Abstract
Objective: To longitudinally evaluate the impact of change in physical activity or change in body mass index (BMI) over time on the risk of developing heart failure (HF) in women without a previous diagnosis of HF.
Design and setting: Longitudinal, observational, prospective study of women in Gothenburg, Sweden. Data on BMI and level of physical activity were collected from examinations 1968–1992 and hospital diagnoses and mortality data were ascertained from 1980 to 2012.
Subjects: Data were obtained from 1749 women included in the Prospective Population Study of Women in Gothenburg.
Main outcome measures: Hazard ratio (HR) for HF was calculated, using a Cox regression model.
Results: Women with stable high physical activity during 1968–1980 and 1980–1992 reduced their risk of subsequent HF compared to the non-active women (for 1968–1980 HR 0.66, 95% Confidence Interval (CI) 0.44–0.99 and for 1980–1992 HR 0.47, 95% CI 0.29–0.74). Women with increasing levels of physical activity during 1980–1992 reduced their risk of HF compared to the non-active women (HR 0.40, 95% CI 0.22–0.72). Increase in BMI from overweight to obesity during 1968–1980 predicted increased risk of developing HF (HR 1.93, 95% CI 1.18–3.14).
Conclusions: Reduced risk of future HF in healthy women may be achieved by remaining physically active from young middle age and throughout life or by increasing the level of physical activity. This is particularly important for sedentary women in middle age. The role of physical activity in preventing the development of obesity must be taken into account.
Key points
A sedentary lifestyle and obesity are risk factors for developing heart failure (HF) in women.
The risk of developing HF may be reduced by increasing the level of activity in sedentary middle-aged women.
For younger women, avoiding obesity is most important to reduce the risk of later HF.
Primary care has a key role in guiding women towards the most effective lifestyle changes to prevent development of HF.
Keywords: Women, heart failure, obesity, physical activity, cardiovascular disease
Introduction
Heart failure (HF) is a severe and progressive condition with poor prognosis [1]. In high-income countries, the prevalence of HF is ∼2% [2]. In 2008, the prevalence of HF in the United States was 2.4% [3]. The Rotterdam study showed an overall HF prevalence of 3.9% [4]. A recent Swedish study found a prevalence of 2.2% in the Stockholm area [5]. The HF prevalence increases with age, up to 8.4% for those aged 75 years or older [6]. HF imposes a great burden on hospitalization [7] and on national economy [8], thus emphasizing the importance of the condition.
Obesity is a risk factor for HF [9] and causality has been established [10]. For women, this has recently been shown to be especially the case regarding obesity present from younger and middle age [11]. A meta-analysis gives evidence that a sedentary lifestyle is a major risk factor for later HF [12], and today a large number of people live a sedentary life. According to the validated Saltine-Grimby scale, a sedentary lifestyle is defined as less than four hours per week of light physical activity. The risk of developing HF has been shown to be reduced in women who are physically active [13].
In 2019, the Public Health Agency of Sweden reported an increase in obesity frequency in the population during 2006 to 2018 from 5% to 7% and from 6% to 10%, respectively, for Swedish women and men aged 16–29 years, and from 11% to 14% for both women and men aged 30–44 years [14]. Physical activity in a Swedish middle-aged female population showed an increase between 1980 and 2004 according to a cohort study [15].
Women are not always adequately represented in HF studies. Investigators may not pose the questions or study the variables relevant to women’s cardiac health due to the history of focusing on male cardiac disease. When echocardiography is performed, more women than men tend to have preserved left ventricular systolic function [16], commonly because at middle age, more men than women are prone to suffer from coronary heart disease that is closely linked to reduced left ventricular systolic function. In the early stages of obesity in women, without any other pathological conditions, subclinical left ventricular diastolic dysfunction is present and correlates with body mass index (BMI) [17].
However, many studies describe an obesity paradox [18], in which overweight and obese patients with established HF have lower mortality than lean patients with HF. It has also been shown that in HF patients with a high cardiorespiratory fitness (CRF), survival was very good regardless of BMI; thus the obesity paradox was not present [19]. Exercise training in women with HF reduced the risk of all-cause mortality or hospitalization compared to women with HF and no exercise training [20]. The level of CRF, determined as metabolic equivalent, is shown to be important as high CRF level resulted in lower risk of all-cause mortality in women independently of BMI [21], but whether this is true also for the risk of developing HF in women is not thoroughly investigated.
HF with preserved left ventricular systolic function is more common in women, and according to the 2016 European Society of Cardiology (ESC) Guidelines for the diagnosis and treatment of acute and chronic HF, there is yet no medical treatment available proven to reduce mortality in this group of HF patients [22]. Thus, prevention of the condition becomes highly important, implicating the need for a focus on preventable risk factors.
