Abstract
Background
Human albumin solutions are used for a range of medical and surgical problems. Licensed indications are the emergency treatment of shock and other conditions where restoration of blood volume is urgent, such as in burns and hypoproteinaemia. Human albumin solutions are more expensive than other colloids and crystalloids.
Objectives
To quantify the effect on mortality of human albumin and plasma protein fraction (PPF) administration in the management of critically ill patients.
Search methods
We searched the Cochrane Injuries Group Specialised Register (searched 31 May 2011), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2), MEDLINE (Ovid) (1948 to week 3 May 2011), EMBASE (Ovid) (1980 to Week 21 2011), CINAHL (EBSCO) (1982 to May 2011), ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to May 2011), ISI Web of Science: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990 to May 2011), PubMed (www.ncbi.nlm.nih.gov/sites/entrez/) (searched 10 June 2011, limit: last 60 days). Reference lists of trials and review articles were checked, and authors of identified trials were contacted.
Selection criteria
Randomised controlled trials comparing albumin or PPF with no albumin or PPF or with a crystalloid solution in critically ill patients with hypovolaemia, burns or hypoalbuminaemia.
Data collection and analysis
We collected data on the participants, albumin solution used, mortality at the end of follow up, and quality of allocation concealment. Analysis was stratified according to patient type.
Main results
We found 38 trials meeting the inclusion criteria and reporting death as an outcome. There were 1,958 deaths among 10,842 trial participants.
For hypovolaemia, the relative risk of death following albumin administration was 1.02 (95% confidence interval (CI) 0.92 to 1.13). This estimate was heavily influenced by the results of the SAFE trial, which contributed 75.2% of the information (based on the weights in the meta‐analysis). For burns, the relative risk was 2.93 (95% CI 1.28 to 6.72) and for hypoalbuminaemia the relative risk was 1.26 (95% CI 0.84 to 1.88). There was no substantial heterogeneity between the trials in the various categories (Chi2 = 26.66, df = 31, P = 0.69). The pooled relative risk of death with albumin administration was 1.05 (95% CI 0.95 to 1.16).
Authors' conclusions
For patients with hypovolaemia, there is no evidence that albumin reduces mortality when compared with cheaper alternatives such as saline. There is no evidence that albumin reduces mortality in critically ill patients with burns and hypoalbuminaemia. The possibility that there may be highly selected populations of critically ill patients in which albumin may be indicated remains open to question. However, in view of the absence of evidence of a mortality benefit from albumin and the increased cost of albumin compared to alternatives such as saline, it would seem reasonable that albumin should only be used within the context of well concealed and adequately powered randomised controlled trials.
Plain language summary
What is the effect of giving human albumin compared to saline to replace lost blood in critically ill or injured people
Trauma, burns or surgery can cause people to lose large amounts of blood. Fluid replacement, giving fluids intravenously (into a vein), is used to help restore blood volume and hopefully reduce the risk of dying. Blood products (including human albumin), non‐blood products or combinations can be used. This review of 38 trials found no evidence that albumin reduces the risk of dying. Albumin is very expensive, in which case it may be better to use cheaper alternatives such as saline for fluid replacement.
Background
In patients with acute and chronic illness, serum albumin concentration is inversely related to mortality risk. A systematic review of cohort studies meeting specified criteria estimated that, for each 2.5 g/L decrement in serum albumin concentration, the risk of death increases by between 24% and 56% (Goldwasser 1997). The association persists after adjusting for other known risk factors and pre‐existing illness, suggesting a direct protective effect of the albumin molecule (Goldwasser 1997). Largely as a result of these observations, human albumin solutions are now used in the management of a diverse range of medical and surgical problems. Published indications for human albumin solution include the emergency treatment of shock and other conditions where restoration of blood volume is urgent, the acute management of burns, and clinical situations associated with hypoproteinaemia (ABPI 1998).
In comparison with other colloidal solutions and with crystalloid solutions, human albumin solutions are expensive (McClelland 1990). Volume for volume human albumin solution is twice as expensive as hydroxyethyl starch, and over 30 times more expensive than crystalloid solutions such as sodium chloride or Ringer's lactate. Because of the high cost and limited availability of human albumin, it is particularly important that its use should be restricted to the indications for which it has been shown to be effective. To assess the effectiveness and safety of human albumin solutions in the management of critically ill patients, particularly those with hypovolaemia from injury or surgery, burns and hypoproteinaemia, a systematic review of randomised controlled trials was conducted.
Objectives
To quantify the effect on mortality of human albumin administration in the management of critically ill patients.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all randomised controlled trials of human albumin or plasma protein fraction (PPF) administration (albumin or PPF versus no albumin or PPF, or a crystalloid solution).
Types of participants
Critically ill patients with hypovolaemia, burns or hypoproteinaemia. Trials involving patients receiving pre‐operative volume loading or haemodilution, and trials of albumin administration during paracentesis, were excluded.
