Abstract
Background
Bronchiolitis is an acute, viral lower respiratory tract infection affecting infants and is sometimes treated with bronchodilators.
Objectives
To assess the effects of bronchodilators on clinical outcomes in infants (0 to 12 months) with acute bronchiolitis.
Search methods
We searched CENTRAL 2013, Issue 12, MEDLINE (1966 to January Week 2, 2014) and EMBASE (1998 to January 2014).
Selection criteria
Randomized controlled trials (RCTs) comparing bronchodilators (other than epinephrine) with placebo for bronchiolitis.
Data collection and analysis
Two authors assessed trial quality and extracted data. We obtained unpublished data from trial authors.
Main results
We included 30 trials (35 data sets) representing 1992 infants with bronchiolitis. In 11 inpatient and 10 outpatient studies, oxygen saturation did not improve with bronchodilators (mean difference (MD) ‐0.43, 95% confidence interval (CI) ‐0.92 to 0.06, n = 1242). Outpatient bronchodilator treatment did not reduce the rate of hospitalization (11.9% in bronchodilator group versus 15.9% in placebo group, odds ratio (OR) 0.75, 95% CI 0.46 to 1.21, n = 710). Inpatient bronchodilator treatment did not reduce the duration of hospitalization (MD 0.06, 95% CI ‐0.27 to 0.39, n = 349).
Effect estimates for inpatients (MD ‐0.62, 95% CI ‐1.40 to 0.16) were slightly larger than for outpatients (MD ‐0.25, 95% CI ‐0.61 to 0.11) for oximetry. Oximetry outcomes showed significant heterogeneity (I2 statistic = 81%). Including only studies with low risk of bias had little impact on the overall effect size of oximetry (MD ‐0.38, 95% CI ‐0.75 to 0.00) but results were close to statistical significance.
In eight inpatient studies, there was no change in average clinical score (standardized MD (SMD) ‐0.14, 95% CI ‐0.41 to 0.12) with bronchodilators. In nine outpatient studies, the average clinical score decreased slightly with bronchodilators (SMD ‐0.42, 95% CI ‐0.79 to ‐0.06), a statistically significant finding of questionable clinical importance. The clinical score outcome showed significant heterogeneity (I2 statistic = 73%). Including only studies with low risk of bias reduced the heterogeneity but had little impact on the overall effect size of average clinical score (SMD ‐0.22, 95% CI ‐0.41 to ‐0.03).
Sub‐analyses limited to nebulized albuterol or salbutamol among outpatients (nine studies) showed no effect on oxygen saturation (MD ‐0.19, 95% CI ‐0.59 to 0.21, n = 572), average clinical score (SMD ‐0.36, 95% CI ‐0.83 to 0.11, n = 532) or hospital admission after treatment (OR 0.77, 95% CI 0.44 to 1.33, n = 404).
Adverse effects included tachycardia, oxygen desaturation and tremors.
Authors' conclusions
Bronchodilators such as albuterol or salbutamol do not improve oxygen saturation, do not reduce hospital admission after outpatient treatment, do not shorten the duration of hospitalization and do not reduce the time to resolution of illness at home. Given the adverse side effects and the expense associated with these treatments, bronchodilators are not effective in the routine management of bronchiolitis. This meta‐analysis continues to be limited by the small sample sizes and the lack of standardized study design and validated outcomes across the studies. Future trials with large sample sizes, standardized methodology across clinical sites and consistent assessment methods are needed to answer completely the question of efficacy.
Keywords: Humans; Infant; Infant, Newborn; Acute Disease; Albuterol; Albuterol/therapeutic use; Ambulatory Care; Ambulatory Care/statistics & numerical data; Bronchiolitis; Bronchiolitis/blood; Bronchiolitis/drug therapy; Bronchodilator Agents; Bronchodilator Agents/therapeutic use; Hospitalization; Hospitalization/statistics & numerical data; Oxygen; Oxygen/blood; Randomized Controlled Trials as Topic
Plain language summary
Bronchodilators for bronchiolitis for infants with first‐time wheezing
What is bronchiolitis?
Bronchiolitis is an acute, highly contagious, viral infection of the lungs that is common in infants 0 to 12 months of age. It occurs every year in the winter months. It causes the small airways in the lungs to become inflamed and fill with debris. The airways are narrowed and this leads to blocking of the free passage of air. The infant has a harsh cough, runny nose and usually a fever. S/he can become breathless, wheezy and short of oxygen.
Why review bronchodilators?
Bronchodilators are drugs often used as aerosols to widen the air passages by relaxing the bronchial muscle. They are effective in helping older children and adults with asthma. However, unlike asthmatics, infants with bronchiolitis are usually wheezing for the first time. They are wheezing for a different reason, that is to say, because their airways are clogged with debris. Therefore, infants with bronchiolitis are less likely to respond to bronchodilators.
Study characteristics
We reviewed the evidence about the effect of bronchodilators in infants with bronchiolitis. We found 30 trials that included a total of 1922 infants, in several countries. The evidence is current up to January 2014. We analyzed studies done in outpatient and inpatient settings separately. All bronchodilators were included in the review except for epinephrine because it is reviewed in another Cochrane review. Albuterol (otherwise known as salbutamol) is commonly used in studies, so we also reviewed this bronchodilator as a subgroup.
Key results
We found no effect of bronchodilators on oxygen saturation. Infants hospitalized for bronchiolitis showed no significant benefit of bronchodilator treatment. This review also found that bronchodilators do not reduce the need for hospitalization, do not shorten the length of stay in hospital and do not shorten the length of the illness at home. Reviewing the subgroup of studies using albuterol (salbutamol), we found no effect of this bronchodilator on oxygen saturation or clinical scores. Side effects of bronchodilators include rapid heart beat, decrease in oxygen and shakiness. Given these side effects, little evidence that they are effective and the expense associated with these treatments, bronchodilators are not helpful in the management of bronchiolitis.
Quality of the evidence
This review is limited by the small number of studies that use the same measures and methods. For example, only 22 studies included only infants wheezing for the first time. Older studies included children who had wheezed before and may have had asthma. Thus these older studies favor the use of bronchodilators. Newer studies that excluded infants with prior wheezing and had a better study design do not show a benefit of bronchodilators. This review is also limited by the small number of infants included in each study. Lastly, clinical scores used to measure the effect of the bronchodilators in some studies may vary from one observer to the next, making this measure unreliable. Studies that include more infants, use better measures and have a stronger study design are needed to define the effectiveness of these medications.
Background
Description of the condition
Bronchiolitis is an acute, highly communicable lower respiratory tract infection characterized by "cough, coryza (runny nose), fever, expiratory wheezing, grunting, tachypnea (fast breathing), retractions and air trapping" (Welliver 1992). Infants with bronchiolitis are wheezing for the first time, unlike asthmatics in whom bronchospasm causes recurrent wheezing. It should be emphasized that definitions of bronchiolitis vary between countries. Bronchiolitis refers to an illness starting as an upper respiratory infection followed by signs of acute respiratory distress and diffuse bilateral crepitations or rales, in addition to signs of bronchiolar obstruction such as air trapping, wheezing and high‐pitched rhonchi (Disney 1960).
Largely caused by respiratory syncytial virus (RSV), bronchiolitis results in significant morbidity and mortality on a global scale (Nair 2010). While the average RSV hospitalization rate is 5.2 per 1000 children under 24 months old in the US, infants younger than two months of age have a much higher hospitalization rate of 17.9 per 1000 children (Hall 2009; Hall 2013). The estimated cost of hospitalization in the US increased by 24% from USD 1.2 billion in 2000 to USD 1.5 billion in 2006, despite the fact that length of stay decreased slightly from 2.4 to 2.3 days (Wilson 2010). Combined with other medical encounters (outpatient and emergency department visits), the total cost of bronchiolitis in the US likely exceeds the year 2000 estimate of USD 652 million (Paramore 2004), because hospitalization rates have increased both in the US and Canada (Langley 2003; Shay 1999; Shay 2001).
Description of the intervention
Bronchodilators have been commonly used in the management of bronchiolitis. However, bronchodilator efficacy for this illness is not universally accepted and bronchodilators are seldom used to treat bronchiolitis in the United Kingdom (Goodman 1993). Significant practice variation in the treatment of infants admitted for bronchiolitis or RSV pneumonia has been documented in the US (Christakis 2005; Florin 2013; Wilson 2001), Europe (Barben 2003; de Bilderling 2003), Canada (Plint 2004), Australia and New Zealand (Babl 2008; Vogel 2003). Significant practice variation in emergency department bronchodilator use and bronchodilator prescription at discharge has also been documented in the US and Canada (Johnson 2013; Plint 2004).
How the intervention might work
Bronchodilators work by reversing bronchoconstriction of the airways due to bronchospasm induced by asthma triggers, viruses, exposure to toxic inhalants, etc. Infants with bronchiolitis present with wheezing, a hallmark of asthma, therefore bronchodilators have been used to manage wheezing.
Why it is important to do this review
Given the considerable cost of hospitalization and significant degree of practice variation documented in various parts of the world, an evidenced‐based approach to bronchiolitis management is indicated. This review focuses on a broad class of bronchodilators, which includes the most commonly used agents, albuterol and salbutamol (β2‐adrenergic agonists). Epinephrine, a bronchodilator with both alpha‐adrenergic and beta‐adrenergic effects, is meta‐analyzed in a separate Cochrane review (Hartling 2011a; Hartling 2011b). Randomized controlled trials (RCTs) of bronchodilators in bronchiolitis, whether for ambulatory or hospitalized children, have yielded variable results. Prior meta‐analyses (Flores 1997; Kellner 1996) and systematic reviews (Hartling 2011b; King 2004; Wainwright 2010) suggest that bronchodilators may improve clinical symptom scores for outpatients but they do not affect disease resolution or length of hospital stay. Some evidence‐based clinical reviews and practice guidelines conflict regarding their recommendations about the use of bronchodilators. Several recommend that bronchodilators should not be used routinely to treat bronchiolitis (DeNicola 2013; Guia Salud 2010; SIGN 2006; Wagner 2009; Wainwright 2010; Zorc 2010), while others suggest the option of a single trial of bronchodilator inhalation with careful assessment of response (AAP 2006; CCHMC 2010).
Cincinnati guidelines suggest that neither bronchodilators, steroids, antivirals nor antibacterial agents should be routinely used (CCHMC 2010). In particular, use of antibiotics and steroids should be strongly discouraged, whereas administration of bronchodilators or epinephrine are considered as an option, particularly when there is a family history for allergy, asthma or atopy.
Objectives
To assess the effects of bronchodilators on clinical outcomes in infants (0 to 12 months) with acute bronchiolitis.
There is widespread use of bronchodilators despite conflicting evidence regarding their efficacy, therefore we updated this systematic review of all randomized placebo‐controlled trials of bronchodilators for bronchiolitis. We have reviewed the quality of studies and provided a quantitative summary of the effects of bronchodilators. The question addressed by the meta‐analysis was: are bronchodilators better than placebo in the management of bronchiolitis in infants, as measured by improvement in oxygen saturation, clinical scores, admission to hospital, duration of hospitalization, pulmonary function tests or time to resolution of illness?
Methods
Criteria for considering studies for this review
Types of studies
Randomized, placebo‐controlled trials of bronchodilators for bronchiolitis. We examined the methods and results if the title or abstract indicated that patients with bronchiolitis were studied in a prospective randomized clinical trial. Both published and unpublished studies could be included as long as the inclusion criteria were fulfilled.
Types of participants
Infants and young children up to 24 months with bronchiolitis. All trials used the term 'bronchiolitis' to refer to an acute lower respiratory tract infection with wheezing.
Types of interventions
Bronchodilator therapy, including albuterol, salbutamol, terbutaline, ipratropium bromide and adrenergic agents. Studies of inhaled steroids were not included. Routes of administration were: nebulized, oral and subcutaneous. Although included in the original review, we excluded studies of epinephrine in bronchiolitis from the updates since these studies are included in the Cochrane Review 'Epinephrine for bronchiolitis' (Hartling 2011a).
Types of outcome measures
Outcome measures of interest were those that assessed signs or symptoms and were, therefore, considered to have the most clinical relevance: oxygen saturation as measured by pulse oximetry, clinical score, admission to hospital, duration of hospital stay and time to resolution of illness. We added pulmonary function tests as an additional outcome in the 2006, 2010 and 2014 updates.
Primary outcomes
Oxygen saturation, as this outcome often drives the clinical decision to hospitalize an infant with bronchiolitis. This outcome is objectively measured using pulse oximetry.
Secondary outcomes
Improvement in clinical scores.
Admission to hospital.
Duration of hospitalization.
Time to resolution of illness.
Pulmonary function tests.
These outcomes are more subjective and subject to interrater variability. Pulmonary function tests are objective measures of the effect of bronchodilators on airway resistance and compliance.
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 12 (accessed 20 January 2014), which contains the Acute Respiratory Infections Group's Specialized Register, MEDLINE (January 2010 to January week 2, 2014) and EMBASE (March 2010 to January 2014). Details of previous searches are in Appendix 1.
We used the following search strategy to search CENTRAL and MEDLINE. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐ maximizing version (2008 revision); Ovid format (Lefebvre 2011). We adapted these search terms to search EMBASE (see Appendix 2). There were no language or publication restrictions.
MEDLINE (OVID)
1 exp Bronchiolitis/ 2 bronchiolit*.tw. 3 1 or 2 4 exp Bronchodilator Agents/ 5 bronchodilator*.tw,nm. 6 Albuterol/ 7 albuterol.tw,nm. 8 salbutamol.tw,nm. 9 Terbutaline/ 10 terbutaline.tw,nm. 11 Ipratropium/ 12 ipratropium.tw,nm. 13 exp Adrenergic Agents/ 14 adrenergic agent*.tw,nm.
Searching other resources
We scanned the reference lists of identified articles and contacted authors of the identified trials and other experts in the field.
Data collection and analysis
Selection of studies
In the original review, two review authors (AG, AB) independently reviewed the articles. In the 2010 and 2014 updates, two review authors (AG, MS) reviewed the search results and independently reviewed new studies. There was complete agreement between the two review authors regarding the articles selected for inclusion in the review.
Data extraction and management
Both review authors (AG, MS) independently extracted data and achieved consensus on what data to include. We requested unpublished data from trial authors when necessary.
Assessment of risk of bias in included studies
Both review authors (AG, MS) rated the quality of each included trial by assessing whether the following five sources of bias were adequately reported (Higgins 2011): 1) sequence allocation was carried out satisfactorily; 2) allocation to treatment groups was concealed; 3) the trial was double‐blinded (Schulz 1995); 4) incomplete data were addressed; and 5) selective reporting was not present.
Measures of treatment effect
We selected oxygen saturation as measured by pulse oximetry, clinical scores based on a multi‐item clinical scale and admission to hospital to measure the effect of bronchodilators on outpatients. We added duration of hospitalization as an outcome measure for inpatients. We thought these outcomes to be the most clinically relevant and to have the largest amount of experimental data reported. Two longer‐term outpatient studies were published, therefore we also added time to resolution of illness as an outcome measure. Respiratory rate was not selected as an isolated measure because of many uncontrollable factors which influence respiratory rate (Gadomski 1994b ‐ neb).
A number of different scoring systems were used in the included studies (see Characteristics of included studies table). A summary of the components of the most widely used clinical scoring systems can be found in Hartling 2003. Fourteen of 30 included studies utilized the partially validated clinical scoring system, that is to say, the Respiratory Distress Assessment Instrument (RDAI) or the Respiratory Assessment Change Score (RACS). Clinical scores were reported in two ways. In several trials, the results were reported as the proportion of infants and children with an improved score based on an a priori determination of significant clinical improvement (improvement in clinical score, a dichotomous variable). Analysis 1.3 defines events as the proportion of participants who did not meet pre‐determined criteria for clinical score improvement. In eight inpatient and nine outpatient trials, the results were reported as the average score or change in score in each treatment group (average clinical score, a continuous variable (Analysis 1.4).
1.3. Analysis.
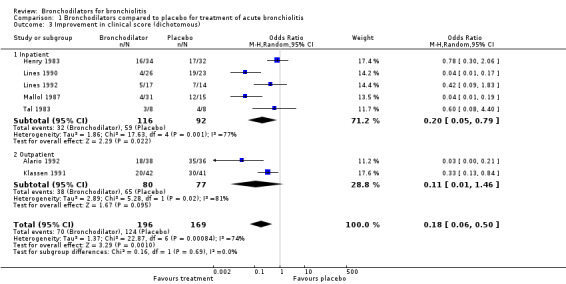
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 3 Improvement in clinical score (dichotomous).
1.4. Analysis.

Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 4 Average clinical score after treatment: by treatment setting (continuous).
Time to resolution of illness (ROI), measured from the period of study enrollment to the time the infant returned to baseline health status, is scored by the primary caregiver at home. ROI comprises parental assessment of degree of improvement of respiratory symptoms scored on a four‐point ordinal scale (worse = 1, same = 2, improved = 3, symptoms resolved = 4) (Cruz 1995).
Duration of hospitalization was measured by length of stay, derived from the time of admission and discharge, as opposed to specific measures of improvement. The exception to this is Dobson 1998, which defined duration as time to reach predetermined discharge criteria.
For the three continuous variables (oxygen saturation, average clinical score and duration of hospitalization) and the ordinal variable of ROI, we determined the effect of treatment compared with placebo using the unbiased estimate of effect size (ES), with its 95% confidence intervals (CI) (Bracken 1989). For oxygen saturation, duration of hospitalization and ROI, we measured effect using the mean difference (MD) between treatment and placebo. We converted the average clinical scores to the standardized mean difference (SMD) because a variety of clinical scoring systems with different ranges were utilized by the included studies. In all scoring systems, higher scores indicate greater severity of illness.
For average clinical score, an ES of less than zero (that is to say, reduction of severity scores) indicates a benefit and an ES of more than zero (that is to say, increased severity scores) indicates that treatment is detrimental. Similarly, for oximetry an ES of less than zero (that is to say, lower mean oxygen saturation with placebo) indicates a beneficial effect of treatment and an ES of more than zero (that is to say, higher mean oxygen saturation with placebo) indicates a detrimental effect.
For the two dichotomous variables (improvement in clinical score and hospital admission), we determined the effect of treatment compared with placebo using the odds ratio (OR). An overall OR of less than one indicates that treatment is beneficial, while an OR of more than one indicates that treatment is detrimental. For improvement in clinical score, an OR of less than one indicates that the odds of not improving were lower in the treatment group compared with the placebo group. For hospital admission, an OR of less than one indicates that the odds of being hospitalized were lower in the treatment group than the placebo group.
