Abstract
Objective:
Complete systems for laboratory-based inhalation toxicology studies are typically not commercially available; therefore, inhalation toxicologists utilize custom-made exposure systems. Here we report on the design, construction, testing, operation and maintenance of a newly developed in vivo rodent ozone inhalation exposure system.
Materials and methods:
Key design requirements for the system included large-capacity exposure chambers to facilitate studies with large sample sizes, automatic and precise control of chamber ozone concentrations, as well as automated data collection on airflow and environmental conditions. The exposure system contains two Hazelton H-1000 stainless steel and glass exposure chambers, each providing capacity for up to 180 mice or 96 rats. We developed an empirically tuned proportional-integral-derivative control loop that provides stable ozone concentrations throughout the exposure period (typically 3h), after a short ramp time (~8min), and across a tested concentration range of 0.2–2ppm. Specific details on the combination of analog and digital input/output system for environmental data acquisition, control and safety systems are provided, and we outline the steps involved in maintenance and calibration of the system.
Results:
We show that the exposure system produces consistent ozone exposures both within and across experiments, as evidenced by low coefficients of variation in chamber ozone concentration and consistent biological responses (airway inflammation) in mice, respectively.
Conclusion:
Thus, we have created a large and robust ozone exposure system, facilitating future studies on the health effects of ozone in rodents.
Keywords: Ozone, air pollution, inhalation, exposure, computer-controlled, exposure system
Introduction
Ozone exposure is associated with significant short and long-term adverse health effects (Ito et al., 2005; Jerrett et al., 2009; Levy et al., 2001, 2005). As a powerful oxidant and respiratory irritant, ozone reacts with airway lining fluid constituents to generate biologically active compounds that cause immediate lung function decrements and acute toxicity (Mudway and Kelly, 2000; Nielsen et al., 1999; Pryor et al., 1995). Within hours of inhalation exposure to ozone, there is an inflammatory response in the respiratory tract characterized by cytokine release and an influx of macrophages and neutrophils in the lung (Aris et al., 1993; Devlin et al., 1996; Mudway and Kelly, 2000). Downstream consequences of ozone exposure include increased susceptibility to respiratory infections and exacerbations of existing airway diseases such as asthma and chronic obstructive airway disease (Ji et al., 2011; Kim et al., 2011; Ko et al., 2007). Long-term exposure to ozone has also been linked to increased incidence of diabetes, cardiovascular disease, neurological disease and mortality (Cleary et al., 2018; Day et al., 2017; Jerrett et al., 2017; Turner et al., 2016).
Although its general biological effects are well studied, the toxicological mechanisms underlying these adverse health effects of ozone remain the subject of ongoing research. Particularly active areas of research include, but are not limited to, identification of specific biologically active products of airway surface liquid ozonation (Speen et al., 2016), the mechanisms of systemic cardiovascular and neurological effects (Miller, Ghio, et al., 2016; Miller, Snow, et al., 2016; Paffett et al., 2015; Tyler et al., 2018) and the influence of ozone on the pathogenesis (versus exacerbation) of respiratory diseases (Herring et al., 2015; Michaudel et al., 2018; Zu et al., 2018). Additionally, the mechanisms underlying sex differences in response to ozone (Cabello et al., 2015; Cho et al., 2019) and genetic modifiers of response (Bauer and Kleeberger, 2010) are also important data gaps that need to be addressed. A combination of epidemiological and experimental approaches (controlled in vivo human and rodent studies, and in vitro systems) will be needed to answer all of these questions. We have focused our work on the use of rodent models to study ozone toxicity, including inter-individual differences in response. In particular, we aim to identify genetic predictors of ozone response using a population of genetically diverse mice, which requires quite large sample sizes (e.g., more than 300 mice). To facilitate such large-scale inhalation studies, we designed and constructed a new large-scale, computer-controlled ozone exposure system for rodents at the University of North Carolina at Chapel Hill.
Inhalation exposure systems typically require a great deal of custom engineering. The design of exposure systems requires the expertise of inhalation toxicologists and engineers, and individual components often need to be acquired from several different commercial sources or fabricated in-house. Because we found these aspects to be true in developing our exposure system, we have written this manuscript to serve as a helpful reference for others considering developing a similar exposure system. The general design as well as many of the components used for the construction of our chambers will likely work well for systems of different sizes implemented by other investigators.
There are several reviews describing guidelines on the best practices for inhalation toxicology studies (Chen and Lippmann, 2015; Cheng and Moss, 1995; Dorato, 1990; MacFarland, 1983; Pauluhn, 2003; Phalen, 1976; Phalen et al., 1984; Wong, 2007). Detailed manuscripts on the design of inhalation exposure systems for gases such as ozone and various other classes of airborne toxicants such as particulate matter, aerosols and vapors have also been published (Goldsmith et al., 2011; Johnson and Fechter, 1996; McKinney and Frazer, 2008; O’Shaughnessy et al., 2003; Wong, 2003). We based the design of our system primarily on these manuscripts and an existing facility at the U.S. EPA’s Inhalation Toxicology Facilities Branch in Research Triangle Park, NC. The EPA’s inhalation facility houses a number of laboratory spaces with various styles of chambers and capabilities for a variety of air pollutant exposures. Our completed system shares several of the same components as the EPA ozone exposure system, namely the H-1000 chambers, airflow parameters, DASYLab® control software and Thermo 49i ozone analyzer. Because of the relatively smaller size of our system (2 versus 4 chambers at the EPA) and laboratory space, we made some decisions to use or construct different components.
Our goal was to design and implement an ozone exposure system that complies with published guidelines (Chen and Lippmann, 2015; OECD, 2018; Phalen, 1976), while at the same time meeting our specific experimental requirements. General design considerations for whole-body inhalation toxicology studies include a delivery system for clean air, compatible/inert construction materials, control and characterization of the exposure atmosphere, and maintenance of suitable environmental conditions without the buildup of waste gases (Phalen, 1976). In addition to these general requirements, we specifically needed the ability to expose a large number of mice to minimize the number of batches (which could confound analyses), an automated control system to maintain precise and reproducible exposures, and automated monitoring and recording of data on several environmental parameters. Here we report on the design, construction and performance of a large-capacity exposure system for rodents that meets these requirements.
Materials and methods
Exposure system design and operation
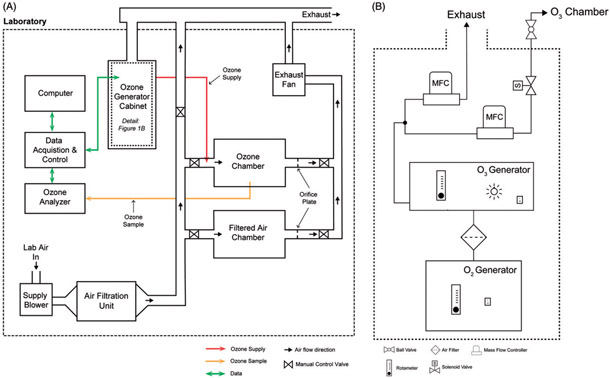
The specific design requirements for our inhalation exposure system were: (1) the ability to expose large numbers of mice, (2) an air-supply and exhaust system to maintain slight negative pressure and 15-air changes per hour, (3) automatic computer-control of chamber ozone concentration, (4) production of reproducible and consistent exposure atmospheres, (5) a safety cutoff system and (6) automated recording of environmental data. We have provided block diagrams of the complete system in Figure 1(A,B) and screen captures from the computer control system in Figure 2(A,B). For reference, we have provided major individual components used to construct the system in Table 1 and detailed wiring diagrams as Supplemental Material.