Currently, there are no studies addressing the impact of change in physical activity or change in BMI overtime on the risk of developing HF in healthy women with no previous diagnosis of HF. We therefore, investigated the possible impact on HF risk by a change in BMI or physical activity, as a guide in primary prevention, to focus on effective means to prevent the development of HF in women.
We hypothesized that an increase in physical activity or a decrease in BMI in without a diagnosis of HF would reduce the risk of developing HF later in life.
The aim was to longitudinally investigate the impact of change in physical activity or change in BMI on the risk of later developing HF in women without a previous diagnosis of HF.
Methods
Study population
Our study population for this report includes 1749 women, originating from the Prospective Population Study of Women in Gothenburg (PPSWG). The women were recruited from the Swedish Population Register based on birth dates and were representative of the female population in Gothenburg in those age groups.
The PPSWG was initiated in 1968–1969 with a cross-sectional examination of 1462 women aged 38, 46, 50, 54 and 60 years. The overall participation rate was 90.1%. All the women were then followed longitudinally [23].
In 1980–1981, a follow-up examination was performed, and two additional groups aged 26 and 38 were included. Also to improve representativeness, women born in 1930 outside Gothenburg, but who had moved into the study area and who fulfilled the inclusion criteria, were invited to the examination 1980–1981. They were then aged 50 years. Participation rate in 1980–1981 was 79% of those originally born in 1908–1930 (Table 1) [24].
Table 1.
Examinations and participation rates of women born in 1908, 1914, 1918, 1922 and 1930 examined in 1968, 1980 and 1992, and women born in 1942 and 1954 examined in 1980 and 1992.
| Year of birth | 1968 examination |
1980 examination |
1992 examination |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | n | % | Age | n | % | Age | n | % | |
| 1954 | – | – | – | 26 | 85 | 66a | 38 | 69 | 72b |
| 1942 | – | – | – | 38 | 122 | 85a | 50 | 99 | 76b |
| 1930 | 38 | 372 | 91c | 50 | 355 | 83d | 62 | 275 | 67d |
| 1922 | 46 | 431 | 90 | 58 | 332 | 77d | 70 | 303 | 63d |
| 1918 | 50 | 398 | 91c | 62 | 325 | 82d | 74 | 213 | 54d |
| 1914 | 54 | 180 | 89c | 66 | 140 | 78d | 78 | 79 | 44d |
| 1908 | 60 | 81 | 84c | 72 | 49 | 60d | 84 | 19 | 23d |
| Total | 38–60 | 1462 | 90c | 26–72 | 1408 | 79e | 38–84 | 1057 | 57e |
Of those sampled in 1980–1981.
Of those participating in 1980–1981.
Of those initially invited.
Of the original women in each age group who participated in the examination 1968–1969.
Of the women born in 1908–1930 who participated in 1968–1969.
In 1992–1993, another follow-up examination took place, with a participation rate of 57.2% of the original 1462 participants in 1968–1969. 33 women, 70 years old, who fulfilled the inclusion criteria and who had moved into the region were added to ensure representativeness. Because 280 (19.2%) had died before the 24-year follow up 1992–1993, the participation rate corresponded to 70.2% of those who had participated in 1968–1969 and who were alive in 1992–1993 [25].
In order to perform the examinations at the time of the women’s age + 6 months, the examination period started in the autumn of the examination year and extended to late spring of the following year. For simplification purposes, the examination year 1968–1969 is indicated as 1968, 1980–1981 as 1980, etc. Women 26 years old were classified as young, 38–60 as middle aged.
Methodology
Participants underwent similar procedures at baseline and at all of the follow-up examinations. The examinations included documentation of socio-economic status based on years of education, marital status and occupation of the woman/husband according to Carlsson’s occupational system [26]. Lifestyle-related exposures such as smoking, alcohol habits and usual level of physical activity were registered. Information concerning medical history, prescription drug use and family history of diseases was collected.
All examinations included body weight, body height, BMI (calculated as weight in kilograms divided by height in meters squared), waist circumference, hip circumference, waist-hip ratio and blood pressure measurements. Body weight was measured to the nearest 0.1 kg using a balance scale, and body height was measured without shoes, in meters to the nearest 0.01 m. Blood samples were drawn, and a urinary sample was collected in the morning. The women were asked not to eat during the preceding night, although they were allowed to drink water. Levels of total s-cholesterol, s-triglycerides and b-glucose were analysed according to standard methods of the laboratory of the Sahlgrenska University Hospital, Gothenburg. A standardized protocol was used for general physical examinations at all surveys and systolic and diastolic blood pressure measurement was conducted using a mercury manometer, cuff width 12 cm. Blood pressure was measured to the nearest 2 mm Hg in the sitting position after 5 min rest. A standard 12-lead ECG was taken.
For this particular study, we included only women without any previous history, sign or diagnosis of HF prior to 1968.