Pulmonary function test (PFT) data are objective measures but changes in PFT measures may achieve statistical significance while having little clinical significance. In this update, we found one additional PFT study (Scarlett 2012), bringing the total number of PFT studies to 10. However, seven of these studies did not fulfill the inclusion criteria and only three studies could be included (Levin 2008; Scarlett 2012; Totapally 2002). However, due to the different PFT measurement techniques used, the outcomes of these studies could not be combined. Therefore, PFT data are not included as outcome measures.
Unit of analysis issues
We stratified results for oxygen saturation and average score (continuous) according to whether the study was conducted in an inpatient or outpatient setting. The rationale for this was that inpatients are more severely ill and, therefore, have a different response profile compared to outpatients. Also the time of outcome assessment varied according to whether the study was an inpatient or outpatient study. Inpatients were usually assessed within 24 hours of admission whereas outpatients were more consistently assessed 30 minutes to six hours after treatment was initiated. In addition, we added oral bronchodilator given at home (ascertained during a 14‐day period following study enrollment) to Analysis 1.6 'Hospital admission after treatment'.
1.6. Analysis.
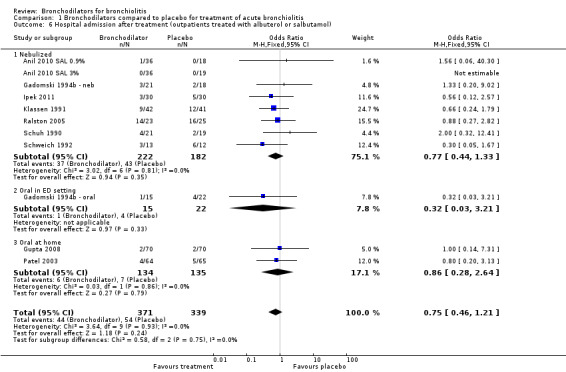
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 6 Hospital admission after treatment (outpatients treated with albuterol or salbutamol).
Cross‐over studies
Three trials employed cross‐over designs (Alario 1992; Ho 1991; Totapally 2002). Pulse oximetry data from Alario 1992, recorded 20 minutes after either nebulized metaproterenol or 0.9% saline first among 74 outpatients (37 in each group), were included in Analysis 1.1. Clinical score data for outpatients aged 12 months or younger (17 in metaproterenol group and 20 in 0.9% saline group) were available and thus included in Analysis 1.3 and Analysis 1.4. Pulse oximetry data from Ho 1991 included 30‐minute readings for 13 inpatients receiving salbutamol first and eight inpatients receiving 0.9% saline first. Cross‐over data were not included. The PFT results presented by Totapally 2002 were not combined with other PFT results because different PFT measurement techniques were used by the PFT studies.
1.1. Analysis.
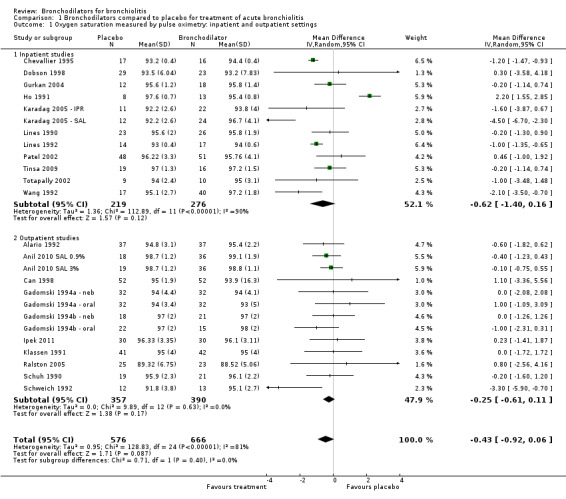
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 1 Oxygen saturation measured by pulse oximetry: inpatient and outpatient settings.
Studies with multiple treatment groups
Some trials had more than one bronchodilator treatment arm, either varying the mode of delivery (nebulized, oral or metered dose inhaler (MDI)) or comparing different bronchodilators (for example, salbutamol and ipratropium), or different diluents (0.9% saline versus 3% saline). In the figures depicting these analyses, the descriptive labels for these trials are annotated to indicate the arm of the trial used in the comparison. For example Gadomski 1994a ‐ neb and Gadomski 1994a ‐ oral are the nebulized and oral treatment arms from the same study (Gadomski 1994a ‐ neb). In a trial that had only one placebo arm but two active treatment groups (Karadag 2005 ‐ IPR), placebo numbers were divided between comparisons to avoid double‐counting of placebo participants. For Ipek 2011, the 3% saline study groups were excluded from analysis.
Dealing with missing data
Given the nature of the clinical trials included in this review (short‐term outpatient or longer‐term inpatient studies), the reported participant drop out rates were low (see Incomplete outcome data). We contacted the trial authors of 11 studies for missing statistics, such as standard deviations.
Assessment of heterogeneity
We assessed statistical heterogeneity visually and with the I2 statistic and the Chi2 test. For meta‐analyses including a small number of studies, we used the I2 statistic.
Assessment of reporting biases
In 2006, an unpublished study was included because it was a RCT of salbutamol, ipratropium and saline that included first‐time wheezing infants admitted to hospital (Karadag 2005 ‐ IPR). This study was later published (Karadag 2008). A second unpublished inpatient study was a RCT comparing salbutamol, placebo and epinephrine (Gurkan 2004). We obtained data for these studies from the trialists. There were two placebo‐controlled studies excluded because they were only available in abstract form (Ferrer 1990; Karaatmaca 2010). Pending clinical trials were sought in the Pediatric Academic Societies abstracts for 2012 and 2013 (none were found). For the original review, seven trial authors provided upon request additional data not stated in their publications (Alario 1992; Gadomski 1994b ‐ neb; Ho 1991; Klassen 1991; Lines 1992; Schuh 1990; Schweich 1992). In the 2006 update, we requested additional data and received these for inclusion from three authors for: duration of hospitalization (Karadag 2005 ‐ IPR), clinical score and oximetry outcomes at 24 hours (Patel 2002) and clinical score and oximetry (Gurkan 2004). In this 2014 update, we requested additional unpublished data and received these from Scarlett 2012. Therefore, the likelihood of publication bias is low.
Data synthesis
We chose a fixed‐effect model initially for the meta‐analysis (Thompson 1991). This model assumes that the true effect of treatment is the same in all trials and that any differences in treatment effect between trials are due to chance. We expected that there would be some heterogeneity in the data due to the different treatment settings and measurement protocols (Thompson 1994). Where there was evidence of significant heterogeneity (I2 statistic greater than 30%), we analyzed the results using both fixed‐effect and random‐effects models. If there was a difference in the results, we used the more conservative random‐effects model.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses include analysis by outpatient or inpatient setting as the severity of illness differs between these two groups. We also analyzed nebulized versus oral bronchodilator studies separately, as well as outpatient versus home settings for oral bronchodilators. They are commonly used, therefore albuterol or salbutamol subgroup analysis was added in this 2014 update. Methods for investigating heterogeneity of effects include comparison of the I2 statistic and the Chi2 test.
Sensitivity analysis
Sensitivity analysis included comparison of the estimates of the effect of bronchodilators in studies with a low risk of bias, studies that specifically included only first‐time wheezers and studies that only included infants younger than or equal to 12 months of age. We defined studies with a low risk of bias as having a ranking of 'low risk' for all five items in the 'Risk of bias' table (see Included studies). In this 2014 update, we included studies using the same clinical score (RDAI) in a sensitivity analysis.
Results
Description of studies
Results of the search
Of the 21 studies identified in the search for this 2014 update, two met the criteria for inclusion. Most of the excluded studies were excluded because they did not include a placebo group (see Characteristics of excluded studies table).
Included studies
From a total of 30 trials (35 data sets) included in this review, 23 trials were in infants wheezing for the first time (Anil 2010 SAL 0.9%; Anil 2010 SAL 3%; Can 1998; Chevallier 1995; Chowdhury 1995; Dobson 1998; Gadomski 1994a ‐ neb; Gadomski 1994b ‐ neb; Goh 1997; Gupta 2008; Gurkan 2004; Ho 1991; Karadag 2008; Klassen 1991; Levin 2008; Lines 1990; Lines 1992; Patel 2002; Patel 2003; Ralston 2005; Schuh 1990; Tinsa 2009; Totapally 2002; Wang 1992). For this 2014 update, we included two new trials, both of which included first‐time wheezing infants (Ipek 2011; Scarlett 2012). We also included five additional trials, in which results from participants with first‐time wheezing could not be separated from those with recurrent wheezing (Alario 1992; Henry 1983; Mallol 1987; Schweich 1992; Tal 1983).
Laboratory methods to identify RSV included direct immunofluorescence microscopy, enzyme immunoassay and serum RSV titers. The range of participants who were RSV‐positive was 3% to 100%, with more than 40% RSV‐positive in 10 trials.
Excluded studies
We excluded a total of 62 studies from this review (see Characteristics of excluded studies table). We made 46 of these exclusions because the trials were not placebo‐controlled (Absar 2008; Abu‐Shukair 2001; Alansari 2013; Barlas 1998; Beck 2007; Bentur 2003; Bertrand 2001; Cengizlier 1997; Chao 2003; Del Vecchio 2012; Fernandez 2009; Florin 2012; Frasson 2012; Goebel 2000; Gomez‐y‐Lopez 2007; Gonzalez 1994; Hammer 1995; John 2006; John 2010; Kadir 2009; Kim 2011; Langley 2005; Luo 2003; Luo 2010; Luo 2012; Mandelberg 2003; Menon 1995; Modaressi 2012; Modl 2005; Mull 2004; Numa 2001; Ozyurek 2002; Ray 2002; Reijonen 1995; Sanchez 1993; Sarrell 2002; Schuh 1992; Sharma 2013; Simsek 2005; Simsek‐Kiper 2011; Soto 1985; Springer 1990; Stokes 1983; Torres 1997; Walsh 2008; Zhou 2001).
We excluded six trials because they contained limited data due to publication in abstract form only (Choong 1998; Ferrer 1990; Karaatmaca 2010; Milner 1995; Ndrepepa 1998; Zhen 2003). We excluded two additional studies as abstract‐only (Ren 2011; Sezer 2010), but the data were later published as included studies in Scarlett 2012 and Ipek 2011, respectively.
We have excluded four trials because they were not RCTs (Brooks 1981; Cortes 1996; Shu 2001; Wankum 2000), one trial because it did not include nebulized delivery of bronchodilators (Ralston 2008), two studies because they did not have clear definitions of bronchiolitis (Sly 1991; Tatochenko 1988) and one study because it published a research protocol only (no outcomes data) (Belcastro 2010). We omitted four trials of epinephrine versus placebo, excluded in previous versions of this review (Hariprakash 2003; Kristjánsson 1993; Lowell 1987; Wainwright 2003), from this 2014 update as they have been addressed by another Cochrane Review (Hartling 2011a).
Risk of bias in included studies
The design and methodological quality features of each study are shown in the Characteristics of included studies table. Generally the studies were of small size. The main problem with older studies was an inability to identify participants who were first‐time wheezers versus recurrent wheezers. Other limitations to study quality included lack of standardized methods for outcome evaluation (timing of assessments, clinical scoring systems used) and lack of standardized intervention (various bronchodilators, drug dosages, routes of administration and nebulization delivery systems) used across the studies. A graphical representation of risk of bias among included studies is shown in Figure 1. A summary of methodological quality among included studies is given in Figure 2
1.
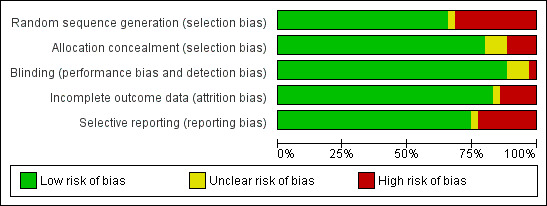
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
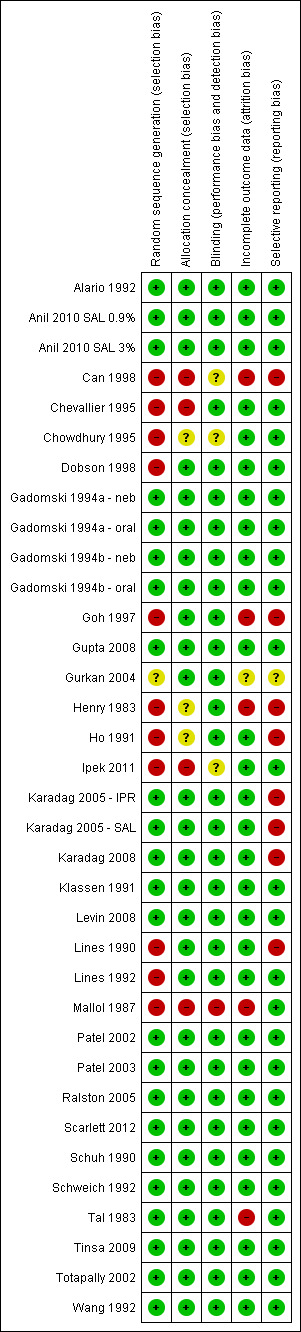
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Methods for sequence generation and allocation concealment were not described in older studies (Can 1998; Chevallier 1995; Chowdhury 1995; Henry 1983; Ho 1991; Lines 1990; Lines 1992; Mallol 1987), or in abstract‐only studies (Gurkan 2004). More recent studies described methods for sequence generation, allocation concealment and use of placebo agents that were indistinguishable from bronchodilator agents. Ipek 2011 allocated infants to the study groups by consecutive order to the short stay unit.
Blinding
Most medical and research staff administering treatment or assessing participants during the trial (or both) are described as being either blinded or masked during the conduct of the studies included in this review, thus reducing the potential for performance, detection or attrition bias. Only one study was described as single‐blind (Mallol 1987). Two studies were described in the abstract as being double‐blind but this was not detailed in the methods (Can 1998; Ipek 2011).
Incomplete outcome data
In the outpatient studies, there tended to be more missing data for follow‐up measurements beyond 60 minutes because many patients were discharged from these settings before 90 or 120‐minute assessments could be done. Bronchodilators have short‐term effects, therefore some outpatient trialists did not include measurement of outcomes longer than 60 minutes post‐treatment. Therefore, the outpatient results are biased towards those data measured at a shorter interval from treatment administration, so sustained outcomes may have been missed.
Details regarding study attrition were often not well described in the included studies. Drop out rates range from 0% to 13% (Gupta 2008; Patel 2003; Scarlett 2012). Few studies included study flow diagrams that could be used to assess differential drop out from the study groups (Anil 2010 SAL 0.9%; Gupta 2008; Patel 2003; Ralston 2005). Few studies employed intention‐to‐treat (ITT) analysis when study participant attrition occurred (Patel 2002; Patel 2003).
Possible attrition bias might be a factor in three studies that excluded participants from analysis because they were 'therapeutic failures' (Tal 1983), or that withdrew participants for other reasons (Dobson 1998; Goh 1997; Scarlett 2012).
Selective reporting
Evidence of selective reporting of outcomes was rare as most studies presented the outcome results that were described in the methods, with one exception, that is, that few studies provided data on heart rate following treatment. Bronchodilators can increase heart rate, therefore it is an important outcome to include, although for most studies this information is included in the description of adverse effects and is not systemically addressed in all studies.
Other potential sources of bias
Adverse effects following treatment were often not systematically addressed in the study design and are not completely described in most studies included in this review.
Effects of interventions
Primary outcome
1. Oxygen saturation
In a random‐effects analysis, bronchodilator recipients did not show a significant improvement in oxygen saturation as measured by pulse oximetry compared to placebo, as reflected by the mean difference (MD) ‐0.43, 95% confidence interval (CI) ‐0.92 to 0.06 (Analysis 1.1).
Nine outpatient studies included treatment protocols that included albuterol or salbutamol nebulization only (Anil 2010 SAL 0.9%; Anil 2010 SAL 3%; Can 1998; Gadomski 1994a ‐ neb; Gadomski 1994b ‐ neb; Ipek 2011; Klassen 1991; Ralston 2005; Schuh 1990; Schweich 1992). When reduced to these nine studies, outpatient oximetry measures showed reduced heterogeneity and also reduced mean differences that were not statistically significant (I2 statistic = 0%; MD ‐0.19, 95% CI ‐0.59 to 0.21; Analysis 1.2).
1.2. Analysis.
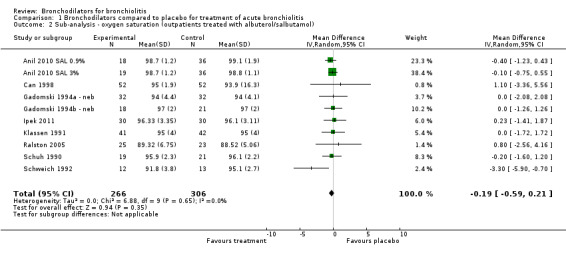
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 2 Sub‐analysis ‐ oxygen saturation (outpatients treated with albuterol/salbutamol).
Secondary outcomes
1. Improvement in clinical scores
In seven trials (five inpatient and two outpatient), the clinical score of 64% of those infants treated with bronchodilators improved compared to 27% with placebo (odds ratio (OR) for no improvement = 0.18, 95% CI 0.06 to 0.50, n = 365), using a random‐effects model (Analysis 1.3). Included in this analysis are three studies that were methodologically weaker than other studies and included older participants who were recurrent wheezers (Alario 1992; Lines 1990; Mallol 1987).
The improvement in overall average clinical score was statistically significant (standardized MD (SMD) ‐0.30, 95% CI ‐0.54 to ‐0.05) (Analysis 1.4), but the small magnitude of this change limits its clinical significance. Inpatients demonstrated no improvement compared to outpatients, underscoring the short‐term effect of bronchodilator treatment as most of the outpatient assessments occurred usually within one hour after treatment compared with longer time points in inpatients (see Subgroup analysis and investigation of heterogeneity). The small magnitude of difference in mean clinical score between bronchodilator and placebo groups is of questionable clinical importance, especially given the differences in scoring systems that were used.
We performed a sub‐analysis among nine outpatient studies with treatment protocols that included albuterol or salbutamol nebulization only (Anil 2010 SAL 0.9%; Anil 2010 SAL 3%; Can 1998; Gadomski 1994a ‐ neb; Gadomski 1994b ‐ neb; Ipek 2011; Klassen 1991; Ralston 2005; Schuh 1990; Schweich 1992). As shown in Analysis 1.5, similar levels of heterogeneity were found but the treatment effect was not significant (I2 statistic = 85%; SMD ‐0.36, 95% CI ‐0.83 to 0.11, P value = 0.13).
