Abstract
Background
Lung disease in preterm infants is often complicated with lung edema.
Objectives
To assess the risks and benefits of administration of a diuretic acting on the loop of Henle (loop diuretic) in preterm infants with or developing chronic lung disease (CLD).
Search methods
Standard search method of the Cochrane Neonatal Review Group was used. Initial search included the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2003), MEDLINE (1966 to April 2003), EMBASE (1974 to 1998). In addition, several abstract books of national and international American and European Societies were hand searched. The MEDLINE and the Cochrane Central searches were updated in March 2007 and December 2010. The EMBASE search was completed in April 2007 and December 2010. Additional searches in CINAHL, clinicaltrials.gov and controlled‐trials.com was completed in December 2010.
Selection criteria
Trials in which preterm infants with or developing chronic lung disease and at least five days of age were all randomly allocated to receive a loop diuretic either enterally or intravenously were included in this analysis.
Data collection and analysis
The standard method for the Cochrane Collaboration described in the Cochrane Collaboration Handbook were used. Two investigators extracted, assessed and coded separately all data for each study. Parallel and cross‐over trials were combined and, whenever possible, transformed baseline and final outcome data measured on a continuous scale into change scores using Follmann's formula.
Main results
The only loop diuretic used in the six studies that met the selection criteria was furosemide. Most studies focused on pathophysiological parameters and did not assess effects on important clinical outcomes defined in this review, or the potential complications of diuretic therapy. In preterm infants < 3 weeks of age developing CLD, furosemide administration has either inconsistent effects or no detectable effect. In infants > 3 weeks of age with CLD, a single intravenous dose of 1 mg/kg of furosemide improves lung compliance and airway resistance for one hour. Chronic administration of furosemide improves both oxygenation and lung compliance.
Authors' conclusions
In view of the lack of data from randomized trials concerning effects on important clinical outcomes, routine or sustained use of systemic loop diuretics in infants with (or developing) CLD cannot be recommended based on current evidence. Randomized trials are needed to assess the effects of furosemide administration on survival, duration of ventilatory support and oxygen administration, length of hospital stay, potential complications and long‐term outcome.
Plain language summary
Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease
There is no strong evidence of benefit from routine use of loop diuretics in preterm babies at risk of, or with, chronic lung disease. Lung disease in babies born early (preterm) is often complicated with excess of water. Medications that reduce body water (diuretics) might help the baby recover from lung disease. The review analysed the effects of diuretics working on the deep part of the small kidney tubes (loop diuretics). The review of trials found that diuretics, given from a single dose to one week's treatment, inconsistently improved lung function and oxygen levels in the blood. There was not enough evidence to show any improvement in long‐term outcome.
Background
Early stages of chronic lung disease (CLD) of prematurity are associated with lung edema. This review is part of a group of three closely related reviews on diuretics in preterm infants with (or developing) CLD (Brion 1999a; Brion 1999b; Brion 1999c). The present review describes the evidence about systemic administration of loop diuretics (i.e., those acting on the loop of Henle) in preterm infants with or developing CLD. The other two reviews discuss the use of aerosolized loop diuretics and the use of diuretics acting on distal segments of the renal tubule (e.g., thiazides and spironolactone).
Description of the condition
Early stages of bronchopulmonary dysplasia (BPD) or CLD of prematurity are associated with alveolar and interstitial edema (Brown 1978; Edwards 1979; Northway 1967; Reid 1979). Factors involved in this edema include increased capillary permeability resulting from lung injury, congestive heart failure due to patent ductus arteriosus, and fluid overload (Brown 1978; Zimmerman 1995). This edema could not only reduce lung compliance (and thus tidal volume if using a pressure‐limited ventilator) but also increase airway resistance by narrowing terminal airways (Northway 1967).
Description of the intervention
1. Diuretics may accelerate lung fluid reabsorption and improve pulmonary mechanics in patients with lung edema via two types of mechanisms: 1.1 An immediate, diuresis‐independent lung fluid reabsorption: This may result (1) from systemic venodilatation, which increases systemic vein capacitance (Dikshit 1973), (2) from decreased pulmonary shunt in the absence of any change in wedge or oncotic pressure (as described in oleic acid‐induced pulmonary edema) (Ali 1979), (3) from pulmonary venodilation which decreases wedge pressure, thereby increasing transpulmonary fluid absorption (Demling 1978) or decreasing fluid filtration, and in turn decreasing lung lymph flow (Bland 1978). Furosemide‐induced pulmonary vasodilation may result from an increase in prostaglandins (Lundergan 1988). 1.2. A delayed increase in urine output (Aufricht 1997), which reduces extracellular volume including interstitial volume (O'Donovan 1989), and may reduce afterload (Wilson 1981) and increase oncotic pressure (Sola 1981). The effects of diuretics on the kidney, in contrast with those on the lung, have been studied extensively and well characterized (Brion 1994), and will not be reviewed here. If diuretic administration is repeated for several days, hormonal and renal adaptation mechanisms eventually will limit the diuretic response. Addition of a diuretic acting upon another segment of the nephron is often able to overcome this tolerance.
2. Pharmacokinetics of loop diuretics in premature infants: The discussion will be limited to possible causes for systematic bias in relevant studies. First, half‐life of loop diuretics is prolonged in very low birth weight infants (Chemtob 1989; Lopez 1990; Mirochnick 1988; Peterson 1980; Shankaran 1990; Vert 1982 ). In one series, furosemide half‐life, often > 24 hours in patients with a postconceptional age < 31 weeks, decreased to < 12 hours in most infants by 33 weeks and to four hours by term age (Mirochnick 1988). Because of that long half‐life in immature infants, a prolonged washout period (without diuretic administration) is needed if one wishes to eliminate all diuretic effect before initiating a clinical trial or between exposures in cross‐over trials. Second, bioavailability of a single dose of enteral furosemide in preterm infants ranges between 84.3% (range 56 ‐ 106%, postconceptional age 39.1 weeks) (Mirochnick 1988) and less than 20% (postnatal age four months) (Peterson 1980). Nevertheless, average steady state (duration 7 ‐ 14 days) maximum plasma concentrations achieved using 1 mg/kg intravenous doses every 12 hours (23.1 ± 13.8 µg/ml at a postconceptional age of 30.1 ± 6.0 weeks) are similar to those achieved using 2 mg/kg enteral doses (20.0±6.5 µg/ml at a postconceptional age of 29.5 ± 2.0 weeks) (Mirochnick 1988). Thus, an enteral dose of 2 mg/kg furosemide is usually considered equivalent to an intravenous dose of 1 mg/kg in premature infants (Mirochnick 1988, Engelhardt 1986).
3. Potential effects of diuretic administration on other systems include the following: 3.1. Changes in water and electrolyte handling: These may include an increase in urine output and in the excretion of Na, K and Cl, a decrease in extracellular volume and thus changes in water distribution in the body, hypovolemia, increased drug‐induced nephrotoxicity (resulting at least in part from hypovolemia), hyponatremia, hypokalemia, hypochloremia, hyperuricemia and metabolic alkalosis. Hyponatremia is common in very low birth weight infants treated with thiazides (Horgan 1996), which do not affect urinary concentration mechanisms, in contrast with loop diuretics. Hypochloremia and alkalosis have been associated with mortality in premature infants with BPD (Giacoia 1991; Perlman 1986); however, causality has not been proven.
3.2. Changes in mineral reabsorption: Hypercalciuria, associated with the use of many diuretics, is most prominent with loop diuretics and spironolactone. Hypercalciuria may lead to nephrolithiasis, nephrocalcinosis and bone demineralization. A 48 fold increased risk of nephrocalcinosis has been associated with a 10mg/kg cumulative dose of furosemide during a NICU hospitalization (Gimpel 2010). Many infants with nephrocalcinosis have long‐term glomerular and tubular renal dysfunction (Downing 1992; Ezzedeen 1988). In contrast, thiazides, metolazone and K‐sparing diuretics other than spironolactone enhance calcium reabsorption by the renal tubule and thus cause hypocalciuria and even may cause hypercalcemia. Nevertheless, administration of thiazide does not prevent hypercalciuria in patients receiving a loop diuretic (Campfield 1997). An increase in phosphaturia is observed with all diuretics. This phosphaturia may contribute to a negative phosphate balance, which is a major factor involved in osteopenia of prematurity.
3.3. Cardiovascular system: Diuretic administration may have both beneficial and harmful effects on the cardiovascular system. Diuretics are useful to treat pulmonary edema due to congestive heart failure and to treat systemic hypertension, a common complication of BPD. In contrast, administration of furosemide increases the incidence of patent ductus arteriosus compared to thiazide diuretic administration in patients with RDS (Green 1983). In addition, furosemide administration simultaneous with indomethacin therapy results in a trend towards a 10% higher risk of failure of ductal closure in response to indomethacin (Brion 1998).
3.4. Gastrointestinal tract: Cholelithiasis is associated with furosemide administration (Randall 1992).
3.5. Neurosensory hearing loss: This may result from high serum levels of furosemide associated with long half‐life in immature infants.
How the intervention might work
Based on the above, one may speculate that enteral or intravenous administration of furosemide could improve pulmonary mechanics by two separate mechanisms: ‐immediate diuresis‐independent reabsorption of lung fluid ‐delayed reabsorption of lung fluid mediated by a decrease in extracellular volume secondary to increased diuresis. Both these mechanisms would be expected to improve lung compliance (and tidal volume with pressure‐limited ventilation).
Why it is important to do this review
Furosemide is reported to be one of the top 10 reported drugs used in NICU’s treating critically ill neonates (Clark 2006). Despite their widespread use, there are surprisingly few studies looking at short and long term improvement in infants with CLD treated with loop diuretics. In addition, these drugs have many side effects including nephrocalcinosis which can contribute to long term renal morbidity (Downing 1992; Ezzedeen 1988).
Objectives
The aim of this systematic review was to assess the risks and benefits of loop diuretic administration in premature infants with or developing chronic lung disease. Primary objectives were to assess: 1. Short‐term improvement (within one week): changes in mean airway pressure, need for artificial ventilation, need for continuous positive airway pressure, failure to tolerate extubation, and oxygen supplementation 2. Long‐term improvement: mortality, duration of need for oxygen supplementation and respiratory support, need for oxygen supplementation at 28 days of life, chronic lung disease at 36 weeks postmenstrual age (gestational age + postnatal age), length of stay, and number of rehospitalizations during the first year of life. Secondary objectives were to assess the potential complications of diuretic administration and changes in pulmonary mechanics after treatment.
Methods
Criteria for considering studies for this review
Types of studies
Only randomized controlled studies were included in this analysis. Randomization needed to involve the allocation of all patients either to a specific treatment (patients on diuretic vs controls on placebo or another therapy), or to a specific time of administration of the diuretic (diuretic first vs placebo first).
Types of participants
Participants needed to be: (1) Preterm infants (< 37 weeks). (2) With oxygen dependency (> 21% O2 to maintain pulse oximetry > 90% or paO2 > 50 mm Hg) or ventilator dependency secondary to lung disease beyond five days of life. Although BPD is usually defined by the need for oxygen supplementation at four weeks of age, CLD already starts during the first few days of life in patients with RDS (Northway 1967). For the present study, data was collected on all patients in whom lung disease persisted beyond five days of age, to avoid overlap with a systematic review of the use of diuretics in patients with RDS (Brion 1999d).
Types of interventions
The design of the study needed to include a randomized allocation to intravenous or enteral administration of a loop diuretic. Eligible studies were those that assessed either the administration of a loop diuretic compared to placebo (or no treatment), the administration of an additional loop diuretic compared to a single diuretic in controls, the administration of a different loop diuretic from that in controls, or administration of a loop diuretic using another mode compared to control therapy. The plan in the original protocol was to include all studies on diuretics. It turned out that the number of comparisons would be too large to fit into a single review, so the review was separated into three parallel and complementary reviews dealing, respectively, with the following: (1) Intravenous and enteral administration of a loop diuretic (2) Aerosolized administration of a loop diuretic (3) Administration of thiazides and spironolactone This review deals with intravenous and enteral administration of a loop diuretic.
Types of outcome measures
Outcome measures had to include an assessment of the effect of diuretic administration on at least one of the following variables:
Primary outcomes
1. Primary outcome variables: 1.1. Short‐term improvement: changes in mean airway pressure, need for artificial ventilation, need for continuous positive airway pressure, failure to tolerate extubation, and oxygen supplementation 1.2. Long‐term improvement: mortality, duration of need for oxygen supplementation and respiratory support, need for oxygen supplementation at 28 days of life, chronic lung disease at 36 weeks postmenstrual age (gestational age + postnatal age), length of stay, and number of rehospitalizations during the first year of life.
Secondary outcomes
2. Secondary outcomes: 2.1. Potential complications of therapy: hypovolemia, alkalosis, hyponatremia, hypochloremia, bone demineralization, nephrocalcinosis, nephrolithiasis, cholelithiasis, neurosensory hearing loss. 2.2. Pulmonary function: resistance, compliance, tidal volume for patients on pressure‐limited ventilation, expiratory flow.
Search methods for identification of studies
See: Collaborative Review Group search strategy The standard search method of the Cochrane Neonatal Review Group was used.
