, NY, USA). An intra-class correlation coefficient (ICC2,1) was used to test the inter-rater reliability of the midsagittal tissue bridge ratio measure across seven different raters. A Pearson correlation was selected to examine the linear relationship between midsagittal tissue bridge ratios and distance walked in 6 minutes. A P value of <0.05 was considered statistically significant.
Results
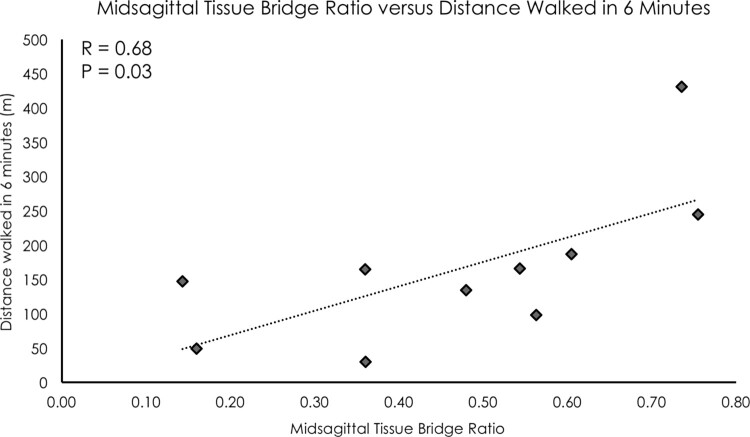
The midsagittal tissue bridge ratio measure demonstrated a high level of inter-rater reliability (ICC2,1 = 0.90). Midsagittal tissue bridge ratios were significantly related to distance walked in six minutes (R = 0.68, P = 0.03, see Fig. 2).
Figure 2.
A significant positive linear relationship was found between midsagittal tissue bridge ratios and distance walked in 6 min (R = 0.68, P = 0.03). Participants were allowed to use assistive devices and orthoses as necessary.
Discussion
In this case series, we uniquely demonstrated that midsagittal tissue bridge ratios were significantly related to a reliable, and valid, discriminative ratio-level measure of walking ability of 10 participants with incomplete SCI. Our findings are in accordance with previous literature that examined this sagittal T2-weighted MRI measure and related to functional outcomes following SCI.18,19 In this present study, we used a continuous variable for our primary outcome, the distance walked within a six-minute time-frame, which provides a more distinguishing measure of an individual’s locomotor ability. While past studies used discrete categorical outcome measures (i.e. motor scores and four categories of walking ability) and related to midsagittal tissue bridges,18,19 our study further expanded upon the predictive potential of this MRI measure to a more discriminative test of walking.
While past correlations using sagittal T2-weighted MRI measures and locomotor abilities have been poor,22 newer studies affirm the importance of these sagittal imaging measures for prognosis of residual motor function.8,18 Spinal cord edema measurement in other planes, such as the axial plane, may also augment prediction of walking and motor recovery after SCI.16,17 As imaging methodology continues to improve, more sophisticated edema measurement techniques, such as machine learning driven approaches, may show potential to better inform and enhance our clinical prognostic abilities.
Limitations
One inherent limitation with a cases series is the smaller sample size. Our future research aims to investigate the prognostic potential of midsagittal tissue bridge measures in a larger dataset of persons with SCI. A second limitation is the subjective nature of this manual measure. However, we found a high level of inter-rater reliability, suggesting this measure is consistent across raters and therefore potentially clinically relevant and useful. A third limitation is that due to the unavailability of data, we did not include other MR sequences or contrasts in this research. Previous literature suggests that short tau inversion recovery (STIR) MRI, in particular, may be more sensitive than T2-weighted MRI in detecting spinal cord lesions in patients with multiple sclerosis, cervical myelopathy, and traumatic SCI.23–28
Conclusion
In this present case series involving 10 participants with incomplete SCI, we uniquely demonstrate that midsagittal tissue bridge ratios were correlated with the ratio-level measure of distance walked in 6 min. These preliminary findings suggest potential for this measure to be considered a prognostic biomarker of residual walking ability following SCI. Improving early and accurate prediction of future locomotor function after SCI is of utmost importance to all stakeholders, including patients, families, and the healthcare team. Early prediction of functional recovery will improve the clinical management and individualized intervention of individuals with SCI. Further research is warranted to apply these methods to a larger dataset in the acute stage of SCI, in order to explore and establish the predictive value of midsagittal tissue bridge measures.