1.5. Analysis.
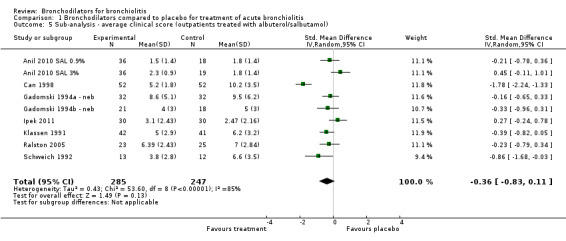
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 5 Sub‐analysis ‐ average clinical score (outpatients treated with albuterol/salbutamol).
2. Admission to hospital
The rate of hospitalization was not significantly reduced in bronchodilator recipients compared with placebo recipients in outpatient studies (11.9% versus 15.9%; OR 0.75, 95% CI 0.46 to 1.21) (Analysis 1.6). Rate of hospitalization was not significantly different between oral bronchodilator or placebo groups followed in longer‐term home‐based studies (4.5% versus 5.2%; OR 0.86, 95% CI 0.28 to 2.64).
3. Duration of hospitalization
There was no difference between bronchodilator and placebo groups in the length of stay (MD 0.06 days, 95% CI ‐0.27 to 0.39) (Analysis 1.7).
1.7. Analysis.

Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 7 Duration of hospitalization (inpatients).
4. Time to resolution of illness
There is no difference between bronchodilator and placebo groups with respect to time to resolution of illness as measured in the two longer‐term home‐based studies by Patel 2003 and Gupta 2008 (MD 0.29, 95% CI ‐0.43 to 1.00, n = 269) (Analysis 1.8). Thus, oral bronchodilators do not shorten the time to resolution of illness among infants treated at home. However, only two studies examined this outcome.
1.8. Analysis.
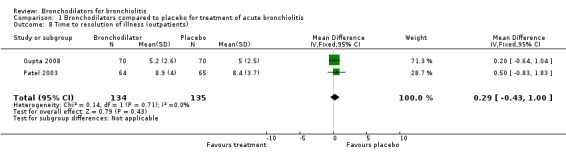
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 8 Time to resolution of illness (outpatients).
5. Pulmonary function tests
In this 2014 update, one placebo‐controlled study utilizing PFT as an outcome met the inclusion criteria (Scarlett 2012). This study utilized tidal breathing analysis using respiratory inductive plethysmography to measure phase angle (thoracoabdominal synchrony). Changes in tidal breathing measures were compared pre‐ and post‐albuterol or saline inhalation for 20 infants hospitalized for bronchiolitis. Totapally 2002 used tidal breathing analysis of flow‐volume loops measured through close‐fitting face masks to compare changes pre‐ and post‐albuterol or saline inhalation for 20 infants with mild RSV‐positive bronchiolitis. Although both studies measured peak expiratory flow to total expiratory time (Tpef/Te), the different PFT techniques used preclude merging these measurements for meta‐analysis. These studies documented no significant changes in tidal breathing and Tpef/Te measures as well as clinical scores between albuterol nebulization and the 0.9% saline study groups after treatment. Scarlett 2012 also documented that the RDAI clinical score did not correlate with phase angle (results included in Analysis 1.4). Levin 2008 measured peak inspiratory pressure and inspiratory system resistance pre‐ and post‐bronchodilator or 0.9% saline nebulization in 22 infants with severe RSV‐positive bronchiolitis who were intubated and ventilated in an ICU setting. Small but statistically significant decreases in peak inspiratory pressure as well as significant increases in heart rate were observed after bronchodilator administration compared to no changes after saline. Interestingly, inspiratory resistance fell after all treatments, including saline. Differences in severity of illness, PFT methodology and outcomes (volume versus pressure) preclude merging the results of these three placebo‐controlled trials that used PFT measures.
Subgroup analyses
Subgroup analysis of oximetry showed no statistically significant effects for either outpatients (MD ‐0.25, 95% CI ‐0.61 to 0.11) or inpatients (MD ‐0.62, 95% CI ‐1.40 to 0.16) (Analysis 1.1).
Subgroup analyses of clinical score showed a slightly greater effect size with bronchodilators in outpatient studies, where there were shorter follow‐up durations than for inpatient studies. This was shown in the analysis of average clinical score where there was a modest effect for outpatient studies (SMD ‐0.42, 95% CI ‐0.79 to ‐0.06) compared to the effect in inpatient studies (SMD ‐0.14, 95% CI ‐0.41 to 0.12) (Analysis 1.4).
However, the magnitude of these differences between inpatient and outpatient studies is of questionable clinical importance (e.g. a MD of ‐0.62 in pulse oximetry is not clinically relevant) and the results of these subgroup analyses should be interpreted with caution. These differences may be due to shorter follow‐up time, inclusion of participants with recurrent wheezing and lesser severity of illness among outpatients.
Subgroup analysis limiting bronchodilators to albuterol or salbutamol among outpatients showed no effect on oxygen saturation (MD ‐0.19, 95% CI ‐0.59 to 0.21) (Analysis 1.2). Nebulized albuterol or salbutamol outpatient treatment had no effect on average clinical score (SMD ‐0.36, 95% CI ‐0.83 to 0.11, Analysis 1.5) or hospital admission after treatment (OR 0.32, 95% CI 0.03 to 3.21, Analysis 1.6). Oral albuterol or salbutamol given at home had no impact on hospital admission after treatment (OR 0.86, 95% CI 0.28 to 2.64, Analysis 1.6).
Heterogeneity
There was evidence of considerable heterogeneity for clinical score measures (dichotomized and average score) and oximetry, but not for hospital admission or duration of hospitalization. Where there was a difference between the effect estimate produced by the random‐ and fixed‐effect models, we chose the more conservative random‐effects model. Therefore, we used a random‐effects model for oximetry and clinical score and a fixed‐effect model for hospital admission, duration of hospitalization and time to resolution of illness outcomes.
For oximetry, use of the fixed‐effect model would have resulted in a slightly larger effect estimate that was statistically significant (‐0.66, 95% CI ‐0.82 to ‐0.49) than the result found with the random‐effects model (‐0.43, 95% CI ‐0.92 to 0.06). There was evidence of considerable heterogeneity with this outcome (P value < 0.00001, I2 statistic = 81%) that may be attributed to measurement differences (Analysis 1.1). The studies measured pulse oximetry at multiple time points. The points selected for pooling were based on times that were most frequently used and were either short‐term, at 60 minutes in outpatient studies, or longer‐term, at one or three days in inpatient studies. These variable time points for assessment reflect the nature of the studies, in that shorter times were used in outpatient studies while longer times were feasible for inpatients. These factors mean that we considered the random‐effects model more appropriate.
Sensitivity analysis
We assessed 16 studies as being at low risk of bias (Alario 1992; Anil 2010 SAL 0.9%; Anil 2010 SAL 3%; Gadomski 1994a ‐ neb; Gadomski 1994a ‐ oral; Gadomski 1994b ‐ neb; Gadomski 1994b ‐ oral; Gupta 2008; Klassen 1991; Levin 2008; Patel 2002; Patel 2003; Ralston 2008; Scarlett 2012; Schuh 1990; Schweich 1992; Tinsa 2009; Totapally 2002; Wang 1992). Including only low risk of bias studies in the analysis significantly reduced the heterogeneity measures for oximetry (I2 statistic = 17%; Analysis 1.9) and average clinical score (I2 statistic = 37%; Analysis 1.10), while having little impact on the overall effect size of oximetry (MD ‐0.38, 95% CI ‐0.75 to 0.00, P value = 0.05; Analysis 1.9) and average clinical score (SMD ‐0.22, 95% CI ‐0.41 to ‐0.03, P value = 0.02; Analysis 1.10). In other words, reducing the heterogeneity by removing studies with higher risk of bias did not uncover a clinically relevant treatment effect or materially change the magnitude of the effect size.
1.9. Analysis.
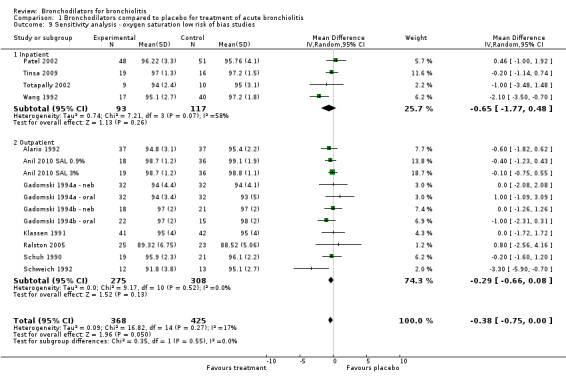
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 9 Sensitivity analysis ‐ oxygen saturation low risk of bias studies.
1.10. Analysis.
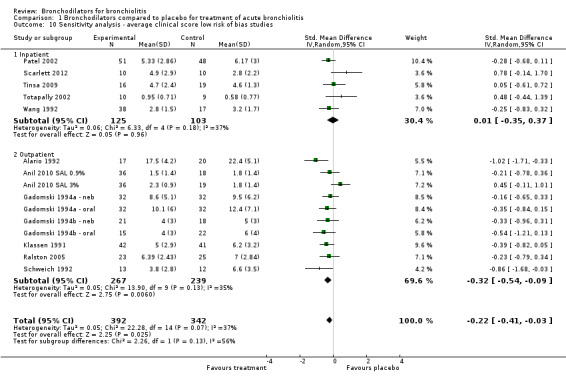
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 10 Sensitivity analysis ‐ average clinical score low risk of bias studies.
Low risk of bias sensitivity analysis did not significantly change the heterogeneity or effect estimates for hospital admission after treatment in an outpatient setting, duration of hospitalization or time to resolution of illness at home.
Fourteen studies included infants aged less than or equal to 12 months (Chevallier 1995; Chowdhury 1995; Gupta 2008; Henry 1983; Ho 1991; Karadag 2008; Levin 2008; Mallol 1987; Patel 2002; Patel 2003; Scarlett 2012; Tal 1983; Tinsa 2009; Totapally 2002). In this sensitivity analysis, the exclusion of several studies did not improve measures of heterogeneity but led to unstable effect size estimates.
Nineteen studies explicitly described inclusion of first‐time wheezing infants (Anil 2010 SAL 0.9%; Chevallier 1995; Chowdhury 1995; Dobson 1998; Gadomski 1994a ‐ neb; Gadomski 1994a ‐ oral; Gadomski 1994b ‐ neb; Gadomski 1994b ‐ oral; Goh 1997; Gupta 2008; Ho 1991; Ipek 2011; Karadag 2008; Levin 2008; Patel 2002; Patel 2003; Ralston 2005; Scarlett 2012; Schuh 1990; Tinsa 2009; Totapally 2002). Limiting the analysis of average clinical score to first‐time wheezers led to a non‐significant treatment effect and also reduced heterogeneity measures and reduced mean differences (I2 statistic = 30%, SMD ‐ 0.10, 95% CI ‐0.28 to 0.08, P value = 0.13). However, no impact was observed for the other outcomes.
Three studies of outpatients utilized identical clinical score measurement, that is, the complete RDAI (Anil 2010 SAL 0.9%; Anil 2010 SAL 3%; Klassen 1991; Ralston 2005) (Analysis 1.11). Limiting the analysis of average clinical score to these three studies showed substantially decreased heterogeneity (I2 statistic = 47%) when compared to all outpatient studies (I2 statistic = 81%). However, there was virtually no change in effect size (SMD ‐0.11, 95% CI ‐0.48 to 0.25, P value = 0.54).
1.11. Analysis.
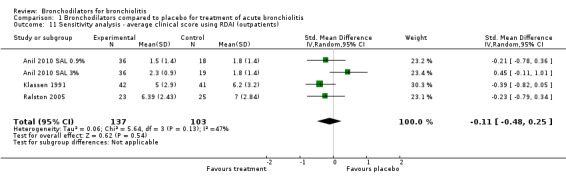
Comparison 1 Bronchodilators compared to placebo for treatment of acute bronchiolitis, Outcome 11 Sensitivity analysis ‐ average clinical score using RDAI (outpatients).
Adverse effects
Where adverse effects were reported, we note that these were exclusively found in the study groups receiving bronchodilators and they included: tachycardia (P value less than 0.05) (Klassen 1991; Lines 1990), decreased oxygen saturation (P value less than 0.05) (Ho 1991; Schweich 1992), flushing (one and four participants, respectively) (Alario 1992; Gadomski 1994b ‐ neb), hyperactivity (three participants) (Gadomski 1994b ‐ neb), tachycardia and prolonged cough (two participants) (Henry 1983) and tremor (one participant each) (Tal 1983; Wang 1992).
Amongst studies added in the 2006 update, tachycardia, mild hypertension and slight tremor were reported by Patel 2002. One infant receiving albuterol was transferred to the intensive care unit for 48 hours but did not require mechanical ventilation. No side effects were noted by Karadag 2005 ‐ IPR, except that one patient in the ipratropium group was subsequently excluded because of deteriorating clinical status. No adverse effects were described by Can 1998 or Totapally 2002.
In the 2010 update, no adverse effects were reported in two studies (Anil 2010 SAL 0.9%; Anil 2010 SAL 3%; Tinsa 2009). Adverse effects including trembling, vomiting and irritability were systematically addressed in the two home studies of oral bronchodilators (Gupta 2008; Patel 2003). While no difference was found in these symptoms between placebo and bronchodilator groups in one study (Patel 2003), more infants in the salbutamol group (six) were reported to have tremors versus the placebo group (none) in the other home study (Gupta 2008). Significant tachycardia (sustained heart rate over 200 beats per minute for more than 30 minutes) was reported in two infants receiving albuterol nebulization (Ralston 2005). Significant increases in heart rate were observed for all nebulized bronchodilators administered to intubated and ventilated infants compared to infants who received normal saline (Levin 2008).
This 2014 update includes Scarlett 2012, who reported a paradoxical response to albuterol in an infant whose phase angle increased after receiving albuterol (the expected response was a decrease).
Discussion
Summary of main results
This 2014 update of the meta‐analysis of trials of bronchodilators to treat infants with bronchiolitis shows no effect on oxygen saturation for outpatients or inpatients. Bronchodilators do not reduce the rate of hospital admission after outpatient treatment, do not shorten the duration of hospitalization and do not shorten the time to resolution of illness in home studies.
The two new studies add to the evidence that β2‐adrenergic agonists, i.e. albuterol (US) or salbutamol (as it is known elsewhere), are not effective for treating bronchiolitis. While they may produce small short‐term improvements in clinical scores for infants treated as outpatients, this short‐term benefit is not justified given the costs and adverse effects of these agents. These bronchodilators cause tachycardia and tremors, therefore routine use of bronchodilators for infants with bronchiolitis is not indicated.
What we learned from new sensitivity analysis. This meta‐analysis is limited by the significant heterogeneity in the analysis of trials that included oximetry and clinical score outcomes. Including only studies at low risk of bias in the meta‐analysis significantly reduced the heterogeneity measures for average clinical score and oximetry, while having little impact on the overall effect size of oximetry and average clinical score outcomes.
Subgroup analyses showed a slightly greater effect size in outpatient studies, where there were shorter follow‐up times and more recurrent wheezers and less severely ill infants included, than in inpatient studies for both oximetry and average clinical score. However, again the effect sizes are small for both settings and are of minimal clinical significance (for oximetry: outpatients mean difference (MD) ‐0.25 versus inpatients ‐0.62; for average clinical score: outpatients standardized MD (SMD) ‐0.42 versus inpatients ‐0.14). These findings may be biased toward showing a difference favoring treatment because older studies included in this analysis included older participants with recurrent wheezing and/or asthma. The inclusion of asthmatic children, who are known to respond to bronchodilators, will falsely increase the apparent level of efficacy in patients with bronchiolitis.
Overall completeness and applicability of evidence
Increased detection of hypoxia by using pulse oximetry has been cited as one of the reasons that, in the US, hospitalization rates for bronchiolitis nearly doubled from 1988 to 1996, with no significant change in mortality during that time period (Shay 2001). Despite other reasons for increased hospitalization rates that include increased daycare attendance at younger ages and increased survival of premature infants (Shay 1999), variable pulse oximetry cut‐off points for hypoxia necessitating oxygen administration probably contribute to increasing hospitalization rates as well as considerable practice variation. Clinically meaningful standardization of pulse oximetry endpoints for hospitalization and definition of what the minimal clinically important difference is for this outcome are now defined in clinical practice guidelines.
The lack of benefit from bronchodilators in preventing hospitalization may be difficult to interpret. In several outpatient studies, the decision to admit was made after the study was completed. This decision was made by non‐study physicians and further treatment may have been given, regardless of the intervention received during the study. Thus, this outcome may reflect other treatments and social considerations, as well as the initial intervention provided in the study.
Similarly, the duration of hospitalization was not altered by receipt of bronchodilators. However, hospital stay is affected by multiple factors other than the clinical status of the patient. Although randomization should balance these factors, length of hospital stay may be an insensitive measure. Among Canadian hospitals, duration of hospitalization did not vary significantly despite significant variation in the types of medications used to treat infants with bronchiolitis (Wang 1996).
The widespread use of bronchodilators in bronchiolitis is likely to be due to the similarity of symptoms and signs of bronchiolitis and asthma. Bronchodilators are effective in the treatment of asthma in older children and adults, where airway obstruction is caused by inflammation, bronchospasm and bronchial hyperreactivity (Levison 1991). However, a Cochrane Review of short‐acting β2‐adrenergic agonists for recurrent wheezing in children under two years of age showed no clear benefit of using bronchodilators in this age group (Chavasse 2002). The pathophysiology of bronchiolitis consists of terminal bronchiolar and alveolar inflammation with airway swelling and luminal debris, therefore the primary mechanism underlying wheezing is airway obstruction and plugging of the small airway diameters rather than bronchospasm (La Via 1992). In addition, it may be difficult to administer the nebulization to young infants effectively. Lastly, the relative lack or immaturity of the β2‐receptor in the bronchial wall smooth muscle in infants further limits the potential effectiveness of β2‐adrenergic agonists. These factors may explain why bronchodilators are not effective for infants with bronchiolitis.
Quality of the evidence
The lack of improvement in oximetry with bronchodilators and the heterogeneity of clinical scoring challenge the utility of these agents. The validity of the clinical score as an indicator of pulmonary status or relevant clinical change has not been proven (Hall 2007). Gadomski and colleagues have suggested that improvement in clinical scores may be due to changes in physiological state (for example, change from asleep to awake) rather than improved respiratory function with bronchodilator therapy (Gadomski 1994a ‐ neb).