1. Published manuscripts: MEDLINE (1966 to 1998), EMBASE (1974 to 1998) and the Cochrane Controlled Trials Register (CCTR) from The Cochrane Library (Issue 3, 1998) in August 1998 were searched. The search was not limited to any language. The following keywords were used: {<bronchopulmonary dysplasia> or <chronic lung disease>} and <explode diuretics>, limited to <human> and limited to <infant, newborn> or <infant>.
A new MEDLINE search was done on August 20, 2000 using the keywords {<bronchopulmonary dysplasia> or <chronic lung disease>} and <diuretics>, limited to <human> and limited to <infant, newborn>, and publications starting January 1, 1998. This search did not yield any additional relevant randomized trials.
An additional search of MEDLINE done using {<bronchopulmonary dysplasia> or <chronic lung disease>} and <diuretic> in April 2003, March 2007 and repeated in December 2010 did not yield any additional eligible studies. An additional EMBASE search in April 2007 and December 2010 did not yield any additional eligible studies.
A new search was completed in December 2010 in CINAHL, clinicaltrials.gov, and controlled‐trials.com. No additional eligible studies were obtained.
2. Published abstracts: The abstracts of the following national or international societies were searched (1991 to 1998 unless otherwise specified): # American Academy of Pediatrics 90‐98 (published in American Journal of Perinatology [1990 to 1995] and in Pediatrics [1996 to 1998]) # American Society of Nephrology (published in Journal of the American Society of Nephrology) # American Thoracic Society 1991 to 1998 (published in American Review of Respiratory Disease [1991 to 1993] and in American Journal of Respiratory and Critical Care Medicine [1994 to 1998]) # British Paediatric Association (now Royal College of Paediatrics and Child Health [RCPCH]) Annual Scientific Meeting # European Respiratory Society # European Society for Pediatric Research (published in Pediatric Research) # Neonatal Society [UK] # Society for Pediatric Research [US] (published in Pediatric Research).
The search was done by hand for most of these societies, and by CD‐ROM where available (American Thoracic Society 1998, [American] Society for Pediatric Research 1997‐8 and American Society of Nephrology 1997‐8). For abstract books or CD‐ROMs with keywords, the following keywords were used: bronchopulmonary dysplasia, bumetanide, chronic lung disease, diuretic, furosemide, lung disease chronic, metolazone, spironolactone, and thiazide.
For abstract books that do not include keywords the search was limited to relevant sections, whenever possible. For the American Academy of Pediatrics the sections on Computer and other Technologies, Critical Care and Perinatal Pediatrics were hand searched. For the American Thoracic Society (1991 to 1993 and 1995 to 1997) sections with a title that included one of the following keywords were searched: airway (pharmacology, dynamics, reactivity, hyperreactivity, hyperresponsiveness), asthma (pharmacology, therapy, treatment), bronchopulmonary dysplasia, BPD, childhood/children, diuretic, edema, fluid, infant, mechanics, neonatal, pediatric(s), pulmonary function tests, or water. For 1994 (no sections) we hand searched all abstracts. For the European Society for Pediatrics 1991 to 1993 and 1997 to 1998 the sections on Clinical Trials, Epidemiology, Neonatology, and Pulmonology were handsearched. For years 1992 to 1993 of the Society for Pediatric Research, (volumes 31 and 33 of Pediatric Research) the sections on Developmental Pharmacology, Neonatology‐General, Neonatal Pulmonology and Nephrology were hand searched.
Hand searching of the Neonatal Society [UK] and RCPCH abstracts in April 2007 did not yield any additional eligible study.
3. Cochrane neonatal specialized register: All publications coded under diuretics as intervention in September, 1998 were screened. Searches of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library) Issue 1, 2003, Issue 1, 2007, and Issue 4, 2010 did not yield any additional eligible study.
4. Selection process: Only randomized controlled trials fulfilling the selection criteria described in the previous section were kept. Selection was done separately by two investigators; any disagreement was resolved by discussion.
Data collection and analysis
The standard method for the Cochrane Collaboration which is described in the Cochrane Collaboration Handbook was used.
Selection of studies
All randomized and quasi‐randomized controlled trials fulfilling the selection criteria described in the Methods section were included. Two investigators reviewed the results of the search and separately selected the studies for inclusion. The review authors resolved any disagreement by discussion.
Data extraction and management
Two investigators extracted, assessed and coded separately all data for each study, using a form that had been designed specifically for this review. Graphical data was transformed into numerical data using a millimetric ruler and an electronic spreadsheet. Any standard error of the mean was replaced by the corresponding standard deviation (SD). All calculations within the spreadsheet were initially entered into the spreadsheet by one review author (LPB) and subsequently checked for accuracy by another review author (RP) (Brion 1999a). As much as possible we homogenized units among studies; in some cases this required using a specific formula to estimate the SD of a ratio or a product (Baird 1995; Armitage 1994). Any disagreement was resolved by discussion. For each study, final data were entered into RevMan by one review author (LPB) and then checked by the other review author (RP).
In December 1998, each author was sent an itemized letter requesting additional information about design, patients, methods, or original outcome data (if missing, incomplete or presented in graphical form).
Conversion of values into SI units: To obtain gas pressure in kPa, multiply the value in cm H2O by 0.10 or that in mm Hg by 0.13 To obtain calcium:creatinine ratio in mM:mM, multiply the ratio in mg:mg by 2.84 To obtain calcium in mM/L, multiply the value in mg/dl by 0.25
For studies in which controls did not receive placebo but another intervention, we arbitrarily considered as 'control' 1) intravenous administration of furosemide, or 2) intermittent administration of a single diuretic.
Assessment of risk of bias in included studies
The standard methods of the Cochrane Neonatal Review Group were used (http://neonatal.cochrane.org/en/index.html) . The methodological quality of the studies was evaluated by assessing the risk for four types of bias (selection, performance, attrition and detection). Each study was assessed separately by two review authors; disagreements were resolved by discussion with the other review authors.
For the update in 2011, review authors independently assessed study quality and risk of bias using the following criteria documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
1) sequence generation: was the allocation sequence adequately generated?
2) allocation concealment: was allocation adequately concealed?
3) blinding of participants, personnel and outcome assessors for each main outcome or class of outcomes: was knowledge of the allocated intervention adequately prevented during the study?
4) incomplete outcome data for each main outcome or class of outcomes: were incomplete outcome data adequately addressed?
5) selective outcome reporting: are reports of the study free of suggestion of selective outcome reporting?
6) other sources of bias: was the study apparently free of other problems that could put it at a high risk of bias? We gave particular attention to completeness of follow up of all randomised infants and to the length of follow‐up studies to identify whether any benefits claimed are robust.
When necessary, we requested additional information and clarification of published data from the authors of individual trials. We assessed each trial for risk of bias based on the criteria listed above and marked as:
a) low risk of bias;
b) unclear risk of bias;
c) high risk of bias.
Measures of treatment effect
The standard method of the Cochrane Neonatal Review Group were used.
Specific problems related to high inter‐individual variability of pulmonary function measurements were encountered. Two types of designs have been used: parallel and cross‐over. In trials with parallel design, most authors have compared serial data in the same patients. In trials with a cross‐over design, many authors have only reported mean change from baseline or percent change from baseline.
As much as possible, outcomes in all patients entered in each trial were assessed on an intent‐to‐treat basis. The total number of patients initially entered in the trial is mentioned as the first item in the column entitled 'participants' in the table of included studies. In some studies however, outcomes were available only in some patients; exclusions are described in the column entitled 'methods.' Some authors have combined all measurements (i.e., many successive values for the same patient) throughout the study to perform their statistical analysis. For this review, the total n in each group was recalculated to reflect the number of randomized patients for which data were provided by the authors.
1. Parallel design: Most authors have used repeated measures analysis of variance, paired Student t‐tests, percentage of the initial value or percentage change from the initial value to assess the effect of diuretics on lung mechanics.
2. Cross‐over design: Evaluation of a cross‐over trial is supposed to include not only the assessment of the effect of the type of medication, but also the assessment of the carry‐over effect (treatment‐period interaction) and of the period effect (effect of the order of administration of the two types of medications). Several studies included a washout period to limit any possible carry‐over effect. For many cross‐over trials, the authors did not present the complete analysis, but instead presented pooled (before and after cross‐over) data for each type of treatment (treatment A vs treatment B), either as per cent changes from baseline, or as mean values in the original unit (e.g., ml/cm H2O/kg). For trials presenting separately the data obtained before and those obtained after the cross‐over, we used data at both exposures if there was no evidence for carry‐over and for period effect. The number of patients in each arm was the total number of patients entered into the trial.
Dealing with missing data
In December 1998, each author was sent an itemized letter requesting additional information about design, patients, methods, or original outcome data (if missing, incomplete or presented in graphical form).
Assessment of heterogeneity
We planned to estimate the treatment effects of individual trials and examine heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the two formal statistics described below.
1) The Chi2 test, to assess whether observed variability in effect sizes between studies is greater than would be expected by chance. Since this test has low power when the number of studies included in the meta‐analysis is small, we planned to set the probability at the 10% level of significance.
2) The I2 statistic to ensure that pooling of data is valid. We planned to grade the degree of heterogeneity as: 0% to 30%: might not be important; 31% to 50%: moderate heterogeneity; 51% to 75%: substantial heterogeneity; 76% to 100%: considerable heterogeneity.
Where there is evidence of apparent or statistical heterogeneity, we planned to assess the source of the heterogeneity using sensitivity and subgroup analysis looking for evidence of bias or methodological differences between trials.
Data synthesis
The meta‐analysis was performed using Review Manager software (RevMan 5), supplied by the Cochrane Collaboration.
Dichotomous variables: We used the relative risk (RR) and risk difference (RD) and their respective 95% confidence intervals (CI). Continuous variables: As much as possible we transformed each variable in various studies into the same unit, and used the weighted mean difference (WMD) and the 95% confidence interval (CI) for meta‐analysis. In addition we transformed all continuous variables into treatment effect defined as change from baseline, in order (1) to take into account baseline differences among studies and (2) to combine as many studies as possible. If individual (or mean and SD or standard error) values of change were not provided by the authors, the variance (var) of change was estimated using Follmann's (Follmann 1992) method, described in version 3.0.2 of the Cochrane Collaboration Handbook (page 213): Var(change)=Var(pretest) + Var(posttest) ‐ 2 x SD(pretest) x SD (posttest) x correlation coefficient (pretest, posttest). For situations in which the value of the pretest‐posttest correlation was not known, we assumed a value of 0.4. Sensitivity analysis was conducted by successively using a correlation coefficient of 0.3 and 0.5. For those studies providing mean and SD of baseline values and of the per cent change from baseline, we obtained the mean change by multiplying per cent change by the mean baseline. The variance of change was calculated using the following formula (derived from Baird 1995 and Armitage 1994): SD per cent change x avg baseline SD baseline x avg per cent change (‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐)^2 ‐ (‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐)^2 100 100 where SD per cent change is the SD of the per cent change, avg is mean and ^2 means square. We combined parallel with cross‐over trials using MetaView, as recommended by the Neonatal CRG.
Subgroup analysis and investigation of heterogeneity
The following comparisons based on type of intervention were planned: 1. Comparison of a loop diuretic versus placebo 2. Comparison of two diuretics versus one diuretic only 3. Comparison of different modes of intravenous administration 4. Comparison of two different diuretics
Subgroup analyses:
1. Mean postnatal age: Within each of the above groups, subgroups were determined based on mean postnatal age. The initial plan was to use a cut‐off value of four weeks, based on the usual definition of bronchopulmonary dysplasia. However, using this value would have made it impossible to classify two of the twenty studies included in this group of three related reviews on diuretics in preterm infants with (or developing) chronic lung disease: (1) Singhal 1983, in which patients had a postnatal age ranging from 23 to 101 days, and (2) Robbins 1993, in which patients in the treatment group had a mean age of 31 days whereas those in the control group had a mean age of 24 days. Therefore, a mean postnatal age of three weeks was selected as the cut‐off for all three reviews.
2. Mean gestational age: Subcategories based on gestational age were planned if differences greater than four weeks were observed among studies.
3. Presence of an endotracheal tube: Sub‐categories were used for intubated patients vs. non‐intubated patients. The presence of an endotracheal tube is expected to increase total resistance (due to a contribution of the endotracheal tube itself) and to decrease dead space (due to contribution of the face mask to the dead space in non‐intubated infants). Patients requiring an endotracheal tube are likely to be sicker and thus to have lower compliance and to require more oxygen than the other patients.
Results
Description of studies
A total of 27 studies were considered for this review. Eighteen studies were eliminated because they did not involve random allocation to administration of a loop diuretic. One study (Engelhardt 1986) was eliminated because it combined a randomized trial with non randomized data. Two studies (Segar 1992, Shankaran 1994) were eliminated because they did not include any information on the outcomes selected for this systematic review. Thus, six studies were included in the present review. Details reported by the authors are provided in the table. Almost all studies included in this review included dynamic measurements of pulmonary mechanics with or without an esophageal balloon. Kao and collaborators (Kao 1983) measured resistance by plethysmography. Main categories (intervention) are shown as first entry in the column labeled 'Interventions.' No subcategories were used based on gestational age, because the maximum range of gestational age within each intervention group was 4 weeks. Main categories (based on intervention) and subcategories (based on postnatal age and mechanical ventilation) are described below: 1. Intravenous or enteral administration of a loop diuretic versus placebo in controls: Five studies were in this group, including two with parallel design (Najak 83, McCann 85) and three with cross‐over design (Kao 83, Rush 90, Singhal 83). Postnatal age ranged between 7 and 101 days; therefore, we classified studies into 2 subcategories based on average postnatal age at study entry: < 3 wk (Najak) vs. > 3 wk (Kao, McCann, Rush, Singhal). Average gestational age ranged between 27 and 30 weeks, so that no subcategories were created for gestational age.