Definition of risk factors
Physical activity was assessed as reported leisure-time physical activity, categorized as follows: non-active (inactive or almost inactive), and active (at least 4 h per week walking, bicycling, gardening, running, dancing, playing golf, tennis or similar activities during the last year, or regular intense training several times per week), according to the validated Saltin-Grimby physical activity level scale [27]. The four-level scale was dichotomized into these two categories because sedentary activity is currently an important lifestyle issue and is shown to be a risk factor for HF [12]. Changes over time in this measure have previously been shown to be associated with total mortality in this cohort [28].
Overweight was classified as 25 ≤ BMI < 30 and obesity was classified as BMI ≥ 30, in accordance with WHO classification [29]. All other subjects were categorized as non-overweight.
Classification of diagnoses
The diagnoses were classified according to the International Classification of Diseases, 9th revision (ICD-9) and from 1997, 10th revision (ICD-10). HF was defined as ICD-9 428A, B or X, or ICD-10 I50.
Follow up data
Hospital care diagnoses and mortality data were continually registered over 32 years, 1980–2012. Data from the Swedish Hospital Discharge Registry (NPR) have been available since 1980, including data about all discharge diagnoses from Swedish hospitals. Ingelsson et al. [30] showed that as a primary discharge diagnosis from Swedish hospitals, the diagnostic accuracy of HF according to the European Society of Cardiology definition was 82–95%. Mortality data were obtained from the Swedish National Board of Health and Welfare register of causes of death from 1 January 1980 to 31 December 2012. The cardiovascular disease risk time was calculated from the day the diagnosis was registered.
Statistical analyses
The endpoint was HF expressed as Hazard Ratio (HR). Covariates were changes in BMI and changes in physical activity. All women were classified into groups of BMI (non-overweight: BMI < 25, overweight: 25 ≤ BMI <30 and obese: BMI ≥ 30), and level of physical activity: non-active (inactive or almost inactive) and active (moderate or high activity). Changes in physical activity and changes in BMI were calculated over time. Cox regression model was used to evaluate the effect of belonging to the different BMI-change and activity-change categories. To examine the assumption of proportional hazard, tests of Schoenfeld residuals were calculated for the total model. In the case of significant result from this test, the follow-up was divided into two periods, the first decade and the second decade and separate effect was reported.
Examinations and participation rates are shown in Table 1.
Results
Incidence and risk time for analysis of incidence of HF for the two groups 1968–2012 and 1980–2012 is shown in Table 2. Kaplan–Meier survival plots of HF, BMI, change in BMI, level of physical activity and change in physical activity are shown in Figure 1. For analysis of incidence and risk time (Kaplan–Meier plot) the women born in 1942 and 1954 were excluded from the analysis because of short follow-up time.
Table 2.
Incidence and risk time analysis of incidence of HF 1968–2012 and 1980–2012.
| Period | N | Cases | Risk years | Risk per 1000 years | 95% CI Risk per 1000 years | Mean age in risk time | Age range |
|---|---|---|---|---|---|---|---|
| 1968–2012 | 1132 | 271 | 26,111 | 10.4 | 9.2–11.7 | 71.2 | 50.3–98.8 |
| 1980–2012 | 773 | 174 | 11,254 | 15.5 | 13.2–17.9 | 77.4 | 62.4–98.8 |
Women born in 1942 and 1954 were excluded.
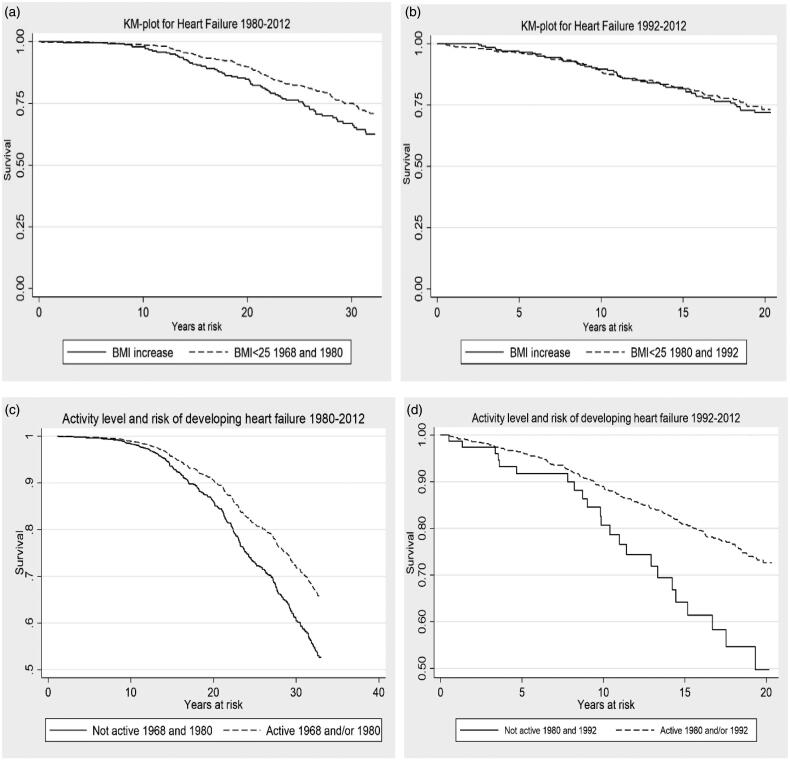
Figure 1.