Figure 1.
Block diagrams of the exposure system. (A) Overview of major components including the clean air supply, chambers, ozone control system, chamber and cabinet exhaust. (B) Detail of oxygen and ozone generator cabinet. Compressed, concentrated oxygen flows from the oxygen generator to the ozone generator then enters a stainless steel manifold attached to the supply and waste mass flow controllers. The waste flow vents into the cabinet exhaust. Downstream of the supply mass flow controller within the gas safety cabinet is a relay actuated solenoid valve. Outside the cabinet is a manual control valve before the ozone supply tubing enters the chamber supply pipe.
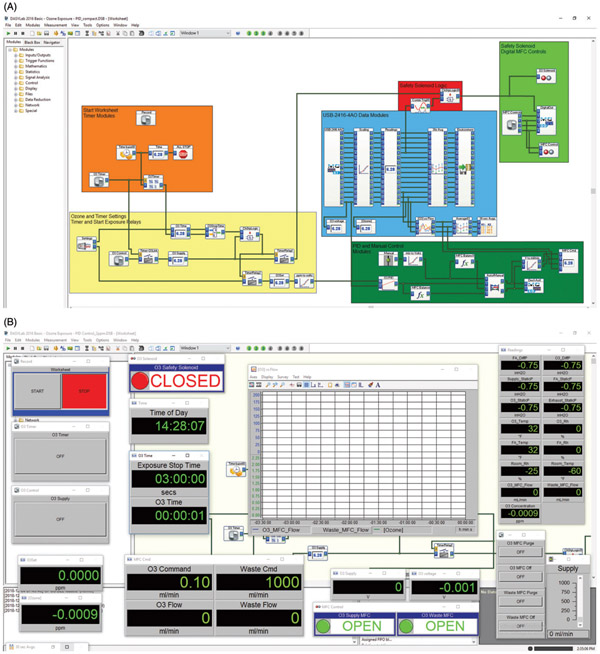
Figure 2.
Screen captures of the DASYLab® computer-control system. (A) Layout of worksheet modules used to acquire and record environmental data, operate the mass flow controllers (manual and automatic), operate the safety shutoff program, and control the duration and concentration of exposures. (B) View of worksheet showing switches, digital displays, chart recorder, manual control slider, and valve status indicators.
Table 1.
List of major exposure system components.
| System component | Product description | Manufacturer | Location |
|---|---|---|---|
| Pressure Blower | Model FPB-70F-M5 | Greenheck | Schofield, WI |
| Chamber Ozone Analyzer | Model 49i Ozone Analyzer | Thermo Scientific | Waltham, MA |
| Chamber T/Rh Transmitters | HX94BV1W | Omega | Norwalk, CT |
| Computer | Optiplex 3040 | Dell | Round Rock, TX |
| Control Software | DASYLab® 2016 Basic V. 14.0 | Measurement Computing | Norton, MA |
| Custom Air Filtration Unit Housing | 4-Stage Side Access | AAF Flanders | Louisville, KY |
| Exhaust Fan | Model FR110 | Fantech | Lenexa, KS |
| Exposure Chamber | Hazelton H-1000 | Lab Products | Seaford, DE |
| Gas Safety Cabinet | 7200 2-Cylinder | Safety Equipment Corporation | Belmont, CA |
| Laboratory Ozone Detector | C-30ZX | EcoSensors | Newark, CA |
| Mass Flow Controllers | Aalborg GFC-17 | Aalborg | Orangeburg, NY |
| Oxygen Generator | Onyx A-016 | A2Z Ozone Inc. | Louisville, KY |
| Ozone Generator | 4G Lab Benchtop | A2Z Ozone Inc. | Louisville, KY |
| Pressure Transmitters | DM-2006-LCD | Dwyer | Michigan City, IN |
| Room T/Rh Transmitter | RHP-2W11-LCD | Dwyer | Michigan City, IN |
| USB Data Acquisition and Control | USB-2416-4AO | Measurement Computing | Norton, MA |
T: temperature; Rh: relative humidity.
Exposure chambers
We designed the system to provide controlled, filtered air and air plus ozone exposure atmospheres using two commercially available Hazelton H-1000 stainless steel and glass whole-body inhalation exposure chambers (Lab Products) (Brown and Moss, 1981). Two doors at the front and rear of the chambers provide access to the animal cages and visual monitoring of animals in the chamber. Within the chambers, three levels of wire mesh cage racks are mounted on sliding tracks with pans to collect excreta beneath each cage rack. Cage racks accommodate up to 60 mice each (larger cages are available for rats and other laboratory animals). The cage racks feature removable food trays and an automatic watering system to facilitate animal housing and/or multiple days of exposure. The H-1000 chambers have several ports in each glass door and in the top of the steel chamber body. We used three ports on each chamber to monitor: (1) static air pressure relative to the laboratory, (2) ozone concentration and (3) temperature and relative humidity. The top and bottom of the chambers have 3-inch diameter clamp-style sanitary fittings that we connected to the stainless steel air supply ducts and CPVC exhaust piping, respectively.
Air supply and exhaust system
At the top of the chambers, we connected a filtered air supply system. Filtered, temperature, and humidity conditioned laboratory air, supplied by a pressure blower, was passed through a custom air filtration unit made by AAF Flanders (AAF, Smithfield, NC). The filtration unit, consisting of a side access filter housing and four stages of filters (Stage 1: 2” Minimum Efficiency Reporting Value (MERV) 8 prefilter, Stage 2: 12”-SAAF™ gas filtration cassette, Stage 3: MERV 8 prepost filter and Stage 4: MERV 14 Final filter) removed ambient particulate matter, ozone and other gases from the air stream. Following the filtration unit, air entered a 3-inch diameter stainless steel ducted supply manifold. The supply manifold divided the filtered air into an excess supply vent (to exhaust) and two chamber drops. We installed manual control valves at the excess supply vent and chamber drops to adjust supply manifold pressure and chamber inlet airflow, respectively. Immediately downstream of each of the chambers were 3-inch CPVC exhaust lines with orifice plates and individual flow control valves (chamber flow and static pressure balance) that combine at an exhaust manifold and then pass through an inline duct fan to enter the laboratory’s exhaust system.