1.1. Postnatal age < 3 weeks Najak 1983: parallel design. This study included both intubated and non‐intubated patients. Patients were randomized to receive either a single daily dose of 1 mg/kg of furosemide for four days, or placebo. No washout period was documented. Individual change scores were provided for alveolar‐arterial O2 gradient. 1.2. Postnatal age > 3 weeks 1.2.1. Non‐intubated patients: Kao 1983: cross‐over design with pooled data. Patients were randomized to receive either a single dose of 1 mg/kg of furosemide followed by a 48‐hour washout period and then placebo, or vice‐versa. Prolonged washout period before the study is also documented. Change scores were estimated from the % change from baseline using Baird and Armitage's method. For tidal volume, the change score (ml/cm H2O) needed then to be converted into ml/cm H2O/kg using Baird and Armitage's method. Rush 1990: cross‐over design with pooled data. Patients were randomized to receive, enterally, either alternate‐day doses of 4 mg/kg of furosemide for eight days followed by a 48‐hour washout period and then placebo for eight days, or vice‐versa. Prolonged washout period before the study and at cross‐over is documented. Change scores for pulmonary function were estimated using Follmann's formula, while those for % inspired O2 and weight gain were provided in the manuscript. No baseline data were provided for calciuria. 1.2.2. Non‐intubated and intubated patients: McCann 1985: parallel design. Patients were randomized to receive either 1 mg/kg of furosemide every 12 hours for seven days, or placebo. Prolonged washout period before the study and at cross‐over is documented. Change scores were estimated using Follmann's formula. 1.2.3. No information provided about respiratory support: Singhal 1983: cross‐over design with pooled data. This study was available as abstract only. Patients were randomized to receive either 1.5 mg/kg of furosemide every 12 hours for three doses followed by placebo, or vice‐versa. No washout period was documented. Change scores for pulmonary function were estimated using Follmann's formula. 2. Administration of furosemide by continuous intravenous infusion versus intermittent intravenous administration in controls: Reiter 1998: parallel design. Eighteen of thirty patients required mechanical ventilation at study entry. Patients were randomly allocated to receive a single dose of furosemide, either (1) by infusion, which included a small bolus of 0.1 mg/kg over two minutes followed by a six‐hour infusion of 0.9 mg/kg, or (2) by intravenous bolus of 1 mg/kg over two minutes followed by a six‐hour infusion of placebo. Prolonged washout period before the study is documented. Change scores were provided in the manuscript.
Risk of bias in included studies
Specific assessments of all four types of bias are described in the table of included studies.
1. Intravenous or enteral administration of a loop diuretic, versus placebo in controls: Of the five studies included in this group, none appeared to be bias‐free.
In Kao's study (Kao 1983) blinding was not documented; this could have yielded an increase in the effect of furosemide.
In McCann's study (McCann 1985) blinding was well documented but attrition bias is likely to have affected the data. Of 24 infants entered into the study, seven were removed from the analysis: in the control group, two infants had clinical deterioration. In the furosemide group, one had severe hypochloremia, two had sepsis, one had a surgical shunt for hydrocephalus, and one had CLD following meconium aspiration syndrome. Removing control patients from analysis because of clinical deterioration could have biased the data towards decreasing the effect of furosemide, whereas removing one treated patient from the analysis because of hypochloremia could have biased the results toward decreasing the incidence of electrolyte abnormalities in treated patients.
In Najak's study (Najak 1983) blinding was not documented; this could have yielded an increase in the effect of furosemide. Two of twelve patients randomized to furosemide were subsequently removed from the study because they did not have typical RDS; it is not clear which effect this could have had on the results.
In Rush's study (Rush 1990) blinding was well documented. Of 16 eligible infants, 13 were enrolled (consent signed); two patients were later excluded because of documented infection, as planned in the authors' protocol.
In Singhal's study (Singhal 1983) blinding was well documented.
2. Administration of furosemide by continuous intravenous infusion versus intermittent intravenous administration in controls: Only one study (Reiter 1998) is in this group. Blinding was well documented. All patients were followed; nevertheless, FiO2 is only provided for patients requiring mechanical ventilation.
Effects of interventions
1. Limitations of the scope of the studies available for this review: Most studies focused on pathophysiological parameters (pulmonary mechanics) and failed to assess the primary outcomes defined in this review (e.g., need for and duration for mechanical ventilation or oxygen supplementation, BPD, mortality) or the potential complications of diuretic therapy. No studies reported on need for continuous positive airway pressure, chronic lung disease at 36 weeks postmenstrual age, mortality, length of stay, number of rehospitalizations during the first year of life, alkalosis, hyponatremia, bone demineralization, nephrocalcinosis, nephrolithiasis, cholelithiasis, or expiratory flow. Furthermore, for most outcomes, only one or two studies provided data that could be merged into a meta‐analysis, so that only a small number of patients was included in each analysis. Therefore, it is possible that real differences due to furosemide administration could have been missed. For each analysis we report the studies and the number of patients in which the particular outcome is reported.
2. Value of the pretest‐posttest correlation coefficient: Limited data are available in the literature about the value of this correlation coefficient for pulmonary mechanics in preterm infants. Data from Kugelman (Kugelman 1997) yield a strong pretest‐posttest correlation for dynamic compliance (0.8 for a one or a two hour period). This value was used for calculating the change score in Singhal's study.
3. Effects of furosemide in preterm infants with or developing CLD 3.1. Intravenous or enteral administration of a loop diuretic, versus placebo in controls: 3.1.1. Oxygenation, oxygen requirement and BPD: Among patients entered at a postnatal age < 3 weeks, daily furosemide administration improved the change in alveolar‐arteriolar gradient within two hours after therapy inconsistently (n = 20) (Najak 1983). The alveolar‐arterial oxygen gradient improved more within two hours after the second dose of furosemide than in controls (WMD ‐16.0 mm Hg, 95%CI ‐2.8 to ‐29.2) but not after the first, third or fourth dose of furosemide. Among patients with a postnatal age > 3 weeks, a two‐day furosemide course did not affect oxygen requirement (n = 17) (McCann 1985). In contrast, a seven to eight day course of furosemide significantly improved the percent inspired O2 (WMD ‐2.7%, 95%CI ‐1.0 to ‐4.4, n = 39) (McCann 1985; Rush 1990). No data were available on total duration of O2 administration, incidence of BPD or chronic lung disease at 36 weeks postmenstrual age.
3.1.2. Failure to wean mechanical ventilation: Furosemide tended to decrease the risk of failure to extubate within a week (RR 0.53, 95%CI 0.17 to 1.68; RD ‐0.35, 95%CI ‐0.87 to +0.17, n = 13) (McCann 1985) and the risk of failure to either wean off the ventilator or to decrease the ventilatory settings (RR 0.12, 95%CI 0.01 to 1.69; RD ‐0.75, 95%CI ‐1.18 to ‐0.32, n = 13). Furosemide tended to improve the change in peak inspiratory pressure (WMD ‐11 cm H2O, 95%CI ‐29 to +7.4, n = 8) and the change in rate of mechanical ventilation (WMD ‐16 cycles/min, 95%CI ‐41 to +9, n = 8) (McCann 1995).
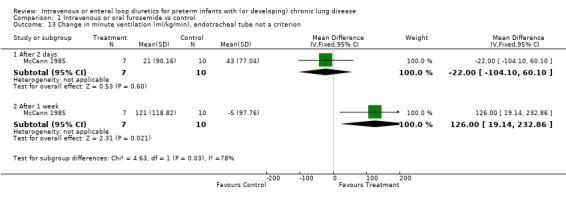
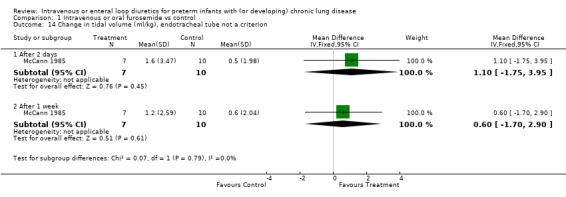
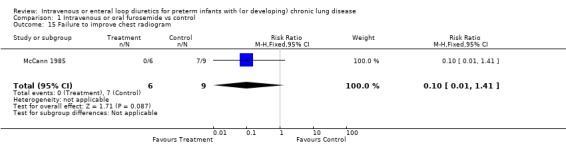
3.1.3. Pulmonary mechanics: The effect of a single dose of furosemide was analyzed in two studies: Kao (1983), in which only non intubated patients were studied, and Singhal (1983), in which tracheal intubation was not an entry criterion. Furosemide improved compliance more than placebo after one hour (WMD 0.5 ml/cm H2O/kg, 95%CI 0.3 to 0.7, n = 20) (Kao 1983). This effect was transient: there was no significant difference after two hours (Kao 1983; Singhal 1983) (WMD 0.09, 95%CI ‐0.17 ml/cm H2O/kg to +0.35), obtained using a pretest‐posttest correlation of 0.8 for calculating the change score in Singhal's study. Sensitivity analysis using a pretest‐posttest correlation of 0.4 yielded a WMD of 0.05 ml/cm H2O/kg, 95%CI ‐0.24 to 0.33. Furosemide significantly improved resistance after one hour (WMD ‐25 cm H2O/L/sec, 95%CI ‐5 to ‐45, n = 20) (Kao 1983); the difference at two hours did not reach significance anymore (WMD ‐10.27 cm H2O/L/sec, 95%CI ‐24.93 to +3.49, n = 20) (Kao 1983). A one to two day course of furosemide tended to improve pulmonary compliance (WMD 0.17 ml/cm H2O/kg, 95%CI ‐0.09 to +0.44, n = 59) (Rush 1990, McCann 1985; Singhal 1983), tidal volume (n = 17) (McCann 1985) and resistance (WMD ‐49 cm/L/sec, 95%CI ‐128 to +33, n = 39) (Mc Cann 1985, Rush 1990) but none of these comparisons reached significance. Furosemide did not affect minute ventilation or alveolar ventilation (n = 17) (McCann 1985). A 4‐6 day course tended to improve compliance and resistance (n = 22) (Rush 1990) but this did not reach statistical significance. Patients treated for seven to eight days with furosemide had a significant improvement in pulmonary compliance (WMD 0.4 ml/cm H2O/kg, 95%CI 0.1 to 0.7, n = 39) (Mc Cann 1985, Rush 1990) and minute ventilation (WMD 126 ml/kg/min, 95%CI 19 to 233, n = 17) (McCann 1985) than controls. Furosemide tended to improve alveolar ventilation (WMD 67 ml/kg/min, 95%CI ‐8 to +143, n = 17) (McCann 1985) and resistance (WMD ‐61.5 cm/L/sec, 95%CI ‐134.2 to + 11.2, n = 39) (McCann 1985; Rush 1990) but did not affect tidal volume (WMD 0.6 ml/kg, 95%CI ‐1.7 to +2.9, n = 17) (McCann 1985). Furosemide tended to decrease the failure to improve the chest radiogram (RR 0.1, 95%CI 0.01 to 1.4, n = 17) (McCann 1985).
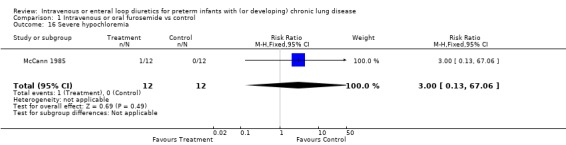
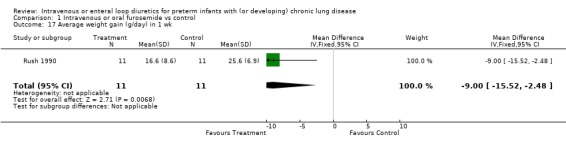
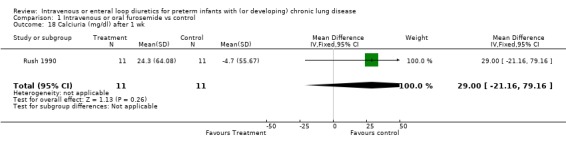
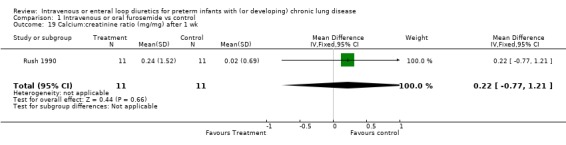
3.1.4. Fluids, electrolytes, minerals, kidney: Severe hypochloremia was observed in 1/12 treated infants and none of 12 controls in one study (McCann 1985). There is no information on the incidence of other electrolyte abnormalities, dehydration, nephrocalcinosis, or nephrolithiasis. A one‐week furosemide administration reduced weight gain (‐9 g, 95%CI ‐15 to ‐2, n = 11) and tended to increase the amount of calciuria (+29 mg/dl, 95%CI ‐21 to +79, n = 11) but not the calcium/creatinine ratio (Rush 1990).