Kaplan–Meier survival plots for HF and change in BMI, level of physical activity and change in physical activity 1968–1980 and 1980–1992. (a,b) Change in BMI, 1968–1980 and 1980–1992. (c,d) Level of physical activity and change in physical activity 1968–1980 and 1980–1992.
Periods 1968–1980 and 1980–1992
Baseline characteristics are shown in Table 3. Mean age at baseline in the examinations 1968 to 1980 for overweight and obese women was 48 years (SD 6 years) and 49 years (SD 6 years) respectively, and in the examinations 1980 to 1992 for overweight and obese women, mean age at baseline was 58 years (SD 8 years for overweight women, SD 9 years for obese women); thus the women in the examination period 1980–1992 were on the average 10 years older than the women in the examination period 1968–1980. The mean age of the women with overweight or obesity was consistently higher than the rest of the sample (Table 3). The development of BMI could be followed in 1132 women who participated in examinations both in 1968 and 1980, and 932 women could be followed from 1980 to 1992. For development of physical activity, 1132 women who participated both in 1968 and 1980, and 932 women in 1980 and 1992, could be followed during these respective follow-up periods (Table 4).
Table 3.
Baseline characteristics for women participating in examinations 1968, 1980 and 1992.
| 1968 |
1980 |
1992 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI |
BMI |
BMI |
|||||||
| <25, N = 817, ma, (SD) | 25–<30, N = 310, ma, (SD) | >30, N = 82, ma, (SD) | <25, N = 826, ma, (SD) | 25–<30, N = 445, ma, (SD) | >30, N = 135, ma, (SD) | <25, N = 483, ma, (SD) | 25–<30, N = 366, ma, (SD) | >30, N = 180, ma, (SD) | |
| Age (years) | 46 (6) | 48 (6) | 49 (6) | 53 (12) | 58 (8) | 58 (9) | 64 (12) | 66 (10) | 68 (8) |
| SBP (mmHg) | 129 (19) | 136 (20) | 148 (27) | 136 (24) | 146 (23) | 151 (24) | 145 (27) | 153 (23) | 160 (23) |
| DBP (mmHg) | 83 (10) | 87 (10) | 94 (13) | 86 (11) | 90 (10) | 94 (9) | 79 (11) | 84 (11) | 87 (10) |
| B-Glucose (mmol/l) | 4.1 (0.7) | 4.2 (0.8) | 4.5 (0.8) | 4.1 (1.1) | 4.4 (1.1) | 4.9 (1.7) | 4.4 (1.0) | 4.7 (1.4) | 5.1 (1.2) |
| Cholesterol (mmol/l) | 6.8 (1.3) | 6.9 (1.0) | 7.3 (1.2) | 6.8 (1.3) | 7.0 (1.4) | 6.8 (1.3) | 6.1 (1.2) | 6.3 (1.1) | 6.3 (1.1) |
| Triglycerides (mmol/l) | 1.1 (0.6) | 1.3 (0.5) | 1.5 (0.6) | 1.2 (0.6) | 1.5 (0.8) | 1.6 (0.7) | 1.2 (0.6) | 1.5 (0.8) | 1.8 (0.8) |
| |
(%) |
(%) |
(%) |
(%) |
(%) |
(%) |
(%) |
(%) |
(%) |
| Physical Activity: Non-active | 16 | 21 | 21 | 27 | 29 | 42 | 18 | 21 | 28 |
| Smokersb | 44 | 33 | 24 | 39 | 28 | 22 | 24 | 20 | 14 |
| Alcoholc | 56 | 47 | 35 | 45 | 32 | 29 | 52 | 51 | 38 |
Mean value.
Women who smoked or had stopped smoking during the previous year.
More than once a month.
Table 4.
