Abstract
The low- and middle-income countries bear the highest burden of typhoid fever in the world. India, along with other South Asian countries, has a significant incidence of typhoid fever among young children though there is a paucity of published data on community burden. In spite of the availability of Vi-polysaccharide (Vi-PS) and conjugated Vi-PS vaccines, these are not adequately utilized in India and in the neighbouring countries. To address many shortcomings of the unconjugated Vi-PS vaccines, typhoid conjugate vaccines (TCVs) are developed by conjugating Vi-PS with different carrier proteins. Three such vaccines using tetanus toxoid as a carrier protein are already licensed in India. Several other Vi-PS conjugates are currently in various stages of development. The current review provides an update on the existing and upcoming new TCVs along with a detailed discussion on the various issues involved with their clinical use and limitations.
Keywords: Conjugate Vi-polysaccharide vaccines, typhoid, typhoid vaccines, Vi-polysaccharide vaccines
Typhoid fever is a significant health problem of young children in many low- and middle-income (LMI) countries of Asia1. According to a model-based estimate, 17.8 million cases of typhoid fever occur each year in LMI countries2. In 2016, India had 6.6 million typhoid cases (499 cases/100,000 population) and 66,439 typhoid deaths, more than half were in children below 15 yr of age3. A systematic review from India estimated 9.7 per cent [95% confidence interval (CI): 5.7-16%] prevalence of laboratory-confirmed typhoid among individuals with fever across all hospital studies, with children aged 2-4 yr having the highest incidence4. In a study of typhoid fever in five Asian countries, the mean age of typhoid was significantly lower in Pakistan and India than that in other countries5. In another study conducted in the southern coastal area of Pakistan, the incidence of typhoid bacteraemia in children less than two years of age was 443.1/100,000 child-years, whereas it was 405.1/100,000 for children less than five years6. Similar high incidence of typhoid in younger children was noticed in the studies from Bangladesh7,8. These studies underline the significant burden of typhoid fever in young children under five years of age and a need for a vaccine to be used in vaccination programmes targeting this age group, particularly in South Asian countries.
Available typhoid vaccines
Currently, three different types of typhoid vaccines are available globally: an oral live attenuated vaccine, Ty21a; a Vi-capsular antigen-based unconjugated polysaccharide (Vi-PS) vaccine, and typhoid conjugate vaccines (TCVs). While the first two vaccines were already licensed and recommended by the WHO9, the one licensed TCV (Typbar-TCV™) has also been approved and endorsed by the WHO in 2018 after attaining WHO pre-qualification (PQ) in January 201810.
Ty21a is an orally administered vaccine available in two formulations: liquid formulation for children above two years of age and an enteric-coated capsule for administration to older children9. Ty21a is a gal E mutant of Salmonella Typhi which cannot synthesize Vi-PS capsule11. This vaccine stimulates serum and mucosal antibodies to O, H and other surface antigens and elicits strong cell-mediated immunity (CMI), but cannot stimulate Vi antibody production because the antigen is lacking. Ty21a also offers some protection against infection from Salmonella Paratyphi A and B12. Ty21a is a moderately effective vaccine with an efficacy of 53-78 per cent against culture-proven typhoid fever in large efficacy trials, conducted in Chile9. The liquid formulation of Ty21a is licensed for use in individuals aged two years and above, whereas the enteric-coated capsule is available for individuals aged five years and above. The Vi-PS is a subunit vaccine developed from wild-type S. Typhi strain Ty2 by non-denatured purification of the Vi-PS. The injectable Vi-PS vaccine contains 25 μg of the antigen and is given as a single dose either by intramuscular or subcutaneous route9. This is a safe vaccine; fever and local side effects such as pain, redness and induration at the injection site are the most common adverse events. Rarely, allergic reactions and rashes have been observed9. The Vi-PS vaccine provides around 55-72 per cent protection lasting for about three years after a single intramuscular dose13,14,15,16,17.
The latest group of typhoid vaccines consists of TCVs, in which Vi-capsular PS is conjugated with tetanus toxoid (TT) at different doses. Two such vaccines, PedaTyph™, and Typbar-TCVTM are licensed in India for children aged 3 and 6 months, respectively18. Another TCV, Zyvac TCV™, having almost similar technical characteristics as Typbar-TCV™, has also been licensed in India19. There are several candidate TCVs in the pipeline globally, in various stages of the development process20 (Fig.1).
Fig. 1.
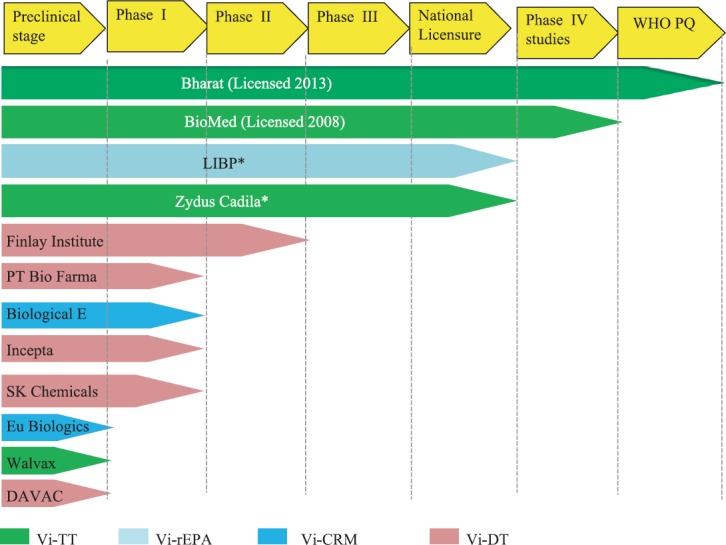
Typhoid conjugate vaccine pipeline: Different licensed and candidate typhoid conjugate vaccines in various phases of development and licensure. *Under review for national licensure. WHO PQ, WHO pre-qualification; LIBP, Lanzhou Institute of Biological Products Co. Ltd., PR China; Vi-TT, Vi conjugated with tetanus toxoid; Vi-rEPA, Vi recombinant exoprotein antigen; Vi-CRM, Vi conjugated with cross reacting material; Vi-DT, Vi conjugated with diphtheria toxoid. Source: Adapted from Ref. 20.
Efficacy/effectiveness and limitations of Vi-PS typhoid vaccines
The Vi-PS vaccines do not generate immune responses in children aged less than two years21. Studies conducted among children above two years of age in Nepal13, South Africa14, China15, India16 and Pakistan17 demonstrated protective vaccine effectiveness (VE) ranging from 31 per cent in Pakistan to 72 per cent in Nepal in different age groups13,14,15,16,17. These vaccines have shown a reasonable duration of protection against typhoid fever that ranges from 2-3 yr22. Many different formulations are available, but only one, Typhim-Vi™, is WHO prequalified9.
