Abstract
Background
Emergency contraception (EC) is using a drug or copper intrauterine device (Cu‐IUD) to prevent pregnancy shortly after unprotected intercourse. Several interventions are available for EC. Information on the comparative effectiveness, safety and convenience of these methods is crucial for reproductive healthcare providers and the women they serve. This is an update of a review previously published in 2009 and 2012.
Objectives
To determine which EC method following unprotected intercourse is the most effective, safe and convenient to prevent pregnancy.
Search methods
In February 2017 we searched CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, Popline and PubMed, The Chinese biomedical databases and UNDP/UNFPA/WHO/World Bank Special Programme on Human Reproduction (HRP) emergency contraception database. We also searched ICTRP and ClinicalTrials.gov as well as contacting content experts and pharmaceutical companies, and searching reference lists of appropriate papers.
Selection criteria
Randomised controlled trials including women attending services for EC following a single act of unprotected intercourse were eligible.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary review outcome was observed number of pregnancies. Side effects and changes of menses were secondary outcomes.
Main results
We included 115 trials with 60,479 women in this review. The quality of the evidence for the primary outcome ranged from moderate to high, and for other outcomes ranged from very low to high. The main limitations were risk of bias (associated with poor reporting of methods), imprecision and inconsistency.
Comparative effectiveness of different emergency contraceptive pills (ECP)
Levonorgestrel was associated with fewer pregnancies than Yuzpe (estradiol‐levonorgestrel combination) (RR 0.57, 95% CI 0.39 to 0.84, 6 RCTs, n = 4750, I2 = 23%, high‐quality evidence). This suggests that if the chance of pregnancy using Yuzpe is assumed to be 29 women per 1000, the chance of pregnancy using levonorgestrel would be between 11 and 24 women per 1000.
Mifepristone (all doses) was associated with fewer pregnancies than Yuzpe (RR 0.14, 95% CI 0.05 to 0.41, 3 RCTs, n = 2144, I2 = 0%, high‐quality evidence). This suggests that if the chance of pregnancy following Yuzpe is assumed to be 25 women per 1000 women, the chance following mifepristone would be between 1 and 10 women per 1000.
Both low‐dose mifepristone (less than 25 mg) and mid‐dose mifepristone (25 mg to 50 mg) were probably associated with fewer pregnancies than levonorgestrel (RR 0.72, 95% CI 0.52 to 0.99, 14 RCTs, n = 8752, I2 = 0%, high‐quality evidence; RR 0.61, 95% CI 0.45 to 0.83, 27 RCTs, n = 6052, I2 = 0%, moderate‐quality evidence; respectively). This suggests that if the chance of pregnancy following levonorgestrel is assumed to be 20 women per 1000, the chance of pregnancy following low‐dose mifepristone would be between 10 and 20 women per 1000; and that if the chance of pregnancy following levonorgestrel is assumed to be 35 women per 1000, the chance of pregnancy following mid‐dose mifepristone would be between 16 and 29 women per 1000.
Ulipristal acetate (UPA) was associated with fewer pregnancies than levonorgestrel (RR 0.59; 95% CI 0.35 to 0.99, 2 RCTs, n = 3448, I2 = 0%, high‐quality evidence).
Comparative effectiveness of different ECP doses
It was unclear whether there was any difference in pregnancy rate between single‐dose levonorgestrel (1.5 mg) and the standard two‐dose regimen (0.75 mg 12 hours apart) (RR 0.84, 95% CI 0.53 to 1.33, 3 RCTs, n = 6653, I2 = 0%, moderate‐quality evidence).
Mid‐dose mifepristone was associated with fewer pregnancies than low‐dose mifepristone (RR 0.73; 95% CI 0.55 to 0.97, 25 RCTs, n = 11,914, I2 = 0%, high‐quality evidence).
Comparative effectiveness of Cu‐IUD versus mifepristone
There was no conclusive evidence of a difference in the risk of pregnancy between the Cu‐IUD and mifepristone (RR 0.33, 95% CI 0.04 to 2.74, 2 RCTs, n = 395, low‐quality evidence).
Adverse effects
Nausea and vomiting were the main adverse effects associated with emergency contraception. There is probably a lower risk of nausea (RR 0.63, 95% CI 0.53 to 0.76, 3 RCTs, n = 2186 , I2 = 59%, moderate‐quality evidence) or vomiting (RR 0.12, 95% CI 0.07 to 0.20, 3 RCTs, n = 2186, I2 = 0%, high‐quality evidence) associated with mifepristone than with Yuzpe. levonorgestrel is probably associated with a lower risk of nausea (RR 0.40, 95% CI 0.36 to 0.44, 6 RCTs, n = 4750, I2 = 82%, moderate‐quality evidence), or vomiting (RR 0.29, 95% CI 0.24 to 0.35, 5 RCTs, n = 3640, I2 = 78%, moderate‐quality evidence) than Yuzpe. Levonorgestrel users were less likely to have any side effects than Yuzpe users (RR 0.80, 95% CI 0.75 to 0.86; 1 RCT, n = 1955, high‐quality evidence). UPA users were more likely than levonorgestrel users to have resumption of menstruation after the expected date (RR 1.65, 95% CI 1.42 to 1.92, 2 RCTs, n = 3593, I2 = 0%, high‐quality evidence). Menstrual delay was more common with mifepristone than with any other intervention and appeared to be dose‐related. Cu‐IUD may be associated with higher risks of abdominal pain than mifepristone (18 events in 95 women using Cu‐IUD versus no events in 190 women using mifepristone, low‐quality evidence).
Authors' conclusions
Levonorgestrel and mid‐dose mifepristone (25 mg to 50 mg) were more effective than Yuzpe regimen. Both mid‐dose (25 mg to 50 mg) and low‐dose mifepristone(less than 25 mg) were probably more effective than levonorgestrel (1.5 mg). Mifepristone low dose (less than 25 mg) was less effective than mid‐dose mifepristone. UPA may be more effective than levonorgestrel.
Levonorgestrel users had fewer side effects than Yuzpe users, and appeared to be more likely to have a menstrual return before the expected date. UPA users were probably more likely to have a menstrual return after the expected date. Menstrual delay was probably the main adverse effect of mifepristone and seemed to be dose‐related. Cu‐IUD may be associated with higher risks of abdominal pain than ECPs.
Plain language summary
Methods of emergency contraception
Review question
The aim of this Cochrane Review was to evaluate the effectiveness and safety of different methods of emergency contraception to prevent pregnancy following unprotected intercourse.
Background
Emergency contraception (EC) is using a drug or copper intrauterine device (Cu‐IUD) to prevent pregnancy shortly after unprotected intercourse. Several interventions are available for EC. Information on the comparative effectiveness, safety and convenience of these methods is crucial for reproductive healthcare providers and the women they serve. Researchers in Cochrane collected and analyzed all relevant studies to answer this question.
Study characteristics
We searched 10 English‐language and three Chinese‐language databases for published studies in any language, in February 2017. We also searched grey literature databases and websites and contacted experts and authors for eligible studies. Studies had to report information on interventions to prevent pregnancy after a single act of unprotected intercourse. We included 115 randomized controlled trials with 60,479 women in this review. Ninety‐two trials were conducted in China. The evidence is up‐to‐date to February 2017.
Key results
The studies compared 25 different interventions of different types of emergency contraception. The studies showed the following.
Levonorgestrel and mifepristone were more effective than Yuzpe regimen (estradiol‐levonorgestrel combination). Our findings suggest that if 29 women per 1000 become pregnant with Yuzpe, between 11 and 24 women per 1000 will do so with the levonorgestrel, and that if 25 women per 1000 become pregnant with Yuzpe, between one and 10 women per 1000 will do so with mifepristone.
Mid‐dose mifepristone (25 mg to 50 mg) was probably more effective than levonorgestrel. Low‐dose mifepristone (less than 25 mg) was probably less effective than mid‐dose mifepristone, but both were more effective than levonorgestrel (two doses of 0.75 mg). Ulipristal acetate (UPA) was also more effective than levonorgestrel.
Levonorgestrel users had fewer side effects than Yuzpe users, and might be more likely to resume menstruation before the expected date. UPA users were probably more likely to resume menstruation after the expected date. Menstrual delay was probably the main adverse effect of mifepristone and seemed to be dose‐related. Cu‐IUD may be associated with higher risks of abdominal pain than mifepristone.
Quality of the evidence
The quality of the evidence for the primary outcome (observed number of pregnancies) ranged from moderate to high, and for other outcomes ranged from very low to high. The main limitations were risk of bias (associated with poor reporting of methods), imprecision and inconsistency.
Summary of findings
Summary of findings for the main comparison. Levonorgestrel compared to Yuzpe for emergency contraception.
| Levonorgestrel compared to Yuzpe for emergency contraception | ||||||
| Patient or population: Women seeking emergency contraception Setting: China (3), Italy (2), multinational (1); family planning clinics Intervention: Levonorgestrel Comparison: Yuzpe | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Yuzpe | Risk with Levonorgestrel | |||||
| Observed number of pregnancies (all women) | 29 per 1,000 | 17 per 1,000 (11 to 24) | RR 0.57 (0.39 to 0.84) | 4750 (6 RCTs) | ⊕⊕⊕⊕ HIGH | |
| Any side effect | 681 per 1,000 | 545 per 1,000 (511 to 586) | RR 0.80 (0.75 to 0.86) | 1955 (1 RCT) | ⊕⊕⊕⊕ HIGH | |
| Specific side effects ‐ Nausea | 447 per 1,000 | 179 per 1,000 (161 to 197) | RR 0.40 (0.36 to 0.44) | 4750 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Specific side effects ‐ Vomiting | 254 per 1,000 | 74 per 1,000 (61 to 89) | RR 0.29 (0.24 to 0.35) | 3640 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Specific side effects ‐ Spotting/bleeding after treatment | 87 per 1,000 | 158 per 1,000 (119 to 210) | RR 1.82 (1.37 to 2.41) | 1614 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | |
| Menses ‐ Early | 119 per 1,000 | 137 per 1,000 (103 to 182) | RR 1.15 (0.86 to 1.52) | 1310 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | |
| Menses ‐ Delay | 103 per 1,000 | 127 per 1,000 (99 to 162) | RR 1.23 (0.96 to 1.57) | 1988 (3 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The quality of evidence was downgraded by one level for “inconsistency” because of high heterogeneity in the meta‐analysis
2 The quality of evidence was downgraded by one level for “imprecision” because the 95% CI overlaps no effect and CI fails to exclude important benefit or important harm.
3 The quality of evidence was downgraded by one level for “ serious risk of bias” associated with poor reporting of randomization methods
4 The quality of evidence was downgraded by one level for “imprecision” because the total (cumulative) sample size is lower than the calculated optimal information size (OIS)
Summary of findings 2. Mid‐dose mifepristone (25 mg‐50 mg) versus levonorgestrel 1.5 mg for emergency contraception.
| Mid‐dose mifepristone (25 mg‐50 mg) compared to levonorgestrel 1.5 mg for emergency contraception | ||||||
| Patient or population: women seeking emergency contraception Setting: China (27); family planning clinics Intervention: mifepristone, mid‐dose (25 mg‐50 mg) Comparison: levonorgestrel 1.5 mg | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with levonorgestrel 1.5 mg | Risk with mid‐dose mifepristone (25 mg‐50 mg) | |||||
| Observed number of pregnancies (all women) | 35 per 1000 | 21 per 1000 (16 to 29) | RR 0.61 (0.45 to 0.83) | 6052 (27 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Any side effect | 202 per 1000 | 111 per 1000 (81 to 150) | RR 0.55 (0.40 to 0.74) | 4352 (18 RCTs) | ⊕⊕⊝⊝ Low1,2 | |
| Specific side effect ‐ nausea | 80 per 1000 | 65 per 1000 (39 to 109) | RR 0.81 (0.48 to 1.36) | 713 (4 RCTs) | ⊕⊕⊝⊝ Low1,3 | |
| Specific side effect ‐ vomiting | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Specific side effect ‐ spotting/bleeding after treatment | 77 per 1000 | 47 per 1000 (32 to 68) | RR 0.61 (0.42 to 0.88) | 1796 (9 RCTs) | ⊕⊕⊝⊝ Low1,3 | |
| Menses ‐ early | 94 per 1000 | 68 per 1000 (47 to 97) | RR 0.72 (0.50 to 1.03) | 1324 (7 RCTs) | ⊕⊕⊝⊝ Low1,3 | |
| Menses ‐ delay | 108 per 1000 | 139 per 1000 (117 to 166) | RR 1.29 (1.09 to 1.54) | 3615 (17 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded the quality of evidence by one level for serious risk of bias associated with poor reporting of randomization methods. 2We downgraded the quality of evidence by one level for inconsistency because of high heterogeneity in the meta‐analysis. 3We downgraded the quality of evidence by one level for imprecision because the total (cumulative) sample size was lower than the calculated optimal information size.
Summary of findings 3. Low‐dose mifepristone (< 25 mg) versus levonorgestrel 1.5 mg for emergency contraception.
| Low‐dose mifepristone (< 25 mg) versus levonorgestrel 1.5 mg for emergency contraception | ||||||
| Patient or population: women seeking emergency contraception Setting: China (12), UK (1), multinational (1); family planning clinics Intervention: mifepristone, low dose (< 25 mg) Comparison: levonorgestrel 1.5 mg | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with levonorgestrel 1.5 mg | Risk with low‐dose mifepristone (< 25 mg) | |||||
| Observed number of pregnancies (all women) | 20 per 1000 | 15 per 1000 (10 to 20) | RR 0.72 (0.52 to 0.99) | 8752 (14 RCTs) | ⊕⊕⊕⊕ High | |
| Any side effect | 342 per 1000 | 89 per 1000 (58 to 130) | RR 0.26 (0.17 to 0.38) | 609 (3 RCTs) | ⊕⊕⊝⊝ Low1,2 | |
| Specific side effect ‐ nausea | 133 per 1000 | 126 per 1000 (112 to 145) | RR 0.95 (0.84 to 1.09) | 6384 (5 RCTs) | ⊕⊕⊕⊝ Moderate3 | |
| Specific side effect ‐ vomiting | 15 per 1000 | 19 per 1000 (8 to 41) | RR 1.22 (0.55 to 2.68) | 6085 (3 RCTs) | ⊕⊕⊝⊝ Low3,4 | |
| Specific side effect ‐ spotting/bleeding after treatment | 284 per 1000 | 173 per 1000 (153 to 196) | RR 0.61 (0.54 to 0.69) | 4598 (5 RCTs) | ⊕⊕⊕⊕ High | |
| Menses ‐ early | 182 per 1000 | 82 per 1000 (64 to 108) | RR 0.45 (0.35 to 0.59) | 1800 (5 RCTs) | ⊕⊕⊝⊝ Low1,2 | |
| Menses ‐ delay | 66 per 1000 | 113 per 1000 (98 to 131) | RR 1.70 (1.48 to 1.97) | 7520 (9 RCTs) | ⊕⊕⊝⊝ Low1,4 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded the quality of evidence by one level for serious risk of bias associated with poor reporting of randomization methods. 2We downgraded the quality of evidence by one level for imprecision because the total (cumulative) sample size was lower than the calculated optimal information size. 3We downgraded the quality of evidence by one level for imprecision because the 95% CI overlaps no effect and CI fails to exclude important benefit or important harm. 4We downgraded the quality of evidence by one level for inconsistency because of high heterogeneity in the meta‐analysis.
Summary of findings 4. Mifepristone (all doses) versus Yuzpe for emergency contraception.
| Mifepristone (all doses) versus Yuzpe for emergency contraception | ||||||
| Patient or population: women seeking emergency contraception Setting: UK (3); family planning clinics Intervention: mifepristone (all doses) Comparison: Yuzpe | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Yuzpe | Risk with mifepristone (all doses) | |||||
| Observed number of pregnancies (all women) | 25 per 1000 | 3 per 1000 (1 to 10) | RR 0.14 (0.05 to 0.41) | 2144 (3 RCTs) | ⊕⊕⊕⊕ High | |
| Any side effect | 735 per 1000 | 610 per 1000 (566 to 647) | RR 0.83 (0.77 to 0.88) | 1693 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Specific side effects ‐ nausea | 424 per 1000 | 267 per 1000 (225 to 322) | RR 0.63 (0.53 to 0.76) | 2186 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Specific side effects ‐ vomiting | 135 per 1000 | 16 per 1000 (9 to 27) | RR 0.12 (0.07 to 0.20) | 2186 (3 RCTs) | ⊕⊕⊕⊕ High | |
| Specific side effects ‐ abdominal pain | 276 per 1000 | 210 per 1000 (168 to 262) | RR 0.76 (0.61 to 0.95) | 1000 (1 RCT) | ⊕⊕⊕⊝ Moderate2 | |
| Specific side effects ‐ spotting/bleeding after treatment | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Menses ‐ delay | 110 per 1000 | 311 per 1000 (253 to 381) | RR 2.83 (2.30 to 3.47) | 1924 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded the quality of evidence by one level for inconsistency because of high heterogeneity in the meta‐analysis. 2We downgraded the quality of evidence by one level for imprecision because the total (cumulative) sample size was lower than the calculated optimal information size.
Summary of findings 5. Ulipristal acetate (all doses) versus levonorgestrel for emergency contraception.
| Ulipristal acetate (all doses) versus levonorgestrel for emergency contraception | ||||||
| Patient or population: women seeking emergency contraception Setting: multinational (2); family planning clinics Intervention: ulipristal acetate (all doses) Comparison: levonorgestrel | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with levonorgestrel | Risk with ulipristal acetate (all doses) | |||||
| Observed number of pregnancies (all women) | 22 per 1000 | 13 per 1000 (8 to 22) | RR 0.59 (0.35 to 0.99) | 3448 (2 RCTs) | ⊕⊕⊕⊕ High | |
| Any side effect | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Specific side effects ‐ nausea | 79 per 1000 | 90 per 1000 (74 to 112) | RR 1.14 (0.93 to 1.41) | 3770 (2 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Specific side effects ‐ vomiting | 3 per 1000 | 3 per 1000 (0 to 18) | RR 1.00 (0.14 to 7.07) | 1549 (1 RCT) | ⊕⊕⊕⊝ Moderate2 | |
| Specific side effects ‐ spotting/bleeding after treatment | 9 per 1000 | 6 per 1000 (2 to 20) | RR 0.71 (0.23 to 2.24) | 1549 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | |
| Menses ‐ early | 256 per 1000 | 110 per 1000 (95 to 128) | RR 0.43 (0.37 to 0.50) | 3593 (2 RCTs) | ⊕⊕⊕⊝ Moderate3 | |
| Menses ‐ delay | 126 per 1000 | 208 per 1000 (179 to 241) | RR 1.65 (1.42 to 1.92) | 3593 (2 RCTs) | ⊕⊕⊕⊕ High | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded the quality of evidence by one level for imprecision because the 95% CI overlaps no effect and CI fails to exclude important benefit or important harm. 2We downgraded the quality of evidence by one level for imprecision because the total (cumulative) sample size is lower than the calculated optimal information size. 3We downgraded the quality of evidence by one level for inconsistency because of high heterogeneity in the meta‐analysis.
Summary of findings 6. Single‐dose levonorgestrel versus split‐dose levonorgestrel for emergency contraception.
| Single‐dose levonorgestrel versus split‐dose levonorgestrel for emergency contraception | ||||||
| Patient or population: women seeking emergency contraception Setting: multinational (3); family planning clinics Intervention: levonorgestrel, single‐dose Comparison: levonorgestrel, split‐dose | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with split‐dose levonorgestrel | Risk with single‐dose levonorgestrel | |||||
| Observed number of pregnancies (all women) | 12 per 1000 | 10 per 1000 (6 to 16) | RR 0.84 (0.53 to 1.33) | 6653 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Any side effect | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Specific side effects ‐ nausea | 195 per 1000 | 189 per 1000 (171 to 208) | RR 0.97 (0.88 to 1.07) | 6804 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Specific side effects ‐ vomiting | 58 per 1000 | 58 per 1000 (48 to 70) | RR 1.01 (0.83 to 1.22) | 6804 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Specific side effects ‐ spotting/bleeding after treatment | 313 per 1000 | 313 per 1000 (282 to 351) | RR 1.00 (0.90 to 1.12) | 2720 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | |
| Menses ‐ early | 299 per 1000 | 200 per 1000 (162 to 245) | RR 0.67 (0.54 to 0.82) | 1118 (1 RCT) | ⊕⊕⊕⊕ High | |
| Menses ‐ delay | 77 per 1000 | 91 per 1000 (74 to 112) | RR 1.18 (0.96 to 1.46) | 3784 (2 RCTs) | ⊕⊕⊝⊝ Low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded the quality of evidence by one level for imprecision because the 95% CI overlaps no effect and CI fails to exclude important benefit or important harm. 2We downgraded the quality of evidence by one level for inconsistency because of high heterogeneity in the meta‐analysis.
Summary of findings 7. Mid‐dose mifepristone (25 mg‐50 mg) versus low‐dose mifepristone (< 25 mg) for emergency contraception.
| Mid‐dose mifepristone (25 mg‐50 mg) versus low‐dose mifepristone (< 25 mg) for emergency contraception | ||||||
| Patient or population: women seeking emergency contraception Setting: China (25); family planning clinics Intervention: mifepristone, mid‐dose (25 mg‐50 mg) Comparison: mifepristone, low‐doses (< 25 mg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with low‐dose mifepristone (< 25 mg) | Risk with mid‐dose mifepristone (25 mg‐50 mg) | |||||
| Observed number of pregnancies (all women) | 17 per 1000 | 12 per 1000 (9 to 16) | RR 0.73 (0.55 to 0.97) | 11914 (25 RCTs) | ⊕⊕⊕⊕ High | |
| Any side effect | 88 per 1000 | 115 per 1000 (89 to 149) | RR 1.31 (1.01 to 1.70) | 2464 (11 RCTs) | ⊕⊕⊕⊝ Moderate1 | |
| Specific side effects ‐ nausea | 104 per 1000 | 114 per 1000 (100 to 128) | RR 1.10 (0.97 to 1.24) | 7948 (13 RCTs) | ⊕⊕⊕⊝ Moderate2 | |
| Specific side effects ‐ vomiting | 6 per 1000 | 7 per 1000 (4 to 13) | RR 1.22 (0.68 to 2.17) | 6082 (6 RCTs) | ⊕⊕⊕⊝ Moderate2 | |
| Specific side effects ‐ spotting/bleeding after treatment | 69 per 1000 | 127 per 1000 (107 to 151) | RR 1.85 (1.55 to 2.20) | 5078 (11 RCTs) | ⊕⊕⊕⊕ High | |
| Menses ‐ early | 118 per 1000 | 129 per 1000 (103 to 160) | RR 1.09 (0.87 to 1.36) | 2136 (7 RCTs) | ⊕⊕⊝⊝ Low1,2 | |
| Menses ‐ delay | 117 per 1000 | 150 per 1000 (130 to 172) | RR 1.28 (1.11 to 1.47) | 11282 (21 RCTs) | ⊕⊕⊕⊝ Moderate3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded the quality of evidence by one level for risk of bias because we judged allocation concealment to be inadequate in the meta‐analysis. 2We downgraded the quality of evidence by one level for imprecision because the 95% CI overlaps no effect and CI fails to exclude important benefit or important harm. 3We downgraded the quality of evidence by one level for inconsistency because of high heterogeneity in the meta‐analysis.
Summary of findings 8. Copper intrauterine device versus mifepristone (all doses) for emergency contraception.
| Copper intrauterine device versus mifepristone (all doses) for emergency contraception | ||||||
| Patient or population: women seeking emergency contraception Setting: China (2); family planning clinics Intervention: copper intrauterine device Comparison: mifepristone (all doses) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with mifepristone (all doses) | Risk with copper intrauterine device | |||||
| Observed number of pregnancies (all women) | 12 per 1000 | 4 per 1000 (0 to 34) | RR 0.33 (0.04 to 2.74) | 395 (2 RCTs) | ⊕⊕⊝⊝ Low1,2 | |
| Any side effect | 84 per 1000 | 5 per 1000 (0 to 83) | RR 0.06 (0.00 to 0.99) | 285 (1 RCT) | ⊕⊕⊝⊝ Low1,2 | |
| Specific side effects ‐ nausea | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Specific side effects ‐ vomiting | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Specific side effects ‐ lower abdominal pain | 0 per 1000 | 0 per 1000 (0 to 0) | RR 73.61 (4.48 to 1208.50) | 285 (1 RCT) | ⊕⊕⊝⊝ Low1,2 | |
| Specific side effects ‐ spotting/bleeding after treatment | See comment | ‐ | ‐ | ‐ | No study reported this outcome | |
| Menses ‐ delay | 180 per 1000 | 41 per 1000 (16 to 115) | RR 0.23 (0.09 to 0.64) | 284 (1 RCT) | ⊕⊕⊝⊝ Low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded the quality of evidence by one level for serious risk of bias associated with poor reporting of randomization method. 2We downgraded the quality of evidence by one level for imprecision because the total (cumulative) sample size was lower than the calculated optimal information size.
Background
Description of the condition
Unwanted pregnancy is a common problem. Worldwide, over 40 million pregnancies end in abortion each year (Sedgh 2012; Sedgh 2014). The standard approach to this problem has been primary prevention (contraception), backed up by induced abortion. However, for a long time, 'contraception' has generally been understood to mean only anticipatory contraception. The definition of the primary prevention of unintended pregnancy could and should be expanded to include post hoc contraception (Grimes 1997).
Description of the intervention
Emergency contraception (EC) is defined as the use of a drug or device as an emergency measure to prevent pregnancy after unprotected intercourse. From this definition it follows that methods of EC are used after coitus but before pregnancy occurs, and that they are intended as a back‐up for occasional use rather than a regular form of contraception (Van Look 1993). Although the terms 'morning after pill' and 'after‐sex pill' are also used to describe the same approach, these can cause confusion regarding the timing and purpose, and are best avoided. EC implies something not to be used routinely (there are far more effective methods for regular contraception) but which can still prevent pregnancy if other options have failed or regular contraception was not used (Webb 1995).
To date, no contraceptive method is 100% reliable and few people use their method perfectly each time they have sexual intercourse, in particularly the short‐acting contraceptives like oral pills and condoms. Furthermore, EC is useful in cases of sexual assault. EC is especially important for outreach to the 4.6 million women at risk of pregnancy but not using a regular method by providing a bridge to use of an ongoing contraceptive method (Trussell 2012).
EC is widely available in Western Europe and in China. However, use of this method is rising rapidly in low‐ and middle‐income countries. For example, the 2008 to 2009 Demographic and Health Survey (DHS) data showed that 22% of unmarried sexually active women in Albania had used EC. In Colombia, Kenya and Nigeria, according to data from DHS, 10% to 16% of unmarried, sexually active women used EC (ICEC 2012a, ICEC 2012b, ICEC 2012c). This proportion in Peru was 35% in 2010 (INEI 2011). However, EC is largely under‐utilised in many other countries. Examining data from 45 countries surveyed between 2000 and 2012, in 16 countries, fewer than 10% of women aged 15 to 49 years had heard of EC; in 36 countries, the rate of use of EC was less than 3% among women who had ever had sex (Palermo 2014). The low awareness of emergency contraceptive pills (ECPs) and the lack of access to EC may subject women to unsafe abortions, which contribute significantly to maternal mortality and morbidity.
Although attempted throughout history, EC methods only started to become effective in the 1960s when hormonal regimens were first introduced. Following the introduction of high‐dose oestrogens, the so‐called Yuzpe regimen, involving the combined use of oestrogen (ethinyl oestradiol 100 μg) and progestogen (levonorgestrel 0.5 mg or dl‐norgestrel 1 mg), repeated once 12 hours apart, with the first dose given within 72 hours of unprotected intercourse, became popular in the late 1970s and early 1980s (Yuzpe 1977).
Since the 1990s, there have been several different interventions available for EC (Glasier 1997). Interest in the development of alternative regimens has led to trials of the progestogen levonorgestrel, the anti‐gonadotropin danazol, and the anti‐progestins mifepristone and ulipristal acetate (UPA) (Trussell 2012). Like the Yuzpe regimen, these methods are recommended for use within 72 hours of unprotected intercourse although levonorgestrel and mifepristone had been tested up to 120 hours (five days) after unprotected intercourse, for research purposes Glasier 2010. The postcoital insertion of a copper intrauterine device (Cu‐IUD) is an option that can be used up to five days after the estimated time of ovulation and can be left in the uterus as a long‐term regular contraceptive method.
The main side effects caused by hormonal emergency contraceptives are nausea and vomiting, which seem to be more frequent with oestrogen‐containing regimens such as Yuzpe regimen and high‐dose oestrogen alone compared to progestogen or anti‐progestogen treatment. Mifepristone can cause menstrual delay, while levonorgestrel may cause earlier menses. IUD insertion can cause discomfort and requires trained staff and facilities. It is generally recommended that the Cu‐IUD be avoided in women at high risk of sexually transmitted diseases.
How the intervention might work
EC can prevent pregnancy after unprotected intercourse but it does not always work effectively. Many factors may affect the effectiveness of EC, and different methods of EC may have different effectiveness. The risk of failure of a less effective method of EC is the major factor to be taken into account when estimating the risk of pregnancy.
For all ECs, the risk of pregnancy is related to the cycle day of intercourse. Women who have intercourse the day before estimated day of ovulation have a fourfold increased risk of pregnancy (odds ratio (OR) 4.42, 95% confidence interval (CI), 2.33 to 8.20; P < 0.0001) compared with women having sex outside the fertile window (Glasier 2011). Time elapsed since intercourse (coitus‐treatment interval) and further acts of intercourse during the same cycle in which EC was used are two other factors affecting the success of EC. It is suggested that emergency contraception may be less effective among obese women, though clinical data are sparse (Jatlaoui 2016a). This is biologically plausible, as there is evidence that among women taking the same dose of levonorgestrel, the serum concentration of levonorgestrel is 50% lower in those who are obese (BMI of 30 kg/m2 or more) than in those with a normal or low BMI (less than 25 kg/m2) (Edelman 2016).
Levonorgestrel is used in ECPs, both in a combined Yuzpe regimen which includes estrogen, and as a levonorgestrel‐only method. The primary mechanism of action of levonorgestrel as a progestogen‐only emergency contraceptive pill is to prevent fertilisation by inhibition of ovulation (Brache 2013; Gemzell‐Danielsson 2004) and thickening of cervical mucus (Lalitkumar 2013). levonorgestrel can disrupt or inhibit ovulation in 96% of cycles if it is given in the presence of an ovarian follicle measuring 12 mm to 17 mm in diameter. Once the luteinising hormone (LH) surge has started levonorgestrel has no effect on ovulation. Review of the evidence suggests that levonorgestrel ECPs cannot prevent implantation of a fertilised egg. This explains the need to take levonorgestrel as soon as possible after intercourse, especially within 72 hours.
Ulipristal acetate (UPA) is a selective progesterone receptor modulator and also works by delaying or inhibiting ovulation. UPA remains reasonably effective even if given after the LH surge has started, delaying ovulation in 79% of cycles at this time, while levonorgestrel delays ovulation in only 14% (and placebo in 10%). Once LH has reached its peak, UPA no longer has any effect on ovulation. When UPA is given before the start of the LH surge, follicle rupture is delayed or inhibited in 100% of cycles (Baird 2015). UPA can be used up to 120 hours after intercourse (Fine 2010a), but should be taken as soon as possible after intercourse (since if the woman has not yet ovulated, the longer she delays using EC the more likely she will be close to ovulation).
Mifepristone, another selective progesterone receptor modulator, has an effect on the endometrium and can both inhibit implantation and induce abortion (Gemzell‐Danielsson 2004).
The Cu‐IUD used for EC may prevent an oocyte from being fertilised if inserted before fertilisation has occurred but will also prevent implantation if it is inserted later (Cleland 2012).
Why it is important to do this review
Information on the comparative effectiveness, safety and convenience of an EC method is crucial for reproductive healthcare providers and the women they serve. The present review aims to search systematically for, and combine, all evidence from randomized controlled trials (RCTs) and controlled clinical trials relating to the effectiveness of different EC methods in order to supply the best evidence currently available on which to base recommendations for clinical practice and further research.
In the previous version of this Cochrane Review, 100 RCTs and 25 comparisons were included. The findings included evidence that intermediate‐dose mifepristone was superior to LNG and Yuzpe regimens and that low‐dose mifepristone and UPA might possibly be more effective than LNG. As this version of the review was published 5 years ago (2012), and failed to evaluate the quality of the evidence using GRADE methods, we considered that with more recent evidence and updated methods our conclusions might be changed.
Objectives
To determine which EC method following unprotected intercourse is the most effective, safe and convenient to prevent pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
We considered RCTs comparing different EC methods, or comparing one method with expectant management or placebo for inclusion. The unit of randomization in all these studies was the individual. Only trials measuring clinical outcomes were considered for inclusion.
Types of participants
Women with regular menses requesting EC following unprotected intercourse. Women attending clinics for 'once‐a‐month' contraception in the form of luteal phase contraceptives and menstrual regulation using mifepristone and prostaglandin analogues were not eligible for inclusion in this review.
Types of interventions
To be included, the intervention had to be applied to women seeking EC following unprotected intercourse. Those studies in which similar interventions were used by women as regular postcoital contraception were not eligible. Comparisons of different delivery systems such as advance provision or over‐the‐counter delivery, and any kind of educational interventions, were not eligible for inclusion in this review.
Trials evaluating the following interventions were included in this review:
Any regimen versus no intervention/placebo. Please note that this comparison is now considered unethical and is included only for completeness.
Hormonal ECPs: comparison of different regimens
IUD compared with ECP
Combination treatments and comparison of these with other treatments alone or in combination were considered for inclusion when such data were available, including different doses.
Types of outcome measures
Primary outcomes
Observed number of pregnancies (all women), including number of ectopic pregnancies (if reported)
Secondary outcomes
-
Side effects:
any side effect,
nausea,
vomiting,
headache,
dizziness,
fatigue,
breast tenderness,
diarrhoea,
spotting or bleeding,
abdominal pain,
others
Menses: early (return before the expected date), delayed (return after the expected date)
Search methods for identification of studies
We searched for all published and unpublished RCTs using the following search strategy, without language or date restriction and in consultation with the Information Specialists of both Cochrane Gynaecology and Fertility, and Cochrane Fertility Regulation. We identified relevant trials from electronic databases and other resources.
Electronic searches
We searched the following electronic databases, trials registers and websites from their inception to 22 February 2017.
The Cochrane Central Register of Studies (CENTRAL; 2017, issue 2) via the Cochrane Register of Studies Online (CRSO) (Appendix 1)
-
English language electronic databases:
Ovid MEDLINE (from 1946 to 22 February 2017) (Appendix 2);
Ovid Embase (from 1980 to 22 February 2017) (Appendix 3);
Ovid PsycINFO (from 1806 to 22 February 2017) (Appendix 4); and
EBSCO CINAHL (from inception to 22 February 2017) (Appendix 5).
-
We ran searches of other databases including:
the Database of Chinese Scientific Journals (from inception to February 2017) (左炔诺孕酮 or 米非司酮 or RU486 or UPA or ulipristal acetate or 乌利司他 or 醋酸优力司特 or 醋酸乌利司他 or Yuzpe or 紧急避孕药 or 毓婷 or 宫内节育器 or IUD or 环) and 紧急避孕 and (临床试验 or 随机对照 or 比较 or 对比) (Appendix 6);
Popline (inception to February 2017);
LILACS (inception to February 2017);
PubMed (inception to February 2017);
-
and the clinical trials registers (to 22 February 2017):
ClinicalTrials.gov (Appendix 7)
The World Health Organization (WHO) International Clinical Trials Registry Platform search portal (ICTRP) (Appendix 7).
We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying RCTs, in Chapter 6, Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We combined the Embase search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN).
Searching other resources
World Health Organization (WHO) RESOURCES (February 2017). We contacted HRP (Human Reproduction Program)/WHO to seek any published or unpublished trials we had missed.
Emergency Contraception Website (February 2017). We checked the Emergency Contraception World Wide Web server operated by the Office of Population Research at Princeton University, USA, to identify any relevant publications.
Pharmaceutical companies (February 2017). We contacted the pharmaceutical companies (Bayer, Beijing Zizhu Pharmaceutical Co., Biopharm Chemical Company, Gador SA, Gedeon Richter, Laboratoire HRA Pharma, Shanghai New Hualian Pharmaceutical Co., Shenyang No. 1 Pharmaceutical Co., Teva, Xianju Pharmaceutical Co.) that are marketing dedicated products for EC to check if they knew of any unpublished trials that were eligible for inclusion in the review. All Chinese companies, and Bayer, Laboratoire HRA Pharma, and Teva responded but they did not have information on, or knowledge of, other trials.
Others (February 2017). We performed the usual steps in the searches of a systematic review, such as searching the reference lists of published articles and contacting investigators active in this area.
Data collection and analysis
Selection of studies
We initially checked the trials identified by our search strategy for duplicates and relevance for the review by looking at the titles and abstracts. If it was not possible to exclude a publication by looking at the title or the abstract, we retrieved the full paper. Two review authors independently selected trials for inclusion and resolved any differences by discussion and consultation of other review authors if needed. Trials were excluded if the loss to follow‐up was greater than 20%. There were no language preferences in the search or the selection of articles.
Data extraction and management
We systematically extracted data from each trial for the following variables.
Intervention and control treatment. Because of the large variation in mifepristone doses, we categorised the doses arbitrarily (before data extraction) as high (more than 50 mg), mid (25 mg to 50 mg) and low (less than 25 mg). We also conducted separate meta‐analyses to validate our groupings of the different doses.
Clinical outcomes: observed number of pregnancies, ectopic pregnancies, side effects (any, nausea, vomiting, headache, dizziness, fatigue, breast tenderness, spotting/bleeding, diarrhoea, others), timing of menses, coitus‐treatment interval, high‐/low‐risk behaviour.
Methodology: random allocation techniques, blinding, post‐randomisation exclusions, loss to follow‐up.
Demographics: type of healthcare setting, city, country, total number of women included, and inclusion and exclusion criteria.
For articles written in English, two review authors independently carried out data extraction and another review author checked the data entry.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias using the Cochrane tool for assessing risk of bias (Higgins 2011) to assess: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias. We assigned judgements as recommended in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion. We described all judgements fully and presented the conclusions in the 'Risk of bias' table, which we incorporated into the interpretation of review findings as part of the GRADE assessment of the evidence (Summary of findings tables) (Characteristics of included studies).
Measures of treatment effect
We used Review Manager 5 (RevMan5) (RevMan 2014) to calculate treatment effects using risk ratio (RR) estimates with 95% confidence intervals (CI). We used risk difference (RD) to analyze effects when there were very few or no events and the number of participants was large. We presented 95% CIs for all outcomes.
Unit of analysis issues
Analysis was per woman.
Dealing with missing data
We attempted to extract data from all studies that would allow intention‐to‐treat (ITT) analysis. For outcomes with loss to follow‐up, the number of women with outcome data was taken as the denominator (available case analysis). In the levonorgestrel versus Yuzpe comparison and levonorgestrel versus mid‐dose mifepristone, we imputed outcomes for missing participants under two extreme scenarios (i.e. all missing in one arm had an event and all missing in the other arm did not have an event and vice versa).
Assessment of heterogeneity
We reviewed heterogeneity in the setting, interventions, and outcomes of included studies in order to make a qualitative assessment of the extent to which the included studies were similar to each other. We examined the forest plots visually to assess the levels of heterogeneity. We considered meta‐analyses with a P value for the Chi2 test of less than 0.1 to have considerable statistical heterogeneity (Deeks 2011). We used an I2 statistic of 50% or more to quantify the level of statistical heterogeneity (Higgins 2003).
Assessment of reporting biases
The comprehensive search strategy for this review helped to reduce the risk of reporting bias. If there were 10 or more studies in an analysis, we used a funnel plot to explore the possibility of small study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We performed meta‐analysis using a fixed‐effect model, where two or more trials with suitable data and homogeneity existed (I2 greater than 50%). In case of heterogeneity (P < 0.10), we used the random‐effects model to produce summary estimates (except when heterogeneity occurred in subgroup analyses where it was not possible to conduct separate analyses).
We planned to make the following comparisons.
Any regimen versus no intervention/placebo
-
Hormonal ECPs: comparison of different regimens
levonorgestrel versus Yuzpe
levonorgestrel versus anordrin
mifepristone versus levonorgestrel
mifepristone versus Yuzpe
mifepristone versus anordrin
mifepristone versus mifepristone + anordrin
mifepristone versus mifepristone + misoprostol
mifepristone versus mifepristone + tamoxifen
mifepristone versus mifepristone + methotrexate
mifepristone versus danazol
mifepristone versus gestrinone
High‐dose oestrogen versus Yuzpe
danazol versus Yuzpe
UPA versus levonorgestrel
drug/dose comparisons
others
IUD comparisons to ECPs
We have produced 'Summary of findings' tables to present each outcome for the main comparisons.
Assessment of quality of evidence
Two review authors independently rated the overall quality of evidence (high, moderate, low or very low) for each of our seven main outcomes using the GRADE system, with any disagreements resolved via consensus or, if required, by consulting a third review author (GRADEpro GDT 2014).
The GRADE system defines the quality of the body of evidence for each review outcome regarding the extent to which one can be confident in the review findings. GRADE criteria include:
risk of bias;
consistency of effect;
imprecision;
indirectness; and
publication bias.
With respect to assessment of imprecision, we made the judgement based on published guidance for the use of GRADE (Guyatt 2011).
"If the optimal information size (OIS) criterion is not met, rate down for imprecision, unless the sample size is very large (at least 2,000 and perhaps 4,000 patients)"
"If the OIS criterion is met and the 95% CI excludes no effect (i.e. CI around RR excludes 1.0) precision adequate"
"If OIS is met, and CI overlaps no effect (i.e. CI includes RR of 1.0) rate down if CI fails to exclude important benefit or important harm."
If there were very few or no events and the number of participants was large, we made judgements about imprecision based on the absolute (rather than the relative) effect measures. Wide CIs around a relative risk effect estimate may translate to clinically small differences in absolute effects. We consulted Figure five in Guyatt 2011 to estimate the OIS, as an alternative to calculating the OIS. For comparisons in which most of the studies failed to provide adequate details of their randomization methods, we did not downgrade the evidence for risk of bias if the analysis also included a large study at low risk of bias which had findings consistent with the smaller studies.
We used the four GRADEpro GDT 2014 quality ratings to describe the quality of the body of evidence for each outcome and we included these in the 'Summary of findings' table:
High quality: we are very confident that the true effect lies close to that of the estimate of the effect
Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect
Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
There are eight main comparisons in our review (levonorgestrel versus Yuzpe, levonorgestrel single versus split dose, mifepristone mid‐dose versus levonorgestrel, mifepristone low‐dose versus levonorgestrel, UPA versus levonorgestrel, mifepristone mid‐dose versus mifepristone low‐dose, mifepristone versus Yuzpe, Cu‐IUD versus mifepristone). For each comparison, the primary outcome measure was the pregnancy rate in women receiving different regimens (or control). Each of the 'Summary of findings' tables lists the following seven outcomes.
Observed number of pregnancies (all women)
Any side effect
Specific side effects ‐ nausea
Specific side effects ‐ vomiting
Specific side effects ‐ spotting/bleeding after treatment
Specific side effects ‐ abdominal pain
Menses ‐ early/delayed
We justified, documented, and incorporated judgements into reporting of results for each outcome, describing our reasons for downgrading in particular.
Subgroup analysis and investigation of heterogeneity
Several factors may affect the success of EC and we considered subgroup analyses when there were sufficient data in an appropriate format to allow such analyses. We considered the following categories for subgroup analyses.
-
Time elapsed since intercourse (coitus‐treatment interval)
24 hours or less
more than 24 hours to 48 hours
more than 48 hours to 72 hours
more than 72 hours to 120 hours
more than 120 hours
-
Risk status
high‐risk: women who had further acts of intercourse during the same cycle in which EC was used
low‐risk: women without further acts of coitus during that cycle
-
BMI
obese: BMI 30 kg/m2 or above
overweight: BMI 25 kg/m2 to 30 kg/m2
lower BMI: less than 25 kg/m2
We considered meta‐analyses with a P value for the Chi2 test of less than 0.1 to have considerable statistical heterogeneity (Deeks 2011). We used an I2 statistic of 50% or more to quantity the level of statistical heterogeneity (Higgins 2003). Where the I2 statistic was over 50%, we considered whether there were any methodological or clinical differences between the studies that might explain the inconsistency in findings.
Sensitivity analysis
No sensitivity analyses were planned.
Results
Description of studies
Results of the search
We included 115 studies with 60,479 women in this review (Figure 1). Ninety‐two trials were conducted in China. All Chinese trials were relatively recent (earliest trial published in 1993) indicating the interest in EC research in China. Except for the Ellertson 2003a, Glasier 2010; von Hertzen 2002; WHO 1998; WHO 1999 trials, all had been conducted in a single country, although some were multicenter trials. WHO trials were multinational involving large numbers of diverse populations (see Characteristics of included studies).
1.
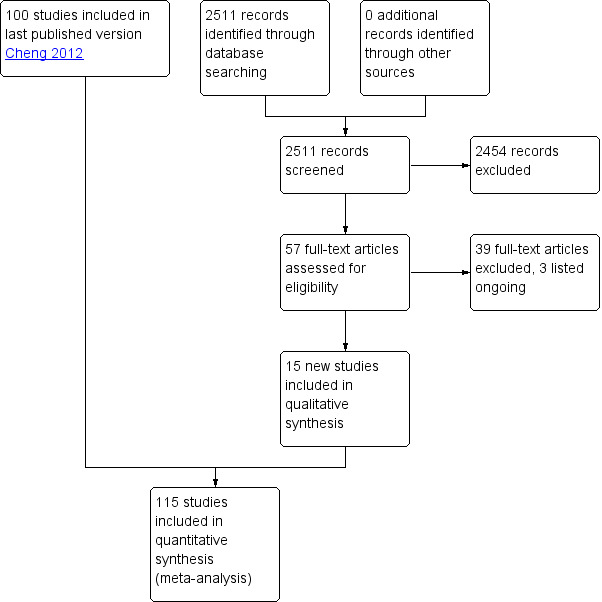
Study flow diagram
Three trials are ongoing, all of which are registered in ClinicalTrials.gov (NCT01539720; NCT02175030; NCT02577601). They compared ulipristal acetate versus levonorgestrel IUS; Cu‐IUD versus levonorgestrel IUD; ulipristal acetate versus levonorgestrel/ethinyl estradiol birth control pill (see Characteristics of ongoing studies).
Included studies
Design
All the included studies were RCTs.
We included Askalani 1987 in the review because random allocation was explicitly mentioned. Unfortunately, no other methodological details were available for this trial. One trial (Webb 1992) was stopped early for effectiveness reasons. Sixteen trials reported appropriate power calculations for the sample size (Arowojolu 2002; Ashok 2002; Creinin 2006; Dada 2010; Ellertson 2003a; Glasier 2010; Hamoda 2004; Hoseini 2013; Ngai 2005; Sang 1999; von Hertzen 2002; Webb 1992; WHO 1998; WHO 1999; Wu 2010; Xiao 2002).
Participants
The inclusion and exclusion criteria were similar with some minor differences between trials. In general, women attending after 72 hours (after 120 hours in Cu‐IUD, some mifepristone and levonorgestrel trials), with multiple episodes of unprotected intercourse, with irregular menstrual periods and those using hormonal contraception were excluded. All trials except Sang 1999 started the intervention as soon as the women came to the clinic. Sang 1999 included only women who had had unprotected intercourse 24 to 96 hours before attending the clinic.