The clinical scoring systems used in the studies included in this review varied considerably. Few have been tested for validity, reliability or compared to a physiologic standard or proven to correlate with clinically significant improvement (Mull 2004; Scarlett 2012; Zorc 2010), or predict the need for oxygen (McCallum 2013) or hospital admission from the emergency department (Destino 2010). Interrater variability of current scoring methods can be high. The most commonly used score, the RDAI, has low intraclass correlation, poor construct and discriminative validity (Destino 2010; Destino 2012; Walsh 2008). Sensitivity analysis of studies that used the RDAI show substantially decreased heterogeneity but no treatment effect (Analysis 1.11).
During this 2014 update, we found few new randomized, placebo‐controlled clinical trials. The adequacy of the outcome measures used to measure infant response to bronchodilators remains limited. The number of studies using similar outcome measures remains small, which limits the reliability of the effect size estimation. Most of the outcome effect estimates are small or show no difference from placebo. The estimates are imprecise as reflected by wide confidence intervals. Therefore, this meta‐analysis continues to be limited by the small sample sizes and the lack of standardized study design and reliable outcome assessment across the studies. Thus, randomized controlled trials (RCTs) with large sample sizes, standardized methodology across clinical sites and consistent assessment methods are needed to answer completely the question of efficacy.
A more objective alternative to these outcomes is pulmonary function testing (PFT) as performed by Levin 2008, although limited to infants with severe disease. Although the number of bronchiolitis studies utilizing PFTs has increased to 10, the methods and outcomes for measuring PFTs vary, thereby precluding comparability. In addition, only three studies employed a placebo‐controlled RCT design comparing measures pre‐ and post‐treatment with a bronchodilator. Future PFT studies should employ a placebo‐controlled RCT design as well as standardized methods so that outcome data can be merged.
Potential biases in the review process
One of the authors is a trialist and a member of the American Academy of Pediatrics Subcommittee on the Diagnosis and Management of Bronchiolitis.
Agreements and disagreements with other studies or reviews
The results of this meta‐analysis concur with recent reviews (Hartling 2011b; Wainwright 2010; Zorc 2010), which underscore the limited effectiveness of bronchodilators, particularly as they relate to β2‐adrenergic agonists in the outpatient management of bronchiolitis. This review is also consistent with these prior reviews in the conclusion that there is no significant treatment effect of bronchodilators for infants hospitalized with bronchiolitis (Hartling 2011b).
Authors' conclusions
Implications for practice.
Given their high cost, adverse effects and lack of effect on oxygen saturation and other outcomes included in this meta‐analysis, bronchodilators are not effective in the routine management of first‐time wheezers who present with the clinical findings of bronchiolitis, in either inpatient or outpatient settings.
Implications for research.
Prior to conducting further treatment trials, an objective outcome measure that correlates with pulmonary function tests and is independent of the level of alertness of the infant needs to be developed and validated. Measures such as need for hospital admission and duration of hospital stay, while important from a health service utilization perspective, may not be adequately sensitive to measure the improvement that may occur from treatment (Hall 2004; Hall 2007). Pulmonary function testing outcomes should be standardized so that outcome data can be merged across studies. Interrater variability as well as validity studies of the current scoring methods are needed to choose the most reliable and valid scoring system, if clinical scoring is used.
Treatment trials need to be conducted using placebo controls. RCTs with large sample size and standardized methodology across clinical sites are needed to answer completely the question of efficacy. Exclusion criteria must be consistently applied to exclude infants with recurrent wheezing, asthma or other pulmonary disease.
Feedback
Bronchodilators for bronchiolitis, 16 November 2014
Summary
It's not clear to me if bronchiolitis is affecting infants less than 12 months old, why the studies inclusion criteria was infants less than 24 months old? Older infants may have more bronchospasm than younger and they should be analyzed separately.
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Renato Cutrera MD, PhD Chief Pediatric Respiratory Unit Ospedale Pediatrico Bambino Gesù
What's new
| Date | Event | Description |
|---|---|---|
| 3 March 2015 | Feedback has been incorporated | Feedback comment published |
History
Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 20 January 2014 | New search has been performed | Searches updated. We included two new studies (Ipek 2011; Scarlett 2012) and excluded 17 new trials (Absar 2008; Alansari 2013; Barlas 1998; Belcastro 2010; Del Vecchio 2012; Florin 2012; Frasson 2012; Gonzalez 1994; John 2006; Karaatmaca 2010; Kim 2011; Luo 2012; Modaressi 2012; Ren 2011; Sezer 2010; Sharma 2013; Simsek‐Kiper 2011). |
| 20 January 2014 | New citation required but conclusions have not changed | We expanded the sensitivity analyses to include studies using the Respiratory Distress Assessment Instrument. A subgroup analysis of albuterol and salbutamol did not change our conclusions. Four previously excluded studies of epinephrine versus placebo were omitted from this update (Hariprakash 2003; Kristjánsson 1993; Lowell 1987; Wainwright 2003), due to the Cochrane Review 'Epinephrine for bronchiolitis' (Hartling 2011a). |
| 27 May 2010 | New citation required and conclusions have changed | A new review author joined the lead author to complete this update; additional outcome measures included; conclusions changed. |
| 19 March 2010 | New search has been performed | Searches conducted. Added to this update: five new studies were included, one previously excluded study was included and 12 new studies were excluded. |
| 22 August 2008 | Amended | Converted to new review format. |
| 19 October 2005 | New search has been performed | This review was first published in 1998. The update process began in 2004 and was completed in 2006. Searches of the literature were conducted during 2005. Authors of published abstracts were contacted. In the update it was decided to include pulmonary function tests as an additional measure but there were insufficient studies with this measure that met all inclusion criteria. Five new trials were added to the update, a relatively small number given the time since the last update. For two outcomes, average clinical score and oximetry, the analyses were stratified according to treatment setting (inpatient or outpatient) rather than by drug delivery mechanism (oral or nebulized) as in the original review. |
| 1 June 1998 | New search has been performed | Searches conducted. |
Acknowledgements
We wish to thank Drs A Alario, T Klassen, L Landau, D Lines, S Schuh, P Schweich and C Ren for providing unpublished data from their studies. We are also grateful to Mr Derek Stephens and Ms Terri Myhr, of Toronto for their assistance with the statistical analyses performed for the original review. The original review was presented in part at the annual meeting of the Ambulatory Pediatric Association: May 1994, Seattle, Washington. The 2010 update was presented at the Pediatric Academic Societies meeting: May 2011, Denver, Colorado.
We would like to acknowledge the contributions of three of the original co‐authors, JD Kellner, A Ohlsson and EEL Wang in conducting the original work for this review on which this update is based (Kellner 1998). Drs F Gurkan, B Karadag and H Patel generously provided unpublished data from their studies for the 2006 update. Drs. Tinsa and S Ralston graciously provided unpublished data from their studies for the 2010 update. The assistance of Sarah Thorning in conducting the searches for the update is also appreciated.
Taixiang Wu provided invaluable assistance in assessing eligibility of trials published in the Chinese language. The support of the National Prescribing Service (NPS), Australia allowed A Bhasale to contribute to this update and is appreciated. Reviews were received with thanks from the following peer referees: Alison Thomas, Craig Mellis, Rob Ware, Juan Lozano, Amanda Young, David Isaacs, Nelcy Rodriguez‐Malagon, Inge Axelsson, Conor Teljeur and Anne Lyddiatt. Finally we would like to acknowledge with thanks the support and guidance of Liz Dooley, Managing Editor for the Cochrane ARI Group, throughout the 2006, 2010 and 2014 updates.
Appendices
Appendix 1. Details of previous searches
In 1998, three computerized bibliographic databases were searched for all publications in all languages examining bronchodilator therapy of bronchiolitis: the National Library of Medicine MEDLINE database (1966 to September 1994); the Excerpta Medica database (1974 to November 1994); and Reference Update® (Research Information Systems, Carlsbad, California) (November 8, 1993, June 29, 1994 and April 26, 1995). The MEDLINE search was repeated June 2, 1998. The search terms "explode bronchiolitis" and "albuterol" or "ipratropium" or "adrenergic agents" or "bronchodilator agents" were used. In addition, the bibliographies of all articles selected were searched for relevant studies.
For the 2010 updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2010, Issue 1) which contains the Acute Respiratory Infections Group's Specialized Register, MEDLINE (1966 to Week 2, March 2010), EMBASE (1998 to March 2010) and reference lists of articles. In addition, we reviewed the files of one author (AG) and conducted a handsearch of reference lists of new studies. We searched presentations given at the Pediatric Academic Societies meetings in 2009 and 2010 for pending studies and found no clinical trials.
We searched MEDLINE and CENTRAL using the following keywords and MeSH terms. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format (Lefebvre 2009) These search terms were adapted to search EMBASE.com.
MEDLINE (OVID)
1 exp BRONCHIOLITIS/ 2 bronchiolit$ 3 or/1‐2 (2208) 4 exp Bronchodilator Agents/ 5 bronchodilator$ 6 exp ALBUTEROL/ 7 albuterol 8 salbutamol 9 exp IPRATROPIUM/ 10 ipratropium 11 exp Adrenergic Agents/ 12 adrenergic agent$ 13 or/4‐12 14 3 and 13
Embase.com
17. #13 AND #16 16. #14 OR #15 15. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((doubl* OR singl*) NEAR/2 (mask* OR blind*)):ab,ti 14. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 13. #3 AND #12 12. #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 11. 'adrenergic agent':ab,ti OR 'adrenergic agents':ab,ti 10. 'adrenergic receptor stimulating agent'/exp 9. ipratropium:ab,ti 8. 'ipratropium bromide'/de 7. albuterol:ab,ti OR salbutamol:ab,ti 6. 'salbutamol'/exp 5. bronchodilat*:ab,ti 4. 'bronchodilating agent'/exp 3. #1 OR #2 2.
*:ab,ti 1. 'bronchiolitis'/exp
Appendix 2. Embase.com search strategy
#17 #3 AND #8 AND #16 #16 #11 NOT #15 #15 #12 NOT #14 #14 #12 AND #13 #13 'human'/de #12 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #11 #9 OR #10 #10 random*:ab,ti OR placebo*:ab,ti OR trial:ti OR allocat*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR (doubl* NEXT/1 blind*):ab,ti #9 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #8 #4 OR #5 OR #6 OR #7 #7 albuterol:ab,ti OR salbutamol:ab,ti OR terbutaline:ab,ti OR ipratropium:ab,ti OR 'adrenergic agent':ab,ti OR 'adrenergic agents':ab,ti #6 'salbutamol'/de OR 'terbutaline'/de OR 'ipratropium bromide'/de OR 'adrenergic receptor stimulating agent'/exp #5 bronchodilator*:ab,ti #4 'bronchodilating agent'/exp #3 #1 OR #2 #2 bronchiolit*:ab,ti #1 'bronchiolitis'/exp
Data and analyses
Comparison 1. Bronchodilators compared to placebo for treatment of acute bronchiolitis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oxygen saturation measured by pulse oximetry: inpatient and outpatient settings | 25 | 1242 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.92, 0.06] |
| 1.1 Inpatient studies | 12 | 495 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.40, 0.16] |
| 1.2 Outpatient studies | 13 | 747 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.61, 0.11] |
| 2 Sub‐analysis ‐ oxygen saturation (outpatients treated with albuterol/salbutamol) | 10 | 572 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.59, 0.21] |
| 3 Improvement in clinical score (dichotomous) | 7 | 365 | Odds Ratio (M‐H, Random, 95% CI) | 0.18 [0.06, 0.50] |
| 3.1 Inpatient | 5 | 208 | Odds Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.79] |
| 3.2 Outpatient | 2 | 157 | Odds Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.46] |
| 4 Average clinical score after treatment: by treatment setting (continuous) | 21 | 1086 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.54, ‐0.05] |
| 4.1 Inpatient studies | 9 | 416 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.41, 0.12] |
| 4.2 Outpatient studies | 12 | 670 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.79, ‐0.06] |
| 5 Sub‐analysis ‐ average clinical score (outpatients treated with albuterol/salbutamol) | 9 | 532 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.83, 0.11] |
| 6 Hospital admission after treatment (outpatients treated with albuterol or salbutamol) | 11 | 710 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.21] |
| 6.1 Nebulized | 8 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.44, 1.33] |
| 6.2 Oral in ED setting | 1 | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.03, 3.21] |
| 6.3 Oral at home | 2 | 269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.28, 2.64] |
| 7 Duration of hospitalization (inpatients) | 6 | 349 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.27, 0.39] |
| 8 Time to resolution of illness (outpatients) | 2 | 269 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.43, 1.00] |
| 9 Sensitivity analysis ‐ oxygen saturation low risk of bias studies | 15 | 793 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.75, 0.00] |
| 9.1 Inpatient | 4 | 210 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.77, 0.48] |
| 9.2 Outpatient | 11 | 583 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.66, 0.08] |
| 10 Sensitivity analysis ‐ average clinical score low risk of bias studies | 15 | 734 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.41, ‐0.03] |
| 10.1 Inpatient | 5 | 228 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.35, 0.37] |
| 10.2 Outpatient | 10 | 506 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.54, ‐0.09] |
| 11 Sensitivity analysis ‐ average clinical score using RDAI (outpatients) | 4 | 240 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.48, 0.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alario 1992.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study | |
| Participants | Outpatients less than 36 months old with acute wheezing and or respiratory distress less than 48 hours. N = 73. Mean age 16.1 months, 68% male, no underlying cardiac or lung disease Country: USA | |
| Interventions | Group 1: metaproterenol sulfate 10 mg (0.2 ml of a 5% solution). Group 2: 0.2 ml normal saline. Both diluted in 2 ml normal saline administered by nebulizer without oxygen via face mask. 20 to 25 minutes after initial treatment, participants crossed over. Children received nebulized metaproterenol, either as an initial treatment or after a control treatment with normal saline solution. Only initial treatment results are included | |
| Outcomes | Respiratory rate, RDI score (color, wheezing, accessory muscle use, flaring, grunting, distress), oxygen saturation, side effects (tremors, vomiting, extreme irritability). RDI results were available for 37 infants aged < 12 months | |
| Notes | Included asthmatic participants or recurrent wheezers. “Responders to metaproterenol therapy” included 40% of those aged 12 months or younger versus 52% of those aged 24 months or older | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Anil 2010 SAL 0.9%.
| Methods | Randomized, double blind, placebo‐controlled trial | |
| Participants | Enrolled 186 children ages 1.5 to 24 months, treated as outpatients in a pediatric ED. Mean age 9.5 months, 65.1% male. Inclusion criterion was mild bronchiolitis (clinical score between 1 and 9). Exclusions were prior history of wheezing, previous treatment with bronchodilators and/or steroids and lung or cardiac disease Country: Turkey | |
| Interventions | All groups were pre‐treated with 8 ml of nebulized normal saline. Treatment was 2.5 mg of salbutamol in 4 ml of 0.9% saline at 0 and 30 minutes. The placebo group received a 4 ml 0.9% saline solution nebulization. 2 other study groups received epinephrine | |
| Outcomes | Clinical score (RDAI), pulse oximetry and heart rate at 0, 30, 60 and 120 minutes, and hospital admission | |
| Notes | All participants were reassessed for recurrent wheezing attacks in the following 6 months (by phone) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Anil 2010 SAL 3%.
| Methods | Randomized, double blind, placebo‐controlled trial | |
| Participants | Enrolled 186 children ages 1.5 to 24 months, treated as outpatients in a pediatric ED. Mean age 9.5 months, 65.1% male. Inclusion criterion was mild bronchiolitis (clinical score between 1 and 9). Exclusions were prior history of wheezing, previous treatment with bronchodilators and/or steroids and lung or cardiac disease Country: Turkey | |
| Interventions | All groups were pre‐treated with 8 ml of normal saline. Treatment was 2.5 mg of salbutamol in 4 ml of 3% saline at 0 and 30 minutes. The placebo group received a 4 ml 0.9% saline solution nebulization. 2 other study groups received epinephrine | |
| Outcomes | Clinical score (RDAI), pulse oximetry and heart rate at 0, 30, 60 and 120 minutes, and hospital admission | |
| Notes | All participants were reassessed for recurrent wheezing attacks in the following 6 months (by phone) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Can 1998.
| Methods | Double‐blind, randomized, placebo‐controlled trial | |
| Participants | Outpatient (emergency department) study of 156 infants with acute bronchiolitis. Mean age 7.1 months. Excluded infants who were pre‐term, had chronic disease, prior bronchodilator treatment, history of previous attack, symptoms for more than 1 week, HR more than 200 beats per minute, lethargy or RDS score more than 5 Country: Turkey | |
| Interventions | Group 1: salbutamol nebulized 0.15 mg/kg in 2 ml saline Group 2: saline nebulized Group 3: mist tent Intervention was repeated at 30 minutes if RDS score more than 5 | |
| Outcomes | Outcomes: heart rate, oximetry, RDS score at 0, 30 and 60 minutes and percentage of participants with RDS score more than 5 at 30 and 60 minutes. Chest X‐ray and laboratory studies (hemoglobin, hematocrit, leucocyte, neutrophils, eosinophils and IgE) were also compared | |
| Notes | Subgroup analysis of infants less than 6 months versus those more than 6 months showed similar changes in RDS at 30 and 60 minutes. No differences in laboratory values noted among the 3 study groups. Chest X‐ray findings consistent with bronchiolitis higher in Group 1 (88%) compared with 69% in Group 2 and 73% in Group 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No detail provided in the methods section of the manuscript; but abstract mentions "double blind" in the methods |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete |
| Selective reporting (reporting bias) | High risk | Non‐significant findings on heart rate not presented |
Chevallier 1995.
| Methods | Double‐blind, randomized controlled trial | |
| Participants | Inpatients aged 1 to 6 months hospitalized with first episode of bronchiolitis. N = 104. Mean age 3.1 months, 67% male, no underlying lung/cardiac disease, preceding bronchodilator/steroids in the past 48 hours also excluded. 78% RSV‐positive Country: France | |
| Interventions | Nebulized salbutamol (0.15 mg/kg/dose) or saline placebo administered using oxygen propellant 3 times at intervals of 1 hour | |
| Outcomes | Respiratory rate, clinical scoring system (4‐point score for each of retractions and wheezing), oximetry (used value taken at 30 minutes) | |
| Notes | All participants less than 12 months of age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Chowdhury 1995.
