Abstract
Study Design.
Test the effect of Cartilage Oligomeric Matrix Protein (COMP) on enhancing rhBMP-2 induced spinal fusion in a prospective 8-week interventional trial of spinal fusion in rats.
Objective:
To determine whether the amount of BMP-2 required to achieve spinal fusion in a pre-clinical model can be reduced by the addition of COMP.
Summary of Background Data:
Bone morphogenetic proteins (BMPs) are applied clinicallyat supraphysiological doses to promote spinal fusion by inducing osseous growth, but dose-related limitations include ectopic bone formation and local inflammatory reactions. COMP is a matricellular BMP-binding protein expressed during endochondral ossification and fracture healing. In-vitro studies demonstrate enhanced activity of BMP bound to COMP. We hypothesized that BMP bound to COMP could achieve equivalent spinal fusion rates at lower doses and with fewer complications.
Methods.
Posterolateral intertransverse process spinal fusion at L4–L5 was performed in 36 Lewis rats. COMP (10μg) was tested with or without “low-dose” rhBMP-2 (2μg), and the results were compared with the “low dose “ (2μg rhBMP-2) and “high-dose” (10μg rhBMP-2) groups. All groups utilized insoluble collagen bone matrix carrier (ICBM). Fusion was evaluated by radiology, histology, and manual palpation. BMP release kinetics were evaluated in-vitro.
Results:
Fusion grading of microCT images demonstrated that the fusion rate with the COMP+LoBMP was statistically equivalent to HiBMP, and significantly better than LoBMP without COMP. These results were confirmed with radiographs and manual palpation. BMP release kinetics suggest that COMP increased local concentrations of BMP due to decreased growth factor retention on the scaffold.
Conclusions.
COMP enhances BMP-induced bone formation, enabling lower doses of BMP to achieve the same level of spinal fusion. COMP may function by affecting the availability and biological presentation of BMP-2. A decrease of BMP-2 required for fusion may reduce dose-related adverse effects, surgical costs, and improve clinical outcomes.
Keywords: Spinal Fusion, Rat Model, Bone Morphogenetic Matrix Protein, BMP-2, INFuse, Cartilage Oligomeric Matrix Protein, COMP, Bone Formation, Extracellular Matrix Proteins, Matricellular Protein, Growth Factor Delivery, Dose Reduction
Introduction
Spinal fusion is a widely accepted procedure first described by Hibbs1 and Albee2 in 1911. Between 1998 and 2008, the annual number of spinal fusion discharges in the United States has increased 2.4-fold (137%) from 174,223 to 413,171 and the national bill for spinal fusion increased7.9-fold.3 However, one significant complication in spinal fusions are non-unions (pseudoarthroses),4 which may account for up to 23% of revision lumbar spinal surgeries.5 Surgical strategies to minimize the incidence of non-union include instrumentation and improved bioactivity of the graft material. Iliac crest bone graft (ICBG) represents the gold standard for graft material but is limited by both harvesting morbidity and availability.6 Osteobiologics such as bone morphogenetic protein-2 (BMP-2) can be superior to ICBG in achieving spinal fusion.7,8 FDA-approved indications for BMP-2 use are restricted to fusion of the lumbar spine for skeletally mature patients with degenerative disc disease at one level from L2-S1. However, the success of BMP-2 in promoting spinal fusion led to its widespread “off-label” use. Adverse side effects including heterotopic bone formation, local infection and swelling, nerve pain, impotence and cancer have been reported,9–11 and in fact the FDA issued a 2008 public health notification concerning life-threatening complications associated with “off-label” BMP use in cervical spine fusion12. Although some of these complications may not be solely attributed to the off label use of BMP,13–15 there is a clinical need to minimize the large doses of BMPs and expand its use in spinal fusion surgeries.
In a clinical setting, purified rhBMP-2 (INFUSE, Medtronic) is reconstituted with sterile water and placed on an absorbable carrier (bovine collagen sponge) that is then applied into the surgical site. Thus, the BMP is presented in the surgical site primarily as a soluble growth factor, and supraphysiological doses of 3.5 to 20mg per fusion level are commonly applied to induce adequate bone formation.16 This is in stark contrast to endogenous BMPs, which are present in very low amounts (2–30μg per kilogram bone17,18) and tightly bound to instructive matrix molecules that help limit their bioavailability and direct appropriate cellular responses. Perhaps by more closely mimicking the biological context surrounding endogenous BMPs, we can lower the adverse effects of recombinant BMPs, reduce the dose necessary for achieving spinal fusion, and possibly also lower costs associated with high doses of recombinant human proteins.
Matricellular proteins have a modular composition and function by concurrently binding to structural proteins in the matrix, cell surface receptors, remodeling proteinases, and cytokines.19,20 Cartilage Oligomeric Matrix Protein (COMP) is a matricellular protein involved in the assembly of collagen fibrils and other structural matrix components. COMP interacts with structural matrix components including collagens and proteoglycans, cell surface receptors such as integrins and CD47, remodeling proteinases such as MMPs and ADAMTSs, and growth-factors including TGF-beta21 and BMPs (reviewed in Acharya et al22). COMP is expressed by hypertrophic chondrocytes and osteoblasts during endochondral ossification,23 and its expression is increased during fracture healing.24 Recent work from our group demonstrated that COMP binds to BMP-2, and such binding increases the osteogenic activity of BMP-2 both in-vitro, and in an ectopic bone formation assay in-vivo.25
Based on this evidence, our hypothesis is that BMPs bound to COMP will better approximate the biological context of endogenous BMPs to more efficiently direct cellular differentiation towards osteogenesis. The goal of this study is to determine whether COMP, together with BMP-2, will reduce the dose of BMP-2 required for spinal fusion in a clinically relevant rat model.
Materials and Methods
Implant preparation
Recombinant human COMP was prepared from stably transduced 293 T cells as described previously.21,26 Inactive collagenous demineralized bone matrix (ICBM) was kindly provided by Dr. A. Hari Reddi.27,28 ICBM alone is unable to induce endochondral bone differentiation in the absence of additional growth factors29 and was used as a substratum in every group. Recombinant human BMP-2 was purchased in the form of INFUSE® Bone Graft (Medtronic, Memphis, TN). Infuse is not labeled for the use under discussion. BMP-2 and COMP were diluted with PBS to a final volume of 200μl. The negative control group was composed of 200μl PBS. The growth factor solutions were pipetted onto 25mg of ICBM, allowed to bind 30 minutes, snap-frozen in liquid nitrogen, and lyophilized overnight.
Treatment groups differed only by the materials added to the ICBM carrier (Table 1). These doses are based on previous experience in our group suggesting that the 10μg BMP-2 leads to near 100% fusion, while the 2μg BMP induces new bone but consistently incomplete fusion.