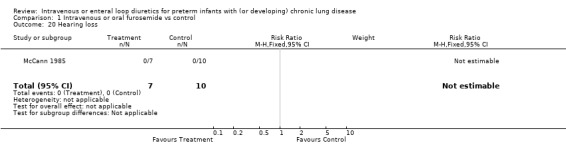
3.1.5. Long term outcome: No hearing loss was detected in seven furosemide treated infants and 10 controls (McCann 1985). None of the other long term outcomes, including the primary outcomes defined for this review, were assessed in these studies.
3.2. Administration of furosemide by continuous intravenous infusion versus bolus intravenous administration in controls: The single available study (Reiter 1998) showed that a slow infusion of furosemide did not improve mean airway pressure compared with a rapid injection of furosemide (n = 18). No patient in the bolus group had any change in mean airway pressure (both mean and SD were 0 at each time point). Among patients in the slow infusion group, furosemide administration yielded no (clinically or statistically) significant changes in mean airway pressure (‐0.24±0.81 cm H2O [‐0.18±0.60 mm Hg] at 1‐4 hours and ‐0.62±1.12 cm H2O [‐0.46±0.83 mm Hg] at 4‐12 hours). RevMan is unable to calculate a WMD for these data because SD in control (bolus) group is zero. The slow infusion tended to improve the need for supplemental oxygen (n = 18), but this did not reach statistical significance.
Discussion
1. Limitations 1.1. Limitations of the studies included in this review: Some studies had possible or likely bias. In the study of McCann, two patients were eliminated from the study because of clinical deterioration in the control group (possibly yielding an underestimation of the effect of furosemide) and one patient in the treatment group was eliminated because of severe hypochloremia (possibly yielding an underestimation of the complications of furosemide).
1.2. Limitations of this review: Outcomes analyzed: Most studies focused on pathophysiological parameters, e.g., pulmonary mechanics, and did not assess the most important outcomes defined in this review or the potential complications of diuretic therapy.
Methods used for the analysis: The methods recommended by the Cochrane Neonatal Review Group were used. For almost all studies, we did not have access to the original data. Therefore, multiple transformations using formulae established by or derived from Follmann, Baird, and Armitage were used. Calculations using Baird and Armitage overestimate the SD of the final variable if the numerator is related to the denominator, because they assume lack of correlation between them. For calculations using Follman's formula, we assumed a pretest‐posttest correlation of 0.4 and did a sensitivity analysis using a correlation coefficient of 0.3 ‐ 0.5. Discussion relevant specifically to pulmonary mechanics is given in section 1.3.
Heterogeneity: Within each subgroup, chi‐square analysis was used to test for statistical evidence of heterogeneity among studies. When chi‐square analysis was significant, differences (in patient selection, baseline values, bias, design and methods) among studies that could possibly explain the heterogeneity were analyzed. Some of these post‐hoc comparisons were used to suggest additional randomized trials. Because of the long half‐life of loop diuretics in immature infants, a prolonged washout period is required to eliminate all diuretic effect before initiating a study or between exposures in a cross‐over study. However, a prolonged washout period may not be possible or ethically acceptable for patients considered clinically to require diuretics. Several studies had a short or no washout period, thereby possibly decreasing the apparent effect of diuretic administration on short and long term outcome. All cross‐over trials failed to provide information that would rule out a carry‐over effect (possibly yielding an underestimation of the real effect of diuretic administration) and a period effect.
The studies available for this review spanned more than a decade (1982‐1998). It is possible that the incidence of lung edema in premature infants may have decreased as a result of changes in therapy introduced in the last decade. In a blinded randomized trial, Gortner has shown that surfactant administration resulted in halving of the utilization of diuretics during postnatal weeks two to four (Gortner 1991). Furthermore, administration of dexamethasone from day eight through day 40 was found to increase urine output in premature infants with BPD (Sonntag 1996).
Sample size: Because of small sample size in most of the subgroups, any real effects of furosemide may have remained undetected.
1.3. Variability of measurement of pulmonary mechanics and change scores: Some of the differences among studies may result from methodologic heterogeneity (Gerhardt 1989). Static measurements are expected to yield lower values for compliance and higher values for resistance than dynamic measurements, because static measurements include the chest wall. These differences may be reduced in very low birth weight infants because of high chest wall compliance and at high respiratory rates because of underestimation of dynamic compliance (Gerhardt 1989). Measurement of airway compliance and resistance requires measurement of transpleural pressure, which assumes that esophageal pressure equals intrapleural pressure. This assumption may not be correct if the balloon placement is not adequate, if there is gastroesophageal reflux or if there is chest wall distortion (LeSouef 1983). In sick low birthweight infants, values of esophageal pressure and dynamic compliance are poorly reproducible and correlate poorly with total respiratory system compliance (Thomson 1983). Computerized two‐factor least square analysis (Abbasi 1990) allows the selection of adequate breaths by visual inspection of the tracing, a process which yields the mean of several breaths (Goyal 1995) but might yield outcome bias. Because of limitations of esophageal pressure measurement, several authors have measured dynamic measurements of compliance and resistance of the total respiratory system without an esophageal balloon (Kugelman 97). Measurements of static compliance and resistance by the passive expiratory occlusion technique do not have the limitations mentioned for dynamic determinations (LeSouef 1984). Despite the above considerations, one study suggests that static and dynamic methods of pulmonary function tests yield similar results when assessing serial changes in pulmonary mechanics (Kugelman 1996). Furthermore, using change scores (rather than absolute values at the end of the study period) may minimize heterogeneity between studies using dynamic measurements of pulmonary mechanics and studies using static (passive) measurements of pulmonary mechanics. Data obtained (Kugelman 1996) in patients with a mean gestational age of 27 weeks, birth weight of 860 g and postnatal age of 13 days was analyzed. Baseline values of compliance differed by 50 per cent: dynamic compliance, 0.32 ± 0.07 ml/cm H2O, vs static compliance 0.54 ± 0.17 ml/cm H2O (p < 0.01, n = 12, Student t‐test). Similarly, dynamic compliance after dexamethasone therapy (0.61 ± 0.14 ml/cm H2O) was significantly different from static compliance (0.88 ± 0.24 ml/cm H2O, p < 0.01), yielding a significant WMD of ‐0.27 ml/cm H2O, 95%CI ‐0.43 to ‐0.11, z = 3.37). Dexamethasone treatment resulted in an increase in dynamic compliance of 0.34 ± 0.22 ml/cm H2O, and an increase in static compliance (of the total respiratory system) of 0.29 ± 0.12 ml/cm H2O. This yielded a non significant WMD of ‐0.05 ml/cm H2O (95%CI ‐0.19 to +0.09), assuming a pretest‐posttest correlation coefficient of 0.3 (selected to yield maximum sensitivity). Limited data in the literature (Kugelman 1997) suggest that the value of 0.4 for the pretest‐posttest correlation is a conservative estimate in comparison with observed values correlation for dynamic compliance and even dynamic compliance. Other values for this correlation coefficient were only used if they were available for preterm infants for an identical test (e.g., compliance) performed at similar time intervals and using the same method. A high mean correlation coefficient (Cronbach alpha) was found for serial dynamic measurements of tidal volume (r = 0.72), minute ventilation (r = 0.84), compliance (r = 0.92) and resistance (r = 0.80) obtained in full‐term infants over a period of 66 hours (Goyal 1995). We are unaware of such values in preterm infants. Therefore, the SD of change scores for lung compliance may have been overestimated. Consequently, relevant results are likely to be conservative, i.e., the confidence intervals are wider than they would have been if primary data on compliance and resistance had been available. Yet, using a correlation coefficient of 0.8 did not affect the results of the analysis of short‐term changes in compliance (see results section).
2. Summary of the results Only limited data are available on the effect of furosemide on the duration of ventilatory support and oxygen supplementation, or long‐term outcome. In preterm infants < 3 wk of age developing CLD, a single daily dose of furosemide improves oxygenation inconsistently. In patients > 3 wk of age with CLD, pulmonary mechanics transiently improve in non‐intubated patients after a single furosemide dose. Pulmonary mechanics and oxygenation improve in all patients after a week of furosemide.
Authors' conclusions
Implications for practice.
Only limited data are available about the primary outcomes defined for this systematic review. Most of the outcome variables have been reported only in one or two studies and in a limited number of patients. Therefore, some effects of furosemide may have been undetected. In preterm infants < 3 weeks of age developing CLD, intravenous furosemide administration has either inconsistent effects or no detectable effect. In infants > 3 weeks of age with CLD, a single intravenous dose of 1 mg/kg of furosemide transiently improves pulmonary mechanics. Chronic enteral or intravenous furosemide administration improves both oxygenation and pulmonary mechanics. There is little evidence to support any benefit of furosemide administration on need for ventilatory support, length of hospital stay, survival or long‐term outcome. In view of the lack of data from randomized trials concerning effects on important clinical outcomes, routine or sustained use of systemic loop diuretics in infants with (or developing) CLD cannot be recommended based on current evidence.
Implications for research.
Further studies need to assess the effects of diuretics on survival, duration of O2‐ and ventilator‐dependency, length of stay and long‐term outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 4 January 2011 | New search has been performed | This review updates the review "Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease" published in The Cochrane Database of Systematic Reviews (Brion 2008).
The review was updated in December 2010. This included an updated search of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2010), MEDLINE and EMBASE. In addition, CINAHL, clinicaltrials.gov and controlled‐trials.com were searched. This did not yield any additional eligible studies. No change to conclusions. |
| 4 January 2011 | New citation required but conclusions have not changed | New authorship |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 5 March 2008 | Amended | Converted to new review format. |
| 5 April 2007 | New search has been performed | This review updates "Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease" published in Issue 4, 2003 of The Cochrane Library (Brion 2003). The review was updated in March/April 2007. This included an updated search of MEDLINE in March 2007, EMBASE in April 2007 and the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 1, 2007). This did not yield any additional eligible studies. Hand searching of the Neonatal Society [UK] and RCPCH abstracts in April 2007 did not yield any additional eligible study. |
| 20 August 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
This review is part of a group of three closely related reviews on diuretics in preterm infants with (or developing) chronic lung disease (Brion 1999 a,b,c). Angel Rios and Jean‐Yves Pauchard participated in the editing of the protocol used in these three reviews. The reader is also referred to a systematic review on furosemide in indomethacin‐treated patients (Brion 1998) and a systematic review on furosemide in preterm infants with respiratory distress syndrome (Brion 1999d).
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Intravenous or oral furosemide vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in alveolar‐arterial pO2 gradient (mm Hg) 2 hours after last dose, PNA < 3 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 After 1 dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐12.80 [‐32.13, 6.53] |
| 1.2 After 1 day of treatment | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐16.0 [‐29.18, ‐2.82] |
| 1.3 After 2 days of treatment | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐15.90, 1.90] |
| 1.4 After 3 days of treatment | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.21 [‐11.99, 18.41] |
| 2 Change in percent inspiratory oxygen after 1 wk, non‐intubated patients | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.17, ‐0.83] |
| 3 Change in percent inspiratory oxygen, all patients | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 After 2 days | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐11.70, 11.70] |
| 3.2 After 1 week | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐4.35, ‐1.05] |
| 4 Failure to extubate after 1 week | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.17, 1.68] |
| 5 Failure to wean ventilatory settings or to extubate within 1 wk | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 1.69] |
| 6 Ventilator peak pressure (cm H2O) after 1 week | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐25.71, 13.71] |
| 7 Ventilator frequency after 1 week | 1 | 8 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐37.70, 9.70] |
| 8 Change in compliance (ml/cm H2O/kg), non‐intubated patients | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 1 hour after a single dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.32, 0.66] |
| 8.2 2 hours after a single dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.28, 0.32] |
| 8.3 After 1‐2 days of treatment | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.24, 0.68] |
| 8.4 After 4 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.26, 0.62] |
| 8.5 After 6 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.04, 1.02] |
| 8.6 After 7‐8 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [0.12, 1.18] |
| 9 Change in compliance (ml/cm H2O/kg), all patients | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 1 hour after a single dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [0.32, 0.66] |
| 9.2 2 hours after a single dose | 2 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.17, 0.35] |
| 9.3 After 1‐2 days of treatment | 3 | 59 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.09, 0.44] |
| 9.4 After 4 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐0.26, 0.62] |
| 9.5 After 6 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.04, 1.02] |
| 9.6 After 7‐8 days | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [0.14, 0.69] |
| 10 Change in resistance (cm/L/sec), non‐intubated patients | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 1 hour after a single dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐25.14 [‐45.37, ‐4.91] |
| 10.2 2 hours after a single dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐10.72 [‐24.92, 3.48] |
| 10.3 After 1‐2 days of treatment | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐31.58 [‐168.68, 105.52] |
| 10.4 After 4 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐24.47 [‐133.16, 84.22] |
| 10.5 After 6 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐33.81 [‐156.42, 88.80] |
| 10.6 After 7‐8 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐81.85 [‐196.98, 33.28] |
| 11 Change in resistance (cm/L/sec), all patients | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 1 hour after a single dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐25.14 [‐45.37, ‐4.91] |
| 11.2 2 hours after a single dose | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐10.72 [‐24.92, 3.48] |
| 11.3 After 1‐2 days of treatment | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐47.56 [‐128.17, 33.05] |
| 11.4 After 4 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐24.47 [‐133.16, 84.22] |
| 11.5 After 6 days | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐33.81 [‐156.42, 88.80] |
| 11.6 After 7‐8 days | 2 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐61.49 [‐134.15, 11.18] |
| 12 Change in alveolar ventilation (ml/kg/min), endotracheal tube not a criterion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 After 2 days | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐60.69, 60.69] |
| 12.2 After 1 week | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 67.0 [‐8.08, 142.08] |
| 13 Change in minute ventilation (ml/kg/min), endotracheal tube not a criterion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 After 2 days | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | ‐22.0 [‐104.10, 60.10] |
| 13.2 After 1 week | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 126.00 [19.14, 232.86] |
| 14 Change in tidal volume (ml/kg), endotracheal tube not a criterion | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 After 2 days | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐1.75, 3.95] |
| 14.2 After 1 week | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.6 [‐1.70, 2.90] |
| 15 Failure to improve chest radiogram | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.41] |
| 16 Severe hypochloremia | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 67.06] |
| 17 Average weight gain (g/day) in 1 wk | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐9.0 [‐15.52, ‐2.48] |
| 18 Calciuria (mg/dl) after 1 wk | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 29.0 [‐21.16, 79.16] |
| 19 Calcium:creatinine ratio (mg/mg) after 1 wk | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐0.77, 1.21] |
| 20 Hearing loss | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.