Interventions
Eighteen out of 115 trials had two or more treatment arms. Fifty‐six trials involved dose‐comparison studies of mifepristone in doses from 5 mg to 600 mg. Forty‐one trials compared levonorgestrel with mifepristone. Six trials compared levonorgestrel with the Yuzpe regimen. Three trials (Arowojolu 2002; Dada 2010; von Hertzen 2002) compared a split dose with a single dose of levonorgestrel and one trial compared a 24‐hour with a 12‐hour double‐dose regimen of levonorgestrel. Two trials (Creinin 2006; Glasier 2010) compared UPA, a second‐generation progesterone receptor modulator, with levonorgestrel. One trial (Wu 2010) compared mifepristone with gestrinone. Other interventions were high‐dose oestrogen, danazol and Cu‐IUD. Anordrin is a steroid hormone with weak oestrogenic effects and is only used in China as a visiting‐contraceptive pill (a type of oral pill that is used for couples who do not cohabit but visit home for a short period. It can start at any day during a menstrual cycle, one pill a day continuing no less than 14 days). In Chinese EC trials, investigators used locally manufactured mifepristone and levonorgestrel.
Two studies (Su 2001; Wang 2000a) had three treatment arms (levonorgestrel versus mifepristone versus Cu‐IUD) but the Cu‐IUD comparison was not randomized. Hence, we excluded this comparison and included only the mifepristone vs levonorgestrel comparison.
Outcomes
Most of the trials reported observed number of pregnancies in comparison to expected number of pregnancies according to estimated probability of pregnancy on the day of the menstrual cycle when unprotected intercourse took place. This information is provided in the Characteristics of included studies table without a formal summary analysis.
In general, side effects were assessed by women themselves on diary charts.
Excluded studies
For this update of the review we excluded 2454 records after initial screening (Figure 1). We excluded 39 studies after examining 57 full‐text articles. Most of these were case‐series, reports without a comparison group, EC education or meta‐analysis. Six studies (Dong 2007; Li 2005a; Liu 2002a; Tian 2000; Turok 2010; Zhang 1999a) compared Cu‐IUDs versus mifepristone with or without levonorgestrel by informed choice (i.e. not randomly allocated). Two studies (Polakow 2013, Shaaban 2013) compared EC with lactational amenorrhoea method (LAM) (see Characteristics of excluded studies).
Risk of bias in included studies
Allocation
Twenty‐five trials (Arowojolu 2002; Ashok 2002; Carbonell 2015; Cheng 1999a; Creinin 2006; Dada 2010; Ellertson 2003a; Glasier 2010; Hamoda 2004; He 2002; Hoseini 2013; Liu 2000; Ngai 2005; Qi 2000a; Van Santen 1985a; von Hertzen 2002; Wang 2001; Webb 1992; WHO 1998; WHO 1999; Wu 1999a; Wu 2002; Wu 2010; Zhang 2012; Zuo 1999) had detailed explanation of randomization and we rated them as low risk of bias (see 'Risk of bias' tables in Characteristics of included studies). Most of the remaining trials had insufficient information on randomization and concealment of allocation, and only used terms such as 'randomly allocated', which we rated as unclear risk of bias (Characteristics of included studies; Figure 2; Figure 3).
2.
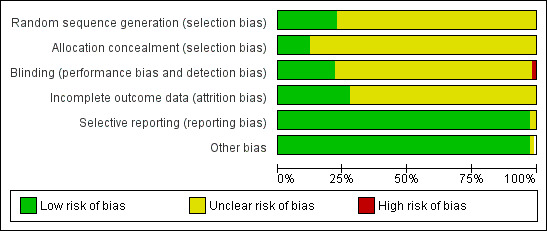
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
We included Askalani 1987 in the review because they explicitly mentioned random allocation. Unfortunately, no other methodological details were available for this trial. One trial (Webb 1992) was stopped early for effectiveness reasons. Sixteen trials reported appropriate power calculations for the sample size (Arowojolu 2002; Ashok 2002; Creinin 2006; Dada 2010; Ellertson 2003a; Glasier 2010; Hamoda 2004; Hoseini 2013; Ngai 2005; Sang 1999; von Hertzen 2002; Webb 1992; WHO 1998; WHO 1999; Wu 2010; Xiao 2002).
Blinding
Twenty‐three trials were reported as double‐blinded (Arowojolu 2002; Carbonell 2015; Creinin 2006; Dada 2010; Ellertson 2003a; He 2002; Hoseini 2013; Lin 2000; Liu 2000; Ngai 2005; Qi 2000a; Van Santen 1985a; von Hertzen 2002; Wang 2001; Wei 2002a; WHO 1998; WHO 1999; Wu 1999a; Wu 2002; Wu 2010; Xiao 2002; Zhang 2005; Zuo 1999) and two as single‐blinded (Glasier 2010; Sang 1999) (Characteristics of included studies; Figure 2; Figure 3).
Incomplete outcome data
ITT analysis was available (or possible) for the Creinin 2006; Glasier 2010; Ho 1993; Ngai 2005; WHO 1998; WHO 1999; Xiao 2002 trials and not mentioned in the other studies. Thirty trials (Arowojolu 2002; Ashok 2002; Carbonell 2015; Chen 2002a; Cheng 1999a; Creinin 2006; Ding 2005; Ellertson 2003a; Fan 2001a; Farajkhoda 2009; Glasier 1992; Glasier 2010; Hamoda 2004; He 2002; Ho 1993; Lai 2004; Liu 2000; Ngai 2005; Rowlands 1983; Sang 1999; von Hertzen 2002; Wang 2003; WHO 1998; WHO 1999; Wu 1999a; Wu 2002; Wu 2010; Xiao 2002; Zhang 1998; Zuo 1999) reported the number of lost follow‐up or post‐randomisation exclusions. The average proportion of loss to follow‐up or post‐randomisation exclusion was 3.3% (range 0.2% to 16.9%). Although several trials did not mention post‐randomisation exclusions, these studies did not explicitly mention ITT analyses either. As there were only a few reported pregnancies, it was possible that some pregnancies could well have been excluded after randomization (Webb 1992) (Characteristics of included studies; Figure 2; Figure 3).
Selective reporting
We used a funnel plot to explore the possibility of reporting bias when the intervention included more than eight studies. Funnel plots for the primary outcomes (observed number of pregnancies) of the comparison between low‐dose mifepristone and levonorgestrel showed asymmetric features, indicating possible reporting bias. We rated all the other studies as at low risk of selective reporting bias.
Other potential sources of bias
No other potential source of bias was identified and all studies were rated as at low risk in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
1. Any regimen versus no intervention/placebo
1.1 IUD versus expectant management
Askalani 1987 compared Cu‐IUD (Cu‐T 200) insertion with expectant management in women requesting EC within four days of unprotected intercourse.
1.1.1 Observed number of pregnancies
Notwithstanding the ethical aspects of this trial, the report was brief and only reported data on number of pregnancies. The evidence suggested a lower number of pregnancies in the IUD group (RR 0.09, 95% CI 0.03 to 0.26,1 RCT, n = 300) (Analysis 1.1). This indicated that IUD use would significantly decrease the number of pregnancies compared to expectant management, which suggests that if the observed number of pregnancies following expectant management is assumed to be 220 per 1000 women, the number following IUD would be between 7 to 57 per 1000 women.
1.1. Analysis.
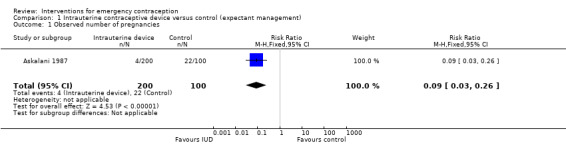
Comparison 1 Intrauterine contraceptive device versus control (expectant management), Outcome 1 Observed number of pregnancies.
1.1.2 Side effects
No data were available.
1.1.3. Effects on menses
No data were available.
2. Hormonal ECPs: comparison of different regimens
2.1 Levonorgestrel versus Yuzpe regimen
Six trials (three Chinese (Ho 1993; Sheng 2008; Sun 2007), two Iranian (Farajkhoda 2009; Hoseini 2013) and one multinational (WHO 1998)) compared the Yuzpe regimen with levonorgestrel 0.75 mg given twice, 12 hours apart. The six studies recruited a total of 4750 women.
2.1.1 Observed number of pregnancies
The levonorgestrel regimen was associated with fewer pregnancies than the Yuzpe regimen (RR 0.57, 95% CI 0.39 to 0.84, 6 RCTs, n = 4750, I2 = 23%, high‐quality evidence) (Figure 4; Analysis 2.1; Table 1). The evidence suggests that if the chance of pregnancy using Yuzpe is assumed to be 29 per 1000 women, the chance of pregnancy using levonorgestrel would be between 11 to 24 per 1000 women.
4.
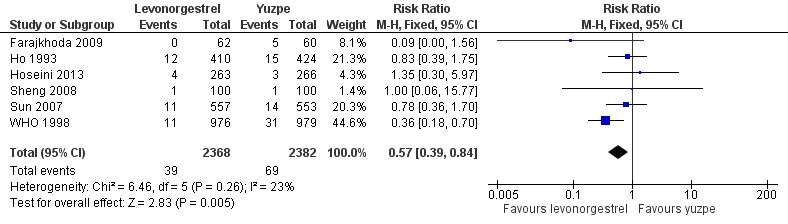
Forest plot of comparison 2.1: levonorgestrel versus Yuzpe, outcome 2.1.1 Observed number of pregnancies (all women)
2.1. Analysis.
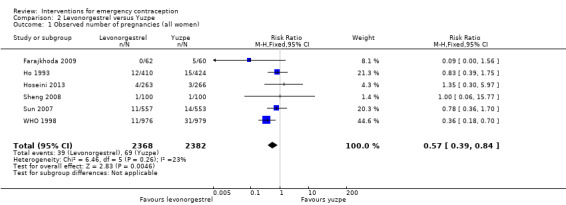
Comparison 2 Levonorgestrel versus Yuzpe, Outcome 1 Observed number of pregnancies (all women).
2.1.2. Side effects
Levonorgestrel was associated with fewer overall side effects than Yuzpe (RR 0.80, 95% CI 0.75 to 0.86, 1 RCT, n = 1955, high‐quality evidence) (Table 1) and probably with fewer complaints of nausea (RR 0.40, 95% CI 0.36 to 0.44, 6 RCTs, n = 4750, I2 = 82%, moderate‐quality evidence), vomiting (RR 0.29, 95% CI 0.24 to 0.35, 5 RCTs, n = 3640, I2 = 78%, moderate‐quality evidence), spotting/bleeding (RR 1.82, 95% CI 1.37 to 2.41, 2 RCTs, n = 1614 , I2 = 60%, moderate‐quality evidence), headache (RR 0.82, 95% CI 0.71 to 0.94, 3 RCTs, n = 2606, I2 = 63%), dizziness (RR 0.74, 95% CI 0.65 to 0.85, 3 RCTs, n = 3318, I2 = 0%) and fatigue (RR 0.67, 95% CI 0.60 to 0.74, 6 RCTs, n = 4750, I2 = 57%). There was no conclusive evidence of a difference between the groups for breast tenderness (RR 0.90, 95% CI 0.76 to 1.06, 3 RCTs, n = 3318, I2 = 39%) or abdominal pain (RR 0.84, 95% CI 0.70 to 1.01), although findings suggested a benefit for levonorgestrel. There was insufficient evidence to determine whether there was a difference between the groups for other reported side effects, which included diarrhoea, hot flushes, stomach pain and "nose spot" (Analysis 2.6; Table 1).
2.6. Analysis.
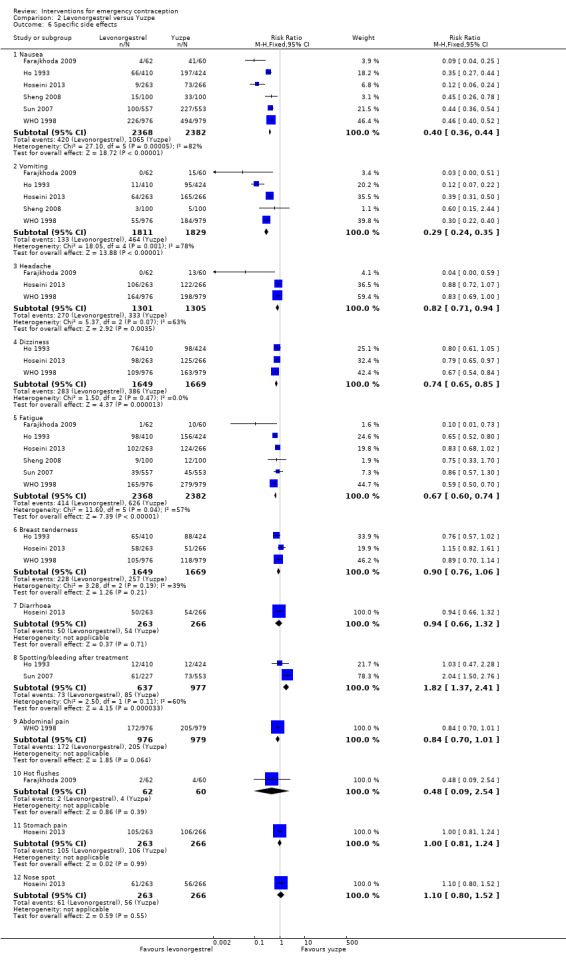
Comparison 2 Levonorgestrel versus Yuzpe, Outcome 6 Specific side effects.
2.1.3. Effects on menses
There was insufficient evidence to determine whether there was a difference between the groups in the rates of early menses (RR 1.15, 95% CI 0.86 to 1.52; 2 RCTs, n = 1310; low‐quality evidence) or menstrual delay (RR 1.23, 95% CI 0.96 to 1.57; 3 RCTs, n = 1988; I2 = 0%, low‐quality evidence) (Table 1).
2.2 Levonorgestrel versus anordrin
One trial from China (Xu 2000a) compared levonorgestrel split‐dose regimen with anordrin (7.5 mg, two doses, 12 hours apart, then 7.5 mg per day for eight days) in 172 women.
2.2.1 Observed number of pregnancies
The data were too imprecise to determine whether there was a difference between the two regimens in pregnancy rates (RR 0.67, 95% CI 0.11 to 3.89, 1 RCT, n = 172) (Analysis 3.1).
3.1. Analysis.
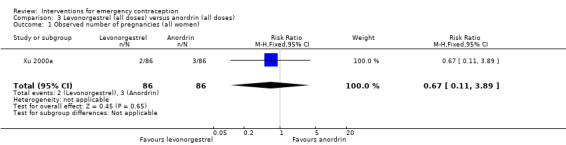
Comparison 3 Levonorgestrel (all doses) versus anordrin (all doses), Outcome 1 Observed number of pregnancies (all women).
2.2.2 Side effects
The data were too imprecise to determine whether there was a difference between the two regimens in overall side effects (RR 0.75, 95% CI 0.27 to 2.07, 1 RCT, n = 172) (Analysis 3.2).
3.2. Analysis.
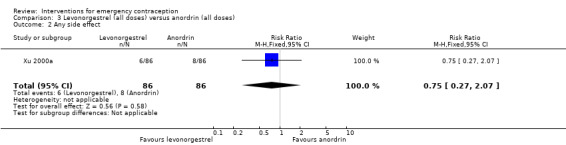
Comparison 3 Levonorgestrel (all doses) versus anordrin (all doses), Outcome 2 Any side effect.
No data were available on any of our other secondary outcomes
2.3 Mid‐dose mifepristone (25 mg to 50 mg) versus levonorgestrel
Twenty‐seven trials (Cao 2011; Chen 2008; Chen 2013; Chen 2015; Cheng 2009; Gan 2007; Han 1999a; Hu 2003; Jin 2012; Li 2000a; Li 2005b; Liang 2001; Liao 2003; Liu 2009; Qi 2003; Shao 2010; Su 2001; Sun 2000; Sun 2003; Tao 2014; Wang 2000b; Wang 2003; Xu 2000a; Xu 2000b; Ye 2013; Zhang 2000; Zhang 2014), all conducted in China, compared levonorgestrel (2939 women), all used a 12‐hour split‐dose regimen with mid‐dose mifepristone (3113 women).
2.3.1 Observed number of pregnancies
Overall, the effectiveness of mid‐dose mifepristone was probably better than that of levonorgestrel, with lower pregnancy rates (RR 0.61, 95% CI 0.45 to 0.83, 27 RCTs, n = 6052, I2 = 0%, moderate‐quality evidence) (Analysis 4.1; Table 2). The evidence suggested that if the chance of pregnancy following levonorgestrel is assumed to be 35 per 1000 women, the chance of pregnancy following mid‐dose mifepristone would be between 16 to 29 per 1000 women.
4.1. Analysis.
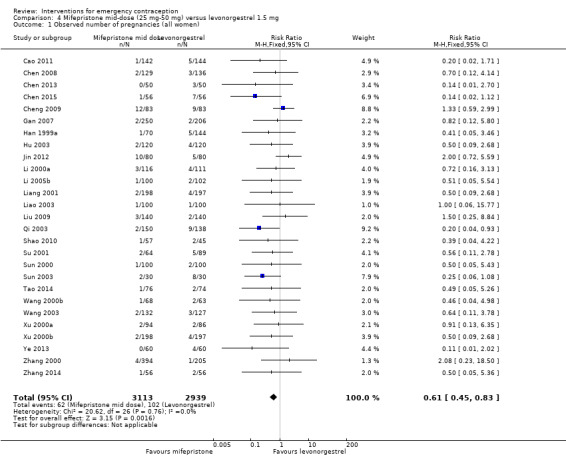
Comparison 4 Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg, Outcome 1 Observed number of pregnancies (all women).
Su 2001 reported a case of ectopic pregnancy in the levonorgestrel group.
This result was confirmed with simulated ITT analyses. When we assumed that all missing participants had the event in the levonorgestrel group, but none in the mifepristone group, the estimated RR was 0.50 (95% CI 0.32 to 0.77) and when we assumed that no missing women had an event in the levonorgestrel group, but that all the women in the mifepristone group did, the estimated RR was 0.57 (95% CI 0.37 to 0.88).
Funnel plots for the observed number of pregnancies between mid‐dose mifepristone and levonorgestrel did not suggest reporting bias.
2.3.2. Side effects
Eighteen trials reported the overall side‐effect rate and suggested that mifepristone may be more tolerable than levonorgestrel (RR 0.55, 95% CI 0.40 to 0.74, 18 RCTs, n = 4352, I2 = 72%, low‐quality evidence) (Analysis 4.3; Table 2).
4.3. Analysis.
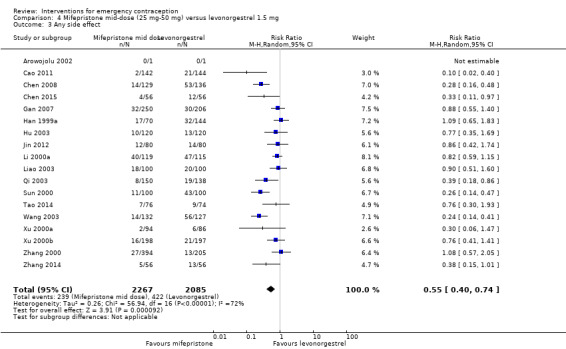
Comparison 4 Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg, Outcome 3 Any side effect.
The data were too imprecise to determine whether there was a difference between the groups for specific types of side effects, including nausea, headache, dizziness, breast tenderness or abdominal pain (Analysis 4.4, low‐quality evidence) except that spotting/bleeding after treatment appeared to be less common in the mifepristone group (RR 0.61, 95% CI 0.42 to 0.88, 9 RCTs, n = 1796, I2 = 29%, low‐quality evidence) (Analysis 4.4; Table 2).
4.4. Analysis.
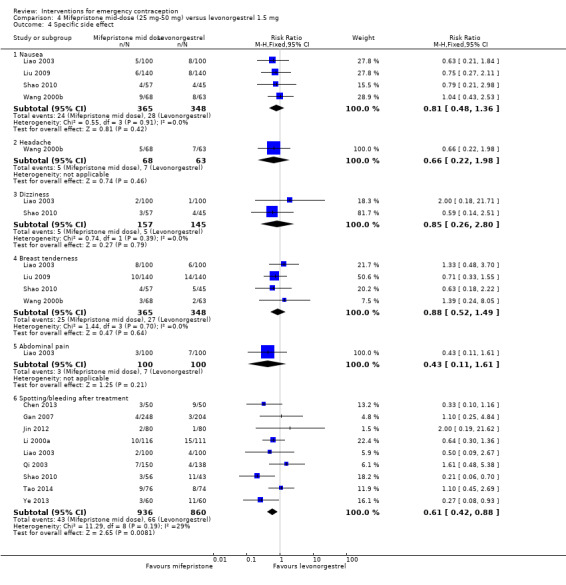
Comparison 4 Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg, Outcome 4 Specific side effect.
2.3.3. Effects on menses
Women who took mifepristone were probably more likely to have a delay in menses than those who took levonorgestrel (RR 1.29, 95% CI 1.09 to 1.54, 17 RCTs, n = 3615, I2 = 31%, moderate‐quality evidence) (Analysis 4.5). There was no clear evidence of a difference between the groups in rates of early menses (RR 0.72, 95% CI 0.50 to 1.03, 7 RCTs, n = 1324, low‐quality evidence) (Table 2).
4.5. Analysis.
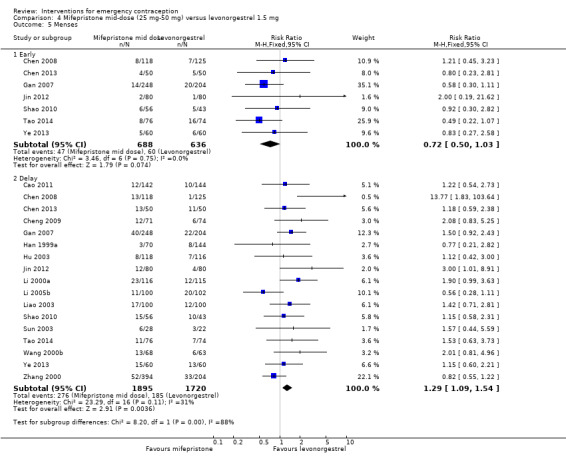
Comparison 4 Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg, Outcome 5 Menses.
2.4 Low‐dose mifepristone (less than 25 mg) versus levonorgestrel
Twelve Chinese studies (Bu 2006; Dong 2009; Li 2002a; Lei 2013; Lin 2000; Liu 2000; Pei 2001; Sheng 2002; Wang 2012; Wang 2000a; Wu 1999a; Zhang 2012), one UK study (Hamoda 2004) and one multinational WHO study (von Hertzen 2002) compared low‐dose mifepristone (3688 women) versus levonorgestrel (5064 women).
2.4.1 Observed number of pregnancies
When we pooled all the studies, there was evidence that there was a difference in effectiveness between low‐dose mifepristone regimens and levonorgestrel, with fewer pregnancies in the low‐dose mifepristone group (RR 0.72, 95% CI 0.52 to 0.99, 14 RCTs, n = 8752, I2 = 0%, high‐quality evidence) (Analysis 5.1; Table 3). Funnel plots for the primary outcomes (observed number of pregnancies) showed asymmetry, suggesting possible reporting bias (Figure 5). The evidence suggested that if the chance of pregnancy following levonorgestrel is assumed to be 20 per 1000 women, the chance of pregnancy following low‐dose mifepristone would be between 10 to 20 per 1000 women.
5.1. Analysis.
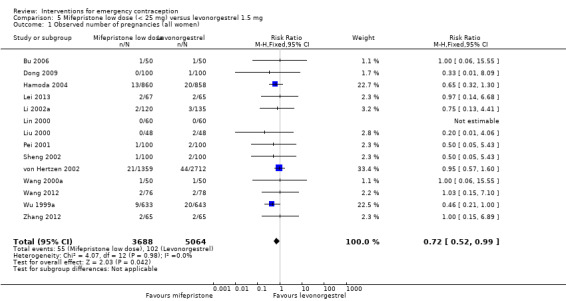
Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 1 Observed number of pregnancies (all women).
5.
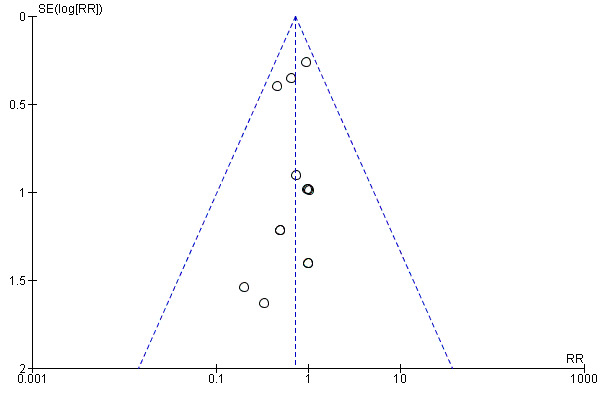
Funnel plot of comparison 2.4: Low‐dose mifepristone (< 25 mg) versus levonorgestrel 1.5 mg, outcome 2.4.1 Observed number of pregnancies (all women)
Additional analysis of data from one trial (von Hertzen 2002) indicated that the above conclusions were not modified by whether women abstained from further acts of intercourse or not (P = 0.14 for the interaction test), or by the time elapsed (within or after 72 hours) from intercourse to treatment administration (P = 0.99 for the interaction test) (Hamoda 2004; von Hertzen 2002). When we assumed that all women lost to follow‐up in the levonorgestrel group became pregnant, whereas none of those lost to follow‐up in the mifepristone group did, results indicated that mifepristone was associated with significantly lower risk of pregnancy than levonorgestrel (RR 0.70; 95% CI 0.50 to 0.98) (Figure 6; Analysis 5.7). However, there was no evidence of a difference between the groups when we assumed that none of the women lost to follow‐up in the levonorgestrel group became pregnant but all those in the mifepristone group did (RR 0.90, 95% CI 0.76 to 1.05) (Analysis 5.8).
6.
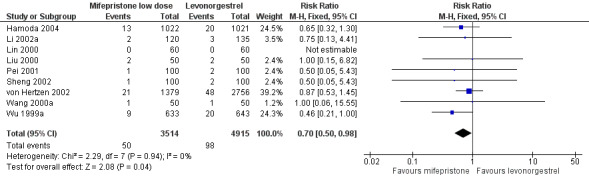
Forest plot of comparison 2.4: Low‐dose mifepristone (< 25 mg) versus levonorgestrel 1.5 mg, outcome 2.4.1 ITT (all loss follow‐up as pregnancy in levonorgestrel, and no pregnancy in mifepristone)
5.7. Analysis.
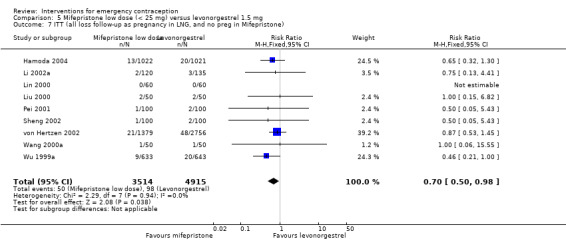
Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 7 ITT (all loss follow‐up as pregnancy in LNG, and no preg in Mifepristone).
5.8. Analysis.
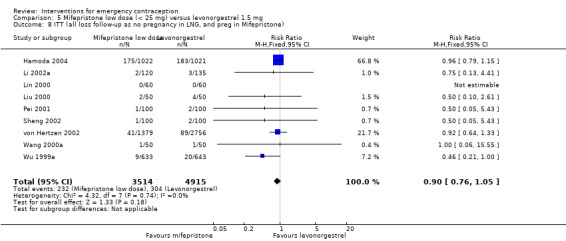
Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 8 ITT (all loss follow‐up as no pregnancy in LNG, and preg in Mifepristone).
2.4.2 Side effects
The low‐dose mifepristone group appeared to have fewer overall side effects than the levonorgestrel group (RR 0.26, 95% CI 0.17 to 0.38, 3 RCTs, n = 609, I2 = 0%, low‐quality evidence) (Analysis 5.4; Table 3). There was no evidence of a difference between the groups in rates of nausea or abdominal pain (Analysis 5.5; Table 3). There was insufficient evidence to determine whether there was a difference between the groups for other specific side effects such as vomiting, headache, dizziness, fatigue, breast tenderness, diarrhoea, or hot flushes, but low‐dose mifepristone was associated with a lower risk of spotting or bleeding after treatment (RR 0.61, 95% CI 0.54 to 0.69, 5 RCTs, n = 4598, I2 = 0%, high‐quality evidence) (Analysis 5.5, Table 3).
5.4. Analysis.
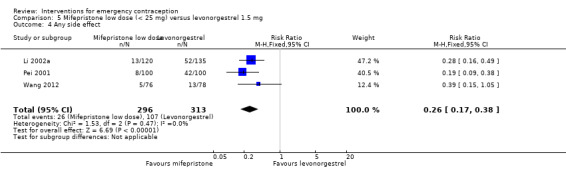
Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 4 Any side effect.
5.5. Analysis.
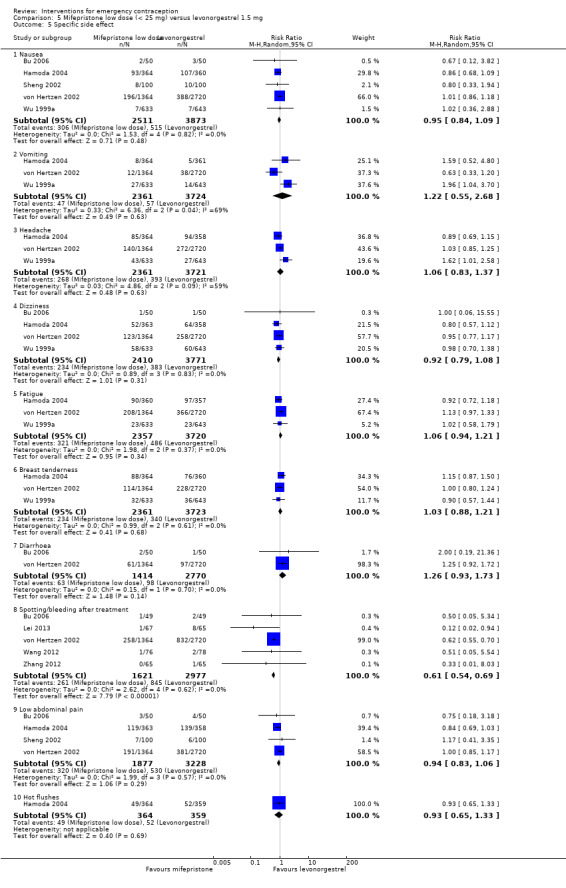
Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 5 Specific side effect.
2.4.3 Effects on menses
Early return of menstruation was probably less frequent in the mifepristone group than in the levonorgestrel group (RR 0.45, 95% CI 0.35 to 0.59, 5 RCTs, n = 1800, I2 = 0%, low‐quality evidence). Delay in menstruation was probably more frequent in the mifepristone group (RR 1.70, 95% CI 1.48 to 1.97, 9 RCTs, n = 7520, I2 = 51%, low‐quality evidence) (Analysis 5.6, Table 3).
5.6. Analysis.

Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 6 Menses.
There were no trials that compared high‐dose (more than 50 mg) mifepristone with levonorgestrel.
2.5 Mifepristone versus Yuzpe regimen
Three trials conducted in the UK compared high‐dose mifepristone (100 mg and 600 mg) with the Yuzpe regimen (Ashok 2002 (100 mg); Glasier 1992 (600 mg); and Webb 1992 (600 mg)). Webb 1992 included danazol as a third arm. This trial was stopped early because mifepristone showed higher effectiveness than the Yuzpe regimen and danazol (number of pregnancies: 0/195 with mifepristone vs 5/191 with Yuzpe and 9/193 with danazol). One trial investigated whether the effectiveness was influenced by high‐ or low‐risk behaviour (Glasier 1992). No pregnancy was observed among women who abstained from further intercourse, but the sample size of this study was relatively small.
2.5.1 Observed number of pregnancies
The risk of pregnancy among mifepristone users was significantly lower than that among the Yuzpe users (RR 0.14; 95% CI 0.05 to 0.41, 3 RCTs, n = 2144 , I2 = 0%, high‐quality evidence) (Analysis 6.1; Table 4). This suggests that if the chance of pregnancy following Yuzpe is assumed to be 25 per 1000 women, the chance following mifepristone would be between 1 to 10 per 1000 women.
6.1. Analysis.
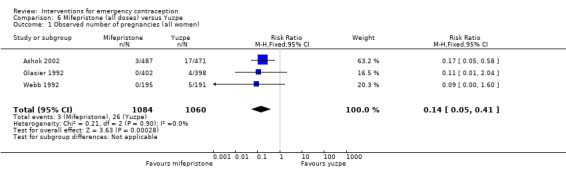
Comparison 6 Mifepristone (all doses) versus Yuzpe, Outcome 1 Observed number of pregnancies (all women).
2.5.2 Side effects
Overall side effects were less common in the mifepristone group (RR 0.83, 95% CI 0.77 to 0.88, 2 RCTs, n = 1693, I2 = 94%, moderate‐quality evidence). Heterogeneity was very high, but the direction of effect was consistent.
Women receiving mifepristone reported fewer complaints of nausea (RR 0.63, 95% CI 0.53 to 0.76, 3 RCTs, n = 2186, I2 = 59%, moderate‐quality evidence), vomiting (RR 0.12, 95% CI 0.07 to 0.20, 3 RCTs, n = 2186, I2 = 0%, high‐quality evidence), headache (RR 0.75, 95% CI 0.61 to 0.91, 2 RCTs, n = 1800, I2 = 48%), dizziness (RR 0.58, 95% CI 0.42 to 0.80), fatigue (RR 0.81, 95% CI 0.68 to 0.95 ), hot flushes RR 0.58, 95% CI 0.40 to 0.83) or abdominal pain (RR 0.76, 95% CI 0.61 to 0.95, 1 RCT, n = 1000, moderate‐quality evidence) (Analysis 6.6; Table 4). There was no clear evidence of a difference between the groups in rates of breast tenderness or lethargy.
6.6. Analysis.
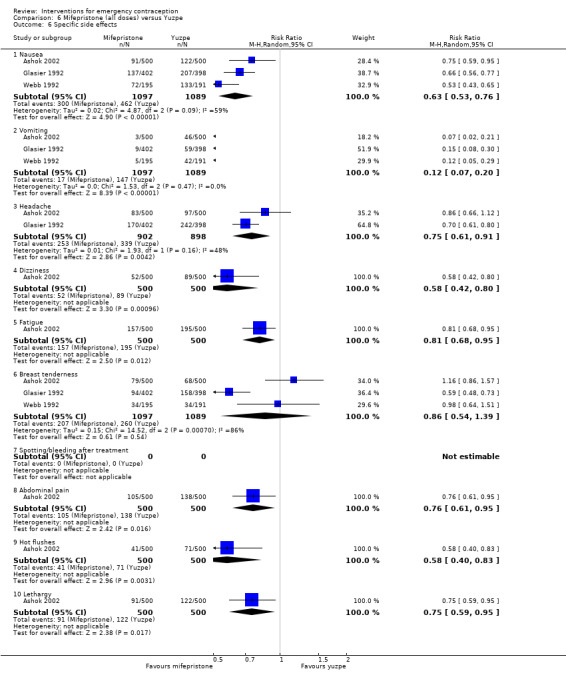
Comparison 6 Mifepristone (all doses) versus Yuzpe, Outcome 6 Specific side effects.
2.5.3 Effects on menses
Delay in menses appeared to be more frequent in women receiving mifepristone than in those who used the Yuzpe regimen (RR 2.83; 95% CI 2.30 to 3.47, 3 RCTs, n = 1924, I2 = 85%, moderate‐quality evidence) (Analysis 6.7; Table 4).
6.7. Analysis.
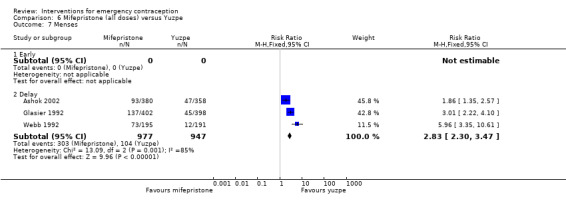
Comparison 6 Mifepristone (all doses) versus Yuzpe, Outcome 7 Menses.
2.6 Mifepristone versus anordrin
Seven trials (Chen 2001; Fu 2000; Han 1995; Liu 2001; Wang 1999; Xu 2000aYang 2001) compared mid‐dose mifepristone with anordrin in different regimens (the total dosage ranging from 15 mg to 90 mg).
2.6.1 Observed number of pregnancies
Mifepristone may be more effective in preventing pregnancy than anordrin (RR 0.26, 95% CI 0.11 to 0.63, 7 RCTs, n = 1035, I2 = 0%, Analysis 7.1). This suggests that if the risk of pregnancy following anordrin is assumed to be 40 per 1000 women, the risk of pregnancy following mifepristone would be between 4 to 25 per 1000 women.
7.1. Analysis.
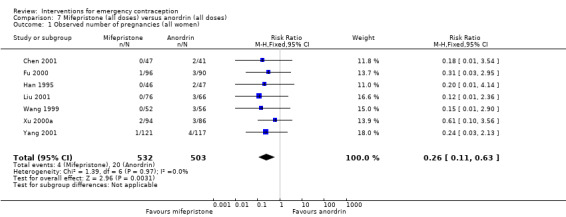
Comparison 7 Mifepristone (all doses) versus anordrin (all doses), Outcome 1 Observed number of pregnancies (all women).
2.6.2 Side effects
Mifepristone may have fewer overall side effects than anordrin (RR 0.62, 95% CI 0.43 to 0.91, 4 RCTs, n = 746, I2 = 66%) (Analysis 7.2). There was insufficient evidence to determine whether there was a difference between the groups in rates of spotting/bleeding (RR 1.82, 95% CI 0.69 to 4.77, 2 RCTs, n = 331, I2 = 0%) (Analysis 7.3).
7.2. Analysis.
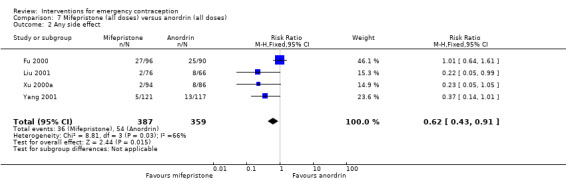
Comparison 7 Mifepristone (all doses) versus anordrin (all doses), Outcome 2 Any side effect.
7.3. Analysis.
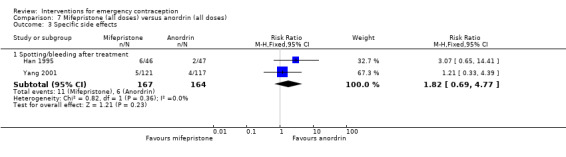
Comparison 7 Mifepristone (all doses) versus anordrin (all doses), Outcome 3 Specific side effects.
2.6.3 Effects on menses
There was no clear evidence of a difference in menses' changes between mifepristone and anordrin (RR 1.14, 95% CI 0.78 to 1.68, 4 RCTs, n = 667, I2 = 85%) (Analysis 7.4).
7.4. Analysis.
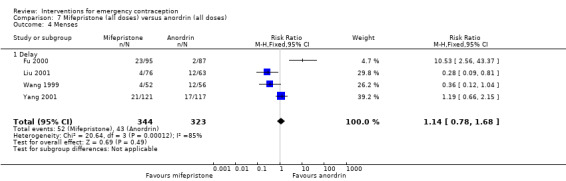
Comparison 7 Mifepristone (all doses) versus anordrin (all doses), Outcome 4 Menses.
2.7 Low‐or mid‐dose mifepristone versus mifepristone with anordrin
Five trials (Han 1995; Han 1996; Lou 2005; Sang 1999; Zhang 2002a) compared low‐ or mid‐dose mifepristone versus mifepristone combined with anordrin.
2.7.1 Observed number of pregnancies
It was unclear whether there was a difference between the groups in the risk of pregnancy (RR 1.32, 95% CI 0.73 to 2.41, 5 RCTs, n = 3038, I2 = 0%) (Analysis 8.1). This suggests that if the chance of pregnancy using mifepristone and anordrin is assumed to be 12 per 1000 women, the chance by using mifepristone alone would be between 9 to 30 per 1000 women.
8.1. Analysis.
Comparison 8 Mifepristone alone (low or mid dose) versus mifepristone + anordrin (all doses), Outcome 1 Observed number of pregnancies (all women).
2.7.2 Side effects
There was no clear evidence of a difference between the groups in overall rates of side‐effects (RR 0.83, 95% CI 0.49 to 1.41, 2 RCTs, n = 442, I2 = 0%) (Analysis 8.2).
8.2. Analysis.
Comparison 8 Mifepristone alone (low or mid dose) versus mifepristone + anordrin (all doses), Outcome 2 Any side effect.
The mifepristone‐only regimen was associated with lower rates of nausea (RR 0.53; , CI 0.44 to 0.65, 1 RCT, n = 2387) (Analysis 8.3), vomiting (RR 0.26, 95% CI 0.14 to 0.50, 1 RCT, n = 2387) and fatigue (RR 0.66, 95% CI 0.49 to 0.89, 1 RCT, n = 2387) , but higher rates of spotting/bleeding (RR 1.80; 95% CI 1.33 to 2.43, 5 RCTs, n = 3038) than the combination regimen (Analysis 8.3). There was no clear evidence of any difference between the groups in rates of headache, dizziness, breast tenderness or abdominal pain.
8.3. Analysis.
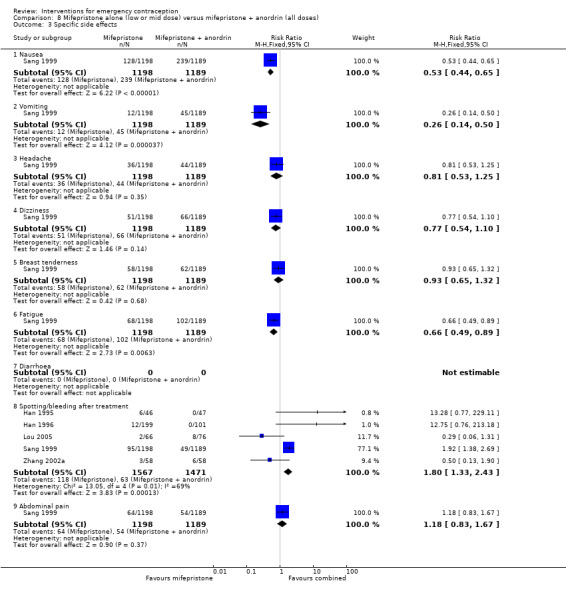
Comparison 8 Mifepristone alone (low or mid dose) versus mifepristone + anordrin (all doses), Outcome 3 Specific side effects.
2.7.3 Effects on menses
The mifepristone‐only regimen was associated with a lower risk of menstrual delay (RR 0.79, 95% CI 0.65 to 0.97, 3 RCTs, n = 2781, I2 = 3%) (Analysis 8.4).
8.4. Analysis.
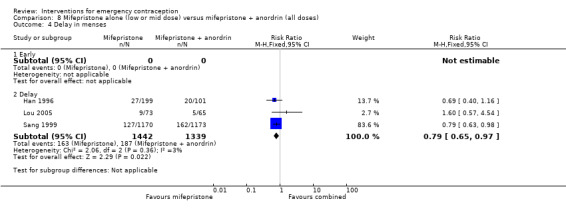
Comparison 8 Mifepristone alone (low or mid dose) versus mifepristone + anordrin (all doses), Outcome 4 Delay in menses.
2.8 Low‐dose mifepristone versus mifepristone with misoprostol (200 μg)
Wu 2002 compared low‐dose mifepristone with mifepristone combined with misoprostol (200 μg).
2.8.1. Observed number of pregnancies
There was insufficient evidence to determine whether there was a difference between the groups in pregnancy rates (RR 3.49, 95% CI 0.73 to 16.65; n = 599) (Analysis 9.1).
9.1. Analysis.
Comparison 9 Mifepristone versus mifepristone + misoprostol (all doses), Outcome 1 Observed number of pregnancies (all women).
2.8.2 Side effects
This study did not report overall rates of side effects.
With regard to specific side effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, headache, dizziness, fatigue, breast tenderness, diarrhoea or spotting/bleeding after treatment, but the mifepristone‐alone regimen is probably associated with a lower risk of abdominal pain than the combination regimen (RR 0.31, 95% CI 0.10 to 0.93) (Analysis 9.2).
9.2. Analysis.
Comparison 9 Mifepristone versus mifepristone + misoprostol (all doses), Outcome 2 Specific side effect.
2.8.3 Effects on menses
No studies reported on this outcome.
2.9 Low‐dose mifepristone versus mifepristone with tamoxifen (20 mg)
One double‐blind trial (He 2002) compared low‐dose mifepristone with mifepristone combined with tamoxifen (20 mg).
2.9.1. Observed number of pregnancies
There was insufficient evidence to determine whether there was a difference between the groups in the risk of pregnancy (RR 3.00, 95% CI 0.31 to 28.60, 1 RCT, n = 400) (Analysis 10.1).
10.1. Analysis.
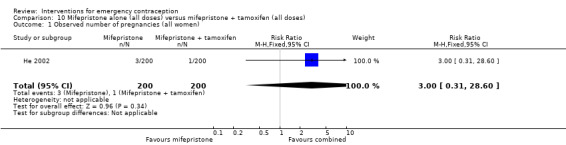
Comparison 10 Mifepristone alone (all doses) versus mifepristone + tamoxifen (all doses), Outcome 1 Observed number of pregnancies (all women).
2.9.2 Side effects
Rates of overall side‐effects were not reported by this study.
With regard to specific side‐effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, headache, dizziness, fatigue, breast tenderness, diarrhoea, spotting/bleeding after treatment, or abdominal pain (Analysis 10.2).
10.2. Analysis.
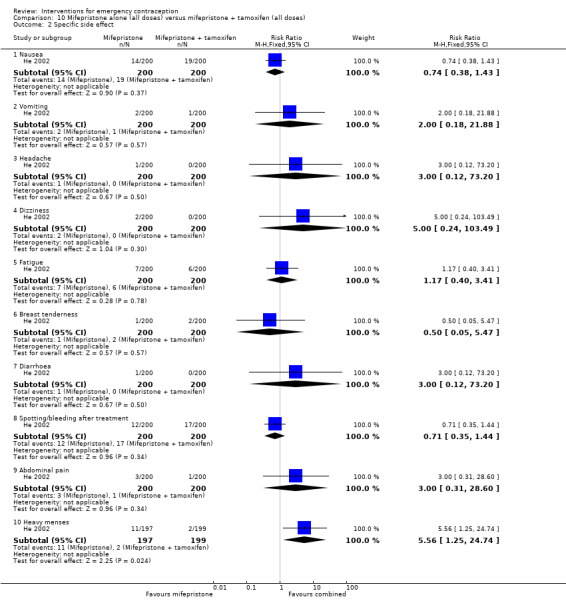
Comparison 10 Mifepristone alone (all doses) versus mifepristone + tamoxifen (all doses), Outcome 2 Specific side effect.
2.9.3 Effects on menses
Heavy menses was more common in the mifepristone‐only group (RR 5.56, 95% CI 1.25 to 24.74, 1 RCT, n = 396) (Analysis 10.2).
There was no evidence of a difference between the groups in the rate of delayed menses (RR 1.79, 95% CI 0.93 to 3.43, 1 RCT, n = 396) (Analysis 10.3).