Table 1:
Experimental groups and animal numbers for each group.
| Group | Implant Composition | ||
|---|---|---|---|
| ICBM (n=4) | – | – | ICBM |
| COMP (n=6) | 10μg COMP | – | ICBM |
| LoBMP (N=10) | – | 2μg BMP | ICBM |
| HiBMP (n=6) | – | 10μg BMP | ICBM |
| COMP+LoBMP (n=10) | 10μg COMP | 2μg BMP | ICBM |
Surgical Procedure
Animal protocols were approved by the UC Davis Animal Use and Care Advisory Committee, and surgeries were performed according to the UC Davis IACUC policy on survival surgery and all National Institutes of Health animal handling protocols. 36 male Lewis rats (10–12 weeks old) were randomly divided into 5 subgroups. A posterior midline incision was made from L4-L7. Fascial incisions were made 2 to 3 cm on each side of the midline, and the transverse process of L4 to L5 and the intertransverse membrane were exposed. The dorsal aspects of L4 to L5 transverse processes were decorticated using a high-speed burr. Grafts were placed bilaterally in the paraspinal muscle bed, between and touching the transverse processes of L4 and L5. The rats were managed with subcutaneous injections of buprenorphine (0.05mg/kg) for the control of perioperative and postoperative pain, and allowed to eat and drink ad libitum.
Graft Harvest and Manual Palpation
Eight weeks post-surgery, rats were euthanized by carbon dioxide inhalation and their lumbar spines were harvested whole. Spines were visually examined and manually palpated to assess the mechanical integrity of the fusion qualitatively. The extent of fusion was graded by 3 orthopaedic surgeons according to the following scale: Grade 0=no fusion; Grade 1=partial fusion; Grade 2=complete fusion.
Micro-CT Analysis
After dissection, the spine tissue was fixed in 10% neutral-buffered formalin for five days and stored in 70% ethanol. Spines were scanned using micro-computed tomography (microCT, ScanCo μCT 35, Bassersdorf, Switzerland), with imaging settings according to the guidelines for analysis of rodent bone structure, at a resolution of 37μm in all dimensions, with 55kVp, 145μAmp, and 3 averages of 300ms integration time.30 The extent of spinal fusion was assessed from dorsal and ventral view 3D renderings for each rat by three blinded graders using the following scale: Grade 0=no bone present between the transverse processes bilaterally; Grade 1=bone mass on one side only; Grade 2=bone mass bilaterally with lucency bilaterally; Grade 3=bone mass bilaterally with lucency on one side only; Grade 4=bridging bone with no gaps or lucent lines bilaterally.
Quantitative parameters were evaluated from the microCT by creating a region of interest (ROI) contoured to L4 and L5 transverse processes on left and right sides separately. Bone volume (BV), bone volume fraction (BVF) and tissue mineral density (TMD) were calculated in software (ScanCo). BV is a measure of total volume of mineralized tissue in the ROI (“mineralized” defined as density > 375mgHA/cc). BVF is the ratio of bone volume to the total volume of the ROI. TMD is the mean density of the mineralized tissue.
Radiographic Assessment of Fusion
Fusion was assessed every other week using posteroanterior radiographs, which were graded by 3 trained blinded observers using the following scale: Grade 0=no bone present between the transverse processes bilaterally; Grade 1=minimal fusion; Grade 2=unilateral fusion; Grade 3=bilateral fusion.
Histology
After microCT analysis, samples were decalcified with 10% formic acid in citrate, and processed for standard paraffin embedding. Serial sagittal sections were cut from the center of the fusion mass (4-μm thick slices). The sectioned tissues were stained sequentially with hematoxylin and eosin (H&E) and evaluated for endochondral ossification. Photomicrographs were acquired using a Leica model EZ4D stereomicroscope, and multiple images assembled using the Photomerge function of Adobe Photoshop CS5.5.
BMP Release Kinetics from COMP/ICBM
BMP-2 (2 or 10μg per tube) in the presence or absence of COMP was added to ICBM as described above and lyophilized overnight (n=3 per condition). The lyophilized product was suspended in 1 mL PBS and maintained at 37°C. The entire volume of PBS was collected and refreshed at designated time points over 21 days and frozen until analysis. The release of BMP-2 was quantified using a human protein specific ELISA kit (R&D Systems, Minneapolis, MN) per manufacturer’s instructions. Data were normalized to the total amount of BMP-2 released over the 3-week study duration for each condition.
Statistical Analysis
CT and palpation grades were compared between groups using Welch’s ANOVA, which allows for unequal variances, followed by Games-Howell post hoc testing. Quantitative CT parameters were compared using an ANOVA with fixed effects for group, side (left and right) and a random effect for rat. X-ray data were analyzed using a longitudinal model including fixed effects for group, time, and their interaction and a random effect for rat. Tukey pairwise comparisons of groups at each timepoint were conducted as contrasts within this model. Statistical analyses were conducted using the statistical software environment R, version 3.1.0 (R Core Team, 2014). Longitudinal modelling was conducted using the R package nlme, version 3.1–11731. Significance was set at p<0.05.
Results
Of the 36 rats in the study, 34 were included for the data analysis, and two rats were excluded because fusion occurred at different levels. All rats survived the surgical procedure with no wound complications, abnormal behavior, or deaths noted. There were no obvious adverse reactions to COMP. None of the rats showed any neurologic deficits before or after the surgical procedure, or during the 8-week follow-up period.
The extent of spinal fusion was most accurately determined from high-resolution 3-dimensional microCT reconstructions, and graded as shown in Figure 1. Essentially complete fusion was observed in the HiBMP group, in agreement with our previous experience using this dose of BMP2 in spinal fusion. The low dose of BMP induced limited new bone formation and resulted in partial fusion in all animals. COMP significantly enhanced the extent of fusion induced by LoBMP, to the same extent seen in the positive control group (Figure 2A). No bone formation was observed in the ICBM or COMP groups. These observations suggest that COMP can augment the activity of BMP to enhance spinal fusion, but COMP alone does not induce bone formation.