| Methods | Randomized controlled trial | |
| Participants | Inpatients aged 23 days to 11 months, admitted with moderate bronchiolitis. Mean age 3.85 months, 73% male. No previous history of wheeze or bronchodilator use, no underlying lung/cardiac disease. 58% RSV‐positive Country: Saudi Arabia | |
| Interventions | Group 1: salbutamol respiratory solution (ventolin 5 mg/ml) 0.15 mg/kg; Group 2: ipratropium bromide (0.025% solution) 12.5 micrograms/kg; Group 3: combination of above two at doses stated; Group 4: normal saline 0.3 ml/kg. All mixed with 2 ml normal saline and delivered with 100% oxygen at 6 to 7 L/min using nebulizer. 6 hourly for 36 hours | |
| Outcomes | Modified RDAI Clinical Score (5‐point score for each of wheezing: expiratory, inspiratory, location; retraction: supraclavicular, intercostal, subcostal; respiratory rate) | |
| Notes | All participants less than 12 months of age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear; insufficient detail provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Investigators blinded up to 36 hours, single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Dobson 1998.
| Methods | Double‐blind, randomized, placebo‐controlled trial | |
| Participants | Inpatients aged less than 24 months admitted to inpatient unit with first episode of wheezing with moderately severe bronchiolitis defined as pulse oximetry < 94% and clinical score > 1. Mean age 5.6 months, 48% male, no underlying lung/cardiac disease. 81% RSV‐positive Country: USA | |
| Interventions | Albuterol: 1.25 mg for patients less than 10 kg and 2.5 mg for patients more than 10 kg in normal saline for total volume of 3 ml or normal saline: 3 ml. Both administered with nebulized aerosol every 2 hours for first 24 hours, then every 4 hours for next 48 hours | |
| Outcomes | Oxygen saturation, clinical score (5‐point score for general appearance, 4‐point score for each of accessory muscle use and wheezing), duration of hospitalization (defined as time to each predetermined discharge criteria) | |
| Notes | 86% of the study population is less than 12 months of age. Adverse effects were compared between study groups. 3 participants were withdrawn from the albuterol group due to worsening hypoxemia. Subgroup analysis of results for infants less than 12 months was done but results not published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Gadomski 1994a ‐ neb.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | Outpatients and emergency department participants less than 18 months old with first‐time wheezing. Mean age 5.9 months. No underlying lung/cardiac disease Country: Egypt | |
| Interventions | Group 1: nebulized salbutamol (0.15 mg/kg/dose), Group 2: nebulized saline solution, Group 3: orally administered salbutamol (0.15 mg/kg/dose), Group 4: orally administered placebo. Nebulized groups received 2 treatments 30 minutes apart and oral‐treated groups received 1 treatment. Nebulization performed within 10 to 12 minutes with flow rate 4 to 6 L/min using a foot‐pump nebulizer, with room air, up‐mist nebulizer and pediatric face mask | |
| Outcomes | Respiratory rate, oxygen saturation, change in state of infant, study‐specific clinical score (34‐point scale for each of degree of grunting, nasal flaring, supraclavicular retractions, intercostal retraction, chest indrawing, air entry, air hunger, wheezing, general appearance) | |
| Notes | Nebulized treatment group: in order to represent the results from the 2 bronchodilator treatment arms (nebulized and oral), this study is listed twice. Each treatment group had its own placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Gadomski 1994a ‐ oral.
| Methods | See Gadomski 1994a ‐ neb | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Oral treatment group: in order to represent the results from the 2 bronchodilator treatment arms (nebulized and oral), this study is listed twice. Each treatment group had its own placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Gadomski 1994b ‐ neb.
| Methods | Randomized, double‐blind, placebo‐controlled clinical trial | |
| Participants | Outpatients less than 15 months old, with first episode of wheezing. Median age 5.5 months, 56% male, no underlying lung/cardiac disease. 48% RSV‐positive Country: USA | |
| Interventions | Group 1: nebulized salbutamol in 3 ml saline, Group 2: nebulized saline placebo in 3 ml saline, Group 3: oral salbutamol, Group 4: oral saline placebo. Dose of salbutamol 0.15 mg/kg/dose. Nebulized group received 2 nebulizations 30 minutes apart and oral groups received 1 dose. Nebulization with compressed air at 6 L/min via Up‐mist nebulizer with pediatric face mask. Infants 7 kg or less received 1 unit dose of 1 mg salbutamol solution for inhalation (5 mg/ml) or 1 oral dose of 2.5 ml | |
| Outcomes | Respiratory rate, heart rate, clinical score (4‐point score for each of grunting, nasal flaring, supraclavicular and intercostal retractions, air entry, air hunger, duration of wheeze in respiratory cycle, location of wheeze, general appearance), oxygen saturation, infant's state. Side effects: flushing of face, hyperactivity, increased coughing, tremors | |
| Notes | Nebulized treatment group: in order to represent the results from the 2 bronchodilator treatment arms (nebulized and oral), this study is listed twice. Each treatment group had its own placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Gadomski 1994b ‐ oral.
| Methods | Oral arm ‐ see Gadomski 1994b ‐ neb | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Oral treatment group: in order to represent the results from the 2 bronchodilator treatment arms (nebulized and oral), this study is listed twice. Each treatment group had its own placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Goh 1997.
| Methods | Randomized, blinded trial | |
| Participants | Inpatients less than 24 months old admitted for signs and symptoms consistent with clinical diagnosis of bronchiolitis. Mean age 5.2 to 7.4 months, 72% male. No history of previous wheezing, no underlying lung/cardiac disease. 42% RSV‐positive Country: Singapore | |
| Interventions | Group 1: salbutamol 2.5 mg/ml; Group 2: ipratropium bromide 250 µg/ml; Group 3: normal saline; Group 4: humidified oxygen without nebulization. Administered over 10 to 15 minutes by face masks driven by oxygen flow rate of 6 to 8 L/min. Nebulized at 4 to 6‐hourly intervals. Less than 6 months: 0.3 ml solution, more than 6 months: 0.6 ml solution in 2 ml saline for nebulizations | |
| Outcomes | Duration of hospitalizations, clinical score (5‐point score for each of respiratory rate, subcostal retractions, presence of wheeze and 2‐point score for each of presence of crepitations, oxygen requirement, nebulization, intravenous infusion). Used day 3 clinical scores | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Low risk | Fourth study group receiving humidified oxygen was studied 1 year later without blinding or allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For 3 treatment groups (salbutamol, ipratropium and normal saline) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 participants excluded without information about which group they were assigned to |
| Selective reporting (reporting bias) | High risk | Length of hospitalization not provided for the randomized groups; oximetry data not provided |
Gupta 2008.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | Outpatients less than 1 year of age, with clinical diagnosis of acute bronchiolitis defined as first episode of wheezing with evidence of an acute viral respiratory tract infection. Included only if mild disease (RR <= 70 breaths/min, SpO2 >= 95% in room air, no or mild accessory muscle use and RDAI score <= 10). Exclusions: dehydration, lethargy, chronic cardiopulmonary disease, or prior bronchodilator use Country: (North) India | |
| Interventions | Group 1: oral salbutamol (0.1 mg/kg/dose) 3 times daily for a maximum of 7 days or until symptoms resolved. Group 2: oral placebo given 3 times daily for a maximum of 7 days or until symptoms resolved | |
| Outcomes | Time to resolution of illness (ROI), defined as time from study enrolment to the time the infant returned to baseline health status, as determined by the principal caregiver on a 4‐point scale. Time to resolution of individual symptoms that comprised the ROI also included. Outcomes were determined at 3, 7 and 14 days. Hospitalization was also reported | |
| Notes | RDAI was used only at baseline. A total of 10 participants were lost to follow‐up, 7 (10%) in the salbutamol group and 3 (4.3%) in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Gurkan 2004.
| Methods | Randomized, placebo‐controlled study | |
| Participants | Inpatients aged between 2 and 24 months, with moderate acute viral bronchiolitis. Mean age 7.2 months, 68% male. 1. Diagnostic criteria were: an acute infection of the lower respiratory tract preceded by or accompanied by fever and/or rhinitis and characterized by tachypnea, expiratory wheezing and increased respiratory effort, as per Dobson 1998. Exclusions: infants with history of more than 1 hospitalization from wheezing; history of personal or familial atopy or presence of atopic dermatitis; chronic cardiac or pulmonary diseases; diagnosed immune deficiency disorder; recent use of corticosteroid or bronchodilator agent; concomitant severe diseases (pneumonia, meningitis, sepsis, etc.) Country: Turkey | |
| Interventions | Group 1: salbutamol 0.15 mg/kg dose. Group 2: adrenaline 0.5 mg (1 ml). Group 3: nebulized saline placebo ‐ 4 ml. All groups received routine supportive management | |
| Outcomes | Clinical score (adapted from Schuh 1990), heart rate, respiratory rate and oxygen saturation, temperature. (Clinical score included 4‐point scale for each of general appearance, accessory muscle use, wheezing). Evaluations were conducted at admission and 30 minutes, 1, 3, 12, 24 and 48 hours. 24‐hour data were used to be as consistent as possible with other data | |
| Notes | Unpublished data and study details provided by e‐mail from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear; not published |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
Henry 1983.
| Methods | Randomized, double‐blind trial | |
| Participants | Inpatients less than 1 year old with acute bronchiolitis. Mean age 4.3 months, 61% male, 68% RSV‐positive Country: UK | |
| Interventions | 6‐hourly nebulized solutions of 250 µg of ipratropium bromide in 2 ml saline (n = 34) or normal saline alone (n = 32) | |
| Outcomes | Day to improvement in study specific clinical score. 4‐point score for each of heart rate, respiratory rate, cough, rhinitis, nasal flaring, cyanosis, hyperinflation, tracheal tug, intercostal recession, subcostal recession, respiratory distress, crepitations and rhonchi. Side effects: increased heart rate, persistent coughing | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete data |
| Selective reporting (reporting bias) | High risk | Yes |
Ho 1991.
| Methods | Randomized, double‐blind, cross‐over trial | |
| Participants | Setting: inpatients Hospitalized participants less than 6 months old with first episode of cough and wheeze due to acute bronchiolitis. Mean age 3 months, 52% male, no underlying heart/lung disease. All RSV‐positive Country: Australia | |
| Interventions | Nebulized salbutamol (2 to 5 mg/2ml) or normal saline placebo (2 ml). Administered with nebulizer run from compressed gas supply with flow of 6 L/min. 30 to 40 minutes after initial treatment, participants crossed over | |
| Outcomes | Oxygen saturation up to 30 minutes after each treatment | |
| Notes | Short follow‐up after intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Unclear; insufficient detail provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | |
Ipek 2011.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | Setting: short‐stay unit of a pediatric emergency department Infants between 1 and 23 months of age, seen for moderate bronchiolitis, first episode of wheezing. Patients with history of preceding viral URI, followed by wheezing and crackles on auscultation and a clinical bronchiolitis severity score (CBSS) of 4 to 8. Mean age 7.96 ± 3.91 months. Exclusion criteria were: prematurity; birth weight < 2500 g; chronic neurological or cardiopulmonary disease, including asthma; infants younger than 1 month old and greater than 2 years; proven immune deficiency, consolidation or atelectasis on CXR; oxygen saturation < 85% on room air Country: Turkey | |
| Interventions | Group 1: nebulized salbutamol 0.15 mg/kg plus normal saline (NS) every 20 minutes for 3 doses Group 2: nebulized salbutamol 0.15 mg/kg plus hypertonic saline (HS) every 20 minutes for 3 doses Group 3: hypertonic saline every 20 minutes for 3 doses Group 4: normal saline every 20 minutes for 3 doses |
|
| Outcomes | Changes in CBSS, respiratory rate, oxygen saturation, heart rate assessed at 0, 20, 40 and 60 minutes; corticosteroid need, hospitalization ratios | |
| Notes | Sezer 2010 was a published abstract using the same trial data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Consecutive patients enrolled |
| Allocation concealment (selection bias) | High risk | Consecutive allocation to treatment groups; insufficient detail provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data; no withdrawals |
| Selective reporting (reporting bias) | Low risk | |
Karadag 2005 ‐ IPR.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | Setting: inpatients Infants less than 1 year of age, hospitalized for moderate to severe bronchiolitis, first episode of wheezing. Chest X‐ray compatible with bronchiolitis. Mean age 5.1 ± 2.7 months. No prematurity; chronic neurological or cardiopulmonary disease, including asthma; proven or suspected acute bacterial infection; previous treatment with bronchodilators or corticosteroids; infants younger than 4 weeks old and who needed ventilation at neonatal period; symptoms present for more than 7 days; fever higher than 38.5 °C; or infants with mild bronchiolitis. Country: Turkey | |
| Interventions | Group 1: nebulized salbutamol solution (Ventolin, Glaxo) plus saline solution (0.9%) 2.5 ml every 6 hours. Group 2: ipratropium bromide (Atrovent, Boehringer Ingelheim) 250 µg/2 ml plus 3 ml saline solution every 6 hours Group 3: normal saline received 5 ml every 6 hours | |
| Outcomes | Changes in the oxygen saturation rates, clinical scores and duration of hospital stay. Adverse effects were recorded, i.e. tachycardia and tremor after nebulization of each medication The clinical score system was based on respiratory rate, degree of wheezing, degree of accessory muscle use and general condition, described by Wang 1992. Improvement was defined as a decrease by 2 points in the total clinical score | |
| Notes | Ipratropium (IPR) treatment group: in order to represent the results from the 2 bronchodilator treatment arms (ipratropium and salbutamol), this study is listed twice. The placebo group was divided between comparisons to avoid double‐counting of placebo participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | Data on heart rate mentioned but not presented in results |
Karadag 2005 ‐ SAL.
| Methods | See Karadag 2005 ‐ IPR | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Salbutamol (SAL) treatment group: in order to represent the results from the 2 bronchodilator treatment arms (ipratropium and salbutamol), this study is listed twice. The placebo group was divided between comparisons to avoid double‐counting of placebo participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | See Karadag 2005 ‐ IPR |
Karadag 2008.
| Methods | Same as Karadag 2005 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This published manuscript describes the same study as Karadag 2005 that was published as an abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | See Karadag 2005 ‐ IPR |
Klassen 1991.
| Methods | Randomized, double‐blind, placebo‐controlled clinical trial | |
| Participants | Outpatients treated in emergency department, aged less than 24 months old, with first episode of wheezing. Mean age 7.2 months, 57% male, no underlying lung/cardiac disease or previous bronchodilator use Country: Canada | |
| Interventions | 2 treatments at 30‐minute intervals of either nebulized salbutamol (0.10 mg/kg in 2 ml normal saline) or similar volume (0.02 ml/kg) normal saline placebo. Administered for 5 to 8 minutes through updraft nebulizer with continuous flow of oxygen for 5 to 6 L/min | |
| Outcomes | Respiratory rate, heart rate, oxygen saturation, RDAI score (5‐point score for each of wheezing: expiration, inspiration, location; retractions: supraclavicular, intercostal, subcostal) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Levin 2008.
| Methods | Randomized, placebo‐controlled, blinded prospective study | |
| Participants | 22 infants with respiratory syncytial virus bronchiolitis who were in respiratory failure and intubated and ventilated in a pediatric ICU. Only first‐time wheezers were included. Mean age 8.1 weeks, 64% male, with no underlying lung or cardiac disease Country: United States | |
| Interventions | Randomized to 4 groups: albuterol (3 ml of 0.083%, 2.5 mg/3 ml), levalbuterol (3 ml of 1.25 mg/3 ml), norepinephrine (0.5 ml of 2.25% solution) and normal saline. Nebulized every 6 hours by the endotracheal tube. Each participants acted as their own control | |
| Outcomes | Peak inspiratory pressure, inspiratory respiratory system resistance and heart rate measured before and 20 minutes after treatment | |
| Notes | Participants recruited from December 2001 to March 2007. Study documented a significant increase in heart rate for all 3 bronchodilator treatment groups but not for the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Lines 1990.
| Methods | Double‐blind, controlled study | |
| Participants | Inpatients less than 18 months old admitted to hospital with bronchiolitis. Mean age 6.2 months, 73% male, no underlying lung/cardiac disease Country: Australia | |
| Interventions | 2 doses given at 2‐hour intervals. Either 0.2 ml salbutamol (5 mg/ml) or 0.2 ml saline in 4 ml of physiological saline given over 10 minutes with oxygen at 8 L/min through a Hudson mask | |
| Outcomes | RDAI, oximetry, RACS (wheezing, retraction, respiratory rate), pulse rate | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | High risk | |
Lines 1992.
| Methods | Randomized, double‐blind, controlled, prospective clinical study | |
| Participants | Inpatients less than 18 months old admitted with acute bronchiolitis. No underlying lung/cardiac disease Country: Australia | |
| Interventions | 2 doses (with 2‐hour interval) of nebulized ipratropium bromide 1 ml (250 µg) in 4 ml saline or 5 ml saline placebo | |
| Outcomes | Oxygen saturation, RACS, respiratory rate, heart rate | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Mallol 1987.
| Methods | Randomized trial | |
| Participants | Inpatients less than 1 year old admitted with acute wheezing. Mean age 5.9 months, 67% male, no underlying lung/cardiac disease Country: Chile | |
| Interventions | Group 1: nebulized fenoterol plus ipratropium bromide. Group 2: fenoterol. Group 3: fenoterol plus steroids. Group 4: aminophylline, IV, plus steroids and oral fenoterol (FNT). Group 5: nebulized normal saline (control). Pediatric nebulizers used with the bronchodilator and saline amounting to 4 ml. A flow of 6 L/min of compressed air, or occasionally, oxygen was used. Warm saline used. Dosage of drugs: nebulized FNT ‐ 0.04 ml/kg/dose every 6 hours (0.5% solution), nebulized IB ‐ 250 µg/dose every 6 hours (0.025% solution), oral or IV aminophylline ‐ less than 6 months (age in weeks *0.3 + 8 = mg/kg/day, 4 equal doses every 6 hours) or more than 6 months (15 mg/kg/day, 4 equal doses every 6 hours), steroids: dexamethasone (IV or IM, 0.3 mg/kg/dose initially, 0.3 mg/kg/day, 3 equal doses every 8 hours) or prednisone (oral 2 mg/kg/day, 3 equal doses every 8 hours) | |
| Outcomes | Clinical score same as with Tal 1983. No adverse side effects | |
| Notes | No distinction made between asthma and bronchiolitis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blinding only |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants whose scores did not decrease at 24 hours were excluded from the study as "failures" |
| Selective reporting (reporting bias) | Low risk | |
Patel 2002.