Limitations
It is well known that these unconjugated Vi-PS vaccines are not effective in children below two years of age; however, their efficacy in the age group of 2-5 years is also not uniformly demonstrated17,21. Two cluster-randomized effectiveness trials of Vi-PS typhoid vaccine in the low socio-economic areas of Kolkata, India (2004-2006)16, and Karachi, Pakistan (between 2002 and 2007)17, were conducted. While in Kolkata16, the vaccine was found highly effective [VE: 80% (95% CI, 53, 91)] in 2-5 yr old children with reasonably good herd protection, in Karachi17, the same vaccine failed to provide any protection [VE: 38% (95% CI: −192, 35%)] in the younger age group and no herd effect was noticed.
There are several other limitations of Vi-PS vaccines. Being purely PS vaccine, these do not induce T-cell immunity; hence, there is no immune memory, and frequent re-vaccinations with extra doses are needed23. The antibody response following the PS vaccine results in low titres of poor affinity IgG antibodies. Further, there is a possibility of hypo-responsiveness with subsequent doses of Vi-PS vaccines23,24. The Vi-PS vaccine is also not fit for co-administration with other routine childhood vaccines provided under Expanded Programme on Immunization.
Typhoid conjugate vaccines
To overcome the limitations of Vi-PS vaccines, the Vi capsular PS, derived either from Salmonella enterica subspecies enterica serovar Typhi (S. Typhi), or from Citrobacter freundii sensu lato (C. freundii s. l.) or other bacterial or plant sources, is covalently linked to different carrier proteins such as TT25,26,27. This conjugation process converts T-independent PS to T-dependent antigen, which results in high-affinity antibodies that last longer than antibodies induced by unconjugated Vi-PS vaccine in young children23. The TCV can also be safely co-administered in combination with measles-containing vaccines (MCVs) [measles, mumps and rubella (MMR)]9. On the whole, the TCVs may demonstrate (i) superior efficacy and effectiveness than unconjugated Vi-PS vaccines; (ii) longer duration of protection; (iii) immunogenicity amongst younger children, including infants; (iv) reasonably good herd immunity; and (v) induction of immune memory23,28. Table I enumerates key differences between unconjugated pure Vi-PS and conjugated Vi-PS typhoid vaccines23, and Table II offers a comparative analysis of a few key TCVs.
Table I.
Key differences between an unconjugated polysaccharide and a protein-polysaccharide conjugate vaccine
| Characteristic | Unconjugated polysaccharide vaccine | Conjugated protein-polysaccharid vaccine |
|---|---|---|
| Cells stimulated | B cells | B and T cells |
| Antibody titres, type | Low, IgM | High, IgG |
| Quality of antibody(avidity) | Low | High |
| Cell-mediated immunity | Absent | Present |
| Duration of response | Short lived | Long lived |
| Immune memory | Poor | Strong |
| Booster response | Poor | Strong |
| Hyporesponsiveness(on repeated doses) | May be present | No |
| Effective ages | >2 yr | All ages |
Source: Ref. 23
Table II.
Comparative analysis of some key typhoid conjugate vaccines
| Vaccine attributes | Typbar-TCV™ | PedaTyph™ | Vi-rEPA | Vi-CRM 197 | Vi-DT |
|---|---|---|---|---|---|
| Developer/manufacturer | Bharat Biotech International Ltd., India | BioMed Pvt Ltd., India | National Institutes of Health (NIH), USA | Novartis Vaccines Institute for Global Health, Italy | International Vaccine Institute, Korea |
| Vi-PS dose (µg) | 25 | 5 | 25 | 5 | 25 |
| Carrier protein | TT | TT | rEPA | CRM 197, nontoxic mutant of diphtheria toxin | DT |
| Source of Vi-PS | Ty2 strain of Salmonella Typhi | S. Typhi | S. Typhi | Citrobacter freundii | S. Typhi strain from India (C6524) |
| Conjugate linker scheme | ADH | ADH | ADH | ADH | ADH |
| Study group | Six months-45 yr | Three months-12 yr | Two years to adults; infants (unpublished) | Six weeks-45 yr | 2-45 yr (phase II and III trials ongoing) |
| Dose schedule | Single dose | Two doses | Two doses | Two-three doses | Two doses |
| Trial under two years of age | Yes | Yes | Yes (unpublished) | Yes | No |
| Efficacy/effectiveness study | Yes | Yes | Yes | No | No |
| Long-term protection | Up to five years | Up to 2.5 yr | Up to four years in 2-5 yr old children | Not examined | Not examined |
| Booster responses: Elicited | Yes | Not studied | Yes (unpublished, NIH trials) | No, titres decreased after booster dose | Not studied |
| Antibody quality tested (avidity) | Yes (high avidity IgG) | Not studied | Not studied | Not studied | Not studied |
| Licensure | In 2013 in India (for children six months of age and above) | In 2009 in India (for children three months of age and above) | Not licensed | Not licensed | Not licensed |
| WHO pre-qualification (PQ) | Yes | No | NA | Interest expressed by future developers to apply for WHO PQ | Interest expressed by future developers to apply for WHO PQ |
| Current status | Licensed in India and Nepal M/s Cadila Healthcare Limited, India, has developed a similar product Zyvac TCV™, based on Vi-TT conjugation employing 25 µg of Vi-PS; got national licensure and market authorization in 2018 in India | Licensed in India; No interest shown in WHO PQ | Technology transfer to LIBP, China LIBP has completed phase III in adults, preschool and school-aged children, submitted for licensure for use in persons >2 yr old | Technology transfer to Biological E. Ltd., India Biological E. Ltd., India and Eubiologics, South Korea are developing this vaccine; BE with 25 µg dose of Vi-PS. Interest expressed toapply for WHO PQ | Technology transfer to four different manufacturers i.e., Shantha Biotechnic (India), PT Bio Farma (Indonesia), SK Chemicals (Korea) and Incepta (Bangladesh); DAVAC (Vietnam) and Finlay Institute (Cuba), are also developing Vi-DT conjugate, Shantha Biotechnic (India) has stopped development, Finlay Institute (Cuba) in most advanced stage |
| Reference | 19,29 | 30,31,32,33 | 28,34,35,36,37,38 | 39,40 | 41 |
P. aeruginosa, Pseudomonas aeruginosa; Vi-PS, Vi polysaccharide; rEPA, recombinant exoprotein A from P. aeruginosa; CRM, cross-reactive material; DT, diphtheria toxoid; TT, tetanus toxoid; ADH, adipic acid dihydrazide; LIBP, Lanzhou Institute of Biological Products Co. Ltd., PR China; TCV, typhoid conjugate vaccine
Issues related to typhoid conjugate vaccines
Three TCVs are licensed and in use in the private sector of India. The WHO Global Advisory Committee on Vaccine Safety did not find any serious safety signal with the currently used TCVs42. Although a preliminary report of the first efficacy trial of Typbar-TCV™ from Nepal43, the field estimate of the seroefficacy of Typbar-TCV™44 based on a previously published trial29, and a school-based cluster randomized efficacy trial of PedaTyph™30 are published, yet there are certain specific issues related to their clinical efficacy, particularly in young children, which deserve further exploration.