Figure 1:
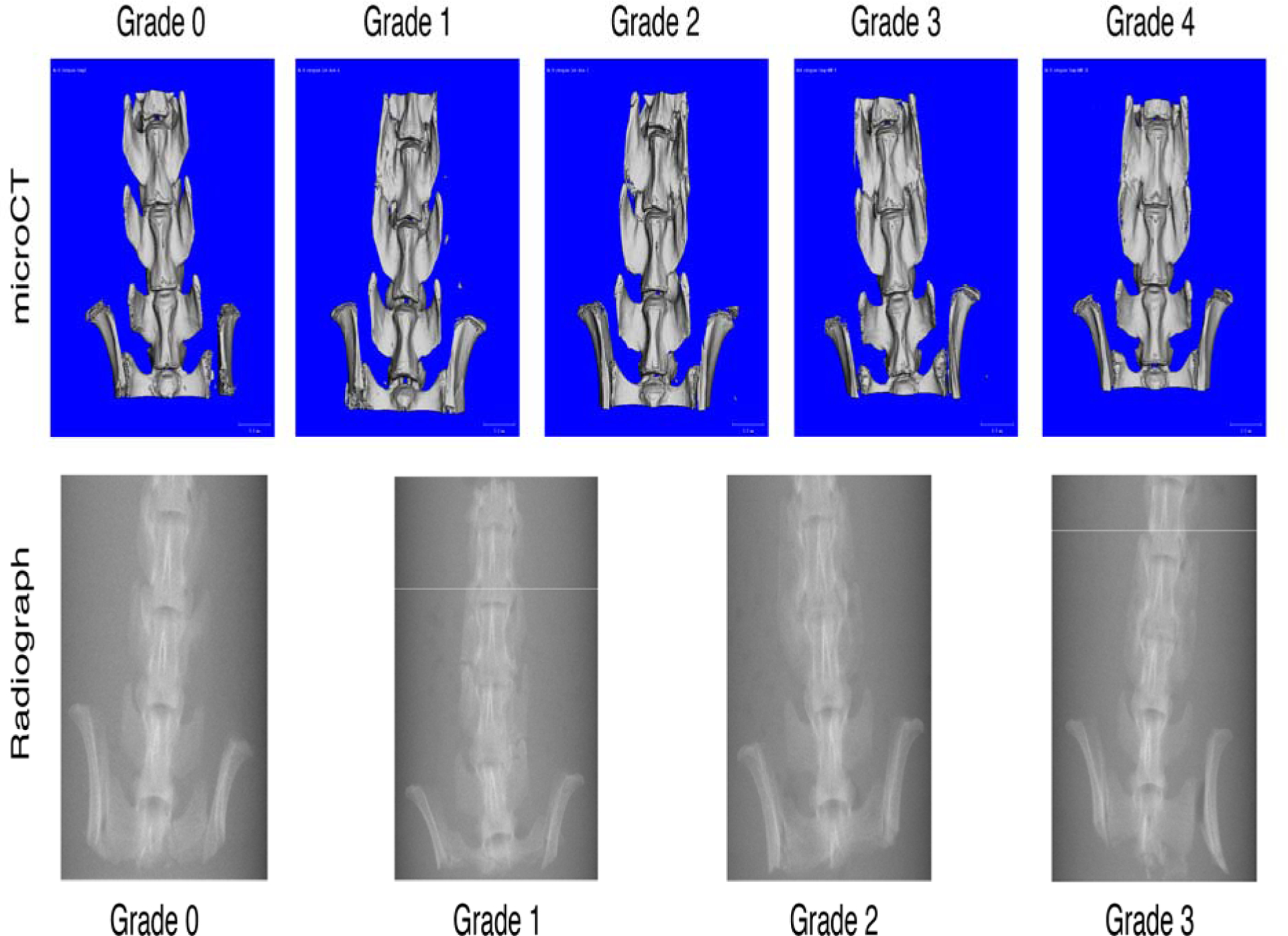
Grading Scale for Assessing Fusion by MicroCT and Plain Radiographs. For the representative images shown, the grades were agreed upon by all blinded evaluators.
Figure 2.
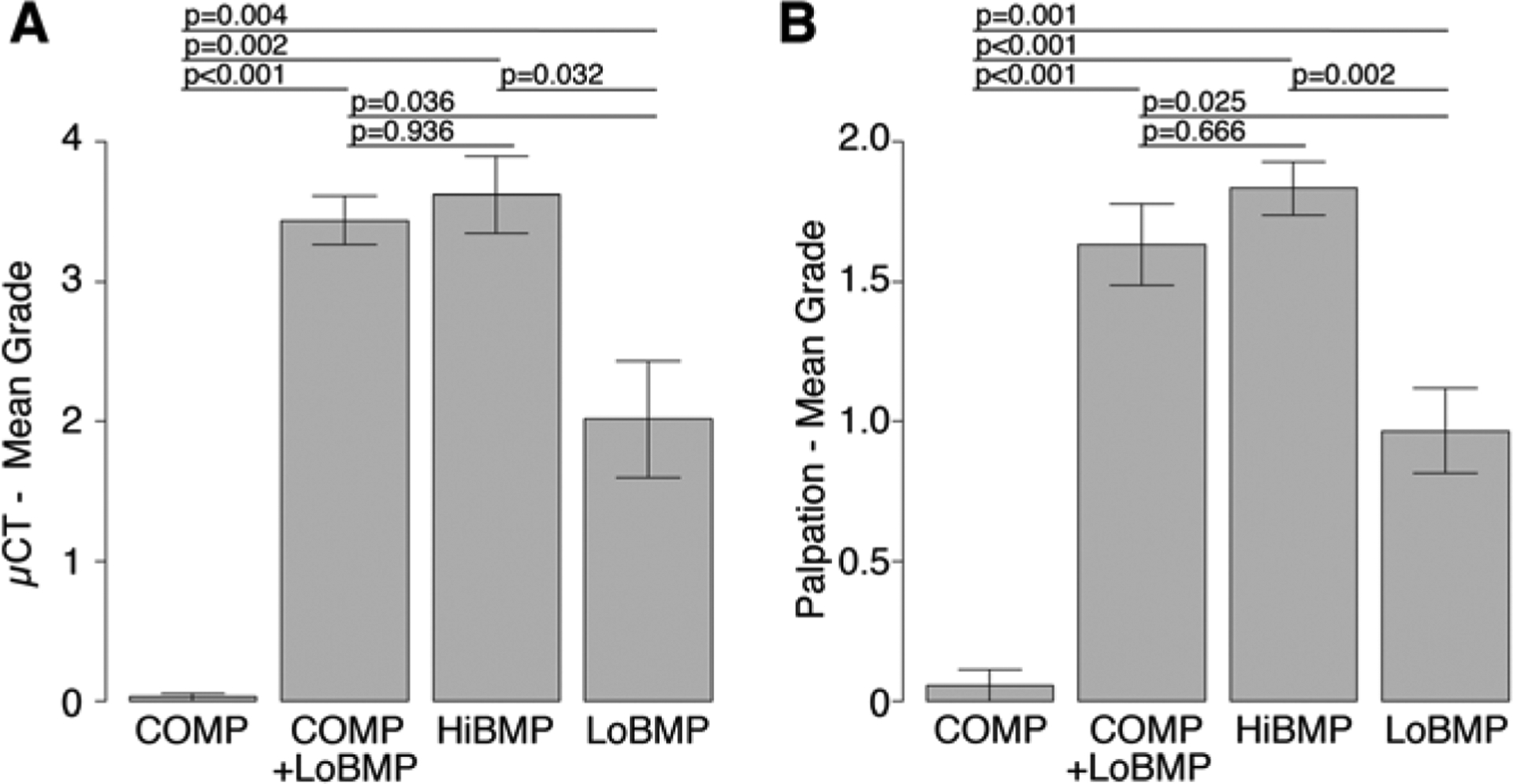
A) Spinal Fusion assessed at 8 weeks by microCT. COMP alone did not promote spinal fusion. HiBMP resulted in almost complete fusion, while LoBMP resulted in partial fusion. COMP+LoBMP resulted in fusion to a similar extent as HiBMP. B) Manual palpation at 8 weeks shows HiBMP and COMP+LoBMP having similar mechanical resistance to bending. Data are mean ± standard deviation
To assess the mechanical integrity of the spinal fusions semi-quantitatively, the dissected spines were palpated manually. The results confirm that the positive control (HiBMP) and the experimental group (LoBMP+COMP) had a similar extent of bone formation and resistance to bending articulation, which was greater than that observed in the LoBMP and COMP groups (Figure 2B).