Disclaimer statements
Funding Dr. Weber is supported by the Interdisciplinary Research Training in Pain and Substance Use Disorders funded by the National Institute on Drug Abuse under award number T32DA035165. Dr. Elliott is supported by the National Institutes of Health Award R01HD079076. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest The authors have no conflicts of interest to disclose.
ORCID
Kenneth A. Weber http://orcid.org/0000-0002-0916-9174
James M. Elliott http://orcid.org/0000-0002-8890-6012
David P. Cummins http://orcid.org/0000-0002-4416-2541
Katherine A. Heller http://orcid.org/0000-0002-0317-436X
Andrew C. Smith http://orcid.org/0000-0001-5020-8094
References
- 1.Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M.. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci 2014;8:141. doi: 10.3389/fnhum.2014.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simpson LA, Eng JJ, Hsieh JTC, Wolfe DL, the Spinal Cord Injury Rehabilitation Evidence Scire Research Team . The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma 2012;29(8):1548–55. doi: 10.1089/neu.2011.2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns AS, Ditunno JF.. Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine 2001;26(24 Suppl):S137–45. doi: 10.1097/00007632-200112151-00023 [DOI] [PubMed] [Google Scholar]
- 4.Boland RA, Lin CS-Y, Engel S, Kiernan MC.. Adaptation of motor function after spinal cord injury: novel insights into spinal shock. Brain 2011;134(Pt 2):495–05. doi: 10.1093/brain/awq289 [DOI] [PubMed] [Google Scholar]
- 5.Bozzo A, Marcoux J, Radhakrishna M, Pelletier J, Goulet B.. The role of magnetic resonance imaging in the management of acute spinal cord injury. J Neurotrauma 2011;28(8):1401–11. doi: 10.1089/neu.2009.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns AS, Marino RJ, Flanders AE, Flett H.. Clinical diagnosis and prognosis following spinal cord injury. Handb Clin Neurol 2012;109:47–62. doi: 10.1016/B978-0-444-52137-8.00003-6 [DOI] [PubMed] [Google Scholar]
- 7.Hu R, Zhou J, Luo C, Lin J, Wang X, Li X, et al. Glial scar and neuroregeneration: histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J Neurosurg Spine 2010;13(2):169–80. doi: 10.3171/2010.3.SPINE09190 [DOI] [PubMed] [Google Scholar]
- 8.Aarabi B, Sansur CA, Ibrahimi DM, Simard JM, Hersh DS, Le E, et al. Intramedullary lesion length on postoperative magnetic resonance imaging is a strong predictor of ASIA impairment scale grade conversion following decompressive surgery in cervical spinal cord injury. Neurosurgery 2017;80(4):610–20. doi: 10.1093/neuros/nyw053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flanders A, Schaefer D, Doan H, Mishkin M, Gonzalez C, Northrup B.. Acute cervical spine trauma: correlation of MR imaging findings with degree of neurologic deficit. Radiology 1990;177:25–33. doi: 10.1148/radiology.177.1.2399326 [DOI] [PubMed] [Google Scholar]
- 10.Flanders A, Spettell C, Tartaglino L, Friedman D, Herbison G.. Forecasting motor recovery after cervical spinal cord injury: value of MR imaging. Radiology 1996;201:649–55. doi: 10.1148/radiology.201.3.8939210 [DOI] [PubMed] [Google Scholar]
- 11.Boldin C, Raith J, Fankhauser F, Haunschmid C, Schwantzer G, Schweighofer F.. Predicting neurologic recovery in cervical spinal cord injury with postoperative MR imaging. Spine 2006;31(5):554–9. doi: 10.1097/01.brs.0000201274.59427.a4 [DOI] [PubMed] [Google Scholar]
- 12.Lundell H, Barthelemy D, Skimminge A, Dyrby TB, Biering-Sørensen F, Nielsen JB.. Independent spinal cord atrophy measures correlate to motor and sensory deficits in individuals with spinal cord injury. Spinal Cord 2011;49(1):70–5. doi: 10.1038/sc.2010.87 [DOI] [PubMed] [Google Scholar]