Optimal source of Vi-PS with the optimal carrier protein
The source of Vi-PS in different TCVs is either S. Typhi or C. freundii (Table II). As the enormous molecular weight of Vi made the filtration and conjugation process difficult, attempts are being made to employ plant-based PSs, which are structurally similar but immunologically unrelated as a replacement for Vi-PS of bacterial origin45. Pectin purified from plants or fruit has been successfully utilized as a source of Vi-PS27. Plant-based Vi-PS is advantageous due to its significantly lower molecular weight when compared to the conventional sources, which makes the process of developing a Vi PS-protein conjugate easier. However, there may be considerable regulatory hurdles that would be expected using plant source PS rather than true Vi-PS from a bacterial source.
Vi capsular PS is a linear homopolymer of (1→4) alpha-D-galacturonic acid with N- and O-acetylation at its O2 and O3 positions28,45. The degree of O-acetylation, which may be variable in different Vi- PS preparations, influences the immunogenicity of a glycoconjugate the most46. Therefore, it is necessary to quantify the optimal level of O-acetylation that can provide adequate antigenic stimulation46. The immunodominant epitopes of Vi-PS molecule are the two hydrophobic groups, O-acetyl and N-acetyl, which overhang on both sides of the PS, whereas the carboxyl groups are less exposed; hence, they remain an insignificant determinant of Vi-PS immunogenicity. The carboxyl group is, therefore, selected as the linking site for carrier protein47.
The most critical step in the development of a Vi-PS protein conjugate is the selection of a correct ‘linker scheme’. In clinical settings, the two commonly employed schemes are hetero-bi-functional cross-linker N-succinimidyl-3-(2-pyridyldithio) propionate (SPDP) and homo-bi-functional linker adipic acid dihydrazide (ADH)48,49. The protein-ADH scheme consistently elicited a higher amount of anti-Vi IgG antibodies than the SPDP scheme48. Four different carrier proteins have been employed in the production of different TCVs so far28. These include recombinant exoprotein A from Pseudomonas aeruginosa (rEPA), TT, diphtheria toxoid (DT) and cross-reactive material (CRM 197), a non-toxic mutant of diphtheria toxin28,41 (Table II). The immunogenicity of a glycoconjugate is affected more by the degree of O-acetylation and conjugation scheme rather than the carrier protein used or the source of Vi-PS28,46. Using a plant-based Vi-PS source may eliminate some of the technical difficulties associated with the production of Vi-PS conjugate. There is a concern that a ‘pre-exposure’ or ‘co-exposure’ of a carrier protein containing TT or DT can adversely affect the immunogenicity of the carbohydrate moiety to which conjugation is done through a phenomenon referred to as ‘epitope suppression’50. However, as stated above, the type of carrier protein is not the sole criteria, and many other factors such as chemical linking, PS size, the degree of O-acetylation and presence of a spacer affect the final immunogenicity of glycoconjugate vaccines51. It needs to be emphasized that the making of a Vi-protein conjugate is a complex process and every Vi conjugate product is distinct.
Optimal dose of Vi-PS for an ideal TCV
The two licensed TCVs, the Typbar-TCV™ and PedaTyph™, contain 25 and 5 μg of Vi-PS, respectively. The two experimental TCVs, the Vi-rEPA and Vi-CRM, also have a different amount of Vi-PS (Table II). The amount of PS in the currently used other conjugate vaccines ranges from 2 μg/injection for pneumococcal conjugate vaccines to 10 μg/ml for Haemophilus influenzae type b. The first, experimental TCV, Vi-rEPA employed 25 μg of PS. The dose of 25 μg was selected on the basis of the amount of PS present in the licensed Vi-PS vaccine28.
As per the WHO guidelines to vaccine manufacturers52, it is mandatory to determine an adequate dose and schedule of a candidate TCV, and extrapolation must be avoided even if the same carrier protein is employed52. The immunogenicity of the Vi-PS conjugate vaccines is found to be dose dependent. In a dose-escalating trial of the experimental Vi-rEPA vaccine in 2-5 yr old Vietnamese children, a dose-dependent increase in anti-Vi IgG titres was noticed, with 25 μg eliciting the highest immune response34. However, the non-availability of a reliable serological correlate of protection (CoP) has compounded the determination of an exact dose. Adopting 4.3 μg/ml [or 3.52 ELISA Unit (EU)] as the putative protective cut-off level, 12.5 μg was found to be the most optimum dose in the above trial, whereas only 77 per cent of patients with 5 µg dose were seroprotected at 52 wk34 (Table III). When the protective cut-off level was lowered to 2.0 μg/ml, the newly estimated serocorrelate based on re-examination of Vietnam's efficacy trial results of Vi-rEPA, 100 per cent children with 5 μg dose were found seroprotected at 52 wk and no difference was seen among the three dose strengths of the vaccine35 (Table III). A very low antigen dose of 1.25 μg of Vi-PS in TCV was found as immunogenic or even better than 25 μg/dose of unconjugated Vi-PS vaccine in a trial of another TCV employing CRM197 as a carrier protein39.
Table III.
Percentage of individuals above the different seroprotective cut-offs and the anti-Vi-IgG levels at different strengths of polysaccharide employed in an experimental V-recombinant exoprotein A from Pseudomonas aeruginosa typhoid conjugate vaccine trial in 2-5 yr old children
| Strength of Vi-PS | Seroprotective level (µg/ml) | |||||
|---|---|---|---|---|---|---|
| >4.3 | 2.0 | |||||
| 0 wk | 10 wk | 52 wk | 0 wk | 10 wk | 52 wk | |
| 25 µg (n=77-78) (%) | 0 | 100 | 95 | 0 | 100 | 99 |
| Anti-Vi-IgG levels (GM, µg/ml) | 0.16 | 126.90 | 16.45 | 0.16 | 126.90 | 16.45 |
| 12.5 µg (n=79-80) (%) | 0 | 100 | 95 | 0 | 100 | 100 |
| Anti-Vi-IgG levels (GM, µg/ml) | 0.18 | 92.58 | 14.02 | 0.18 | 92.58 | 14.02 |
| 5 µg (n=75-76) (%) | 0 | 100 | 77 | 3 | 100 | 100 |
| Anti-Vi-IgG levels (GM, µg/ml) | 0.21 | 53.29 | 7.97 | 0.21 | 53.29 | 7.97 |
GM, geometric mean. Source: Reproduced with permission from Ref. 35
The issue of the exact dose of Vi-PS in a TCV is still unsettled. Most of the manufacturers of TCVs have adopted a high-end dose, 25 μg of Vi-PS, in their upcoming products (Table II). However, more studies are needed, mainly, on long-term effectiveness trials, to get a final answer.