To assess the rate of fusion over time, biweekly radiographs were graded. Bone growth progressed to near complete fusion in the HiBMP and LoBMP+COMP groups (Figure 3). The differences between these groups were not significant at any time point. LoBMP induced bone formation but incomplete fusion, and the extent of fusion in this group was statistically less than the HiBMP and LoBMP+COMP groups at weeks 4 and later. COMP alone had an insignificant effect on spinal fusion. These results confirm the microCT data that COMP reduces the amount of BMP required to induce spinal fusion at 8 weeks, with the added insight that fusion progresses on a similar time scale as the HiBMP group.
Figure 3.
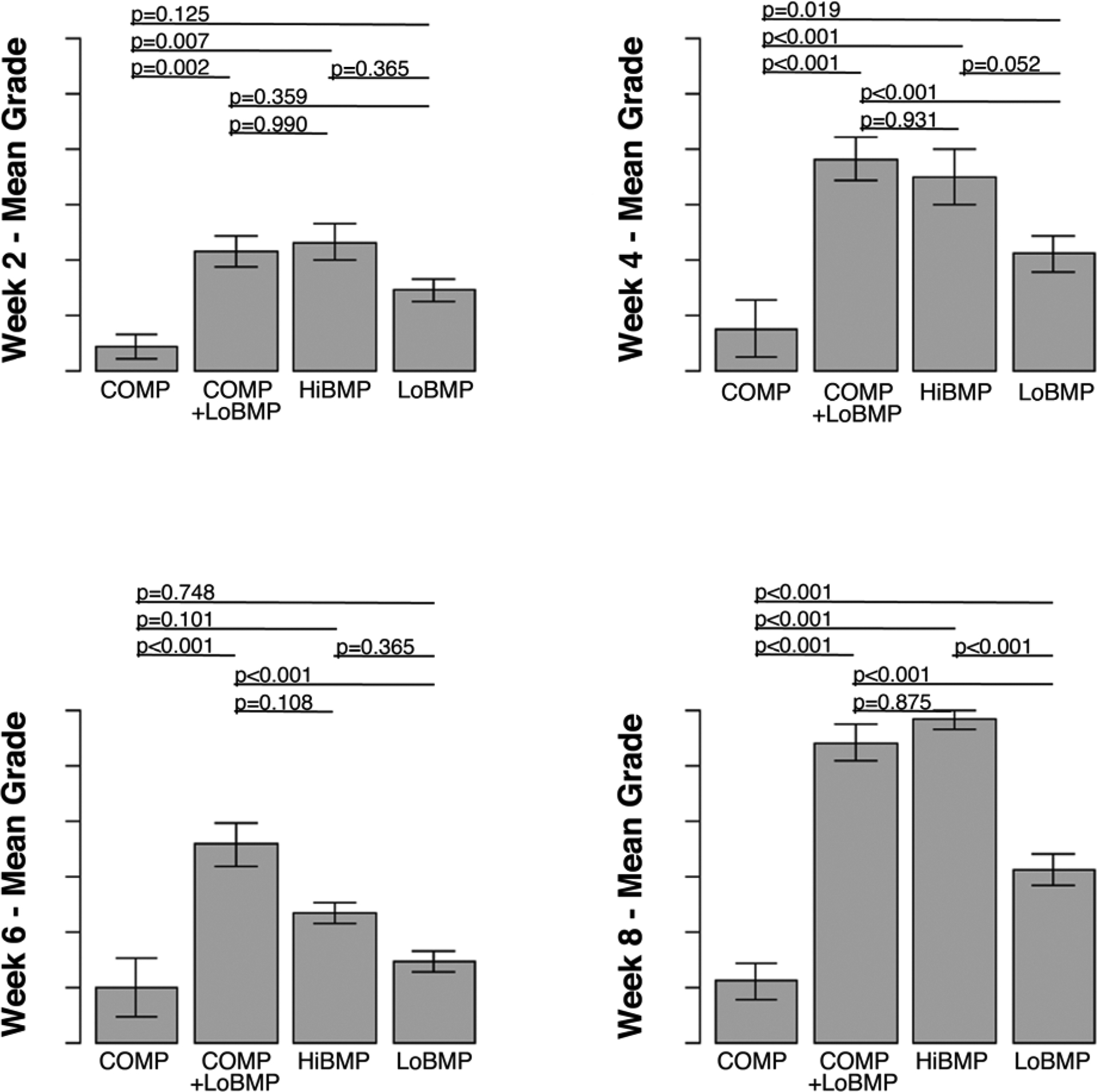
Time course of fusion by plain X-rays at 2-week intervals. LoBMP resulted in significantly greater fusion in the presence of COMP at 4 week and later time points. Fusion grade in the LoBMP+COMP group and was not distinguishable from the extent of fusion in the HiBMP group at 8 weeks, and significantly greater than the LoBMP alone group. Data are mean ± standard deviation.
In addition to determining the extent of fusion between adjacent vertebrae, we also examined the quality of the newly formed bone from the microCT scans. The quantitative measure of bone volume (BV) showed that HiBMP and LoBMP were significantly higher than COMP alone (Figure 4). There was a trend (p<0.10) that the bone volume was less in COMP+LoBMP than either HiBMP or LoBMP. The tissue mineral density was less in the COMP+LoBMP group than in the COMP or HiBMP group. There was no difference in Bone Volume Fraction between any of the groups with BMP, and differences in TMD did not reach statistical significance.
Figure 4.