Chamber balancing/airflow calibration
We designed the system to have a nominal airflow rate of 250 L/min (15-air changes/hour/chamber) and a slight negative chamber pressure relative to the room to minimize the possibility of ozone entering the laboratory. We achieved these parameters by sequentially adjusting the supply and exhaust fan speeds and the system of control valves. While adjusting the system of fans and valves, we measured the static and differential pressures at specific locations of the air supply, chambers and exhaust. First, the operating pressure ranges of the chamber supply and exhaust manifolds were determined by running the supply blower and exhaust fans while the valves to the chambers were closed. After we achieved a sufficient difference between the supply and exhaust manifold static pressures (relative to the laboratory), we opened the chamber valves and adjusted them until the measurement of an orifice flow meter installed in the chamber exhaust corresponded to 250 L/min. The orifice flow meter we constructed consists of a flow restrictive orifice plate and a pressure transmitter connected to taps in the exhaust pipe to measure the differential pressure between up and downstream of the orifice. To generate a calibration curve for the orifice flow meter, we made airflow measurements with a pitot tube placed several feet downstream of the orifice and plotted these measurements against differential pressure measurements at a series of exhaust and supply valve settings. The calibration curve enables the maintenance of airflow at 250 L/min based on the corresponding orifice flow meter reading. To enable the monitoring of pressure readings directly (without the computer control software open), we mounted all of the static and differential pressure transmitters (6 units) in the door of a wall cabinet where they are visible. We also installed electrical conduit with wires to carry 4–20 mA current signals from the pressure transmitters to the opposite side of the laboratory. We terminated the wires at a USB microcontroller, which converts the 4–20 mA analog signal to a digital signal enabling visualization and recording on a computer.
Ozone generation and analysis
Ozone generation equipment was housed in a compressed gas safety cabinet (Figure 1(B)). The cabinet has an air vent on the front door and an exhaust on the top of the cabinet, which was connected to the laboratory’s exhaust ventilation and maintained a negative pressure inside the cabinet to prevent leaks. A medical oxygen concentrator was chosen for the system due to its safety, and lower cost over time compared to compressed oxygen. The oxygen generator (A2Z Ozone, Louisville, KY) was placed in the lower section of the cabinet, and connected with Tygon S3™ E-3603 (Saint-Gobain, Malvern, PA) tubing through an in-line Whatman HEPA-Vent particle filter to the ozone generator. We maintained the flow of oxygen to the ozone generator at the manufacturer’s recommended flow of 2 L/min. At this flow, the ozone generator is capable of generating up to 5 g/h of ozone via silent corona arc discharge. Downstream of the generator, we maintained a constant 1L/min flow of ozone by using the generator’s built-in rotameter (units: 1 L/min, range: 0–3 L/min). Ozonated air flowed through fluorinated ethylene propylene (Teflon FEP) tubing into a 316 stainless steel manifold, which we connected to two Aalborg GFC17 mass flow controllers (MFC), designated supply and waste. The MFCs controlled the flow of ozone into the ozone exposure chamber (supply) and metered any excess ozone (waste) into the laboratory exhaust via the gas cylinder cabinet vent. The ozone supply MFC and the waste MFC were adjusted proportionally via an analog voltage signal (0–5V DC) from a PC-based data-acquisition and control system. Downstream of the ozone supply MFC, we installed a relay-actuated solenoid safety valve and a manually operated cutoff valve. Following the control valves, we connected the ¼”−316 stainless steel ozone supply line to the stainless steel filtered air supply duct via a ¼” tube size stainless steel threaded LET-LOK compression fitting (HAM-LET Group, Missouri City, TX). This fitting was welded into the duct in between the control valve and the designated ozone chamber’s air inlet on the chamber drop duct. A stem was mounted extending into the center of duct that opens counterflow into the oncoming filtered air stream, which aids in mixing the ozone and filtered air streams as they enter the chamber. The H-1000 chambers have a Stairmand disk (circular plate centered below the chamber inlet) that aids in uniformly distributing the incoming air or air-ozone mixture. We measured chamber ozone concentration with a Thermo 49i UV photometric ozone analyzer, which continuously drew chamber air at a flow rate of 3 L/min through 1/4 inch FEP Teflon tubing inserted into the middle of the front door of the ozone chamber through a compression fitting. The sampling tube extended several inches into the chamber above the middle cage rack. The Thermo 49i analyzer then determined the chamber ozone concentration every 10 s at a resolution and lower limit of detection of 0.001 ppm. For personnel safety, we mounted an EcoSensors (Newark, CA) C30-ZX ozone monitor with an audible alarm and an LED light-bar graph display in the laboratory.
Environmental data acquisition
Data from the ozone analyzer and environmental sensors (pressure, temperature, relative humidity and chamber airflow) were continuously transmitted to a multifunction USB-based 24-bit data acquisition device (MCC-DAQ 2416-4AO) via a 4–20 mA or 0–5V DC signal then displayed and recorded (30 s averages) by a computer. The MCC-DAQ 2416-4AO features 16 analog voltage input channels, to which the transmitter signals were passed. Additionally, the interface hardware included digital in/out and four 0–5V DC analog out channels. For transmitters that output an analog current of 4–20 mA, we converted the signal to a 0–5V DC voltage with a 250-ohm resistor placed in the circuit. The pressure transmitters had a measurement range of 0–3 inches of H2O, corresponding to a signal output of 4–20 mA. To allow for measurements of both positive and negative pressure, the transmitters had high and low-pressure connections. To measure positive pressure (e.g. supply manifold), we used the high-pressure connection and the low-pressure connection was left open to the laboratory air. For low-pressure measurements (e.g. exhaust manifold or chamber static), the opposite was true. Differential pressure measurements for airflow required the use of both connections. We monitored the chamber temperature and relative humidity using combination temperature and relative humidity sensors inserted into ports on the rear door of each chamber to ensure that these parameters stayed within OECD recommended ranges of 22 ±3 °C and 30–70% relative humidity (OECD, 2018). We also installed a temperature and relative humidity transmitter on a wall in the laboratory. The Thermo 49i analyzer had analog voltage outputs, which we used to transmit the ozone concentration signal to the USB microcontroller. Serial port or Ethernet-based TCP/IP connections were also available for monitoring data from the Thermo 49i instrument.