- 13.Wilson JR, Grossman RG, Frankowski RF, Kiss A, Davis AM, Kulkarni AV, et al. A clinical prediction model for long-term functional outcome after traumatic spinal cord injury based on acute clinical and imaging factors. J Neurotrauma 2012;29(13):2263–71. doi: 10.1089/neu.2012.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellingson BM, Salamon N, Holly LT.. Imaging techniques in spinal cord injury. World Neurosurg 2014;82(6):1351–8. doi: 10.1016/j.wneu.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyanji F, Furlan J, Aarabi B, Arnold P, Fehlings M.. Acute cervical traumatic spinal cord injury: MR imaging findings correlated with neurologic outcome - prospective student with 100 consecutive patients. Radiology 2007;243(3):820–7. doi: 10.1148/radiol.2433060583 [DOI] [PubMed] [Google Scholar]
- 16.Smith AC, Weber KA, Parrish TB, Hornby TG, Tysseling VM, McPherson JG, et al. Ambulatory function in motor incomplete spinal cord injury: a magnetic resonance imaging study of spinal cord edema and lower extremity muscle morphometry. Spinal Cord 2017;55(7):672–8. doi: 10.1038/sc.2017.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AC, Weber KA, O’Dell DR, Parrish TB, Wasielewski M, Elliott JM.. Lateral corticospinal tract damage correlates with motor output in incomplete spinal cord injury. Arch Phys Med Rehabil 2018;99(4):660–6. doi: 10.1016/j.apmr.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber E, Lachappelle P, Sutter R, Curt A, Freund P.. Are midsagittal tissue bridges predictive of outcome after cervical spinal cord injury? Ann Neurol 2017;81(5):740–8. doi: 10.1002/ana.24932 [DOI] [PubMed] [Google Scholar]
- 19.Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V.. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma 2000;17(1):1–17. doi: 10.1089/neu.2000.17.1 [DOI] [PubMed] [Google Scholar]
- 20.Scivoletto G, Tamburella F, Laurenza L, Foti C, Ditunno JF, Molinari M.. Validity and reliability of the 10-m walk test and the 6-min walk test in spinal cord injury patients. Spinal Cord 2011;49(6):736–40. doi: 10.1038/sc.2010.180 [DOI] [PubMed] [Google Scholar]
- 21.van Hedel HJA, Dietz V, Curt A.. Assessment of walking speed and distance in subjects with an incomplete spinal cord injury. Neurorehabil Neural Repair 2007;21(4):295–301. doi: 10.1177/1545968306297861 [DOI] [PubMed] [Google Scholar]
- 22.Flanders A, Spettell C, Friedman D, Marino RJ, Herbison G.. The relationship between the functional abilities of patients with cervical spinal cord injury and the severity of damage revealed by MR imaging. AJNR Am J Neuroradiol 1999;20:926–34. [PMC free article] [PubMed] [Google Scholar]
- 23.Mascalchi M, Dal Pozzo G, Bartolozzi C.. Effectiveness of the short TI inversion recovery (STIR) sequence in MR imaging of intramedullary spinal lesions. Magn Reson Imaging 1993;11(1):17–25. doi: 10.1016/0730-725X(93)90407-5 [DOI] [PubMed] [Google Scholar]
- 24.Philpott C, Brotchie P.. Comparison of MRI sequences for evaluation of multiple sclerosis of the cervical spinal cord at 3 T. Eur J Radiol 2011;80(3):780–5. doi: 10.1016/j.ejrad.2010.09.031 [DOI] [PubMed] [Google Scholar]
- 25.Campi A, Pontesilli S, Gerevini S, Scotti G.. Comparison of MRI pulse sequences for investigation of lesions of the cervical spinal cord. Neuroradiology 2000;42(9):669–75. doi: 10.1007/s002340000368 [DOI] [PubMed] [Google Scholar]
- 26.Rocca MA, Mastronardo G, Horsfield MA, et al. Comparison of three MR sequences for the detection of cervical cord lesions in patients with multiple sclerosis. AJNR Am J Neuroradiol 1999;20(9):1710–16. [PMC free article] [PubMed] [Google Scholar]
- 27.Alcaide-Leon P, Pauranik A, Alshafai L, Oh J, Montanera W, Leung G, et al. Comparison of sagittal FSE T2, STIR, and T1-weighted phase-sensitive inversion recovery in the detection of spinal cord lesions in MS at 3T. AJNR Am J Neuroradiol 2016;37(5):970–5. doi: 10.3174/ajnr.A4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nayak NB, Salah R, Huang JC, Hathout GM.. A comparison of sagittal short T1 inversion recovery and T2-weighted FSE sequences for detection of multiple sclerosis spinal cord lesions. Acta Neurol Scand 2014;129(3):198–203. doi: 10.1111/ane.12168 [DOI] [PubMed] [Google Scholar]