Number of doses needed for a primary immunization schedule
The issue of the exact number of doses required for a primary series of Vi-TCV is not yet fully elucidated. Some earlier trials of TCVs have employed more than a single primary dose in their protocols30,36,40. In Vietnam, after 46 months of vaccination, both one- and two-dose recipients of Vi-rEPA vaccine, showed comparable point estimates of efficacy (87.7 and 89%, respectively)36,37. No difference in the geometric mean titres (GMTs) of anti-Vi IgG antibodies was found in one- and two-dose recipients after three years of the first dose in an earlier trial of the same vaccine38. Although four weeks after the second injection the GMTs were significantly higher in children who received two doses than those who received only one dose, however the antibody gap between the one- and two-dose recipients steadily narrowed down considerably after three years38. In the multicentric trial of experimental Vi-CRM in three countries, the second dose in the primary series did not have any incremental effect on GMTs in children and older infants40. In the post-licensure cluster-randomized effectiveness study of PedaTyph™, no case of culture-positive typhoid was found throughout the 12 months among 140 individuals who had received only a single dose30. Furthermore, in a subgroup analysis of 62 individuals, 100 per cent individuals seroconverted at six weeks following a single dose of the vaccine30. In a pre-licensure study of the same vaccine, a single dose seroconverted 100 per cent of the study individuals aged three months to two years31. A post-licensure study of PedaTyph™ on 163 individuals in Chennai found 83 per cent seroconversion following a single dose32. A subset analysis of one- and two-dose recipients did not find any significant advantage of two doses after 30 months post-vaccination33.
On analysis of the available data, it seems that a single dose is sufficient for the induction of adequate immune responses and a closely spaced second dose is not going to confer higher immunity in a primary series. However, as some amount of waning of antibody titres after 6 to 12 months of immunization was observed in some studies30,38,40, a booster dose, especially in young children (<2 yr of age), may be needed. The WHO-SAGE Working Group on Typhoid Vaccines has recommended only a single dose of the TCV at any time between 6 and 23 months of age in the endemic countries9,24.
Immune responses and correlate of protection (CoP)
Antibodies, produced in response to both typhoid infection and vaccination, are generally used as the gold standard for measuring vaccine immunogenicity although their role in the clearance of S. Typhi infections is not properly understood53. The protection is primarily conferred by the higher level of anti-Vi antibodies as suggested by both the earlier trials of Vi-PS vaccine13,14 and later trials of TCVs29,30,36,37,40,44. Some experts have suggested that serial measurement of Vi antibodies may serve as a marker of typhoid exposure44,54. However, serum IgG titres were found to be poor correlates of protection for Vi-PS vaccines in some communities. In a human challenge study of Typbar-TCVTM55, no significant difference in the titres of anti-Vi IgG antibodies was found between individuals who were diagnosed with typhoid fever and those who did not. This observation suggests that antibody functionality is equally important for protection as the total antibody quantity55. There may be a role of subclasses of IgG antibodies (IgG1-IgG4), and functional Vi-antibodies such as those involved with neutralization, opsonization and/or antibody-dependent cellular cytotoxicity activity and they may become more important determinant of protection56,57. In the trials of Vi-PS vaccine, IgG2 titres were found to be the main determinant of protection57. The intensity of the anamnestic response is also determined by the avidity of elicited antibodies29.
Immunity to S. Typhi is complex and involves both systemic antibodies (against O, H, Vi and other S. Typhi antigens) and local (IgA) antibodies along with cell-mediated immunity (CMI)53. The role of CMI in the elimination of S. Typhi infection and prevention of carrier stage becomes crucial as the organism may remain intracellularly. Some reports suggest a more dominant role of CMI in protection against typhoid through the active participation of CD4+ helper and CD8+ cytotoxic T cells58,59. The trials of oral Ty21a vaccine reveal that CMI responses comprise both Th1 and cytotoxic T cell type responses that are associated with lymphoproliferation53,58. However, no association was observed in humoral and cellular responses53.
The role of gut-homing, circulating IgA antibody-secreting cells (ASCs) in protecting against the typhoid was also studied in Ty21a trials. The extent of the IgA ASC responses against O antigen correlated with efficacy, but the boost in IgA ASC levels failed to show any association with serum anti-S. Typhi lipopolysaccharide (LPS) O responses60.
The human immune responses to S. Typhi following immunization are complex. However, the immunologic CoP remains mostly indeterminate. Identification of a reliable immune CoP is essential for the proper evaluation of new typhoid vaccines. In the past, several attempts were made to decide a reliable CoP for TCV. However, these attempts were limited by the lack of efficacy trials with TCV because only one large efficacy trial of any TCV has been conducted so far36. During the Vi-rEPA efficacy trial, the CoP was first proposed to be 8.7 μg/ml of anti-Vi IgG level based on the 27 months of active surveillance and subsequently lowered to 4.3 μg/ml (equal to 3.52 EUs) at 46 months36,37. Later, in a re-analysis of the anti-Vi IgG levels in different age groups of children in the Vietnam efficacy trial37, a much lower estimate (in the range of 1.4-2.0 μg/ml) was described35. The WHO has also analyzed the clinical data of Vi-rEPA and concluded that it is not possible to identify a cut-off based on the old NIH-sponsored trial data24.
Need for a standardized international reference and validation of ELISA kits
Before any cut-off based on protective antibody level is applied to a new candidate TCV, it is of paramount importance to calibrate ELISA kits used by different vaccine manufacturers in their trials. To evaluate new TCVs, it is essential to quantify anti-Vi IgG antibodies in serum accurately. Currently, the antibody levels are expressed in EUs assigned arbitrarily by different laboratories. However, the assignment of EU varies extensively among different developers with no common reference to calibrate. This shortcoming rendered the comparison of clinical results of different trials nearly impossible. A standardized human reference is essential to estimate and compare the immune responses of a candidate TCV with the existing known levels28.
Long-term persistence of immune responses and need for booster doses
The Vi-rEPA has been observed to provide sustained protection against typhoid for at least 46 months in young children aged 2-5 yr after two doses (based on efficacy data)36,37. In school-age children (5-14 yr) and adults, the immunity persisted for 8 and 10 yr, respectively28,37,38. In the follow up evaluation of the Vi-rEPA efficacy trial36,37, a subset of children between five and eight years received only one injection of the test vaccine. Seventy five randomly selected children from this cohort were found to have high GMT levels of anti-Vi IgG antibodies (17.7 μg/ml), and 84 per cent had higher than the estimated seroprotective level eight years after the vaccination28.