| Methods | Randomized, double‐blind, parallel‐group, controlled trial | |
| Participants | Inpatients less than 12 months old with clinical diagnosis of bronchiolitis. Mean age 4 months. No previous wheeze or bronchodilator use, prematurity, underlying chronic disease, immunocompromise, RSV immunoprophylaxis or parents not fluent in English or French Country: Canada | |
| Interventions | Group 1: epinephrine (0.03 ml/kg/dose of a 2.25% solution) Group 2: nebulized albuterol (0.03 ml/kg of a 5 mg/ml solution) Group 3: saline (0.03 ml/kg/dose of 0.9% solution of 0.9% sodium chloride) | |
| Outcomes | Duration of hospitalization (LOS) was defined as time between study entry and time that infant left the inpatient ward, time from admission to normal hydration, oxygenation and minimal respiratory distress RDAI (Lowell 17‐point categorical score) | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Patel 2003.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 129 infants, mean age 5.3 months, 60% male, seen in an emergency department setting for mild to moderate bronchiolitis, defined as first episode of wheezing in an infant with evidence of URI. Upon discharge, randomized to receive either oral albuterol or placebo. Exclusions were age older than 12 months, prior wheezing, prior bronchodilator use, underlying lung or cardiac disease, or admission to hospital Country: Canada | |
| Interventions | First dosage of medication was given in the ED before discharge. Oral albuterol was dosed at 0.1 mg/kg per dose given 3 times per day for 7 days. Placebo was also given 3 times per day for 7 days | |
| Outcomes | Time to resolution of illness (ROI), measured on a daily basis by telephone interview until the score of 4 was documented. Secondary outcomes included time to normal feeding, normal sleeping, quiet breathing, resolved cough and resolved coryza. Hospitalization was also recorded | |
| Notes | RDAI was used only at baseline. More infants in the albuterol group who did not complete 7 days of therapy as compared to placebo (8 in albuterol and 2 in placebo). There were 2 withdrawals from each study group. Total drop out for this study was 10.8% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Ralston 2005.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | 65 participants ages 6 weeks to 24 months, outpatients with acute bronchiolitis seen in an urgent care setting. Mean age 7.6 months, 55% male. Inclusion criteria were RDAI score between 4 and 14. Exclusion criteria were prior wheezing or asthma, lung or cardiac disease, systemic steroid use or physiologic instability at presentation Country: United States, high altitude (5000 feet) |
|
| Interventions | Treatment was 5 mg of racemic albuterol in 3 ml of normal saline administered at 0 and 30 minutes, compared to 3 ml placebo nebulization of 0.9% saline. (A third group received 5 mg racemic epinephrine) | |
| Outcomes | Need for hospitalization or home oxygen. RDAI and oxygen saturation at 60 minutes were included as unpublished data | |
| Notes | Participants recruited from January 2000 to March 2004 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Low risk | Clinical scores and oximetry data that were not published were obtained from the author for the 2010 update |
Scarlett 2012.
| Methods | Randomized, double‐blind, placebo‐controlled trial | |
| Participants | Inpatients younger than or equal to 12 months of age with a first episode of wheezing due to RSV bronchiolitis. Mean age in placebo group 8.8 weeks, albuterol 20.1 weeks (despite randomization, albuterol‐treated infants were significantly older than placebo‐treated infants, P value < 0.0001). Excluded preterm infants, underlying chronic lung disease, previous history of wheezing or treatment with bronchodilators before current illness, previous treatment with RSV prophylaxis therapy, history of respiratory infection 3 weeks before enrollment, hemodynamically significant congenital heart disease, immune‐compromised state, albuterol therapy within 6 hours of administration of the study drug, gastro‐esophageal reflux requiring medical therapy, neurodevelopmental delay, severe bronchiolitis requiring admission to pediatric intensive care Country: USA |
|
| Interventions | Group 1: albuterol nebulized (0.1 mg/kg in 3 ml saline). Group 2: saline (3 ml) | |
| Outcomes | Respiratory inductive plethysmography (RIP) for a total of 30 breaths, ratio of time to peak expiratory flow to total expiratory time (Tpef/Te), RDAI, oxygen saturation, respiratory rate | |
| Notes | Only infants with a 10% increase in heart rate were included in the final analysis. 3 participants excluded because they did not maintain quiet sleep. Ren 2011 was a published poster abstract using the same trial data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Performed by research pharmacy (unpublished information) |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Schuh 1990.
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Outpatients in emergency department, 6 weeks to 24 months old. Mean age 5.7 months. No prior history of wheeze or bronchodilators, no underlying lung/cardiac disease Country: Canada | |
| Interventions | Group 1: 3 doses of 0.5% nebulized salbutamol, 0.15 mg/kg/dose at 1‐hour intervals, Group 2: 2 doses of nebulized saline solution, followed by 1 dose of 0.5% nebulized salbutamol, 0.15 mg/kg/dose, 1 hour apart. All doses suspended in 3 ml normal saline solution and delivered for 15 minutes by face mask and nebulizer, driven by oxygen at flow rate of 6 to 7 L/min | |
| Outcomes | Respiratory rate, heart rate, accessory muscle score, wheezing score, transcutaneous oxygen saturation | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Schweich 1992.
| Methods | Double‐blind, placebo‐controlled trial | |
| Participants | Outpatients admitted to emergency department, aged less than 24 months old with wheezing. Mean age 7.35 months old, 48% male, no underlying cardiac/lung disease. 3 infants in each study group had prior wheezing Country: USA | |
| Interventions | 2 doses of nebulized salbutamol (0.15 mg/kg in 3 ml normal saline) or placebo (0.03 ml/kg normal saline in 3 ml normal saline). Both administered with continuous‐flow oxygen at 6 liters/min at interval of about 30 minutes | |
| Outcomes | Respiratory rate, heart rate, wheeze score (5‐point score for each of expiration, inspiration, location), retraction score (5 point score for each of supraclavicular, intercostal, subcostal), oxygen saturation) | |
| Notes | Included recurrent wheezers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Tal 1983.
| Methods | Randomized, double‐blind trial | |
| Participants | Inpatients aged 1 to 12 months, hospitalized with bronchiolitis, asthma or WARI. Mean age 5.4 months, 62.5% male Country: USA | |
| Interventions | Intramuscular dexamethasone or placebo (double‐blind) and salbutamol (oral and inhaled) or none (open) in all 4 possible combinations. Dexamethasone (4 mg/ml) or placebo (normal saline) administered intramuscularly, 0.075 ml/kg on admission and 0.025 ml/kg every 8 hours for next 3 days. Also, half of these patients were given salbutamol (via 2 routes simultaneously) or no additional treatment. Salbutamol: inhalation (0.5 ml salbutamol respiratory solution with 2 ml water) given on admission and subsequently every 6 hours, oral (salbutamol syrup, 0.15 mg/kg) every 8 hours | |
| Outcomes | Study‐specific clinical scoring system (4‐point scale for each of respiratory rate, wheezing, cyanosis, use of accessory muscles). Measurements of arterial blood gases, blood pressure. Side effects: tremors | |
| Notes | Included asthmatic patients and recurrent wheezers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 relative therapeutic failures and 2 complete therapeutic failures were excluded from analysis |
| Selective reporting (reporting bias) | Low risk | |
Tinsa 2009.
| Methods | Prospective, randomized, placebo‐controlled, double‐blind clinical trial | |
| Participants | 36 first‐time wheezing infants ages 3 to 12 months admitted to hospital for moderate severity bronchiolitis. Inclusion criterion was RDAI score between 4 and 15. Excluded were children with underlying lung or cardiac disease, concurrent bronchodilator or corticosteroid treatment and recurrent wheezing Country: Tunisia | |
| Interventions | Treatment was nebulized terbutaline at 0.15 mg/kg in 4 ml of normal saline every 4 hours. Placebo group received 4 ml of normal saline nebulized | |
| Outcomes | RDAI score, respiratory rate, pulse oximetry and heart rate at 0, 30, 60 and 120 minutes after the first treatment and duration of hospitalization | |
| Notes | 1 participant withdrawn from placebo group due to worsening clinical status, necessitating transfer to the ICU | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Totapally 2002.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study | |
| Participants | Inpatients less than 12 months old with a first episode of wheezing due to RSV bronchiolitis. Mean age 5.8 months. Excluded preterm infants, underlying chronic disease or infants with grunting Country: USA | |
| Interventions | Group 1: albuterol nebulized (0.15 mg/kg in 3 ml saline). Group 2: saline (3 ml). All infants treated first with chloral hydrate. Participants crossed over at 6‐hour intervals in random order | |
| Outcomes | Tidal breathing flow loops, wheeze score, heart rate, respiratory rate, pulse oximetry, ratio of time to peak expiratory flow to total expiratory time (Tpef/Te) | |
| Notes | Wheeze score was: 0 for none 1 for end exp 2 for audible with stethoscope 3 for audible without stethoscope | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Wang 1992.
| Methods | Randomized, double‐blind, factorial trial | |
| Participants | Setting: inpatients Infants 2 to 24 months of age hospitalized for the first time with mild bronchiolitis. 55% male, no underlying cardiac/lung disease Country: Canada | |
| Interventions | Group 1: salbutamol at 0.15 mg/kg/dose in 2 ml saline followed 1 hour later by 0.5 ml or 1 ml saline placebo. Group 2: 0.03 ml/kg saline in 2 ml saline followed by either 125 µg ipratropium bromide if less than 6 months old or 250 µg ipratropium bromide if older than 6 months. Group 3: both salbutamol and ipratropium bromide in doses indicated. Group 4: saline placebos in same volumes indicated | |
| Outcomes | Oxygen saturation, study‐specific clinical assessment (4‐point score for each of respiratory rate, wheezing, retractions, general condition) | |
| Notes | Infants with prior use of bronchodilators were included (1 in salbutamol, 2 in ipratropium, 4 in saline). 4 participants withdrawn from trial due to worsening: 1 in Group 1, 2 in Group 3 and 1 in Group 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
CBSS = clinical bronchiolitis severity score CXR = chest X‐ray ED = emergency department HR = heart rate hr = hour HS = hypertonic saline ICU = intensive care unit IM = intramuscular IV = intravenous L/min = liters per minute MDI = metered dose inhaler NS = normal saline RACS = Respiratory Assessment Change Score RDAI = Respiratory Distress Assessment Instrument RDI = Respiratory Distress Index RDS = Respiratory Distress Score RR = respiratory rate RSV = respiratory syncytial virus SpO2 = oxygen saturation URI = upper respiratory infection WARI = wheeze associated acute respiratory infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Absar 2008 | No placebo group |
| Abu‐Shukair 2001 | No placebo group |
| Alansari 2013 | No placebo group |
| Barlas 1998 | No placebo group |
| Beck 2007 | No placebo group |
| Belcastro 2010 | Research protocol only ‐ no patient outcomes data |
| Bentur 2003 | No placebo group |
| Bertrand 2001 | No placebo group |
| Brooks 1981 | Not a randomized controlled trial |
| Cengizlier 1997 | Control group was not given placebo |
| Chao 2003 | Groups were stratified by age but no equivalent aged placebo group for the bronchodilator (terbutaline) group, therefore no comparison could be made |
| Choong 1998 | Poster abstract only |
| Cortes 1996 | Not clearly randomized, insufficient information provided in brief report |
| Del Vecchio 2012 | Not a randomized controlled trial, no placebo group |
| Fernandez 2009 | Study compared heliox versus oxygen to drive albuterol or epinephrine. No placebo group |
| Ferrer 1990 | Only available in abstract form |
| Florin 2012 | No placebo group |
| Frasson 2012 | Abstract only; testing method of nebulization; no placebo group |
| Goebel 2000 | No placebo group |
| Gomez‐y‐Lopez 2007 | No placebo group |
| Gonzalez 1994 | No placebo group |
| Hammer 1995 | Not a RCT; no placebo group |
| John 2006 | No placebo group |
| John 2010 | No placebo group |
| Kadir 2009 | No placebo group and not blinded |
| Karaatmaca 2010 | Abstract only |
| Kim 2011 | No placebo group |
| Langley 2005 | No placebo group |
| Luo 2003 | No placebo group; quasi‐experimental; not fully randomized |
| Luo 2010 | No placebo group |
| Luo 2012 | No placebo group |
| Mandelberg 2003 | No placebo group |
| Menon 1995 | No placebo group |
| Milner 1995 | Data not provided |
| Modaressi 2012 | No placebo group |
| Modl 2005 | Not randomized or placebo‐controlled |
| Mull 2004 | No placebo group |
| Ndrepepa 1998 | Poster abstract only, available only in Turkish |
| Numa 2001 | Not a RCT; no placebo group; epinephrine only |
| Ozyurek 2002 | No placebo group |
| Ralston 2008 | Nasal phenylephrine, not used as a bronchodilator |
| Ray 2002 | No placebo group |
| Reijonen 1995 | No placebo group |
| Ren 2011 | Poster abstract only |
| Sanchez 1993 | Not a RCT; no placebo group |
| Sarrell 2002 | No placebo group |
| Schuh 1992 | No placebo group |
| Sezer 2010 | Abstract only |
| Sharma 2013 | No placebo group |
| Shu 2001 | Not randomized |
| Simsek 2005 | No placebo group; abstract only |
| Simsek‐Kiper 2011 | Nebulized epinephrine versus salbutamol; no placebo group |
| Sly 1991 | Patients did not clearly have bronchiolitis |
| Soto 1985 | Not a RCT; salbutamol only ‐ no placebo group |
| Springer 1990 | Results and analysis focused on pulmonary function tests |
| Stokes 1983 | Excluded from original review as results and analysis focused on pulmonary function tests. Excluded from update as not clearly randomized and water not a valid placebo |
| Tatochenko 1988 | Criteria for diagnosis unclear |
| Torres 1997 | No placebo group |
| Walsh 2008 | Compared 3 doses of albuterol to 1 dose of epinephrine plus 2 saline nebulizers and therefore was not placebo‐controlled |
| Wankum 2000 | Results and analysis focused on pulmonary function tests. Only 3 infants studied. Author contacted but no response |
| Zhen 2003 | Poster abstract only |
| Zhou 2001 | No placebo group |
RCT: randomized controlled trial
Differences between protocol and review
Oxygen saturation was designated as the primary outcome in the 2010 update. Two review authors assessed risk of bias and completed the 'Risk of bias' table for all studies in the 2010 update. We completed sensitivity analysis for low risk of bias studies, studies including only first‐time wheezers and studies including only infants less or equal to 12 months of age in the 2010 update. We completed sensitivity analysis for studies using RDAI in the 2014 update. We added subgroup analyses limited to studies using either albuterol or salbutamol in 2014.
Contributions of authors
For the 2006 update, Anne Gadomski (AG) reviewed all the searches, selected studies and contacted authors to request unpublished data. AG identified outcomes of trials relevant for inclusion, reviewed the results and wrote the discussion and conclusions. Alice Bhasale (AB) assisted with some of the searches and selection of studies, data entry and analyses.
For the 2010 and 2014 updates, AG and Melissa (Brower) Scribani (MS) reviewed all the searches, selected studies, reviewed the included studies for risk of bias as well as outcomes and reviewed meta‐analysis results. MS performed the meta‐analysis and sensitivity analysis. AG contacted authors to request unpublished data and updated the text of the review.
Sources of support
Internal sources
National Prescribing Service Pty Ltd, Australia.
External sources
No sources of support supplied
Declarations of interest
Ann Gadomski is a trialist in included studies and is a member of the AAP Subcommittee on Diagnosis and Management of Bronchiolitis. Melissa Scribani: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Alario 1992 {published data only}
- Alario AJ, Lewander WJ, Dennehy P, Seifer R, Mansell AL. The efficacy of nebulized metaproterenol in wheezing infants and young children. American Journal of Diseases of Children 1992;146:412‐8. [DOI] [PubMed] [Google Scholar]
Anil 2010 SAL 0.9% {published data only}
- See Anil 2010 SAL 3.0%.
Anil 2010 SAL 3% {published data only}
- Anil AB, Anil M, Saglam AB, Cetin N, Bal A, Aksu N. High volume normal saline alone is as effective as nebulized salbutamol‐normal saline, epinephrine‐normal saline, and 3% saline in mild bronchiolitis. Pediatric Pulmonology 2010;45:41‐7. [DOI] [PubMed] [Google Scholar]
Can 1998 {published data only}
- Can D, Inan G, Yendur G, Oral R, Günay I. Salbutamol or mist in acute bronchiolitis. Acta Paediatrica Japonica 1998;40(3):252‐5. [DOI] [PubMed] [Google Scholar]
Chevallier 1995 {published data only}
- Chevallier B, Aegerter P, Parat S, Bidat E, Renaud C, Lagardere B. Comparative study of nebulized sambutol against placebo in the acute phase of bronchiolitis in 33 infants aged 1 to 6 months [Etude comparative des nebulisations de salbutamol contre placebo a la phase aigue d'une bronchiolite chez 33 nourrissons de 1 a 6 mois]. Archives de Pédiatrie 1995;2(1):11‐7. [DOI] [PubMed] [Google Scholar]
Chowdhury 1995 {published data only}
- Chowdhury D, Al Howasi M, Khalil M, Al‐Frayh AS, Chowdhury S, Ramia S. The role of bronchodilators in the management of bronchiolitis: a clinical trial. Annals of Tropical Paediatrics 1995;15:77‐84. [DOI] [PubMed] [Google Scholar]
Dobson 1998 {published data only}
- Dobson JV, Stephen‐Groff SM, McMahon SR, Stemmler MM, Brallier SL, Bay C. The use of albuterol in hospitalized infants with bronchiolitis. Pediatrics 1998;101:361‐8. [DOI] [PubMed] [Google Scholar]
Gadomski 1994a ‐ neb {published data only}
- Gadomski AM, Aref GH, Din OB, Sawy IH, Khallaf N, Black RE. Oral versus nebulized albuterol in the management of bronchiolitis in Egypt. Journal of Pediatrics 1994;124:131‐8. [DOI] [PubMed] [Google Scholar]
Gadomski 1994a ‐ oral {published data only}
- See Gadomski 1994a ‐ neb.
Gadomski 1994b ‐ neb {published data only}
- Gadomski AM, Lichenstein R, Horton L, King J, Keane V, Permutt T. Efficacy of albuterol in the management of bronchiolitis. Pediatrics 1994;93:907‐12. [PubMed] [Google Scholar]
Gadomski 1994b ‐ oral {published data only}
- See Gadomski 1994b ‐ neb.