Distribution of BMI categories, change in BMI, physical activity levels, change in physical activity levels and cases of HF for women participating in the examinations 1968–1980 and 1980–1992.
| BMI | 1968–1980, n = 1132 |
1980–1992, n = 932 |
||
|---|---|---|---|---|
| 1968 | 1980 | 1980 | 1992 | |
| BMI <25, n (%) | 769 (67.9) | 611 (54.0) | 571 (61.3) | 446 (47.9) |
| ≤25 BMI <30, n (%) | 289a (25.5) | 404b (35.7) | 287b (30.8) | 327c (35.1) |
| BMI ≥30, n (%) | 74d (6.5) | 117b (10.3) | 74b (7.9) | 159e (17.1) |
| BMI change | 1968–1980, n = 1132 |
1980–1992, n = 932 |
||
| BMI increase from ≤25 BMI <30 to BMI ≥30, n (%) | 59 (5.2) | 87 (9.3) | ||
| BMI decrease from BMI ≥30 to ≤25 BMI <30, n (%) | 18 (1.6) | 12 (1.3) | ||
| No change; ≤25 BMI <30 | 197 (17.4) | 168 (18.0) | ||
| No change; BMI ≥30, n (%) | 54 (4.8) | 60 (6.4) | ||
| Cases of HF, n (%) | 271 (23.9) | 187 (20.1) | ||
| Physical activity | 1968–1980, n = 1132 |
1980–1992, n = 932 |
||
| 1968 |
1980 |
1980 |
1992 |
|
| Non-active, n (%) | 194 (17.1) | 328 (29.0) | 255 (27.4) | 199 (21.4) |
| Active, n (%) | 938 (82.9) | 804 (71.0) | 677 (72.6) | 733 (78.6) |
| Physical activity change | 1968–1980, n = 1132 |
1980–1992, n = 932 |
||
| Activity increase from non-active to active, n (%) | 92 (8.1) | 171 (18.3) | ||
| Activity decrease from active to non-active n (%) | 226 (20.0) | 115 (12.3) | ||
| No change; active, n (%) | 712 (62.9) | 562 (60.3) | ||
| Cases of HF, n (%) | 271 (23.9) | 187 (20.1) | ||
Mean age 48 years.
Mean age 58 years.
Mean age 66 years.
Mean age 49 years.
Mean age 68 years.
Observations from 1968 to 1980
From 1968 to 1980, BMI could be followed in 1132 women, of whom 271 (23.9%) developed HF by 2012. The majority, 769 women (67.9%) in the 1968 examination and 611 women (54.0%) in the 1980 examination, were classified as non-overweight at respective examinations, whereas 289 women (25.5%) in 1968 and 404 women (35.7%) in 1980 were overweight at respective examinations. A smaller proportion, 74 women (6.5%) in 1968 and 117 women (10.3%) in 1980, were obese at respective examinations. Overall, there was a small shift towards a higher BMI in 1980 (Table 4).
For the 59 women (5.2%), who increased their BMI from overweight to obesity between 1968 and 1980, the risk of future HF increased significantly with a HR of 1.93 (95% CI 1.18–3.14), compared to the reference group with BMI <25, both in 1968 and 1980 (Table 5).
Table 5.
Hazard ratios for HF and change in physical activity and BMI, adjusted for age.
| HR | p Value | 95 % CI | |
|---|---|---|---|
| Change in BMI between 1968 and 1980 | |||
| Increase in BMI from normal to overweight | 1.26 | 0.17 | 0.90–1.76 |
| Increase in BMI from overweight to obese | 1.93 | 0.008 | 1.18–3.14 |
| Decrease in BMI from obese to overweight | 0.49 | 0.32 | 0.12–2.00 |
| Decrease in BMI from overweight to non-overweight | 1.30 | 0.45 | 0.66–2.59 |
| No change; obese both in 1968 and in 1980 | 1.21 | 0.46 | 0.73–1.99 |
| No change; overweight both in 1968 and in 1980 | 1.26 | 0.18 | 0.90–1.72 |
| BMI <25 in 1968 and 1980 (reference group) | 1.00 | ||
| Change in BMI between 1980 and 1992 | |||
| Increase in BMI from normal to overweight | 1.07 | 0.78 | 0.67–1.70 |
| Increase in BMI from overweight to obese | 1.10 | 0.71 | 0.66–1.85 |
| Decrease in BMI from obese to overweight | 1.47 | 0.59 | 0.36–6.07 |
| Decrease in BMI from overweight to non-overweight | 1.62 | 0.20 | 0.77–3.42 |
| No change; obese both in 1980 and in 1992 | 1.27 | 0.41 | 0.72–2.21 |
| No change; overweight both in 1980 and in 1992 | 1.01 | 0.96 | 0.66–1.54 |
| BMI <25 in 1980 and 1992 (reference group) | 1.00 | ||
| Change in physical activity between 1968 and 1980 | |||
| Increase from non-active to active | 0.71 | 0.26 | 0.40–1.28 |
| Decrease from active to non-active | 0.71 | 0.15 | 0.44–1.13 |
| No change; active both in 1968 and in 1980 | 0.66 | 0.04 | 0.44–0.99 |
| Non-active in 1968 and 1980 (reference group) | 1.00 | ||
| Change in physical activity between 1980 and 1992 | |||
| Increase from non-active to active | 0.40 | 0.002 | 0.22–0.72 |
| Decrease from active to non-active | 0.73 | 0.28 | 0.41–1.29 |
| No change; active both in 1980 and in 1992 | 0.47 | 0.001 | 0.29–0.74 |
| Non-active in 1980 and 1992 (reference group) | 1.00 |
Reference groups: BMI < 25, 1968–1980 and 1980–1992; non-active, 1968–1980 and 1980–1992.