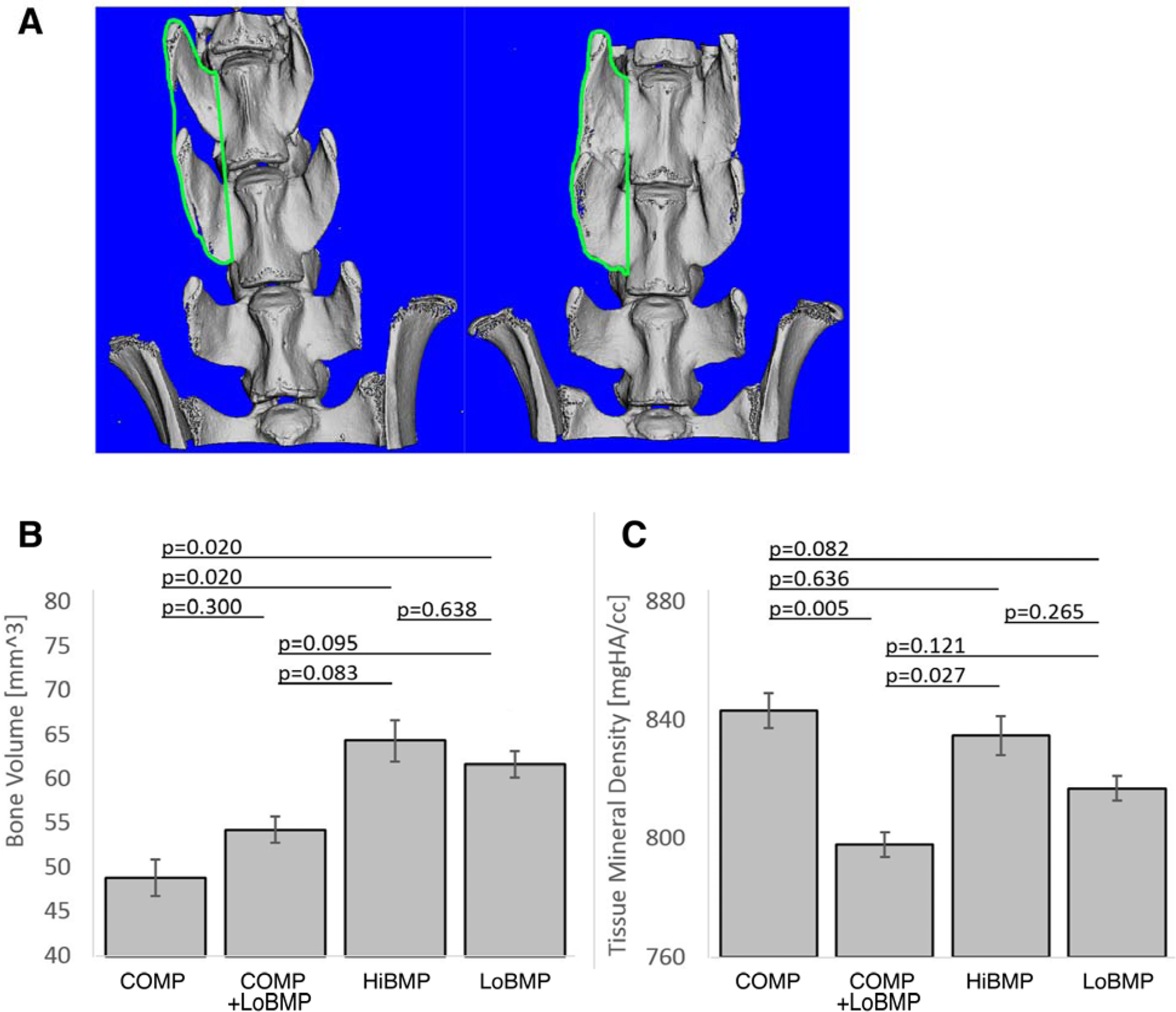
The quality of the bone formed as assessed at 8 weeks by quantitative microCT of a ROI surrounding L4 and L5. A) Depiction of the region of interest analyzed in the quantitative analysis of microCT. ROI is shown in a representative case treated with COMP alone showing no fusion (left) and HiBMP showing complete fusion (right). B) Mean bone volume and C) mean tissue mineral density. Data are mean ± standard deviation
Finally, we examined whether COMP affects the in vitro release kinetics of BMP-2 from ICBM to provide insight into the causes of difference in bone formation. Based on total eluted mass, BMP-2 was released much faster when complexed with COMP than when directly adsorbed to ICBM (Figure 6). As expected, initial loading of ICBM with more BMP-2 yielded an increase in the total amount of BMP-2 released. As a percentage of total initial dose, we observed similar relative mass of BMP-2 released from ICBM when complexed with COMP (7% of 2ug BMP, 11% of 10ug BMP). However, the adsorption of free BMP to ICBM resulted in large differences in total BMP released as a function of initial loading dose (0.4% of 2ug, 18% of 10ug) under the conditions studied.
Figure 6:
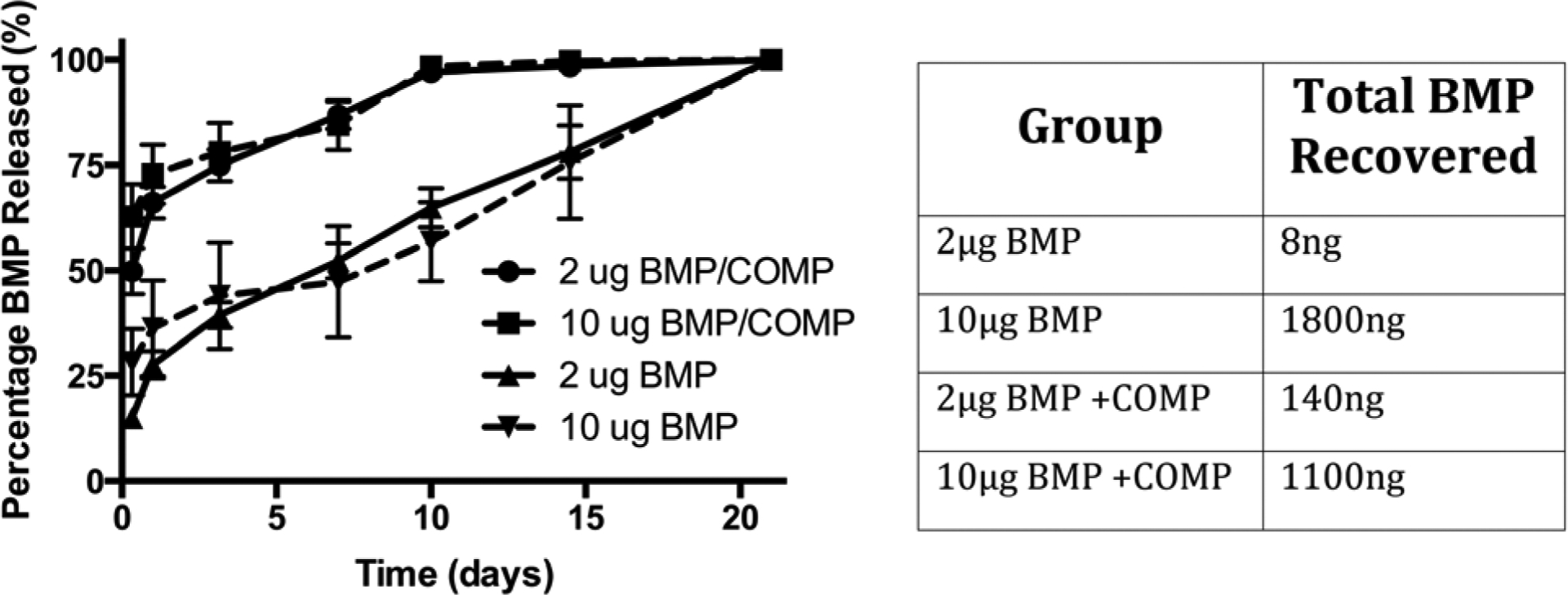
Elution kinetics of BMP-2 from ICBM with or without COMP. The presence of COMP significantly enhanced the elution kinetics of BMP-2, as well as the total mass of BMP eluted from the ICBM at the lower dose. Data are mean ± standard deviation, n=3 per condition.
Discussion
Bone formation in spinal fusion involves a coordinated interplay between an osteoconductive scaffold, osteoprogenitor cells, and growth factors such as BMPs. The objectives of this study were to determine whether providing a more biologically relevant context for exogenous BMP-2, by binding it to the matricellular protein COMP, would result in a higher biological activity of BMP-2 and reduce the dose required for spinal fusion in a rat model. The results demonstrate that this approach was successful. In the presence of COMP, the delivery of 2μg BMP-2 achieved a similar outcome to 10μg of BMP-2 alone.