Computer-control system
We developed a data acquisition and control system using a Windows-based PC that we could operate from within the exposure facility or remotely from our main lab via a Windows remote desktop connection. The system utilizes DASYLab®, an icon-based software that allows users to quickly develop customized data acquisition, analysis and control applications without needing to write code. We employed a single worksheet in DASYLab® containing a series of icons/modules to facilitate the acquisition and recording of environmental data, control of the MFCs (manual and automatic), operation of a safety shutoff program and control the duration of exposures (Figure 2). We used DASYLab® modules to scale voltage measurements from each analog input channel (e.g. 0–5V DC = 0–5 ppm ozone), display real-time data onscreen, calculate averages for data reduction and record the data in a text file. We copied specific data streams and passed them to other modules to achieve four main objectives: (1) monitor and automatically control chamber ozone concentration at a desired setpoint during exposures, (2) control the duration of exposure, (3) provide a safety routine to automatically shut off the flow of ozone and (4) monitor, display and record all chamber environmental condition data. For initial chamber testing, we controlled the flow of ozone manually using slider modules connected to analog voltage outputs (MFC control signal). For automatic control during exposures, we used a proportional-integral-derivative (PID) control module in the DASYLab® worksheet. We passed an analog voltage input from the ozone analyzer and a virtual-voltage from a signal generator module corresponding to the desired setpoint (e.g. 2 ppm setpoint = 2V DC output) to the PID module. The PID module operated by continuously determining the deviation between the ozone analyzer voltage and the setpoint voltage to calculate an appropriate control signal output to the ozone supply MFC to minimize the deviation. We adjusted the gain settings (proportional, integral, derivative) and the maximum control output within the PID module to achieve an optimum exposure profile (Figure 3). We designed a series of modules that operated as a timer and a relay to control the duration of exposure and control the safety solenoid valve. Following the desired exposure time, the system turned off the ozone supply MFC output and closed the safety solenoid valve. We designed a safety routine using a trigger module to monitor the ozone chamber static pressure data stream and control a relay-operated, normally closed solenoid valve (safety solenoid valve). An increase in ozone chamber pressure indicating a leak or other problem with the air balance, would turn off the trigger module and thus the flow of ozone to the chambers. We set the trigger module so that it would only open the safety solenoid when both the static pressure was below a threshold setting (<0.10 inches H2O) and the timer-relay module was on.
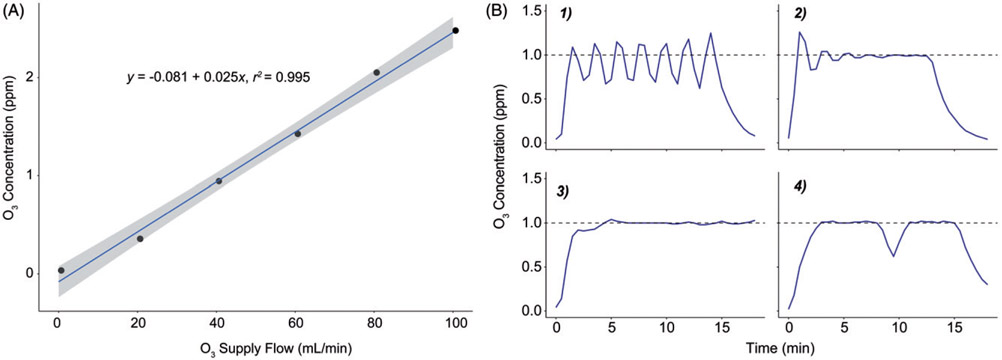
Figure 3.
Initial manual testing and abridged PID control tuning. (A) Linear correlation between ozone supply MFC flow rate setting and resulting chamber ozone concentration. These data were used to determine the range and estimate the capacity of the generation system. The shaded area represents a 95% confidence interval for a linear regression model (see Equation). (B) Panels illustrating the progression of the Edisonian process used to determine optimal gain settings for the DASYLab® PID control module. Panel 1: steady oscillations induced by increasing the proportional gain. Panel 2: improved settling time and offset resulting from increasing the integral and derivative gains. Panel 3: an appropriately damped system with optimal PID gain settings. Panel 4: system response to turning off the ozone generator for 1 min. Dashed line indicates the desired concentration setpoint of 1 ppm during initial testing.
Chamber maintenance
While most of the components of the system are durable (e.g. chambers, valves, ducts, etc.) and require infrequent attention, three aspects of maintenance should be discussed, namely the air filtration unit, the ozone generation system and the ozone analyzer. The service life of the diluent air filtration stages will vary by frequency of use and ambient environmental conditions. For our system, we implemented general replacement schedules and guidelines for evaluating the function of the filtration unit. The MERV 8 filters are replaced every 30 exposure-hours or if noticeably soiled upon visual inspection (before every exposure). The MERV 14 filter has a longer service life and is replaced every 60 exposure-hours. We estimated the function of the gas filtration cassette by observing the magnitude of the decrease in pre-exposure chamber ozone concentration from ambient levels. We plan to replace the cassette when it can no longer decrease the chamber ozone concentration to less than 20% of the ambient level prior to the start of an exposure. We monitored the function of the ozone generator and oxygen generator by the use of a handheld oxygen sensor, observing the average level of flow required to maintain ozone concentrations at the setpoint. A significant departure from the oxygen generator’s typical output of 90% or in the average level of ozone supply flow compared to initial test exposures can indicate problems with the ozone generation system. The manufacturer’s manuals for both the ozone and oxygen generators contain troubleshooting guides in case there is a loss of function. We conducted zero and span checks prior to each exposure, using a zero air source and an ozonator within the instrument (the internal ozonator is an optional feature). Calibration of the ozone analyzer was conducted on a monthly basis with a Thermo 49i-PS transfer standard photometer. The transfer standard photometer was calibrated annually against the SRP-1 at the U.S. EPA Office of Research and Development in Research Triangle Park, NC.
Concentration uniformity assay
To evaluate the concentration uniformity of ozone in the chamber, we utilized an assay developed by Flamm in which the oxidation of a 0.1 M boric acid-buffered 1% potassium iodide indicator solution (BKI), measured by the absorbance of BKI at 352 nm in a UV-transparent 96 well plate, is used as a surrogate of ozone concentration (Flamm, 1977). We distributed 35-mm polystyrene tissue culture dishes containing 3 mL of BKI throughout the ozone chamber (five dishes in each of the three cage racks, one in each corner and one in the center, see Supplemental Figure 1). In the control chamber, we placed one dish in the middle of each rack. We conducted a 30-minute computer-controlled 2-ppm ozone exposure of the dishes. Finally, we calculated the standard deviation and coefficient of variation (CV) statistics of absorbance values across all of the dishes as an indicator of heterogeneity of the ozone concentration.
Animal chamber and cage sanitation
The H-1000 chambers, trays and cage racks were cleaned and sanitized prior to exposures. A solution of 70% ethanol was used to spray and wipe clean the inside surfaces of the H-1000 animal chambers. Cage racks and trays were removed from the chambers and cleaned using a tunnel-style cage washer operated by the Department of Comparative Medicine at the University of North Carolina at Chapel Hill.
Animal exposures and phenotyping
We conducted four animal exposure experiments using 8-to 10-week-old female C57BL/6J mice (Total n = 29 ozone, 28 control) obtained from The Jackson Laboratory. In a separate exposure experiment, 50 mice [surplus C57BL/6NJ mice from a colony established in our laboratory, with variation at the Bpifb1 gene (Donoghue et al., 2017)] were exposed at one time to test whether the system could handle a large number of mice at once (Supplemental Figure 3). All mice were housed in an AAALAC approved facility and all procedures received approval by the Institutional Animal Care and Use Committee (IACUC) at the University of North Carolina Chapel Hill. Mice were housed over ALPHA-Dri bedding (Shepard) under standard 12 h lighting conditions, with ad libitum food and water.