Bold figures: statistically significant, level of significance p ≤ 0.05.
For the 18 women (1.6%) who decreased their BMI from obesity to overweight and for the 54 women (4.8%) who remained obese between 1968 and 1980, there was no significant association with risk of future HF, compared to the reference group whose BMI remained <25 (Table 5). Only two women decreased their BMI from obesity to non-overweight.
Level of physical activity could be followed in 1132 women participating in examinations both in 1968 and 1980, and 271 women (23.9%) developed HF by 2012. In 1968, there were 194 women (17.1%) who were non-active, and in 1980, there were 328 women (29.0%) who were non-active. Overall, more women became less active during the time period (Table 4).
Ninety-two women (8.1%) increased their level of activity, and 226 women (20.0%) decreased their activity level between 1968 and 1980, but neither of these groups showed a significant change in the risk of later developing HF. The 712 women (62.9%) who remained active during the time period had a reduced risk of developing HF of HR equal to 0.66 (95% CI 0.44–0.99) (Table 5).
Observations from 1980 to 1992
BMI could be followed in 932 women from 1980 to 1992, of whom 187 women (20.1%) later developed HF. Between 1980 and 1992, there was an overall decrease in the number of women who were non-overweight, from 571 (61.3%) in 1980 to 446 (47.9%) in 1992, a small increase in the number of women who were overweight, from 287 (30.8%) to 327 (35.1%), and also an increase in the number of women who were obese, from 74 women (7.9%) in 1980 to 159 women (17.1%) in 1992 (Table 4).
Eighty-seven women (9.3%) increased their BMI from overweight to obesity between 1980 and 1992, and 12 women (1.3%) decreased their BMI from obesity to overweight, but neither an increase nor a decrease in BMI for these women showed any significant change in the risk of HF development later in life. Only two women decreased their BMI from obesity to non-overweight. Also, there was no significant association with future HF for the women who remained obese throughout 1980 to 1992, compared with women whose BMI remained <25 during the same time period (Table 5).
Level of physical activity could be followed in 932 women participating in the examinations 1980 and 1992, and 187 women (20.1%) developed HF until 2012. A slight decrease in the number of non-active women, from 255 (27.4%) to 199 (21.4%), was observed between 1980 and 1992, whereas there was a small increase in the number of active women, from 677 (72.6%) to 733 (78.6%). Overall, more women became active during this time period (Table 4).
The 171 women (18.3%) who increased their level of activity from non-active to active during 1980 to 1992 significantly reduced their risk of HF to HR equal to 0.40 (95% CI 0.22–0.72). Further, 562 women (60.3%) who remained active throughout the time period significantly reduced their risk of developing HF later in life, HR 0.47 (95% CI 0.29–0.74). A decrease in activity level did not significantly alter the risk for HF (Table 5).
We also performed a complementary analysis where we excluded women who were diagnosed with myocardial infarction before they were diagnosed with HF, which did not substantially affect the results. However, results concerning physical activity were strengthened in the period 1968–1980 and indicated significantly reduced risk for those women who were physically active, especially in younger ages. In a second complementary analysis, we excluded women who suffered a myocardial infarction prior to their HF diagnosis and also adjusted for s-triglycerides, s-cholesterol, smoking and hypertension. In this analysis, we still reached significantly lower risk for HF in the younger women who were continuously active, whereas for the older women there was still a reduced risk, though not significant, for the continuously active women and for the women who increased their activity. The most important confounding factor for both groups was smoking.
Discussion
In this study, we followed healthy women from the PPSWG taking part in the examinations 1968, 1980 and 1992 with regard to change in BMI or change in levels of physical activity during that period and the risk of later in life developing HF. The mean age for overweight and obese women in the examinations 1980 to1992 was 58 years, and the mean age for overweight and obese women in the examinations 1968 to1980 were 48 and 49 years, respectively. That is, women in the 1980 to1992 examinations were on the average 10 years older than the women in the 1968 to 1980 examinations. Thus, with regard to the risk of developing HF later in life, it seems that gaining weight in early middle age is more harmful than gaining weight later in life, which is consistent with the results in our earlier study on obesity and risk of HF in women [11]. On the other hand, we could not show significant impact of the risk on future HF with weight reduction from obese to overweight in young middle-aged women, which could be an effect of the relatively small number of women in this category. As for the women in later middle age, although our results were not significant, they suggest that a reduction in BMI from overweight to normal weight may increase the risk of future HF and raise the question of whether overweight in later middle-aged women is protective of the development of HF compared to women of normal weight. This could mimic the obesity paradox where a normal BMI in HF patients resulted in higher mortality compared to HF patients with overweight or obesity, but for HF patients with high CRF, survival was very good regardless of BMI. With regard to the development of HF, one could thus argue that it may be preferable for late middle-aged women to focus on fitness instead of weight reduction. Using only BMI for prediction of health outcome may be misleading unless CRF is taken into consideration according to one study on women [21], where the authors showed an almost linear relationship between BMI and moderate or high level of fitness. Around 90% of women with BMI 18.5–21 had a moderate or high level of CRF, whereas around 50% of women with BMI 31.1–33.0 and only 20% of women with BMI >37 had a moderate or high CRF, thus indicating that BMI may be a marker of fitness. Yet all-cause mortality was lower among the women with moderate or high CRF regardless of BMI, which could be parallel to our results in later middle-aged women, showing that a change in BMI per se does not affect the risk of developing HF, but rather that the level of physical activity or a change in the physical activity level is of greater importance.