10.3. Analysis.
Comparison 10 Mifepristone alone (all doses) versus mifepristone + tamoxifen (all doses), Outcome 3 Menses.
2.10 Mifepristone (25 mg) versus mifepristone with methotrexate (5 mg)
Two trials (Chen 2002b; Zeng 2007) compared a mid‐dose mifepristone (25 mg) regimen with a regimen of mifepristone combined with methotrexate (5 mg).
2.10.1. Observed number of pregnancies
Two women were pregnant in the mifepristone‐alone group, and none in the combination group. There was insufficient evidence to determine whether there was a difference between mifepristone alone and mifepristone with methotrexate (RR 3.00, 95% CI 0.32 to 28.36, 2 RCTs, n = 200, I2 = 0%) (Analysis 11.1). Since only 100 women were recruited in each arm, the non‐significant result may be due partly to the small sample sizes of these two trials (Analysis 11.1).
11.1. Analysis.
Comparison 11 Mifepristone alone (all doses) versus mifepristone + methotrexate (all doses), Outcome 1 Observed number of pregnancy (all women).
2.10.2 Side effects
There was insufficient evidence to determine whether there was a difference between the groups in the overall incidence of side effects (RR 0.75, 95% CI 0.33 to 1.70, 2 RCTs, n = 200, I2 = 0%, Analysis 11.2).
11.2. Analysis.
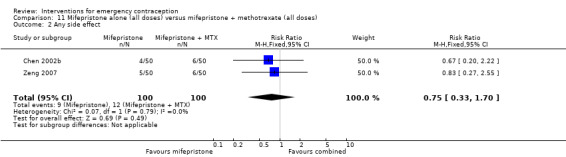
Comparison 11 Mifepristone alone (all doses) versus mifepristone + methotrexate (all doses), Outcome 2 Any side effect.
2.10.3 Effects on menses
There was insufficient evidence to determine whether there was a difference between two regimens in rates of early menses (RR 1.50, 95% CI 0.26 to 8.60, 1 RCT, n = 100) or menstrual delay (RR 0.91, 95% CI 0.60 to 1.39, 2 RCTs, n = 199, I2 = 0%) (Analysis 11.3).
11.3. Analysis.
Comparison 11 Mifepristone alone (all doses) versus mifepristone + methotrexate (all doses), Outcome 3 Menses.
2.11 Mifepristone versus danazol
Two trials (Webb 1992; Yang 2001) compared mifepristone (50 mg or 600 mg) with danazol (400 mg or 600 mg, repeated after 12 hours).
2.11.1 Observed number of pregnancies
Mifepristone is probably associated with a lower risk of pregnancy than danazol (RR 0.10, 95% CI 0.02 to 0.55, 2 RCTs, n = 629, I2 = 0%) (Analysis 12.1). This suggests that if the chance of pregnancy using danazol is assumed to be 45 per 1000 women, the chance of pregnancy using mifepristone would be between 1 to 25 per 1000 women.
12.1. Analysis.
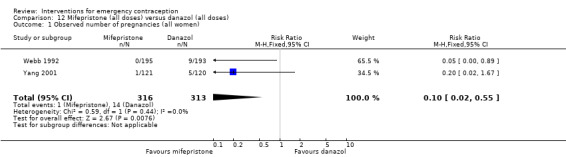
Comparison 12 Mifepristone (all doses) versus danazol (all doses), Outcome 1 Observed number of pregnancies (all women).
2.11.2 Side effects
Mifepristone was associated with fewer overall side effects than danazol (RR 0.35, 95% CI 0.13 to 0.95, 1 RCT, n = 241) (Analysis 12.2).
12.2. Analysis.
Comparison 12 Mifepristone (all doses) versus danazol (all doses), Outcome 2 Any side effect.
With respect to specific side‐effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, breast tenderness or other (unspecified) side effects (Analysis 12.3).
12.3. Analysis.
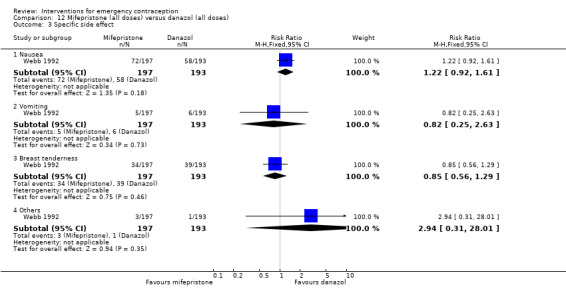
Comparison 12 Mifepristone (all doses) versus danazol (all doses), Outcome 3 Specific side effect.
2.11.3 Effects on menses
It was unclear whether there was a difference between the groups in rates of delayed menses (RR 2.39, 0.56 to 10.27, 2 RCTs, n = 621, I2 = 91%) (Analysis 12.4). Heterogeneity was very high, but the direction of effect was consistent.
12.4. Analysis.
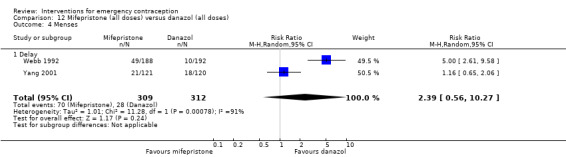
Comparison 12 Mifepristone (all doses) versus danazol (all doses), Outcome 4 Menses.
2.12 Mifepristone versus gestrinone
Wu 2010 conducted a randomized, double‐blind, multicentre clinical trial (996 women) comparing mifepristone 10 mg with gestrinone 10 mg (a 19‐nortestosterone derivative with anti‐progestagenic, anti‐oestrogenic and anti‐gonadotropic properties).
2.12.1. Observed number of pregnancies
It was unclear whether there was a difference between the groups in pregnancy rates (RR 0.75, 95% CI 0.32 to 1.76, 1 RCT, n = 996) (Analysis 13.1).
13.1. Analysis.
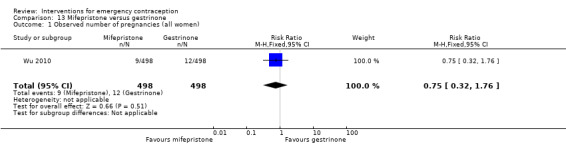
Comparison 13 Mifepristone versus gestrinone, Outcome 1 Observed number of pregnancies (all women).
2.12.2 Side effects
Overall rates of side‐effects were not reported.
With regard to specific side‐effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, headache, dizziness, fatigue, breast tenderness, diarrhoea, spotting/bleeding after treatment, or lower abdominal pain (Analysis 13.2).
13.2. Analysis.
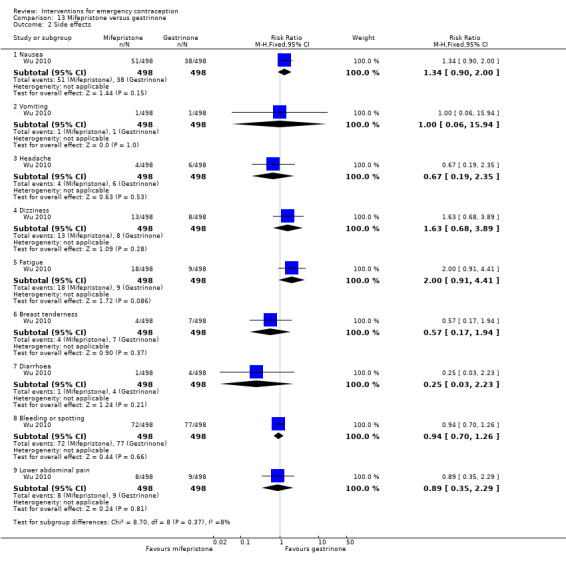
Comparison 13 Mifepristone versus gestrinone, Outcome 2 Side effects.
2.12.3 Effects on menses
Mifepristone was probably associated with higher risk of menstrual delay than gestrinone (RR 1.37, 95% CI 1.03 to 1.82, 1 RCT, n = 975) (Analysis 13.3) and a lower risk of early return to next menses (RR 0.37, 95% CI 0.20 to 0.69, 1 RCT, n = 975) (Analysis 13.3).
13.3. Analysis.
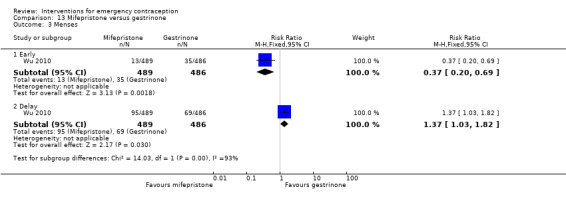
Comparison 13 Mifepristone versus gestrinone, Outcome 3 Menses.
2.13 High‐dose oestrogen versus Yuzpe regimen
One trial conducted in the early 1980s compared a five‐day ethinyl oestradiol 5 mg regimen (standard treatment at that time) versus Yuzpe in a double‐blind trial (Van Santen 1985a).
2.13.1. Observed number of pregnancies
There was insufficient evidence to determine whether there was any difference between the groups in the risk of pregnancy (RR 2.17, 95% CI 0.20 to 23.77, 1 RCT, n = 384) (Analysis 14.1).
14.1. Analysis.
Comparison 14 High‐dose oestrogens versus Yuzpe, Outcome 1 Observed number of pregnancies (all women).
2.13.2 Side effects
No studies reported this outcome.
2.13.3 Effects on menses
No studies reported this outcome.
2.14 Danazol versus Yuzpe regimen
Danazol was compared to the Yuzpe regimen in one trial (Rowlands 1983) and to the Yuzpe regimen and mifepristone (600 mg) in a three‐arm trial (Webb 1992).
2.14.1. Observed number of pregnancies
There was insufficient evidence to determine whether there was any difference in risk of pregnancy between danazol and the Yuzpe regimen (RR 1.78, 95% CI 0.61 to 5.22, 2 RCTs, n = 485) (Analysis 15.1).
15.1. Analysis.
Comparison 15 Danazol (all doses) versus Yuzpe, Outcome 1 Observed number of pregnancies (all women).
2.14.2 Side effects
Overall rates of side effects were not reported.
With regard to specific side effects, danazol is probably associated with lower rates of nausea (RR 0.38, 95% CI 0.30 to 0.47, 2 RCTs, n = 538, I2 = 81%) and vomiting (RR 0.13, 95% CI 0.06 to 0.27, 2 RCTs, n = 538, I2 = 0%). There was very high heterogeneity for the outcome of nausea, but the direction of effect was consistent. There was insufficient evidence to determine whether there was any difference between the groups in rates of breast tenderness (RR 1.14, 95% CI 0.75 to 1.72, 1 RCT, n = 384) (Analysis 15.2).
15.2. Analysis.
Comparison 15 Danazol (all doses) versus Yuzpe, Outcome 2 Specific side effects.
2.14.3 Effects on menses
There was insufficient evidence to determine whether there was any difference between the groups in rates of delay of menses (RR 1.53, 95% CI 0.74 to 3.18, 1 RCT, n = 384) (Analysis 15.3).
15.3. Analysis.
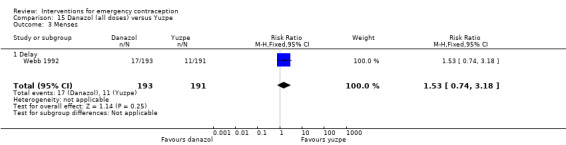
Comparison 15 Danazol (all doses) versus Yuzpe, Outcome 3 Menses.
2.15 UPA (all doses) versus levonorgestrel
UPA is a second‐generation progesterone receptor modulator. Creinin 2006 compared levonorgestrel split‐dose regimen with UPA unmicronised, 50 mg single‐dose, orally within 72 hours of unprotected intercourse. Glasier 2010 compared levonorgestrel single‐dose regimen with UPA micronised, 30 mg, single‐dose, orally within 120 hours of unprotected intercourse. Since both the European Medicines Agency (EMA) and the Food and Drug Administration (FDA, USA) accepted the bioequivalence of the two regimens, we combined data from the two trials for meta‐analysis in this review.
2.15.1 Observed number of pregnancies
UPA was associated with fewer pregnancies than levonorgestrel within 120 hours after unprotected intercourse (RR 0.59, 95% CI 0.35 to 0.99, 2 RCTs, n = 3448, I2 = 0%, high‐quality evidence) (Analysis 16.1; Table 5). The evidence suggests that if the chance of pregnancy using levonorgestrel is assumed to be 22 per 1000 women, the chance of pregnancy using UPA would be between 8 to 22 per 1000 women.
16.1. Analysis.
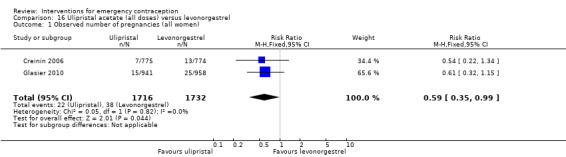
Comparison 16 Ulipristal acetate (all doses) versus levonorgestrel, Outcome 1 Observed number of pregnancies (all women).
2.15.2 Side effects
Overall rates of side effects were not reported.
With regard to specific side effects, there was no evidence of a difference between the groups in rates of nausea, vomiting, headache, dizziness, fatigue, breast tenderness, diarrhoea, spotting/bleeding after treatment, overall abdominal pain, lower abdominal pain, upper abdominal pain, back pain or dysmenorrhoea. (Analysis 16.5, moderate‐quality evidence, Table 5).
16.5. Analysis.
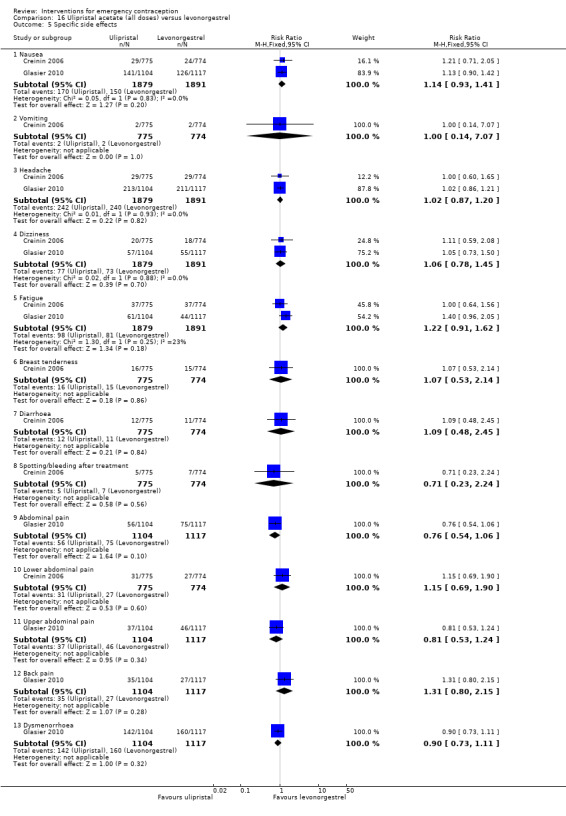
Comparison 16 Ulipristal acetate (all doses) versus levonorgestrel, Outcome 5 Specific side effects.
2.15.3 Effects on menses
Women who took UPA were less likely to have earlier return of menses, compared with those who received levonorgestrel (RR 0.43, 95% CI 0.37 to 0.50, 2 RCTs, n = 3593, I2 = 72%, moderate‐quality evidence) and UPA users were more likely to have delayed return of next menses than those who received levonorgestrel (RR 1.65, 95% CI 1.42 to 1.92, 2 RCTs, n = 3593, I2 = 0%, high‐quality evidence) (Analysis 16.6; Table 5).
16.6. Analysis.
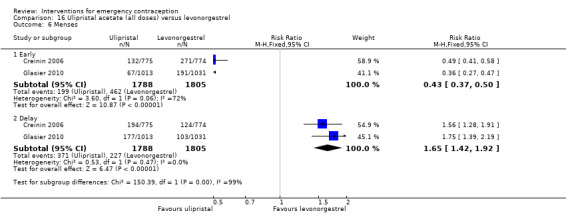
Comparison 16 Ulipristal acetate (all doses) versus levonorgestrel, Outcome 6 Menses.
2.16 Split‐dose levonorgestrel: 24 hours versus 12 hours
One double‐blind, randomized, multicenter trial conducted in China (Ngai 2005) compared levonorgestrel split‐dose in two different regimens (24 hours versus 12 hours apart).
2.16.1. Observed number of pregnancies
There was no clear evidence of any difference between the two regimens (RR 0.98, 95% CI 0.53 to 1.82, 1 RCT, n = 2060) (Analysis 17.1). This conclusion was not modified by whether or not the women abstained from further acts of intercourse (Analysis 17.2).
17.1. Analysis.
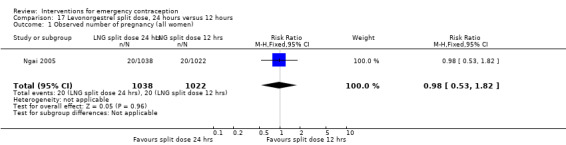
Comparison 17 Levonorgestrel split dose, 24 hours versus 12 hours, Outcome 1 Observed number of pregnancy (all women).
17.2. Analysis.
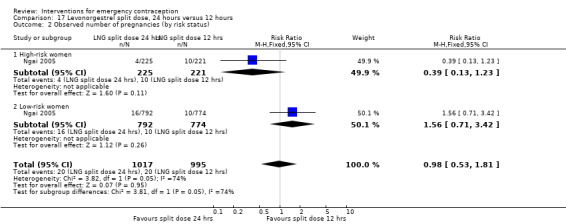
Comparison 17 Levonorgestrel split dose, 24 hours versus 12 hours, Outcome 2 Observed number of pregnancies (by risk status).
2.16.2 Side effects
Overall rates of side effects were not reported.
With regard to specific side effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, headache, dizziness, breast tenderness, or lower abdominal pain (Analysis 17.3).
17.3. Analysis.
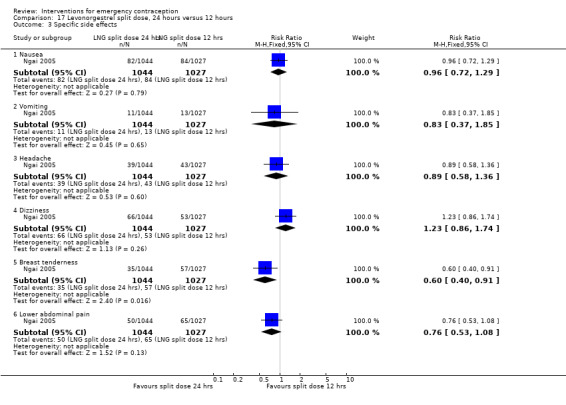
Comparison 17 Levonorgestrel split dose, 24 hours versus 12 hours, Outcome 3 Specific side effects.
2.16.3. Effects on menses
There was no clear evidence of a difference between the two regimens in rates of delayed menses (RR 0.79, 95% CI 0.53 to 1.17, 1 RCT, n = 1978) (Analysis 17.4).
17.4. Analysis.
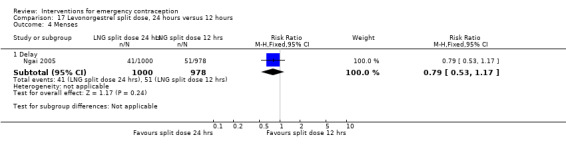
Comparison 17 Levonorgestrel split dose, 24 hours versus 12 hours, Outcome 4 Menses.
2.17 Single‐dose levonorgestrel versus split‐dose levonorgestrel
Three trials compared regimens of levonorgestrel 1.5 mg single dose with levonorgestrel 0.75 mg two doses, 12 hours apart. Arowojolu 2002 recruited 1160 women who had a single act of unprotected intercourse within 72 hours, and von Hertzen 2002 recruited 4136 and Dada 2010 recruited 3022 women attending clinics within 120 hours of unprotected intercourse.
2.17.1 Observed number of pregnancies
There was no evidence of a difference between the groups in pregnancy rates (RR 0.84; 95% CI 0.53 to 1.33, 3 RCTs, n = 6653, I2 = 0%, moderate‐quality evidence) (Analysis 18.1; Table 6). Additional analysis of the von Hertzen 2002 trial data indicated that this conclusion was not modified by whether the women abstained from further acts of intercourse or not (P = 0.18 for the interaction test), or by the time elapsed (within or after 72 hours) from intercourse to treatment administration (P = 0.90 for the interaction test).
18.1. Analysis.
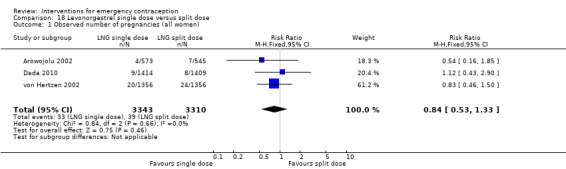
Comparison 18 Levonorgestrel single dose versus split dose, Outcome 1 Observed number of pregnancies (all women).
von Hertzen 2002 reported one case of ectopic pregnancy in the split‐dose group.
2.17.2 Side effects
Overall rates of side‐effects were not reported.
With regard to specific side‐effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, dizziness, fatigue, breast tenderness, diarrhoea, spotting/bleeding after treatment, or lower abdominal pain (Analysis 18.4, moderate‐quality evidence, Table 6). Headache was more common in the single‐dose group (RR 1.14, 95% CI 1.01 to 1.30, 3 RCTs, n = 6804, I2 = 57%).
18.4. Analysis.
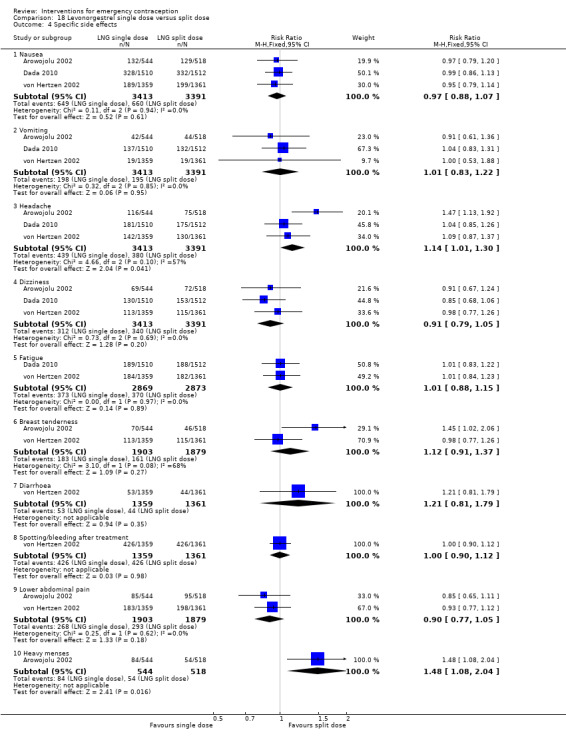
Comparison 18 Levonorgestrel single dose versus split dose, Outcome 4 Specific side effects.
2.17.3. Effects on menses
Those who took single‐dose levonorgestrel were probably more likely to experience heavy menses (RR 1.48, 95% CI 1.08 to 2.04, 1 RCT, n = 1062) (Analysis 18.4) and delayed menses (RR 1.18, 95% CI 0.96 to 1.46, 2 RCTs, n = 3784, I2 = 50%, low‐quality evidence) (Analysis 18.5; Table 6).
18.5. Analysis.
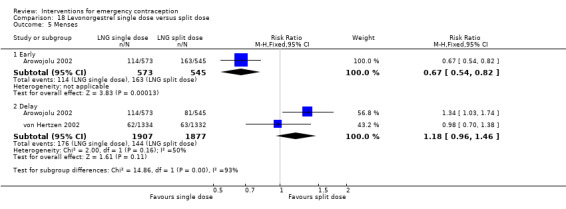
Comparison 18 Levonorgestrel single dose versus split dose, Outcome 5 Menses.
2.18 Low‐dose mifepristone (10 mg) versus low‐dose mifepristone (5 mg)
Two Chinese trials (Lan 2006; Zhang 1998) and two Cuban trials (Carbonell 2015; Miras 2014) compared the effectiveness of mifepristone 10 mg to that of mifepristone 5 mg, among 3110 women.
2.18.1 Observed number of pregnancies
There was no clear evidence of a difference between the groups (RR 0.65, 95% CI 0.33 to 1.31, 4 RCTs, n = 3110, I2 = 0%) (Analysis 19.1).
19.1. Analysis.
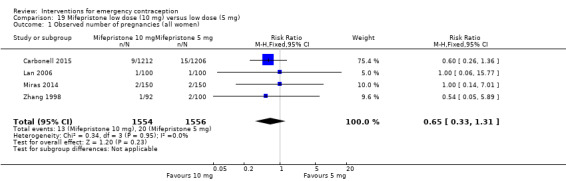
Comparison 19 Mifepristone low dose (10 mg) versus low dose (5 mg), Outcome 1 Observed number of pregnancies (all women).
2.18.2 Side effects
Overall rates of side‐effects were not reported.
With regard to specific side‐effects, there was no clear evidence of a difference between the groups in rates of nausea, headache, dizziness, fatigue, breast tenderness, or lower abdominal pain (Analysis 19.2).
19.2. Analysis.
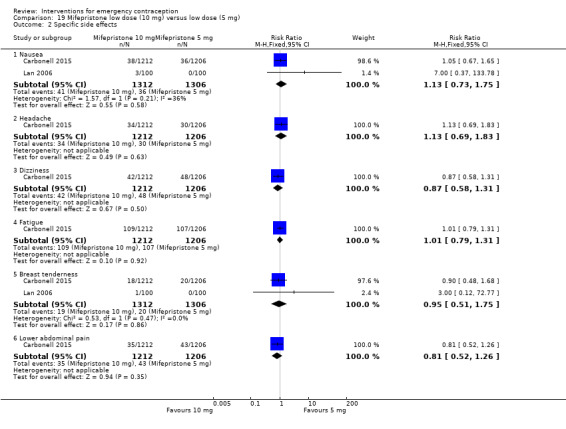
Comparison 19 Mifepristone low dose (10 mg) versus low dose (5 mg), Outcome 2 Specific side effects.
2.18.3 Effects on menses
Those who took 10 mg mifepristone were more likely to experience delayed menses (RR 2.91, 95% CI 2.51 to 3.38, 1 RCT, n = 2418) and less likely to experience early menses (RR 0.62, 95% CI 0.50 to 0.76, 1 RCT, n = 2418) (Analysis 19.3)
19.3. Analysis.
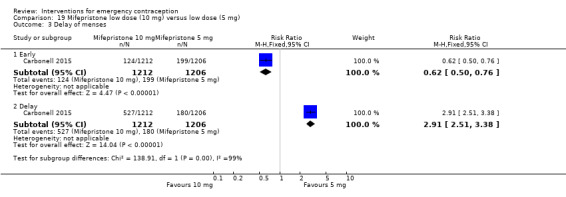
Comparison 19 Mifepristone low dose (10 mg) versus low dose (5 mg), Outcome 3 Delay of menses.
2.19 Low dose mifepristone (10 mg) versus split low dose mifepristone (10 mg x2)
One Chinese trial (Zhang 2005) compared regimens of mifepristone 10mg single dose with mifepristone 10mg two doses, 12 hours apart.
2.19.1 Observed number of pregnancies
There was no clear evidence of a difference between the groups in the risk of pregnancy (RR 0.96, 95% CI 0.06 to 15.22; 1 RCT, n= 220) (Analysis 20.1)
20.1. Analysis.
Comparison 20 Low dose mifepristone (10 mg) versus split low dose mifepristone (10 mg x2), Outcome 1 Observed number of pregnancies (all women).
2.19.2 Side effects
There was no conclusive evidence of a difference between the groups in rates of overall side effects. (RR 1.48, 95% CI 0.82 to 2.68; 1 RCT,n = 220) (Analysis 20.2)
20.2. Analysis.
Comparison 20 Low dose mifepristone (10 mg) versus split low dose mifepristone (10 mg x2), Outcome 2 Any side effect.
Specific side‐effects were not reported.
2.19.3 Effects on menses
There was insufficient evidence to determine whether there was a difference between two regimens in rates of early menses (RR 1.29, 95% CI 0.29 to 5.61; 1 RCT, n = 220) or menstrual delay (RR 0.58, 95% CI 0.22 to 1.54; RCT,n = 220) (Analysis 20.3)
20.3. Analysis.
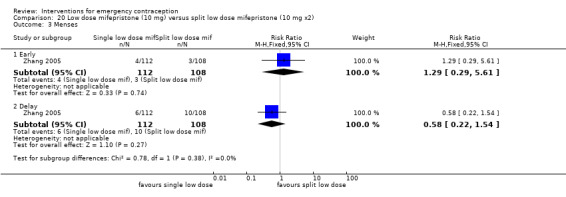
Comparison 20 Low dose mifepristone (10 mg) versus split low dose mifepristone (10 mg x2), Outcome 3 Menses.
2.20 Mid‐dose mifepristone (25 mg to 50 mg) versus low‐dose mifepristone (less than 25 mg)
Twenty‐five trials were included in this comparison. Seventeen trials were two‐arm comparisons of mifepristone 25 mg versus mifepristone 10 mg (Chen 2009; Du 2002; Fan 2001a; Han 2001a; Lai 2004; Qi 2000a; Sang 1999; Wang 2001; Wang 2004; Wang 2006a; Wang 2008; Wei 2002a; Wei 2011; Xiao 2002; Xie 2010; Zeng 2008; Zuo 1999). Seven trials had three arms (Cheng 1999a; Ding 2005; Tan 2003; WHO 1999; Zhang 1998; Zhang 2002b; Zhao 2003) and one trial had four comparisons (Cao 1999). Except for the WHO trial (WHO 1999), all trials were conducted in China.
2.20.1 Observed number of pregnancies
The pooled data showed that the mid‐dose regimen was more effective than the low‐dose regimen (RR 0.73, 95% CI 0.55 to 0.97, 25 RCTs, n = 11,914, I2 = 0%, high‐quality evidence) (Analysis 21.1; Table 7). This suggests that if the chance of pregnancy following low‐dose mifepristone is assumed to 17 per 1000 women, that chance following mid‐dose would be between 9 to 16 per 1000 women.
21.1. Analysis.
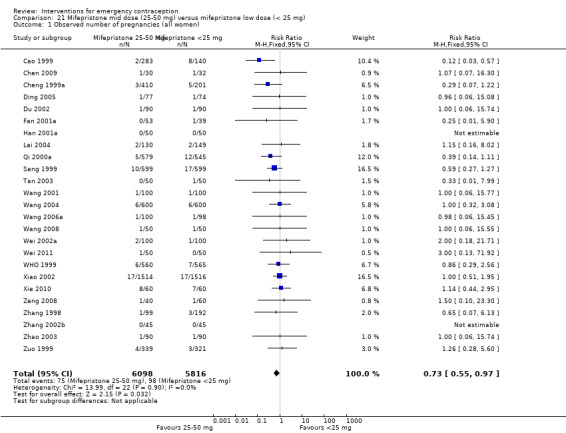
Comparison 21 Mifepristone mid dose (25‐50 mg) versus mifepristone low dose (< 25 mg), Outcome 1 Observed number of pregnancies (all women).
WHO 1999 reported two cases of ectopic pregnancy in the 50 mg mifepristone group and Sang 1999 reported one ectopic pregnancy in the 10 mg mifepristone group.
Funnel plots for the primary outcomes (live birth and ongoing pregnancy) did not suggest reporting bias.
2.20.2 Side effects
Mid‐dose mifepristone was associated with a higher overall rate of side effects than low‐dose mifepristone (RR 1.31, 95% CI 1.01 to 1.70, 11 RCTs, n =2464 , I2 = 9%, moderate‐quality evidence) (Analysis 21.3).
21.3. Analysis.

Comparison 21 Mifepristone mid dose (25‐50 mg) versus mifepristone low dose (< 25 mg), Outcome 3 Any side effect.
With regard to specific side effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, headache, dizziness, fatigue, breast tenderness, diarrhoea, or abdominal pain, but mid‐dose mifepristone was associated with higher risks of spotting/bleeding (RR 1.85, 95% CI 1.55 to 2.20, 11 RCTs, n = 5078 , I2 = 41%, high‐quality evidence) (Analysis 21.4; Table 7).
21.4. Analysis.
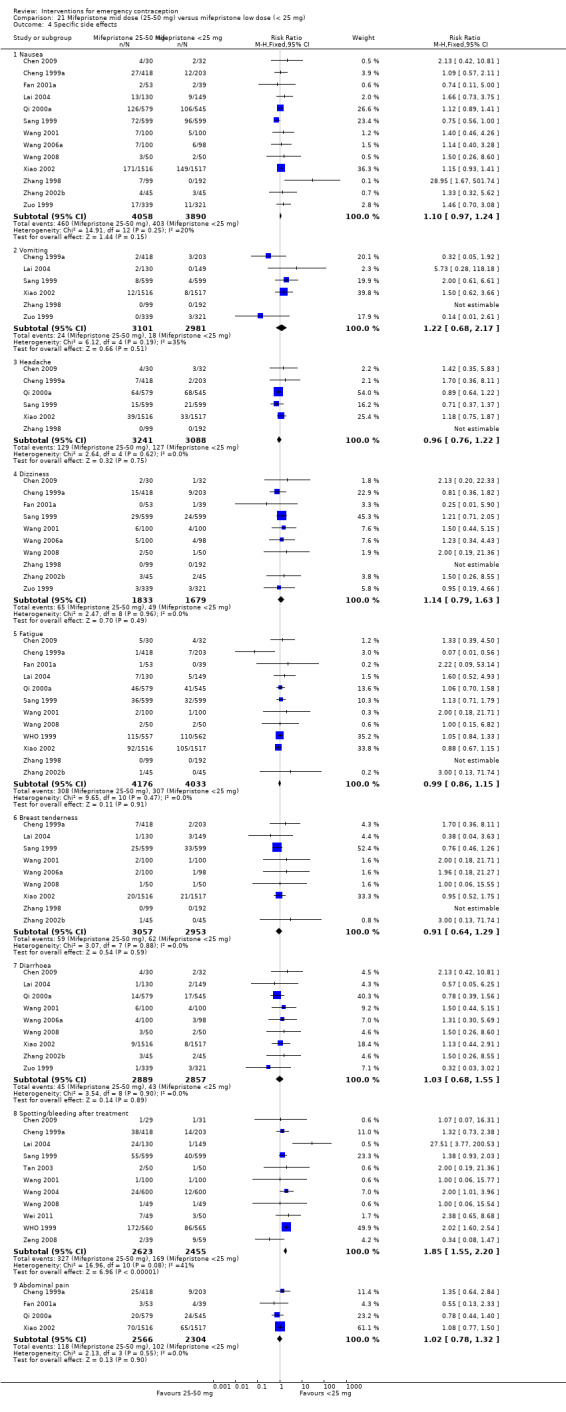
Comparison 21 Mifepristone mid dose (25‐50 mg) versus mifepristone low dose (< 25 mg), Outcome 4 Specific side effects.
2.20.3 Effects on menses
Women in the mid‐dose group may be more likely to experience menstrual delay (RR 1.28; 95% CI 1.11 to 1.47, 21 RCTs, n = 11,282, I2 = 56%, moderate‐quality evidence) than low‐dose mifepristone (Analysis 21.5; Table 7). There was no clear evidence of a difference between the groups in rates of early menses (RR 1.09, 95% CI 0.87 to 1.36, 7 RCTs, n = 2136, I2 = 0%, low‐quality evidence).
21.5. Analysis.
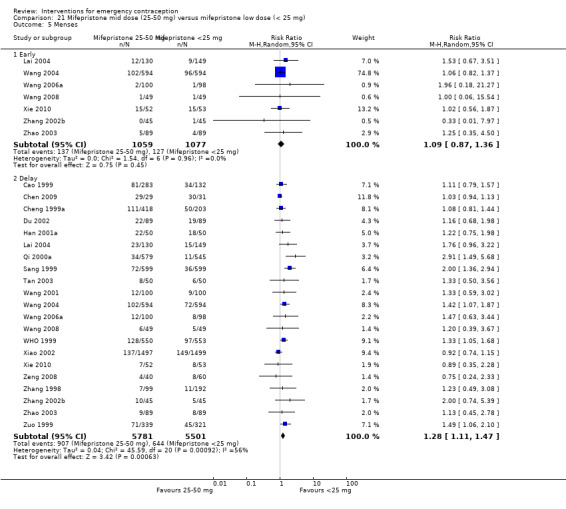
Comparison 21 Mifepristone mid dose (25‐50 mg) versus mifepristone low dose (< 25 mg), Outcome 5 Menses.
2.21 Mid‐dose mifepristone (50 mg) versus mid‐dose mifepristone (25 mg)
Thirteen Chinese trials (Cao 1999; Chen 2002a; Cheng 1999a; Fang 2000; Han 1996; Li 2000b; Li 2000c; Lou 2002; Tan 1999; Xie 1998; Yang 2003; Zhang 2000; Zhao 2003 ) compared mifepristone 50 mg with mifepristone 25 mg.
2.21.1 Observed number of pregnancies
There was no conclusive evidence of a difference in pregnancies between the groups receiving 50 mg mifepristone and 25 mg mifepristone (RR 0.72, 95% CI 0.41 to 1.27, 13 RCTs, n = 3123, I2 = 0%) (Analysis 22.1).
22.1. Analysis.
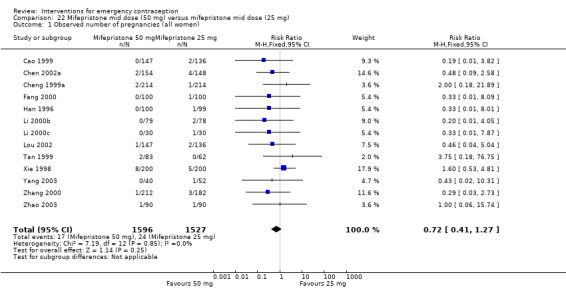
Comparison 22 Mifepristone mid dose (50 mg) versus mifepristone mid dose (25 mg), Outcome 1 Observed number of pregnancies (all women).
Funnel plots for the primary outcomes (observed number of pregnancies) did not suggest reporting bias.
2.21.2 Side effects
The 50 mg dose of mifepristone was associated with a higher overall rate of side effects than the 25 mg dose (RR 1.79, 95% CI 1.39 to 2.31, 6 RCTs, n = 1465, I2 = 5%) (Analysis 22.2).
22.2. Analysis.
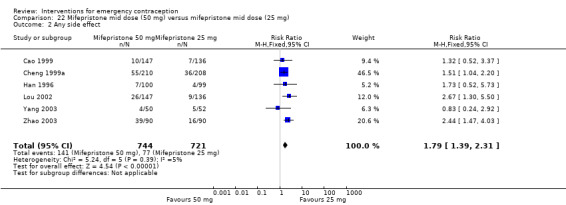
Comparison 22 Mifepristone mid dose (50 mg) versus mifepristone mid dose (25 mg), Outcome 2 Any side effect.
With regard to specific side effects, there was no clear evidence of a difference between the groups in rates of nausea, vomiting, headache, dizziness, fatigue, breast tenderness, abdominal pain, or spotting/bleeding after treatment (Analysis 22.3).
22.3. Analysis.
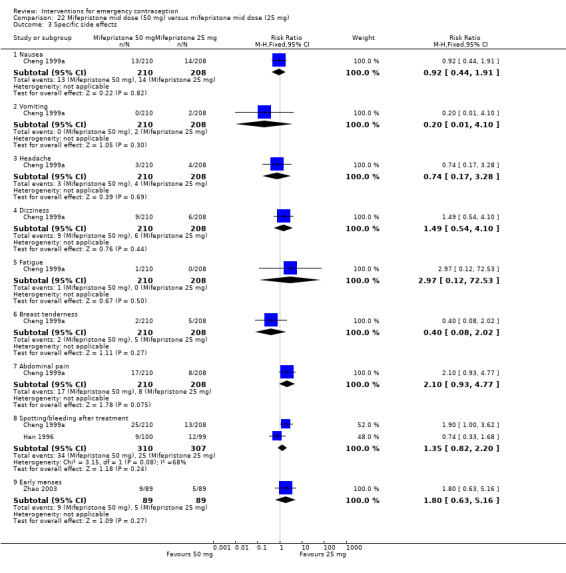
Comparison 22 Mifepristone mid dose (50 mg) versus mifepristone mid dose (25 mg), Outcome 3 Specific side effects.
2.21.3 Effects on menses
There was evidence that the 50 mg regimen may be associated with a higher probability of menstrual delay than the 25 mg regimen (RR 1.32, 95% CI 1.12 to 1.56, 8 RCTs, n = 1945 , I2 = 0%) (Analysis 22.4).
22.4. Analysis.
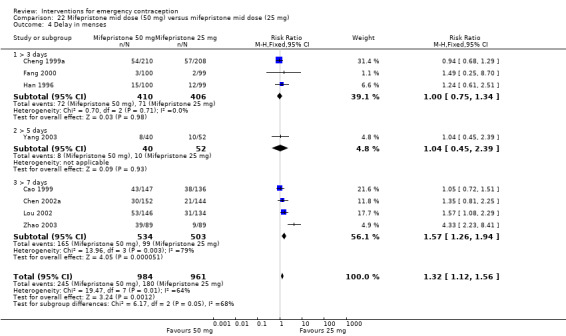
Comparison 22 Mifepristone mid dose (50 mg) versus mifepristone mid dose (25 mg), Outcome 4 Delay in menses.
2.22 Mid dose mifepristone split dose comparisons
One trial (Zhang 1999b) compared three different regimens of mifepristone (1) mifepristone 25 mg orally two doses 12 hours apart; (2) mifepristone 10 mg/day for five days and (3) mifepristone 10mg/day for three days.
2.22.1 Observed number of pregnancies
There was no conclusive evidence of a difference in pregnancies between the groups receiving 50 mg mifepristone (25 mg orally two doses ) versus 30 mg mifepristone (10mg/day for three days) (RR 1.93, 95% CI 0.18 to 21.03; 1 RCT,n= 236); the groups receiving 50 mg mifepristone (25 mg orally two doses ) versus 50 mg mifepristone (10mg/day for five days) (RR 4.92, 95% CI 0.24 to 101.35; 1 RCT, n = 238); or the groups receiving 30 mg mifepristone (10mg/day for three days) versus 50 mg mifepristone (10mg/day for five days) (RR 3.05, 95% CI 0.13 to 74.14; 1 RCT, n = 234) (Analysis 23.1).
23.1. Analysis.
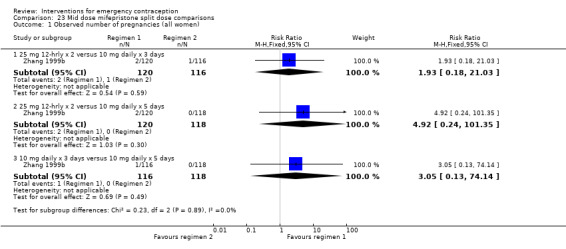
Comparison 23 Mid dose mifepristone split dose comparisons, Outcome 1 Observed number of pregnancies (all women).
2.22.2 Side effects
There were only 14 events in this trial, all of which occurred in the group receiving the 50 mg dose split into two 25 mg doses, suggesting that this regimen was associated with a higher risk of side effects than the 50 mg dose split into five 10 mg doses (RR 28.52, 95% CI 1.72 to 472.69; 1 RCT, n = 238), or the 30 mg dose (10mg/day for three days) (RR 28.04, 95% CI 1.69 to 464.70; 1 RCT, n = 236) (Analysis 23.2).
23.2. Analysis.
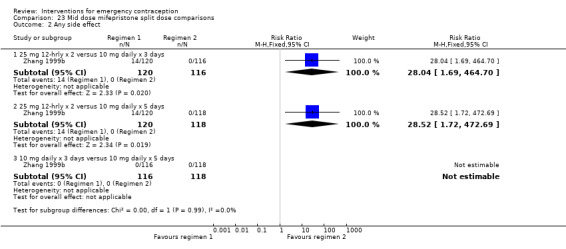
Comparison 23 Mid dose mifepristone split dose comparisons, Outcome 2 Any side effect.
2.22.3 Effects on menses
There was no clear evidence of a difference in rates of early menses between the groups receiving 50 mg mifepristone (25 mg orally two doses ) versus 30 mg mifepristone (10mg/day for three days) (RR 1.05, 95% CI 0.63 to 1.77; 1 RCT,n= 236); the groups receiving 50 mg mifepristone (25 mg orally two doses ) versus 50 mg mifepristone (10mg/day for five days) (RR 1.07, 95% CI 0.64 to 1.80; 1 RCT, n = 238); or the groups receiving 30 mg mifepristone (10mg/day for three days) versus 50 mg mifepristone (10mg/day for five days) (RR 1.02, 95% CI 0.60 to 1.73; 1 RCT, n = 234) (Analysis 23.3).
23.3. Analysis.

Comparison 23 Mid dose mifepristone split dose comparisons, Outcome 3 Early menses.
Delay of subsequent menses was more common in the group receiving 50 mg mifepristone (25 mg orally two doses ) than in those receiving 30 mg mifepristone (10mg/day for three days) (RR 1.86, 95% CI 1.03 to 3.37; 1 RCT, n = 236). There was no clear evidence of a difference in rates of delayed menses between the groups receiving 50 mg mifepristone (25 mg orally two doses ) versus 50 mg mifepristone (10mg/day for five days) (RR 1.15 95% CI 0.70 to 1.89; 1 RCT, n = 238); or the groups receiving 30 mg mifepristone (10mg/day for three days) versus 50 mg mifepristone (10mg/day for five days) (RR 0.62, 95% CI 0.34 to 1.14; 1 RCT, n = 234) (Analysis 23.4).
23.4. Analysis.
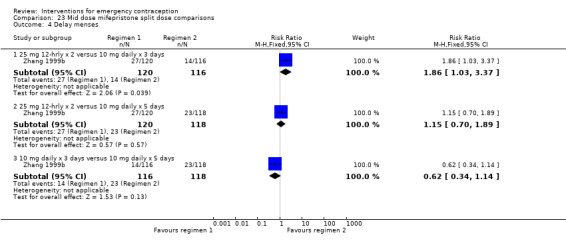
Comparison 23 Mid dose mifepristone split dose comparisons, Outcome 4 Delay menses.
2.23 High‐dose mifepristone (more than 50 mg) versus low‐dose mifepristone (less than 25 mg)
Five trials compared high‐ versus low‐dose mifepristone, one with four treatment arms (Cao 1999; 100 mg, 50 mg, 25 mg, 10 mg) and four with three (WHO 1999: 600 mg, 50 mg, 10 mg; Ding 2005: 75 mg, 50 mg, 10 mg; Tan 2003: 150 mg, 50 mg, 12.5 mg; Zhang 2002b: 100 mg, 50 mg, 10 mg).
2.23.1 Observed number of pregnancies
There was no conclusive evidence of a difference in the risk of pregnancy between high‐dose and low‐dose mifepristone (RR 0.52, 95% CI 0.23 to 1.17; 5 RCTs, n = 1726 , I2 = 20%, Analysis 24.1 ). This suggests that if the risk of pregnancy following low‐dose mifepristone is assumed to be 19 per 1000 women, the risk following high‐dose would be between 4 and 23 per 1000 women.
24.1. Analysis.
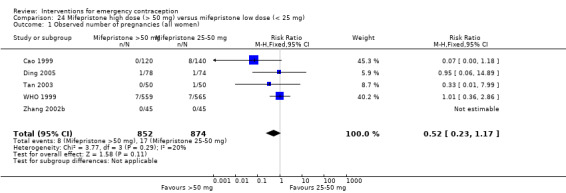
Comparison 24 Mifepristone high dose (> 50 mg) versus mifepristone low dose (< 25 mg), Outcome 1 Observed number of pregnancies (all women).