The clinical success of rhBMP-2 in anterior spinal fusion32 has increased its off-label use, but with significant complications11,33,34 and culminating in a series of articles calling for further research to evaluate the early- and long-term complications.33,35 The doses of BMP-2 (2.5–20mg) used for adult spinal deformity are high compared to the BMP amounts in endogenous bone (2–30μg/kg bone),17,18,36,37 which may contribute to the complications.
Several approaches are being tested to minimize complications by lowering doses of BMP-2 while still maintaining its effectiveness. One approach mixes BMP-2 with additional growth factors, for example NEL-like molecule-1 (Nell-1) has been successful in pre-clinical studies.38–40 A second approach increases the in-vivo half-life and local retention of BMP-2 using “slow-release” agents such as synthetic BMP Binding Peptide41–43 or creating a synthetic fusion protein of rhBMP-2 with a collagen-binding domain.44 However, using heparin-conjugated fibrin as a slow-release delivery vehicle was not successful at reducing the BMP dose.45 A third approach is the addition of bulking agents, such as biphasic calcium phosphate ceramic/collagen compression resistant matrix to BMP.46–48 Similarly, bone marrow aspirate can improve the activity of low dose BMPs.49 Mixed results were obtained by reducing endogenous BMP antagonists using noggin siRNA delivered at the site of spinal fusion.50
Many of these studies provide BMP as an unbound growth factor, a context that does not occur naturally. Our approach was to more closely mimic the biological context surrounding endogenous BMPs, by providing the growth factor pre-bound to an instructive matrix molecule (COMP). While we do not expect COMP to directly modify osteogenic gene expression, we hypothesized that COMP affects BMP bioavailability and directs the appropriate cellular responses. We observed that while COMP itself did not induce bone formation, adding COMP to 2μg BMP2 increased the fusion rate statistically similar to that of the 10μg BMP2 group. The results of manual palpation and microCT correlated well with radiographic outcomes at the conclusion of the study, and histological analysis at 8-weeks showed normal new bone formation.
The quality of bone within a region-of-interest (ROI) drawn between adjacent vertebrae was semi-quantitatively assessed by microCT, with the unexpected observation of somewhat lower bone volume and tissue mineral density in the COMP+LoBMP group than the HiBMP group. We were unable to consistently differentiate new bone from old bone, thus the ROI includes a combination of both. Therefore the BV and TMD measurements reflect a combination of the bone quality in both native and newly formed bone, and are affected by the amount of new bone growth between processes. While this limitation may help explain the unexpected observations, these assays of bone quality are independent of the successful fusion of adjacent vertebrae.
The in-vitro elution kinetics were compared between BMP-2 loaded directly on ICBM versus BMP-2+COMP on ICBM. COMP increased the elution kinetics of BMP-2, with a much greater percentage released during the first few days. BMP-2 was completely eluted by day 10 in the COMP groups, while it continued to elute from the ICBM groups. At the lower concentration, COMP greatly enhance the total amount of BMP-2 recovered. In all cases, the majority of BMP-2 was not recovered from the ICBM. It is unknown whether the BMP-2 remained on the ICBM, or was denatured to the extent that it could not be detected by the ELISA assay employed to quantify the BMP-2. One limitation of the ELISA assay is that it is insensitive to the biological activity of the BMP. Differences in elution may be related to growth factor binding to the substrate. It is likely that BMP binds more effectively to ICBM than the BMP/COMP complex, resulting in differences in adsorption and desorption kinetics from the matrix. Based on the promising results in this preclinical model, we intend to examine these differences as the subject of future work.
Conclusion:
Taken together, these data indicate that COMP enhances that biological activity of BMP and achieves statistically improved rates of fusion in this animal model of posterolateral spinal fusion. These findings illustrate the potential for COMP combined with a lower dose of BMP-2 to achieve similar fusion outcomes as a high dose of BMP-2 alone. COMP may allow for lower costs and fewer BMP dose-related side effects. Furthermore, COMP may be useful when used in conjunction with a number of other growth factors relevant in orthopedic surgery.
Figure 5:
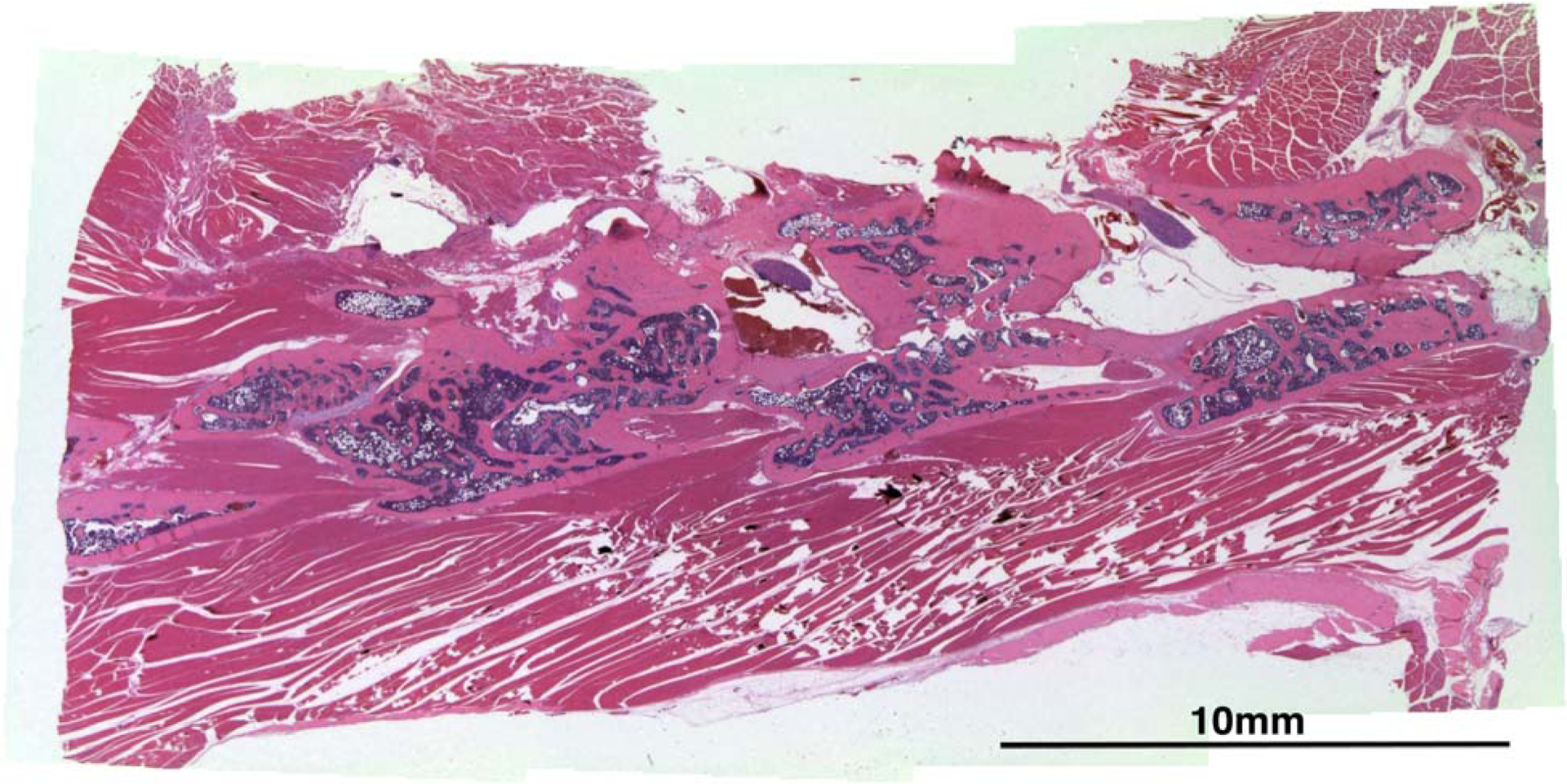
Representative histological section stained with H&E shows bone formation between vertebral bodies from a rat in the COMP+LoBMP group.