Our study showed that staying physically active significantly reduced the risk of HF in women, both in the observations 1968 to 1980 and in the observations 1980 to1992. We also found that women who increased their level of physical activity from non-active to active among those participating 1980 to 1992 significantly reduced their risk of developing HF later in life. For the women examined between 1968 and 1980, there was no significant change in risk of HF despite an increase in physical activity from non-active to active. However, this could be an effect of the low number of women in this category. It may also be an effect of possible differences in fitness as well as age at baseline among the cohorts. There was no significant change in risk of HF in women with a decrease in physical activity from active to non-active.
When taking other confounding factors into consideration and excluding women who suffered a myocardial infarction prior to the HF diagnosis, the strong effect of physical activity in reducing the risk for later HF still remained in the younger group, which again emphasizes the importance of being active in younger ages. Also, the significantly higher risk for future HF remained among those women who increased their BMI from overweight to obese in the younger group. Adjusting for s-triglycerides, s-cholesterol, smoking and hypertension in this analysis did not affect the results concerning physical activity to any major extent. As for the older women, the association between high or increased level of physical activity and significantly reduced risk for later HF remained strong and robust when we excluded the women with a myocardial infarction prior to HF. When also adjusting for s-triglycerides, s-cholesterol, smoking and hypertension, the effect of physical activity was weakened, and the most important confounding factor was smoking. The smaller effect could be a result of the relatively small size of the sample, with insufficient power for performing sub-analyses. It may also be a result of the severely harmful effect of long-term smoking on the cardiovascular system that cannot be sufficiently reversed by the positive effect of physical activity.
A strength of this study is the prospective design and long observation time, 44 years for the women participating in the examinations 1968 to1980, and 32 years for the women participating in the examinations 1980 to1992. Another strength is the generally high participation rate and population-based sampling, which enable us to generalize our results to the female population of those ages. However the study is not without limitations including the fact that few women reduced their BMI, and few women increased their level of activity in the group examined in 1968 to 1980. This may explain the non-significant outcomes for these categories.
Being overweight or obese is a well-known risk factor for cardiovascular disease. Having normal weight, i.e. BMI < 25 in younger years and young middle age, greatly reduces the risk of developing cardiovascular diseases; the same is true for individuals having a high level of physical activity. Physical activity is now an obvious part of the treatment for patients suffering from HF and cardiovascular disease and is recommended in the European guidelines for treatment of HF and cardiovascular disease [22]. Yet, the obesity paradox implicates that when patients with established HF show reduction of BMI, they are at higher risk of premature death [18]. However, the possible impact on risk for later HF by a change in BMI or change in physical activity in overweight or obese but otherwise healthy women has not been thoroughly studied, which was the aim of this study where we show that lifestyle changes and preventive actions are very important to reduce the risk of future development of HF in women. We also show that the effect of change in various preventive actions and lifestyle may differ between women of different ages.
Conclusions
Reduced risk of future HF in healthy women may be achieved by remaining physically active from young middle age and throughout life or by increasing the level of physical activity. Increasing the level of physical activity is particularly important for sedentary women in middle age. The role of physical activity in preventing the development of obesity must be taken into account.
Funding Statement
This work was supported by grants from the Swedish Council for Health, Working Life and Welfare – FORTE (EpiLife 2006-1506, WISH 2007-1958) and by grants from the Swedish state under the agreement between the Swedish government and the country councils, the ALF-agreement [68771 and 30411].