2.23.2 Side effects
High‐dose mifepristone was associated with a higher overall rate of side effects than low‐dose mifepristone (RR 13.04, 95% CI 5.13 to 33.15, 3 RCTs, n = 512, I2 = 55%) (Analysis 24.3).
24.3. Analysis.
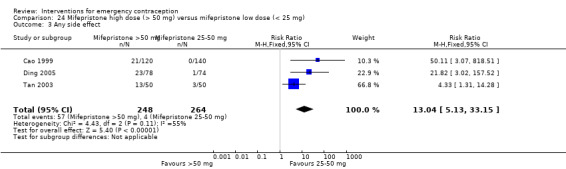
Comparison 24 Mifepristone high dose (> 50 mg) versus mifepristone low dose (< 25 mg), Outcome 3 Any side effect.
With regard to specific side effects, there was no clear evidence of a difference between the groups in rates of nausea, dizziness, fatigue, breast tenderness or diarrhoea. There was evidence that spotting/bleeding is probably more frequent with use of the high‐dose mifepristone (RR 2.36, 95% CI 1.89 to 2.95, 2 RCTs, n = 1224, I2 = 0%) (Analysis 24.4).
24.4. Analysis.
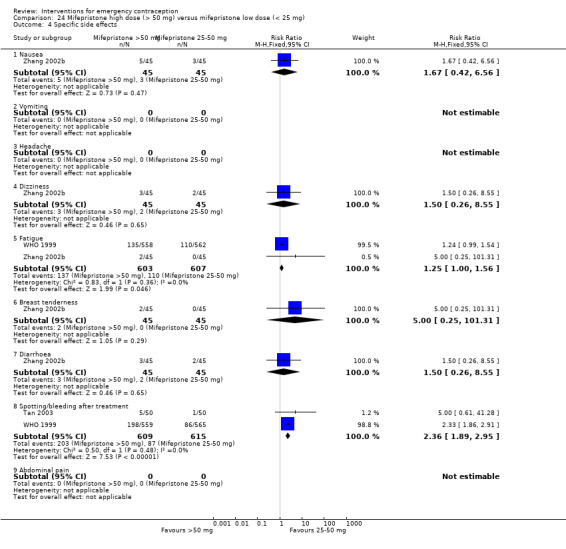
Comparison 24 Mifepristone high dose (> 50 mg) versus mifepristone low dose (< 25 mg), Outcome 4 Specific side effects.
2.23.3 Effects on menses
Delay of subsequent menses (RR 1.98, 95% CI 1.66 to 2.37, 4 RCTs, n = 1574 , I2 = 47%) (Analysis 24.5) appeared to be more frequent in the high‐dose mifepristone group.
24.5. Analysis.
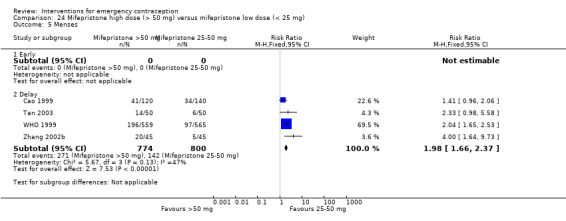
Comparison 24 Mifepristone high dose (> 50 mg) versus mifepristone low dose (< 25 mg), Outcome 5 Menses.
2.24 High‐dose mifepristone (more than 50 mg) versus mid‐dose mifepristone (25 mg to 50 mg)
Eight Chinese trials (Cao 1999; Ding 2005; Li 2000c; Qian 1999; Tan 2003; Xie 1998;Zhang 2002b; Zheng 2005) and one WHO trial (WHO 1999) were included in this comparison. The WHO trial had three study arms (600 mg, 50 mg, 10 mg).
2.24.1 Observed number of pregnancies
There was no conclusive evidence of a difference between the high‐ and mid‐dose groups in the risk of pregnancy (RR 0.93, 95% CI 0.50 to 1.72, 9 RCTs, n = 3009, I2 = 0%) (Analysis 25.1). Funnel plots for this outcome did not suggest reporting bias.
25.1. Analysis.
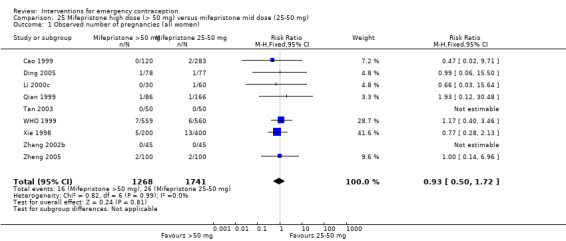
Comparison 25 Mifepristone high dose (> 50 mg) versus mifepristone mid dose (25‐50 mg), Outcome 1 Observed number of pregnancies (all women).
2.24.2 Side effects
High dose mifepristone was associated with a higher overall rate of side‐effects than mid dose mifepristone (RR 2.64 95% CI 1.57 to 4.43, 5 RCTs, n = 1310, I2 = 58%) (Analysis 25.2).
25.2. Analysis.
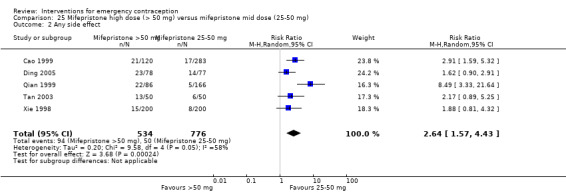
Comparison 25 Mifepristone high dose (> 50 mg) versus mifepristone mid dose (25‐50 mg), Outcome 2 Any side effect.
With regard to specific side effects, there was no clear evidence of a difference between the groups in rates of nausea, dizziness, fatigue, breast tenderness or diarrhoea. Bleeding episodes appear to be more frequent with the high‐dose regimen (RR 1.32, 95% CI 1.12 to 1.56, 4 RCTs, n = 1509, I2 = 78%, Analysis 25.3). There was high heterogeneity, but the direction of effect was consistent.
25.3. Analysis.
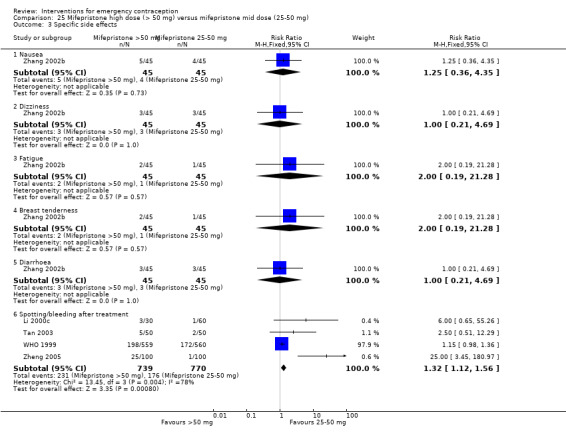
Comparison 25 Mifepristone high dose (> 50 mg) versus mifepristone mid dose (25‐50 mg), Outcome 3 Specific side effects.
2.24.3 Effects on menses
Delays in subsequent menses (RR 1.53, 95% CI 1.34 to 1.75, 8 RCTs, n = 2854, I2 = 0%) were probably more frequent in the high‐dose regimen group. Evidence suggested that the high‐dose regimen was also associated with a higher rate of early menses (RR 10.00, 95% CI 1.30 to 76.66, 2 RCTs, n = 290) (Analysis 25.4).
25.4. Analysis.
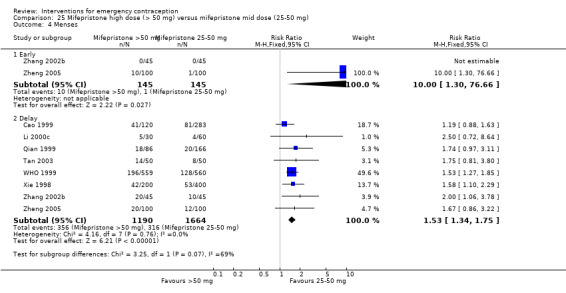
Comparison 25 Mifepristone high dose (> 50 mg) versus mifepristone mid dose (25‐50 mg), Outcome 4 Menses.
2.25 Half‐dose Yuzpe regimen versus standard Yuzpe regimen
Ellertson 2003a compared the standard Yuzpe regimen (of two doses, 12 hours apart) to a half dose given only once, and to a standard regimen replacing norgestrel with norethindrone in a three‐arm trial.
2.25.1. Observed number of pregnancies
It was unclear whether there was a difference between the groups in the risk of pregnancy (RR 1.41, 95% CI 0.76 to 2.61, 1 RCT, n = 1323) (Analysis 26.1).
26.1. Analysis.
Comparison 26 Half‐dose Yuzpe versus standard Yuzpe, Outcome 1 Observed number of pregnancies (all women).
2.25.2 Side effects
The side‐effect profile was improved with the half dose (any side effect: RR 0.85, 95% CI 0.77 to 0.93, 1 RCT, n = 1288; nausea: RR 0.86, 95% CI 0.77 to 0.97, 1 RCT, n = 1288; vomiting: RR 0.50, 95% CI 0.36 to 0.69, 1 RCT, n = 1288), though there was no evidence of a difference between the groups in rates of headache, dizziness or abdominal pain (Analysis 26.3).
26.3. Analysis.
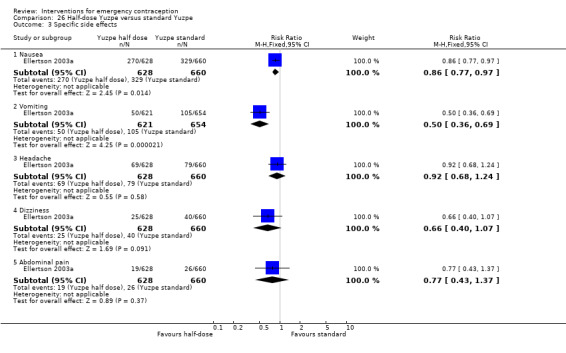
Comparison 26 Half‐dose Yuzpe versus standard Yuzpe, Outcome 3 Specific side effects.
2.25.3 Effects on menses
No studies reported this outcome.
3. IUD versus emergency contraceptive pills
3.1 Cu‐IUD versus mifepristone
Two Chinese trials (Liu 2002b, Tian 2013) compared Cu‐IUD with mifepristone 50 mg.
3.1.1. Observed number of pregnancies
There was insufficient evidence to determine whether there was a difference in the risk of pregnancy between the Cu‐IUD group and the mifepristone group (RR 0.33, 95% CI 0.04 to 2.74; 2 RCTs, n = 395, I2 = 0%, low‐quality evidence) (Analysis 27.1; Table 8).
27.1. Analysis.
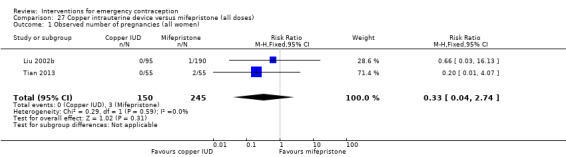
Comparison 27 Copper intrauterine device versus mifepristone (all doses), Outcome 1 Observed number of pregnancies (all women).
3.1.2 Side effects
The evidence suggested that the overall risk of side effects was lower in the Cu‐IUD group (RR 0.06, 95% CI 0.00, 0.99, 1 RCT, n = 285), but the risk of abdominal pain was higher (RR 73.61, 95% CI 4.48 to 1208.50; 1 RCT, n = 285, low‐quality evidence) (Analysis 27.3).
27.3. Analysis.
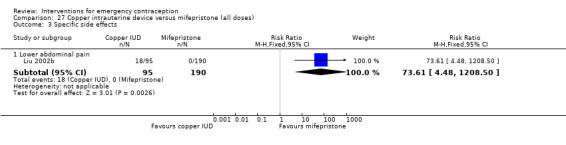
Comparison 27 Copper intrauterine device versus mifepristone (all doses), Outcome 3 Specific side effects.
3.1.3 Effects on menses
Cu‐IUD users may be more likely to experience delay of menses than mifepristone users (RR 0.23, 95% CI 0.09 to 0.64, 1 RCT, n = 284, low‐quality evidence) (Analysis 27.4; Table 8).
27.4. Analysis.
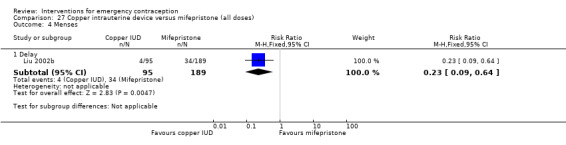
Comparison 27 Copper intrauterine device versus mifepristone (all doses), Outcome 4 Menses.
Subgroup analyses
Time elapsed since intercourse
In comparisons of shorter versus longer time elapsed between intercourse and treatment, times compared varied from a minimum of under 24 hours to a maximum of over 72 hours.
For levonorgestrel there were seven relevant studies. The risk of pregnancy was lower if levonorgestrel was given within 72 hours of intercourse than if it was given later than this (RR 0.51, 95% CI 0.31 to 0.84, 4 RCTs, n = 7453, I2 = 59%). There was moderate heterogeneity, but the direction of effect was consistent.
For mifepristone there were three relevant studies, but there were insufficient data to determine whether there was a difference between the groups.
For the Yuzpe regimen there were three relevant studies. The risk of pregnancy was lower in women receiving treatment within 24 hours than in those who received treatment at 24 hours to 48 hours (RR 0.47, 95% CI 0.26 to 0.88, 3 studies, n = 1527, I2 = 0%) or at 48 hours to 72 hours (RR 0.41, 95% CI 0.18 to 0.89, 2 studies, n = 863, I2 = 0%). Findings were inconclusive in the comparison of treatment between 24 hours and 48 hours after intercourse versus between 48 hours and 72 hours.
For UPA there were two relevant studies, but there were insufficient data to determine whether there was a difference between the groups.
High‐risk women versus low‐risk women
When we combined all studies of hormonal interventions, women defined as 'high risk' (who had further acts of intercourse during the same cycle in which EC was used) were at higher risk of pregnancy than women defined as 'low risk' (those without further acts of coitus during that cycle) (RR 2.67, 95% CI 2.11 to 3.39, 11 studies, n = 19,700, I2 = 66%). There was moderate heterogeneity, but the direction of effect was consistent.
BMI
No randomized data were reported by the included studies.
Discussion
Summary of main results
A total of 115 trials with 60,479 women met our inclusion criteria and were included in this review. Our main findings were as follows.
Evidence from six trials in 4750 women showed that the levonorgestrel regimen is more effective in preventing pregnancy than the Yuzpe regimen. However, since the Yuzpe regimen is a well‐established method for emergency contraception, and in many countries is the only available emergency contraception method, Yuzpe should continue to be provided in these specific settings.
Evidence from 27 studies in 6052 women showed that effectiveness of mid‐dose mifepristone regimens was probably better than that of levonorgestrel regimens to prevent unwanted pregnancy. Moreover, based on data from 14 trials in 8752 women, low‐dose mifepristone (less than 25 mg) regimens were more effective than levonorgestrel regimens.
Evidence from 25 trials in 11,914 participants indicated that the mid‐dose mifepristone regimens are more effective than the low‐dose mifepristone regimens.
Three trials compared a 1.5 mg, single‐dose levonorgestrel regimen with two doses of 0.75 mg levonorgestrel, 12 hours apart. The pooled data from 6653 women suggested that the effectiveness of the two regimens was similar.
One double‐blinded, randomized, multicenter trial compared levonorgestrel split‐dose 24 hours apart with levonorgestrel split‐dose 12 hours apart. Findings from 2060 participants indicated that the effectiveness between the two split‐dose regimens was probably similar.
Two trials compared effectiveness of UPA with that of levonorgestrel regimens. The UPA regimen was associated with fewer pregnancies than the levonorgestrel regimen.
There was insufficient evidence (data from two trials in 395 women) to determine the relative effectiveness of Cu‐IUD compared with mifepristone. The evidence suggests that Cu‐IUD may be more effective, but more data are required for this comparison.
Evidence from one trial in 300 women showed (as would be expected) that there was a higher number of pregnancies in the expectant management group than in the group of IUD users. This type of comparison is no longer considered ethical.
Altogether there were five cases of ectopic pregnancy out of 1153 pregnancies reported overall in the included 115 trials (0.4%). These occurred as follows: WHO 1999 reported two cases after 50 mg mifepristone; Sang 1999 reported one case after 10 mg mifepristone; and Su 2001 and von Hertzen 2002 reported one case each after split‐dose of levonorgestrel. Eleven healthy infants were reported to be delivered following the use of ECPs (Arowojolu 2002; Glasier 2010; Webb 1992): seven mothers used levonorgestrel, two used the Yuzpe regimen, one used danazol and one used mifepristone.
Nausea and vomiting occurred with oestrogen‐containing EC methods. Progestogen and anti‐progestogen methods caused changes in subsequent menses. All methods of EC appeared safe, and no serious adverse events were identified among EC users.
Overall completeness and applicability of evidence
We have added 13 trials to the previous version of the review (Cheng 2012), and the number of included women in these studies has increased to 60,479 from 55,666 in Cheng 2012. All the included studies reported observed pregnancies as their primary outcome. However, studies often failed to report important secondary outcomes such as breast tenderness, headache, dizziness, fatigue, lower abdominal pain, diarrhoea, and spotting or bleeding after treatment.
We only identified three trials evaluating the effects of Cu‐IUD to prevent unwanted pregnancy, and we rated their data quality as low. As pregnancy appears to be rare among women using Cu‐IUD for EC and the side effects of Cu‐IUD are well documented, Cu‐IUD would seem to be particularly appropriate for women presenting too late for ECPs, provided they are not at risk of sexually transmitted diseases and prefer long‐term contraception. Nevertheless, we need more well‐designed studies to enable us to make a solid conclusion with regard to this.
We decided to exclude trials comparing emergency contraception pills with lactational amenorrhoea method (LAM). In practice, women who are breastfeeding are a unique group who are at high risk of unplanned pregnancy. Several trials have been undertaken that compare emergency contraception pills with LAM and that were excluded from our review. This comparison could be a useful topic for a future Cochrane Review.
We aim for the findings of this review to be applicable to women seeking services for EC following a single act of unprotected intercourse, and the included studies include women with wide range of ages, and ethnicities, from countries including Egypt, Italy, UK, Cuba and China. But there is a problem in that the methods and pills for emergency contraception were provided in differing manners by different countries and regions, and not all methods are universally available. In many countries and regions, the traditional Yuzpe regimen is still the only option available for emergency contraception. Problems with the availability and distribution of emergency contraception pills are the main factors limiting the applicability of our findings.
With respect to the influence of BMI on the effectiveness of EC, no data were reported by the primary studies. However, secondary analyses of randomized data from two RCTs included in our review (Creinin 2006; Glasier 2010) suggested a fourfold increased risk of pregnancy after use of levonorgestrel ECP among obese women, compared with normal or underweight women (Glasier 2011). In women weighing about 80 kg, the rate of pregnancy rose above 6%, which is the estimated pregnancy probability without contraception (Kapp 2015). However secondary analysis of data from three other trials in our review (Dada 2010; von Hertzen 2002; WHO 1998) did not confirm that the efficacy of levonorgestrel decreased with increasing BMI (Gemzell‐Danielsson 2015). With respect to ulipristal, a secondary analysis (Moreau 2012), which pooled data from one of the RCTs in our review (Glasier 2010), and a non‐randomised study (Fine 2010a), suggested that pregnancy rates in obese women might be double those in non‐obese women, though confidence intervals were wide and the findings were not statistically significant. The findings of all these secondary analyses are discussed in a recent systematic review (Jatlaoui 2016a).
For this current update, we searched trials registers in order to identify new ongoing trials. We scrutinised reference lists of the new trials, only to find two trials that we had already included and three ongoing trials awaiting classification. Two of the ongoing trials compare ulipristal acetate with levonorgestrel IUS; and one compares Cu‐IUD versus levonorgestrel IUD (see NCT01539720; NCT02175030).
Quality of the evidence
We assessed the primary outcome and secondary outcomes with GRADEpro GDT 2014 criteria. All the included trials were RCTs, which minimises the risk of bias associated with study design. The quality of the evidence for the primary outcome ranged from moderate to high, and for other outcomes ranged from very low to high. The main limitations were risk of bias (associated with poor reporting of randomization methods), imprecision and inconsistency.
For details of the quality of the evidence for each comparison, see Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; and Table 8.
We graded fewer than 15% of the studies (14/115) as being at low risk of bias randomization methods: these studies had detailed description of methods of sequence generation and allocation concealment. Moreover, we have included 92 Chinese trials. However, most of the trials only mentioned 'randomization' and did not report randomization methods in detail, making us uncertain about the possible risk of bias, which caused us to downgrade the quality of the evidence.
Another potentially important source of bias in these studies was loss to follow‐up and missing outcome data. Only 31 studies, with an attrition of about 30%, clearly reported the reasons for attrition. Poor reporting of losses to follow‐up is problematic, even with lower levels of attrition, particularly where loss is not balanced across different arms of trials.
We included a large number of trials (115) with a wide array of comparisons (26). The availability of several recent large multicenter trials was helpful in increasing the power and the generalisability of the study findings. However, RCTs with large sample sizes were relatively few. In addition, most of the included trials were conducted in China, in particular those evaluating the effects of mifepristone regimens. This is probably because few countries have approved mifepristone for use as EC, and China is the largest one. Since it was evident that ECs were effective, most recent studies on EC aimed to reduce dosage or times of medication administration so that clients' compliance or cost of treatment, or both, could be improved. Because of this reason, most recent EC trials were designed as equivalence rather than superiority studies (trying to show that two treatments are as good as each other rather than one is more effective than the other). It is common to claim an equivalent effectiveness when the difference between two treatment groups is not statistically significant. Nevertheless, few trials in this review calculated their sample sizes on the basis of an equivalence approach that usually requires a large study sample.
As noted above, for comparisons in which most of the studies failed to provide adequate details of their randomization methods, we did not downgrade the evidence for risk of bias if the analysis also included a large study at low risk of bias which had findings consistent with the smaller studies.
We did not downgrade the evidence for the primary outcome (pregnancy) for lack of blinding. Since pregnancy is an objective outcome, lack of blindness probably has little influence on evaluation of this outcome. We extracted information from the trials about other potential sources of bias in line with the Cochrane 'Risk of bias' tool.
Potential biases in the review process
There may be potential biases at any stage of the review process. In order to minimise the risk of bias, two review authors independently screened studies for inclusion and any disagreements were resolved by a third review author. One review author performed data extraction and assessed risk of bias and a second review author checked them. Again, any discrepancies were resolved by a third review author. In this updated review, we assessed risk of bias for all the included 115 trials.
The key strengths of this review include exhaustive searches for literature about EC trials (including searches of the Chinese databases) and restriction to RCTs. Limiting the review to RCTs reduced the number of studies available, because, except for some regions where women have no alternatives for emergency contraception pills because of poor availability, most women do have a choice, and this tends to increase the difficulties of randomization. Hence, we found many observational trials, comparing the effectiveness of different ECPs. If this review had had broader inclusion criteria in relation to the type of study design, we would have included additional studies. However, we decided not to change our previous decision to exclude observational trials trials, to minimise the risk of bias in our review. Observational trials are at high risk of selection bias, which could influence the applicability of the result.
Another strength of this review is that we conducted additional analyses in order to assess problems associated with attrition. This consisted of simulated ITT analyses for the comparison of mid‐dose mifepristone (25 mg to 50 mg) with levonorgestrel, assuming on the one hand that all women lost to follow‐up in the levonorgestrel group had had an event, but none in the mifepristone group; or on the other hand assuming that the opposite was the case.
Agreements and disagreements with other studies or reviews
The main conclusions about emergency contraception in our review are similar to those of other published articles and reviews (Jatlaoui 2016b; Lalitkumar 2013; Milosavljevic 2014; Mozzanega 2014; Shohel 2014).
Authors' conclusions
Implications for practice.
Levonorgestrel and mid‐dose mifepristone (25 mg to 50 mg) were more effective than Yuzpe regimen (estradiol‐levonorgestrel combination). Both mid‐dose (25 mg to 50 mg) and low‐dose mifepristone (less than 25 mg) were probably more effective than levonorgestrel (1.5 mg). Low‐dose mifepristone (less than 25 mg) was less effective than mid‐dose mifepristone. Ulipristal acetate (UPA) may be more effective than levonorgestrel.
Levonorgestrel users had fewer side effects than Yuzpe users, and appeared to be more likely to have a menstrual return before the expected date. UPA users were probably more likely to have a menstrual return after the expected date. Menstrual delay was probably the main adverse effect of mifepristone and seemed to be dose‐related. Copper intrauterine device (Cu‐IUD) may be associated with higher risks of abdominal pain than emergency contraceptive pills (ECPs).
Emergency contraception (EC) should be offered to all women requesting this service. Women should start the method as soon as possible to maximise effectiveness, preferably within 72 hours of intercourse.
Implications for research.
In order to demonstrate the relative effectiveness of UPA against levonorgestrel more data are needed. The effectiveness of levonorgestrel, UPA and mifepristone in relation to time since unprotected intercourse is not confirmed and more studies are needed. Moreover, the effectiveness of Cu‐IUD for EC should be further evaluated. There is also a need to compare the effectiveness and safety of UPA and mifepristone (in countries where it is applicable) in order to provide evidence for clients and service providers. Studies are needed that investigate whether BMI influences the effectiveness of EC, and a review of the evidence on emergency contraception versus lactational amenorrhoea method would also be useful. Trial protocols should clearly state when equivalence is sought and trials should be powered accordingly. Most of the trials included in this review did not have sufficiently detailed reporting to enable satisfactory assessment of risk of bias. Future trials should report their methods in sufficient detail to allow this assessment.
Feedback
Comment by Dr. Paul Beirne
Summary
Comment: These comments relate to the Full(1888K) pdf version of the Review. I have been using this review for teaching purposes (with our undergraduate public health students) and I have noticed problems with the labelling of some Figures in the review and with the text accompanying these Figures. Specifically, on page 12, Section 5 is entitled "Mid‐dose mifepristone (25‐50mg) versus LNG". However, in the accompanying Figure 4, the Forest Plot is labelled as follows: "Forest Plot of comparison: 5 Levonorgestrel 1.5mg versus mifepristone mid‐dose (25‐50mg)." This label appears to incorrectly imply that the data presented in the Forest Plot for the "Treatment group" in each trial relates to Levonorgestrel (rather than to mid‐dose mifepristone). This mis‐labelling could potentially result in the reader misinterpreting the pooled estimate on the Forest Plot. Could the review authors please examine Figure 4 and consider changing the label to: "Forest plot of comparison: 5 Mifepristone mid‐dose (25‐50mg) versus Levonorgestrel 1.5mg". On page 12, Section 6 is entitled "low‐dose mifepristone (<25mg) versus LNG". However, in the accompanying Figure 5, the Forest Plot is labelled as follows: "Forest Plot of comparison: 6 Levonorgestrel 1.5mg versus mifepristone low dose (<25mg)." Could the review authors examine Figure 5 and consider changing the label to: "Forest plot of comparison: 6 Mifepristone low dose (<25mg) versus Levonorgestrel 1.5mg". In the text accompanying section 6 "Low‐dose mifepristone (<25mg) versus LNG" the following sentences appear: "Nine Chinese....one UK....and one multinational WHO trial....compared LNG (4856 women) with low‐dose mifepristone (3480 women). There was a statistically significant difference in effectiveness between LNG and low‐dose mifepristone regimens when all studies were included (RR 0.70; 95% CI 0.50 to 0.97)". It is my understanding that, when presenting comparisons in the text of the review, the 'Treatment' group should be compared with the 'Control' group (i.e. low‐dose mifepristone should be compared with LNG and not vice‐versa). The statements that currently appear in the text accompanying section 6 incorrectly imply that levonorgestrel is more effective than low‐dose mifepristone, whereas the opposite is in fact the case. Could the review authors examine the text in Section 6 and consider altering the text as follows: "Nine Chinese....one UK....and one multinational WHO trial....compared low‐dose mifepristone (3480 women) with LNG (4856 women). There was a statistically significant difference in effectiveness between low‐dose mifepristone and LNG regimens when all studies were included (RR 0.70; 95% CI 0.50 to 0.97)". I have not examined all figures and the accompanying text throughout the entire review but perhaps the review authors could consider double‐checking all Figures and any accompanying text to ensure that no similar issues arise elsewhere in the review. Best wishes Paul I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Dear Anja and Frans,
Thanks very much for your email and the comments from Paul. Paul's comments is complete right. There are mistakes in the Caption of Forest plot of comparison in Figure 4 ‐ 9.
According the suggestion of peer reviewer, we had to use LNG as the control for all another EC‐drug study. So that we rewrote Section 5‐7 in the text and re‐enter the data for statistical analysis, but we forgot change the Caption of Forest plot of comparison in Figue 5 ‐ 9. I am terrible sorry for those mistakes. We are happy to double‐check all the Figures and any accompanying test again to ensure that no similar mistakes arise elsewhere in our review. Would we do it by Archie system? Please send our sincere thanks to Paul.
With all the best.
Linan
Contributors
LC contributed to all sections of the revision.
Comment by Dr. Stefanie Schenk, 25 April 2018
Summary
With interest but also with concern I read the updated Cochrane review “Interventions for emergency contraception”, published in Issue 8, 2017. Without presenting any new data you changed your conclusion about the effectiveness of ulipristal acetate (UPA) from “UPA may be more effective than LNG” (= levonorgestrel) in the former version (published 2012) to “UPA was more effective than levonorgestrel” in the actual review. I suppose that this is based on a mistake in the results section of the new version:
a. On page 23 in the full pdf of the updated review under item 2.15.1 you state that “UPA was associated with fewer pregnancies than levonorgestrel ‘within 72 hours after unprotected intercourse’ (RR 0.59, 95% CI 0.35 to 0.99, 2 RCTs, n = 3448…)” refering to Analysis 16.1. In the abstract you present this result even without mentioning the time elapsed since intercourse. In Analysis 16.1, however, all the five‐day data (i.e. within 120 hours) from the Glasier 2010 trial were combined with the three‐day data from the Creinin 2006 trial.
b. The correct analysis for the comparison UPA versus LNG within 72 hours after unprotected intercourse is Analysis 16.4 which reveals a statistically non‐significant difference (RR 0.63, 95% CI 0.37 to 1.07). This was the first comparison described in the results section of the former review (published 2012) and the only one mentioned in the former abstract and there are good reasons prefering it: The risk of pregnancy is significantly lower if LNG is administered within 72 hours of unprotected intercourse than if it is given later than this (RR 0.51, 95% CI 0.31 to 0.84, Analysis 28.4). In Europe as well as in the USA LNG is approved for use within 72 hours of unprotected intercourse only. In the discussion of the former review you also stated that “since the Creinin 2006 trial did not recruit participants who had unprotected intercourse after 72 hours, the rationale of combining all five‐day data from the Glasier 2010 trial in the analysis is debatable” (page 19 in the full pdf of the version from 2012). The current review lacks such critical considerations – the thought that “we may need more trials concucted by independent investigators to make a firm conclusion” (page 19, version from 2012) has disappeared as well.
2. The appraisal of ulipristal acetate in the actual review assessing interventions for emergency contraception unfortunately provides only a flawed subset of information and a misleading presentation and interpretation of results. It’s my great hope that you will change these parts of your review as soon as possible.
I don’t have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment.
Reply
We thank Dr. Schenk for her diligence in bringing these concerns to our attention. Our responses are outlined in the table below:
| Dr. Schenk comments | Author / FRG responses |
| With interest but also with concern I read the updated Cochrane review “Interventions for emergency contraception”, published in Issue 8, 2017. Without presenting any new data you changed your conclusion about the effectiveness of ulipristal acetate (UPA) from “UPA may be more effective than LNG” (= levonorgestrel) in the former version (published 2012) to “UPA was more effective than levonorgestrel” in the actual review. I suppose that this is based on a mistake in the results section of the new version: | We edited the conclusion to say “UPA may be more effective than LNG” in the Authors’ conclusions in the abstract and main text |
| On page 23 in the full pdf of the updated review under item 2.15.1 you state that “UPA was associated with fewer pregnancies than levonorgestrel ‘within 72 hours after unprotected intercourse’ (RR 0.59, 95% CI 0.35 to 0.99, 2 RCTs, n = 3448…)” referring to Analysis 16.1. In the abstract you present this result even without mentioning the time elapsed since intercourse. In Analysis 16.1, however, all the five‐day data (i.e. within 120 hours) from the Glasier 2010 trial were combined with the three‐day data from the Creinin 2006 trial. | We edited section 2.15.1 to say ‘within 120 hours after unprotected intercourse’ |
| The correct analysis for the comparison UPA versus LNG within 72 hours after unprotected intercourse is Analysis 16.4 which reveals a statistically non‐significant difference (RR 0.63, 95% CI 0.37 to 1.07). This was the first comparison described in the results section of the former review (published 2012) and the only one mentioned in the former abstract and there are good reasons preferring it: The risk of pregnancy is significantly lower if LNG is administered within 72 hours of unprotected intercourse than if it is given later than this (RR 0.51, 95% CI 0.31 to 0.84, Analysis 28.4). In Europe as well as in the USA LNG is approved for use within 72 hours of unprotected intercourse only. In the discussion of the former review you also stated that “since the Creinin 2006 trial did not recruit participants who had unprotected intercourse after 72 hours, the rationale of combining all five‐day data from the Glasier 2010 trial in the analysis is debatable” (page 19 in the full pdf of the version from 2012). The current review lacks such critical considerations – the thought that “we may need more trials conducted by independent investigators to make a firm conclusion” (page 19, version from 2012) has disappeared as well. | No changes were made, the abstract will remain as: Ulipristal acetate (UPA) was associated with fewer pregnancies than levonorgestrel (RR 0.59; 95% CI 0.35 to 0.99, 2 RCTs, n = 3448, I2 = 0%, high‐quality evidence). |
Follow up comment from Dr. Schenk, 31 October 2018
In my first comment, I did not only refer to a misquoted analysis, that is to a discrepancy between the stated time elapsed since intercourse in 2.15.1 ("within 72 hours after unprotected intercourse") and the denoted risk reduction corresponding to a period of 120 hours. But I also argued for the time window of 72 hours as adequate comparison of ulipristal acetate (UPA) versus levonorgestrel (LNG): "This was the first comparison described in the results section of the former review (published 2012) and the only one mentioned in the former abstract and there are good reasons preferring it: The risk of pregnancy is significantly lower if LNG is administered within 72 hours of unprotected intercourse than if it is given later than this (RR 0.51, 95% CI 0.31 to 0.84, Analysis 28.4). In Europe as well as in the USA LNG is approved for use within 72 hours of unprotected intercourse only. In the discussion of the former review you also stated that “since the Creinin 2006 trial did not recruit participants who had unprotected intercourse after 72 hours, the rationale of combining all five‐day data from the Glasier 2010 trial in the analysis is debatable” (page 19 in the full pdf of the version from 2012).” For this reason I do not agree with the way my comment was handled: ‐ The time window in 2.15.1 was simply converted to 120 hours. Furthermore, in the abstract the result is still presented without mentioning the time elapsed since intercourse. ‐ There is no discussion why the former approach to the comparison UPA versus LNG was dropped and not even a mention that it was changed. In my opinion a discussion about these points is essential in a frank, open and transparent debate about health evidence.
Response: We thank Dr. Schenk for her comments, we have re‐reviewed the most recent publication and have decided we will not make additional changes to the review.
Contributors
Dr. Yan Che, Dr. Jeanne‐Marie Guise, Dr. Maria Rodriguez
What's new
| Date | Event | Description |
|---|---|---|
| 19 December 2018 | Amended | A typo in the 'What's new' section was corrected. |
| 19 December 2018 | Feedback has been incorporated | Feedback received prompted the authors and review group to make edits to the Authors' conclusions, and section 2.15.1. |
| 19 December 2018 | New citation required but conclusions have not changed | The amendments made did not change the conclusions. |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 19 December 2018 | Amended | We are reverting to the original content as changes were made without a new citation version. |
| 25 July 2017 | New citation required and conclusions have changed | The addition of 15 new trials and application of Cochrane methods led to a change in the conclusions of this review |
| 7 March 2017 | New search has been performed | The number of included studies in this updated review increased to 115. The search was updated to February 2017. In this update, we assessed all the included studies for risk of bias using the Cochrane 'Risk of bias' tool, and completed 'Risk of bias' tables. We graded major outcomes and comparisons of this review following GRADEpro GDT 2014 guidelines. These two processes changed our conclusions. |
| 15 February 2012 | New citation required and conclusions have changed | The number of included studies in this updated review increased from 81 to 100. Compared with levonorgestrel, the risk ratio of pregnancy with mid‐dose (25 mg‐50 mg) mifepristone was slightly increased from 0.50 (95% CI 0.32 to 0.79) to 0.64 (95% CI 0.45 to 0.92) in this update. Ulipristal acetate appeared more effective than levonorgestrel, but more data are needed to confirm this association. Ulipristal acetate users were more likely to experience a menstrual return after the expected date than levonorgestrel users did. However, levonorgestrel was associated with higher risk of early menstrual return than ulipristal acetate. Gestrinone was included in this review for the first time. It appeared to have similar effectiveness and overall side effects as mifepristone. The latter was associated with higher risk of menstrual delay than gestrinone. |
| 18 February 2008 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are grateful to Drs A Glasier, J Guillebaud, PC Ho, S Rowlands, A Webb, Xiao Bilian, A Templeton and H von Hertzen who responded to our requests for information about their (ongoing) trials. We are particularly indebted to Mr A Peregoudov for providing additional data from the WHO 1998 trial. We thank Dr R Guidotti for his assistance with translation, Dr C van Oel for her work on the initial review and Dr H von Hertzen for her comments on earlier drafts. In the 2007 update of the review David Grimes, Laureen Lopez and Carol Manion made substantive contributions to the review by updating the literature searches, extraction of duplicates and re‐appraisal of allocation concealment scores for all trials. We own great gratitude to Drs Gilda GP Piaggio, Enrique E Ezcurra, Paul PFA van Look and A Metin Gülmezoglu, who were co‐authors of previous versions of this review and made a valuable contribution to the success of this review. In the 2008 review update, Ms Carol Manion and Mr Dongbing Chen did the literature searches, and Dr Qiuju Chen duplicated the data extraction. In the 2012 review update, Paul V Beirne commented and revised the draft.
In this 2017 update, Carol Manion and Frans M Helmerhorst helped with searching Embase. This 2017 update received funding for Knowledge to Action (or Implementation) Seed Grants from Canadian Institutes of Health Research. Funder award number: CIHR NC 122929, which is under the Knowledge Translation Program awarded by Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, Canada.
Appendices
Appendix 1. CENTRAL Register of Studies Online (CRSO) search strategy
From inception to 22 February 2017
Web platform
#1 MESH DESCRIPTOR Contraception, Postcoital EXPLODE ALL TREES 41
#2 (emergency adj3 contracept*):TI,AB,KY 156
#3 (emergency adj3 post?coit*):TI,AB,KY 4
#4 (postcoit* adj3 contracept*):TI,AB,KY 51
#5 (post‐coit* adj3 contracept*):TI,AB,KY 6
#6 (post?coit* adj3 intercept*):TI,AB,KY 2
#7 (morning after pill*):TI,AB,KY 2
#8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 180
Appendix 2. MEDLINE search strategy
From 1946 to 22 February 2017
OVID platform
1 exp Contraception, Postcoital/ (885) 2 post?coital fertility control*.tw. (5) 3 (emergency adj3 contracept*).tw. (2339) 4 (emergency adj3 post?coit*).tw. (72) 5 (postcoit* adj3 contracept*).tw. (484) 6 (post coit* adj3 contracept*).tw. (152) 7 (post?coit* adj3 interrupt*).tw. (1) 8 (post?coit* adj3 intercept*).tw. (22) 9 morning after pill*.tw. (210) 10 day after pill*.tw. (9) 11 morning after contracept*.tw. (17) 12 or/1‐11 (3096) 13 randomized controlled trial.pt. (448501) 14 controlled clinical trial.pt. (91935) 15 randomized.ab. (388859) 16 randomised.ab. (76875) 17 placebo.tw. (188737) 18 clinical trials as topic.sh. (181403) 19 randomly.ab. (271532) 20 trial.ti. (174346) 21 (crossover or cross‐over or cross over).tw. (72893) 22 or/13‐21 (1153262) 23 exp animals/ not humans.sh. (4323444) 24 22 not 23 (1064067) 25 12 and 24 (283)
Appendix 3. Embase search strategy
From 1980 to 22 February 2017
OVID platform
1 exp emergency contraception/ or exp postcoitus contraceptive agent/ (46775) 2 post?coit* fertility control*.tw. (1) 3 (emergency adj contracept*).tw. (2995) 4 (emergency adj2 post?coit*).tw. (60) 5 postcoit* contracept*.tw. (373) 6 post coit* contracept*.tw. (172) 7 post?coit* intercept*.tw. (11) 8 morning after pill*.tw. (259) 9 day after pill*.tw. (14) 10 morning after contracept*.tw. (12) 11 or/1‐10 (47170) 12 Clinical Trial/ (1036381) 13 Randomized Controlled Trial/ (479359) 14 exp randomization/ (85088) 15 Single Blind Procedure/ (29618) 16 Double Blind Procedure/ (140111) 17 Crossover Procedure/ (55225) 18 Placebo/ (328250) 19 Randomi?ed controlled trial$.tw. (155221) 20 Rct.tw. (23370) 21 random allocation.tw. (1657) 22 randomly.tw. (346282) 23 randomly allocated.tw. (27234) 24 allocated randomly.tw. (2229) 25 (allocated adj2 random).tw. (849) 26 Single blind$.tw. (19137) 27 Double blind$.tw. (175914) 28 ((treble or triple) adj blind$).tw. (688) 29 placebo$.tw. (252374) 30 prospective study/ (400066) 31 or/12‐30 (2034601) 32 case study/ (95853) 33 case report.tw. (330045) 34 abstract report/ or letter/ (999642) 35 or/32‐34 (1416155) 36 31 not 35 (1981905) 37 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5788120) 38 36 not 37 (1856590) 39 11 and 38 (8446)
Appendix 4. PsycINFO search strategy
From 1806 to 22 February 2017
OVID platform
1 exp Oral Contraceptives/ or exp Birth Control/ or exp Contraceptive Devices/ (7869) 2 emergency.tw. (21187) 3 post?coit*.tw. (62) 4 2 or 3 (21243) 5 1 and 4 (239) 6 (emergency adj3 contracept*).tw. (269) 7 (emergency adj3 post?coit*).tw. (0) 8 (postcoit* adj3 contracept*).tw. (7) 9 (post coit* adj3 contracept*).tw. (4) 10 morning after pill*.tw. (16) 11 day after pill*.tw. (1) 12 morning after contracept*.tw. (1) 13 or/5‐12 (312) 14 random.tw. (49000) 15 control.tw. (379558) 16 double‐blind.tw. (20440) 17 clinical trials/ (10180) 18 placebo/ (4801) 19 exp Treatment/ (675062) 20 or/14‐19 (1043437) 21 13 and 20 (125)
Appendix 5. CINAHL search strategy
From inception to 22 February 2017
EBSCO platform
| # | Query | Results |
| S25 | S12 AND S24 | 510 |
| S24 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 | 1,112,233 |
| S23 | TX allocat* random* | 6,013 |
| S22 | (MH "Quantitative Studies") | 15,390 |
| S21 | (MH "Placebos") | 10,010 |
| S20 | TX placebo* | 43,326 |
| S19 | TX random* allocat* | 6,013 |
| S18 | (MH "Random Assignment") | 42,512 |
| S17 | TX randomi* control* trial* | 118,648 |
| S16 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 876,075 |
| S15 | TX clinic* n1 trial* | 198,873 |
| S14 | PT Clinical trial | 79,974 |
| S13 | (MH "Clinical Trials+") | 209,925 |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 | 2,206 |
| S11 | TX morning after contracept* | 19 |
| S10 | TX day after pill* | 10 |
| S9 | TX morning after pill* | 89 |
| S8 | TX post‐coit* intercept* | 2 |
| S7 | TX postcoit* intercept* | 3 |
| S6 | TX post‐coit* contracept* | 26 |
| S5 | TX postcoit* contracept* | 1,711 |
| S4 | TX emergency post‐coit* | 7 |
| S3 | TX emergency postcoit* | 27 |
| S2 | TX emergency contracept* | 1,403 |
| S1 | (MM "Contraceptives, Postcoital+") | 1,303 |
Appendix 6. Chinese database search strategy
From inception to February 2017
(左炔诺孕酮 or 米非司酮 or RU486 or UPA or ulipristal acetate or 乌利司他 or 醋酸优力司特 or 醋酸乌利司他 or Yuzpe or 紧急避孕药 or 毓婷 or 宫内节育器 or IUD or 环) and 紧急避孕 and (临床试验 or 随机对照 or 比较 or 对比)
Appendix 7. Clinicaltrials search strategy
International Clinical Trials Registry Platform (ICTRP), Word Health Organization (WHO) http://www.who.int/ictrp/en/ (searched 27 February 2017)
("postcoital contraceptives" OR "postcoital contraception" OR "postcoital contracept*" OR "emergency contraceptives" OR "emergency contraception" OR "morning after pill" OR "day after pill" OR "Yuzpe") AND ("advance*" OR "home" OR "over the counter" OR "OTC" OR "behind the counter" OR "health services accessibility" OR "community pharmacy services" OR "access")
ClinicalTrials.gov, US National Institutes of Health (NIH) http://clinicaltrials.gov/ (searched 27 February 2017)
("postcoital contraceptives" OR "postcoital contraception" OR "postcoital contracept*" OR "emergency contraceptives" OR "emergency contraception" OR "morning after pill" OR "day after pill" OR "Yuzpe") AND ("advance*" OR "home" OR "over the counter" OR "OTC" OR "behind the counter" OR "health services accessibility" OR "community pharmacy services" OR "access")
Appendix 8. Search strategies used from inception until 31st May 2016
1. Central/Cochrane Controlled Trials Register (the Cochrane Library, Issue 12, 2014)
2. PubMed: to 31 May 2016
(contraceptives, postcoital OR contraception, postcoital OR postcoital contracept* OR "emergency contraceptives" OR "emergency contraception" OR "morning after pill" OR "day after pill" OR Yuzpe) AND (advance* OR home OR over the counter OR OTC OR behind the counter OR health services accessibility OR community pharmacy services OR access) limited to human and English
3. "Biosis/Embase: to 31 May 2016
(“postcoitus contraceptive agent” OR “postcoital contraceptive agent”OR “postcoital contraceptive” OR “postcoital contraceptives” OR “postcoital contraception” OR “emergency contraceptive” OR “emergency contraceptives” OR “emergency contraception” OR “morning after pill” OR Ru‐486 OR Yuzpe OR postcoital NEAR/1 insert* OR “unprotected intercourse” OR mifepristone OR danazol OR anordrin) NOT (“prenatal diagnosis” OR “chromosome aberration” OR menopause OR infertility OR neoplasm OR “spontaneous abortion” OR “rheumatoid arthritis”) AND (“clinical study” OR “clinical trial” OR “double blind procedure”OR “crossover procedure”OR placebo) AND humans AND (2011:py OR 2012:py OR 2013:py OR 2014:py)
4. Popline: to 31 May 2016
(emergency contracept* / postcoital contracept* / morning after pill* / morning after contracept* / morning‐after pill* / morning‐after contracept* / day after pill* / day after contracept* / day‐after pill* / day‐after contracept* / Yuzpe) & (advance* prov* / self administ* / self‐administ* / home / over the counter / over‐the‐counter /otc/ behind the counter / advance prescript*/advance prescib* / pharmac* prov*/ access*) limited to English
5. CINAHL: to 31 May 2016
(contraceptives or emergency contraceptive or morning after pill or Yuzpe or postcoital insertion or unprotected intercourse or mifepristone or danazol or anordrin or Ru‐486 or Ru486 or Ru 486) AND (clinical and (article or study or trial or studies or trials) or controlled study or randomised controlled trial or randomised controlled trial or clinical study or single blind or phase 3 clinical study or phase 4 clinical study or crossover or placebo or placebos or allocated or allocation or allocate or assign or assigned or blinded or comparative or comparison or factorial or follow up or prospective or random or randomised or randomised or masked or masking or versus or vs) NOT (prenatal diagnosis or chromosome aberration or menopause or infertility or neoplasm or spontaneous abortion or rheumatoid arthritis)
6. LILACS: to 31 May 2016
contraception, postcoital or anticonception postcoital or anticoncepcao pos‐coito or contraceptives, postcoital or anticonceptivos poscoito or anticoncepcionais pos‐coito or contraceptives, postcoital, hormonal or postcoital contraceptives or postcoital contraception or postcoital contraceptive or emergency contraception or emergency contraceptives or emergency contraceptive or morning after pill or Yuzpe or postcoital insertion or unprotected intercourse or mifepristone or danazol or anordrin or Ru‐486 or Ru486 or Ru 486
7. Database of Chinese Scientific Journal (to 31 May 2016)
(左炔诺孕酮 or 米非司酮 or RU486 or UPA or ulipristal acetate or 乌利司他 or 醋酸优力司特 or 醋酸乌利司他 or Yuzpe or 紧急避孕药 or 毓婷 or 宫内节育器 or IUD or 环) and 紧急避孕 and (临床试验 or 随机对照 or 比较 or 对比)
8. Trials Registries
International Clinical Trials Registry Platform (ICTRP), Word Health Organization (WHO) http://www.who.int/ictrp/en/ (searched 8 Aug 2016)
("postcoital contraceptives" OR "postcoital contraception" OR "postcoital contracept*" OR "emergency contraceptives" OR "emergency contraception" OR "morning after pill" OR "day after pill" OR "Yuzpe") AND ("advance*" OR "home" OR "over the counter" OR "OTC" OR "behind the counter" OR "health services accessibility" OR "community pharmacy services" OR "access")
ClinicalTrials.gov, US National Institutes of Health (NIH) http://clinicaltrials.gov/ (searched 8 Aug 2016)
("postcoital contraceptives" OR "postcoital contraception" OR "postcoital contracept*" OR "emergency contraceptives" OR "emergency contraception" OR "morning after pill" OR "day after pill" OR "Yuzpe") AND ("advance*" OR "home" OR "over the counter" OR "OTC" OR "behind the counter" OR "health services accessibility" OR "community pharmacy services" OR "access")
Appendix 9. response to Dr Beirne's comments
Comments on: update of Review Number 0003 – Interventions for emergency contraception
1) Many of the Summary of Findings (SoF) Tables contain more than the recommended number of outcomes (some SoF tables contain 20 outcomes). The Cochrane Handbook (Section 11.5.6.2) recommends that SoF Tables should contain a maximum of seven outcomes[1]. The review authors will need to decide what outcomes are essential for decision‐making and will need to omit the less important outcomes from the SoF tables. Where feasible and appropriate, the review authors may wish to consider including a composite outcome for side effects (i.e. “any side effect”) in the SoF tables rather than reporting on individual side effects.