Acknowledgments
Professor A. Hari Reddi provided ICBM, the Department of the Army award W81XWH-10-1-0956 to JKL; the UC Davis Academic Senate Committee on Research for pilot award to DRH; and the Denny and Jeanene Dickinson Orthopaedic Research Fellowship, the UC Davis Center for Clinical and Translational Science supported by the National Center for Advancing Translational Sciences (NCATS, through grant #UL-TR000002) for statistical analysis funds were received in support of this work.
Relevant financial activities outside the submitted work: consultancy, grants, payment for lectures, patents.
Footnotes
Publisher's Disclaimer: The manuscript discusses using rhCOMP for augmenting the activity of Medtronic’s Infuse rhBMP2 for spinal fusions, in a rat model. The product is not labeled for the use under discussion in humans or rats, and the use of rhCOMP is not FDA approved
References
- 1.Hibbs RA. An operation for progressive spinal deformities. A preliminary report of three cases from the service of the orthopaedic hospital. N. Y. State J. Med 1911;92:1013–6. [DOI] [PubMed] [Google Scholar]
- 2.Albee FH. Transplantation of a portion of the tibia into the spine for pott’s disease. JAMA 1911;57:885–6. [DOI] [PubMed] [Google Scholar]
- 3.Rajaee SS, Bae HW, Kanim LE, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine (Phila Pa 1976) 2012;37:67–76. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Revision surgery following operations for lumbar stenosis. J Bone Joint Surg Am 2011;93:1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin BI, Mirza SK, Comstock BA, Gray DT, Kreuter W, Deyo RA. Reoperation rates following lumbar spine surgery and the influence of spinal fusion procedures. Spine (Phila Pa 1976) 2007;32:382–7. [DOI] [PubMed] [Google Scholar]
- 6.Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 1996:300–9. [DOI] [PubMed] [Google Scholar]
- 7.Zhang H, Wang F, Ding L, Zhang Z, Sun D, Feng X, An J, Zhu Y. A meta analysis of lumbar spinal fusion surgery using bone morphogenetic proteins and autologous iliac crest bone graft. PLoS One 2014;9:e97049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HJ, Buchowski JM, Zebala LP, Dickson DD, Koester L, Bridwell KH. RhBMP-2 is superior to iliac crest bone graft for long fusions to the sacrum in adult spinal deformity: 4- to 14-year follow-up. Spine (Phila Pa 1976) 2013;38:1209–15. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto S, Takaoka K, Yonenobu K, Ono K. Ossification of the ligamentum flavum induced by bone morphogenetic protein. An experimental study in mice. The Journal of bone and joint surgery 1992;74:279–83. [DOI] [PubMed] [Google Scholar]
- 10.Epstein NE. Pros, cons, and costs of INFUSE in spinal surgery. Surgical neurology international 2011;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein NE. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surgical neurology international 2013;4:S343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA. FDA Public Health Notification: LIfe-Threatening Complications Associated with Recombinant Human Bone Morphogenetic Protein in Cerfical Spine Fusion. Online 2008;http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm062000.htm.
- 13.Michielsen J, Sys J, Rigaux A, Bertrand C. The effect of recombinant human bone morphogenetic protein-2 in single-level posterior lumbar interbody arthrodesis. J Bone Joint Surg Am 2013;95:873–80. [DOI] [PubMed] [Google Scholar]
- 14.Bess S, Line BG, Lafage V, Schwab F, Shaffrey CI, Hart RA, Boachie-Adjei O, Akbarnia BA, Ames CP, Burton DC, Deverin V, Fu KM, Gupta M, Hostin R, Kebaish K, Klineberg E, Mundis G, O’Brien M, Shelokov A, Smith JS, International Spine Study Group I. Does recombinant human bone morphogenetic protein-2 use in adult spinal deformity increase complications and are complications associated with location of rhBMP-2 use? A prospective, multicenter study of 279 consecutive patients. Spine (Phila Pa 1976) 2014;39:233–42. [DOI] [PubMed] [Google Scholar]
- 15.Crandall DG, Revella J, Patterson J, Huish E, Chang M, McLemore R. Transforaminal lumbar interbody fusion with rhBMP-2 in spinal deformity, spondylolisthesis, and degenerative disease--part 2: BMP dosage-related complications and long-term outcomes in 509 patients. Spine (Phila Pa 1976) 2013;38:1137–45. [DOI] [PubMed] [Google Scholar]
- 16.Dawson J, Kiner D, Gardner W 2nd, Swafford R, Nowotarski PJ. The Reamer Irrigator Aspirator (RIA) as a Device for Harvesting Bone Graft Compared with Iliac Crest Bone Graft (ICBG): Union Rates and Complications. J Orthop Trauma 2014. [DOI] [PubMed] [Google Scholar]
- 17.Sampath TK, Muthukumaran N, Reddi AH. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A 1987;84:7109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang EA, Rosen V, Cordes P, Hewick RM, Kriz MJ, Luxenberg DP, Sibley BS, Wozney JM. Purification and characterization of other distinct bone-inducing factors. Proc Natl Acad Sci U S A 1988;85:9484–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bornstein P DIVERSITY OF FUNCTION IS INHERENT IN MATRICELLULAR PROTEINS - AN APPRAISAL OF THROMBOSPONDIN-1. J. Cell Biol 1995;130:503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy-Ullrich JE, Helene Sage E. Revisiting the matricellular concept. Matrix Biol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haudenschild DR, Hong E, Yik JH, Chromy B, Morgelin M, Snow KD, Acharya C, Takada Y, Di Cesare PE. Enhanced activity of transforming growth factor beta1 (TGF-beta1) bound to cartilage oligomeric matrix protein. J Biol Chem 2011;286:43250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acharya C, Yik JH, Kishore A, Van Dinh V, Di Cesare PE, Haudenschild DR. Cartilage oligomeric matrix protein and its binding partners in the cartilage extracellular matrix: interaction, regulation and role in chondrogenesis. Matrix Biol 2014;37:102–11. [DOI] [PubMed] [Google Scholar]
- 23.Di Cesare PE, Fang C, Leslie MP, Tulli H, Perris R, Carlson CS. Expression of cartilage oligomeric matrix protein (COMP) by embryonic and adult osteoblasts. J Orthop Res 2000;18:713–20. [DOI] [PubMed] [Google Scholar]
- 24.Heiner DE, Meyer MH, Frick SL, Kellam JF, Fiechtl J, Meyer RA Jr. Gene expression during fracture healing in rats comparing intramedullary fixation to plate fixation by DNA microarray. J Orthop Trauma 2006;20:27–38. [DOI] [PubMed] [Google Scholar]
- 25.Ishida K, Acharya C, Christiansen BA, Yik JH, DiCesare PE, Haudenschild DR. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone 2013;55:23–35. [DOI] [PubMed] [Google Scholar]
- 26.Tan K, Duquette M, Joachimiak A, Lawler J. The crystal structure of the signature domain of cartilage oligomeric matrix protein: implications for collagen, glycosaminoglycan and integrin binding. Faseb J 2009;23:2490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz RW, Felthousen GC, Reddi AH. Radiation-sterilized insoluble collagenous bone matrix is a functional carrier of osteogenin for bone induction. Calcif Tissue Int 1990;47:183–5. [DOI] [PubMed] [Google Scholar]
- 28.Moazzaz P, Gupta MC, Gilotra MM, Gilotra MN, Maitra S, Theerajunyaporn T, Chen JL, Reddi AH, Martin RB. Estrogen-dependent actions of bone morphogenetic protein-7 on spine fusion in rats. Spine (Phila Pa 1976) 2005;30:1706–11. [DOI] [PubMed] [Google Scholar]
- 29.Reddi AH, Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A 1972;69:1601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 2010;25:1468–86. [DOI] [PubMed] [Google Scholar]
- 31.Pinheiro J, Bates D, DebRoy S, Sarkar D. nlme: Linear and Nonlinear Mixed Effects Models. R Package version 3.1–117. http://CRAN.R-project.org/package=nlme 2014.