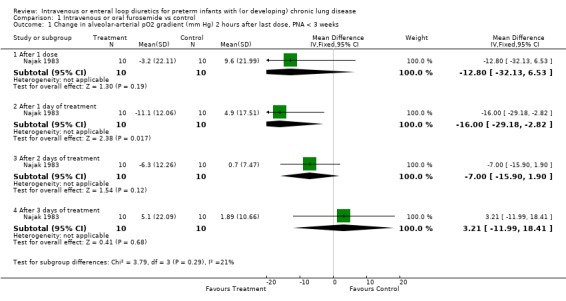
Comparison 1 Intravenous or oral furosemide vs control, Outcome 1 Change in alveolar‐arterial pO2 gradient (mm Hg) 2 hours after last dose, PNA < 3 weeks.
1.2. Analysis.
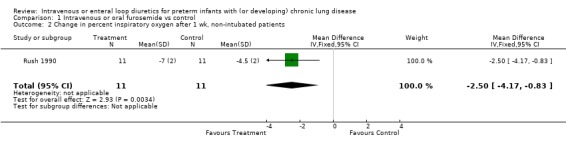
Comparison 1 Intravenous or oral furosemide vs control, Outcome 2 Change in percent inspiratory oxygen after 1 wk, non‐intubated patients.
1.3. Analysis.
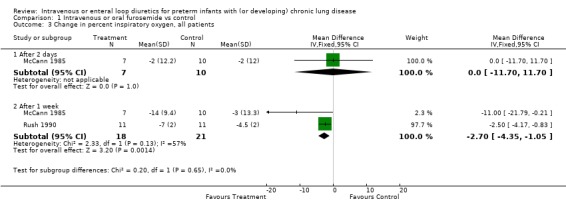
Comparison 1 Intravenous or oral furosemide vs control, Outcome 3 Change in percent inspiratory oxygen, all patients.
1.4. Analysis.
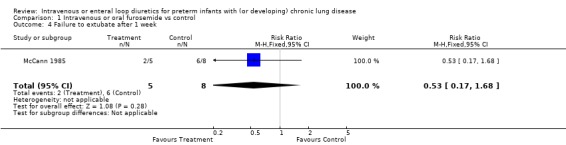
Comparison 1 Intravenous or oral furosemide vs control, Outcome 4 Failure to extubate after 1 week.
1.5. Analysis.
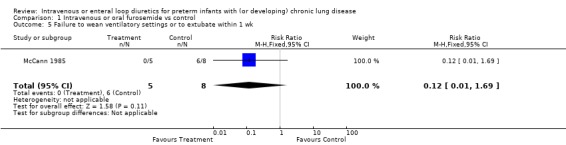
Comparison 1 Intravenous or oral furosemide vs control, Outcome 5 Failure to wean ventilatory settings or to extubate within 1 wk.
1.6. Analysis.
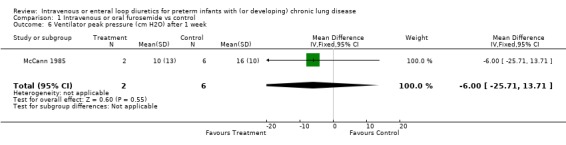
Comparison 1 Intravenous or oral furosemide vs control, Outcome 6 Ventilator peak pressure (cm H2O) after 1 week.
1.7. Analysis.
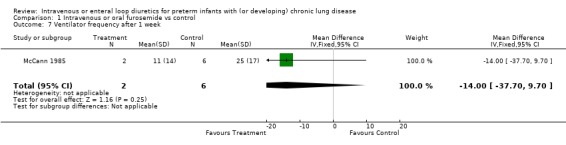
Comparison 1 Intravenous or oral furosemide vs control, Outcome 7 Ventilator frequency after 1 week.
1.8. Analysis.
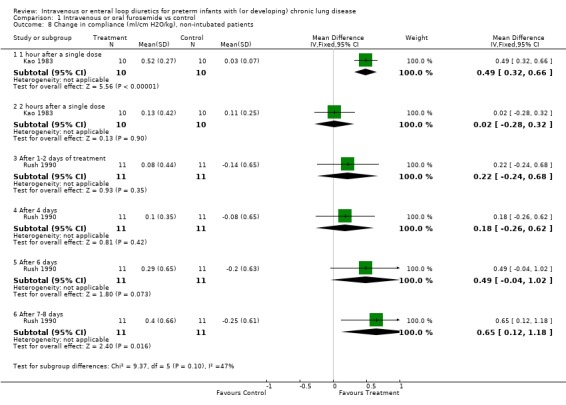
Comparison 1 Intravenous or oral furosemide vs control, Outcome 8 Change in compliance (ml/cm H2O/kg), non‐intubated patients.
1.9. Analysis.
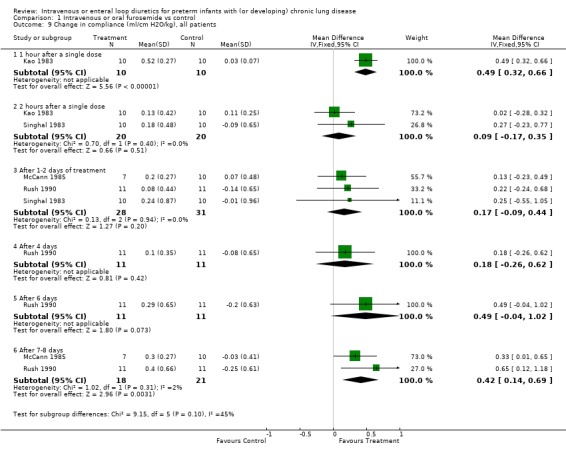
Comparison 1 Intravenous or oral furosemide vs control, Outcome 9 Change in compliance (ml/cm H2O/kg), all patients.
1.10. Analysis.
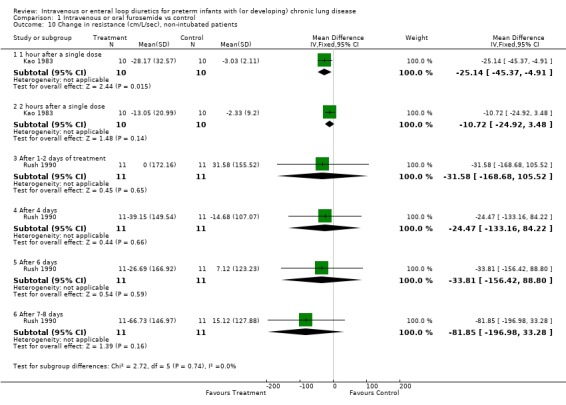
Comparison 1 Intravenous or oral furosemide vs control, Outcome 10 Change in resistance (cm/L/sec), non‐intubated patients.
1.11. Analysis.
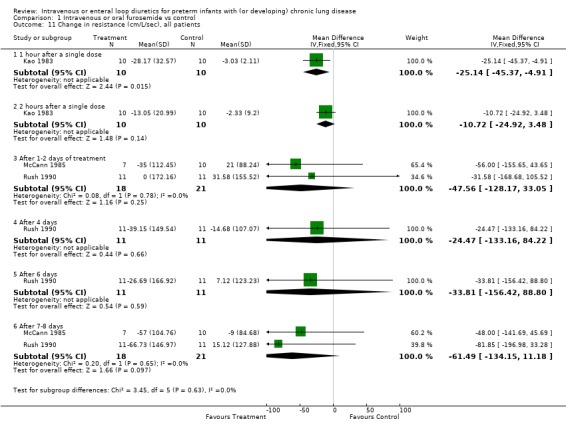
Comparison 1 Intravenous or oral furosemide vs control, Outcome 11 Change in resistance (cm/L/sec), all patients.
1.12. Analysis.
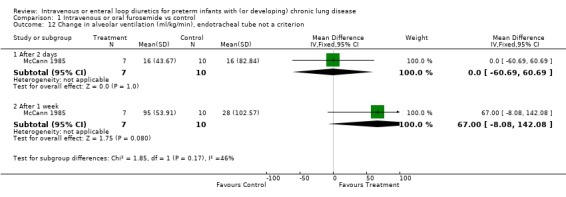
Comparison 1 Intravenous or oral furosemide vs control, Outcome 12 Change in alveolar ventilation (ml/kg/min), endotracheal tube not a criterion.
1.13. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 13 Change in minute ventilation (ml/kg/min), endotracheal tube not a criterion.
1.14. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 14 Change in tidal volume (ml/kg), endotracheal tube not a criterion.
1.15. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 15 Failure to improve chest radiogram.
1.16. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 16 Severe hypochloremia.
1.17. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 17 Average weight gain (g/day) in 1 wk.
1.18. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 18 Calciuria (mg/dl) after 1 wk.
1.19. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 19 Calcium:creatinine ratio (mg/mg) after 1 wk.
1.20. Analysis.
Comparison 1 Intravenous or oral furosemide vs control, Outcome 20 Hearing loss.
Comparison 2. Furosemide infusion vs furosemide by intravenous bolus.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in % inspiratory O2 | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 After 1‐4 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐4.99 [‐15.51, 5.53] |
| 1.2 After 4‐12 hours | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐10.61 [‐24.86, 3.64] |
2.1. Analysis.
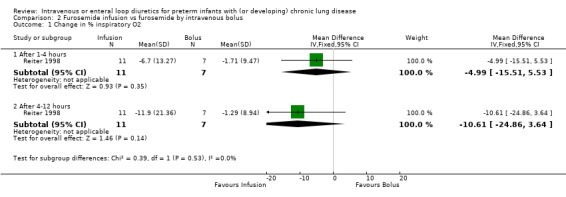
Comparison 2 Furosemide infusion vs furosemide by intravenous bolus, Outcome 1 Change in % inspiratory O2.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kao 1983.
| Methods | Blinding of randomization: not mentioned ‐ 'random sequence' Blinding of intervention: not mentioned Complete follow up: yes Blinding of outcome: not mentioned Randomized controlled trial, cross‐over design with pooled data Washout period: 48 hours before study, 48 hours at cross‐over | |
| Participants | Patients entered into the study: n=10 No mechanical ventilation Entry criteria: gestational age < 34 weeks, history of RDS (typical chest radiograph, need for >40% O2, need for CPAP or mechanical ventilation), continued requirement for mechanical ventilation and 30% FiO2 at 30 days of age, radiographic evidence of stage 3 or 4 of BPD by one month of age. Exclusion criteria: patent ductus arteriosus, mechanical ventilation. Ten patients were included into the study. Mean birth weight was 1.37±0.63 kg, gestational age 30.0±3.2 weeks, average postnatal age 11 weeks, postconceptional age 41.0±3.2 weeks and weight 2.28±0.38 kg. There was no difference between furosemide and placebo periods in baseline values of lung volume and resistance. Baseline values for compliance tended to be 27% lower during the furosemide period than during the placebo period, and those for conductance were 26 % lower (p<0.05 by unpaired Student t‐test). | |
| Interventions | Furosemide vs placebo Patients were randomly allocated, either to receive 1 dose of furosemide 1 mg/kg iv followed after 48 hours by placebo (10% glucose in water), or vice versa. Fluid intake was similar on furosemide and on placebo (175.4±38.0 vs 175.5±30.4 ml/kg/day). | |
| Outcomes | No changes in urine output or pulmonary function occurred versus baseline after placebo administration. Furosemide resulted in a significantly higher increase than placebo for urine output during the first period (0‐1 hr, +226±85% vs ‐9±49%, respectively, p<0.001) and the second period (1‐3 hr, +58±74% vs 0±22%, p<0.05). Furosemide administration resulted in a significantly better improvement than placebo for the following variables: compliance at 1 hour (+54±41% vs +2±5% with placebo, p<0.01), airway conductance both at 1/2 hour (+54±52% vs ‐18±6% with placebo, p<0.001) and at 1 hour (+84±70% vs +3±8% with placebo, p<0.01) and resistance at 1/2 hour (‐30±37% vs +2±3%, p<0.05) and at 1 hour (‐35±41% vs ‐5±3%, p<0.05. The authors state there was no significant change in thoracic gas volume; data are not provided. | |
| Notes | Measurements of pulmonary function and urine were obtained at 1/2, 1,2,4,6 and 24 hours after medication administration. Patients were lightly sedated with chloral hydrate (5‐10 mg/kg). The authors measured airway resistance by plethysmography and dynamic compliance using an esophageal balloon. Urine output was measured over the following periods: 0‐1, 1‐3,3‐6,6‐16 and 16‐24 hours. Values are presented as % of baseline. Some values of pulmonary function measurements calculated from the figures were overridden to match those in the text. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized controlled trial, cross‐over design with pooled data Blinding of randomization: not mentioned ‐ 'random sequence' |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomization: not mentioned ‐ 'random sequence' |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: not mentioned Blinding of outcome: not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes |
McCann 1985.