Response:
We kept the essential outcomes and omitted the less important outcomes according to the main results. Now every SoF tables contains no more than 7 outcomes.
As for the side effects, we can’t make a composite outcome for side effects (i.e. “any side effect”) for all the comparisons because we wanted to keep the main observation of the original articles. Some articles reported on detailed individual side effects. But, we omitted the less important side effects and only kept the main side effects in the SoF tables.
2) The updated review currently contains 31 Summary of Findings (SoF) Tables and there is a serious risk of overwhelming the reader with information. I would suggest that the review authors consider only including SoF tables for the main comparisons made in the review (e.g. for the comparisons that are specified in the Abstract of the review).
Response:
We changed the 31 comparisons to 18 comparisons, remaining the main results.
3) The review authors have not used a consistent format for the Summary of Findings (SoF) Tables. For example, in SoF Tables 1, 8 and 9 (and in several other tables) the Relative Effect (95% CI) appears in the second column of the Table. However, the relative effect is presented in a different column in Tables 2, 3 and 4 (and in several other tables). To avoid confusing the reader, it would be advisable to use the same format for all of the SoF Tables (I would suggest using the format adopted in Tables 2, 3 and 4).
Response:
We re‐checked all the SoF tables and formatted all the tables.
4) Incorrect symbols for the GRADEpro GDT 2014 ratings have been used in several of the Summary of Findings (SoF) Tables (e.g. SoF tables 6, 8, 9 and many other tables in the pdf version of the review) . The review authors should use the following symbols consistently in all SoF tables.
| ⊕⊝⊝⊝ | ⊕⊕⊝⊝ | ⊕⊕⊕⊝ | ⊕⊕⊕⊕ |
| very low | low | moderate | high |
Response:
We re‐checked all the SoF tables and formatted all the tables.
5) In Summary of Findings Table 6 ‐ Mifepristone low dose (<25mg) versus Levonorgestrel 1.5mg ( pages 89‐93 of the pdf version of the review) the results presented for the primary outcome do not match the results presented in the Abstract, the main text of the review and in the Forest plot (Figure 5.1). The relative risk estimate presented in the Summary of Findings Table is 1.44 (95% CI 1.03 to 2.01) and is apparently based on 11 trials with 8336 participants. The correct relative risk estimate (according to other sections of the review) is 0.72 (95% CI 0.52 to 0.99) and is based on a meta‐analysis of 14 trials with 8752 participants. I would advise the review authors to re‐check all of the relative risk estimates in all other Summary of Findings Tables to ensure that no similar errors have occurred elsewhere.
Response:
Well‐checked and made the revision.
6) The footnotes in the Summary of Findings (SoF) Tables are insufficiently clear and require some additional detail to enhance clarity (several footnotes consist of a single word). For example, one reason for downgrading is given in several tables as “ACS” (e.g. see SoF table 10 [outcome 1 – observed number of pregnancies]). I could not find any definition of the abbreviation “ACS” in the review, but I presume it may refer to Allocation Concealment Score. The footnotes should include sufficient detail to allow the reader to understand why the review team have downgraded the body of evidence for a specific outcome (see the attached documents from the Cochrane Editorial Unit). Furthermore, the reasons for downgrading should be directly linked to the GRADEpro GDT 2014 factors that can reduce the quality of evidence (risk of bias, inconsistency of results, indirectness of evidence, imprecision, publication bias). In this context, instead of saying “ACS” in SoF table 10 for outcome 1, a more appropriate and sufficiently informative footnote would be something like “The quality of evidence was downgraded by one level for “risk of bias” because allocation concealment was judged to be adequate in only six trials included in the meta‐analysis”
Response:
We made the revision of all the footnotes.
7) Several footnotes cite “Sample Size” and “CI overlaps 1.00” as reasons for downgrading the body of evidence. Sample size and confidence intervals for relative effect measures including the value of ‘no effect’ are not sufficient reasons for downgrading the quality of evidence for imprecision. The GRADEpro GDT 2014 manual suggests that review authors should consider downgrading for imprecision if either 1) the total (cumulative) sample size is lower than the calculated optimal information size (OIS) or 2) the 95% confidence interval around the pooled or best estimate of effect includes both a) no effect andb) “appreciable benefit” or “appreciable harm”. The suggested GRADEpro GDT 2014 threshold for “appreciable benefit” or “appreciable harm” that should be considered for downgrading is a relative risk reduction or relative risk increase greater than 25%. However,if there are very few or no events and the number of participants is large, judgements about imprecision may be based on the absolute (rather than the relative) effect measures. Wide confidence intervals around a relative risk effect estimate may translate to clinically small differences in absolute effects. For example, in a trial with 500 participants in the experimental group and 500 participants in the control group, if 2 events occur in the experimental group (event rate 0.4%) and if three events occur in the control group (event rate 0.6%), then the relative risk (RR) will be 0.67 (95% CI 0.11, 3.97) but the risk difference (RD) (or absolute risk reduction ) will be ‐0.2% (95% CI ‐1%, +1%). In this example, the confidence interval around the absolute effect measure may be deemed sufficiently precise to warrant not downgrading the evidence (if review authors are happy that a 1% absolute difference in either direction would not constitute “appreciable benefit” or “appreciable harm”).
In the review of emergency contraceptives several trials have reasonably large sample sizes and few events and therefore I would suggest that the review authors consider basing their decisions about imprecision, where appropriate, on the absolute effect measures rather than the relative effect measures.
Response:
For those observations with few events such as “the observed pregnancies in all women”, we graded about imprecision on the absolute effect measures rather than the relative effect measures.
8) The reasons for downgrading (and not downgrading) the quality of evidence for outcomes do not appear to be consistent throughout the review. For example, in Summary of Findings Table 1, p78 of the pdf file, (IUD versus expectant management), a “high” quality of evidence rating is given for the outcome “observed number of pregnancies”. This finding is based on one trial (Askalani 1987) which was judged to have a ‘high risk of bias’ due to ‘inadequate allocation concealment’(see p18 of pdf file). In other Summary of Findings Tables the quality of evidence for the outcome “observed number of pregnancies” is downgraded for ‘risk of bias’ due to inadequate allocation concealment. The review authors should re‐check their ratings of the quality of evidence for all outcomes to ensure that GRADEpro GDT 2014 is applied consistently throughout the review.
Response:
We double‐checked all the evidence for all outcomes and for sure that GRADEpro GDT 2014 is applied consistently throughout the review.
9) The review authors do not mention GRADEpro GDT 2014 in the Abstract. In the “Data collection and analysis” section of the Abstract the review authors should state that they rated the quality of evidence using the GRADEpro GDT 2014 system. In addition, the GRADEpro GDT 2014 ratings should be incorporated into the “Main results” section of the Abstract. The review authors may find the attached pdf. document from the Cochrane Editorial Unit helpful when redrafting the Results section of the Abstract The document describes how GRADEpro GDT 2014 can be incorporated into various sections of a Cochrane review, including the Abstract, and provides some “good practice examples” from completed Cochrane reviews. I have also attached a worksheet developed by the Cochrane EPOC group which gives helpful suggestions regarding the language that review authors can use when describing the results of their review in the Abstract (and in other sections of a Cochrane review) ‐ they recommend use of the term ‘probably’ for moderate‐quality evidence and ‘may’ for ‘low‐quality evidence’ [An example that incorporates the suggestions made by the CEU and the EPOC group would be: Mid‐dose mifepristone (25‐50 mg) probablyprevents more pregnancies than levonorgestrel (RR 0.64, 95% CI 0.47 to 0.88, 26 trials, 5940 participants, moderate‐quality evidence)].
Response:
We incorporated all the GRADEpro GDT 2014 ranting into the “main result” and “Abstract” sections.
10) In the Abstract the review authors state that “Low dose‐mifepristone was less effective than mid‐dose mifepristone (25 trials; RR 0.73 (95% CI 0.55 to 0.97)”. The relative risk estimate presented by the review authors relates to the comparison between mid‐dose mifepristone versus low‐dose mifepristone (not low‐dose versus mid‐dose) and therefore it would be more appropriate to state that mid‐dose mifepristone was more effective than low‐dose mifepristone (NOTE: also see comments above regarding the incorporation of GRADEpro GDT 2014 ratings into the Abstract).
Response:
We made the relevant revision.
11) In the Abstract it is stated that: “Cu‐IUD were significantly more effective than mid‐dose Miferpistone (2 trials, RR 3.01, 95% CI 0.36 to 24.76) and LNG (1 trial, RR 1.89, 95% CI 1.05 to 3.38)” [sic]. There are several problems with this sentence. Firstly, the difference between Cu‐IUD and mid‐dose Mifepristone was not statistically significant. Secondly, for the comparison between Cu‐IUD and LNG, the review authors judged the quality of evidence to be “very low” for the outcome of pregnancy. If the quality of the evidence is “very low” this means that there is considerable uncertainty over the effect estimate and therefore no conclusions can be drawn about the relative effectiveness of the two interventions. Finally, as the outcome is pregnancy, the manner in which the results are presented suggest that the Cu‐IUD resulted in more pregnancies than mid‐dose Mifepristone and LNG (both RRs are >1.00). I would suggest that the review authors amend comparison 21 (page 13 of the pdf file) and any accompanying analyses to compare Cu‐IUD versus mid‐dose mifepristone (and not mid‐dose mifepristone versus Cu‐IUD). This will ensure that the calculated relative risk estimates are <1.00 and will ensure consistency with the format of presentation of other results in the Abstract (i.e. the entry in the Abstract should be: Cu‐IUD may prevent more pregnancies than mid‐dose mifepristone (RR 0.33, 95% CI 0.04 to 2.74); two trials, 395 participants, low‐quality evidence). The review authors should also amend comparison 26 to compare Cu‐IUD versus LNG.
Response:
We revised the explanation on Cu‐IUD after deep discussion and also amended the comparison 21 and 26 as suggested.
13) In the Authors’ conclusions section of the Abstract it is stated that “The copper IUD was the most effective EC method….”. This conclusion does not appear to be justified based on the evidence presented in the “Main results” section in the Abstract.
Response:
Agree. We rephrased the conclusion on Cu‐IUD.
14) The review authors could improve the format of the Discussion (pages 14‐16 of the pdf file) by using the standard headings recommended for the discussion section of Cochrane reviews (i.e. Summary of main results; Overall completeness and applicability of the evidence; Quality of the evidence; Potential biases in the review process; Agreements and disagreements with other studies or reviews).
Response:
We have rewritten the discussion section using the standard headings recommended.
15) In the “Implications for Practice” section of the review (page 16 of the pdf file) the review authors should delete the phrase ‘strong recommendation’ which appears on two occasions in this section of the review. Grading of the strength of recommendations should only be done by guideline development groups after considering the balance of desirable and undesirable consequences, quality of evidence, values and preferences and resource issues. These matters are beyond the remit of a systematic review and the Cochrane handbook (section 12.7.2) advises that “Authors of Cochrane reviews should not make recommendations”.
Response:
We have rewritten this section.
16) In the Background it is stated that “Examining data from 45 countries who most recent survey occurred between 2000 and 2012, in 16 countries, the percentage of women aged 15‐49 was less than 10%; in 36 countries, the rate of use of EC was less than 3% among women who had ever had sex (Palermo 2014)” [sic] . The first part of this sentence (highlighted in red) is very unclear and the review authors will need to rephrase it to enhance clarity.
Response:
This sentence was corrected.
Paul Beirne, 10‐12‐15
[1] Section 11.5.6.2 states that “The rows of a Summary of Findings Table should include all desirable and undesirable outcomes (listed in order of importance), that are essential for decision –making, up to a maximum of seven outcomes. If there are an excessive number of outcomes in the review authors will need to omit the less important outcomes”.
Data and analyses
Comparison 1. Intrauterine contraceptive device versus control (expectant management).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.26] |
Comparison 2. Levonorgestrel versus Yuzpe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 6 | 4750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.39, 0.84] |
| 2 Observed number of pregnancies (by risk status) | 2 | 2781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.31, 0.82] |
| 2.1 High‐risk women | 2 | 888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.19, 0.80] |
| 2.2 Low‐risk women | 2 | 1893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.32, 1.26] |
| 3 Observed number of pregnancies (time from intercourse) | 2 | 2632 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.28, 0.82] |
| 3.1 Within 24 h | 2 | 1343 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.19, 1.34] |
| 3.2 25‐48 h | 2 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.19, 0.94] |
| 3.3 49‐72 h | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.19, 1.77] |
| 4 Need for extra dose | 1 | 1955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.38, 0.75] |
| 5 Any side effect | 1 | 1955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.75, 0.86] |
| 6 Specific side effects | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Nausea | 6 | 4750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.36, 0.44] |
| 6.2 Vomiting | 5 | 3640 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.24, 0.35] |
| 6.3 Headache | 3 | 2606 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.71, 0.94] |
| 6.4 Dizziness | 3 | 3318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.65, 0.85] |
| 6.5 Fatigue | 6 | 4750 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.60, 0.74] |
| 6.6 Breast tenderness | 3 | 3318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.06] |
| 6.7 Diarrhoea | 1 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.32] |
| 6.8 Spotting/bleeding after treatment | 2 | 1614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.37, 2.41] |
| 6.9 Abdominal pain | 1 | 1955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.01] |
| 6.10 Hot flushes | 1 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.09, 2.54] |
| 6.11 Stomach pain | 1 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.24] |
| 6.12 Nose spot | 1 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.80, 1.52] |
| 7 Menses | 3 | 3298 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.99, 1.44] |
| 7.1 Early | 2 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.86, 1.52] |
| 7.2 Delay | 3 | 1988 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.96, 1.57] |
2.2. Analysis.
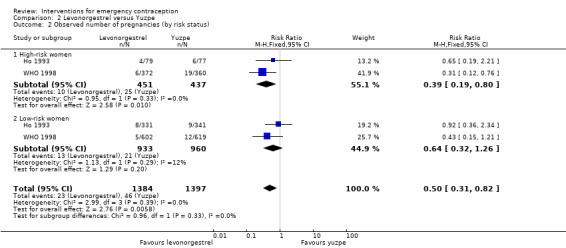
Comparison 2 Levonorgestrel versus Yuzpe, Outcome 2 Observed number of pregnancies (by risk status).
2.3. Analysis.
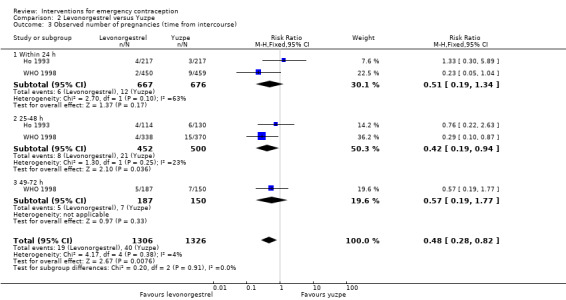
Comparison 2 Levonorgestrel versus Yuzpe, Outcome 3 Observed number of pregnancies (time from intercourse).
2.4. Analysis.
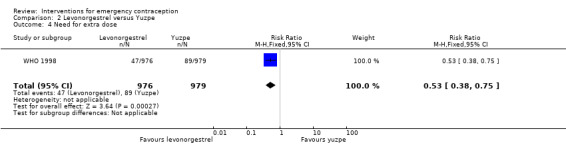
Comparison 2 Levonorgestrel versus Yuzpe, Outcome 4 Need for extra dose.
2.5. Analysis.
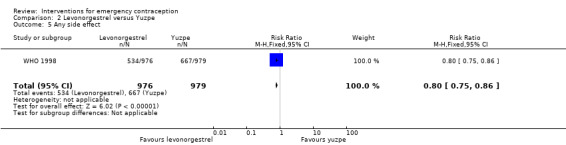
Comparison 2 Levonorgestrel versus Yuzpe, Outcome 5 Any side effect.
2.7. Analysis.
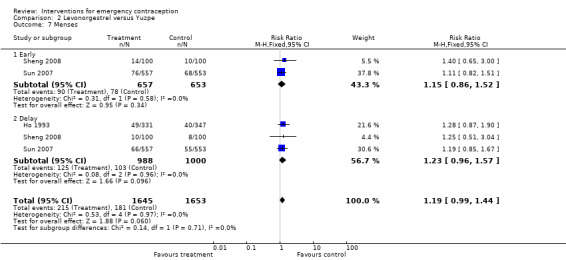
Comparison 2 Levonorgestrel versus Yuzpe, Outcome 7 Menses.
Comparison 3. Levonorgestrel (all doses) versus anordrin (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.89] |
| 2 Any side effect | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.27, 2.07] |
Comparison 4. Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 27 | 6052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.45, 0.83] |
| 2 Observed number of pregnancies (by risk status) | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.26, 10.24] |
| 2.1 High‐risk women | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.11, 12.05] |
| 2.2 Low‐risk women | 1 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.12, 53.29] |
| 3 Any side effect | 18 | 4352 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.40, 0.74] |
| 4 Specific side effect | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 4 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.48, 1.36] |
| 4.2 Headache | 1 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.22, 1.98] |
| 4.3 Dizziness | 2 | 302 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.26, 2.80] |
| 4.4 Breast tenderness | 4 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.52, 1.49] |
| 4.5 Abdominal pain | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.61] |
| 4.6 Spotting/bleeding after treatment | 9 | 1796 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.88] |
| 5 Menses | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Early | 7 | 1324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.03] |
| 5.2 Delay | 17 | 3615 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.09, 1.54] |
| 6 ITT (all loss follow‐up as pregnancy in LNG, and no preg in Mife) | 15 | 3758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.77] |
| 7 ITT (all loss follow‐up as no pregnancy in LNG, and preg in Mife) | 15 | 3758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.37, 0.88] |
4.2. Analysis.
Comparison 4 Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg, Outcome 2 Observed number of pregnancies (by risk status).
4.6. Analysis.
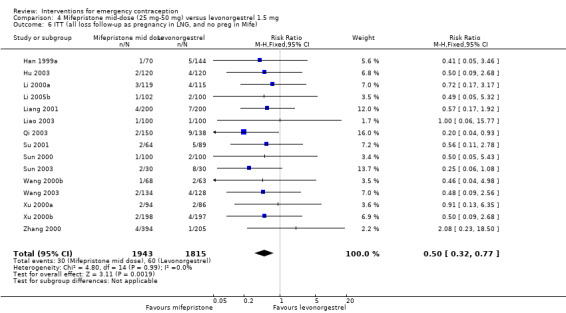
Comparison 4 Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg, Outcome 6 ITT (all loss follow‐up as pregnancy in LNG, and no preg in Mife).
4.7. Analysis.
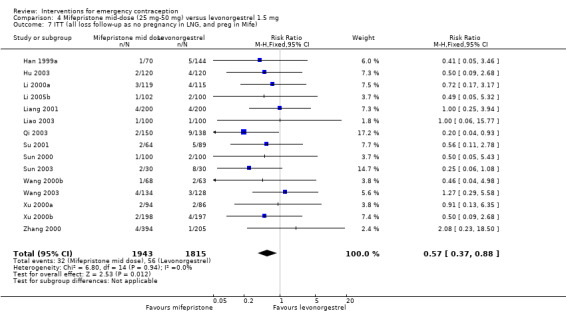
Comparison 4 Mifepristone mid‐dose (25 mg‐50 mg) versus levonorgestrel 1.5 mg, Outcome 7 ITT (all loss follow‐up as no pregnancy in LNG, and preg in Mife).
Comparison 5. Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 14 | 8752 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.52, 0.99] |
| 2 Observed number of pregnancies (by risk status) | 1 | 4071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.55, 1.55] |
| 2.1 High‐risk women | 1 | 1235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.67, 2.60] |
| 2.2 Low‐risk women | 1 | 2836 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.25, 1.35] |
| 3 Observed number of pregnancies (time from intercourse) | 2 | 6074 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.28] |
| 3.1 Within 72 h | 2 | 5553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.27] |
| 3.2 Later than 72 h | 2 | 521 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.35, 3.57] |
| 4 Any side effect | 3 | 609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.17, 0.38] |
| 5 Specific side effect | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Nausea | 5 | 6384 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.09] |
| 5.2 Vomiting | 3 | 6085 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.55, 2.68] |
| 5.3 Headache | 3 | 6082 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.37] |
| 5.4 Dizziness | 4 | 6181 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.79, 1.08] |
| 5.5 Fatigue | 3 | 6077 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.94, 1.21] |
| 5.6 Breast tenderness | 3 | 6084 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.88, 1.21] |
| 5.7 Diarrhoea | 2 | 4184 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.93, 1.73] |
| 5.8 Spotting/bleeding after treatment | 5 | 4598 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.54, 0.69] |
| 5.9 Low abdominal pain | 4 | 5105 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.83, 1.06] |
| 5.10 Hot flushes | 1 | 723 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.65, 1.33] |
| 6 Menses | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Early | 5 | 1800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.35, 0.59] |
| 6.2 Delay | 9 | 7520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.48, 1.97] |
| 7 ITT (all loss follow‐up as pregnancy in LNG, and no preg in Mifepristone) | 9 | 8429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.98] |
| 8 ITT (all loss follow‐up as no pregnancy in LNG, and preg in Mifepristone) | 9 | 8429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.05] |
5.2. Analysis.
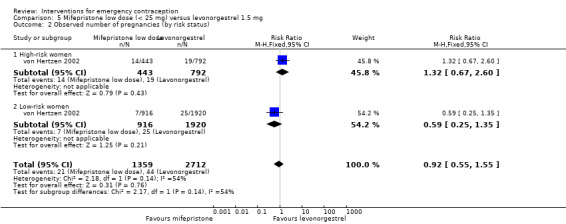
Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 2 Observed number of pregnancies (by risk status).
5.3. Analysis.
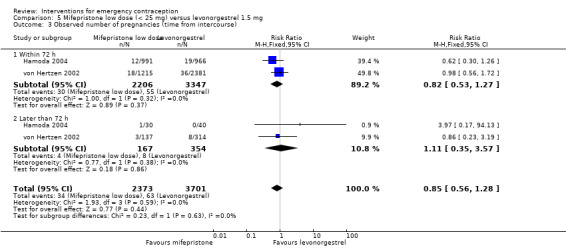
Comparison 5 Mifepristone low dose (< 25 mg) versus levonorgestrel 1.5 mg, Outcome 3 Observed number of pregnancies (time from intercourse).
Comparison 6. Mifepristone (all doses) versus Yuzpe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 3 | 2144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.05, 0.41] |
| 2 Observed number of pregnancies (by risk status) | 1 | 800 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.90] |
| 2.1 High‐risk women | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.90] |
| 2.2 Low‐risk women | 1 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Observed number of pregnancies (time from intercourse) | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.06, 0.59] |
| 3.1 within 24 h | 1 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.72] |
| 3.2 25‐48 h | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.18] |
| 3.3 49‐72 h | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.05, 1.16] |
| 4 Need for extra dose | 1 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.03, 0.49] |
| 5 Any side effect | 2 | 1693 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.77, 0.88] |
| 6 Specific side effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Nausea | 3 | 2186 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.53, 0.76] |
| 6.2 Vomiting | 3 | 2186 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.07, 0.20] |
| 6.3 Headache | 2 | 1800 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.61, 0.91] |
| 6.4 Dizziness | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.80] |
| 6.5 Fatigue | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.95] |
| 6.6 Breast tenderness | 3 | 2186 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.54, 1.39] |
| 6.7 Spotting/bleeding after treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.8 Abdominal pain | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.95] |
| 6.9 Hot flushes | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.40, 0.83] |
| 6.10 Lethargy | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.59, 0.95] |
| 7 Menses | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Early | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Delay | 3 | 1924 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [2.30, 3.47] |
6.2. Analysis.
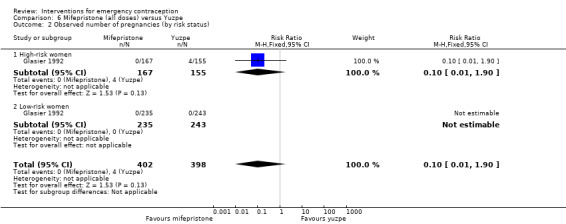
Comparison 6 Mifepristone (all doses) versus Yuzpe, Outcome 2 Observed number of pregnancies (by risk status).
6.3. Analysis.
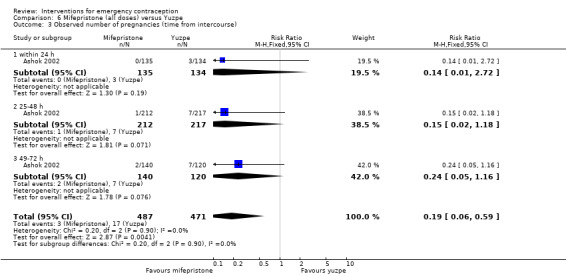
Comparison 6 Mifepristone (all doses) versus Yuzpe, Outcome 3 Observed number of pregnancies (time from intercourse).
6.4. Analysis.
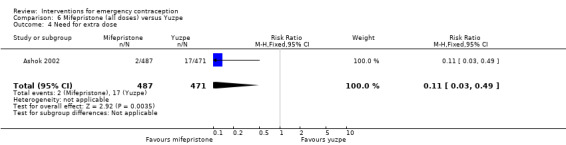
Comparison 6 Mifepristone (all doses) versus Yuzpe, Outcome 4 Need for extra dose.
6.5. Analysis.
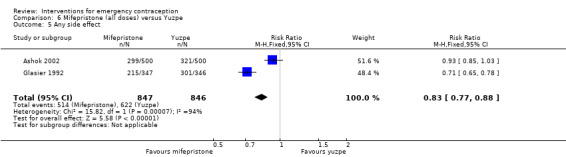
Comparison 6 Mifepristone (all doses) versus Yuzpe, Outcome 5 Any side effect.
Comparison 7. Mifepristone (all doses) versus anordrin (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 7 | 1035 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.63] |
| 2 Any side effect | 4 | 746 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.43, 0.91] |
| 3 Specific side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Spotting/bleeding after treatment | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.69, 4.77] |
| 4 Menses | 4 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.78, 1.68] |
| 4.1 Delay | 4 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.78, 1.68] |
Comparison 8. Mifepristone alone (low or mid dose) versus mifepristone + anordrin (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 5 | 3038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.73, 2.41] |
| 2 Any side effect | 2 | 442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.49, 1.41] |
| 3 Specific side effects | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.44, 0.65] |
| 3.2 Vomiting | 1 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.50] |
| 3.3 Headache | 1 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.25] |
| 3.4 Dizziness | 1 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.54, 1.10] |
| 3.5 Breast tenderness | 1 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.32] |
| 3.6 Fatigue | 1 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.49, 0.89] |
| 3.7 Diarrhoea | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.8 Spotting/bleeding after treatment | 5 | 3038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.33, 2.43] |
| 3.9 Abdominal pain | 1 | 2387 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.83, 1.67] |
| 4 Delay in menses | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Early | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Delay | 3 | 2781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.65, 0.97] |
Comparison 9. Mifepristone versus mifepristone + misoprostol (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [0.73, 16.65] |
| 2 Specific side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.56] |
| 2.2 Vomiting | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.47] |
| 2.3 Headache | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.05, 5.47] |
| 2.4 Dizziness | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.09, 2.70] |
| 2.5 Fatigue | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.86] |
| 2.6 Breast tenderness | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.13] |
| 2.7 Diarrhoea | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.22] |
| 2.8 Spotting/bleeding after treatment | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.35, 1.06] |
| 2.9 Abdominal pain | 1 | 599 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.93] |
Comparison 10. Mifepristone alone (all doses) versus mifepristone + tamoxifen (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.31, 28.60] |
| 2 Specific side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.38, 1.43] |
| 2.2 Vomiting | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.88] |
| 2.3 Headache | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.20] |
| 2.4 Dizziness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 103.49] |
| 2.5 Fatigue | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.41] |
| 2.6 Breast tenderness | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.47] |
| 2.7 Diarrhoea | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.20] |
| 2.8 Spotting/bleeding after treatment | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.35, 1.44] |
| 2.9 Abdominal pain | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.31, 28.60] |
| 2.10 Heavy menses | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.56 [1.25, 24.74] |
| 3 Menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Delay | 1 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.93, 3.43] |
Comparison 11. Mifepristone alone (all doses) versus mifepristone + methotrexate (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancy (all women) | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.36] |
| 2 Any side effect | 2 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.33, 1.70] |
| 3 Menses | 2 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.63, 1.43] |
| 3.1 Early | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.60] |
| 3.2 Delay | 2 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.39] |
Comparison 12. Mifepristone (all doses) versus danazol (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 2 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.55] |
| 2 Any side effect | 1 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.13, 0.95] |
| 3 Specific side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.92, 1.61] |
| 3.2 Vomiting | 1 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.25, 2.63] |
| 3.3 Breast tenderness | 1 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.29] |
| 3.4 Others | 1 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.94 [0.31, 28.01] |
| 4 Menses | 2 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [0.56, 10.27] |
| 4.1 Delay | 2 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [0.56, 10.27] |
Comparison 13. Mifepristone versus gestrinone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.32, 1.76] |
| 2 Side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.90, 2.00] |
| 2.2 Vomiting | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.94] |
| 2.3 Headache | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.19, 2.35] |
| 2.4 Dizziness | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.68, 3.89] |
| 2.5 Fatigue | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.91, 4.41] |
| 2.6 Breast tenderness | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.94] |
| 2.7 Diarrhoea | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.23] |
| 2.8 Bleeding or spotting | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.70, 1.26] |
| 2.9 Lower abdominal pain | 1 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.35, 2.29] |
| 3 Menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Early | 1 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.20, 0.69] |
| 3.2 Delay | 1 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.03, 1.82] |
Comparison 14. High‐dose oestrogens versus Yuzpe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.20, 23.77] |
Comparison 15. Danazol (all doses) versus Yuzpe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 2 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.61, 5.22] |
| 2 Specific side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.30, 0.47] |
| 2.2 Vomiting | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.06, 0.27] |
| 2.3 Breast tenderness | 1 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.75, 1.72] |
| 3 Menses | 1 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.74, 3.18] |
| 3.1 Delay | 1 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.74, 3.18] |
Comparison 16. Ulipristal acetate (all doses) versus levonorgestrel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 2 | 3448 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 0.99] |
| 2 Observed number of pregnancies (by risk status) | 2 | 3445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.97] |
| 2.1 High‐risk women | 2 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.25, 2.46] |
| 2.2 Low‐risk women | 2 | 3274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.97] |
| 3 Observed number of pregnancies (time from intercourse) | 2 | 3447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.37, 1.00] |
| 3.1 Within 24 h | 2 | 1185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.15, 1.05] |
| 3.2 24‐48 h | 2 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.59, 3.00] |
| 3.3 > 48‐72 h | 2 | 846 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.06] |
| 3.4 > 72‐96 h | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.73] |
| 3.5 > 96‐120 h | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.68] |
| 4 Observed number of pregnancies within 0‐72 h | 2 | 3245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.37, 1.07] |
| 5 Specific side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Nausea | 2 | 3770 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.93, 1.41] |
| 5.2 Vomiting | 1 | 1549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.07] |
| 5.3 Headache | 2 | 3770 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.87, 1.20] |
| 5.4 Dizziness | 2 | 3770 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.78, 1.45] |
| 5.5 Fatigue | 2 | 3770 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.91, 1.62] |
| 5.6 Breast tenderness | 1 | 1549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.53, 2.14] |
| 5.7 Diarrhoea | 1 | 1549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.48, 2.45] |
| 5.8 Spotting/bleeding after treatment | 1 | 1549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.24] |
| 5.9 Abdominal pain | 1 | 2221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.54, 1.06] |
| 5.10 Lower abdominal pain | 1 | 1549 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.69, 1.90] |
| 5.11 Upper abdominal pain | 1 | 2221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.24] |
| 5.12 Back pain | 1 | 2221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.80, 2.15] |
| 5.13 Dysmenorrhoea | 1 | 2221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.11] |
| 6 Menses | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Early | 2 | 3593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.37, 0.50] |
| 6.2 Delay | 2 | 3593 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.42, 1.92] |
16.2. Analysis.
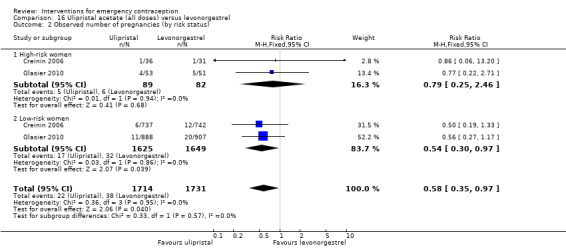
Comparison 16 Ulipristal acetate (all doses) versus levonorgestrel, Outcome 2 Observed number of pregnancies (by risk status).
16.3. Analysis.
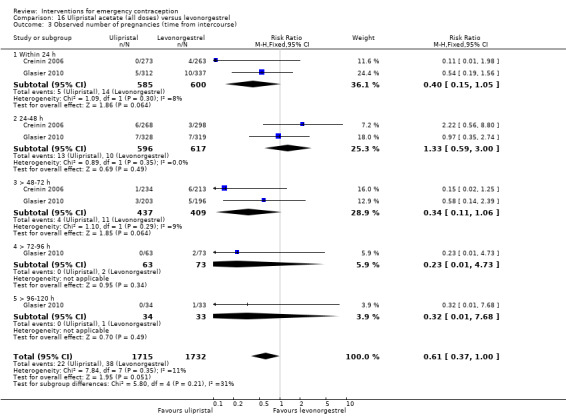
Comparison 16 Ulipristal acetate (all doses) versus levonorgestrel, Outcome 3 Observed number of pregnancies (time from intercourse).
16.4. Analysis.
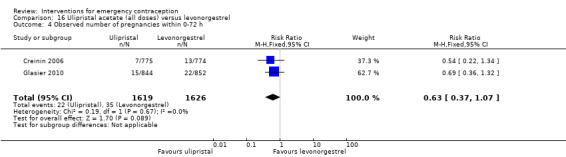
Comparison 16 Ulipristal acetate (all doses) versus levonorgestrel, Outcome 4 Observed number of pregnancies within 0‐72 h.
Comparison 17. Levonorgestrel split dose, 24 hours versus 12 hours.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancy (all women) | 1 | 2060 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.82] |
| 2 Observed number of pregnancies (by risk status) | 1 | 2012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.81] |
| 2.1 High‐risk women | 1 | 446 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.13, 1.23] |
| 2.2 Low‐risk women | 1 | 1566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.71, 3.42] |
| 3 Specific side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.29] |
| 3.2 Vomiting | 1 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.85] |
| 3.3 Headache | 1 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.36] |
| 3.4 Dizziness | 1 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.86, 1.74] |
| 3.5 Breast tenderness | 1 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.40, 0.91] |
| 3.6 Lower abdominal pain | 1 | 2071 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.53, 1.08] |
| 4 Menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Delay | 1 | 1978 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.17] |
Comparison 18. Levonorgestrel single dose versus split dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 3 | 6653 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 2 Observed number of pregnancies (by risk status) | 1 | 2712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.49] |
| 2.1 High‐risk women | 1 | 792 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.22, 1.41] |
| 2.2 Low‐risk women | 1 | 1920 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.51, 2.40] |
| 3 Observed number of pregnancies (time from intercourse) | 2 | 5489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.57, 1.57] |
| 3.1 Within 72 h | 2 | 4873 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.54] |
| 3.2 Later than 72 h | 2 | 616 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.46, 3.43] |
| 4 Specific side effects | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 3 | 6804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 4.2 Vomiting | 3 | 6804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.83, 1.22] |
| 4.3 Headache | 3 | 6804 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.01, 1.30] |
| 4.4 Dizziness | 3 | 6804 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.79, 1.05] |
| 4.5 Fatigue | 2 | 5742 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.15] |
| 4.6 Breast tenderness | 2 | 3782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.91, 1.37] |
| 4.7 Diarrhoea | 1 | 2720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.79] |
| 4.8 Spotting/bleeding after treatment | 1 | 2720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.90, 1.12] |
| 4.9 Lower abdominal pain | 2 | 3782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 4.10 Heavy menses | 1 | 1062 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.08, 2.04] |
| 5 Menses | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Early | 1 | 1118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.82] |
| 5.2 Delay | 2 | 3784 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.96, 1.46] |
18.2. Analysis.
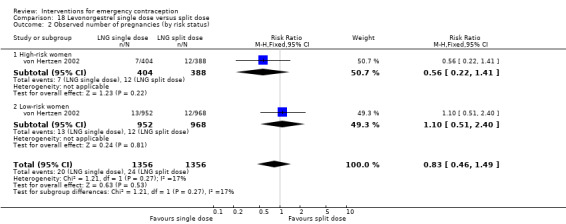
Comparison 18 Levonorgestrel single dose versus split dose, Outcome 2 Observed number of pregnancies (by risk status).
18.3. Analysis.
Comparison 18 Levonorgestrel single dose versus split dose, Outcome 3 Observed number of pregnancies (time from intercourse).
Comparison 19. Mifepristone low dose (10 mg) versus low dose (5 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 4 | 3110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.33, 1.31] |
| 2 Specific side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea | 2 | 2618 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.73, 1.75] |
| 2.2 Headache | 1 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.69, 1.83] |
| 2.3 Dizziness | 1 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.58, 1.31] |
| 2.4 Fatigue | 1 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.79, 1.31] |
| 2.5 Breast tenderness | 2 | 2618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.51, 1.75] |
| 2.6 Lower abdominal pain | 1 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.52, 1.26] |
| 3 Delay of menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Early | 1 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.50, 0.76] |
| 3.2 Delay | 1 | 2418 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [2.51, 3.38] |
Comparison 20. Low dose mifepristone (10 mg) versus split low dose mifepristone (10 mg x2).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 15.22] |
| 2 Any side effect | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.82, 2.68] |
| 3 Menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Early | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.29, 5.61] |
| 3.2 Delay | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.22, 1.54] |
Comparison 21. Mifepristone mid dose (25‐50 mg) versus mifepristone low dose (< 25 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 25 | 11914 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.97] |
| 2 Observed number of pregnancies (by risk status) | 3 | 4715 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.38] |
| 2.1 High‐risk women | 3 | 1544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.36, 1.42] |
| 2.2 Low‐risk women | 3 | 3171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.45, 2.17] |
| 3 Any side effect | 11 | 2464 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.01, 1.70] |
| 4 Specific side effects | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 13 | 7948 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.97, 1.24] |
| 4.2 Vomiting | 6 | 6082 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.68, 2.17] |
| 4.3 Headache | 6 | 6329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.22] |
| 4.4 Dizziness | 10 | 3512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.79, 1.63] |
| 4.5 Fatigue | 12 | 8209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.15] |
| 4.6 Breast tenderness | 9 | 6010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.29] |
| 4.7 Diarrhoea | 9 | 5746 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.55] |
| 4.8 Spotting/bleeding after treatment | 11 | 5078 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.55, 2.20] |
| 4.9 Abdominal pain | 4 | 4870 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.32] |
| 5 Menses | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Early | 7 | 2136 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.87, 1.36] |
| 5.2 Delay | 21 | 11282 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.11, 1.47] |
21.2. Analysis.
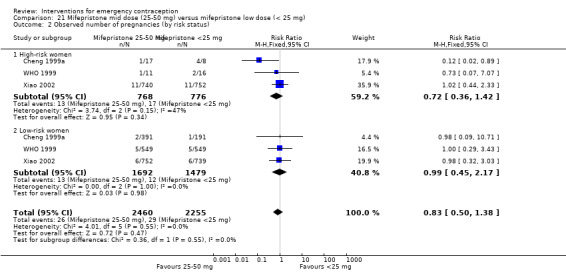
Comparison 21 Mifepristone mid dose (25‐50 mg) versus mifepristone low dose (< 25 mg), Outcome 2 Observed number of pregnancies (by risk status).
Comparison 22. Mifepristone mid dose (50 mg) versus mifepristone mid dose (25 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 13 | 3123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.41, 1.27] |
| 2 Any side effect | 6 | 1465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [1.39, 2.31] |
| 3 Specific side effects | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.44, 1.91] |
| 3.2 Vomiting | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.10] |
| 3.3 Headache | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.17, 3.28] |
| 3.4 Dizziness | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.54, 4.10] |
| 3.5 Fatigue | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [0.12, 72.53] |
| 3.6 Breast tenderness | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.08, 2.02] |
| 3.7 Abdominal pain | 1 | 418 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.93, 4.77] |
| 3.8 Spotting/bleeding after treatment | 2 | 617 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.82, 2.20] |
| 3.9 Early menses | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.63, 5.16] |
| 4 Delay in menses | 8 | 1945 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.12, 1.56] |
| 4.1 > 3 days | 3 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.75, 1.34] |
| 4.2 > 5 days | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.45, 2.39] |
| 4.3 > 7 days | 4 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.26, 1.94] |
Comparison 23. Mid dose mifepristone split dose comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 25 mg 12‐hrly x 2 versus 10 mg daily x 3 days | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [0.18, 21.03] |
| 1.2 25 mg 12‐hrly x 2 versus 10 mg daily x 5 days | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.92 [0.24, 101.35] |
| 1.3 10 mg daily x 3 days versus 10 mg daily x 5 days | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.13, 74.14] |
| 2 Any side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 25 mg 12‐hrly x 2 versus 10 mg daily x 3 days | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 28.04 [1.69, 464.70] |
| 2.2 25 mg 12‐hrly x 2 versus 10 mg daily x 5 days | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 28.52 [1.72, 472.69] |
| 2.3 10 mg daily x 3 days versus 10 mg daily x 5 days | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Early menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 25 mg 12‐hrly x 2 versus 10 mg daily x 3 days | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.77] |
| 3.2 25 mg 12‐hrly x 2 versus 10 mg daily x 5 days | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.64, 1.80] |
| 3.3 10 mg daily x 3 days versus 10 mg daily x 5 days | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.60, 1.73] |
| 4 Delay menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 25 mg 12‐hrly x 2 versus 10 mg daily x 3 days | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.03, 3.37] |
| 4.2 25 mg 12‐hrly x 2 versus 10 mg daily x 5 days | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.70, 1.89] |
| 4.3 10 mg daily x 3 days versus 10 mg daily x 5 days | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.34, 1.14] |
Comparison 24. Mifepristone high dose (> 50 mg) versus mifepristone low dose (< 25 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 5 | 1726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.23, 1.17] |
| 2 Observed number of pregnancies (by risk status) | 1 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.29, 3.41] |
| 2.1 High‐risk women | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low‐risk women | 1 | 1102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.29, 3.41] |
| 3 Any side effect | 3 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.04 [5.13, 33.15] |
| 4 Specific side effects | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.56] |
| 4.2 Vomiting | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Headache | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Dizziness | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.55] |
| 4.5 Fatigue | 2 | 1210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.00, 1.56] |
| 4.6 Breast tenderness | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.31] |
| 4.7 Diarrhoea | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.55] |
| 4.8 Spotting/bleeding after treatment | 2 | 1224 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.89, 2.95] |
| 4.9 Abdominal pain | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Menses | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Early | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Delay | 4 | 1574 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.66, 2.37] |
24.2. Analysis.
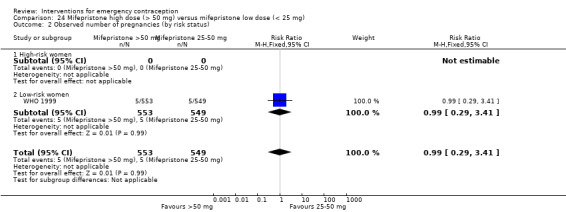
Comparison 24 Mifepristone high dose (> 50 mg) versus mifepristone low dose (< 25 mg), Outcome 2 Observed number of pregnancies (by risk status).