Goh 1997 {published data only}
- Goh A, Chay OM, Foo AL, Ong EK. Efficacy of bronchodilators in the treatment of bronchiolitis. Singapore Medical Journal 1997;38:326‐8. [PubMed] [Google Scholar]
Gupta 2008 {published data only}
- Gupta P, Aggarwal A, Gupta P, Sharma KK. Oral salbutamol for symptomatic relief in mild bronchiolitis: a double blind randomized placebo controlled trial. Indian Pediatrics 2008;45:547‐53. [PubMed] [Google Scholar]
Gurkan 2004 {published and unpublished data}
- Gurkan F, Tuzun H, Ece A, Haspolat K, Bosnak M, Dikici B. Comparison of treatments with nebulized salbutamol and epinephrine in acute bronchiolitis [Abstract]. European Respiratory Journal 2004;24(Suppl 48):344. [Google Scholar]
Henry 1983 {published data only}
- Henry RL, Milner AD, Stokes GM. Ineffectiveness of ipratropium bromide in acute bronchiolitis. Archives of Disease in Childhood 1983;58:925‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 1991 {published data only}
- Ho L, Collis G, Landau LI, Souf PN. Effect of salbutamol on oxygen saturation in bronchiolitis. Archives of Disease in Childhood 1991;66:1061‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ipek 2011 {published data only}
- Ipek IO, Yalcin EU, Sezer RG, Bozaykut A. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol/hypertonic saline combination in moderate bronchiolitis. Pulmonary Pharmacology and Therapeutics 2011;24:633‐7. [DOI] [PubMed] [Google Scholar]
Karadag 2005 ‐ IPR {published and unpublished data}
- Karadag B, Ceran O, Dursun E, Guven G, Ozahi I, Karakoc F, et al. Efficacy of bronchodilators in acute bronchiolitis [Abstract]. European Respiratory Journal 2000;16(Suppl 31):562. [CN‐00415977] [Google Scholar]
- Karadag B, Ceran O, Guven G, Dursun E, Ozahi I, Karakoc F, et al. The role of bronchodilators in the management of acute bronchiolitis. Unpublished manuscript 2005.
- Karadag B, Cerna O, Dursun E, Guven G, Ozahi I, Karakoc F, et al. Comparison of bronchodilators in acute bronchiolitis [Abstract]. European Respiratory Society Annual Congress. 2002. [CN‐00429340]
Karadag 2005 ‐ SAL {published and unpublished data}
- See Karadag 2005 ‐ IPR.
Karadag 2008 {published data only}
- Karadag B, Ceran O, Guven G, Dursun E, Ipek IO, Karakoc F, et al. Efficacy of salbutamol and ipratropium bromide in the management of acute bronchiolitis ‐ a clinical trial. Respiration 2008;76:283‐7. [DOI] [PubMed] [Google Scholar]
Klassen 1991 {published data only}
- Klassen TP, Rowe PC, Sutcliffe T, Ropp LJ, McDowell IW, Li MM. Randomized trial of salbutamol in acute bronchiolitis. Journal of Pediatrics 1991;118:807‐11. [DOI] [PubMed] [Google Scholar]
Levin 2008 {published data only}
- Levin DL, Garg A, Hall LJ, Slogic S, Jarvis JD, Leiter JC. A prospective randomized controlled blinded study of three bronchodilators in infants with respiratory syncytial virus bronchiolitis on mechanical ventilation. Pediatric Critical Care Medicine 2008;9:598‐604. [DOI] [PubMed] [Google Scholar]
Lines 1990 {published data only}
- Lines DR, Kattampallil JS, Liston P. Efficacy of nebulized salbutamol in bronchiolitis. Pediatric Reviews & Communications 1990;5:121‐9. [Google Scholar]
Lines 1992 {published data only}
- Lines DR, Bates ML, Rechtman AR. Efficacy of nebulized ipratropium bromide in acute bronchiolitis. Pediatric Reviews & Communications 1992;6:161‐7. [Google Scholar]
Mallol 1987 {published data only}
- Mallol J, Barrueto L, Girardi G, Munoz R, Puppo H, Ulloa V, et al. Use of nebulized bronchodilators in infants under 1 year of age: analysis of four forms of therapy. Pediatric Pulmonology 1987;3:298‐303. [DOI] [PubMed] [Google Scholar]
Patel 2002 {published and unpublished data}
- Patel H, Platt RW, Pekeles GS, Ducharme FM. A randomized, controlled trial of the effectiveness of nebulized therapy with epinephrine compared with albuterol and saline in infants hospitalized for acute viral bronchiolitis. Journal of Pediatrics 2002;141(6):818‐24. [DOI] [PubMed] [Google Scholar]
Patel 2003 {published data only}
- Patel H, Gouin S, Platt RW. Randomized, double‐bind, placebo‐controlled trial of oral albuterol in infants with mild‐to‐moderate acute viral bronchiolitis. Journal of Pediatrics 2003;142:509‐14. [DOI] [PubMed] [Google Scholar]
Ralston 2005 {published and unpublished data}
- Ralston S, Hartenberger C, Anaya T, Qualls C, Kelly HW. Randomized, placebo‐controlled trial of albuterol and epinephrine at equipotent beta‐2 agonist doses in acute bronchiolitis. Pediatric Pulmonology 2005;40:292‐9. [DOI] [PubMed] [Google Scholar]
Scarlett 2012 {published data only}
- Scarlett EE, Walker S, Rovitelli A, Ren CL. Tidal breathing responses to albuterol and normal saline in infants with viral bronchiolitis. Pediatric Allergy, Immunology, and Pulmonology 2012;25:220‐5. [Google Scholar]
Schuh 1990 {published data only}
- Schuh S, Canny G, Reisman JJ, Kerem E, Bentur L, Petric M, et al. Nebulized albuterol in acute bronchiolitis. Journal of Pediatrics 1990;117:633‐7. [DOI] [PubMed] [Google Scholar]
Schweich 1992 {published data only}
- Schweich PJ, Hurt TL, Walkley EI, Mullen N, Archibald LF. The use of nebulized albuterol in wheezing infants. Pediatric Emergency Care 1992;8:184‐8. [DOI] [PubMed] [Google Scholar]
Tal 1983 {published data only}
- Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71:13‐8. [PubMed] [Google Scholar]
Tinsa 2009 {published data only}
- Tinsa F, Rhouma AB, Ghaffari H, Zouari B, Brini I, Karboul L, et al. A randomized, controlled trial of nebulized terbutaline for the first acute bronchiolitis in infants less than 12‐months‐old. Tunisie Medicale 2009;87:200‐3. [PubMed] [Google Scholar]
Totapally 2002 {published data only}
- Totapally BR, Demerci C, Zureikat G, Nolan B. Tidal breathing flow‐volume loops in bronchiolitis in infancy: the effect of albuterol. Critical Care (London) 2002;6(2):160‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1992 {published data only}
- Wang EEL, Milner R, Allen U, Maj H. Bronchodilators for treatment of mild bronchiolitis: a factorial randomised trial. Archives of Disease in Childhood 1992;67:289‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Absar 2008 {published data only}
- Absar SMN, Jahan S, Khanum S. Efficacy of nebulized ipratropium bromide versus salbutamol in infants with acute bronchiolitis. Bangladesh Medical Research Council Bulletin 2008;34(3):112. [DOI] [PubMed] [Google Scholar]
Abu‐Shukair 2001 {published data only}
- Abu‐Shukair M, El‐Tal Y, Al‐Rawabdeh E. Salbutamol versus epinephrine in the treatment of acute bronchiolitis. Journal of the Bahrain Medical Society 2001;13(3):143‐6. [Google Scholar]
Alansari 2013 {published data only}
- Alansari K, Sakran M, Davidson BL, Ibrahim K, Alrefai M, Zakaria I. Oral dexamethasone for bronchiolitis: a randomized trial. Pediatrics 2013;132(1):e810‐6. [DOI] [PubMed] [Google Scholar]
Barlas 1998 {published data only}
- Barlas C, Kiper N, Gocmen A, Ozcelik U, Dilber E, Anadol D, et al. Racemic adrenaline and other treatment regimens in mild and moderate bronchiolitis [Hafif ve orta siddetteki bronsiolit vakalarinda rasemik adrenalin ve diger tedavi yontemlerinin karsilastirilmasi]. Cocuk Sagligi Ve Hastaliklari Dergisi 1998;41:155–65. [Google Scholar]
Beck 2007 {published data only}
- Beck R, Elias N, Shoval S, Tov N, Talmon G, Godfrey S, et al. Computerized acoustic assessment of treatment efficacy of nebulized epinephrine and albuterol in RSV bronchiolitis. BMC Pediatrics 2007;7:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belcastro 2010 {published data only}
- Belcastro MR, Backes CR, Chila AG. Bronchiolitis: a pilot study of osteopathic manipulative treatment, bronchodilators, and other therapy. Journal of the American Osteopathic Association 2010;83(9):672‐6. [PubMed] [Google Scholar]
Bentur 2003 {published data only}
- Bentur L, Beck R, Shoval S, Elias N, Naveh T, Godfrey S. Nebulized epinephrine versus albuterol for treatment of RSV bronchiolitis. American Thoracic Society 99th International Conference. 2003:C093 Poster 703.
Bertrand 2001 {published data only}
- Bertrand P, Aranibar H, Castro E, Sanchez I. Efficacy of nebulized epinephrine versus salbutamol in hospitalized infants with bronchiolitis. Pediatric Pulmonology 2001;31:284‐8. [DOI] [PubMed] [Google Scholar]
Brooks 1981 {published data only}
- Brooks LJ, Cropp GJA. Theophylline therapy in bronchiolitis. American Journal of Diseases of Children 1981;135:934‐6. [DOI] [PubMed] [Google Scholar]
Cengizlier 1997 {published data only}
- Cengizlier R, Saraclar Y, Adalioglu G, Tuncer A. Effect of oral and inhaled salbutamol in infants with bronchiolitis. Acta Paediatrica Japonica 1997;39:61‐3. [DOI] [PubMed] [Google Scholar]
Chao 2003 {published data only}
- Chao LC, Lin YZ, Wu WF, Huang FY. Efficacy of nebulized budesonide in hospitalized infants and children younger than 24 months with bronchiolitis. Acta Paediatrica Taiwanica 2003;44(6):332‐5. [PubMed] [Google Scholar]
Choong 1998 {published data only}
- Choong CS, Huang YF, Liew KL, Liu PN, Tsai DH, Cheng IY, et al. The efficacy of nebulized beta‐2 agonist bronchodilator therapy in the treatment of acute bronchiolitis. Tsu Chi Medical Journal 1998;10(2):95‐101. [Google Scholar]
Cortes 1996 {published data only}
- Cortes ME, Graniel GJ. Administration of nebulized salbutamol versus placebo in infants with bronchiolitis. Practica Pediatrica 1996;5(7):18‐9. [Google Scholar]
Del Vecchio 2012 {published data only}
- Vecchio MT, Doerr LE, Gaughan JP. The use of albuterol in young infants hospitalized with acute RSV bronchiolitis. Interdisciplinary Perspectives on Infectious Diseases 2012;2012:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandez 2009 {published data only}
- Fernandez CI, Fernandez BH, Navarro CM, Soler MG, Lopez PV, Pardillo RM. Heliox‐driven bronchodilator nebulization in the treatment of infants with bronchiolitis. Annals of Pediatrics 2009;70:40‐4. [DOI] [PubMed] [Google Scholar]
Ferrer 1990 {published data only}
- Ferrer C, Gaig P, Marin A, et al. Bromuro de ipratropio en las bronquiolitis. Revista Espanola de Alergologia e Immunologia Clinica 1990;5(Suppl 1):83‐4. [Google Scholar]
Florin 2012 {published data only}
- Florin TA, Kittick M, Shaw KN, Zorc JJ. A randomized trial of nebulized hypertonic saline for bronchiolitis in the emergency department. Pediatric Emergency Care 2012;28(9):1096. [DOI] [PubMed] [Google Scholar]
Frasson 2012 {published data only}
- Frasson T, Nascimento‐Junior G, Frasson F, Rossi M, Rodrigues A, Goncalves W, et al. Increase in induced expiratory flow reduces peripheral hypoxemia in children with acute bronchiolitis. Pediatric Critical Care Medicine 2012;13(5):618. [Google Scholar]
Goebel 2000 {published data only}
- Goebel J, Estrada B, Quinonez J, Nagji N, Sanford D, Boerth RC. Prednisolone plus albuterol versus albuterol alone in mild to moderate bronchiolitis. Clinical Pediatrics 2000;39(4):213‐20. [DOI] [PubMed] [Google Scholar]
Gomez‐y‐Lopez 2007 {published data only}
- Gomez‐y‐Lopez RE, Hernandez‐Sierra JF, Torres‐Ruvalcaba BA, Martinez‐Puente E, Carmen Martinez‐Garcia M. Comparative clinical study of dexamethasone vs. nebulized salbutamol in acute bronchiolitis. Gaceta Medica de Mexico 2007;142(3):189‐92. [PubMed] [Google Scholar]
Gonzalez 1994 {published data only}
- Gonzalez PYE, Ruiz BA, Garate AJ, Romero IC, Bone CJ, Arranz AL, et al. A multicenter randomized, open, parallel group study comparing different treatments for hospitalized infants with acute wheezy bronchitis. Anales Espanoles de Pediatria 1994;14(5):315‐9. [Google Scholar]
Hammer 1995 {published data only}
- Hammer J, Numa A, Newth CJL. Albuterol responsiveness in infants with respiratory failure caused by respiratory syncytial virus infection. Pediatric Pharmacology and Therapeutics 1995;127(3):485‐90. [DOI] [PubMed] [Google Scholar]
John 2006 {published data only}
- John BM, Patnaik SK, Prasad PL. Efficacy of nebulized epinephrine versus salbutamol in hospitalized children with bronchiolitis. Medical Journal Armed Forces India 2006;62:354‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
John 2010 {published data only}
- John BM, Singh D. Comparison of nebulised salbutamol and L‐epinephrine in first time wheezy children. Medical Journal Armed Forces India 2010;66:9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kadir 2009 {published data only}
- Kadir MA, Mollah AH, Basak R, Choundhury AM, Ahmed S. Comparative efficacy of combined nebulized salbutamol with ipratropium bromide and nebulized adrenaline to treat children with acute bronchiolitis. Mymensingh Medical Journal 2009;18(2):208‐14. [PubMed] [Google Scholar]
Karaatmaca 2010 {published data only}
- Karaatmaca B, Aydogan M, Piraneci R, Bicer S. Comparison of the effectiveness of nebulized budesonide, salbutamol and adrenaline in the treatment of acute bronchiolitis: a randomized double blind placebo controlled clinical trial. Allergy 2010;65(Suppl 92):716. [Google Scholar]
Kim 2011 {published data only}
- Kim IK, Phrampus E, Sikes K, Pendleton J, Saville A, Corcoran T, et al. Helium‐oxygen therapy for infants with bronchiolitis: a randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2011;165(12):1115‐22. [DOI] [PubMed] [Google Scholar]
Langley 2005 {published data only}
- Langley JM, Smith MB, LeBlanc JC, Joudrey H, Ojah CR, Pianosi P. Racemic epinephrine compared to salbutamol in hospitalized young children with bronchiolitis; a randomized controlled clinical trial. BMC Pediatrics 2005;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luo 2003 {published data only}
- Luo AW, Ma HH, Zhou W, Xie XA. The clinical observation of budesonide and terbutaline aerosol on the treatment of bronchiolitis. Chinese Journal of Hospital Pharmacy 2003;23(9):549‐50. [Google Scholar]
Luo 2010 {published data only}
- Luo A, Lui E, Luo J, Li S, Zeng F, Yang X, et al. Nebulized hypertonic saline/salbutamol solution treatment in hospitalized children with mild to moderate bronchiolitis. Pediatrics International 2010;52:199‐202. [DOI] [PubMed] [Google Scholar]
Luo 2012 {published data only}
- Luo Z, Liu E, Luo J, Li S, Zeng F, Yang X, et al. Nebulized hypertonic saline/salbutamol solution treatment in hospitalized children with mild to moderate bronchiolitis. Pediatrics International 2010;52(2):199‐202. [DOI] [PubMed] [Google Scholar]
Mandelberg 2003 {published data only}
- Mandelberg A, Tal G, Witzling M, Someck E, Houri S, Balin A, et al. Nebulized 3% hypertonic saline solution treatment in hospitalized infants with viral bronchiolitis. Chest 2003;123:481‐7. [DOI] [PubMed] [Google Scholar]
Menon 1995 {published data only}
- Menon K, Sutcliffe T, Klassen TP. A randomized trial comparing the efficacy of epinephrine with salbutamol in the treatment of acute bronchiolitis. Journal of Pediatrics 1995;126(6):1004‐7. [DOI] [PubMed] [Google Scholar]
Milner 1995 {published data only}
- Milner A. [Role des anticholinergiques dans la bronchiolite aigue du nourisson]. Archives de Pédiatrie 1995;2(Suppl 2):159‐62. [DOI] [PubMed] [Google Scholar]
Modaressi 2012 {published data only}
- Modaressi MR, Asadian A, Faghihinia J, Arashpour M, Mousavinasab F. Comparison of epinephrine to salbutamol in acute bronchiolitis. Iranian Journal of Pediatrics 2012;22:241‐4. [PMC free article] [PubMed] [Google Scholar]
Modl 2005 {published data only}
- Modl M, Eber E, Malle‐Scheid D, Weinhandl E, Zach MS. Does bronchodilator responsiveness in infants with bronchiolitis depend on age?. Journal of Pediatrics 2005;147(5):617‐21. [DOI] [PubMed] [Google Scholar]
Mull 2004 {published data only}
- Mull CC, Scarfone RJ, Ferri LR, Carlin T, Salvaggio C, Bechtel KA, et al. A randomized trial of nebulized epinephrine vs albuterol in the emergency department treatment of bronchiolitis. Archives of Pediatrics & Adolescent Medicine 2004;158(2):113‐8. [DOI] [PubMed] [Google Scholar]
Ndrepepa 1998 {published data only}
- Ndrepepa A, Sinamti J, Vevcka E, Kote L. Comparative study of inhaled bronchodilators in infants less than 18 months old with acute bronchiolitis. European Respiratory Journal 1998;12(Suppl 28):4085. [Google Scholar]
Numa 2001 {published data only}
- Numa AH, Williams GD, Dakin CJ. The effect of nebulized epinephrine on respiratory mechanics and gas exchange in bronchiolitis. American Journal of Respiratory and Critical Care Medicine 2001;164(1):86‐91. [DOI] [PubMed] [Google Scholar]
Ozyurek 2002 {published data only}
- Ozyurek H, Uyan AP, Keskin M, Afsar Y, Kocabay K. Comparison of two different bronchodilators in the treatment of acute bronchiolitis. Cocuk Sagligi ve Hastaliklari Dergisi 2002;45(4):298‐303. [Google Scholar]
Ralston 2008 {published data only}
- Ralston S, Roohi M. A randomized, controlled trial of nasal phenylephrine in infants hospitalized for bronchiolitis. Journal of Pediatrics 2008;153:795‐8. [DOI] [PubMed] [Google Scholar]
Ray 2002 {published data only}
- Ray MS, Singh V. Comparison of nebulized adrenaline versus salbutamol in wheeze associated respiratory tract infection in infants. Indian Pediatrics 2002;39:12‐22. [PubMed] [Google Scholar]
Reijonen 1995 {published data only}
- Reijonen T, Korppi M, Pitkakangas S, Tenhola S, Remes K. The clinical efficacy of nebulized racemic epinephrine and albuterol in acute bronchiolitis. Archives of Pediatrics & Adolescent Medicine 1995;149:686‐92. [DOI] [PubMed] [Google Scholar]
Ren 2011 {published data only}
- Ren CL, Scarlett EE. Effect of inhaled albuterol on tidal breathing patterns in infants hospitalized for viral bronchiolitis. American Journal of Respiratory and Critical Care Medicine 2011;183:A1889. [DOI: 10.1164/ajrccm-conference.2011.183.1_MeetingAbstracts.A1889A1889] [DOI] [Google Scholar]
Sanchez 1993 {published data only}
- Sanchez I, Koster J, Powell RE, Wolstein R, Chernick V. Effect of racemic epinephrine and salbutamol on clinical score and pulmonary mechanics in infants with bronchiolitis. Journal of Pediatrics 1993;122:145‐51. [DOI] [PubMed] [Google Scholar]
Sarrell 2002 {published data only}
- Sarrell EM, Tal G, Witzling M, Someck E, Houri S, Cohen HA, et al. Nebulized 3% hypertonic saline solution treatment in ambulatory children with viral bronchiolitis decreases symptoms. Chest 2002;122:2015‐20. [DOI] [PubMed] [Google Scholar]
Schuh 1992 {published data only}
- Schuh S, Johnson D, Canny G, Reisman J, Shields M, Kovesi T, et al. Efficacy of adding nebulized ipratropium bromide to nebulized albuterol therapy in acute bronchiolitis. Pediatrics 1992;90:920‐3. [PubMed] [Google Scholar]
Sezer 2010 {published data only}
- Sezer GR, Bozaykut A, Ipek IO, Uyur E, Seren PL, Paketci C. The efficacy of nebulized salbutamol, hypertonic saline and salbutamol/hypertonic saline combination in first bronchiolitis attack. Acta Paediatrica, International Journal of Paediatrics 2010;99:114. [Google Scholar]
Sharma 2013 {published data only}
- Sharma BS, Gupta MK, Rafik SP. Hypertonic (3%) saline vs 0.9% saline nebulization for acute viral bronchiolitis: a randomized controlled trial. Indian Pediatrics 2013;50(14):743‐7. [DOI] [PubMed] [Google Scholar]
Shu 2001 {published data only}
- Shu L, Long Z, Pi GX. Therapeutic effect of ipratropine combined with heparin aerosol inhalation on children with bronchiolitis. Chinese Journal of Contemporary Pediatrics 2001;3(5):555‐6. [Google Scholar]
Simsek 2005 {unpublished data only}
- Simsek PO, Aslan AT, Kiper N, Yalcin E, Dogru D, Ozcelic U, et al. A randomized trial of the effectiveness of nebulized therapy with epinephrine compared with salbutamol in the treatment of acute viral bronchiolitis [Abstract]. European Respiratory Society Annual Congress. 18 September 2005:Poster P865.