| Methods | Blinding of randomization: yes. Medications were prepared in the pharmacy. Blinding of intervention: yes Complete follow up: no. Of 24 infants entered into the study, 7 were removed from the analysis. In the control group, 2 infants had clinical deterioration. In the treatment group, 1 had severe hypochloremia, 2 had sepsis, 1 had a surgical shunt for hydrocephalus, and 1 had CLD following meconium aspiration syndrome. Blinding of outcome: yes Randomized controlled trial, parallel design Washout period: 72 hours | |
| Participants | Patients entered into the study: n=24 (12 in each group) The need for mechanical ventilation was not used as a criterion. Entry criteria: (1) CLD defined as >30% O2 and chest radiograph findings of coarse interstitial markings with areas of hyperaeration (2) following mechanical ventilation for respiratory distress. Exclusion criteria: renal failure, sepsis, necrotizing enterocolitis, hyperbilirubinemia, current administration of aminoglycoside, administration of diuretics within 72 hours. A final number of 17 patients were available for this analysis, including 7 in the furosemide group and 10 in the placebo group. The average birth weight was 947 g (range 620‐1510) in the treatment group and 859 g (650‐1030) in the placebo group. The average gestational age was 27 weeks (24‐32) and 27 weeks (range 24‐29), respectively. Postnatal age was 49 days (36‐72) and 38 days (23‐82), respectively, and postconceptional age 34.0 and 32.4 weeks, respectively. The two groups had similar severity of respiratory distress, history of PDA, history of pulmonary interstitial emphysema, serum electrolytes, alveolar ventilation, minute ventilation, venous admixture, blood pH and pCO2, and urine output. Patients in the furosemide group tended to have a 90% higher dead space than those in the control group and lesser trends for other pulmonary function measurements. Five of 7 patients in the treatment group and 8 of 10 in the control group were on mechanical ventilation at the time of recruitment. Mean FiO2 was 0.45±0.10 in the treatment group and 0.45±0.11 in the control group. | |
| Interventions | Furosemide vs placebo. Patients were randomly allocated to receive, either furosemide (1 mg/kg iv or 2 mg/kg orally q 12 hours) and KCl for 7 days, or similar placebo solutions. The dose of furosemide was doubled if no diuretic response (50% increase in urine output over 12 hours) was observed after 48 hours. The KCl dose was adjusted to keep serum K > 3.5 mEq/L and serum Cl > 95 mEq/L. Fluid intake was limited to 100‐150 ml/kg/day. Six of seven infants received furosemide orally. | |
| Outcomes | Main outcome: risk and benefits of furosemide including physical findings, fluid and Na balance, serum electrolytes, ventilator and O2 requirements, blood gases, and pulmonary function. One patient in the furosemide group had no detectable diuresis after the first dose and the dose was doubled. In the furosemide group, 3 infants were extubated and 2 infants needed less ventilatory support after therapy; in the control group, 2 were extubated and 6 were not weaned. Six patients in the furosemide group had clinical improvement (chest examination) compared with 2 in the control group. Similarly, 6 patients in the furosemide group had an improvement in lung aeration on the chest radiograph compared with 2 in the control group. The authors analyzed the results based on a total number of 7 patients in the furosemide group and 10 in the control group and found significant differences between groups. However, analysis of relative risk showed no significant difference, whether using the same total numbers as the authors did, or whenever possible using an intention‐to treat basis (11 furosemide‐treated patients and 12 controls). The authors reported that after 7 days patients in the furosemide group, but not those in the control group, had significant improvements in minute ventilation, alveolar ventilation, dynamic compliance, ventilator peak pressure and ventilator frequency. Nevertheless, there was no significant difference between the 2 groups in any of these variables by Student t‐test (using numbers provided by the authors for ventilated patients). Comparison of change scores showed a significant difference only for % inspired O2, compliance and minute ventilation after 1 week of treatment. Patients in the furosemide group had a transient increase in urine output and a decrease in serum chloride concentration; their urine output was higher than that in controls on the first day of therapy (p<0.01) and their serum chloride concentration was lower (p<0.05) after 48 hours of therapy. | |
| Notes | One infant in the furosemide group received the medication for 5 days only; study medication was stopped when serum creatinine concentration increased to 2.5 mg/dl. The authors did not provide any additional information about this patient's renal failure (e.g., pre‐renal vs intrinsic renal), nor about the potential role of furosemide. Pulmonary function was measured at baseline, after 2 days and after 7 days; they were obtained at variable times (range 1.5‐7 hours) after furosemide administration. The authors measured dynamic compliance and resistance using an esophageal balloon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized controlled trial, parallel design |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes. Medications were prepared in the pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no. Of 24 infants entered into the study, 7 were removed from the analysis. In the control group, 2 infants had clinical deterioration. In the treatment group, 1 had severe hypochloremia, 2 had sepsis, 1 had a surgical shunt for hydrocephalus, and 1 had CLD following meconium aspiration syndrome. |
Najak 1983.
| Methods | Blinding of randomization: no Blinding of intervention: no Complete follow‐up: no. Two of twelve patients randomized to furosemide were subsequently removed from the study because they did not have typical RDS. All ten control patients were kept in the study. Blinding of outcome: no Randomized controlled trial, parallel design. No washout period documented | |
| Participants | Patients entered into the study: n=22 Mechanical ventilation or oxygen requirement. Entry criteria included: prematurity, RDS, 7th postnatal day, either FiO2 > 0.30 for last 24 hours or continued ventilatory assistance. Exclusion criteria: cardiac failure, renal disease, clinically significant PDA by echocardiography (left atrium/aorta ratio <1.0). A total of 20 patients were available for the analysis, 10 in each group. The average birth weight was 1019±269 g in the treatment group and 1271±553 g in the control group. The average gestational age was 29±1.4 weeks and 30±2.9 weeks, respectively. The postnatal age was 7‐13 days and 6‐11 days, respectively, and postconceptional age 30.4 and 31.2 weeks, respectively. Baseline FiO2 was 0.43±0.09 and 0.40±0.22, respectively. Baseline A‐aDO2 gradient and PCO2 were similar in both groups. Average compliance tended to be 32% lower in the treatment group than in controls. Four infants in the furosemide group were ventilated. | |
| Interventions | Furosemide vs no medication. Patients were randomly allocated to receive either furosemide at a dose of 1 mg/kg/day intravenously for 4 days or no treatment. Average fluid intake was similar in both groups (147 ml/kg/day, range 86‐280 in treatment group, vs 153 ml/kg/day, range 97‐243 in control group). | |
| Outcomes | The authors report that 1) compliance improved more often in the treatment group than in controls, and that compliance improved significantly in 4 ventilated patients treated with furosemide. 2) AaDO2 improved more in the treatment group than in the controls, and 3) urine output did not increase. Using the original data but with a total n equalling the number of enrolled infants, we found no difference in AaDO2 between the groups on days 1, 3 and 4, but a significant difference on day 2 (‐11.1±12.06 mm Hg in treatment group vs +4.9±17.51 mmHg in controls, p<0.05); identical results were obtained using either Student t‐test or Wilcoxon test. Urine output was similar on day 1 in both groups, but was higher in the treatment group than in controls on day 4 (28±7 ml/kg/6 hr vs 21±6 ml/kg/6 hr, respectively, p<0.05, unpaired Student t‐test). | |
| Notes | Measurements were obtained each of the 4 days of the study. Dynamic compliance was measured using an esophageal balloon in the furosemide group at 1,2,4, and 6 hours after furosemide but is not reported in the control group. The AaDo2 was measured 2 hours after furosemide, and urine output was measured for 6 hours after furosemide. Unfortunately, for most of the outcomes, data cannot be used for meta‐analysis for one of the following reasons: Only mean values are given, only data in the treatment group are provided, or more than one test was reported per baby and used for statistical analysis (n=30‐39 for 10 infants). Statistics were recalculated using a total n of 10 (1 data per patient per day). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized controlled trial, parallel design. |
| Allocation concealment (selection bias) | High risk | Blinding of randomization: no |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no Blinding of outcome: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow‐up: no. Two of twelve patients randomized to furosemide were subsequently removed from the study because they did not have typical RDS. All ten control patients were kept in the study |
Reiter 1998.
| Methods | Blinding of randomization: yes Blinding of intervention: yes Complete follow up: yes, except for FiO2 (only documented in patients requiring mechanical ventilation) Blinding of outcome: yes Randomized clinical trial, parallel design Washout period: 24 hours | |
| Participants | Patients entered into the study: n=30 The need for mechanical ventilation was not used as a criterion. Entry criteria: (1) Significant lung disease defined as oxygen or ventilator support with chest radiograph compatible with BPD, and (2) need for furosemide for fluid overload management after a red blood cell transfusion. Thirty infants were randomized, 14 to into the infusion (treatment) group and 16 into the bolus (control) group. The average birth weight was 1120±467 g in the treatment group and 1107±423 g in the control group. Average gestational age was 29±2.5 weeks in the treatment group and 29±2.7 weeks in the control group. At the time of the study, postnatal age was 21.2±13.6 days and 15.4±11.4 days, respectively, postconceptional age 32.0±3.1 and 31.2±3.2 weeks, respectively, and weight 1279±504 g and 1232±607 g, respectively. Both groups had similar incidence of need for artificial ventilation or for methlyxanthines, and similar mean %O2 (57±27% vs 49±19%), mean airway pressure (8.7±2.6 vs 6.9±1.5 cm H2O), mean arterial pressure, fluid intake (132 ml/kg/day in both groups the day before the study), urine output, and fractional excretion of sodium. | |
| Interventions | Furosemide infusion vs bolus. Patients were randomly allocated to receive 1 single dose of furosemide either (1) by infusion, including a small bolus of 0.1 mg/kg over 2 minutes followed by a 6‐hour infusion of 0.9 mg/kg, or (2) by intravenous bolus of 1 mg/kg over 2 minutes followed by a 6‐hour infusion of placebo. | |
| Outcomes | Main outcomes: Urine output, fractional excretion of sodium, concentrations of serum electrolytes, FiO2, mean airway pressure, and arterial blood pressure. FiO2 is only provided in patients requiring artificial ventilation, i.e. patients randomized to furosemide bolus and 11 randomized to furosemide infusion. Furosemide administration yielded an increase in urine output and fractional excretion of sodium in both groups, but no significant change in any of the other target variables. Average urine output was similar in both groups (˜3.6 and 3.5 ml/kg/hr, respectively), although urine output at 4 hr was lower in the first group (3.4±1.5 ml/kg/hr vs 6.0±2.1 ml/kg/hr, respectively). Fractional excretion of sodium after 8 hours tended to be lower in the infusion group (6.2%, SEM not provided, vs 10.5±14.2% in the control group). Both groups had similar mean arterial pressure, serum concentrations of Na, calcium and creatinine, mean airway pressure, and FiO2. | |
| Notes | Mean blood pressure and urine output were measured every other hour for 24 hours. Fractional excretion of sodium was measured 8 hours after furosemide. Mean airway pressure and FiO2 were averaged at 1‐4 hours and 4‐12 hours after furosemide. The standard error was converted into standard deviation for this analysis. No standard error was reported for fractional excretion of sodium in the treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized clinical trial, parallel design |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome: yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow up: yes, except for FiO2 (only documented in patients requiring mechanical ventilation) |
Rush 1990.