Comparison 25. Mifepristone high dose (> 50 mg) versus mifepristone mid dose (25‐50 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 9 | 3009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.50, 1.72] |
| 2 Any side effect | 5 | 1310 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.57, 4.43] |
| 3 Specific side effects | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.36, 4.35] |
| 3.2 Dizziness | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.69] |
| 3.3 Fatigue | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.28] |
| 3.4 Breast tenderness | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.28] |
| 3.5 Diarrhoea | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.21, 4.69] |
| 3.6 Spotting/bleeding after treatment | 4 | 1509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.12, 1.56] |
| 4 Menses | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Early | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.30, 76.66] |
| 4.2 Delay | 8 | 2854 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.34, 1.75] |
Comparison 26. Half‐dose Yuzpe versus standard Yuzpe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 1 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.76, 2.61] |
| 2 Any side effect | 1 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.93] |
| 3 Specific side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea | 1 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.77, 0.97] |
| 3.2 Vomiting | 1 | 1275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.36, 0.69] |
| 3.3 Headache | 1 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.24] |
| 3.4 Dizziness | 1 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.40, 1.07] |
| 3.5 Abdominal pain | 1 | 1288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.43, 1.37] |
26.2. Analysis.
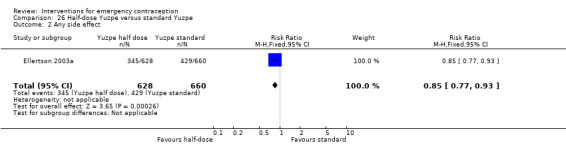
Comparison 26 Half‐dose Yuzpe versus standard Yuzpe, Outcome 2 Any side effect.
Comparison 27. Copper intrauterine device versus mifepristone (all doses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies (all women) | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.74] |
| 2 Any side effect | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.99] |
| 3 Specific side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Lower abdominal pain | 1 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 73.61 [4.48, 1208.50] |
| 4 Menses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Delay | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.09, 0.64] |
27.2. Analysis.
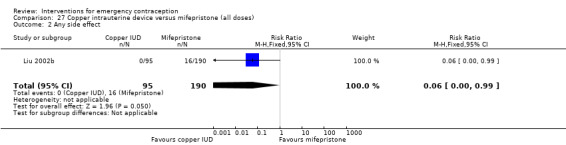
Comparison 27 Copper intrauterine device versus mifepristone (all doses), Outcome 2 Any side effect.
Comparison 28. Time elapsed since intercourse in levonorgestrel.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≤ 24 h vs > 24‐48 h | 4 | 2336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.50, 1.73] |
| 2 ≤ 24 h vs > 48‐72 h | 3 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.19] |
| 3 > 24‐48 h vs > 48‐72 h | 3 | 1551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.11] |
| 4 < 72 h vs > 72 h | 4 | 7453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.31, 0.84] |
28.1. Analysis.

Comparison 28 Time elapsed since intercourse in levonorgestrel, Outcome 1 ≤ 24 h vs > 24‐48 h.
28.2. Analysis.
Comparison 28 Time elapsed since intercourse in levonorgestrel, Outcome 2 ≤ 24 h vs > 48‐72 h.
28.3. Analysis.
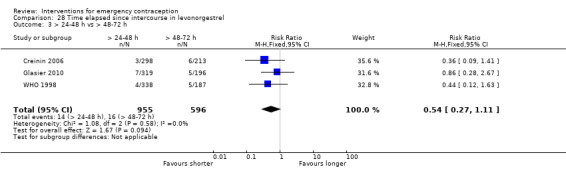
Comparison 28 Time elapsed since intercourse in levonorgestrel, Outcome 3 > 24‐48 h vs > 48‐72 h.
28.4. Analysis.
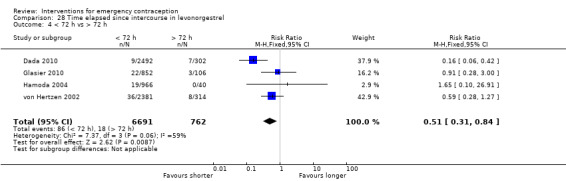
Comparison 28 Time elapsed since intercourse in levonorgestrel, Outcome 4 < 72 h vs > 72 h.
Comparison 29. Time elapsed since intercourse (coitus‐treatment interval) in mifepristone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≤ 24 h vs > 24‐48 h | 2 | 1136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.29, 3.54] |
| 2 ≤ 24 h vs > 48‐72 h | 2 | 841 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.13, 1.53] |
| 3 > 24‐48 h vs > 48‐72 h | 2 | 979 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.13, 1.42] |
| 4 < 72 h vs > 72 h | 2 | 2373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.21, 1.67] |
29.1. Analysis.
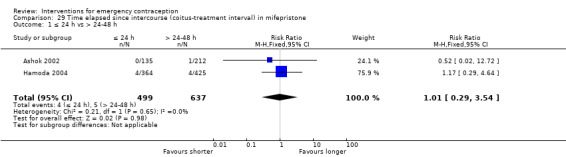
Comparison 29 Time elapsed since intercourse (coitus‐treatment interval) in mifepristone, Outcome 1 ≤ 24 h vs > 24‐48 h.
29.2. Analysis.
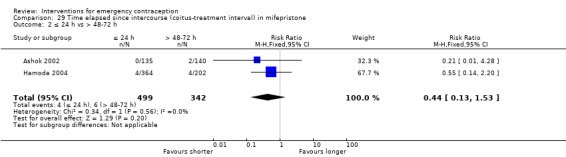
Comparison 29 Time elapsed since intercourse (coitus‐treatment interval) in mifepristone, Outcome 2 ≤ 24 h vs > 48‐72 h.
29.3. Analysis.
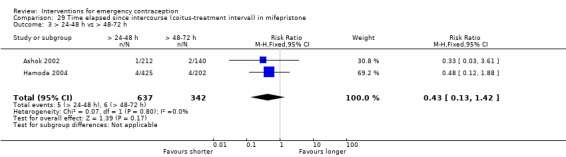
Comparison 29 Time elapsed since intercourse (coitus‐treatment interval) in mifepristone, Outcome 3 > 24‐48 h vs > 48‐72 h.
29.4. Analysis.
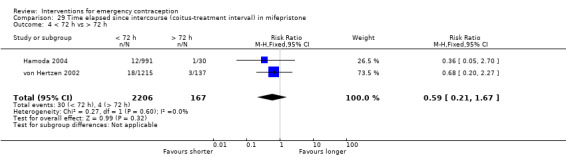
Comparison 29 Time elapsed since intercourse (coitus‐treatment interval) in mifepristone, Outcome 4 < 72 h vs > 72 h.
Comparison 30. Time elapsed since intercourse in Yuzpe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≤ 24 h vs > 24‐48 h | 3 | 1527 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.26, 0.88] |
| 2 ≤ 24 h vs > 48‐72 h | 2 | 863 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.18, 0.89] |
| 3 > 24‐48 h vs > 48‐72 h | 2 | 857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.37, 1.39] |
30.1. Analysis.

Comparison 30 Time elapsed since intercourse in Yuzpe, Outcome 1 ≤ 24 h vs > 24‐48 h.
30.2. Analysis.
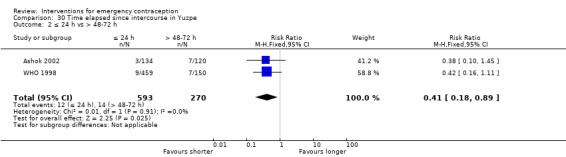
Comparison 30 Time elapsed since intercourse in Yuzpe, Outcome 2 ≤ 24 h vs > 48‐72 h.
30.3. Analysis.
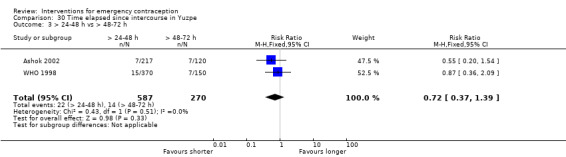
Comparison 30 Time elapsed since intercourse in Yuzpe, Outcome 3 > 24‐48 h vs > 48‐72 h.
Comparison 31. Time elapsed since intercourse in ulipristal acetate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≤ 24 h vs > 24‐48 h | 2 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.12] |
| 2 ≤ 24 h vs > 48‐72 h | 2 | 1022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.24, 2.95] |
| 3 > 24‐48 h vs > 48‐72 h | 2 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.77, 6.82] |
| 4 < 72 h vs > 72 h | 1 | 970 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [0.28, 77.39] |
31.1. Analysis.
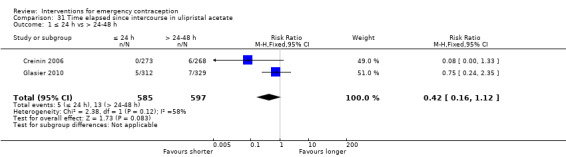
Comparison 31 Time elapsed since intercourse in ulipristal acetate, Outcome 1 ≤ 24 h vs > 24‐48 h.
31.2. Analysis.
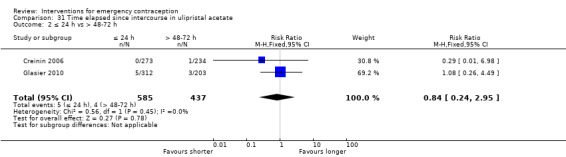
Comparison 31 Time elapsed since intercourse in ulipristal acetate, Outcome 2 ≤ 24 h vs > 48‐72 h.
31.3. Analysis.
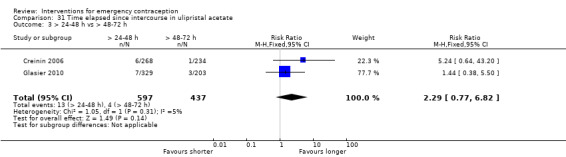
Comparison 31 Time elapsed since intercourse in ulipristal acetate, Outcome 3 > 24‐48 h vs > 48‐72 h.
31.4. Analysis.
Comparison 31 Time elapsed since intercourse in ulipristal acetate, Outcome 4 < 72 h vs > 72 h.
Comparison 32. High‐risk women versus low‐risk women (all hormonal methods).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Observed number of pregnancies | 11 | 19700 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [2.11, 3.39] |
32.1. Analysis.
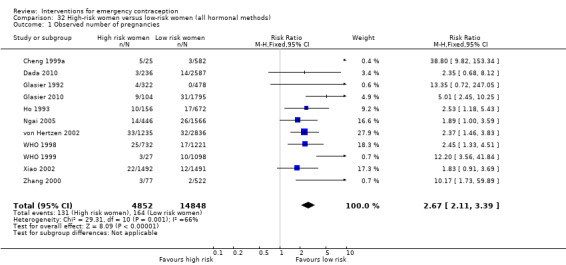
Comparison 32 High‐risk women versus low‐risk women (all hormonal methods), Outcome 1 Observed number of pregnancies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arowojolu 2002.
| Methods | Randomised, double‐blind, multicentre trial. Random number generation done centrally Similar looking placebos were used |
|
| Participants | 1160 healthy women recruited into the study from family planning clinics, University College Hospital, Ibadan, and Planned Parenthood Federation of Nigeria, Ikolaba, Ibadan Included women with regular menstrual periods (21‐35 days), who had attended the clinic within 72 h of a single act of unprotected intercourse Excluded women who were not available for follow‐up, were pregnant, on hormonal contraception in the current cycle and those who had contraindications to the use of hormonal contraceptive pills. 1118 into efficacy analysis, 1062 into safety analysis |
|
| Interventions | LNG 0.75 mg, 2 doses, 12 h apart orally (split dose) vs LNG 1.5 mg (single dose) | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "participants were randomised into two groups (A and B) using a computer generated random table." |
| Allocation concealment (selection bias) | Unclear risk | No explanation of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "These were administered by a family planning nurse who was blind to the contents in the boxes." "The medications were packed in similar boxes, each tagged with the users name, and containing two tablets. Group A took the box containing one 0.75 mg levonorgestrel tablet and one similarly looking, inactive placebo tablet" Double‐blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Explained loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Ashok 2002.
| Methods | Women randomised into 2 groups by opening sequentially‐numbered, sealed, opaque envelopes that were prepared using random number tables The study was not blinded and the clinician and patient were both aware of the treatment allocation |
|
| Participants | 1000 women attending family planning clinics in Aberdeen, UK. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 100 mg orally vs Yuzpe regimen (2 tablets each with ethinyl oestradiol 50 μg and LNG 0.25 mg) orally, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects, change in menstrual pattern and patient acceptability | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Women were randomized into two groups by opening sequentially numbered, sealed opaque envelopes which were prepared using random number tables." |
| Allocation concealment (selection bias) | Low risk | "Women were randomized into two groups by opening sequentially numbered, sealed opaque envelopes which were prepared using random number tables." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The study was not blinded and the clinician and patient were both aware of the treatment allocated since patient acceptability was an outcome measure." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was used "women allocated to a method of treatment were attributed to that method for the purpose of analysis, whether or not they had the particular method of treatment." |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were reported |
| Other bias | Low risk | None detected |
Askalani 1987.
| Methods | 'randomly allocated' women to 2 groups. The numbers enrolled in 2 groups were 2:1 between treatment and control. Although 2:1 randomisation was not specifically mentioned, the trial was included because it is explicitly stated that the allocation was random No details of allocation concealment or other methodological aspects were mentioned |
|
| Participants | 300 women attending the family planning clinic of the Al‐Azhar University, Cairo, Egypt Included women who had had unprotected intercourse around the time of ovulation and attended the clinic within 4 days of unprotected intercourse |
|
| Interventions | Cu‐IUD (Cu‐T 200) vs control (no treatment) | |
| Outcomes | Pregnancy rates | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not explained |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned or method explained |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Any losses to follow‐up or exclusion were not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Bu 2006.
| Methods | Women were randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 100 women attending Fulaerji District Hospital, Qiqihaer, Helongjiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 10 mg single‐dose orally vs LNG 0.75 mg, 2 doses, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Any losses to follow‐up or exclusion were not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Cao 1999.
| Methods | Women 'randomly allocated' to 4 groups. Method of randomisation not reported | |
| Participants | 543 women (aged 18‐47 years old) attending the outpatient clinic of the No. 477 Military Hospital, China. Women had regular menstrual periods and unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife (single dose) 100 mg vs Mife 50 mg vs Mife 25 mg vs Mife 10 mg, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Any losses to follow‐up or exclusion were not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Cao 2011.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 286 women attending the gyn clinic in a general hospital, Tianjin, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, single dose orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of allocation concealment not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Carbonell 2015.
| Methods | Randomised, double‐blind trial. Random number generation done with a random computer‐generated list Same appearance, colour and shape capsules were used |
|
| Participants | 2418 healthy women recruited into the study from Eusebio Hernandez Hospital, Havana, Cuba Included women with regular menstrual cycles (24‐36 days), who had a single act of unprotected intercourse in the last 6 days of attending the hospital and no wish to be pregnant Excluded women who were not available for follow‐up, were pregnant or lactated, using hormonal contraceptives in the last 3 months and those who using any contraindication for mifepristone 1206 into 5 mg analysis, 1212 into 10 mg analysis |
|
| Interventions | Mife 5 mg versus 10 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assignation to the treatment groups was done by compiling a random computer generated list." |
| Allocation concealment (selection bias) | Low risk | "People not participating in the study prepared sealed, opaque envelopes containing a card bearing the treatment group to which the patient would be assigned" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double blinded." "Once the subject had been evaluated in line with the inclusion and exclusion criteria and had signed the informed consent, the envelope corresponding to the subjects numbered incorporation into the study was opened and she was included in the treatment group indicated on the card contained in the envelope: 'mifepristone A' or 'mifepristone B', where A corresponded to one dose and B to the other. This code was opened once data processing had finished; neither the doctors nor the subjects knew which group the subjects had been assigned to." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions reported‐ "Did not attend the visit n=2 and were excluded from data analysis" |
| Selective reporting (reporting bias) | Low risk | Planned variables were reported |
| Other bias | Low risk | None detected |
Chen 2001.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 88 women attending the gyn clinic in a general hospital, Guangxi, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs anordrin 7.5 mg, 2 doses, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Chen 2002a.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 312 women attending the clinic in 4 family planning centres, Guangdong, China Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic |
|
| Interventions | Mife 50 mg vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions and loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Chen 2002b.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 100 women attending the gyn clinic in a general hospital, Fujian, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 25 mg + MTX 5 mg vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Chen 2008.
| Methods | Women were randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 273 women attending in a family planning clinic, Tongxiang, Zhejiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 25 mg, single dose vs LNG 0.75 mg, 2 doses | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Chen 2009.
| Methods | Women were randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 62 women attending in a family planning clinic, Liaoning Province. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 25 mg vs Mife 10 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Chen 2013.
| Methods | Women were randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 100 women attending in an obs/gyn clinic, Guangdong, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | LNG 0.75 mg in 2 doses vs Mife 25 mg single dose | |
| Outcomes | Observed number of pregnancies and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Chen 2015.
| Methods | Women were randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 112 women attending in an obs/gyn clinic, Zhengzhou, Henan Province, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | LNG 0.75 mg in 2 doses vs Mife 25 mg, single dose | |
| Outcomes | Observed number of pregnancies and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Cheng 1999a.
| Methods | Women 'randomly allocated' to 3 groups. Random number table used to generate the allocation sequence There was no concealment of allocation and no blinding Side effects were assessed by women on a chart |
|
| Participants | 639 women in Shanghai, China, attending 17 district MCH hospitals Included if they had regular menstrual periods (21‐35 days), aged 18‐45 years, with a single act of unprotected intercourse within 120 h of attending the clinic Excluded women on oral contraceptives, with contraindications to Mife and those that were considered difficult to follow‐up |
|
| Interventions | Mife, single dose (Chinese domestic product): 50 mg vs Mife 25 mg vs Mife 10 mg | |
| Outcomes | Observed number of pregnancies, side effects, changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women 'randomly allocated' to 3 groups. Random number table used to generate the allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Cheng 2009.
| Methods | Women were randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 166 women attending in an obs/gyn clinic, Huadu District Hospital, Guangzhou, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | LNG 0.75 mg in 2 doses vs Mife 25 mg single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Creinin 2006.
| Methods | Randomised, double‐blinded, non‐inferiority trial
Study drug was supplied in sequentially‐numbered, sealed packages containing 2 opaque capsules. The packages either contained a single opaque capsule with UPA (CDB‐2914) 50 mg plus an identical placebo capsule or 2 opaque capsules, each with a tablet of LNG 0.75 mg The identification of the contents of the capsules was unknown to the investigators and the subjects |
|
| Participants | 1672 healthy women aged at least 18 years of age not using any hormonal contraception who requested EC within 72 h after unprotected intercourse as a result of using no contraception, condom breakage or slippage, or failure of another barrier method To be eligible for enrolment, they were required to have had a recent history of regular menstrual cycles (24‐42 days). At least 1 normal menstrual cycle (2 menses) was required after delivery, abortion or discontinuation of hormonal contraceptive |
|
| Interventions | Women randomly assigned to receive a single dose of UPA (CDB‐2914) 50 mg plus a placebo 12 h later or 2 doses of LNG 0.75 mg taken 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed in blocks of eight such that, within each block of study drug, the chance of getting each treatment was equal." |
| Allocation concealment (selection bias) | Low risk | "The study drug was supplied in sequentially numbered sealed packages containing two opaque capsules. The packages either contained a single opaque capsule with 50 mg of CDB‐2914 plus an identical placebo capsule, or two opaque capsules, each with a tablet of 0.75 mg of levonorgestrel. The identification of the contents of the capsules was unknown to the investigators and the subjects. A portion of the label on each package of study drug was affixed to the case report form. This portion of the label had a removable opaque panel to allow for emergency unblinding. Once removed, these labels could not be replaced." "If the woman’s urine pregnancy test was negative, the next sequentially numbered envelope was then opened and the first dose of study drug taken in the office." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study drug was supplied in sequentially numbered sealed packages containing two opaque capsules. The packages either contained a single opaque capsule with 50 mg of CDB‐2914 plus an identical placebo capsule, or two opaque capsules, each with a tablet of 0.75 mg of levonorgestrel. The identification of the contents of the capsules was unknown to the investigators and the subjects. A portion of the label on each package of study drug was affixed to the case report form. This portion of the label had a removable opaque panel to allow for emergency unblinding. Once removed, these labels could not be replaced." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Explains loss to follow‐up. Used ITT analysis |
| Selective reporting (reporting bias) | Low risk | Planned outcomes in the protocol were reported in paper |
| Other bias | Low risk | None detected |
Dada 2010.
| Methods | Women were randomly allocated to 2 groups. Method of randomisation of double‐blind trial was mentioned in the paper |
|
| Participants | 3022 Nigerian women with regular menstrual cycles (24–42 days' duration with variation of no more than 5 days) Desired EC within 120 h after a single act of unprotected coitus during the present menstrual cycle, agreed to abstain from further acts of intercourse during that cycle or to use a condom or diaphragm if this was not possible Available for follow‐up over the next 6 weeks |
|
| Interventions | 2‐dose LNG: participants received 2 doses of LNG 0.75 mg administered 12 h apart Single‐dose LNG: participants received 1 dose of LNG 1.5 mg and 1 LNG placebo 12 h apart |
|
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation to study group (two dose or single dose) was determined through a random‐number sequence, which was computer generated by WHO in fixed blocks of eight." |
| Allocation concealment (selection bias) | Low risk | "A sealed bag containing the polyethylene bags (of treatment) was labelled with the name of the center, participant number and tablet expiration date. The sealed bags were used sequentially in the order of subject numbers. The allocation sequence was concealed from investigators and WHO staff in Geneva." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double blinding was maintained throughout for the participants, trial clinicians and outcome evaluators." "Levonorgestrel tablets and levonorgestrel placebo were provided" ; placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Did not use ITT analysis however still compared baseline characteristics for all women including those lost to follow‐up whereas pregnancy rate analysis used a different sample size |
| Selective reporting (reporting bias) | Low risk | Reports planned outcomes |
| Other bias | Low risk | None detected |
Ding 2005.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 240 women attending the clinic in an MCH hospital, Henan, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 75 mg vs Mife 50 mg vs Mife 10 mg, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Dong 2009.
| Methods | Women allocated to 2 groups. Method of randomisation not reported | |
| Participants | 200 women attending in a family planning clinic, Yuhuan, Zhejiang, China. Women had regular menstrual periods, and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 10 mg, 2 doses, 12 h apart, orally vs LNG 0.75 mg, 2 doses, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Du 2002.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 180 women attending a general hospital, Henan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs Mife 10 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Ellertson 2003a.
| Methods | Randomised, double‐blind, controlled trial Each dose of therapy was inserted in opaque gelatin capsules and then packaged in opaque envelopes labelled either "first dose" or "second dose" Following computer‐generated randomisation the pairs were inserted into sequentially‐numbered, opaque envelopes and sealed |
|
| Participants | 2041 women at 5 centres in the US and UK within 72 h of a single, unprotected intercourse that occurred between 10 days before and 6 days after the estimated day of ovulation Included women aged 16‐45 years, willing to abstain further in the current cycle, could attend follow‐ups, keep a diary of side effects and refused the insertion of Cu‐IUDs Excluded women who had used hormonal contraception during the past 2 months, had not had 2 normal periods in the previous 2 cycles, breastfeeding and those who had a positive pregnancy test |
|
| Interventions | Standard 2‐dose Yuzpe regimen vs modified Yuzpe using norethindrone 2.0 mg instead of norgestrel 1.0 mg vs single dose of the standard Yuzpe regimen (followed 12 h later by a placebo) | |
| Outcomes | Observed number of pregnancies, side effects, changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation" |
| Allocation concealment (selection bias) | Low risk | "Population council staff enclosed each dose of each course of therapy in opaque gelatin capsules, and then packaged these capsules in opaque envelopes labeled either first dose or second dose. After computer generated randomisation, we inserted pairs of these envelopes into larger sequentially numbered envelopes, which we then sealed. Clinic staff simply gave each enrolled woman the next envelope in the sequence at that site." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "each dose of each course of therapy [placed] in opaque gelatin capsules". "Neither the clinic staff nor the women knew which regimens had been taken by which women until we broke the code at the end of the trial." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was used |
| Selective reporting (reporting bias) | Low risk | All planned outcomes reported |
| Other bias | Low risk | None detected |
Fan 2001a.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 103 women attending an MCH hospital, Hubei, China. Women had regular menstrual periods and a single act of unprotected intercourse within 96 h of attending the clinic | |
| Interventions | Mife 25 mg vs Mife 10 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation exclusion and loss to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Fang 2000.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 200 women attending an MCH clinic in Guangzhou, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 50 mg vs Mife 25 mg orally single dose | |
| Outcomes | Observed number of pregnancies, side effects, changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Farajkhoda 2009.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | Prospective, randomised, comparative study, including 124 healthy volunteers who, in the observed cycle, had had only 1 act of unprotected intercourse within 72 h of treatment Randomly allocated to LNG (n = 62) and Yuzpe (n = 62) |
|
| Interventions | Yuzpe: involved 2 doses of combined oestrogen/progestin pills, with each dose containing 100 μg of ethinyl oestradiol and 500 μg of LNG LNG: LNG 0.75 mg taken within 72 h of unprotected coitus and LNG 0.75 mg taken 12 h later |
|
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomisation was done by randomisation schedules"‐ doesn't clarify what kind of schedule or how it was generated |
| Allocation concealment (selection bias) | Unclear risk | Does not state if there was any allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Does not state if blinding was present |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two women were excluded from the study because they were lost to follow‐up" Explains the lost participants as loss to follow‐up. Analysed all others |
| Selective reporting (reporting bias) | Unclear risk | The primary and secondary planned outcomes are not clearly stated in the methods and there is no protocol available to check. |
| Other bias | Low risk | None detected |
Fu 2000.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 186 women attending an MCH hospital, Qinghai, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Anordrin 7.5 mg, twice daily, 12 h apart, for 2 days vs Mife 50 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Gan 2007.
| Methods | Women randomly allocated to 2 groups. The method of randomisation not reported | |
| Participants | 456 women attending in an obs/gyn clinic, Boluo County Hospital, Guangdong, China. Women had regular menstrual periods, and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 25 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy rate was reported |
| Other bias | Low risk | None detected |
Glasier 1992.
| Methods | Randomly allocated women to 2 treatment groups within predefined age groups (16‐25 years, 26‐34 years, 35‐45 years). Cards with the treatment names on were put in sealed envelopes and allocation was made by shuffling the cards. There was no blinding, placebos were not used. Side effects were assessed by women |
|
| Participants | 800 women attending a family planning clinic and an accident and emergency department in Edinburgh, UK Included women with regular menstrual periods, aged 16‐45 years who had attended the clinic within 72 h of a single act of unprotected intercourse Excluded women on oral contraceptives, regular prescription drugs, with medical contraindications, who were difficult to follow up and who would continue with the pregnancy in case of a failure |
|
| Interventions | Yuzpe (ethinyl oestradiol 100 μg + norgestrel 1 mg, repeated after 12 h) vs Mife 600 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Explanation for method of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not explained |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions reported "26 women were lost to follow up" "A total of 693 women returned completed diary charts" (and were hence analysed) |
| Selective reporting (reporting bias) | Low risk | Planned variables were reported |
| Other bias | Low risk | None detected |
Glasier 2010.
| Methods | Enrolled women randomly assigned to receive UPA 30 mg or LNG 1.5 mg orally. Randomisation schedule stratified by site and time from unprotected sexual intercourse to treatment (within 72 h and 72–120 h) with a block size of 4 Single‐blind (women masked to treatment assignment, whereas those giving the interventions and study investigators were not, since the study drugs differed in appearance (different tablet size and blister pack)) |
|
| Participants | Women with regular menstrual cycles who presented to a participating family planning clinic requesting emergency contraception within 5 days of unprotected sexual intercourse were eligible for enrolment Randomised, multicentre, non‐inferiority trial 2221 women randomly assigned to UPA (CDB‐2914) (n = 1104) or LNG (n = 1117) |
|
| Interventions | UPA 30 mg vs LNG 1.5 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization schedule was stratified by site and time from unprotected sexual intercourse to treatment with a block size of four." Computer generated |
| Allocation concealment (selection bias) | Low risk | "allocation concealment by identical opaque boxes labelled with a unique treatment number" "Only after registration and request for randomization did the system allocate a treatment number to the participant from the lot available on site, according to the randomization schedule." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study was single blind‐ i.e. participants were masked to treatment assignment, whereas those giving the interventions and study investigators were not, since the study drugs differed in appearance. Study drug blister packs were packaged individually in identical opaque boxes labelled with a unique treatment number". "The investigator or nurse took the appropriate treatment pack from storage, removed the tablet from the blister pack out of sight of the participant, and gave it to the participant under direct supervision". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used and participant withdrawals explained |
| Selective reporting (reporting bias) | Low risk | Planned outcomes from protocol reported |
| Other bias | Low risk | None detected |
Hamoda 2004.
| Methods | Women presenting within 72 h of unprotected intercourse enrolled. Women presenting beyond 72 h and up to 120 h were offered a Cu‐IUD insertion as the first treatment choice. Those declining IUD insertion were randomised to receive Mife 10 mg single tablet or 2 LNG 750 μg tablets, 12 h apart, by opening sequentially‐numbered, opaque, sealed envelopes prepared using random number tables. The randomisation envelopes were prepared in the Family Planning Clinic in Aberdeen, UK by a healthcare assistant not involved in the recruitment or data collection The study was not blinded, and both medical staff and participants were aware of the treatment assigned |
|
| Participants | Eligible participants were women > 16 years of age with regular menstrual cycles (21‐35 days), who requested EC within 120 h of unprotected sexual intercourse. Advice was given to women to avoid further episodes of unprotected sexual intercourse within that cycle. Women with more than 1 episode of unprotected sexual intercourse within 120 h of presentation were also included in the study 2065 women recruited; 2043 women included in the data analysis. Mife: 1022 women; LNG: 1021 women. Treatment outcome for women was known for 860 women (84.2%) in the Mife group and 858 (84.1%) in the LNG group | |
| Interventions | Mife 10 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number tables" |
| Allocation concealment (selection bias) | Low risk | "Women were randomized to receive a single tablet of mifepristone 10 mg or 2 tablets of levonorgestrel, 750 ug given 12 hours apart, by opening sequentially numbered opaque sealed envelopes prepared using random number tables. The randomization envelopes were prepared in the Family Planning Clinic in Aberdeen by a health care assistant not involved in the recruitment or data collection." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "The study was not blinded, and both medical staff and patients were aware of the treatment assigned." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up explained |
| Selective reporting (reporting bias) | Low risk | Planned outcomes of pregnancy, adverse effects and bleeding reported |
| Other bias | Low risk | None detected |
Han 1995.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 139 women attending the outpatient clinic of a hospital in Beijing, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, orally, 2 doses, 12 h apart vs anordrin 7.5 mg, orally, 2 doses, 12 h apart vs Mife 25 mg + anordrin 7.5 mg, orally, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome was reported |
| Other bias | Low risk | None detected |
Han 1996.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 300 healthy women in Beijing, China, with regular menstrual periods, aged 18‐48 years, with attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, orally, 2 doses, 12 h apart vs Mife 25 mg, orally, single dose, vs Mife 25 mg + anordrin 7.5 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Planned outcome was reported |
| Other bias | Low risk | None detected |
Han 1999a.
| Methods | Women 'randomly allocated' into 2 groups in a 2:1 ratio. Method of randomisation not reported | |
| Participants | 214 women aged 21‐45 years attending the obs/gyn clinic Chao Yang Hospital, Beijing, China. Women had regular menstrual periods and unprotected intercourse within 72 h of attending the clinic | |
| Interventions | LNG 0.75 mg, 2 doses, 12 h apart vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome was reported |
| Other bias | Low risk | None detected |
Han 2001a.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 100 women attending a hospital clinic in Shanghai, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife single dose 25 mg vs Mife 10 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss to follow‐up and exclusions reported |
| Selective reporting (reporting bias) | Low risk | Planned outcome was reported |
| Other bias | Low risk | None detected |
He 2002.
| Methods | Randomised, double‐blind, multicentre trial. Random number generation done centrally, double‐blinded by use of identical placebos | |
| Participants | 400 healthy women recruited into study from family planning clinics in Shanghai, China Included women with regular menstrual periods (24‐42 days), who had a single act of unprotected intercourse within 120 h of attending the clinic, and they were willing to avoid further acts of unprotected coitus during that cycle and willing to have an induced abortion if pregnancy was diagnosed following intake of the study drug during the study period Excluded women: current pregnancy or breastfeeding, on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period and no contraindication to use of Mife or tamoxifen |
|
| Interventions | Mife (single dose) 10 mg + placebo vs Mife 10 mg + tamoxifen 20 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were allocated randomly to one of the two treatment groups using a computer‐generated random number table". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Neither the participants nor the investigator knew which treatment was received. The tablets of mifepristone and placebo or tamoxifen were swallowed in the presence of a member of the study team who could record the date and time when they were taken." "Double blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Explained loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Ho 1993.
| Methods | Women 'randomly allocated' to 2 groups. A random number table used to generate the allocation sequence and allocation was done by sealed envelopes. Placebos were not used. Side effects were recorded by women | |
| Participants | 880 healthy women attending Family Planning Association clinics in Hong Kong Included women with regular menstrual periods (21‐35 days), aged 18‐45 years, with a single act of unprotected intercourse within 48 h of attending the clinic |
|
| Interventions | Yuzpe (ethinyl oestradiol 100 μg + norgestrel 1 mg, repeated after 12 h) vs LNG 0.75 mg, orally, 2 doses 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were 'randomly allocated' into 2 groups ‐ doesn't state how |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported loss to follow‐up‐ "Of these, 16 subjects in the Yuzpe group and 30 subjects in the levonorgestrel group were lost to follow up after the first visit and the results of treatment were not known. These subjects were excluded from the analysis." |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were reported |
| Other bias | Low risk | None detected |
Hoseini 2013.
| Methods | Women were included in the double‐blind, controlled trial and randomly assigned into 2 groups | |
| Participants | 529 participants aged 15‐49 having regular menses (having regular menstrual cycles of 24‐42 days) and one act of unprotected intercourse within 72h were included in the trial in 2006‐2007 in Iran; breast‐feeding women were also included provided that their baby was older than 6 months. Exclusion criteria were breast‐feeding women with their baby younger than 6 months, hormonal contraindication in their current cycle, use of hormonal contraceptives, uncertainty about the time of last menstrual period, and suspected pregnancy |
|
| Interventions | LNG 0.75 mg, 2‐dose regimen vs Yuzpe regimen | |
| Outcomes | Observed number of pregnancies, specific side effects and changes in menstrual pattern | |
| Notes | 1. Observed pregnancy/total number of women: Yuzpe 3/266; LNG 4/263 2. Balanced block randomisation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were then classified according to balanced block randomisation." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Neither the obstetrics, nor the participants were aware of the type of tablets in each set (double blind). Only the person responsible for randomization was aware of the contents of the sets according to the serial numbers stuck on the sets." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of any loss to follow‐up or how many were analysed |
| Selective reporting (reporting bias) | Low risk | Planned outcomes in the protocol were reported |
| Other bias | Low risk | None detected |
Hu 2003.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not recorded | |
| Participants | 240 women attending the clinic in a general hospital, Zhejiang, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | LNG 0.75 mg, 2‐dose regimen vs Mife 25 mg single‐dose orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Jin 2012.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 160 women attending the family planning service site, Anhui, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the service site | |
| Interventions | Mife 25 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes | 1. Post‐randomisation exclusions or loss to follow‐up not reported 2. Observed pregnancy/total number of women: LNG 5/80; Mife 10/80 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Lai 2004.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 300 women attending the gyn clinic in a general hospital, Qinghai, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 10 mg vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation exclusion and loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Lan 2006.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 200 women attending in obs/gyn clinic, No. 8 People's Hospital, Wenzhou, Zhejiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending clinic | |
| Interventions | Mife 5 mg vs Mife 10 mg single dose orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Lei 2013.
| Methods | Women randomly allocated into 2 groups. Method of randomisation not reported | |
| Participants | 132 women attending a maternity and child health hospital, Chengdu, Sichuan, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the hospital | |
| Interventions | Mife 10 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Li 2000a.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 234 women attending the clinic in an MCH hospital, Hainan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, single dose vs LNG 0.75 mg, 2‐dose regimen, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Li 2000b.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 160 women attending a family planning clinic in Tianjing, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 50 mg vs Mife 25 mg single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Li 2000c.
| Methods | Women 'randomly allocated' to 2 groups | |
| Participants | 90 women attending a clinic in Heilongjiang, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 150 mg vs Mife 50 mg vs Mife 25 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and change in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Li 2002a.
| Methods | Women 'randomly allocated' to 2 groups | |
| Participants | 255 women attending the family planning clinics in Guizhou, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 10 mg orally, single dose vs LNG 0.75 mg orally, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and change in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Li 2005b.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 202 women attending the gyn clinic in a general hospital, Guangxi, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs LNG 0.75 mg, 2‐dose regimen, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
Liang 2001.
| Methods | Women 'randomly allocated' to 2 groups | |
| Participants | 400 women attending an MCH hospital clinic in Heilongjiang, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg orally vs LNG 0.75 mg orally, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies and side effects | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Liao 2003.
| Methods | Women 'randomly allocated' to 2 groups | |
| Participants | 200 women attending a reproductive medical clinic in Wuhan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg orally vs LNG 0.75 mg orally, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and change in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Lin 2000.
| Methods | Double‐blind randomised trial. Method of randomisation not reported | |
| Participants | 120 women attending a family planning clinic in Tianjing, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg + placebo, 12 h apart vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Liu 2000.
| Methods | Randomised, double‐blind, multicentre trial. Random number generation done centrally, double‐blinded by use of identical placebos | |
| Participants | 100 healthy women recruited in the study from Henan Research Institute for family planning Included women with regular menstrual periods, who had had a single act of unprotected intercourse or had had multi‐intercourse but attended the clinic within 72 h of the first one Excluded women who were breastfeeding, on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period |
|
| Interventions | Mife (single dose) 10 mg vs LNG 0.75 mg, 2 doses, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation done centrally |
| Allocation concealment (selection bias) | Low risk | Randomised, double‐blind, multicentre trial. Random number generation done centrally, double‐blinded by use of identical placebos |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Liu 2001.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 142 women attending the gyn clinic in a general hospital, Sichuan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, 2‐doses, 12 h apart vs anordrin 7.5 mg, 12 h later repeat 1 dose, then 7.5 mg per night for 10 days | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Liu 2002b.
| Methods | Women 'randomly allocated' into 2 groups in a 2:1 ratio. Method of randomisation not reported | |
| Participants | 285 women attending the gyn clinic in a general hospital, Hubei, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 50 mg orally vs Cu‐IUD | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Liu 2009.
| Methods | Women randomly allocated to 2 groups. The method of randomisation was not described | |
| Participants | 280 women attending a family planning clinic, Wangdu, Hebei, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 25 mg, single dose vs LNG 0.75 mg, 2 doses, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Lou 2002.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 283 women attending the gyn clinic in a general hospital, Zhejiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 50 mg vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Lou 2005.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 142 women attending the gyn clinic in a general hospital, Sichuan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg + anordrin 5 mg vs Mife 10 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Miras 2014.
| Methods | A prospective, longitudinal, observational study. Women were randomly selected into 2 groups. The study was performed double blind. | |
| Participants | 300 women attending Eusebio Hernandez Teaching Hospital in Havana from 1 January 2011 to 1 September 2012 Criteria for inclusion and exclusion were clear (detailed in France) |
|
| Interventions | Mife 10 mg vs Mife 5 mg, both single dose, orally | |
| Outcomes | Observed number of pregnancies | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Finally, a sample of 300 women was chosen by simple random sampling without replacement"‐ doesn't elaborate on method. Also claims to be a longitudinal, observational study but also randomised |
| Allocation concealment (selection bias) | Unclear risk | Doesn't explain allocation concealment method |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States that it is double‐blinded but doesn't elaborate how/who |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Doesn't appear to refer to loss to follow‐up etc |
| Selective reporting (reporting bias) | Unclear risk | Doesn't state planned outcomes in methods to check in results |
| Other bias | Unclear risk | Hard to assess as paper is in Spanish |
Ngai 2005.
| Methods | The pharmacy department in Queen Mary Hospital generated the randomisation sequence by computer programme. Drug package was prepared by the pharmacy department according to the randomisation list. Clinicians and participants were unaware of the drug assignment. The pharmacy kept the randomisation list and it was revealed only at the final analysis. LNG and placebo were supplied by the WHO. Placebo was identical in colour, shape and size to LNG | |
| Participants | 2071 healthy women aged > 16 years were recruited from 5 sites in China (Beijing, Hong Kong, Nanjing, Shanghai and Shenzhen). All women had regular menstrual cycles (every 24‐42 days) and requested EC within 120 h of a single act of unprotected intercourse; they were willing to abstain from further acts of unprotected intercourse and were available for follow‐up over the next 6 weeks. Exclusion criteria: post‐abortion or post‐partum women whose period had not yet returned, regular use of prescription drugs before admission to the study and intercourse during the treatment cycle > 120 h before admission into the study. Women satisfying these criteria were admitted into the study after they had given written informed consent. 2060 women into efficacy analysis, 2071 women into safety analysis |
|
| Interventions | LNG 0.75 mg, 2 doses, 24 h apart orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The pharmacy department in Queen Mary Hospital generated the randomization sequence by computer program." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method wasn't explained |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The levonorgestrel and the placebo was supplied by the World Health Organization. The placebo was identical in colour, shape and size to the levonorgestrel." "Both the clinicians and the participants were unaware of the drug assignment. The pharmacy kept the randomization list and it was revealed only at the final analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were reported |
| Other bias | Low risk | None reported |
Pei 2001.
| Methods | Women 'randomly allocated' to 2 groups | |
| Participants | 200 women attending a hospital clinic in Shanxi, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg orally vs LNG 0.75 mg orally, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and change in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Qi 2000a.
| Methods | Double‐blind, randomised, multicentre trial Random number generation done centrally. Double‐blinded by use of identical placebos | |
| Participants | 1209 women attending the family planning clinics in 11 provinces of China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs Mife 10 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation done centrally |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Qi 2003.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 288 women attending the gyn clinic in a general hospital, Qinghai, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, single dose vs LNG 0.75 mg, 2‐dose regimen, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusions or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Qian 1999.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 252 women attending a family planning clinic in Shenzhen, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 150 mg orally, single dose vs Mife 50 mg vs Mife 25 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Rowlands 1983.
| Methods | Women randomly allocated to 2 treatments. Side effects assessed through interviews with the women | |
| Participants | 101 healthy women attending a family planning clinic (Margaret Pyke Centre) in London, UK Included women who had had unprotected intercourse within 120 h (included some women who had had multiple acts of unprotected intercourse) |
|
| Interventions | Yuzpe (ethinyl oestradiol 100 μg + norgestrel 1 mg, repeated after 12 h) vs danazol 400 mg, repeated after 12 h | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not stated "Patients were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No explanation of blinding was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "One woman was lost to follow‐up. Data for six further women are incomplete as they were not seen at follow‐up; contact was made by telephone or post." Explains lost numbers |
| Selective reporting (reporting bias) | Unclear risk | Planned outcomes to report were not clearly outlined in the methods and no protocol available. |
| Other bias | Low risk | None detected |
Sang 1999.
| Methods | Single‐blind randomised trial. Power calculation reported | |
| Participants | 2400 women attending urban hospital and family planning clinics in 5 cities in China Included only women who came after 24 h to 96 h of unprotected intercourse Excluded women who had irregular menstrual periods, multiple acts of intercourse, who had been using other oral contraceptives and whose normal menses had not resumed after an abortion or delivery |
|
| Interventions | Mife 25 mg vs Mife 25 mg + anordrin 7.5 mg vs Mife 10 mg + anordrin 5 mg vs Mife 10 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation exclusions and loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Shao 2010.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 102 women attending in a Chinese traditional medicine hospital, Tonglu, Zhejiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 25 mg single dose vs LNG 0.75 mg 2‐dose regimen | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Sheng 2002.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 200 women attending a family planning centre, Jiangsu, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg single dose vs LNG 0.75 mg, 2‐dose regimen, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Sheng 2008.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 200 women attending in a family planning clinic, Tongxiang, Zhejiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | LNG‐COC, 4 tablets (total ethinyl oestradiol 0.12 mg and LNG 0.6 mg) 2‐dose, 12 h apart, orally vs LNG 0.75 mg, 2‐dose, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Su 2001.
| Methods | Women who had had unprotected intercourse within the preceding 72 h were 'randomly allocated' to Mife or LNG groups, and women who had had unprotected intercourse within the previous 72 h‐120 h were assigned to an IUD group. Randomisation took place between 2 types of pills | |
| Participants | 315 women attending a hospital clinic, Baotou, China. Women had regular menstrual periods and unprotected intercourse once within 72 to 120 h (in the case of IUDs) | |
| Interventions | Mife 25 mg, single dose vs LNG 0.75 mg, twice, orally vs Cu‐IUD This study had three treatment arms, but the Cu‐IUD comparison was not randomized. Hence, we excluded this comparison and included only the mifepristone vs levonorgestrel comparison |
|
| Outcomes | Observed number of pregnancies | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomization but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Sun 2000.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 200 women attending a family planning clinic in Haerbing, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, single dose, orally vs LNG 0.75 mg, orally, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Sun 2003.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 60 women attending the clinic in a general hospital, Hubei, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs LNG 0.75 2‐dose 12 h apart orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Sun 2007.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 1100 women attending in a village clinic, Miyun county, Beijing, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | LNG 0.75 mg, 2‐dose, 12 h apart, orally vs LNG‐COC 4 tablets (total ethinyl oestradiol 0.12 mg and LNG 0.6 mg), 2‐dose, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Tan 1999.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 145 women (aged 18‐47 years) attending the family planning clinics in Guangzhou, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 12.5 mg orally, 2 doses, 12 h apart vs Mife 25 mg orally, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Tan 2003.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 150 women attending the clinic in a general hospital, Hubei, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 12.5 mg vs Mife 25 mg, 2‐dose, 12 h apart vs Mife 150 mg orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Tao 2014.
| Methods | Women randomly allocated into 2 groups. Method of randomisation not reported | |
| Participants | 150 Women attending the maternity and child health hospital in Hubei Included sexually active women aged 18‐45 years having regular menstrual cycles of 30 ± 7 days. Women had a single act of unprotected intercourse within 120 h of attending the hospital Exclusion criteria were breast‐feeding women, hormonal contraindication in their current cycle, use of hormonal contraceptives, having irregular menstrual cycles |
|
| Interventions | Mife 30 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Tian 2013.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 110 women attending the family planning service centre, Anyang, Henan, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the service centre | |
| Interventions | Mife 50 mg, single dose, orally vs IUD | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Van Santen 1985a.
| Methods | Randomised, double‐blind trial. Random number sequence generated from a random number table. A numbered strip containing the capsules given to participating women. Masking achieved by giving each woman the active and corresponding placebo treatments. Side effects were assessed by women | |
| Participants | 465 healthy women attending Utrecht State University Hospital, the Netherlands Included women with regular menstrual periods, who had had a single act of unprotected intercourse Excluded women who were breastfeeding, on medications and difficult to follow up |
|
| Interventions | Yuzpe (ethinyl oestradiol 100 μg + norgestrel 1 mg, repeated after 12 h) on day 1 + placebo capsules for 4 days vs ethinyl oestradiol 5 mg dose followed by a placebo capsule 12 h later followed by ethinyl oestradiol 5 mg single daily dose for 4 days | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "By using a table of random numbers, 465 women were given one of the two treatments." |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not explained |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double blinded". "In the high‐dose estrogen treatment, the second capsule was inert, whereas in the EE + NG combination treatment, the capsules to be taken after the first day were placebo. In this fashion strips of wrapped capsules with a similar appearance were used throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Exact number of loss to follow‐up is not clear |
| Selective reporting (reporting bias) | Low risk | Planned outcome of pregnancy and side effects were reported and I am unclear on how they chose how many they analysed in each outcome |
| Other bias | Low risk | None detected |
von Hertzen 2002.