- 32.Ong KL, Villarraga ML, Lau E, Carreon LY, Kurtz SM, Glassman SD. Off-label use of bone morphogenetic proteins in the United States using administrative data. Spine (Phila Pa 1976) 2010;35:1794–800. [DOI] [PubMed] [Google Scholar]
- 33.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 2011;11:471–91. [DOI] [PubMed] [Google Scholar]
- 34.Rihn JA, Makda J, Hong J, Patel R, Hilibrand AS, Anderson DG, Vaccaro AR, Albert TJ. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: a clinical and radiographic analysis. Eur Spine J 2009;18:1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carragee EJ, Ghanayem AJ, Weiner BK, Rothman DJ, Bono CM. A challenge to integrity in spine publications: years of living dangerously with the promotion of bone growth factors. Spine J 2011;11:463–8. [DOI] [PubMed] [Google Scholar]
- 36.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542–7. [DOI] [PubMed] [Google Scholar]
- 37.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27:2662–73. [DOI] [PubMed] [Google Scholar]
- 38.Yuan W, James AW, Asatrian G, Shen J, Zara JN, Tian HJ, Siu RK, Zhang X, Wang JC, Dong J. NELL-1 based demineralized bone graft promotes rat spine fusion as compared to commercially available BMP-2 product. J Orthop Sci 2013;18:646–57. [DOI] [PubMed] [Google Scholar]
- 39.Zhu S, Song D, Jiang X, Zhou H, Hu J. Combined effects of recombinant human BMP-2 and Nell-1 on bone regeneration in rapid distraction osteogenesis of rabbit tibia. Injury 2011;42:1467–73. [DOI] [PubMed] [Google Scholar]
- 40.Shen J, James AW, Zara JN, Asatrian G, Khadarian K, Zhang JB, Ho S, Kim HJ, Ting K, Soo C. BMP2-induced inflammation can be suppressed by the osteoinductive growth factor NELL-1. Tissue Eng Part A 2013;19:2390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alanay A, Chen C, Lee S, Murray SS, Brochmann EJ, Miyazaki M, Napoli A, Wang JC. The adjunctive effect of a binding peptide on bone morphogenetic protein enhanced bone healing in a rodent model of spinal fusion. Spine (Phila Pa 1976) 2008;33:1709–13. [DOI] [PubMed] [Google Scholar]
- 42.McGovern SC, Fong W, Wang JC. Can bone morphogenetic protein binding peptide increase efficiency of bone formation? Spine (Phila Pa 1976) 2010;35:1655–9. [DOI] [PubMed] [Google Scholar]
- 43.Sintuu C, Simon RJ, Miyazaki M, Morishita Y, Hymanson HJ, Taghavi C, Brochmann EJ, Murray SS, Wang JC. Full-length spp24, but not its 18.5-kDa proteolytic fragment, inhibits bone-healing in a rodent model of spine fusion. J Bone Joint Surg Am 2011;93:1022–32. [DOI] [PubMed] [Google Scholar]
- 44.Han XL, Zhang W, Gu J, Zhao H, Ni L, Han JJ, Zhou Y, Gu YN, Zhu XS, Sun J, Hou XL, Yang HL, Dai JW, Shi Q. Accelerated Postero-Lateral Spinal Fusion by Collagen Scaffolds Modified with Engineered Collagen-Binding Human Bone Morphogenetic Protein-2 in Rats. Plos One 2014;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koo KH, Lee JM, Ahn JM, Kim BS, La WG, Kim CS, Im GI. Controlled Delivery of Low-Dose Bone Morphogenetic Protein-2 Using Heparin-Conjugated Fibrin in the Posterolateral Lumbar Fusion of Rabbits. Artif. Organs 2013;37:487–94. [DOI] [PubMed] [Google Scholar]
- 46.Barnes B, Boden SD, Louis-Ugbo J, Tomak PR, Park JS, Park MS, Minamide A. Lower dose of rhBMP-2 achieves spine fusion when combined with an osteoconductive bulking agent in non-human primates. Spine (Phila Pa 1976) 2005;30:1127–33. [DOI] [PubMed] [Google Scholar]
- 47.Khan SN, Toth JM, Gupta K, Glassman SD, Gupta MC. Early-term and Mid-term Histologic Events During Single-level Posterolateral Intertransverse Process Fusion With rhBMP-2/Collagen Carrier and a Ceramic Bulking Agent in a Nonhuman Primate Model Implications for Bone Graft Preparation. J. Spinal Disord. Tech 2014;27:212–9. [DOI] [PubMed] [Google Scholar]
- 48.Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine 2006;31:2534–9. [DOI] [PubMed] [Google Scholar]
- 49.Bae HW, Zhao L, Kanim LE, Wong P, Marshall D, Delamarter RB. Bone marrow enhances the performance of rhBMP-2 in spinal fusion: a rodent model. J Bone Joint Surg Am 2013;95:338–47. [DOI] [PubMed] [Google Scholar]
- 50.Klineberg E, Haudenschild DR, Snow KD, Garitty S, Christiansen BA, Acharya C, Maitra S, Gupta MC. The effect of noggin interference in a rabbit posterolateral spinal fusion model. Eur Spine J 2014. [DOI] [PubMed] [Google Scholar]