For exposures, we transferred the mice from their normal housing and placed them in individual stainless steel wire mesh cages on the middle rack within the H-1000 chambers. After the doors to the chambers were sealed, we started the automated ozone exposure worksheet and exposure timer module in DASYLab®. We exposed groups of mice to filtered air or 2-ppm ozone for 3 h. After shut down, the chamber ozone concentration returned to ambient levels in approximately 10 min and we returned the mice to their normal housing. At 21 h following exposure, we euthanized the mice by a lethal dose of urethane (2 g/kg, in PBS; U2500, Sigma-Aldrich) followed by exsanguination and bronchoalveolar lavage (BAL) fluid collection. We quantified percent neutrophils in the BAL fluid by standard differential white blood cell counting techniques to phenotype lung inflammation.
Statistical analysis
We compared BAL neutrophil measurements from four independent experiments using ANOVA and pairwise Student’s t-tests with Holm’s method to correct for multiple comparisons. We used ANOVA models to examine the potential effect of placement in the chamber on the biological response to ozone. Position in the chamber was broken into one of four ‘zones’, left front, right front, left back and right back (Supplemental Figure 1).
Results
Chamber testing and operation
Manual testing
Before performing tests with ozone present in the chambers, we evaluated the function of the safety shutoff routine. With the ozone generator unplugged and the rest of the system running, we turned the exhaust fan off or opened the chamber door. Both of these conditions caused the system to close the safety solenoid, confirming that the safety program would function if needed. To evaluate the performance of the exposure system and linearity of the relationship between ozone supply flow and chamber ozone concentration we conducted a manual step test (Figure 3(A)). With the PID module setpoint corresponding to 0 ppm ozone, we used a slider module to increase the flow of ozone manually in steps of 20 mL/min. At each step, we allowed the chamber concentration to reach a plateau (at ~8 min) then remain at steady state for at least 15 min before increasing the output for the next step. We concluded the test one step after reaching 2 ppm ozone, the maximum concentration planned for animal exposures in the lab. From the step test, we determined that we would need a flow rate of approximately 80 mL/min ozone to maintain a 2 ppm exposure.
Automated-control tuning and exposure profiles
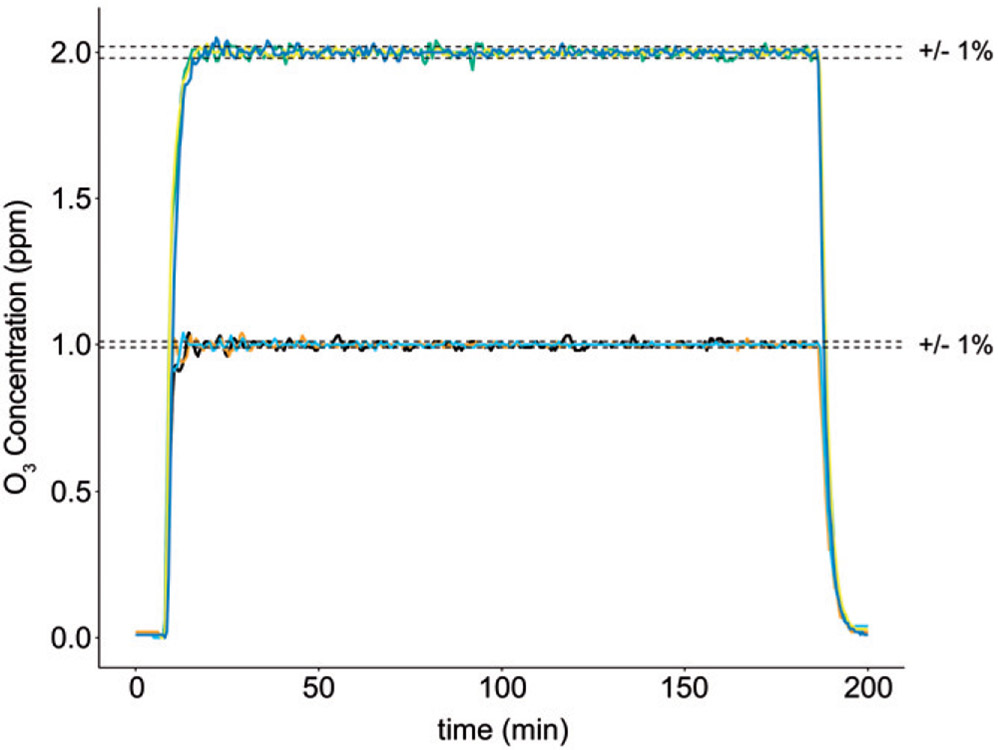
Based on the results of the manual step test, we limited the maximum control output from the PID module to 0.5V DC (100 mL/min) to prevent accidental overproduction of ozone during testing and provide some capacity (20 mL/min) to compensate for ozone loss due to uptake and adsorption by animals in the chamber. There are several published heuristic methods for determining, also known as ‘tuning’, the optimal gain settings of a PID controller (Tehrani and Mpanda, 2012; Astrom and Hägglund, 1995). While these references were helpful for understanding the basics of PID control theory, we ultimately determined the proportional, integral and derivative gains for our PID control module through an Edisonian process. An abridged visualization of the progress of tuning the PID control module is shown in Figure 3(B). First, we adjusted the P, I and D gain settings individually until we determined how each setting modulated the system’s response. Adjusting the P gain upward eventually produced a steady oscillation of the ozone concentration close to the desired setpoint (Figure 3(B-1)). Increasing the D gain caused the oscillations to taper off, and adding in an I-gain setting at this point eliminated a small offset causing the mean value to be below the setpoint (Figure 3(B-2)). We continued to increase the D-gain and decreased the P-gain slightly, which decreased the amount of settling time until the exposure system produced a fast-ramp and maintenance of chamber ozone at the setpoint with minimal overshoot (Figure 3(B-3)). These settings also produced a sufficient response to a brief loss of output from the ozone generator, returning the ozone concentration to the setpoint after we turned off the ozone generator for 1 min (Figure 3(B-4)). The automated PID control loop was able to keep the ozone concentration to within 1% of the setpoint over several exposures, with and without mice in the chambers (Figures 3(B-3) and 4).
Figure 4.
Concentration profiles for several exposures with mice present in the chamber. In exposure experiments conducted at both 1 and 2 ppm, the system maintained the ozone exposure concentration to within 1% of the setpoint for 3h. The number of mice exposed to ozone in the experiments shown ranged from 5 to 21. Plots represent concentration–time profiles (30-second running average) of three exposures at each concentration.
Concentration uniformity
One of the objectives of this project was to support future studies using large numbers of genetically diverse mice. These studies require several exposure batches and variable spatial placement of mice of different strains within the chambers. Although the chamber design, ozone inlet and airflow rate all contribute to mixing and spatial distribution of ozone in the chamber, perfect mixing is unlikely to be achieved. As such, we conducted an evaluation of ozone concentration across several chamber locations to obtain reference data for potential covariate adjustment or serve as the impetus to take measures to improve mixing. The concentration uniformity assay (oxidation of BKI solution, see Materials and methods section) revealed a mean absorbance of 1.149 across all indicator dishes for the ozone chamber and a CV of 14.6% (Table 2). The top rack had the highest mean absorbance value of 1.266 and largest CV of 16.8%. The middle and bottom racks had mean absorbance values of 1.143 and 1.038, and CVs of 7.6% and 7.2%, respectively. Individual sample absorbance values indicate that there is a region of relatively higher concentration in the center of the top rack (Supplemental Figure 2).