| Methods | Randomised, double‐blind, multicentre trial. Random number generation done centrally, double‐blinded by use of identical placebos. Allocation concealment achieved by sealed, sequentially numbered, treatment packs | |
| Participants | 4136 healthy women recruited in the study from 15 family planning clinics in 10 countries Included women with regular menstrual periods, aged 14‐52 years, who had attended the clinic within 120 h of a single act of unprotected intercourse Excluded women who were breastfeeding, on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period |
|
| Interventions | Mife 10 mg, single dose vs LNG 1.5 mg, single dose vs LNG 0.75 mg, 2 doses, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a computer‐generated randomization sequence developed by WHO to assign participants in each centre to one of three treatment groups: single dose mifepristone; single dose levonorgestrel; or two dose levonorgestrel. Each centre received assignments by randomly‐permuted blocks with a fixed block size of 10." |
| Allocation concealment (selection bias) | Low risk | "Allocation was concealed by the use of sealed, sequentially numbered treatment packs, which were filled and labelled in accordance with the list of randomization for each centre by Labatec, Geneva, Switzerland." "only the person who prepared the random lists had access to them." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "women received two 5 mg tablets of mifepristone and two placebo tablets identical in appearance to mifepristone." "Clinicians, participants and investigators were unaware of drug assignments and this double‐blinding was maintained until after the final analysis; only the person who prepared the random lists had access to them." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analysis was by intention to treat" Loss to follow‐up was explained. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes results reported |
| Other bias | Low risk | None detected |
Wang 1999.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 108 women attending the obs/gyn clinic in Tianjing No. 1 People's Hospital, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg orally, 2 doses, 12 h apart vs anordrin 7.5 mg on the first day, 2 doses, 12 h apart, then 7.5 mg/day for 10 days, total dosage of anordrin 90 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2000a.
| Methods | Women were given choice for Cu‐IUD or ECPs and those choosing ECPs were randomly allocated to 2 ECP groups. Method of randomization not reported | |
| Participants | 150 women attending the family planning clinics in Shandong, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 10 mg single dose vs LNG 0.75 mg, 2 doses, 12 h apart As noted above, this study had three treatment arms, but the Cu‐IUD comparison was not randomized. Hence, we excluded this comparison and included only the mifepristone vs levonorgestrel comparison |
|
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomization but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2000b.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 131 women attending an MCH hospital in Guangdong, China Included women who had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse |
|
| Interventions | LNG 0.75 mg, 2 doses, 12 h apart vs Mife 25 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2001.
| Methods | Randomised, double‐blind, multicentre trial. Random number generation done centrally, double‐blinded by use of identical placebos | |
| Participants | 200 healthy women recruited in the study from an obs/gyn clinic in Wuhan, China Included women with regular menstrual periods, aged 22‐42 years, who had attended the clinic within 72 h of a single act of unprotected intercourse Excluded women who were on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period |
|
| Interventions | Mife 10 mg, single dose vs Mife 25 mg, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation done centrally |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2003.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 262 women attending the clinic in an MCH hospital, Shanxi, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs LNG 0.75 mg, 2‐dose regimen, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2004.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 1200 women attending the gyn clinic in a general hospital, Shandong, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 12.5 mg vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2006a.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 198 women attending the gyn clinic in a general hospital, Anhui, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg vs Mife 25 mg, orally, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2008.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 100 women attending in an obs/gyn clinic, No. 5 hospital, Haerbin Medical University, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the clinic | |
| Interventions | Mife 25 mg vs Mife 10 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wang 2012.
| Methods | Women randomly allocated into 2 groups. Method of randomisation not reported | |
| Participants | 154 women attending a family planning service site, Shandong, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the hospital | |
| Interventions | Mife 20 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Webb 1992.
| Methods | Women 'randomly allocated' to 3 groups. Random number generation by computer. Schedule prepared by someone not involved in recruitment and outcome assessment. No blinding or use of placebos reported. Side effects were recorded by women | |
| Participants | 616 healthy women attending a community family planning clinic in Liverpool, UK Included women with regular menstrual periods (21‐35 days), aged 16‐45 years, with attended the clinic within 72 h of a single act of unprotected intercourse |
|
| Interventions | Yuzpe (ethinyl oestradiol 100 μg + norgestrel 1 mg, repeated after 12 h) vs danazol 600 mg, twice, 12 h apart vs Mife 600 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The allocation sequence was constructed by using a computer based pseudo‐random number generator with a uniform distribution." |
| Allocation concealment (selection bias) | Unclear risk | "The schedule was prepared before the start of the study by JR, who did not participate in either the selection or assessment of women." No mention of method of concealment so unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding or placebo reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attempts to explain loss to follow‐up but the numbers actually analysed don't add up to those remaining |
| Selective reporting (reporting bias) | Low risk | Planned outcomes were reported |
| Other bias | Low risk | None detected |
Wei 2002a.
| Methods | Randomised double‐blind trial by use of identical placebos | |
| Participants | 200 women attending the gyn clinic in a general hospital, Hainan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs Mife 10 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wei 2011.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 100 women attending in a clinic, Anhui, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs Mife 10 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
WHO 1998.
| Methods | Randomised, double‐blind, multinational trial. Random number generation done centrally. Double‐blinded by use of identical placebos. Allocation concealment achieved by sealed, sequentially‐numbered, tinted bottles, filled and labelled by the manufacturer | |
| Participants | 1998 healthy women at 21 centres worldwide Included women with regular menstrual periods, aged 18‐45 years, who had attended the clinic within 72 h of a single act of unprotected intercourse Excluded women who were breastfeeding, on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period 1955 women into the final analysis |
|
| Interventions | Yuzpe (ethinyl oestradiol 100 μg + LNG 0.50 mg, repeated after 12 h) vs LNG 0.75 mg, twice, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The unit of randomisation was the individual woman. We used a computer generated randomisation sequence developed in Geneva to assign participants to treatment groups. Each centre received assignments by random permuted blocks with a fixed block size of ten." |
| Allocation concealment (selection bias) | Low risk | "The allocation was concealed by use of sealed, sequentially numbered, tinted pill bottles, which were filled and labelled by the manufacturer." "The allocation sequence was kept in Geneva, and assignments were not revealed to investigators or participants during the trial." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Clinicians and participants were unaware of the next assignment." "Double blinding was maintained throughout the trial. Each pill bottle contained two identical tablets. Bottles containing a levonorgestrel tablet had an identical placebo tablet. The supplier formulated, especially for the trial, tablets containing the Yuzpe regimen, of identical appearance to the levonorgestrel tablets." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We analysed the data by intention to treat." Also explained loss to follow‐up reasons |
| Selective reporting (reporting bias) | Low risk | Reported planned primary and secondary outcomes |
| Other bias | Low risk | None detected |
WHO 1999.
| Methods | Multinational RCT. Randomisation sequence was generated centrally at the WHO and women randomised to 3 groups within centres. Sequentially‐numbered bottles, each containing 3 pills were given to women at the centre. Each bottle contained the active and placebo pills accordingly. However, 200 mg pills were slightly larger and, therefore, not all pills were identical. Power calculation was made | |
| Participants | 1717 women attending family planning clinics in 11 centres in 6 countries Included women with regular menstrual cycles, within 120 h of a single act of unprotected intercourse and who were willing to avoid intercourse for the rest of the current cycle Excluded women who were breastfeeding, with uncertain date of last menstrual period, use of hormonal contraception in the current cycle and those with a contraindication to Mife use. 1684 women included in the final analysis |
|
| Interventions | Mife 600 mg vs Mife 50 mg vs Mife 10 mg. All taken orally as a single dose at the time of enrolment | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used a computer generated randomisation sequence developed by WHO staff to assign participants to treatment groups within centres. Each centre received assignments by randomly permuted blocks with a fixed block size of nine." |
| Allocation concealment (selection bias) | Low risk | "The manufacturer supplied sequentially numbered bottles of pills for each participating centre, according to the randomisation sequence. We attempted to maintain allocation concealment by having three pills in each bottle; two 5 mg tablets plus one placebo tablet for the 10 mg dose; one 50 mg tablet plus two placebo tablets for the 50 mg dose; and three 200 mg tablets for the 600 mg dose. Each bottle was sealed and labelled sequentially with the number of the centre, participant number, and expiry date." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Each pill bottle contained three white tablets. The 200 mg tablets were somewhat larger than the 50 mg and 5 mg tablets or placebos. Clinicians and participants were not told the composition of the three pills." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Allocations were by intention to treat." |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported (unintended confirmed pregnancy, side‐effects and delay in the onset of next menses) |
| Other bias | Low risk | None detected |
Wu 1999a.
| Methods | Double‐blind, randomised trial. Random number generation done centrally. Double‐blinded by use of identical placebos. Allocation concealment achieved by sealed, sequentially numbered, tinted bottles filled and labelled by the manufacturer | |
| Participants | 1324 women in 16 urban family planning clinics in China Included only women who came within 72 h of unprotected intercourse Excluded women with irregular menstrual periods, with multiple acts of intercourse, on oral contraceptives and post‐abortal women whose menstrual periods had not returned to normal |
|
| Interventions | LNG 0.75 mg, 2 doses, 12 h apart vs Mife 10 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation done centrally |
| Allocation concealment (selection bias) | Low risk | Allocation concealment achieved by sealed, sequentially numbered, tinted bottles filled and labelled by the manufacturer |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation exclusion and loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wu 2002.
| Methods | Randomised, double‐blind multicentre trial. Random number generation done centrally, double‐blinded by use of identical placebos. Allocation concealment achieved by sealed, sequentially‐numbered, tinted bottles filled and labelled by manufacturer | |
| Participants | 903 healthy women recruited in the study from 10 clinics in Shanghai, China Included women with regular menstrual periods (22‐42 days), who had a single act of unprotected intercourse within 120 h of attending the clinic and they were willing to avoid further acts of unprotected coitus during that cycle and willing to have an induced abortion if pregnancy was diagnosed following intake of the study drug during the study period Excluded women with current pregnancy or breastfeeding, on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period |
|
| Interventions | Mife 25 mg, 24 h later misoprostol 0.2 mg vs Mife 10 mg, 24 h later misoprostol 0.2 mg vs Mife (single dose) 10 mg + placebo | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation done centrally |
| Allocation concealment (selection bias) | Low risk | Allocation concealment achieved by sealed, sequentially‐numbered, tinted bottles, filled and labelled by manufacturer |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation exclusion and loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Wu 2010.
| Methods | Women randomly allocated to 2 groups. Method of randomisation, double‐blind trial was reported | |
| Participants | 998 healthy women with regular menstrual cycles and negative urine pregnancy tests who were requesting emergency contraception up to 72 h after unprotected coitus to receive single‐dose gestrinone 10 mg or Mife 10 mg | |
| Interventions | Gestrinone: 4 gestrinone 2.5 mg capsules, and 1 placebo tablet identical in appearance to Mife Mife: 1 Mife 10 mg tablet and 4 placebo capsules identical in appearance to gestrinone |
|
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization sequence was computer generated by WHO and stratified by clinic. Randomization was performed using randomized blocks so that the chance of being assigned to either to either treatment group was equal in each block." |
| Allocation concealment (selection bias) | Low risk | "All clinics received sets of opaque, sealed envelopes containing the randomly allocated emergency contraception treatment pack assigned to a given participant number. When a woman was assigned to a participant number, the envelope containing the emergency contraception tablets with the corresponding participant number was given to her." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Each envelope contained one tablet and four capsules. Participants assigned to the gestrinone group received four 2.5 mg gestrinone capsules and one placebo tablet identical in appearance to mifepristone." "The clinicians, participants and investigators were blinded to the drug assignments. Double‐blinding was maintained until after the final analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The final analysis excluded two women (one gestrinone group, one mifepristone group) who were lost to follow‐up; thus, 498 participants in each group were included in the final analysis." |
| Selective reporting (reporting bias) | Low risk | Planned outcomes of pregnancy rate, side effects and menstrual bleeding in protocol were reported |
| Other bias | Low risk | None detected |
Xiao 2002.
| Methods | Randomised, double‐blind, multicentre trial. Random number generation done centrally Double‐blinded by use of identical placebos |
|
| Participants | 3052 healthy women recruited in the study from 10 centres in China Included women with regular menstrual periods, aged 19‐49 years, who had a single act of unprotected intercourse within 120 h of attending the clinic Excluded women who were breastfeeding, on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period 3030 into efficacy analysis, 3033 into safety analysis |
|
| Interventions | Mife (single dose) 10 mg vs Mife 25 mg, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation done centrally |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation exclusion and loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Xie 1998.
| Methods | Women randomly allocated to 3 groups. Method of randomisation not reported | |
| Participants | 600 women attending an urban MCH Hospital in Fuzhou, China Excluded women attending after 72 h, irregular menstrual periods and who had had multiple acts of intercourse |
|
| Interventions | Mife 150 mg vs Mife 50 mg vs Mife 25 mg, all single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion or loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Xie 2010.
| Methods | Women allocated to 3 groups. The method was not reported | |
| Participants | 120 women attending a family planning clinic, Shenzhen, China | |
| Interventions | Mife 25 mg, single dose vs Mife 10 mg, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Xu 2000a.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 266 women attending a family planning centre, Jianfsu, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs anordrin 7.5 mg, 12 h late repeat 1 dose, then 7.5 mg per night for 8 days vs LNG 0.75 mg, 2‐dose regimen | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss of follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Xu 2000b.
| Methods | Women randomly allocated to 2 groups. Method of randomisation not reported | |
| Participants | 400 women attending the family planning clinic in Zhejiang, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, single dose vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss of follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Yang 2001.
| Methods | Women 'randomly allocated' to 4 groups. Method of randomisation not reported | |
| Participants | 358 healthy women recruited into the study from clinics of MCH hospital in Guangzhou, China Included women with regular menstrual periods, aged 17‐46 years, who had attended the clinic within 72 h of a single act of unprotected intercourse and they were willing to use condom for further acts of unprotected coitus during that cycle Excluded women on hormonal contraception in the current cycle and those with uncertain dates of last menstrual period |
|
| Interventions | Mife 25 mg twice, 12 h apart vs anordrin 7.5 mg, twice, 12 h apart vs danazol 400 mg, twice, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Yang 2003.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 92 women attending the clinic in a general hospital, Hunan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs Mife 50 mg orally, single dose | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss of follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Ye 2013.
| Methods | Women randomly allocated into 2 groups. Method of randomisation not reported | |
| Participants | 120 women attending a family planning service site in Zhejiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the service site | |
| Interventions | Mife 25 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zeng 2007.
| Methods | Women allocated to 2 groups. The method of allocation was not reported | |
| Participants | 100 women attending in a county hospital, Zhejiang, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 25 mg + MTX 5 mg vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zeng 2008.
| Methods | Women randomly allocated to 2 groups. The method of randomisation not reported | |
| Participants | 100 women attending in an MCH hospital, Wuhua county, Guangzhou, China. Women had regular menstrual periods and a single act of unprotected intercourse within 120 h of attending the clinic | |
| Interventions | Mife 10 mg vs Mife 25 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 1998.
| Methods | Randomised trial. Method of randomisation not reported | |
| Participants | 309 women attending family planning clinics in Beijing, China Included only women attending within 72 h of an unprotected intercourse Excluded women with irregular menstrual periods, who used oral contraceptives and those who had not resumed normal menses after an abortion or delivery |
|
| Interventions | Mife 25 mg vs Mife 10 mg vs Mife 5 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 1999b.
| Methods | Women 'randomly allocated' into 3 groups. Method of randomisation not reported | |
| Participants | 360 women attending the family planning clinics in Chengwu (a county in Shandong), China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg orally, 2 doses, 12 h apart vs Mife 10 mg, for 5 days vs Mife 10 mg, for 3 days | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 2000.
| Methods | Women 'randomly allocated' into 4 groups | |
| Participants | 782 women attending a hospital clinic in Qinhai, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg, 2 doses, 12 h apart vs LNG 0.75 mg, 2 doses, 12 h apart vs Mife 25 mg, single dose vs Mife 25 mg + LNG 0.75 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 2002a.
| Methods | Women 'randomly allocated' to 2 groups. Method of randomisation not reported | |
| Participants | 116 women attending the gyn clinic in a general hospital, Sichuan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg + anordrin 5 mg vs Mife 25 mg | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 2002b.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 135 women attending the clinic in a general hospital, Henan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 100 mg vs Mife 50 mg vs Mife 10 mg, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 2005.
| Methods | Double‐blind, randomised, single‐centre trial | |
| Participants | 220 women attending the gyn clinic in a general hospital, Guangdong, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg, single dose vs Mife 10 mg, 2‐dose, 12 h apart, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 2012.
| Methods | Women randomly allocated into 2 groups. Randomised digital table method was used for randomisation | |
| Participants | 130 women attending a ob/gyn department in a general hospital in Shanghai, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the hospital | |
| Interventions | Mife 20 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised digital table method was used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of post‐randomisation exclusion and loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhang 2014.
| Methods | Women randomly allocated into 2 groups. Method of randomisation not reported | |
| Participants | 112 women attending a maternity and child health hospital in Liaoning, China. Women had regular menstrual periods and a single act of unprotected intercourse within 72 h of attending the hospital | |
| Interventions | Mife 25 mg, single dose, orally vs LNG 0.75 mg, 2 doses, 12 h apart | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zhao 2003.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 270 women attending the gyn clinic in a general hospital, Shandong, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 50 mg vs Mife 25 mg vs Mife 10 mg, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Unclear risk | None detected |
Zheng 2005.
| Methods | Women 'randomly allocated' to 3 groups. Method of randomisation not reported | |
| Participants | 200 women attending the gyn clinic in a general hospital, Hunan, China. Women had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 25 mg vs Mife 600 mg, single dose, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned randomisation but description not adequate |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion and loss to follow‐up not reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
Zuo 1999.
| Methods | Double‐blind, randomised trial Random number generation done centrally. Double‐blinded by use of identical placebos | |
| Participants | 668 women recruited from 14 family planning clinics in Changsha, China. Women aged < 40 years had regular menstrual periods and attended the clinic within 72 h of a single act of unprotected intercourse | |
| Interventions | Mife 10 mg, single dose vs Mife 25 mg, orally | |
| Outcomes | Observed number of pregnancies, side effects and changes in menstrual pattern | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generation done centrally |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not mentioned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | Reported planned outcomes |
| Other bias | Low risk | None detected |
COC: combined oral contraceptive; Cu‐IUD: copper intrauterine device; EC: emergency contraception; ITT: intention to treat; IUD: intrauterine device; LNG: levonorgestrel; MCH: maternal and child health; Mife: mifepristone; MTX: methotrexate; RCT: randomized controlled trial; UPA: ulipristal acetate; WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ashok 2001 | Not an RCT or quasi‐RCT |
| Ashok 2004 | It is the same clinical trial as Ashok 2002. The objective of this paper was to compare side effects, women's acceptance and satisfaction with Mife 100 mg vs the Yuzpe regimen for EC |
| Ban 2001 | Not an RCT |
| Benagiano G 2010 | Not an RCT |
| Brache 2013 | Pooled data meta‐analysis of LNG vs UPA as EC |
| Byamugisha 2010 | RCT to compare LNG vs Yuzpe in 4 clinics. The primary objective of this study was to determine side effects and acceptability of 2 ECP regimens among users in Kampala, Uganda. There was no effectiveness result in the data and the side effects were assessed on a semi‐quantitative scale |
| Chen 2011 | Phase IV clinical trial |
| Chen 2012 | Not RCT |
| Creinin 1997 | Meta‐analysis, not a clinical trial |
| D'Souza 2003 | An RCT in an outpatient clinic setting. Objective was to assess insertion‐linked pain and the short‐term user‐acceptability and safety of the GyneFix as compared with T‐framed IUDs. No effectiveness result mentioned in this paper |
| Dixon 1980 | Comparative study of ethinyl oestradiol 5 mg/day and conjugated oestrogens at 30 mg/day for 5 days. The study was conducted in 5 centres, 2 of which prescribed the drugs alternately. In these 2 centres, none of the 137 women who received ethinyl oestradiol became pregnant while 6 out of 132 women receiving conjugated oestrogens became pregnant. No other details were available for these centres |
| Dong 2007 | An observational study on Mife vs LNG vs Cu‐IUD for EC, not an RCT |
| Ellertson 2003b | An observational study, not an RCT |
| Espinos 1999 | Not an RCT |
| Fan 1998 | Not an RCT 518 women used Mife 25 mg + anordrin 7.5 mg for EC, 1 observed pregnancy/40 expected pregnancies |
| Fan 2001b | Not an RCT 1013 women used Cu‐IUD for EC, 2 women got pregnant |
| Fasoli 1989 | Review paper |
| Fine 2010b | A prospective, multicentre, open‐label study to evaluate the effectiveness and safety of UPA as EC in women presenting 48‐120 h after unprotected intercourse. 1241 women from 45 planned parenthood clinics were treated with a single dose of UPA 30 mg |
| Gan 1999 | Not an RCT 200 women used Mife 10 mg for EC, 2 observed pregnancies/15 expected pregnancies |
| Gan SX 2001 | No mention of random allocation |
| Gao 2001 | Not an RCT |
| Glasier 2013 | pooled data meta‐analysis of LNG vs UPA for EC |
| Gottardi 1979 | Not an RCT |
| Gottardi 1986 | Not an EC study |
| Gu 2002 | Not an RCT |
| Guillebaud 1983 | Randomised and non‐randomised groups of women analysed together. Randomised groups were published separately and included in this review (Rowlands 1983) |
| Halpern 2010 | A systematic review, not an RCT |
| Han 1999b | Part of Sang 1999 study |
| Han 2001b | Not an RCT 126 women used GyneFix IUD for EC, no one got pregnant/12 expected pregnancies |
| Haspels 1976 | Not an RCT |
| He 1991 | Not an EC study; it is a study on regular postcoital use of LNG |
| Ho 2013 | Review |
| Hoffman 1983 | Not an RCT or quasi‐RCT |
| Jiang 2000 | No mention of random allocation |
| Jiang 2002 | Not an RCTor quasi‐RCT 120 women used R2323 (gestrinone) 5 mg as ECP within 120 h of intercourse |
| Jin 2005 | Part of a large WHO multicentre dose‐finding study of Mife (see WHO 1999) |
| Kesserü 1973 | Not an RCT; also it is a study on regular postcoital contraception |
| Li 2001 | Not an RCT or quasi‐RCT 100 women used Mife 25 mg as ECPs within 72 h of intercourse. 2 women got pregnant |
| Li 2002b | Not an RCT 150 women used Mife 25 mg as ECPs within 72 h of intercourse. 3 women got pregnant |
| Li 2005a | Not an RCT
After introduction of IUD and ECPs, women chose one of the EC methods that they wanted 2 groups (Cu375‐IUD vs Mife 25 mg, single dose, orally). Observed/expected pregnancy/total number of women: IUD 0/12/150; Mife 4/13/150 |
| Lippes 1976 | Not an RCT |
| Lippes 1979 | Not an RCT |
| Liu 2002a | Not an RCT
After introduction of IUD and ECPs, women chose the method that wanted to use. 2 groups (Cu375‐IUD vs Mife 25 mg, single dose, orally). Observed/expected pregnancy/total number of women: IUD 1/8/80; Mife 1/9/80 |
| Luerti 1986 | Not an RCT |
| Ma 2001 | Not an RCT 110 women used Mife 25 mg single dose for EC, 1 got pregnant |
| Mo 2004 | An RCT, but the loss of follow‐up was 20% |
| Mor 2005 | A prospective, open‐label, cross‐over study comparing the physiological effects of vaginally‐ and orally‐administered EC. They concluded the vaginal route of administration of EC regimens may be as efficacious as the oral route |
| Moreau 2012 | Pooled data meta‐analyses of UPA for EC |
| Piaggio 2003a | A meta‐analyses of Mife 10 mg for EC |
| Piaggio 2003b | A meta‐analyses of effectiveness of different dosages of Mife for EC |
| Polakow 2013 | LNG used during LAM. EC was not the main study subject |
| Qi 2000b | Not an RCT 622 women used Mife 25 mg for EC. 5 got pregnant, the effective rate was 91.25% |
| Qiao 2002 | Not an RCT 140 women used Mife 25 mg in combination with MTX 5 mg for EC. No one got pregnant |
| Qin 2000 | Not an RCT |
| Raymond 2000 | An RCT of meclizine to prevent nausea associated with Yuzpe regimen |
| Raymond 2006 | A study to assess how a strategy to maximise access to ECP would affect rates of pregnancy and sexually transmitted infections |
| Roye 2001 | Not an RCT. It is a letter to the editor |
| Ruan 2012 | Not RCT |
| Scarduelli 1998 | Not an RCT |
| Schilling 1979 | Not an RCT |
| Schreiber 2010 | Conducted to assess the role of advanced supply of EC to teenage mothers |
| Scott 2012 | Review |
| Shaaban 2013 | Comparison of LAM and LNG for EC, EC was not the main study subject |
| Shen 2010 | Not an RCT |
| Shochet 2004 | Not an RCT. Investigated side effects after the standard Yuzpe regimen or 2 modifications |
| Song 2007 | Not an RCT |
| Sun 2005 | Review |
| Tian 2000 | Not an RCT
After introduction of IUD and ECPs, women chose one of the two methods that they wanted 2 groups (Cu375‐IUD vs Mife 25 mg, single dose, orally). Observed/expected pregnancy/total number of women: IUD 0/8/80; Mife 2/7/80 |
| Turok 2010 | A prospective observational study, not an RCT |
| Turok 2014 | A prospective observational study, not an RCT |
| Turok 2016 | A prospective observational study, not an RCT |
| Van Santen 1983 | Not an RCT |
| Van Santen 1985b | This study has been excluded because the report includes 1 group of a randomised comparison study published elsewhere and another cohort of women receiving the same treatment (Yuzpe regimen) |
| Virjo 1999 | Not an RCT |
| Wang 2006b | Not an RCT |
| Wei 2002b | Not an RCT 309 women used Mife 25 mg for EC. 209 women had taken the pill within 72 h, and 3 of them got pregnant; 100 women had taken the pill within 72‐120 h and 2 of them got pregnant |
| Wu 1999b | Not an RCT 793 women used Mife 25 mg single dose, 6 observed pregnancies/58 expected pregnancies |
| Wu 2005 | Review |
| Xiao 2004 | Not an RCT A total of 4945 women were recruited in 31 clinical centres in 18 provinces and municipalities in China in a descriptive clinical trial with 1 dose (Mife 10 mg) treatment. 28 cases lost to follow‐up. An analysis of 4917 cases showed a pregnancy rate of 1.4% (95% CI 1.1% to 1.8%) and an effectiveness of prevention of pregnancy of 82.2% (95% CI 77.5% to 86.2%). No trend of increase of pregnancies with delay of treatment was found. Increase of risk of pregnancy in women who had unprotected intercourse after treatment is about 11.1 times higher. Side effects were mild and in small proportion of women, such as nausea and vomiting in 9.2% and other side effects in 0.7% to 3.7% of women. Delay of menstruation over 7 days occurred in 6.5% of women |
| Yang 2002 | Not an RCT 106 women used Mife 10 mg for EC within 72 h of intercourse. Among them, 1 case pregnancy and 1 loss to follow‐up |
| Ye 2014 | No randomised comparison |
| Yu 2001 | Review |
| Yuzpe 1974 | No randomised comparison |
| Yuzpe 1977 | No randomised comparison |
| Yuzpe 1982 | No randomised comparison |
| Zhang 1999a | Not an RCT 200 women were divided into 2 groups (Mife 25 mg or IUD). Women who had unprotected intercourse within 72 h were given Mife and within 72‐120 h given IUD. 0 pregnancy/10 expected pregnancies in IUD group, 2 observed pregnancies/8 expected pregnancies in Mife group |
| Zhang 1999c | Part of Sang 1999 study |
| Zhang 1999d | Results have been included in Sang 1999 |
| Zhang 1999e | Not an RCT 123 women used LNG 0.75 mg orally, 2 doses, 12 h apart, 1 observed pregnancy/13 expected pregnancies |
| Zhao 2006 | Not an RCT A questionnaire survey among 301 women who had LNG EC failure and had abortion |
| Zhao H 2001 | Not an RCT |
| Zhu 1999 | Not an RCT. 17 women used Mife 25 mg + MTX 5 mg for EC, no one got pregnant |
| Zhu 2007 | Not an RCT |
| Zuliani 1990 | Study conducted in Milan, Italy, which started reporting in 1986. The first report refers to an ongoing randomised trial comparing ethinyl oestradiol‐norgestrel combination (Yuzpe regimen) to danazol 800 mg in 835 women. Subsequently, it is reported that 1000 women were randomised and, afterwards, a third group (danazol 1200 mg) comparison was added. There was no report from which the results for the 1000 women randomised to Yuzpe and danazol 800 mg can be extracted. In subsequent reports in 1988 and 1990, the results are reported with randomised and non‐randomised groups together and, therefore, this study has been excluded from analysis |
CI: confidence interval; Cu‐IUD: copper‐intrauterine device; EC: emergency contraception; ECP: emergency contraceptive pill; IUD: intrauterine device; LAM: LNG: levonorgestrel; Mife: mifepristone; MTX: methotrexate; RCT: randomised controlled trial; UPA: ulipristal acetate
Characteristics of ongoing studies [ordered by study ID]
NCT01539720.
| Trial name or title | Levonorgestrel intrauterine system for emergency contraception (LIFE) |
| Methods | Single group assignment |
| Participants |
|
| Interventions | UPA LNG IUS |
| Outcomes | Pregnancy |
| Starting date | 21 February 2012 |
| Contact information | Michele Curran curranm@wudosis.wustl.edu |
| Notes |
NCT02175030.
| Trial name or title | RAPID EC ‐ RCT assessing pregnancy with intrauterine devices for EC |
| Methods | Parallel assignment |
| Participants |
|
| Interventions | Cu‐IUD vs LNG IUD |
| Outcomes | Efficacy of LNG IUD and copper T380 IUD for EC |
| Starting date | 23 Jun 2014 |
| Contact information | Marie Gibson marie.gibson@hsc.utah.edu Jessica Sanders jessica.sanders@hsc.utah.edu Principal Investigator: David K Turok, MD |
| Notes |
NCT02577601.
| Trial name or title | Impact of combined hormonal contraceptives on UPA |
| Methods | Cross‐over assignment |
| Participants |
|
| Interventions | UPA vs LNG |
| Outcomes | Evidence of follicle rupture by day 5 following use of UPA |
| Starting date | 5 August 2015 |
| Contact information | Principal Investigator: Alison Edelman, MD Women's Health Research Unit whru@ohsu.edu |
| Notes |
BMI: body mass index; Cu‐IUD: copper intrauterine device; EC: emergency contraception; IUS: intrauterine system; LNG: levonorgestrel; UPA: ulipristal acetate; UPT: urine pregnancy test
Differences between protocol and review
We added a post‐hoc subgroup analysis by BMI at the suggestion of our peer reviewers. In the event, none of the included studies reported suitable data, but we hope this will become available in future updates of this review.
Contributions of authors
In this updated version (2017), JS screened literature title and abstracts, did data extraction, assessed all the included Chinese studies for risk of bias and contributed to all sections of the update. ES assessed all the included English studies for risk of bias and made the ROB tables. LC had the idea and conducted the previous version of the review. LC and YC commented and helped to revise the updated version. KC conducted literature searches and contributed title and abstract screening and did data extracts.
Sources of support
Internal sources
HRP‐UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
UK Cochrane Centre, NHS R&D Programme, Oxford, UK.
Shanghai Institute of Planned Parenthood Research, China.
External sources
The David and Lucile Packard Foundation, Los Altos, CA, USA.
Declarations of interest
JS: no conflicts of interest. YC: no conflicts of interest KC: no conflicts of interest LC: participated in emergency contraceptive trials included in this review (including Chen 2011). LC did not participate in selecting these studies, or extracting their data. LC has received travel costs and consulting fees from Regenex Pharmaceutical Corporation, China Resources Zizhu Pharmaceutical Co, and Bayer Pharma AG. ES: no conflicts of interest
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Arowojolu 2002 {published data only}
- Arowojolu AO, Okewole LA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception 2002;66:269‐73. [DOI] [PubMed] [Google Scholar]
Ashok 2002 {published data only}
- Ashok PW, Stalder C, Wagaarachchi PT, Flett GM, Melvin L, Templeton A. A randomised study comparing a low dose of mifepristone and the Yuzpe regimen for emergency contraception. BJOG 2002;109:553‐60. [DOI] [PubMed] [Google Scholar]
Askalani 1987 {published data only}
- Askalani AH, Al‐Senity AM, Al‐Agizy HM, Salam HI, Al‐Masry GI, El‐Sadek SM. Evaluation of copper T‐200 as a post‐coital contraceptive. Egyptian Journal of Obstetrics and Gynaecology 1987;13:63‐6. [Google Scholar]
Bu 2006 {published data only (unpublished sought but not used)}
- Bu GY, Yang MY, Cao XL. Clinical study on administration of low dose of mifepristone and levonorgestrel in urgent postcoital contraception [小剂量米非司酮和左炔诺孕酮用于紧急避孕的临床研究]. Progress in Modern Biomedicine 2006;6(2):53‐4. [Google Scholar]
Cao 1999 {published data only}
- Cao P, Li M, Xu J, Li Q. Different doses of mifepristone for emergency contraception [不同剂量米非司酮用于紧急避孕效果的观察与分析]. Journal of Practical Obstetrics and Gynecology 1999;15:295‐6. [Google Scholar]
Cao 2011 {published data only}
- Cao YQ, Yang AH, Zhu XL. A comparative clinical study on mifepristone with levonorgestrel for emergency contraception [米非司酮和左炔诺孕酮在紧急避孕中的临床研究]. Chinese Journal of General Practice 2011;9(7):1087‐8. [Google Scholar]
Carbonell 2015 {published data only}
- Carbonell JL, Garcia R, Gonzalez A. Mifepristone 5 mg versus 10 mg for emergency contraception: double‐blinded randomized clinical trial. International Journal of Women's Health 2015;7:95‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen G. Mifepristone for emergency contraception [米非司酮用于紧急避孕的临床观察]. Journal of Guangxi Traditional Chinese Medical University 2001;4:22‐4. [Google Scholar]
Chen 2002a {published data only}
- Chen R, Li Q, Zhang Y, Huang M, Chen Y, Zhong X, et al. A comparative study of low‐dose mifepristone for emergency contraception [两种不同小剂量米非司酮用于紧急避孕的临床研究]. Shi Yong Yi Xue Zha Zi 2002;10:1028‐9. [Google Scholar]
Chen 2002b {published data only}
- Chen H, Min X. Mifepristone in combination with MTX for emergency contraception [米非司酮加用氨甲喋呤用于紧急避孕的研究]. Strait Pharmaceutical Journal 2002;3:51‐2. [Google Scholar]
Chen 2008 {published data only}
- Chen Y. A comparative study of mifepristone and LNG for emergency contraception [米非司酮、左炔诺孕酮用于紧急避孕的临床效果对比性观察]. China Foreign Medical Treatment 2008;34:60. [Google Scholar]
Chen 2009 {published data only}
- Chen S, An HB, Wang D, Jin FB. Different dose of mifepristone for emergency contraception [不同剂量米非司酮用于紧急避孕的临床观察]. China Health Care Nutrition ‐ Clinical Medicine Journal 2009;2:28‐9. [Google Scholar]
Chen 2013 {published data only}
- Chen SJ, Wen CX, Huang CX. A comparative study on mifepristone with levonorgestrel for emergency contraception [左炔诺孕酮与米非司酮用于紧急避孕的效果比较]. Chinese Journal of Ethnomedicine and Ethnopharmacy 2013;10:105. [Google Scholar]
Chen 2015 {published data only}
- Chen CX. A comparative study on mifepristone with levonorgestrel for emergency contraception [米非司酮与左炔诺孕酮用于紧急避孕临床对比研究]. Medical Forums in Basic 2015;8:1024‐5. [Google Scholar]
Cheng 1999a {published data only}
- Cheng L, Tong CH, Xiao ZH. Low doses of mifepristone for emergency postcoital contraception [不同剂量米非司酮用于紧急避孕的临床多中心研究]. Chinese Journal of Obstetrics and Gynaecology 1999;6:335‐8. [Google Scholar]
Cheng 2009 {published data only}
- Cheng S. Clinical observation on YUTING for emergency contraception [毓婷预防早孕的临床疗效观察]. Chinese Journal of Modern Drug Application 2009;8:147‐8. [Google Scholar]
Creinin 2006 {published data only}
- Creinin MD, Schlaff W, Archer DF, Wan L, Frezieres R, Tomas M, et al. Progesterone receptor modulator for emergency contraception. A randomized controlled trial. Obstetrics & Gynecology 2006;108:1089‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dada 2010 {published data only}
- Dada OA, Godfrey EM, Piaggio G, Hertzen H, Nigerian Network for Reproductive Health Research and Training. A randomized, double‐blind, noninferiority study to compare two regimens of levonorgestrel for emergency contraception in Nigeria. Contraception 2010;82(4):373‐8. [DOI] [PubMed] [Google Scholar]
Ding 2005 {published data only}
- Ding G. Different doses of mifepristone for emergency contraception [不同剂量米非司酮用于紧急避孕的临床观察]. Journal of Practical Diagnosis and Therapy 2005;19:226‐7. [Google Scholar]
Dong 2009 {published data only}
- Dong JF. Two different methods for emergency contraception [两种不同方法用作紧急避孕的临床研究]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine 2009;9(1):58‐9. [Google Scholar]
Du 2002 {published data only}
- Du J. Low dose of mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的临床观察]. Henan Yi Yao Xin Xi 2002;11:14‐5. [Google Scholar]
Ellertson 2003a {published data only}
- Ellertson C, Webb A, Blanchard K, Bigrigg A, Haskell S, Shochet T, et al. Modifying the Yuzpe regimen of emergency contraception: a multicenter randomized controlled trial. Obstetrics and Gynecology 2003;101:1160‐7. [DOI] [PubMed] [Google Scholar]
Fan 2001a {published data only}
- Fan H, Cheng Y, Guo F, Wu S, Tan Y, Chen X, et al. Low dose of mifepristone for emergency contraception. Hubei Yu Fang Yi Xue Zha Zi 2001;23:52. [Google Scholar]
Fang 2000 {published data only}
- Fang Q, Guo X, Pan J, Xiao J, Li Y. A comparative study on different doses of mifepristone for emergency contraception [不同剂量米非司酮用于紧急避孕的临床观察]. Maternal and Child Health Care of China 2000;15:48‐9. [Google Scholar]
Farajkhoda 2009 {published data only}
- Farajkhoda T, Khoshbin A, Enjezab B, Bokaei M, Karimi Zarchi M. Assessment of two emergency contraceptive regimens in Iran: levonorgestrel versus the Yuzpe. Niger Journal of Clinical Practice 2009;12(4):450‐2. [PubMed] [Google Scholar]
Fu 2000 {published data only}
- Fu X, Wang L, Jiang Q, Yang X. Anordrin and mifepristone for emergency contraception [双炔失碳酯或米非司酮用于紧急避孕临床观察]. Journal of Qinghai Medical College 2000;21:43‐4. [Google Scholar]
Gan 2007 {published data only}
- Gan XH, Jiang H, Li LP. A clinical study of low‐dose mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的临床观察]. Modern Medicine Health 2007;23(11):1634‐5. [Google Scholar]
Glasier 1992 {published and unpublished data}
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird D. Postcoital contraception with mifepristone (letter). Lancet 1991;337:1414‐5. [DOI] [PubMed] [Google Scholar]
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high‐dose estrogen and progestogen for emergency postcoital contraception. New England Journal of Medicine 1992;327:1041‐4. [DOI] [PubMed] [Google Scholar]
Glasier 2010 {published data only}
- Glasier AF, Cameron ST, Fine PM, Logan SJ, Casale W, Horn J, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non‐inferiority trial and meta‐analysis. Lancet 2010;375(9714):555‐62. [DOI] [PubMed] [Google Scholar]
Hamoda 2004 {published data only}
- Hamoda H, Ashok PW, Stalder C, Flett GMM, Kennedy E, Templeton A. A randomized trial of mifepristone (10 mg) and levonorgestrel for emergency contraception. Obstetrics & Gynecology 2004;104:1307‐13. [DOI] [PubMed] [Google Scholar]
Han 1995 {published data only}
- Han X, Weng L, Zhang L, Zeng T, Xiao B. Clinical trial of mifepristone and anordrin for emergency contraception [米非司酮,双炔失碳酯单独或联合应用于紧急避孕的临床观察]. Journal of Reproductive Medicine (China) 1995;4:206‐11. [Google Scholar]
Han 1996 {published data only}
- Han X, Weng L, Xiao B. Emergency contraception with mifepristone and anordrin [米非司酮用于紧急避孕的临床观察]. Chinese Journal of Obstetrics and Gynecology 1996;9:526‐9. [PubMed] [Google Scholar]
Han 1999a {published data only}
- Han X, Jin X, Weng L. A comparative study of mifepristone with levonorgestrel for emergency contraception [左炔诺孕酮或米非司酮用于紧急避孕的临床观察]. Journal of Practical Obstetrics and Gynecology 1999;6:294‐5. [Google Scholar]
Han 2001a {published data only}
- Han L, Ma Y, Li H. Low doses of mifepristone for emergency contraception [低剂量米非司酮用于紧急避孕]. Fudan University Journal of Medical Sciences 2001;2:176‐7. [Google Scholar]
He 2002 {published data only}
- He CH, Gui YL, Yang J, Wang BS, Zheng E, Gao ES, et al. A randomized comparative study on mifepristone alone and in combination with tamoxifen for emergency contraception. Contraception 2002;66:221‐4. [DOI] [PubMed] [Google Scholar]
Ho 1993 {published and unpublished data}
- Ho PC, Kwan MSW. A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post‐coital contraception. Human Reproduction 1993;8:389‐92. [DOI] [PubMed] [Google Scholar]
Hoseini 2013 {published data only}
- Hoseini FS, Eslami M, Abbasi M, Noroozi Fashkhami F, Besharati S. A randomized, controlled trial of levonorgestrel vs. the Yuzpe regimen as emergency contraception method among Iranian women. Iranian Journal of Public Health 2013;42(10):1158‐66. [PMC free article] [PubMed] [Google Scholar]