A single dose of Typbar-TCV™ or PedaTyph™ provided good GMTs and seroconversions in the majority of the vaccines at least till 2.5 to 5 years after vaccination24,33. Theoretically, the TCV should provide a longer duration of protection than an unconjugated Vi-PS vaccine in children above two years of age. However, it is the performance of these new TCVs under two years of age which needs a close monitoring. Typically, the immune responses elicited during young age are not as robust as in older children due to the immaturity of the immune system23. This phenomenon has also been observed in the immunogenicity study of Vi-CRM19740 and efficacy trial of Vi-rEPA (Fig. 2)36. There is gradual waning of immune responses following a primary series of TCV. During the efficacy study of Vi-rEPA, the anti-Vi IgG titres decreased to 3.52 and 4.31 EU at 46 months from the peak values of 18.57 and 25.08 EU attained at six months following two doses of the vaccine in 2-3 and 4-5 yr old children, respectively (Fig. 2)37.
Fig. 2.
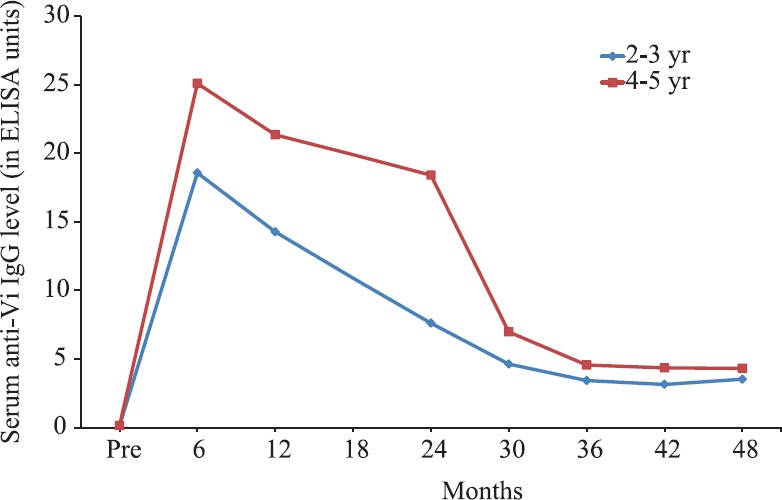
Levels of anti-Vi IgG of 2-5 yr old children in Vi-rEPA efficacy study stratified according to age. Source: Adapted from Ref. 37.
Both licensed TCVs, Typbar-TCVTM and PedaTyph™ have elicited higher immune responses in younger children (<2 yr of age) than in older children and adults29,31. However, it is the durability of these responses that merits attention. In the long-term immunogenicity study of Typbar-TCV™, 72.7 per cent of the younger age group children (<2 yr) had four or more folds seroconversion from the baseline at three years follow up in contrast to 83.6 per cent in older age group despite having higher seroconversion and GMTs in the younger age group24,29. The understanding about the long-term persistence of anti-Vi IgG antibodies is essential to determine the exact timing of a booster dose.
Exact schedule of TCVs and timing of a booster dose
The WHO-SAGE Working Group on Typhoid Vaccines did not find any need for booster doses for children or adults, especially those residing in typhoid-endemic areas24. Deciding the need and the appropriate timing of a booster dose of TCV is somewhat tricky. The schedule may differ among different age groups and populations. The older children may get considerable natural boosting, especially in a highly endemic setting. On the other hand, regular boosters may be required in specific low-endemic regions so that a minimum concentration of antibodies is maintained to confer protection. For older children, a single primary dose may provide long-term protection. As per the available studies with licensed TCVs in India, the single dose should provide adequate protection for at least 2.5 (PedaTyph™) to five years (Typbar-TCV™). However, if the clinical efficacy data of Vi-rEPA are also taken in to account, the protection may last up to 8 to 10 yr in older children and adults, respectively28. Hence, in the endemic regions, a single dose of TCV should provide long-lasting protection to older children.
For young children, particularly those under two years of age, some key issues need to be considered before recommending a 'single-dose, no-booster’ policy. First, as a general rule, immune responses elicited during infancy and young age lack certain key ‘immunological edifices’ needed for providing a long-lasting immunity owing to the immaturity of the immune system23. In some of the trials with candidate TCVs, there is a perceptible drop in the anti-Vi IgG titres at 6-12 months after vaccination following an initial rise in the antibody levels after the first dose29,30,36,37,40. Whether this observation warrants consideration of a booster dose is difficult to determine in the absence of reliable knowledge about the protective antibody levels in different age groups. At last, due to a comparatively lower burden of typhoid infection below two years of age than in 2-5 yr age group, there is limited opportunity to get natural boosting secondary to subclinical infections.
Considering all the limitations and available evidence, the best schedule for TCVs would be to harmonize their administration schedules with measles vaccination under the EPI. The option of TCV co-administration with MCVs seems entirely practicable and also allows flexibility of adopting either one or two-dose schedules. Thus, the first dose of the TCV can be administered at nine months along with Measles, Rubella (MR)/Measles, Mumps, Rubella (MMR) followed by a 2nd dose, if at all it is needed, at 16-18 months along with the 2nd dose of MCV. For the ‘catch-up schedule’ of older children who have missed the primary dose/s (at 9 and 16-18 months), a single dose of TCV can be offered. Typbar-TCV™ has already demonstrated non-interference with co-administered measles and MCV (like MR and MMR), and the other licensed TCVs should also follow suit24,42.
The WHO-SAGE Working Group on Typhoid Vaccines has also ‘tied’ the TCV schedule with measles vaccination for younger children24. However, they have suggested only a single dose of TCV at any time between 6 and 23 months of age in endemic countries9,24. For older children, aged two years and above, the SAGE has indicated their preference for TCV over other two typhoid vaccines, ViPS and Ty21a24. Although the WHO-SAGE had analyzed the data about the clinical trials of all TCVs including Vi-rEPA, Vi-CRM, Typbar-TCV™ and PedaTyph™, they have based their recommendations on the one licensed TCV, Typbar-TCV™.
Limitations of TCVs and future perspectives
The current TCVs are moderately efficacious. There are no large field efficacy data available on the licensed products. A human challenge study with Typbar-TCV™ was conducted in naïve adult volunteers in a non-endemic setting55. The Typbar-TCV™ was found to have an estimated efficacy of 54.6 per cent (95% CI: 26.8-71.8%) based on the original primary endpoint of persistent fever or S. Typhi bacteraemia and 87.1 per cent (95% CI: 47.2-96.9%) based on a post hoc analysis of alternative diagnostic criteria of persistent fever followed by positive blood culture. The respective figures for the comparator Vi-PS vaccine were 52 (23.2-70) and 52.3 per cent (−4.2-78.2)55. Although the human challenge study is cited as an efficacy study to strengthen the case of Typbar-TCV™ as an effective vaccine, still there is a need for a large efficacy trial from an endemic setting. A Cochrane review found very low-certainty evidence for PedaTyph™ and did not offer any recommendation on the Typbar-TCV™ because no efficacy data were available61. The effectiveness data from the Typhoid Vaccine Acceleration Consortium (TyVAC) sponsored trials of TCV in a few LMI countries of Asia and Africa should fill this void43.