Table 2.
Concentration uniformity data.
| Location | Mean absorbance | SD | CV |
|---|---|---|---|
| Control (n = 3) | 0.034 | 0.004 | 13.098 |
| Ozone | |||
| Top rack (n = 5) | 1.266 | 0.212 | 16.768 |
| Middle rack (n = 5) | 1.143 | 0.087 | 7.639 |
| Bottom rack (n = 5) | 1.038 | 0.075 | 7.215 |
| Ozone overall (n = 15) | 1.149 | 0.168 | 14.594 |
SD: standard deviation; CV: coefficient of variation (SD/Mean × 100).
Animal exposures
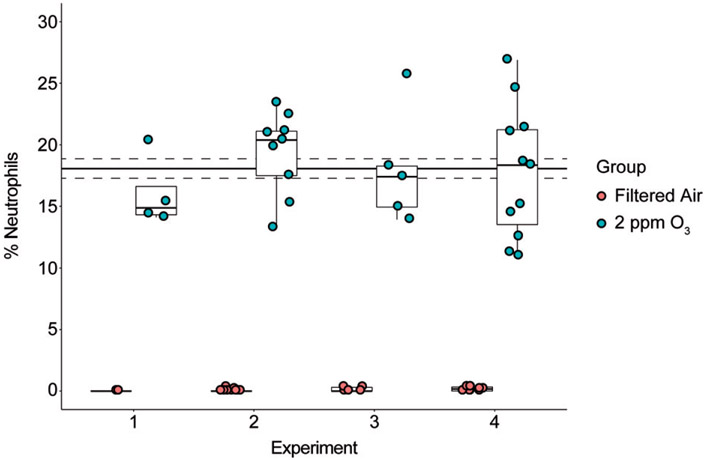
The biological consequences of acute ozone exposure include the induction of airway inflammation, which is in part reflected by the recruitment of neutrophils to the airways. To assess the reproducibility of this outcome in our exposure system, we measured the levels of airway neutrophilia across several experiments using the same strain (and sex) of mice. Data from four experiments in which female C57BL/6J mice were exposed to 2 ppm ozone or filtered air (Figure 5) demonstrate that exposures with our system produced biologically reproducible results. To examine whether the position in the chamber influenced the degree of neutrophilic inflammation, we compared mice placed in different chamber positions (or zones, see Supplemental Figure 1). Overall, we found that position was not associated with the degree of inflammation (F = 0.806, p = 0.504), but we caution that this analysis was based on a relatively small sample size (n = 2–9 mice per zone).
Figure 5.
Reproducibility of ozone-induced airway inflammation in four experiments. Experiments 1–4 were conducted over the span of several months to test the reproducibility of our exposure system by a biological measure. On average, the percent neutrophils was 18.1 ± 0.8% (solid and dashed lines, mean ± SEM). For each experiment there was a significant increase in percent neutrophils (p < 0.05) and the percent neutrophil value was not significantly different across experiments (ANOVA F statistic = 0.132, p = 0.941).
Discussion
We developed a whole-body ozone inhalation exposure system to facilitate studies involving relatively large numbers of mice. With the present design, we achieved a system that performed well and met our major requirements in terms of capacity, environmental conditions, automation, reproducibility, safety and data management. Our safety cutoff system operated correctly, turning off the flow of ozone upon the requisite increase in chamber static pressure. During experiments, the system was straightforward to setup, run and monitor remotely. With the system under automated PID control, we have maintained consistent and repeatable exposure profiles for up to 50 mice. At the time of writing, we have not utilized the total capacity of each chamber (180 mice), but preliminary tests suggest that the system would respond appropriately to the additional demand of larger numbers of mice.
Automated, computer-operated feedback control systems have been employed for inhalation exposure studies since 1978 (Davies et al., 1987; Johnson et al., 1995; Johnson and Fechter, 1996; O’Shaughnessy and Hemenway, 1994; Van Stee and Moorman, 1978, 1984a,b). These early systems enabled inhalation exposure studies without operator intervention and typically achieved control to within 5% of the target concentration. Like our system, some systems included alarms or safety shutoff features when certain parameters were out of range as well as environmental data recording capabilities (Davies et al., 1987; Van Stee and Moorman, 1983, 1984). A primary difference between our system and previous automated systems is the use of modern DasyLab® control software that requires almost no specialized engineering or programing knowledge. Importantly, this control system enabled us to meet or exceed the performance of early systems in terms of control by maintaining the ozone concentration to within 1% of the setpoint. An additional feature of our control system is the ability to monitor the exposure using remote desktop from any location with internet access. Because our main laboratory is in a different building, this simple to implement remote monitoring capability has proven very useful.
There are a few automated exposure systems that have been described within the last two decades (Goldsmith et al., 2011; McKinney et al., 2009; McKinney and Frazer, 2008; Wong, 2003). With regards to computer-control and toxicant type, our system is most similar to an ozone exposure system developed by McKinney and Frazer (2008) at NIOSH. We employed a similar PID-based feedback control loop with continuous sampling; however, McKinney and Frazer’s feedback loop controlled the flow of diluent air to adjust the ozone concentration. Because our chambers are substantially larger, using the diluent air to control the ozone concentration was not feasible. Our diluent air is supplied at a constant rate by an electric pressure blower that pushes laboratory air through a series of filter panels as opposed to dry compressed air. We controlled the ozone supply using two MFCs; one MFC controls the flow of ozone and the other meters the excess supply into the exhaust system. We chose to use a corona arc discharge ozone generator because it has greater output capacity than a mercury lamp-style ozone generator. Additionally, compared to the NIOSH system, temperature and humidity in our chambers are controlled by the building HVAC system and we integrate environmental data recording (pressure, temperature and relative humidity) alongside the feedback control of ozone exposure using DASYLab®. Using this arrangement, we were able to maintain chamber temperature and humidity during and across exposure experiments within the Office of Economic Cooperation and Development (OECD) recommended ranges of 22 ± 3 °C and 30 to 70% relative humidity (OECD, 2018).
As mentioned previously, we modeled our system after an existing system at the US EPA. As in the EPA system, we utilized H-1000 chambers, a Thermo49i ozone analyzer and DasyLab® control software. Additionally, we matched our nominal diluent airflow and pressure characteristics to those of the EPA system. Most of our system’s other features were unique and custom-built to fit the laboratory space, electrical, and HVAC systems available. These features include the stainless steel supply ductwork, CPVC exhaust pipes, the arrangement and organization of manifolds and control valves, remote tubing to monitor pressures, and the power supply and analog signal cable wiring. Different commercially acquired components include the supply and exhaust fans, filtration unit, ozone and oxygen generators, USB DAQ, and mass flow controllers. Despite these differences, we were able to achieve a similar level of performance as the EPA system in terms of automated and reproducible exposures (Higuchi and Walsh, personal communications).