Hu 2003 {published data only}
- Hu X, Lu C. A comparative study of mifepristone with levonorgestrel for emergency contraception [米非司酮、左炔诺孕酮用于紧急避孕的临床效果观察]. Sichuan Medical Journal 2003;24(8):F004. [Google Scholar]
Jin 2012 {published data only}
- Jin YC, Jin YY. A comparative study on efficiency and side effects of mifepristone with levonorgestrel for emergency contraception [毓婷与米非司酮在紧急避孕中的效果及不良反应分析]. Seek Medical and Ask the Medicine 2012;10(2):167‐8. [Google Scholar]
Lai 2004 {published data only}
- Lai Z, Wang J, Zhou Z, Lu H, Song X, Sun J. A comparative study of low‐dose mifepristone for emergency contraception [低剂量米非司酮用于紧急避孕的临床观察]. Maternal and Child Health Care of China 2004;19(2):36‐8. [Google Scholar]
Lan 2006 {published data only}
- Lan XL, Chen LP, Ye QH. Clinical study of low‐dose mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的临床观察]. Clinical Medicine 2006;26(11):68‐9. [Google Scholar]
Lei 2013 {published data only}
- Lei X, Yang G. A comparative observation on mifepristone with levonorgestrel for emergency contraception [米非司酮与左炔诺孕酮用于紧急避孕的效果比较]. Medical Information 2013;26(15):486. [Google Scholar]
Li 2000a {published data only}
- Li A, Zhang Y. Low dose of mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的疗效观察]. Journal of Guangxi Medical University 2000;17:857. [Google Scholar]
Li 2000b {published data only}
- Li Q, Chen R, Zhang Y, Huang M, Chen RX, Zhong X. A comparative study of mifepristone 50 mg and 25 mg for emergency contraception [50mg与25mg米非司酮用于紧急避孕的临床效果比较]. Guangdon Medical Journal 2000;22:884‐5. [Google Scholar]
Li 2000c {published data only}
- Li H, Chang JP, Li J. A study of low‐dose mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的临床观察]. Heilongjiang Medical Journal 2000;23:90. [Google Scholar]
Li 2002a {published data only}
- Li W. A comparative study of mifepristone with levonorgestrel for emergency contraception [米非司酮与左炔诺酮用于紧急避孕的临床效果观察]. Guizhou Journal of Medicine 2002;5:457. [Google Scholar]
Li 2005b {published data only}
- Li J. A comparative study of mifepristone with levonorgestrel for emergency contraception [米非司酮和左炔诺酮用于紧急避孕效果观察]. Anthology of Medicine 2005;5:754. [Google Scholar]
Liang 2001 {published data only}
- Liang JZ, Zhou MR. A randomised comparative study on mifepristone and levonorgestrel for emergency contraception [米非司酮和左炔诺孕酮在紧急避孕中的临床观察]. Heilongjiang Medical Journal 2001;25:594. [Google Scholar]
Liao 2003 {published data only}
- Liao AH, Chang CF, Zhu JW. Randomised controlled prospective studies of mifepristone in small doses and levonorgestrel for emergency contraception [小剂量米非司酮和左炔诺孕酮用于紧急避孕的随机对照研究]. Chinese Journal of Practical Gynaecology and Obstetrics 2003;19:25‐7. [Google Scholar]
Lin 2000 {published data only}
- Lin N, Cheng W, Yang Y, Shao L. A comparative study of mifepristone and LNG for emergency contraception [小剂量米非司酮与左旋18‐甲基炔诺酮单方用于紧急避孕的比较研究]. Tianjing Medical Journal 2000;28:601‐3. [Google Scholar]
Liu 2000 {published data only}
- Liu JL, Liu LH, Li KZ, Liu HL. Comparative study of the efficacy of low‐dose mifepristone and levonorgestrel on the emergency contraception [低剂量米非司酮与左旋18甲基炔谱酮紧急避孕临床效果观察]. Practical Preventive Medicine 2000;7:126‐7. [Google Scholar]
Liu 2001 {published data only}
- Liu L, Wang Z, Li L. Mifepristone and anordrin for emergency contraception [米非司酮、双炔失碳酯用于紧急避孕的临床观察]. Zhong Guo Yiu Sheng Yu Yi Chuan Zha Zi 2001;9:108‐11. [Google Scholar]
Liu 2002b {published data only}
- Liu L, Chen A. A comparative study of mifepristone with Cu‐IUD for emergency contraception [米非司酮与带铜宫内节育器(IUD)用于紧急避孕方法的对比观察]. Journal of Changzhi Medical College 2002;61:198‐9. [Google Scholar]
Liu 2009 {published data only}
- Liu RQ. A comparative study of mifepristone and LNG for emergency contraception [米非司酮和毓婷紧急避孕的有效性和安全性评价]. China Pharmaceuticals 2009;19:68‐9. [Google Scholar]
Lou 2002 {published data only}
- Lou C. Low‐dose mifepristone for emergency contraception [低剂量米非司酮用于紧急避孕的效果]. Xian Dai Shi Yong YI Xue 2002;14:485. [Google Scholar]
Lou 2005 {published data only}
- Lou X, Ma L, Yang Y. Mifepristone and C53 contraceptive in postcoital contraception [米非司酮与双炔失碳酯联合用于紧急避孕的临床观察]. Journal of Chinese Modern Gynaecology and Obstetrics 2005;2:405‐6. [Google Scholar]
Miras 2014 {published data only}
- Miras RG, Sanchez AG, Miras LG, Llibre Guerra JJ, Jesus Delgado González M, Penalver Cruz AM. Mifepristone efficacy for emergency contraception when comparing 5 and 10 milligrams doses [Eficacia de la mifepristona como comtraceptivo de emergencia al comparar dosis de 5 y 10 miligramos]. Revista Cubana de Obstetricia y Ginecologia 2014;40(3):326‐35. [Google Scholar]
Ngai 2005 {published data only}
- Ngai SW, Fan S, Li S, Cheng L, Ding J, Jing X, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Human Reproduction 2005;20:307‐11. [DOI] [PubMed] [Google Scholar]
Pei 2001 {published data only}
- Pei JH, Wang ZX. A randomised comparative study of mifepristone in small doses and levonorgestrel for emergency contraception [小剂量米非司酮与左炔诺孕酮用于紧急避孕的临床观察]. Haerbin Medicine 2001;21:32‐3. [Google Scholar]
Qi 2000a {published data only}
- Qi Y, Zhang J, Cao Y, Zhang Z. A comparative clinical trial on two low doses of mifepristone for emergency contraception [两种低剂量米非司酮用于紧急避孕随机对比临床观察]. Maternal and Child Health Care of China 2000;15:701‐4. [Google Scholar]
Qi 2003 {published data only}
- Qi M, Wang Y, Yan L. A comparative study of low‐dose mifepristone with levonorgestrel for emergency contraception ‐ 288 cases report [小剂量米非司酮与左炔诺孕酮片紧急避孕288例观察]. Journal of Qinghai Medical College 2003;24:255‐6. [Google Scholar]
Qian 1999 {published data only}
- Qian L. Three doses of mifepristone for emergency contraception [三种剂量米非司酮用于紧急避孕的临床观察]. Chinese Journal of Family Planning 1999;7:322‐3. [Google Scholar]
Rowlands 1983 {published data only}
- Rowlands S, Guillebaud J, Bounds W, Booth M. Side effects of danazol compared with an ethinyloestradiol/norgestrel combination when used for postcoital contraception. Contraception 1983;27:39‐49. [DOI] [PubMed] [Google Scholar]
- Rowlands S, Kubba AA, Guillebaud J, Bounds W. A possible mechanism of action of danazol and an ethinylestradiol/norgestrel combination used as postcoital contraceptive agents. Contraception 1986;33:539‐45. [DOI] [PubMed] [Google Scholar]
Sang 1999 {published data only}
- Sang GW, Shao Q, Zhang J, Zhang M, Chen S, Song S, et al. A randomized multicentre clinical trial on different doses of mifepristone alone and in combination with anordrin as emergency contraception [不同剂量米非司酮及合并双炔失碳酯用于紧急避孕的多中心随机临床研究]. Chinese Journal of Obstetrics and Gynaecology 1999;34(6):331‐4. [PubMed] [Google Scholar]
Shao 2010 {published data only}
- Shao XY. Clinical observation on mifepristone for emergency contraception [米非司酮用于紧急避孕的临床观察]. Practical Clinical Journal of Integrated Traditional Chinese and Western Medicine 2010;10(3):55. [Google Scholar]
Sheng 2002 {published data only}
- Sheng A. Clinical observation of the efficacy of mifepristone and levonorgestrel on the emergency contraception [小剂量米非司酮和左炔诺酮用于紧急避孕的疗效观察]. Academic Journal of Jiangsu University (Medicine) 2002;12:246‐9. [Google Scholar]
Sheng 2008 {published data only}
- Sheng SY. A clinical study of LNG‐COC for emergency contraception [复方左炔诺孕酮用于紧急避孕的临床观察]. Asia‐Pacific Traditional Medicine 2008;4(9):93‐4. [Google Scholar]
Su 2001 {published data only}
- Su W, Chui JY, Liu P. A comparative study of IUCD with mifepristone and with levonorgestrel for emergency contraception [米非司酮、 左炔诺孕酮和IUCD用于紧急避孕的临床观察研究]. Journal of Baotou Medicine 2001;25:24. [Google Scholar]
Sun 2000 {published data only}
- Sun Y, Wang X. A clinical comparative study of mifepristone with levonorgestrel for emergency contraception [米非司酮,左炔诺孕酮用于紧急避孕的临床效果观察]. Chinese Journal of Family Planning 2000;8:172‐3. [Google Scholar]
Sun 2003 {published data only}
- Sun P. Mifepristone for emergency contraception [米非司酮用于紧急避孕的研究]. Journal of Chinese Practice Medicine 2003;5:92. [Google Scholar]
Sun 2007 {published data only}
- Sun MX. A clinical comparative study of LNG vs. LNG‐COC for emergency contraception [左炔诺孕酮和复方短效避孕药用于紧急避孕的临床观察]. Chinese Journal of Family Planning 2007;15(6):366‐7. [Google Scholar]
Tan 1999 {published data only}
- Tan K, Mai T, He P, Lin H, Li S. Low doses of mifepristone for emergency contraception [分两次服用小剂量米非司酮用于紧急避孕]. Chinese Journal of Family Planning 1999;7:470‐1. [Google Scholar]
Tan 2003 {published data only}
- Tan L, Zheng G, Li J. Mifepristone for emergency contraception ‐ 150 cases report [150例米非司酮用于紧急避孕的临床观察]. Health Vocational Education 2003;21:138‐9. [Google Scholar]
Tao 2014 {published data only}
- Tao XL, Xiong J. A study on mifepristone for emergency contraception [复方米非司酮应用于紧急避孕150例]. Herald of Medicine 2014;33(6):742‐3. [Google Scholar]
Tian 2013 {published data only}
- Tian ZM. A clinical comparative observation among copper IUD and mifepristone for emergency contraception [Cu‐IUD和米非司酮用于紧急避孕的效果比较]. China Health Care & Nutrition 2013;9:5238. [Google Scholar]
Van Santen 1985a {published data only}
- Santen MR, Haspels AA. A comparison of high‐dose estrogens versus low‐dose ethinylestradiol and norgestrel combination in postcoital interception: a study in 493 women. Fertility and Sterility 1985;43:206‐13. [DOI] [PubMed] [Google Scholar]
- Santen MR, Haspels AA. Comparative randomized double‐blind study of high dosage ethinylestradiol versus ethinylestradiol and norgestrel combination in postcoital contraception. Acta Endocrinologica 1982;99(suppl 246):2. [Google Scholar]
von Hertzen 2002 {published data only}
- Hertzen H, Piaggio G, Ding J, Chen J, Song S, Bartfai G, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception. Lancet 2002;360:1803‐10. [DOI] [PubMed] [Google Scholar]
Wang 1999 {published data only}
- Wang Z, Liu L, Liu Q, Zhang H. A clinical comparative study of mifepristone with anordrin for emergency contraception [米非司酮,双炔失碳酯用于紧急避孕临床效果观察]. Chinese Journal of Family Planning 1999;7:320‐1. [Google Scholar]
Wang 2000a {published data only}
- Wang C, Tian M, Chang Y, Shao M. A clinical comparative observation among copper IUD, lower dose mifepristone and levonorgestrel for emergency contraception [含铜IUD、小剂量米非司酮与左炔诺孕酮用于紧急避孕的临床比较观察]. Journal of Chinese Physician 2000;2:271‐3. [Google Scholar]
Wang 2000b {published data only}
- Wang Q, Li A. A comparative study of levonorgestrel with low dose mifepristone for emergency contraception [左炔诺孕酮和小剂量米非司酮用于紧急避孕的临床观察]. Northwestern Pharmaceutical Journal 2000;15:72. [Google Scholar]
Wang 2001 {published data only}
- Wang SZ, Huang ZK, Li S. Clinical trial of mifepristone in different dose for emergency contraception [不同剂量米非司酮用于紧急避孕的临床比较观察]. Chinese Journal of Practical Gynaecology and Obstetrics 2001;9:534‐6. [Google Scholar]
Wang 2003 {published data only}
- Wang Y, Liu H. A comparative study on low doses of mifepristone with levonorgestrel for emergency contraception [左炔诺孕酮和小剂量米非司酮用于紧急避孕的临床观察]. Chinese Journal of Family Planning 2003;8:505‐6. [Google Scholar]
Wang 2004 {published data only}
- Wang L, Lv Y, Guan D, Zhang H, Yao L. 12.5 mg mifepristone for emergency contraception [12.5 mg 米非司酮用于紧急避孕的临床研究]. Chinese General Practice 2004;7:1477‐8. [Google Scholar]
Wang 2006a {published data only}
- Wang J. A comparative study on different doses of mifepristone for emergency contraception [不同剂量米非司酮用于紧急避孕的临床疗效研究]. Journal Huaihai Medicine 2006;24:19‐20. [Google Scholar]
Wang 2008 {published data only}
- Wang ZW, Qu HW. Two different doses of mifepristone for emergency contraception [两种剂量米非司酮进行紧急避孕比较分析]. Chinese Journal of Misdiagnosis 2008;20:4819‐20. [Google Scholar]
Wang 2012 {published data only}
- Wang CL. A comparative clinical study on mifepristone with levonorgestrel for emergency contraception [左炔诺孕酮和米非司酮用于紧急避孕的临床观察]. Guide of China Medicine 2012;10(5):102‐3. [Google Scholar]
Webb 1992 {published data only}
- Webb AM. Alternative treatments in oral postcoital contraception: interim results. Advances in Contraception 1991;7:271‐9. [DOI] [PubMed] [Google Scholar]
- Webb AMC, Russell J, Elstein M. Comparison of Yuzpe regimen, danazol, and mifepristone (RU486) in oral postcoital contraception. BMJ 1992;305:927‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2002a {published data only}
- Wei RH. Low dose of mifepristone for emergency contraception ‐ 200 cases report [小剂量米非司酮用于紧急避孕200例的临床观察]. Shanghai Sheng Wu Yi Xue Gong Cheng Zha Zi 2002;23:39‐42. [Google Scholar]
Wei 2011 {published data only}
- Wei H, He CB, Liu J. Low dose mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的临床研究]. Chinese and Foreign Women Health 2011;19(3):70. [Google Scholar]
WHO 1998 {published data only}
- WHO Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet 1998;352:428‐33. [PubMed] [Google Scholar]
WHO 1999 {published data only}
- WHO Task Force on Postovulatory Methods of Fertility Regulation. Comparison of three single doses of mifepristone as emergency contraception: a randomised trial. Lancet 1999;353:697‐702. [PubMed] [Google Scholar]
Wu 1999a {published data only}
- Wu S, Wang C, Wang Y, Cheng W, Zuo S, Li H, et al. A randomized, double‐blind, multicentre study on comparing levonorgestrel and mifepristone for emergency contraception [左炔诺孕酮和小剂量米非司酮用于紧急避孕的临床观察]. Chinese Journal of Obstetrics and Gynaecology 1999;34:327‐30. [PubMed] [Google Scholar]
Wu 2002 {published data only}
- Wu XZ, Sao JY, Chen CQ, Yan Y, Fa YY, Liu JH, et al. A comparative study on methods for emergency contraception [两种紧急避孕方法比较性研究]. Reproduction & Contraception (China) 2002;22:152‐5. [Google Scholar]
Wu 2010 {published data only}
- Wu S, Dong J, Cong J, Wang CP, Hertzen H, Godfrey EM. Gestrinone compared with mifepristone for emergency contraception: a randomized controlled trial. Obstetrics and Gynecology 2010;115(4):740‐4. [DOI] [PubMed] [Google Scholar]
Xiao 2002 {published data only}
- Xiao BL, Hertzen H, Piaggio G. A randomized double‐blind comparison of two single doses of mifepristone for emergency contraception. Human Reproduction 2002;17:3084‐9. [DOI] [PubMed] [Google Scholar]
Xie 1998 {published data only}
- Xie X, Liu Y, Lin X. A clinical study on 600 cases of mifepristone for emergency contraception [米非司酮用于紧急避孕600例临床观察]. Reproduction & Contraception (China) 1998;18:224‐6. [Google Scholar]
Xie 2010 {published data only}
- Xie HH, Shang XM, Dai WY. Analysis of emergency contraception use mifepristone by different doses [不同剂量米非司酮用于紧急避孕的疗效分析]. Guide of China Medicine 2010;8(14):34‐5. [Google Scholar]
Xu 2000a {published data only}
- Xu Z. A comparative study of mifepristone, anordrin and levonorgestrel for emergency contraception [米非司酮、双炔失碳酯、左炔诺孕酮用于紧急避孕的临床观察]. Journal of Yichun Medical College 2000;12:248‐9. [Google Scholar]
Xu 2000b {published data only}
- Xu L, Wang Z. A comparative study on low dose mifepristone with levonorgestrel for emergency contraception [低剂量米非司酮、左炔诺孕酮用于紧急避孕的临床观察]. Chinese Journal of Family Planning 2000;8:419‐20. [Google Scholar]
Yang 2001 {published data only}
- Yang LJ. A comparative study on mifepristone, anordrin and danazol for emergency contraception [米非司酮双炔失碳酯丹那唑紧急避孕的临床研究]. Guangzhou Medical Journal 2001;32:12‐3. [Google Scholar]
Yang 2003 {published data only}
- Yang F. A comparative study on two low doses of mifepristone for emergency contraception. Journal of Clinical Research 2003;20:630‐1. [Google Scholar]
Ye 2013 {published data only}
- Ye LQ. A study on the effect of mifepristone for emergency contraception. For all Health 2013;7(1):12‐13. [Google Scholar]
Zeng 2007 {published data only}
- Zeng XY. A clinical study of Mifepristone in combination with MTX for emergency contraception. Practical Clinical Journal of Integrated Chinese and Western Medicine 2007;7(3):62‐3. [Google Scholar]
Zeng 2008 {published data only}
- Zeng MY, Zhu LF, Huang Y. A comparative study of low‐dose mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的临床观察]. International Medicine Health Guidance News 2008;14:68‐70. [Google Scholar]
Zhang 1998 {published data only}
- Zhang Y, Qiao G, Zhu P, Zhang S, Zhang J, Zhu N. Clinical observation of three lower doses of mifepristone for emergency contraception [三种低剂量米非司酮用于紧急避孕的临床观察]. Chinese Journal of Family Planning 1998;6:343‐5. [Google Scholar]
Zhang 1999b {published data only}
- Zhang X, Gao G, Shi J, Qu C, Leng Y. A clinical study on low doses of mifepristone for emergency contraception [低剂量米非司酮用于紧急避孕的临床效果观察]. Chinese Journal of Family Planning 1999;4:175‐6. [Google Scholar]
Zhang 2000 {published data only}
- Zhang JQ. Emergency contraception in high‐land [高原地区紧急避孕临床应用]. Chinese Journal of Family Planning 2000;8:552‐4. [Google Scholar]
Zhang 2002a {published data only}
- Zhang Y, Zhang W, Wang L. Low‐dose of mifepristone and anordrin for emergency contraception: observation of 116 cases [低剂量米非司酮并用双炔失碳酯用于紧急避孕116例分析]. Journal of Qiqihar Medical College 2002;4:415. [Google Scholar]
Zhang 2002b {published data only}
- Zhang Y, Wen L, Li S, Wang Y. Mifepristone for emergency contraception [米非司酮用于紧急避孕的临床研究]. Henan YI Yao Xin XI 2002;8:20‐1. [Google Scholar]
Zhang 2005 {published data only}
- Zhang L, Lai L, Deng X. Single and small dose of Mifepristone for emergency contraception of curative effect observe [单次小剂量米非司酮用于紧急避孕的疗效观察]. Journal of Gannan Medical College 2005;3:328‐30. [Google Scholar]
Zhang 2012 {published data only}
- Zhang H. A comparative clinical study on mifepristone with levonorgestrel for emergency contraception [左炔诺孕酮和米非司酮用于紧急避孕的临床观察]. Guide of China Medicine 2012;10(26):576‐7. [Google Scholar]
Zhang 2014 {published data only}
- Zhang LR. A comparative clinical study on mifepristone with levonorgestrel for emergency contraception [米非司酮与左炔诺孕酮用于紧急避孕的临床对比研究]. Modern Healthcare 2014;7:67. [Google Scholar]
Zhao 2003 {published data only}
- Zhao J, Liu R, Li H, Zhang Y. Different doses of mifepristone for emergency contraception [不同剂量米非司酮用于紧急避孕的临床观察]. Journal of Shandong University (Health Sciences) 2003;41:468. [Google Scholar]
Zheng 2005 {published data only}
- Zheng A. Low‐dose of mifepristone for emergency contraception [小剂量米非司酮用于紧急避孕的临床观察]. Youjiang Medical Journal 2005;4:375‐6. [Google Scholar]
Zuo 1999 {published data only}
- Zuo SH, Wu J, Liu L, Liu J, Gao Y. A clinical trial on two low doses of mifepristone for emergency contraception [两种低剂量米非司酮用于房事后紧急避孕的临床研究]. Reproduction & Contraception (China) 1999;6:352‐6. [Google Scholar]
References to studies excluded from this review
Ashok 2001 {published data only}
- Ashok PW, Wagaarachchi PT, Flett GM, Templeton A. Mifepristone as a late post‐coital contraceptive. Human Reproduction 2001;16:72‐5. [DOI] [PubMed] [Google Scholar]
Ashok 2004 {published data only}
- Ashok PW, Hamoda H, Flett GMM, Templeton A. Mifepristone versus the Yuzpe regimen (PC4) for emergency contraception. International Journal of Gynecology and Obstetrics 2004;87:188‐93. [DOI] [PubMed] [Google Scholar]
Ban 2001 {published data only}
- Ban X, Xiao Y, Fan H, Liu G, Liu Q, Yu L. A comparative clinical study on Tcu380A and Cu‐IUD for emergency contraception. Maternal & Child Health Care of China 2001;16:498‐501. [Google Scholar]
Benagiano G 2010 {published data only}
- Benagiano G, Hertzen H. Towards more effective emergency contraception?. Lancet 2010;375(9714):527‐28. [DOI] [PubMed] [Google Scholar]
Brache 2013 {published data only}
- Brache V, Cochon L, Deniaud M, Croxatto HB. Ulipristal acetate prevents ovulation more effectively than levonorgestral: analysis of pooled data from three randomized trials of emergency contraception regimens. Contraception 2013;88(5):611‐8. [DOI] [PubMed] [Google Scholar]
Byamugisha 2010 {published data only}
- Byamugisha JK, Mirembe FM, Faxelid E, Tumwesigye NM, Gemzell‐Danielsson K. A randomized clinical trial of two emergency contraceptive pill regimens in a Ugandan population. Acta Obstetricia et Gynecologica 2010;89:670‐6. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen QJ, Xiang WP, Zhang DK, et al. Efficacy and safety of a levonorgestrel enteric‐coated tablet as an over‐the‐counter drug for emergency contraception: a Phase IV clinical trial. Human Reproduction (Oxford, England) 2011;26(9):2316‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen DY, Wang JL. A clinical observation on mifepristone and Yuzpe for emergency contraception. Chinese and Foreign Medical Research 2012;10(24):116‐7. [Google Scholar]
Creinin 1997 {published data only}
- Creinin MD. A reassessment of efficacy of the Yuzpe regimen of emergency contraception. Human Reproduction 1997;12:496‐8. [DOI] [PubMed] [Google Scholar]
D'Souza 2003 {published data only}
- D'Souza RE, Masters T, Bounds W, Guillebaud J. Randomised controlled trial assessing the acceptability of GyneFix versus Gyne‐T389S for emergency contraception. Journal of Family Planning and Reproductive Health Care 2003;29:23‐9. [DOI] [PubMed] [Google Scholar]
Dixon 1980 {published data only}
- Dixon GW. Ethinylestradiol and conjugated estrogens as postcoital contraceptives. JAMA 1980;244:1336‐9. [PubMed] [Google Scholar]
Dong 2007 {published data only}
- Dong SZ, Wu SH. Comparing mifepristone, levonorgestrel and the copper IUD for emergency contraception: a report of 268 cases. Journal of Chinese Modern Gynecology and Obstetrics 4;2:127‐9. [Google Scholar]
Ellertson 2003b {published data only}
- Ellertson C, Evans M, Ferden S, Leadbetter C, Spears A, Johnestone K, et al. Extending the time limit for starting the Yuzpe regimen of emergency contraception to 120 hours. Obstetrics & Gynecology 2003;101:1168‐71. [DOI] [PubMed] [Google Scholar]
Espinos 1999 {published data only}
- Espinos JJ, Senosiain R, Vanrell C, Armengol J, Cuberas N, Calaf J. Safety and effectiveness of hormonal postcoital contraception: a prospective study. European Journal of Contraception and Reproductive Health Care 1999;4:27‐33. [DOI] [PubMed] [Google Scholar]
Fan 1998 {published data only}
- Fan Ai, Wang Y, Wang Z. Clinical study on 518 cases of emergency contraception. Chinese Journal of Family Planning 1998;6:408‐9. [Google Scholar]
Fan 2001b {published data only}
- Fan H, Zhou L. Emergency contraception with Multiload Cu 375 SL IUD: a multicentre clinical trial. Journal of Reproductive Medicine (China) 2001;10:70‐7. [DOI] [PubMed] [Google Scholar]
Fasoli 1989 {published data only}
- Fasoli M, Parazzini F, Cecchetti G, Vecchia CL. Post‐coital contraception: an overview of published studies. Contraception 1989;39:459‐69. [DOI] [PubMed] [Google Scholar]
Fine 2010b {published data only}
- Fine P, Mathe H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48‐120 hours after intercourse for emergency contraception. Obstetrics and Gynecology 2010;115(2):247‐63. [DOI] [PubMed] [Google Scholar]
Gan 1999 {published data only}
- Gan SH, Chang M, Hu S, Zhang P, Chang M, Xu X. A clinical study on mifepristone 10mg for emergency contraception. Reproduction and Contraception (China) 1999;19:311‐3. [Google Scholar]
Gan SX 2001 {published data only}
- Gan SX, Li SS, Lu Y. Comparative study of the efficacy of mifepristone and levonorgestrel on the emergency contraception. Chinese Journal of Family Planning 2001;9:178‐81. [Google Scholar]
Gao 2001 {published data only}
- Gao ER, Zhao Sh, Lou CH. Study on the acceptability of emergency contraception among those who underwent induced abortion. Reproduction & Contraception (China) 2001;21:104‐9. [Google Scholar]
Glasier 2013 {published data only}
- Glasier A. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Obstetrical and Gynecological Survey 2013;68(3):208‐10. [DOI] [PubMed] [Google Scholar]
Gottardi 1979 {published data only}
- Gottardi G, Marzi MM, Pozzi S. Postcoital estrogen or IUD? [Oestrogène postcoital ou DIU?]. IPPF Europe Bulletin d'Information Régional 1979;8:7‐8. [PubMed] [Google Scholar]
Gottardi 1986 {published data only}
- Gottardi G, Spreafico A, Orchi L. The postcoital IUD as an effective continuing contraceptive method. Contraception 1986;34:549‐58. [DOI] [PubMed] [Google Scholar]
Gu 2002 {published data only}
- Gu XY, Yie TF. Clinical study of the effect of Multiload 375 SL and levo‐norgestrel on emergency contraception. Chinese Journal of Family Planning 2002;10:740‐2. [Google Scholar]
Guillebaud 1983 {published data only}
- Guillebaud J, Kubba A, Rowlands S, White J, Elder EG. Postcoital contraception with danazol, compared with an ethinyloestradiol‐norgestrel combination or insertion of a intrauterine device. Journal of Obstetrics and Gynaecology 1983;suppl 1:s64‐8. [Google Scholar]
Halpern 2010 {published data only}
- Halpern V, Raymond EG, Lopez LM. Repeated use of pre‐ and postcoital hormonal contraception for prevention of pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007595.pub2] [DOI] [PubMed] [Google Scholar]
Han 1999b {published data only}
- Han X, Wong L, Sun J. A clinical study on mifepristone alone and in combination with anordrin for emergency contraception. Chinese Journal of Family Planning 1999;7:411‐4. [Google Scholar]
Han 2001b {published data only}
- Han Y. The clinical observation of GyneFix IUD for emergency contraception. Journal of Practical Obstetrics and Gynecology 2001;17:171‐2. [Google Scholar]
Haspels 1976 {published data only}
- Haspels AA. Interception: post‐coital estrogens in 3016 women. Contraception 1976;14:375‐81. [DOI] [PubMed] [Google Scholar]
He 1991 {published data only}
- He C, Shi Y, Xu J, Look PFA. A multicenter clinical study on two types of levonorgestrel tablets administered for postcoital contraception. International Journal of Gynecology and Obstetrics 1991;36:43‐8. [DOI] [PubMed] [Google Scholar]
Ho 2013 {published data only}
- Ho PC. Levonorgestrel (LNG)‐development of hormonal methods of emergency contraception. European Journal of Contraception and Reproductive Health Care 2013;18:S44‐45. [Google Scholar]
Hoffman 1983 {published data only}
- Hoffman KOK. Postcoital contraception: experiences with ethinyl oestradiol/norgestrel and levonorgestrel only. In: Harrison RF, Bonnar J, Thompson W editor(s). Fertility and Sterility. Dublin: IFFS Fertility and Sterility, 1983:311‐6. [Google Scholar]
Jiang 2000 {published data only}
- Jiang L, Duan Y, Sun Y. A comparative study of mifepristone with levonorgestrel for emergency contraception. Chinese Journal of Family Planning 2000;8:463‐4. [Google Scholar]
Jiang 2002 {published data only}
- Jiang DX, Wu ER. Effects of gestrinone (R2323) on emergency contraception: a clinical observation of 120 cases. Journal of Reproductive Medicine 2002;11:326‐30. [Google Scholar]
Jin 2005 {published data only}
- Jin J, Weisberg E, Fraser IS. Comparison of three single doses of mifepristone as mifepristone as emergency contraception: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2005;45:489‐94. [DOI] [PubMed] [Google Scholar]
Kesserü 1973 {published data only}
- Kesserü E, Larranaga A, Parada J. Postcoital contraception with d‐norgestrel. Contraception 1973;7:367‐79. [Google Scholar]
Li 2001 {published data only}
- Li XY, Hu LY. A study of low‐dose mifepristone for emergency contraception. Chinese Journal of Practical Gynaecology and Obstetrics 2001;17:619‐20. [Google Scholar]
Li 2002b {published data only}
- Li F, Chen YX, Tang JH. Emergency contraception by low‐dose mifepristone: observation of 150 cases. Journal of First Military Medical University 2002;22:466. [PubMed] [Google Scholar]
Li 2005a {published data only}
- Li F, Qian X, Wu W. A comparative study of mifepristone with Cu‐IUD for emergency contraception. Journal of Practice Medicine 2005;21:2313‐4. [Google Scholar]
Lippes 1976 {published data only}
- Lippes J, Malik T, Tatum HJ, Zielezny M. The postcoital copper‐T. Advances in Planned Parenthood 1976;11:24‐9. [PubMed] [Google Scholar]
Lippes 1979 {published data only}
- Lippes J, Tatum HJ, Maulid D, Zielezny M. Postcoital copper IUD found to be effective in preventing pregnancy. Family Planning Perspectives 1979;11:195. [PubMed] [Google Scholar]
Liu 2002a {published data only}
- Liu Y, Chen X. A comparative study of mifepristone with Cu‐IUD for emergency contraception. Journal of Qiqihar Medical College 2002;23:890‐1. [Google Scholar]
Luerti 1986 {published data only}
- Luerti M, Tonta A, Feria P, Molla R, Santini F. Post‐coital contraception by estrogen‐progestagen combination or IUD insertion. Contraception 1986;33:61‐8. [DOI] [PubMed] [Google Scholar]
Ma 2001 {published data only}
- Ma J. A study on 110 cases of emergency contraception. Chinese Journal of Practical Gynaecology and Obstetrics 2001;17:189. [Google Scholar]
Mo 2004 {published data only}
- Mo Y. A clinical observation on different dose of mifepristone for emergency contraception. Hainan Yi Xue 2004;15:42‐3. [Google Scholar]
Mor 2005 {published data only}
- Mor E, Saadat P, Kives S, White E, Reid RL, Paulson RJ, et al. Comparison of vaginal and oral administration of emergency contraception. Fertility and Sterility 2005;84:40‐5. [DOI] [PubMed] [Google Scholar]
Moreau 2012 {published data only}
- Moreau C, Trussell J. Results from pooled phase III studies of ulipristal scetate for emergency contraception. Contraception 2012;86:673‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piaggio 2003a {published data only}
- Piaggio G, Heng Z, Hertzen H, Bilian X, Linan C. Combined estimates of effectiveness of mifepristone 10mg in emergency contraception. Contraception 2003;68:439‐46. [DOI] [PubMed] [Google Scholar]
Piaggio 2003b {published data only}
- Piaggio G, Hertzen H, Zhao H, Xiao BL, Cheng L. Meta‐analyses of randomized trials comparing different doses of mifepristone in emergency contraception. Contraception 2003;68:447‐52. [DOI] [PubMed] [Google Scholar]
Polakow 2013 {published data only}
- Polakow FS. Levonorgestrel used for emergency contraception during lactation‐A prospective observational cohort study on maternal and infant safety. Journal of Maternal‐Fetal and Neonatal Medicine 2013;26(3):219‐21. [DOI] [PubMed] [Google Scholar]
Qi 2000b {published data only}
- Qi Y, Zhang J, Cao Y, Yan W, Zhang Z. A clinical study on mifepristone at low dose for emergency contraception. Chinese Journal of Family Planning 2000;8:305‐7. [Google Scholar]
Qiao 2002 {published data only}
- Qiao Y. A clinical trial of mifepristone in combination with MTX for emergency contraception. Journal of Jining Medical College 2002;25:44. [Google Scholar]
Qin 2000 {published data only}
- Qin C. A clinical study on 137 cases of emergency contraception with mifepristone. Zhejiang Journal of Clinical Medicine 2000;2:302‐3. [Google Scholar]
Raymond 2000 {published data only}
- Raymond EG, Creinin MD, Barnhart KT, Lovvorn AE, Rountree RE, Trussell J. Meclizine for prevention of nausea associated with use of emergency contraceptive pills: a randomized trial. Obstetrics and Gynecology 2000;95:271‐7. [DOI] [PubMed] [Google Scholar]
Raymond 2006 {published data only}
- Raymond EG, Stewart F, Weaver M, Monteith C, Pol B. Impact of increased access to emergency contraceptive pills. Obstetrics and Gynecology 2006;108(5):1098‐106. [DOI] [PubMed] [Google Scholar]
Roye 2001 {published data only}
- Roye CF. Routine provision of emergency contraception to teens and subsequent condom use: a preliminary study. Journal of Adolescent Health 2001;28:165‐6. [DOI] [PubMed] [Google Scholar]
Ruan 2012 {published data only}
- Ruan JH. A clinical observation on LNG for emergency contraception [复方左炔诺孕酮用于紧急避孕的临床观察]. China Medicine and Pharmacy 2012;2(18):80‐1. [Google Scholar]
Scarduelli 1998 {published data only}
- Scarduelli C, Anselmino M, Caccamo A, Sezzi E, Lombroso Finzi GC. Emergency contraception: a new evaluation of effectiveness. P‐159. Abstracts of the 14th Annual Meeting of the ESHRF, Göteborg. 1998:208‐9.
Schilling 1979 {published data only}
- Schilling LH. An alternative to the use of high dose estrogen for postcoital contraception. Journal of American College of Health Association 1979;27:247‐9. [PubMed] [Google Scholar]
Schreiber 2010 {published data only}
- Schreiber CA, Ratcliffe SJ, Barnhart KT. A randomized controlled trial of the effect of advanced supply of emergency contraception in postpartum teens: a feasibility study. Contraception 2010;81:435‐40. [DOI] [PubMed] [Google Scholar]
Scott 2012 {published data only}
- Scott LJ. Ulipristal acetate: a guide to its use in emergency contraception. Drugs and Therapy Perspectives 2012;28(2):6‐9. [Google Scholar]
Shaaban 2013 {published data only}
- Shaaban OM, Hassen SG, Nour SA, et al. Emergency contraceptive pills as a backup for lactational amenorrhea method (LAM) of contraception: a randomized controlled trial. Contraception 2013;87:363‐369. [DOI] [PubMed] [Google Scholar]
Shen 2010 {published data only}
- Shen HX. A observational study of mifepristone for emergency contraception. Journal of China Traditional Chinese Medicine Information 2010;2(28):163. [Google Scholar]
Shochet 2004 {published data only}
- Shochet T, Blanchard K, King H, Henchcliffe B, Hunt J. Side effects of the Yuzpe regimen of emergency contraception and modifications. Contraception 2004;69:301‐7. [DOI] [PubMed] [Google Scholar]
Song 2007 {published data only}
- Song ZH, Wang Y, Chen P, Wang D, Lu WH. A clinical study of mifepristone and the copper IUD for emergency contraception. Chinese Medicine of Factory and Mine 2007;20(6):630‐1. [Google Scholar]
Sun 2005 {published data only}
- Sun Y, Che Y, Ding Y, Zhou W, Han Y, Fang K, et al. Systematic review of emergency contraception. Chinese Journal of Family Planning 2005;4:217‐22. [Google Scholar]
Tian 2000 {published data only}
- Tian Q. A comparative study of mifepristone with Cu‐IUD for emergency contraception. Journal of Henan Medical College for Staff and Workers 2000;12:51. [Google Scholar]
Turok 2010 {published data only}
- Turok DK, Gurtcheff SE, Handley E, Simonsen SE, Sok C, Murphy P. A pilot study of the copper T380A IUD and oral levonorgestrel for emergency contraception. Contraception 2010;82:520‐5. [DOI] [PubMed] [Google Scholar]
Turok 2014 {published data only}
- Turok DK, Jacobson JC, Dermish AI, Simonsen SE, Gurtcheff S, McFadden M, et al. Emergency contraception with a copper ICD or oral levonorgestrel: an observational study of 1‐year pregnancy rates. Contraception 2014;89(2014):222‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turok 2016 {published data only}
- Turok DK, Sanders JN, Thompson IS. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception 2016;93(6):526‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Santen 1983 {published data only}
- Santen MR, Haspels AA. Postcoital contraception with an IUD [Contraccezione con D.I.U. post‐coitale]. Contraccezione, Fertilita, Sessualita 1983;10:549‐57. [Google Scholar]
Van Santen 1985b {published data only}
- Santen MR, Haspels AA. Interception II: postcoital low‐dose estrogens and norgestrel combination in 633 women. Contraception 1985;31:275‐93. [DOI] [PubMed] [Google Scholar]
Virjo 1999 {published data only}
- Virjo I, Kirkkola AL, Isokoski M, Mattila K. Use and knowledge of hormonal emergency contraception. Advances in Contraception 1999;15:85‐94. [DOI] [PubMed] [Google Scholar]
Wang 2006b {published data only}
- Wang CP, Liu Y, Chang YF, Shao WQ. A comparing study of the copper intrauterine device and low dose of mifepristone for emergency contraception. Journal of Reproductive Medicine 2006;15(4):271‐3. [Google Scholar]
Wei 2002b {published data only}
- Wei R. Low‐dose of mifepristone for emergency contraception: observation of 309 cases. Jiangxi Medical Journal 2002;37:102‐4. [Google Scholar]
Wu 1999b {published data only}
- Wu C, Zhang Y. An extend study on using single dose of mifepriston 25mg for emergency contraception. Chinese Journal of Family Planning 1999;7:358‐60. [Google Scholar]
Wu 2005 {published data only}
- Wu S, Zhou Y. Clinical use of emergency contraception pill. Chinese Journal of Practical Gynaecology and Obstetrics 2005;21:15‐7. [Google Scholar]
Xiao 2004 {published data only}
- Xiao BL. Clinical study of emergency contraception with low‐dose mifepristone. Chinese Journal of Obstetrics and Gynecology 2004;39:35‐8. [PubMed] [Google Scholar]
Yang 2002 {published data only}
- Yang Y, Liang X, Liu X. Low‐dose of mifepristone for emergency contraception: observation of 106 cases. Heilongjiang Medical Journal 2002;26:283. [Google Scholar]
Ye 2014 {published data only}
- Ye JM. A clinical observation on LNG for emergency contraception. Chinese journal of ethnomedicine and ethnopharmacy 2014;9:75. [Google Scholar]
Yu 2001 {published data only}
- Yu MD. A primary discussion of the drugs for emergency contraception. Anhui Medical and Pharmaceutical Journal 2001;5:95‐6. [Google Scholar]
Yuzpe 1974 {published data only}
- Yuzpe AA, Thurlow HJ, Ramzy I, Leushon JL. Postcoital contraception ‐ a pilot study. Journal of Reproductive Medicine 1974;1:53‐8. [PubMed] [Google Scholar]
Yuzpe 1977 {published data only}
- Yuzpe AA, Lancee WJ. Ethinylestradiol and dl‐norgestrel as a postcoital contraceptive. Fertility and Sterility 1977;28:932‐6. [PubMed] [Google Scholar]
Yuzpe 1982 {published data only}
- Yuzpe AA, Percival Smith R, Rademaker AW. A multicentre clinical investigation employing ethinylestradiol combined with dl‐norgestrel as a postcoital contraceptive agent. Fertility and Sterility 1982;37:508‐13. [DOI] [PubMed] [Google Scholar]
Zhang 1999a {published data only}
- Zhang J, Jing X, Wong L. Cu‐IUD versus mifepristone for emergency contraception. Chinese Journal of Obstetrics and Gynecology 1999;34:569‐70. [Google Scholar]
Zhang 1999c {published data only}
- Zhang M, Yang H, Wang Z, Liang X, Wang Y. A study on mifepristone alone and in combination with anordrin for emergency contraception. Zhejiang Journal of Practical Medicine 1999;4:1‐2. [Google Scholar]
Zhang 1999d {published data only}
- Zhang X, Du M, Ying Y. A study on mifepristone alone and in combination with anordrin for emergency contraception. Reproduction and Contraception (China) 1999;19:163‐8. [Google Scholar]
Zhang 1999e {published data only}
- Zhang X, Leng Y, Shi J, Gao G, Xu Y, Sun H. A study on LNG for emergency contraception. Chinese Journal of Family Planning 1999;7:375‐6. [Google Scholar]
Zhao 2006 {published data only}
- Zho H, Han L. Analysis of the reason for failure of emergency contraception. Journal of China‐Japan Friendship Hospital 2006;20:207‐10. [Google Scholar]
Zhao H 2001 {published data only}
- Zhao H, Tang JR, Wu MH, Cheng H. A comparative study of mifepristone with IUCD for emergency contraception. Journal of Capital University of Medical Sciences 2001;22:273‐4. [Google Scholar]
Zhu 1999 {published data only}
- Zhu P, Chai J, Wang N, Li G. An initial observation of mifepristone combined with MTX for the use of emergency contraception. Guangdong Journal of Medicine 1999;20:11‐2. [Google Scholar]
Zhu 2007 {published data only}
- Zhu YH, Ou YL. Clinical observational study of three methods for emergency contraception. Journal of Medical Theory and Practice 2007;20(2):200‐2. [Google Scholar]
Zuliani 1990 {published data only}
- Colombo UF, Zuliani G, Benzi G, Bregozzo T, Viezzoli T. [Contraccezione post coitale ormonale con danazolo: ristati di due differenti schemi posologici]. Pediatric and Adolescent Gynaecology. Paper presented at the III European Symposium on Pediatric and Adolescent Gynaecology; 1987 Oct 7‐10; Florence. Florence, Italy: CIC Edizioni Internazionali, 1987:206‐11.
- Zuliani G, Colombo UF, Luerti M, Casolati E, Viezzoli T. Postcoital contraception with an ethinylestradiol‐norgestrel combination and two different danazol regimens. In: Genazzani AR, Petraglia F, Volpe A, Facchinetti F editor(s). Recent Research on Gynecological Endocrinology. New Jersey: Parthenon Publishing, 1988. [Google Scholar]
- Zuliani G, Colombo UF, Molla R. Hormonal postcoital contraception with an ethinylestradiol‐norgestrel combination and two danazol regimens. European Journal of Obstetrics Gynecology and Reproductive Biology 1990;37:253‐60. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Colombo UF, Molla R, Bregozzo T, Mojana G. [Confronto tra danazolo e etinilestradiolo‐norgestrel utilzzati come intercettori post‐coitali ormonali: studio clinico randomizzato]. Congresso Internazionale di Endocrinologia Ginecologica. Madonna di Campiglio, 1986 16‐22 Mar, Bologna. 1986:341‐4.
References to ongoing studies
NCT01539720 {unpublished data only}
- NCT01539720. Levonorgestrel intrauterine system for emergency contraception (LIFE). clinicaltrials.gov/show/NCT01539720 first received: 21 February, 2012.
NCT02175030 {unpublished data only}
- NCT02175030. RAPID EC ‐ RCT assessing pregnancy with intrauterine devices for EC. clinicaltrials.gov/show/NCT02175030 first received: 23 June , 2014.
NCT02577601 {unpublished data only}
- NCT02577601. Impact of combined hormonal contraceptives on UPA. clinicaltrials.gov/show/NCT02577601 first received: 5 August , 2015.
Additional references
Baird 2015
- Baird DT, Cameron S, Evers JLH, Gemzell‐Danielsson K, Glasier A, Moreau C, et al. Emergency contraception. Widely available and effective but disappointing as a public health intervention: a review. Hum Reprod 2015;30(4):751‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cleland 2012
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience.. Hum Reprod 2012;27:1994‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Edelman 2016
- Edelman AB, Cherala G, Blue SW, Erikson DW, Jensen JT. Impact of obesity on the pharmacokinetics of levonorgestrel‐based emergency contraception: single and double dosing. Contraception 2016;94:52‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fine 2010a
- Fine P, Mathe H, Ginde S, Cullins V, Morfesis J, Gainer E. Ulipristal acetate taken 48–120 hours after intercourse for emergency contraception.. Obstet Gynecol 2010;115:257‐63. [DOI] [PubMed] [Google Scholar]
Gemzell‐Danielsson 2004
- Gemzell‐Danielsson K, Marions L. Mechanisms of action of mifepristone and levonorgestrel when used for emergency contraception. Hum Reprod Update 2004;10:341‐8. [DOI] [PubMed] [Google Scholar]
Gemzell‐Danielsson 2015
- Gemzell‐Danielsson K, Kardos L, Hertzen H. Impact of bodyweight/body mass index on the effectiveness of emergency contraception with levonorgestrel: a pooled‐analysis of three randomized controlled trials. Curr Med Res Opin 2015;31:2241‐8. [DOI] [PubMed] [Google Scholar]
Glasier 1997
- Glasier A. Emergency postcoital contraception. New England Journal of Medicine 1997;337:1058‐64. [DOI] [PubMed] [Google Scholar]
Glasier 2011
- Glasier A, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011;84:363‐7. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 15 March 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grimes 1997
- Grimes DA. Emergency contraception: expanding opportunities for primary prevention. New England Journal of Medicine 1997;337:1078‐9. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICEC 2012a
- International Consortium for Emergency Contraception (ICEC). Knowledge and ever used of emergency contraception in Latin America. www.cecinfo.org/custom‐content/uploads/UserFiles/File/DHS/Emergency%20Contraception%20in%20Latin%20America.pdf (Posted on April 25, 2011).
ICEC 2012b
- ICEC. Knowledge and ever used of emergency contraception in Africa. www.cecinfo.org/custom‐content/uploads/UserFiles/File/DHS/Emergency%20Contraception%20in%20Africa.pdf (Posted on April 25, 2011).
ICEC 2012c
- ICEC. Knowledge and ever used of emergency contraception in Europe and West Asia.. www.cecinfo.org/custom‐content/uploads/UserFiles/File/DHS/Emergency%20Contraception%20in%20Europe%20and%20West%20Asia.pdf (Posted on April 25, 2011).
INEI 2011
- Instituto Nacional De Estadística E Informática (INEI) and ICF Macro. Peru: DHS, 2010 ‐ Final report continuous (2010). ORC Macro / Measure DHS+, May 2011. [Google Scholar]
Jatlaoui 2016a
- Jatlaoui TC, Curtis KM. Safety and effectiveness data for emergency contraceptive pills among women with obesity: a systematic review. Contraception 2016;94:605‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jatlaoui 2016b
- Jatlaoui TC. Safety data for levonorgestrel, ulipristal acetate and Yuzpe regimens for emergency contraception.. Contraception 93;2:93‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapp 2015
- Kapp N, Abitbol JL, Mathe H, Scherrer B, Guillard H, Gainer E, et al. Effect of body weight and BMI on the efficacy of levonorgestrel emergency contraception. Contraception 2015;91(2):97‐104. [DOI] [PubMed] [Google Scholar]
Lalitkumar 2013
- Lalitkumar PGL. Emergency contraception. Best Practice and Research: Clinical Endocrinology and Metabolism 27;1:91‐101. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Milosavljevic 2014
- Milosavljevic J. Mechanism of action, efficacy and safety of emergency hormonal contraception (levonorgestrel and ulipristal acetate) and attitudes of pharmacists. Acta Facultatis Medicae Naissensis 31;3:155‐61. [Google Scholar]
Mozzanega 2014
- Mozzanega B. Ulipristal acetate: critical review about endometrial and ovulatory effects in emergency contraception. Reproductive Sciences 21;6:678‐85. [DOI] [PubMed] [Google Scholar]
Palermo 2014
- Palermo T, Bleck J, Westley E. Knowledge and use of emergency contraception: a multicountry analysis. International Perspectives on Sexual and Reproductive Health 2014;40(2):79‐86. [DOI: 10.1363/4007914] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collabortion. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collabortion, 2014.
Sedgh 2012
- Sedgh G, Singh S, Shah IH, Ahman E, Henshaw SK, Bankole A. Induced abortion: incidence and trends worldwide from 1995 to 2008. Lancet 2012;379(9816):625‐32. [DOI] [PubMed] [Google Scholar]
Sedgh 2014
- Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Studies in Family Planning 2014;45(3):301‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shohel 2014
- Shohel M, Rahman MM, Zaman A, Uddin MMN, Al‐Amin MM, Reza HM. A systematic review of effectiveness and safety of different regimens of levonorgestrel oral tablets for emergency contraception. BMC Women's Health 14;1:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trussell 2012
- Trussell J, Raymond EG. Emergency contraception: a last chance to prevent unwanted pregnancy. April 2012. ec.princeton.edu/questions/ec‐review.pdf (accessed 3 June 2012).
Van Look 1993
- Look PFA, Hertzen H. Emergency contraception. British Medical Bulletin 1993;49:158‐70. [DOI] [PubMed] [Google Scholar]
Webb 1995
- Webb A. Emergency contraception. Fertility Control Reviews 1995;4:3‐7. [Google Scholar]
References to other published versions of this review
Cheng 1999
- Cheng L, Gülmezoglu M, Ezcurra E, Look PFA. Interventions for emergency contraception. Cochrane Database of Systematic Reviews 1999, Issue 7. [DOI: 10.1002/14651858.CD001324.pub4] [DOI] [PubMed] [Google Scholar]
Cheng 2004
- Cheng L, Gülmezoglu M, Oel CJ, Piaggio G, Ezcurra E, Look PFA. Interventions for emergency contraception. Cochrane Database of Systematic Reviews 2004, Issue 7. [DOI: 10.1002/14651858.CD001324.pub4] [DOI] [PubMed] [Google Scholar]
Cheng 2008
- Cheng L, Gülmezoglu M, Piaggio GP, Ezcurra E, Look PFA. Interventions for emergency contraception. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001324.pub4] [DOI] [PubMed] [Google Scholar]
Cheng 2012
- Cheng L, Che Y, Gülmezoglu AM. Interventions for emergency contraception. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD001324.pub4] [DOI] [PubMed] [Google Scholar]