The use of currently available TCVs is limited due to their inability to protect S. Paratyphi A and non-typhoidal Salmonella serotypes. As the current TCVs are based on Vi-antigen, these are ineffective against Vi-negative S. Typhi strains which exist naturally and have caused disease in the past62. With the large-scale use of TCVs, there is a potential threat of outbreaks caused by Vi-negative strains due to natural selection under vaccine pressure63. Furthermore, the existing TCVs do not produce broad immune responses including CMI and fail to induce intestinal secretory IgA response.
Considering all these limitations, there is a need for new typhoid vaccines which are more efficacious and have serotype-independent coverage against all Salmonella strains. There are new advanced technologies underway to develop more effective and broadly protective typhoid vaccines. A few such novel approaches include the use of single or multiprotein subunit vaccines64,65, use of novel linking methods51 and exploration of the dual role of proteins as a carrier and protective antigen66. Although protein subunit vaccines may overcome some of the limitations of TCVs, yet the challenge is to recognize suitable antigens that can be developed into effective human vaccines67. One such protein antigen could be the outer membrane vesicles (OMVs) which are secreted naturally from several Gram-negative bacteria including Salmonella and have already been used in some vaccine development studies68. The OMVs contain LPS and other membrane proteins that act as a natural adjuvant and are found protective against both S. Typhi and Paratyphi A68.
Another exciting development is the exploration of new ways to present carbohydrate antigens to the immune system51. Conventionally, PS antigens are covalently attached to carrier proteins to convert T-independent antigens into T-dependent ones. The evidence is now emerging that the covalent bonding may not be required, and protein carriers can be directly coupled to activated glycans to introduce functional groups for subsequent conjugation51. Genetically modified proteins can also be employed to predetermine the site of linking with PS for in vivo expression. These new advancements along with the use of novel carrier systems such as nanoparticles have been projected as alternative methods to develop new glycoconjugates51.
With the looming threat of Vi-negative strains63, there is a greater focus on antigens universally present in all S. Typhi such as O-specific PSs69. These new typhoid vaccines will be considerably cost-effective than the TCVs at present. Unlike Vi-based vaccines, these would be effective against Vi-negative strains as well as S. Paratyphi A infection.
Conclusions
There is a significant burden of typhoid fever in India, especially among young children under five years of age. There is a need for a large-scale vaccination programme along with other preventive measures to tackle typhoid burden in India4. The existing Vi-PS vaccines are unable to meet this challenge. Despite having limitations, the new-generation TCVs are best suited to fill the existing void. Typbar-TCV™ has attained WHO Pre-Qualification to become eligible for introductions in the Global Alliance for Vaccines and Immunization (GAVI)-eligible countries9. In addition, many new TCVs are under various stages of development20,24. Although the current generation of TCVs are usually considered safe, robust post-marketing surveillance studies with a large number of individuals are still needed. The time is ripe to address some of the key concerns enumerated above so that these more efficient products can be utilized widely to have a significant dent on the burden of typhoid in the highly endemic countries of Asia and Africa70. At the same time, efforts should continue to develop more refined, broadly protective, typhoid vaccines to cover the entire spectrum of Salmonella infections in humans.
Footnotes
Financial support & sponsorship: None
Conflicts of Interest: None
References
- 1.Mogasale V, Maskery B, Ochiai RL, Lee JS, Mogasale VV, Ramani E, et al. Burden of typhoid fever in low-income and middle-income countries: A systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2:e570–80. doi: 10.1016/S2214-109X(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 2.Antillón M, Warren JL, Crawford FW, Weinberger DM, Kürüm E, Pak GD, et al. The burden of typhoid fever in low – And middle-income countries: A meta-regression approach. PLoS Negl Trop Dis. 2017;11:e0005376. doi: 10.1371/journal.pntd.0005376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John J, Van Aart CJ, Grassly NC. The burden of typhoid and paratyphoid in India: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004616. doi: 10.1371/journal.pntd.0004616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochiai RL, Acosta CJ, Danovaro-Holliday MC, Baiqing D, Bhattacharya SK, Agtini MD, et al. A study of typhoid fever in five Asian countries: Disease burden and implications for controls. Bull World Health Organ. 2008;86:260–8. doi: 10.2471/BLT.06.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owais A, Sultana S, Zaman U, Rizvi A, Zaidi AK. Incidence of typhoid bacteremia in infants and young children in Southern Coastal Pakistan. Pediatr Infect Dis J. 2010;29:1035–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Saha SK, Baqui AH, Hanif M, Darmstadt GL, Ruhulamin M, Nagatake T, et al. Typhoid fever in Bangladesh: Implications for vaccination policy. Pediatr Infect Dis J. 2001;20:521–4. doi: 10.1097/00006454-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Brooks WA, Hossain A, Goswami D, Nahar K, Alam K, Ahmed N, et al. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis. 2005;11:326–9. doi: 10.3201/eid1102.040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Typhoid vaccines: WHO position paper – March 2018. Wkly Epidemiol Rec. 2018;93:153–72. [Google Scholar]
- 10.Typbar TCV® from Bharat Biotech, World's First Typhoid Conjugate Vaccine Prequalifeid by WHO. Hyderabad: Genome Valley; 2018. Jan 3, [accessed on September 16, 2018]. Available from: http://www. who.int/medicines/news/2017/Bharat-Biotech-TypbarTCVWHO- PQ-Press-Release-Global-Final.pdf . [Google Scholar]
- 11.Germanier R, Füer E. Isolation and characterization of gal E mutant Ty 21a of Salmonella Typhi: A candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975;131:553–8. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 12.Wahid R, Fresnay S, Levine MM, Sztein MB. Immunization with Ty21a live oral typhoid vaccine elicits crossreactive multifunctional CD8+ T-cell responses against Salmonella enterica serovar Typhi, S Paratyphi A, and S Paratyphi B in humans. Mucosal Immunol. 2015;8:1349–59. doi: 10.1038/mi.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharya IL, Lowe CU, Thapa R, Gurubacharya VL, Shrestha MB, Cadoz M, et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella Typhi. A preliminary report. N Engl J Med. 1987;317:1101–4. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 14.Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella Typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine. 1996;14:435–8. doi: 10.1016/0264-410x(95)00186-5. [DOI] [PubMed] [Google Scholar]
- 15.Yang HH, Wu CG, Xie GZ, Gu QW, Wang BR, Wang LY, et al. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in South-Western China. Bull World Health Organ. 2001;79:625–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, Manna B, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med. 2009;361:335–44. doi: 10.1056/NEJMoa0807521. [DOI] [PubMed] [Google Scholar]
- 17.Khan MI, Soofi SB, Ochiai RL, Habib MA, Sahito SM, Nizami SQ, et al. Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: A cluster randomized trial in Karachi, Pakistan. Vaccine. 2012;30:5389–95. doi: 10.1016/j.vaccine.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Vashishtha VM, Choudhury P, Kalra A, Bose A, Thacker N, Yewale VN, et al. Indian Academy of Pediatrics (IAP) recommended immunization schedule for children aged 0 through 18 years – India, 2014 and updates on immunization. Indian Pediatr. 2014;51:785–800. doi: 10.1007/s13312-014-0504-y. [DOI] [PubMed] [Google Scholar]
- 19.Gupta G. The new generation ‘Indian’ TCV Zyvac-TCV. [accessed December 1, 2019]. Available from: https://www.slideshare.net/gauravg/zyvac-tcvthe- indian-typhoid-conjugate-vaccination-yamunanagaraug- 2018,
- 20.Khan MI, Franco-Paredes C, Sahastrabuddhe S, Ochiai RL, Mogasale V, Gessner BD, et al. Barriers to typhoid fever vaccine access in endemic countries. Res Rep Trop Med. 2017;8:37–44. doi: 10.2147/RRTM.S97309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: A partially answered question. Indian J Med Res. 2012;135:161–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Tacket CO, Levine MM, Robbins JB. Persistence of antibody titres three years after vaccination with Vi polysaccharide vaccine against typhoid fever. Vaccine. 1988;6:307–8. doi: 10.1016/0264-410x(88)90175-2. [DOI] [PubMed] [Google Scholar]
- 23.Siegrist CA. Vaccine immunology. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia: Saunders Elsevier; 2013. pp. 14–33. [Google Scholar]
- 24.World Health Organization. Background Paper on Typhoid Vaccines for SAGE Meeting World Health Organization; October, 2017. [accessed on September 16, 2018]. Available from: http://www.who.int/immunization/sage/ meetings/2017/october/1_Typhoid_SAGE_background_paper_ Final_v3B.pdf .