Ethical approval
The Prospective Population Study of Women in Gothenburg was approved by the ethical review board of the University of Gothenburg. The studies comply with the Declaration of Helsinki and informed consent has been obtained from the subjects.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292(3):344–350. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125(1):188–197. [DOI] [PubMed] [Google Scholar]
- 4.Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20(6):447–455. [PubMed] [Google Scholar]
- 5.Zarrinkoub R, Wettermark B, Wandell P, et al. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail. 2013;15(9):995–1002. [DOI] [PubMed] [Google Scholar]
- 6.Redfield MM, Jacobsen SJ, Burnett JC Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123–1133. [DOI] [PubMed] [Google Scholar]
- 8.Cook C, Cole G, Asaria P, et al. The annual global economic burden of heart failure. Int J Cardiol. 2014;171(3):368–376. [DOI] [PubMed] [Google Scholar]
- 9.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. [DOI] [PubMed] [Google Scholar]
- 10.Fall T, Hagg S, Magi R, et al. ; for the European Network for Genetic and Genomic Epidemiology (ENGAGE) consortium . The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med. 2013;10(6):e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halldin AK, Schaufelberger M, Lernfelt B, et al. Obesity in middle age increases risk of later heart failure in women-results from the prospective population study of women and H70 studies in Gothenburg, Sweden. J Card Fail. 2017;23(5):363–369. [DOI] [PubMed] [Google Scholar]
- 12.Pandey A, Garg S, Khunger M, et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation. 2015;132(19):1786–1794. [DOI] [PubMed] [Google Scholar]
- 13.Rahman I, Bellavia A, Wolk A. Relationship between physical activity and heart failure risk in women. Circ Heart Fail. 2014;7(6):877–881. [DOI] [PubMed] [Google Scholar]
- 14.Public Health Agency of Sweden. Overweight and obesity. Public health reports and statistics [a ccessed 2019 Aug 23]. Available from: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/tolkad-rapportering/folkhalsans-utveckling/halsa/overvikt-och-fetma/
- 15.Waller M, Lissner L, Hange Sund V, et al. Socioeconomic disparities in physical activity among Swedish women and trends over time – the population study of women in Gothenburg. Scand J Prim Health Care. 2018;36(4):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenzen MJ, Rosengren A, Op Reimer WJMS, et al. Management of patients with heart failure in clinical practice: differences between men and women. Heart. 2008;94(3):e10–e10. [DOI] [PubMed] [Google Scholar]
- 17.Pascual M, Pascual DA, Soria F, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89(10):1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oreopoulos A, Padwal R, Kalantar-Zadeh K, et al. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156(1):13–22. [DOI] [PubMed] [Google Scholar]
- 19.Lavie CJ, Cahalin LP, Chase P, et al. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88(3):251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pina IL, Bittner V, Clare RM, et al. Effects of exercise training on outcomes in women with heart failure: analysis of HF-ACTION (Heart Failure-A Controlled Trial Investigating Outcomes of Exercise TraiNing) by sex. JACC Heart Fail. 2014;2(2):180–186. [DOI] [PubMed] [Google Scholar]
- 21.Farrell SW, Braun L, Barlow CE, et al. The relation of body mass index, cardiorespiratory fitness, and all-cause mortality in women. Obes Res. 2002;10(6):417–423. [DOI] [PubMed] [Google Scholar]
- 22.Ponikowski P, Voors AA, Anker SD, Bueno H, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891–975. [DOI] [PubMed] [Google Scholar]
- 23.Bengtsson C, Blohme G, Hallberg L, et al. The study of women in Gothenburg 1968-1969 – a population study. General design, purpose and sampling results. Acta Med Scand. 2009;193(1–6):311–318. [DOI] [PubMed] [Google Scholar]
- 24.Bengtsson C, Gredmark T, Hallberg L, et al. The population study of women in Gothenburg 1980-81 – the third phase of a longitudinal study. Comparison between participants and non-participants. Scand J Soc Med. 1989;17(2):141–145. [DOI] [PubMed] [Google Scholar]
- 25.Bengtsson C, Ahlqwist M, Andersson K, et al. The Prospective Population Study of Women in Gothenburg, Sweden, 1968-69 to 1992-93. A 24-year follow-up study with special reference to participation, representativeness, and mortality. Scand J Prim Health Care. 1997;15(4):214–219. [DOI] [PubMed] [Google Scholar]
- 26.Carlsson G. Social mobility and class structure. Lund (Sweden): CWK Gleerup; 1958. [Google Scholar]
- 27.Grimby G, Borjesson M, Jonsdottir IH, et al. The “Saltin-Grimby Physical Activity Level Scale” and its application to health research. Scand J Med Sci Sports. 2015;25(Suppl 4):119–125. [DOI] [PubMed] [Google Scholar]
- 28.Lissner L, Bengtsson C, Bjorkelund C, et al. Physical activity levels and changes in relation to longevity. A prospective study of Swedish women. Am J Epidemiol. 1996;143(1):54–62. [DOI] [PubMed] [Google Scholar]
- 29.WHO. Obesity and overweight [accessed 2019 Mar 3] . Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html
- 30.Ingelsson E, Arnlov J, Sundstrom J, et al. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7(5):787–791. [DOI] [PubMed] [Google Scholar]