Concentration uniformity, or spatial variation, is a commonly discussed aspect of whole-body inhalation chamber performance and was a concern we sought to address during the testing of our system (Cheng and Moss, 1995; MacFarland, 1983). As expected, due to imperfect mixing we measured differences in the concentration of ozone (as reflected by the BKI assay) between 15 chamber locations. Currently, there is no standard for acceptable concentration uniformity, which depends on the type of test material (e.g. gases, particulates, aerosols, etc.), chamber characteristics (e.g. size, airflow, etc.) and the sampling method (number and location of samples). Of the inhalation chamber studies surveyed by MacFarland, most achieved a spatial variation between 5 and 15% (MacFarland, 1983). Cheng et al. (1989) observed a spatial variation of 7.7% for a 1 μm nickel oxide aerosol in an H-1000 chamber. Using a 210-liter rectangular chamber with a multi-port ozone inlet, Marra and Rombout (1990) observed a spatial variation in ozone concentration of 8%. Our overall spatial variation was higher, at 14.6% but this appears largely driven by top rack and a region of relatively high concentration in the center of this rack. If the top center sample is removed the overall variability is 9.7%. These data suggest that our chamber concentration uniformity is reasonable, but it may be prudent to avoid this top-center region when placing mice in the chamber if possible. Other options that could improve ozone concentration uniformity are increasing chamber airflow or installing a recirculation system (Cheng et al., 1989), especially when conducting studies involving all three racks of the H-1000 chamber. Nonetheless, we did not find that chamber position was associated with variation in the biological response to ozone (as measured by percent neutrophils in BALF) among mice exposed on the second rack of the chamber. Additionally, we measured consistent toxicologic responses to 2 ppm ozone over several exposures, confirming the system’s overall performance and repeatability.
While using the system over time, we identified a few other areas for potential improvement or modifications in the future. There are reports that changes in humidity can alter or interfere with the readings of UV absorbance ozone analyzers (Wilson and Birks, 2006). Because the humidity did not change dramatically during or between experiments, we did not investigate whether water vapor interference was an issue for our system. In either case, installing lengths of Nafion tubing on both the sample and reference lines just prior to the photometer has been shown to eliminate any potential water vapor interference and thus may be a good practice. [Note: care should be taken to make sure calibrations are performed under the same conditions as typical sampling (Wilson and Birks, 2006).] More precise and direct/local control of chamber humidity and temperature using an additional feedback control system may also help to address inter-experimental variability and water vapor interference caused by changes in humidity (Sterner et al., 1991). Finally, we observed that the temperature increases in the safety cabinet because of the heat produced by the equipment therein. Excessive heat may decrease the output of the ozone generator or decrease the life of the equipment housed in the cabinet, therefore it may be desirable to examine this issue in detail in the future.
In conclusion, we have developed a robust, computer-controlled ozone exposure system that can be used to expose large numbers of mice. Our system provides precise and reproducible ozone exposures, maintaining the desired ozone concentration regardless of the number of subjects tested. The system’s data acquisition and control equipment afford minimal operator involvement during exposures and automatic recording of data on environmental conditions. This new system will be invaluable for large-scale studies in mice and facilitate the identification of mechanisms by which ozone causes adverse respiratory and systemic health effects.
Supplementary Material
Acknowledgements
We thank the following individuals who were instrumental to the success of this project: Ian Gilmour at The United States Environmental Protection Agency’s National Health and Environmental Effects Research Laboratory (NHEERL), Chris Gregory from the Department of Genetics at The University of North Carolina at Chapel Hill, and William Robertson and Artie Neese of Facilities Services at The University of North Carolina at Chapel Hill.
Funding
This research was supported by the National Institute of Environmental Health Sciences under award numbers R01 ES024965, T32 ES007126-35, and P30-ES-010126.
Footnotes
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental data for this article can be accessed at https://doi.org/10.1080/08958378.2019.1597222.
References
- Aris RM, Christian D, Hearne PQ, et al. (1993). Ozone-induced airway inflammation in human subjects as determined by airway lavage and biopsy. Am Rev Respir Dis 148:1363–72. [DOI] [PubMed] [Google Scholar]
- Astrom K, Hägglund T. (1995). PID controllers: theory, design and tuning. 2nd ed. Research Triangle Park, NC: International Society for Measurement and Control; p. 343. [Google Scholar]
- Bauer AK, Kleeberger SR. (2010). Genetic mechanisms of susceptibility to ozone-induced lung disease. Ann NY Acad Sci 1203:113–9. [DOI] [PubMed] [Google Scholar]
- Brown MG, Moss OR. (1981). An inhalation exposure chamber designed for animal handling. Lab Anim Sci 31:717–20. [PubMed] [Google Scholar]
- Cabello N, Mishra V, Sinha U, et al. (2015). Sex differences in the expression of lung inflammatory mediators in response to ozone. Am J Physiol Lung Cell Mol Physiol 309:L1150–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LC, Lippmann M. (2015). Inhalation toxicology methods: the generation and characterization of exposure atmospheres and inhalational exposures. Curr Protoc Toxicol 63:24.4.1–24.4.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng YS, Barr EB, Carpenter RL, et al. (1989). Improvement of aerosol distribution in whole-body inhalation exposure chambers. Inhal Toxicol 1:153–66. [Google Scholar]
- Cheng YS, Moss OR. (1995). Inhalation exposure systems. Toxicol. Methods, 5: 161–197. [Google Scholar]
- Cho Y, Abu-Ali G, Tashiro H, Brown TA, Osgood R, Kasahara DI, Huttenhower C, Shore SA. 2019. Sex differences in pulmonary responses to ozone in mice: role of the microbiome. Am J Respir Cell Mol Biol., 60: 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary EG, Cifuentes M, Grinstein G, et al. (2018). Association of low-level ozone with cognitive decline in older adults. J Alzheimers Dis 61:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DW, Walsh LC, Hiteshew ME, et al. (1987). Evaluating the toxicity of urban patterns of oxidant gases. I. An automated chronic gaseous animal inhalation exposure facility. J Toxicol Environ Health 21:89–97. [DOI] [PubMed] [Google Scholar]
- Day DB, Xiang J, Mo J, et al. (2017). Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med 177:1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RB, McDonnell WF, Becker S, et al. (1996). Time-dependent changes of inflammatory mediators in the lungs of humans exposed to 0.4ppm ozone for 2hr: A comparison of mediators found in broncoalveolar lavage fluid 1 and 18 hr after exposure. Toxicol Appl Pharmacol 138:176–85. [DOI] [PubMed] [Google Scholar]