- 25.Daniels EM, Schneerson R, Egan WM, Szu SC, Robbins JB. Characterization of the Salmonella Paratyphi C Vi polysaccharide. Infect Immun. 1989;57:3159–64. doi: 10.1128/iai.57.10.3159-3164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hale C, Bowe F, Pickard D, Clare S, Haeuw JF, Powers U, et al. Evaluation of a novel Vi conjugate vaccine in a murine model of salmonellosis. Vaccine. 2006;24:4312–20. doi: 10.1016/j.vaccine.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szu SC, Lin KF, Hunt S, Chu C, Thinh ND. Phase I clinical trial of O-acetylated pectin conjugate, a plant polysaccharide based typhoid vaccine. Vaccine. 2014;32:2618–22. doi: 10.1016/j.vaccine.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szu SC. Development of Vi conjugate – A new generation of typhoid vaccine. Expert Rev Vaccines. 2013;12:1273–86. doi: 10.1586/14760584.2013.845529. [DOI] [PubMed] [Google Scholar]
- 29.Mohan VK, Varanasi V, Singh A, Pasetti MF, Levine MM, Venkatesan R, et al. Safety and immunogenicity of a Vi polysaccharide-tetanus toxoid conjugate vaccine (Typbar-TCV) in healthy infants, children, and adults in typhoid endemic areas: A multicenter, 2-cohort, open-label, double-blind, randomized controlled phase 3 study. Clin Infect Dis. 2015;61:393–402. doi: 10.1093/cid/civ295. [DOI] [PubMed] [Google Scholar]
- 30.Mitra M, Shah N, Ghosh A, Chatterjee S, Kaur I, Bhattacharya N, et al. Efficacy and safety of Vi-tetanus toxoid conjugated typhoid vaccine (PedaTyph™) in Indian children: School based cluster randomized study. Hum Vaccin Immunother. 2016;12:939–45. doi: 10.1080/21645515.2015.1117715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg P, Garg S, Sharma MK. Clinical trial of tetanus toxoid conjugated Vi polysaccharide typhoid vaccine in infants and young children. Biotechnol Int. 2014;7:90–100. [Google Scholar]
- 32.Chinnasami B, Mangayarkarasi V, Prema A, Sadasivam K, Davis MJ. Safety and immunogenicity of Salmonella Typhi Vi conjugate vaccine (PedaTyph™) in children upto five years. Int J Sci Res Publications. 2013;3:1–5. [Google Scholar]
- 33.Chinnasami B, Sadasivam K, Vivekanandhan A, Arunachalam P, Pasupathy S. A study on longevity of immune response after vaccination with Salmonella Typhi Vi conjugate vaccine (Pedatyph™) in children. J Clin Diagn Res. 2015;9:SC01–3. doi: 10.7860/JCDR/2015/13302.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canh DG, Lin FY, Thiem VD, Trach DD, Trong ND, Mao ND, et al. Effect of dosage on immunogenicity of a Vi conjugate vaccine injected twice into 2- to 5-year-old Vietnamese children. Infect Immun. 2004;72:6586–8. doi: 10.1128/IAI.72.11.6586-6588.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szu SC, Klugman KP, Hunt S. Re-examination of immune response and estimation of anti-Vi IgG protective threshold against typhoid fever-based on the efficacy trial of Vi conjugate in young children. Vaccine. 2014;32:2359–63. doi: 10.1016/j.vaccine.2014.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, et al. The efficacy of a Salmonella Typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–9. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 37.Mai NL, Phan VB, Vo AH, Tran CT, Lin FY, Bryla DA, et al. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Engl J Med. 2003;349:1390–1. doi: 10.1056/NEJM200310023491423. [DOI] [PubMed] [Google Scholar]
- 38.Kossaczka Z, Lin FY, Ho VA, Thuy NT, Van Bay P, Thanh TC, et al. Safety and immunogenicity of Vi conjugate vaccines for typhoid fever in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun. 1999;67:5806–10. doi: 10.1128/iai.67.11.5806-5810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Damme P, Kafeja F, Anemona A, Basile V, Hilbert AK, De Coster I, et al. Safety, immunogenicity and dose ranging of a new Vi-CRM197 conjugate vaccine against typhoid fever: Randomized clinical testing in healthy adults. PLoS One. 2011;6:e25398. doi: 10.1371/journal.pone.0025398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and Southeast Asia: Results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis. 2014;14:119–29. doi: 10.1016/S1473-3099(13)70241-X. [DOI] [PubMed] [Google Scholar]
- 41.Capeding MR, Teshome S, Saluja T, Syed KA, Kim DR, Park JY, et al. Safety and immunogenicity of a Vi-DT typhoid conjugate vaccine: Phase I trial in healthy Filipino adults and children. Vaccine. 2018;36:3794–801. doi: 10.1016/j.vaccine.2018.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Global Advisory Committee on Vaccine Safety 30 November – 1 December 2016. Wkly Epidemiol Rec. 2017;92:13–20. [PubMed] [Google Scholar]
- 43.Shakya M, Colin-Jones R, Theiss-Nyland K, Voysey M, Pant D, Smith N, et al. Phase 3 efficacy analysis of a typhoid conjugate vaccine trial in Nepal. N Engl J Med. 2019;381:2209–18. doi: 10.1056/NEJMoa1905047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voysey M, Pollard AJ. Seroefficacy of Vi polysaccharide-tetanus toxoid typhoid conjugate vaccine (Typbar TCV) Clin Infect Dis. 2018;67:18–24. doi: 10.1093/cid/cix1145. [DOI] [PubMed] [Google Scholar]
- 45.Pilnik W, Voragen AGJ. Pectic substances and other uronides. In: Hulme AC, editor. The biochemistry of Fruits and their Products. Vol. 1. London: Academic Press; 1970. pp. 53–87. [Google Scholar]
- 46.Bystricky S, Szu SC. O-acetylation affects the binding properties of the carboxyl groups on the Vi bacterial polysaccharide. Biophys Chem. 1994;51:1–7. doi: 10.1016/0301-4622(94)00002-6. [DOI] [PubMed] [Google Scholar]