Simsek‐Kiper 2011 {published data only}
- Simsek‐Kiper PO, Kiper N, Hascelik G, Dolgun A, Yalcin E, Dogru‐Ersoz D, et al. Emergency room management of acute bronchiolitis: a randomized trial of nebulized epinephrine. Turkish Journal of Pediatrics 2011;6:651‐60. [PubMed] [Google Scholar]
Sly 1991 {published data only}
- Sly PD, Lanteri CJ, Raven JM. Do wheezy infants recovering from bronchiolitis respond to inhaled salbutamol?. Pediatric Pulmonology 1991;10:36‐9. [DOI] [PubMed] [Google Scholar]
Soto 1985 {published data only}
- Soto ME, Sly PD, Uren E, Taussig LM, Landau LI. Bronchodilator response during acute viral bronchiolitis in infancy. Pediatric Pulmonology 1985;1(2):85‐90. [DOI] [PubMed] [Google Scholar]
Springer 1990 {published data only}
- Springer C, Bar‐Yishay E, Uwayyed K, Avital A, Vilozni D, Godfrey S. Corticosteroids do not affect the clinical or physiological status of infants with bronchiolitis. Pediatric Pulmonology 1990;9:181‐5. [DOI] [PubMed] [Google Scholar]
Stokes 1983 {published data only}
- Stokes GM, Milner AD, Hodges IGC, Henry RL, Elphick MC. Nebulised therapy in acute severe bronchiolitis in infancy. Archives of Disease in Childhood 1983;58:279‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tatochenko 1988 {published data only}
- Tatochenko VK, Shiryaeva IS, Dorokhova NF, et al. Effectiveness of spasmolytic aerosols in infants with obstructive bronchitis. Voprosy Okhrany Materinstva i Detstva 1988;33:31‐3. [Google Scholar]
Torres 1997 {published data only}
- Torres Jr A, Anders M, Anderson P, Heulitt MJ. Efficacy of metered‐dose inhaler administration of albuterol in intubated infants. Chest 1997;112:484‐90. [DOI] [PubMed] [Google Scholar]
Walsh 2008 {published data only}
- Walsh P, Caldwell J, McQuillan KK, Friese S, Robbins D, Rothenberg SJ. Comparison of nebulized epinephrine to albuterol in bronchiolitis. Academic Emergency Medicine 2008;15(4):305‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wankum 2000 {published data only}
- Wankum P, Graff GR, Tobias J. A comparison of the effects of nebulized albuterol, L‐epinephrine, and saline on the pulmonary mechanics in RSV bronchiolitis [Abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(Suppl 3):A340. [CN‐00428970] [Google Scholar]
Zhen 2003 {published data only}
- Zhen KJ, Xiong MH, Wang JH, Lu XX, Wang WW, Lin RM. Fog‐inhalation therapy of Ventolin for bronchiolitis in babies. Medical Journal of Qilu 2003;18(2):180‐1. [Google Scholar]
Zhou 2001 {published data only}
- Zhou XH, Lu M. Clinical outcomes observation of inspiring budesonide and salbutamol on children bronchiolitis. Journal of Nanjing Railway Medical College 2001;20(2):120‐1. [Google Scholar]
Additional references
AAP 2006
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774‐93. [DOI] [PubMed] [Google Scholar]
Babl 2008
- Babl FE, Sheriff N, Neutze J, Borland M, Oakley E. Bronchiolitis management in pediatric emergency departments in Australia and New Zealand: a PREDICT study. Pediatric Emergency Care 2008;24(10):656‐8. [DOI] [PubMed] [Google Scholar]
Barben 2003
- Barben J, Hammer J. Current management of acute bronchiolitis in Switzerland. Swiss Medical Weekly 2003;133(1‐2):9‐15. [DOI] [PubMed] [Google Scholar]
Bracken 1989
- Bracken MB. Statistical methods for analysis of effects of treatment in overviews of randomized trials. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. New York, NY: Oxford University Press, 1989:13‐8. [Google Scholar]
CCHMC 2010
- Cincinnati Children's Hospital Medical Center (CCHMC). Evidence‐based care guideline for management of first time episode bronchiolitis in infants less than 1 year of age. Cincinnati (OH): Cincinnati Children's Hospital Medical Center 2010:16.
Chavasse 2002
- Chavasse RJPG, Seddon P, Bara A, McKean MC. Short acting beta2 agonists for recurrent wheeze in children under two years of age. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christakis 2005
- Christakis DA, Cowan CA, Garrison MM, Molteni R, Marcuse E, Zerr DM. Variation in inpatient diagnostic testing and management of bronchiolitis. Pediatrics 2005;115(4):878‐84. [DOI] [PubMed] [Google Scholar]
Cruz 1995
- Cruz MN, Stewart G, Rosenberg N. Use of dexamethasone in the outpatient management of acute laryngotracheitis. Pediatrics 1995;96:220‐3. [PubMed] [Google Scholar]
de Bilderling 2003
- Bilderling G, Bodart E. Bronchiolitis management by the Belgian paediatrician: discrepancies between evidence‐based medicine and practice. Acta Clinica Belgica 2003;58(2):98‐105. [PubMed] [Google Scholar]
DeNicola 2013
- DeNicola LK. Bronchiolitis treatment & management. Medscape (http://emedicine.medscape.com/article/961963‐treatment) 2013 (accessed 12 September 2013). [Accessed September 12, 2013.http://emedicine.medscape.com/article/961963‐treatment]
Destino 2010
- Destino L, Soung P, Weisgerber M, Bakalarski D, Yan K, Rehborg R, et al. The reliability and validity of the respiratory distress assessment instrument (RDAI) and Children's Hospital of Wisconsin Respiratory Score (CHWRS) in infants with bronchiolitis. Pediatric Academic Societies Meeting, Vancouver, Canada; 2010 May 4. 2010:Abstract #4403.187.
Destino 2012
- Destino L, Weisgerber MC, Soung P, Bakalarski D, Yan K, Rehborg R, et al. Validity of respiratory scores in bronchiolitis. Hospital Pediatrics 2012;2(4):202‐9. [DOI] [PubMed] [Google Scholar]
Disney 1960
- Disney ME, Sandiford BR, Cragg J, Wolff J. Epidemic bronchiolitis in infants. British Medical Journal 1960;1:1407‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flores 1997
- Flores G, Horwitz RI. Efficacy of beta2‐agonists in bronchiolitis: a reappraisal and meta‐analysis. Pediatrics 1997;100:233‐9. [DOI] [PubMed] [Google Scholar]
Florin 2013
- Florin TA, Byczkowski T, Ruddy R, Zorc J, Test M, Sha SS. Variation in the management of infants hospitalized for bronchiolitis persists after the 2006 AAP Bronchiolitis Guidelines. Pediatrics Academic Societies Meeting. 2013:Abstract 1150.5.
Goodman 1993
- Goodman BT, Chambers TL. Bronchodilators for bronchiolitis?. Lancet 1993;341:1380. [DOI] [PubMed] [Google Scholar]
Guia Salud 2010
- Giua Salud. Clinical practice guideline on acute bronchiolitis. Catalan Agency for Health Information, Assessment and Quality ‐ State/Local Government Agency [Non‐U.S.] 2010.
Hall 2004
- Hall CB. Managing bronchiolitis and respiratory syncytial virus. Archives of Pediatrics and Adolescent Medicine 2004;158:111‐2. [DOI] [PubMed] [Google Scholar]
Hall 2007
- Hall CB. Therapy for bronchiolitis: when some becomes none. New England Journal of Medicine 2007;357(4):402‐4. [DOI] [PubMed] [Google Scholar]
Hall 2009
- Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. New England Journal of Medicine 2009;360(6):588‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2013
- Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus‐associated hospitalizations among children less than 24 months of age. Pediatrics 2013;132(2):e341‐8. [DOI] [PubMed] [Google Scholar]
Hariprakash 2003
- Hariprakash S, Alexander J, Carroll W, Ramesh P, Randell T, Turnbull F, et al. Randomized controlled trial of nebulized adrenaline in acute bronchiolitis. Pediatric Allergy and Immunology 2003;14:134‐9. [DOI] [PubMed] [Google Scholar]
Hartling 2003
- Hartling L, Wiebe N, Russell K, Patel H, Klassen TP. A meta‐analysis of randomized controlled trials evaluating the efficacy of epinephrine for the treatment of acute viral bronchiolitis. Archives of Pediatrics & Adolescent Medicine 2003;157(10):957‐64. [DOI] [PubMed] [Google Scholar]
Hartling 2011a
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003123.pub3] [DOI] [PubMed] [Google Scholar]
Hartling 2011b
- Hartling L, Fernandes RM, Bialy L, Milne A, Johnson D, Plint A. Steroids and bronchodilators for acute bronchiolitis in the first two years of life: systematic review and meta‐analysis. BMJ 2011;342:d1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration Available from www.cochrane‐handbook.org. Chichester: Wiley‐Blackwell, 2011. [Google Scholar]
Johnson 2013
- Johnson LW, Robles J, Hudgins A, Osburn S, Martin D, Thompson A. Management of bronchiolitis in the emergency department: impact of evidence‐based guidelines?. Pediatrics 2013;131(Suppl 1):103‐9. [DOI] [PubMed] [Google Scholar]
King 2004
- King VJ, Viswanathan M, Bordley C, Jackman AM, Sutton SF, Lohr KN, et al. Pharmacologic treatment of bronchiolitis in infants and children. A systematic review. Archives of Pediatrics & Adolescent Medicine 2004;158:127‐37. [DOI] [PubMed] [Google Scholar]
Kristjánsson 1993
- Kristjánsson S, Lodrup Carlson KC, Wennergren G, Strannegård I‐L, Carlsen K‐H. Nebulised racemic adrenaline in the treatment of acute bronchiolitis in infants and toddlers. Archives of Disease in Childhood 1993;69:650‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
La Via 1992
- Via WV, Marks MI, Stutman HR. Respiratory syncytial virus puzzle: clinical features, pathophysiology, treatment, and prevention. Journal of Pediatrics 1992;121:503‐10. [DOI] [PubMed] [Google Scholar]
Langley 2003
- Langley JM, LeBlanc JC, Smith B, Wang EE. Increasing incidence of hospitalization for bronchiolitis among Canadian children, 1980‐2000. Journal of Infectious Diseases 2003;188(11):1764‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester: Wiley‐Blackwell, 2011. [Google Scholar]
Levison 1991
- Levison H. Canadian consensus on the treatment of asthma in children. Canadian Medical Association Journal 1991;145:1449‐55. [PMC free article] [PubMed] [Google Scholar]
Lowell 1987
- Lowell DI, Lister G, Koss H, McCarthy P. Wheezing in infants: the response to epinephrine. Pediatrics 1987;79:939‐45. [PubMed] [Google Scholar]
McCallum 2013
- McCallum GB, Morris PS, Wilson CC, Versteegh LA, Ward LM, Chatfield MD, et al. Severity scoring systems: are they internally valid, reliable and predictive of oxygen use in children with acute bronchiolitis?. Pediatric Pulmonology 2013;48(8):797‐803. [DOI] [PubMed] [Google Scholar]
Nair 2010
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta‐analysis. Lancet 2010;375(9725):1545‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paramore 2004
- Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus‐related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22:275‐84. [DOI] [PubMed] [Google Scholar]
Plint 2004
- Plint AC, Johnson DW, Wiebe N, Bulloch B, Pusic M, Joubert G, et al. Practice variation among pediatric emergency departments in the treatment of bronchiolitis. Academic Emergency Medicine 2004;11(4):353‐60. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Shay 1999
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis‐associated hospitalizations among US children, 1980‐1996. JAMA 1999;282(15):1440‐6. [DOI] [PubMed] [Google Scholar]
Shay 2001
- Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis‐associated mortality and estimates of respiratory syncytial virus‐associated deaths among US children, 1979‐1997. Journal of Infectious Diseases 2001;183(1):16‐22. [DOI] [PubMed] [Google Scholar]
SIGN 2006
- Scottish Intercollegiate Guidelines Network (SIGN). Bronchiolitis in children. A national clinical guideline. Edinburgh (Scotland): Scottish Intercollegiate Guidelines Network (SIGN). SIGN publication 2006;91:41. [Google Scholar]
Thompson 1991
- Thompson SG, Pocock SJ. Can meta‐analyses be trusted?. Lancet 1991;338:1127‐30. [DOI] [PubMed] [Google Scholar]
Thompson 1994
- Thompson SG. Why sources of heterogeneity in meta‐analysis should be investigated?. BMJ 1994;309:1351‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vogel 2003
- Vogel AM, Lennon DR, Harding JE, Pinnock RE, Graham DA, Grimwood K, et al. Variations in bronchiolitis management between five New Zealand hospitals: can we do better?. Journal of Paediatrics and Child Health 2003;39(1):40‐5. [DOI] [PubMed] [Google Scholar]
Wagner 2009
- Wagner T. Bronchiolitis. Pediatrics in Review 2009;30(10):386‐95. [DOI] [PubMed] [Google Scholar]
Wainwright 2003
- Wainwright C, Altamirano L, Cheney M, Cheney J, Barber S, Price D. A multicenter, randomized, double blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. New England Journal of Medicine 2003;349(1):27‐35. [DOI] [PubMed] [Google Scholar]
Wainwright 2010
- Wainwright C. Acute viral bronchiolitis in children ‐ a very common condition with few therapeutic options. Paediatric Respiratory Review 2010;11(1):39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1996
- Wang EE, Law BJ, Boucher FD, Stephens D, Robinson JL, Dobson S, et al. Pediatric Investigators Collaborative Network in Infections in Canada (PICNIC) study of admissions and management variation in patients hospitalized with respiratory syncytial virus lower respiratory tract infection. Journal of Pediatrics 1996;1996:390‐5. [DOI] [PubMed] [Google Scholar]
Welliver 1992
- Welliver RC, Cherry JD. Bronchiolitis and infectious asthma. In: Feigin RD, Cherry JD editor(s). Textbook of Pediatric Infectious Diseases. Third Edition. Philadelphia: WB Saunders, 1992:245‐54. [Google Scholar]
Wilson 2001
- Wilson DF, Horn SD, Hendley JO, Smout R, Gassaway J. Effect of practice variation on resource utilization in infants hospitalized for viral lower respiratory illness. Pediatrics 2001;108:851‐5. [DOI] [PubMed] [Google Scholar]
Wilson 2010
- Wilson KM, Blumkin AK, Rauch DA, Szilagyi PG. Trends in cost and length of stay for children hospitalized for bronchiolitis: 2000‐2006. Pediatric Academic Societies Meeting; 2010 May 2; Vancouver, Canada.
Zorc 2010
- Zorc JJ, Hall CB. Bronchiolitis: recent evidence on diagnosis and management. Pediatrics 2010;125(2):342‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gadomski 2006
- Gadomski AM, Bhasale AL. Bronchodilators for bronchiolitis. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001266.pub2] [DOI] [PubMed] [Google Scholar]
Gadomski 2010
- Gadomski AM, Brower MA. Bronchodilators for bronchiolitis. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD001266.pub3] [DOI] [PubMed] [Google Scholar]
Kellner 1996
- Kellner JD, Ohlsson A, Gadomski AM, Wang EEL. Efficacy of bronchodilator therapy in bronchiolitis: a meta‐analysis. Archives of Pediatrics and Adolescent Medicine 1996;150:1166‐72. [DOI] [PubMed] [Google Scholar]
Kellner 1998
- Kellner JD, Ohlsson A, Gadomski AM, Wang EEL. Bronchodilator therapy in bronchiolitis. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001266.pub2] [DOI] [Google Scholar]