- Donoghue LJ, Livraghi-Butrico A, McFadden KM, et al. (2017). Identification of trans protein QTL for secreted airway mucins in mice and a causal role for Bpifb1. Genetics 207:801–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorato MA. (1990). Overview of inhalation toxicology. Environ Health Perspect 85:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith WT, McKinney W, Jackson M, et al. (2011). A computer-controlled whole-body inhalation exposure system for the oil dispersant COREXIT EC9500A. J Toxicol Environ Heal - Part A Curr Issues 74:1368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamm DL (1977). Analysis of ozone at low concentrations with boric acid buffered KI. Environ. Sci. Technol 11:978–983. [Google Scholar]
- Herring MJ, Putney LF, St George JA, et al. (2015). Early life exposure to allergen and ozone results in altered development in adolescent rhesus macaque lungs. Toxicol Appl Pharmacol 283:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, De Leon SF, Lippmann M. (2005). Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology 16: 446–57. [DOI] [PubMed] [Google Scholar]
- Jerrett M, Brook R, White LF, et al. (2017). Ambient ozone and incident diabetes: a prospective analysis in a large cohort of African American women. Environ Int 102:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Pope CA, et al. (2009). Long-term ozone exposure and mortality. N Engl J Med 360:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji M, Cohan DS, Bell ML. (2011). Meta-analysis of the association between short-term exposure to ambient ozone and respiratory hospital admissions. Environ Res Lett 6: 024006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DL, Fechter LD. (1996). Performance of an automatic feedback control vapor generation system during near-continuous inhalation exposures. Inhal Toxicol 8:423–31. [Google Scholar]
- Johnson DL, Hagstrom EG, Fechter LD. (1995). Automatic feedback control of a vapor generation system using off-the-shelf components. Inhal Toxicol 7:1293–303. [Google Scholar]
- Kim BJ, Kwon JW, Seo JH, et al. (2011). Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann Allergy Asthma Immunol 107:214–219.e1. [DOI] [PubMed] [Google Scholar]
- Ko FWS, Tam W, Wong TW, et al. (2007). Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 62:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JI, Carrothers TJ, Tuomisto JT, et al. (2001). Assessing the public health benefits of reduced ozone concentrations. Environ Health Perspect 109:1215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JI, Chemerynski SM, Sarnat JA. (2005). Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology 16: 458–68. [DOI] [PubMed] [Google Scholar]
- MacFarland HN. (1983). Designs and operational characteristics of inhalation exposure equipment-a review. Fundam Appl Toxicol off J Soc Toxicol 3:603–13. [DOI] [PubMed] [Google Scholar]
- Marra M, Rombout P. (1990). Design and performance of an inhalation chamber for exposing laboratory animals to oxidant air pollutants. Inhal Toxicol 2:187–204. [Google Scholar]
- McKinney W, Chen B, Frazer D. (2009). Computer controlled multi-walled carbon nanotube inhalation exposure system MWCNT inhalation exposure system. Inhal Toxicol 21:1053–61. [DOI] [PubMed] [Google Scholar]
- McKinney W, Frazer D. (2008). Computer-controlled ozone inhalation exposure system. Inhal Toxicol 20:43–8. [DOI] [PubMed] [Google Scholar]
- Michaudel C, Fauconnier L, Julé Y, Ryffel B. (2018). Functional and morphological differences of the lung upon acute and chronic ozone exposure in mice. Sci Rep 8:10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Ghio AJ, Karoly ED, et al. (2016). Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am J Respir Crit Care Med 193:1382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Snow SJ, Schladweiler MC, et al. (2016). Acute ozone-induced pulmonary and systemic metabolic effects are diminished in adrenalectomized rats. Toxicol Sci 150:312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudway IS, Kelly FJ. (2000). Ozone and the lung: a sensitive issue. Mol Aspects Med 21:1–48. [DOI] [PubMed] [Google Scholar]
- Nielsen GD, Hougaard KS, Larsen ST, et al. (1999). Acute airway effects of formaldehyde and ozone in BALB/c mice. Hum Exp Toxicol 18:400–9. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PT, Achutan C, O’Neill ME, Thorne PS. (2003). A small whole-body exposure chamber for laboratory use. Inhal Toxicol 15:251–63. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy PT, Hemenway DR. (1994). Computer automation of a dry-dust generating system. Inhal Toxicol 6:95–113. [Google Scholar]
- OECD. (2018). Acute inhalation toxicity: fixed concentration procedure. Available from: https://www.oecd-ilibrary.org/environment/test-no-433-acute-inhalation-toxicity-fixed-concentration-procedure_9789264284166-en. [Last accessed: 24 Oct 2018].
- Paffett ML, Zychowski KE, Sheppard L, et al. (2015). Ozone inhalation impairs coronary artery dilation via intracellular oxidative stress: evidence for serum-borne factors as drivers of systemic toxicity. Toxicol Sci 146:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J (2003). Overview of testing methods used in inhalation toxicity: From facts to artifacts. Toxicol Lett 140–141:183–93. [DOI] [PubMed] [Google Scholar]
- Phalen RF. (1976). Inhalation exposure of animals. Environ Health Perspect 16:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalen RF, Mannix RC, Drew RT. (1984). Inhalation exposure methodology. Environ Health Perspect 56:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA, Squadrito GL, Friedman M. (1995). The cascade mechanism to explain ozone toxicity: the role of lipid ozonation products. Free Radic Biol Med 19:935–41. [DOI] [PubMed] [Google Scholar]
- Speen AM, Kim HH, Bauer RN, et al. (2016). Ozone-derived oxysterols affect liver x receptor (LXR) signaling: a potential role for lipid-protein adducts.291(48):25192–25206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner RT, Johns BE, Crane KA, et al. (1991). An inexpensive humidifying and cooling system for inhalation chambers. Inhal Toxicol 3: 139–44. [Google Scholar]
- Tehrani KA, Mpanda A (2012). PID control theory In: Introduction to PID Controllers - Theory, Tuning and Application to Frontier Areas. In: Panda RC, editor. Croatia: InTech Publisher; p. 213–228. [Google Scholar]
- Turner MC, Jerrett M, Pope CA, et al. (2016). Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med 193:1134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR, Noor S, Young T, et al. (2018). Aging exacerbates neuroinflammatory outcomes induced by acute ozone exposure. Toxicol Sci 163(1):123–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stee EW, Moorman MP. (1978). Monitoring for temperature, humidity and concentration. Proceedings of the Workshop on Inhalation Chamber Technology, Formal Report 51318, Brookhaven National Laboratory, Upton, NY October 16-17, pp.81–87. [Google Scholar]
- Van Stee EW, Moorman MP. (1984a). Calibration of a system for the computer-assisted operation of a small animal inhalation facility. Environ Health Perspect 54:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stee EW, Moorman MP. (1984b). Overview of a system for the computer-assisted operation of a Small animal inhalation facility. Environ Health Perspect 54:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KL, Birks JW. (2006). Mechanism and elimination of a water vapor interference in the measurement of ozone by UV absorbance. Environ Sci Technol 40:6361–7. [DOI] [PubMed] [Google Scholar]
- Wong BA. (2003). Automated feedback control of an inhalation exposure system with discrete sampling intervals: testing, performance, and modeling. Inhal Toxicol 15:729–43. [DOI] [PubMed] [Google Scholar]
- Wong BA. (2007). Inhalation exposure systems: design, methods and operation. Toxicol Pathol 35:3–14. [DOI] [PubMed] [Google Scholar]
- Zu K, Shi L, Prueitt RL, et al. (2018). Critical review of long-term ozone exposure and asthma development. Inhal Toxicol 30: 99–113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.