- 47.Szu SC, Li XR, Stone AL, Robbins JB. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect Immun. 1991;59:4555–61. doi: 10.1128/iai.59.12.4555-4561.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szu SC, Stone AL, Robbins JD, Schneerson R, Robbins JB. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987;166:1510–24. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kossaczka Z, Bystricky S, Bryla DA, Shiloach J, Robbins JB, Szu SC. Synthesis and immunological properties of Vi and di-O-acetyl pectin protein conjugates with adipic acid dihydrazide as the linker. Infect Immun. 1997;65:2088–93. doi: 10.1128/iai.65.6.2088-2093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dagan R, Poolman J, Siegrist CA. Glycoconjugate vaccines and immune interference: A review. Vaccine. 2010;28:5513–23. doi: 10.1016/j.vaccine.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Micoli F, Adamo R, Costantino P. Protein carriers for glycoconjugate vaccines: History, selection criteria, characterization and new trends. Molecules. 2018;23 doi: 10.3390/molecules23061451. pii: E1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. Guidelines on the Quality, Safety and Efficacy of Typhoid Conjugate Vaccines. World Health Organization; 2013. [accessed on September 2, 2018]. Available from: http://www. who.int/biologicals/areas/vaccines/TYPHOID_BS2215_doc_ v1.14_WEB_VERSION.pdf . [Google Scholar]
- 53.Sztein MB. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis. 2007;45(Suppl 1):S15–9. doi: 10.1086/518140. [DOI] [PubMed] [Google Scholar]
- 54.Klugman KP. Usefulness of the serial measurement of Vi antibodies. Clin Infect Dis. 2018;67:25–6. doi: 10.1093/cid/cix1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jin C, Gibani MM, Moore M, Juel HB, Jones E, Meiring J, et al. Efficacy and immunogenicity of a Vi-tetanus toxoid conjugate vaccine in the prevention of typhoid fever using a controlled human infection model of Salmonella Typhi: A randomised controlled, phase 2b trial. Lancet. 2017;390:2472–80. doi: 10.1016/S0140-6736(17)32149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lindow JC, Fimlaid KA, Bunn JY, Kirkpatrick BD. Antibodies in action: Role of human opsonins in killing Salmonella enterica serovar Typhi. Infect Immun. 2011;79:3188–94. doi: 10.1128/IAI.05081-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: From structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sztein MB, Salerno-Goncalves R, McArthur MA. Complex adaptive immunity to enteric fevers in humans: Lessons learned and the path forward. Front Immunol. 2014;5:516. doi: 10.3389/fimmu.2014.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fresnay S, McArthur MA, Magder LS, Darton TC, Jones C, Waddington CS, et al. Importance of Salmonella Typhi-responsive CD8+ T cell immunity in a human typhoid fever challenge model. Front Immunol. 2017;8:208. doi: 10.3389/fimmu.2017.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kantele A, Mäkelä PH. Different profiles of the human immune response to primary and secondary immunization with an oral Salmonella Typhi Ty21a vaccine. Vaccine. 1991;9:423–7. doi: 10.1016/0264-410x(91)90129-t. [DOI] [PubMed] [Google Scholar]
- 61.Milligan R, Paul M, Richardson M, Neuberger A. Vaccines for preventing typhoid fever. Cochrane Database Syst Rev. 2018;5:CD001261. doi: 10.1002/14651858.CD001261.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saha MR, Ramamurthy T, Dutta P, Mitra U. Emergence of Salmonella Typhi Vi antigen-negative strains in an epidemic of multidrug-resistant typhoid fever cases in Calcutta, India. Natl Med J India. 2000;13:164. [PubMed] [Google Scholar]
- 63.Haque A. Significance of Vi negative isolates of Salmonella enterica serovar Typhi. Adv Exp Med Biol. 2018;1052:9–18. doi: 10.1007/978-981-10-7572-8_2. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S, Chakraborty K, Nagaraja T, Basak S, Koley H, Dutta S, et al. An adhesion protein of Salmonella enterica serovar Typhi is required for pathogenesis and potential target for vaccine development. Proc Natl Acad Sci U S A. 2011;108:3348–53. doi: 10.1073/pnas.1016180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Das S, Chowdhury R, Ghosh S, Das S. A recombinant protein of Salmonella Typhi induces humoral and cell-mediated immune responses including memory responses. Vaccine. 2017;35:4523–31. doi: 10.1016/j.vaccine.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 66.Bröker M, Berti F, Schneider J, Vojtek I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency” – Potential and limitations. Vaccine. 2017;35:3286–94. doi: 10.1016/j.vaccine.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 67.Martinez-Becerra FJ, Kumar P, Vishwakarma V, Kim JH, Arizmendi O, Middaugh CR, et al. Characterization and protective efficacy of type III secretion proteins as a broadly protective subunit vaccine against Salmonella enterica serotypes. Infect Immun. 2018;86:e00473–17. doi: 10.1128/IAI.00473-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Howlader DR, Koley H, Sinha R, Maiti S, Bhaumik U, Mukherjee P, et al. Development of a novel S.Typhi and Paratyphi A outer membrane vesicles based bivalent vaccine against enteric fever. PLoS One. 2018;13:e0203631. doi: 10.1371/journal.pone.0203631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Salman M, St Michael F, Ali A, Jabbar A, Cairns C, Hayes AC, et al. First characterization of immunogenic conjugates of Vi negative Salmonella Typhi O-specific polysaccharides with rEPA protein for vaccine development. J Immunol Methods. 2017;450:27–33. doi: 10.1016/j.jim.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Steele AD, Hay Burgess DC, Diaz Z, Carey ME, Zaidi AK. Challenges and opportunities for typhoid fever control: A call for coordinated action. Clin Infect Dis. 2016;62(Suppl 1):S4–8. doi: 10.1093/cid/civ976. [DOI] [PMC free article] [PubMed] [Google Scholar]