| Methods | Blinding of randomization: yes Blinding of intervention: yes Complete follow up: no. Of 16 eligible infants, 13 were enrolled (consent signed); two patients were later excluded because of documented infection. Blinding of outcome: yes Randomized controlled trial, cross‐over design with pooled data Washout 24 hours before the study, 48 hours at cross‐over | |
| Participants | Patients entered into the study: n=13 No mechanical ventilation. Entry criteria: (1) History of respiratory failure requiring O2 and mechanical ventilation at birth, (2) postnatal age > 28 days, (3) persistence of respiratory symptoms and O2 requirement to maintain hemoglobin O2 saturation > 92%, (4) radiographic evidence of parenchymal CLD, (5) no mechanical ventilation at time of enrolment, (6) no drug therapy except for vitamins, (7) no evidence of congenital malformations, infection or congestive heart failure, (8) > 75% enteral feedings. Eleven patients were analyzed. Average birth weight was 962±328 g, mean gestational age 28.0±2.3 weeks, postnatal age 9.4±3.5 weeks, postconceptional age 37.4±4.2 weeks and FiO2 0.45±0.13. Baseline compliance during the furosemide week tended to be 20% lower than that during the placebo week (0.61±0.32 ml/cm/kg vs 0.75±0.67 ml/cm/kg, respectively, NS). | |
| Interventions | Alternate day oral furosemide vs placebo. Patients were randomly allocated to receive either (1) alternate day furosemide (4 mg/kg orally in 2 divided doses) for 8 days followed by a 48‐hour washout period and then alternated day placebo (in 2 divided doses) for 8 days, or (2) placebo followed by a washout period and then furosemide. Fluid intake and caloric intake were identical during furosemide administration and during placebo administration: 134.4±14.0 ml/kg/day vs 135.7±19.8 ml/kg/day, and 106.3±6.8 kcal/kg/day vs 107.2±4.6 kcal/kg/day, respectively). | |
| Outcomes | Main outcome: pulmonary function including compliance, resistance and FiO2 required to maintain pulse oximetry 92‐94% when quiet and awake. Improvement was defined as a 20% increase in compliance, a 20% decrease in resistance, or both, between baseline and day 8. Secondary outcome: body weight, electrolyte and mineral balance. After 8 days of therapy, dynamic compliance on diuretic therapy was 1.01±0.72 ml/cm/kg compared to 0.50±0.32 ml/cm/kg on placebo (p<0.05), and total pulmonary resistance on diuretic was 0.61±0.26 cm/L/sec, compared to 1.02±0.27 cm/L/sec on placebo (<0.01). One infant improved during both periods, 8 improved with furosemide but not with placebo, 1 improved with placebo but not furosemide and 1 did not improve during either treatment (p<0.04 for unmatched pairs, McNemar test). Oxygen requirement decreased by 7±2% during furosemide administration compared to 4.5±2% during placebo administration (furosemide vs placebo, p<0.01). The average weight gain was lower during treatment than during placebo, 16.6±8.6 g/day vs 25.6±6.9 g/day, p<0.04. Urine output did not increase anytime during furosemide administration, and was similar during diuretic treatment and during placebo treatment (79.0±17.4 ml/kg/day vs 72.3±10.6 ml/kg/day). Furosemide treatment did not significantly affect the calcium/creatinine ratio (p>0.10) nor serum electrolytes; serum sodium tended to increase less with furosemide than with placebo (0.1±5.0 vs 3.4±4.0 mEq/L, p=0.07). | |
| Notes | All variables were obtained 12‐18 hours after drug administration. Dynamic pulmonary function tests were obtained at baseline and after 2,4,6, and 8 days. Urine output, weight gain and serum electrolytes concentrations were obtained at baseline and after 8 days. No infant received Na, K or Cl supplementation for correction of serum electrolyte anomalies. There was no difference in fluid and electrolyte variables (blood and urine) between the fist and the second part of the study, suggesting there was no carry‐over effect on fluid balance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized controlled trial, cross‐over design with pooled data |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome: yes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Complete follow up: no. Of 16 eligible infants, 13 were enrolled (consent signed); two patients were later excluded because of documented infection |
Singhal 1983.
| Methods | Blinding of randomization: yes Blinding of intervention: yes Complete follow up: unclear Blinding of outcome: yes Double blind trial; cross‐over design with pooled data No washout period documented | |
| Participants | Patients entered into the study: n=10 Entry criteria: BPD. Ten patients were randomized. Postnatal age ranged between 23 and 101 days, and average FiO2 was 0.27±0.13. Baseline compliance during the furosemide period tended to be 26% lower than during the placebo period (1.14±0.79 ml/cm vs 1.55±1.04 ml/cm, respectively, NS). | |
| Interventions | Furosemide vs placebo Patients were randomly allocated to receive either furosemide 1.5 mg/kg IM q 12 hours for 3 doses, or placebo. | |
| Outcomes | Main outcome: compliance and ventilation. The authors report that furosemide produced a significant increase in compliance, a 6‐fold increase in urine output, an increase in serum solids and no significant change in serum Na and K concentrations. Compliance was 1.38±0.79 ml/cm after the third dose of furosemide, compared with 1.54±0.98 after the third dose of placebo. We found no significant difference between the two periods in compliance expressed in % of baseline. | |
| Notes | (1) Abstract form only; incomplete information available. Standard error was mentioned for compliance but not for other variables. (2) Neither 'random', 'randomized' or 'randomly' is mentioned in the text. Nevertheless, this is a double‐blind clinical trial, so that the we assumed this was a randomized trial. 3) Compliance was measured 2 hours after the third dose of furosemide. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double blind trial; cross‐over design with pooled data |
| Allocation concealment (selection bias) | Low risk | Blinding of randomization: yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome: yes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow up: unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Atkinson 1988 | Not a randomized study |
| Braden 1997 | Not a randomized study |
| Campfield 1997 | Not a randomized study |
| Engelhardt 1986 | Mixes patients in a randomized trial with cross‐over design and non randomized patients. Not exactly a randomized trial. Only 10 of 16 patients were randomly allocated to receive either furosemide for 6‐10 days followed by a 7‐day period with placebo (3/10 patients) or no treatment (7/10 patients), or vice versa. The other 6 infants were included in a non randomized trial of furosemide, without control period. Data on randomized patients were not separately reported in a way permitting us to extract them. The authors do not provide data to assess whether the two groups of patients significantly differ in baseline parameters or in variables measured during furosemide administration. |
| Giacoia 1991 | Not a randomized study |
| Kenney 1988 | Not a randomized study |
| Logvinoff 1985 | Not a randomized study. |
| Mirochnick 1988 | Not a randomized study |
| Mirochnick 1990 | Not a randomized study |
| O'Donovan 1989 | Not a randomized study |
| Patel 1985 | Not a randomized study |
| Perlman 1986 | Not a randomized study |
| Rowe 1989 | Not a randomized study |
| Segar 1992 | This short‐term study includes random allocation of diuretics in premature infants with lung disease, however it does not provide any information on the outcome variables selected for this systematic review. |
| Segar 1997 | This short‐term study includes random allocation either to furosemide and metolazone, or to furosemide only. This study will be discussed in a related review on diuretics acting on the distal segments of the renal tubule. |
| Shankaran 1994 | This short‐term study includes random allocation of diuretics in premature infants with lung disease, however it does not provide any information on the main outcome variables selected for this systematic review. |
| Sniderman 1978 | Not a randomized study |
| Stefano 1990 | No randomized allocation to diuretic administration |
| Verma 1994 | Not a randomized study |
| Vileisis 1990 | No randomized allocation to diuretic therapy. Instead, patients were randomized to receive one of three doses of phosphorus. |
| Yeh 1984 | Not a randomized study |
Contributions of authors
LPB and RAP performed the searches, wrote the review and revised the review.
We would like to thank Dr. Primhak’s for his prior contribution to reviews and revisions.
ALS and LPB revised the most recent review (2011).
Sources of support
Internal sources
Albert Einstein Coll. Med & Montefiore Med. Ctr., Div. Neonatology, USA.
External sources
Grant‐in‐Aid 9650445N of the American Heart Association (LPB), USA.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Kao 1983 {published data only}
- Kao LC, Warburton D, Sargent CW, Platzker AC, Keens TG. Furosemide acutely decreases airways resistance in chronic bronchopulmonary dysplasia. Journal of Pediatrics 1983;103:624‐9. [DOI] [PubMed] [Google Scholar]
McCann 1985 {published data only}
- McCann EM, Lewis K, Deming DD, Donovan MJ, Brady JP. Controlled trial of furosemide therapy in infants with chronic lung disease. Journal of Pediatrics 1985;106:957‐62. [DOI] [PubMed] [Google Scholar]
Najak 1983 {published data only}
- Najak ZD, Harris EM, Lazzara A, Pruitt AW. Pulmonary effects of furosemide in preterm infants with lung disease. Journal of Pediatrics 1983;102:758‐63. [DOI] [PubMed] [Google Scholar]
Reiter 1998 {published data only}
- Reiter PD, Makhlouf R, Stiles AD. Comparison of 6‐hour infusion versus bolus furosemide in premature infants. Pharmacotherapy ‐ The Journal of Human Pharmacology and Drug Therapy 1998;18:63‐8. [PubMed] [Google Scholar]
Rush 1990 {published data only}
- Rush MG, Engelhardt B, Parker RA, Hazinski TA. Double‐blind, placebo‐controlled trial of alternate‐day furosemide therapy in infants with chronic bronchopulmonary dysplasia. Journal of Pediatrics 1990;117:112‐8. [DOI] [PubMed] [Google Scholar]
Singhal 1983 {published data only}
- Singhal N, McMillan DD, Rademaker AW. Furosemide improves lung compliance in infants with bronchopulmonary dysplasia [abstract]. Pediatric Research 1983;17:336A. [Google Scholar]
References to studies excluded from this review
Atkinson 1988 {published data only}
- Atkinson SA, Shah JK, McGee C, Steele BT. Mineral excretion in premature infants receiving various diuretic therapies. Journal of Pediatrics 1988;113:540‐5. [DOI] [PubMed] [Google Scholar]
Braden 1997 {published data only}
- Braden G, Campfield T, Flynn‐Valone P, Bednarek F, Pappagallo M. Nephrocalcinosis in very low birthweight infants: a prospective multicenter study of risk factors and complications [abstract]. Journal of the American Society of Nephrology 1997;8:122A. [Google Scholar]
Campfield 1997 {published data only}
- Campfield T, Braden G, Flynn‐Valone P, Powell S. Effect of diuretics on urinary oxalate, calcium, and sodium excretion in very low birth weight infants. Pediatrics 1997;99:814‐8. [DOI] [PubMed] [Google Scholar]
Engelhardt 1986 {published data only}
- Engelhardt B, Elliott S, Hazinski TA. Short‐ and long‐term effects of furosemide on lung function in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1986;109:1034‐9. [DOI] [PubMed] [Google Scholar]
Giacoia 1991 {published data only}
- Giacoia GP, Pineda R. Diuretics, hypochloremia, and outcome in bronchopulmonary dysplasia patients. Developmental Pharmacology and Therapeutics 1991;4:212‐20. [PubMed] [Google Scholar]
Kenney 1988 {published data only}
- Kenney IJ, Aiken CG, Lenney W. Frusemide‐induced nephrocalcinosis in very low weight infants. Pediatric Radiology 1988;18:323‐5. [DOI] [PubMed] [Google Scholar]
Logvinoff 1985 {published data only}
- Logvinoff MM, Lemen RJ, Taussig LM, Lamont BA. Bronchodilators and diuretics in children with bronchopulmonary dysplasia. Pediatric Pulmonology 1985;1:198‐203. [DOI] [PubMed] [Google Scholar]
Mirochnick 1988 {published data only}
- Mirochnick MH, Miceli JJ, Kramer PA, Chapron DJ, Raye JR. Furosemide pharmacokinetics in very low birth weight infants. Journal of Pediatrics 1988;112:653‐7. [DOI] [PubMed] [Google Scholar]
Mirochnick 1990 {published data only}
- Mirochnick MH, Miceli JJ, Kramer PA, Chadron DJ, Raye JR. Renal response to furosemide in very low birth weight infants during chronic administration. Developmental Pharmacology and Therapeutics 1990;15:1‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
O'Donovan 1989 {published data only}
- O'Donovan BH, Bell EF. Effects of furosemide on body water compartments in infants with bronchopulmonary dysplasia. Pediatric Research 1989;26:121‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Patel 1985 {published data only}
- Patel H, Yeh TF, Jain R, Pildes R. Pulmonary and renal responses to furosemide in infants with stage III‐IV bronchopulmonary dysplasia. American Journal of Diseases of Children 1985;139:917‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perlman 1986 {published data only}
- Perlman JM, Moore V, Siegel MJ, Dawson J. Is chloride depletion an important contributing cause of death in infants with bronchopulmonary dysplasia?. Pediatrics 1986;77:121‐216. [PubMed] [Google Scholar]
Rowe 1989 {published data only}
- Rowe JC, Carey DE, Goetz CA, Adams ND, Horak E. Effect of high calcium and phosphorus intake on mineral retention in very low birth weight infants chronically treated with furosemide. Journal of Pediatric Gastroenterology and Nutrition 1989;9:206‐11. [DOI] [PubMed] [Google Scholar]
Segar 1992 {published data only}
- Segar JL, Robillard JE, Johnson KJ, Bell EF, Chemtob S. Addition of metolazone to overcome tolerance to furosemide in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1992;120:966‐73. [DOI] [PubMed] [Google Scholar]
Segar 1997 {published data only}
- Segar JL, Chemtob S, Bell EF. Changes in body water compartments with diuretic therapy in infants with chronic lung disease. Early Human Development 1997;48:99‐107. [DOI] [PubMed] [Google Scholar]
Shankaran 1994 {published data only}
- Shankaran S, Liang KC, Ilagan N, Fleischmann L. Mineral excretion following furosemide compared with bumetanide therapy in premature infants. Pediatric Nephrology 1995;9:159‐62. [DOI] [PubMed] [Google Scholar]
Sniderman 1978 {published data only}
- Sniderman S, Chung M, Roth R, Ballard R. Treatment of neonatal chronic lung disease with furosemide [abstract]. Clinical Research 1978; Vol. 26:201A.
Stefano 1990 {published data only}
- Stefano JL, Bhutani VK. Role of furosemide therapy after booster‐packed erythrocyte transfusions in infants with bronchopulmonary dysplasia. Journal of Pediatrics 1990;117:965‐8. [DOI] [PubMed] [Google Scholar]
- Stefano JL, Bhutani VK. The effect of furosemide on pulmonary function following booster transfusions in infants with bronchopulmonary dysplasia. American Journal of Perinatology 1990; Vol. 7:393.
Verma 1994 {published data only}
- Verma RP, Fornell JE, Vidyasagar D. Body fluid electrolytes in bronchopulmonary dysplasia and the effects of diuretic therapy. Indian Journal of Pediatrics 1994;61:213‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vileisis 1990 {published data only}
- Vileisis RA. Furosemide effect on mineral status of parenterally nourished premature neonates with chronic lung disease. Pediatrics 1990;85:316‐22. [PubMed] [Google Scholar]
Yeh 1984 {published data only}
- Yeh TF, Jain R, Patel H, Pildes RS. Renal response to furosemide in infants with bronchopulmonary dysplasia [abstract]. Pediatric Research 1984; Vol. 18:340A.
Additional references
Abbasi 1990
- Abbasi S, Bhutani VK. Pulmonary mechanics and energetics of normal, non‐ventilated low birthweight infants. Pediatric Pulmonology 1990;8:89‐95. [DOI] [PubMed] [Google Scholar]
Ali 1979
- Ali J, Chernicki W, Wood LDH. Effect of furosemide in canine low‐pressure pulmonary edema. The Journal of Clinical Investigation 1979;64:1494‐1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armitage 1994
- Armitage P, Berry G. Sampling. In: Armitage P, Berry G editor(s). Statistical Methods in Medical Research. Oxford: Blackwell Scientific, 1994:78‐92. [Google Scholar]
Aufricht 1997
- Aufricht C, Votava F, Marx M, Frenzel K, Simbruner G. Intratracheal furosemide in infants after cardiac surgery: its effects on lung mechanics and urinary output, and its levels in plasma and tracheal aspirate. Journal of Intensive Care Medicine 1997;23:992‐7. [DOI] [PubMed] [Google Scholar]
Baird 1995
- Baird DC. Statistics of observation. In: Baird DC editor(s). Experimentation. An introduction to measurement theory and experiment design. New Jersey: Prentice Hall, 1995:29‐56. [Google Scholar]
Bland 1978
- Bland RD, McMillan DD, Bressack MA. Decreased pulmonary transvascular fluid filtration in awake newborn lambs after intravenous furosemide. The Journal of Clinical Investigation 1978;62:601‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brion 1994
- Brion LP, Satlin LM, Edelmann CM Jr. Renal disease. In: Avery GB, Fletcher MA, MacDonald MG editor(s). Neonatology: Pathophysiology and Management of the Newborn. 4th Edition. Philadelphia: JB Lippincott, 1994:792‐886. [Google Scholar]
Brion 1998
- Brion LP, Campbell DE. Furosemide in indomethacin‐treated infants with symptomatic patent ductus arteriosus (Cochrane Review). Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001148] [DOI] [Google Scholar]
Brion 1999b
- Brion LP, Primhak RA, Yong W. Aerosolized diuretics for preterm infants with (or developing) chronic lung disease (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001694.pub2] [DOI] [PubMed] [Google Scholar]
Brion 1999c
- Brion LP, Primhak RA, Ambrosio‐Perez I. Diuretics acting on the distal renal tubule for preterm infants with (or developing) chronic lung disease (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001817] [DOI] [Google Scholar]
Brion 1999d
- Brion LP, Soll RF. Diuretics for respiratory distress syndrome in preterm infants (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001454] [DOI] [PubMed] [Google Scholar]
Brown 1978
- Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME. Bronchopulmonary dysplasia: Possible relationship to pulmonary edema. Journal of Pediatrics 1978;92:982‐4. [DOI] [PubMed] [Google Scholar]
Campfield 1997
- Campfield T, Braden G, Flynn‐Valone P, Powell S. Effect of diuretics on urinary oxalate, calcium, and sodium excretion in very low birth weight infants. Pediatrics 1997;99:814‐8. [DOI] [PubMed] [Google Scholar]
Chemtob 1989
- Chemtob S, Kaplan BS, Sherbotie JR, Aranda JV. Pharmacology of diuretics in the newborn. The Pediatric Clinics of North America 1989;36:1231‐50. [DOI] [PubMed] [Google Scholar]
Clark 2006
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006;117:1979‐87. [DOI] [PubMed] [Google Scholar]
Demling 1978
- Demling RH, Will JA. The effect of furosemide on the pulmonary transvascular fluid filtration rate. Critical Care Medicine 1978;6:317‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dikshit 1973
- Dikshit K, Vyden JK, Forrester JS, Chatterjee K, Prakash R, Swan HJ. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. New England Journal of Medicine 1973;288:1087‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Downing 1992
- Downing GJ, Egelhoff JC, Daily DK, Thomas MK, Alon U. Kidney function in very low birth weight infants with furosemide‐related renal calcifications at ages 1 to 2 years. Journal of Pediatrics 1992;120:599‐604. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edwards 1979
- Edwards DK, Colby TV, Northway WH. Radiographic‐pathologic correlation in bronchopulmonary dysplasia. Journal of Pediatrics 1979;85:834‐6. [DOI] [PubMed] [Google Scholar]
Ezzedeen 1988
- Ezzedeen F, Adelman RD, Ahlfors CE. Renal calcification in preterm infants: pathophysiology and long‐term sequelae [comment in: J Pediatr 1989;114:1068]. Journal of Pediatrics 1988;113:532‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45:769‐73. [DOI] [PubMed] [Google Scholar]
Gerhardt 1989
- Gerhardt T, Reifenberg L, Duara S, Bancalari E. Comparison of dynamic and static measurements of respiratory mechanics in infants. Journal of Pediatrics 1989;114:120‐5. [DOI] [PubMed] [Google Scholar]
Giacoia 1991
- Giacoia GP, Pineda R. Diuretics, hypochloremia, and outcome in bronchopulmonary dysplasia patients. Developmental Pharmacology and Therapeutics 1991;4:212‐20. [PubMed] [Google Scholar]
Gimpel 2010
- Gimpel C, Krause A, Franck P, Krueger M, Schnakenburg C. Exposure to furosemide as the strongest risk factor for nephrocalcinosis in preterm infants. Pediatrics International 2010;52:51‐6. [DOI] [PubMed] [Google Scholar]
Gortner 1991
- Gortner L, Bernsau U, Hellwege HH, Hieronimi G, Jorch G, Reiter HL. Does prophylactic use of surfactant change drug utilization in very premature infants during the neonatal period?. Developmental Pharmacology and Therapeutics 1991;16:1‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Goyal 1995
- Goyal M, Suresh BR, Reinersman G, Gewolb IH, Brion LP. Evolution and variability of pulmonary mechanics during postnatal transition in full‐term infants. Journal of Perinatology 1995;15:441‐8. [PubMed] [Google Scholar]
Green 1983
- Green TP, Thompson TR, Johnson DE, Lock JE. Furosemide promotes patent ductus arteriosus in premature infants with the respiratory‐distress syndrome. New England Journal of Medicine 1983;308:743‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins 2008. Cochrane Handbook. [Google Scholar]
Horgan 1996
- Horgan MJ. Thiazide‐induced hyponatremia in <1500 gm infants with bronchopulmonary dysplasia [abstract]. Pediatric Research 1996; Vol. 38:216A.
Kugelman 1996
- Kugelman A, McEvoy C, Bowling S, Durand M. Comparison of dynamic and passive measurements of respiratory dynamics during dexamethasone (Dex) treatment in very low birthweight (VLBW) infants [abstract]. American Journal of Respiratory and Critical Care Medicine 1996; Vol. 153:A556.
LeSouef 1983
- LeSouëf PN, Lopes JM, England SJ, Bryan MH, Bryan AC. Influence of chest wall distortion on esophageal pressure. Journal of Applied Physiology 1983;55:353‐8. [DOI] [PubMed] [Google Scholar]
LeSouef 1984
- LeSouëf PN, England SJ, Bryan AC. Passive respiratory mechanics in newborns and children. The American Review of Respiratory Disease 1985;132:694‐709. [PubMed] [Google Scholar]
Lopez 1990
- Lopez AM, Modi MW, Adams JA, Horner GP, Grazia FTR, Goldberg RN. Pharmacokinetics of bumetanide in the newborn infant [abstract]. Pediatric Research 1990; Vol. 27:62A.
Mirochnick 1988
- Mirochnick MH, Miceli JJ, Kramer PA, Chapron DJ, Raye JR. Furosemide pharmacokinetics in very low birth weight infants. Journal of Pediatrics 1988;112:653‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Northway 1967
- Northway WH, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: bronchopulmonary dysplasia. New England Journal of Medicine 1967;276:357‐68. [DOI] [PubMed] [Google Scholar]
O'Donovan 1989
- O'Donovan BH, Bell EF. Effects of furosemide on body water compartments in infants with bronchopulmonary dysplasia. Pediatric Research 1989;26:121‐4. [DOI] [PubMed] [Google Scholar]
Perlman 1986
- Perlman JM, Moore V, Siegel MJ, Dawson J. Is chloride depletion an important contributing cause of death in infants with bronchopulmonary dysplasia?. Pediatrics 1986;77:212‐6. [PubMed] [Google Scholar]
Peterson 1980
- Peterson RG, Simmons MA, Rumack BH, Levine RL, Brooks JG. Pharmacology of furosemide in the premature infant. Journal of Pediatrics 1980;97:139‐43. [DOI] [PubMed] [Google Scholar]
Randall 1992
- Randall LH, Shaddy RE, Sturtevant JE, Reid BS, Molteni RA. Cholelithiasis in infants receiving furosemide: a prospective study of the incidence and one‐year follow‐up. Journal of Perinatology 1992;12:107‐111. [MEDLINE: ] [PubMed] [Google Scholar]
Reid 1979
- Reid L. Bronchopulmonary dysplasia ‐ Pathology. Journal of Pediatrics 1979;85:836‐41. [DOI] [PubMed] [Google Scholar]
Robbins 1993
- Robbins G, Tayaba R, Ochshorn IL, Green RS, Holzman IR. Effects of nebulized furosemide vs normal saline on pulmonary mechanics in bronchopulmonary dysplasia [abstract]. Pediatric Research 1993; Vol. 33:233A.
Shankaran 1990
- Shankaran S, Ilagan N, Liang KC, Bhavsar V, Kauffman R. Bumetanide pharmacokinetics in preterm neonates [abstract]. Pediatric Research 1990; Vol. 27:64A.
Sola 1981
- Sola A, Gregory GA. Colloid osmotic pressure of normal newborns and premature infants. Critical Care Medicine 1981;9:568‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sonntag 1996
- Sonntag J. Effect of dexamethasone and spironolactone therapy on diuresis and creatinine clearance in premature infants with a birth weight below 1,500g. Klinische Padiatrie 1996;208:319‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thomson 1983
- Thomson, Elliott J, Silverman M. Pulmonary compliance in sick low birthweight infants. Archives of Disease in Childhood 1983;58:891‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vert 1982
- Vert P, Broquaire M, Legagneur M, Morselli PL. Pharmacokinetics of furosemide in neonates. European Journal of Clinical Pharmacology 1982;22:39‐45. [DOI] [PubMed] [Google Scholar]
Wilson 1981
- Wilson JR, Reichek N, Dunkman WB, Goldberg S. Effect of diuresis on the performance of the failing left ventricle in man. The American Journal of Medicine 1981;70:234‐9. [DOI] [PubMed] [Google Scholar]
Zimmerman 1995
- Zimmerman JJ. Bronchoalveolar inflammatory pathophysiology of bronchopulmonary dysplasia. Clinical Perinatology 1995;22:429‐56. [PubMed] [Google Scholar]
References to other published versions of this review
Brion 1999a
- Brion LP, Primhak RA. Use of intravenous or oral furosemide (F) in preterm infants with or developing chronic lung disease (CLD): Systematic review and meta‐analysis [abstract]. Pediatric Research 1999; Vol. 45:296A.
Brion 2000
- Brion LP, Primhak RA. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD001453] [DOI] [PubMed] [Google Scholar]
Brion 2001
- Brion LP, Yong SC, Perez IA, Primhak R. Diuretics and chronic lung disease of prematurity. Journal of Perinatology 2001;21:269‐71. [DOI: 10.1002/14651858.CD001453] [DOI] [PubMed] [Google Scholar]
Brion 2002
- Brion LP, Primhak RA. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001453] [DOI] [PubMed] [Google Scholar]
Brion 2003
- Brion LP, Primhak RA. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD001453] [DOI] [PubMed] [Google Scholar]
Brion 2008
- Brion LP, Primhak RA. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD001453] [DOI] [Google Scholar]