Types of interventions
Human albumin solution or plasma protein fraction (PPF).
Types of outcome measures
The principal outcome measure was mortality from all causes assessed at the end of the follow‐up period scheduled for each trial.
Search methods for identification of studies
The latest search was carried out in May to June 2011 using the electronic databases listed below. See Figure 1 for the study identification and selection process for this update.
1.
PRISMA diagram: study identification and selection process‐latest update only
Search strategies used for previous versions of this review can be obtained from the Trials Search Co‐ordinator of the Cochrane Injuries Group.
Electronic searches
We searched the following electronic databases:
Cochrane Injuries Group Specialised Register (searched May 31 2011);
Cochrane Central Register of Controlled Trials (The Cochrane Library 2011, Issue 2);
MEDLINE(Ovid) (1948 to week 3 May 2011);
Embase (Ovid) (1980 to Week 21 2011);
CINAHL (EBSCO) (1982 to May 2011);
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to May 2011);
ISI Web of Science: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990 to May 2011);
PubMed (www.ncbi.nlm.nih.gov/sites/entrez/) (searched 10 June 2011, limit: last 60 days).
Full details of the latest search strategies can be found in Appendix 1.
Searching other resources
For the original searches, trials were identified using BIDS Index to Scientific and Technical Proceedings and by handsearching 29 international journals and the proceedings of several international meetings on fluid resuscitation. To identify unpublished trials we searched the register of the Medical Editors' Trial Amnesty, and contacted the Medical Directors of Bio Products Laboratory (Zenalb), Centeon Limited (Albuminar), and Alpha Therapeutic UK Limited (Albutein).
We have subsequently identified studies by examining reference lists of included studies and previously published reviews. We have also contacted authors of included studies to enquire about other published or unpublished studies that they may be aware of. We did not limit our searches by date, language or publication status.
Data collection and analysis
One author scanned the titles and abstracts of reports identified by electronic searching to produce a list of possibly relevant reports. Two authors (PA and IR) then checked the list to determine which articles to retrieve in full. Disagreements were resolved by discussion.
The same two authors applied the selection criteria, again resolving disagreements by discussion. They both extracted data on study design, allocation concealment, participants, interventions and mortality. One author (IR) entered the data into Review Manager and the other (PA) checked it against his data extraction.
Where clarification on any aspect of a study was needed, one review author contacted the author of the trial.
Relative risks and 95% confidence intervals (CI) for mortality were calculated for each trial on an intention‐to‐treat basis. Heterogeneity between trials was tested using a Chi2 test, where P ≤ 0.05 was taken to indicate significant heterogeneity. As long as statistical heterogeneity did not exist, for dichotomous data summary relative risks and 95% CIs were calculated using a fixed‐effect model. In the event of statistical heterogeneity, if the source of heterogeneity could obviously be related to patient type, or allocation concealment, we stratified the analyses on that dimension.
Results
Description of studies
A total of 38 randomised controlled trials were identified that met the study inclusion criteria. Mortality data were available either from the published report or on contact with the authors of 38 of these trials. The five trials for which mortality data could not be obtained (Ernest 1999; Ernest 2001; McNulty 1993; Oca 1999; Skillman 1975) included a total of 124 randomised patients, comprising 1.14% of the total number of randomised patients in all trials meeting the study inclusion criteria. One of the trials was an unpublished trial registered in the Medical Editors' Trial Amnesty (Woittiez 1998). Further details about this trial, including data on mortality, were obtained directly from the trialist. In six trials there were no deaths in either the intervention or comparison groups. An intention‐to‐treat analysis could not be done for nine of 50 patients in one study (Oca 2003) consequently mortality data is only presented for 41 patients. The trial by Lucas et al was reported in five publications. An early report gave the mortality data for 52 randomised patients, 27 allocated to receive albumin, 25 allocated to receive no albumin (Lucas 1978). Subsequent publications indicated that recruitment to the trial continued until 94 patients were randomised. Mortality data for all the 94 patients were not published, nor were they available on contact with the author. Consequently the outcome data for the 52 patients are presented. For the 32 included trials in which there were one or more deaths in either the intervention or control groups, allocation concealment involved a method that would be expected to reduce the risk of foreknowledge of treatment allocation (pharmacy controlled randomisation or serially numbered sealed opaque envelopes) in 20 trials, was unclear in eight trials, and inadequate in four trials.
Risk of bias in included studies
Bland 1973 Randomised control trial. Therapy cards were randomised in pairs matched for weight. Method of allocation concealment was not described.
Bland 1976 This study is reported as randomised but the method of allocating random numbers and method of allocation concealment are unknown.
Boldt 1993 Randomised controlled trial. Allocation concealment was by the use of sealed opaque envelopes.
Boutros 1979 The study is reported as randomised but the method of randomisation and allocation concealment are unknown.
Brown 1988 The random sequence was generated using random number tables. No allocation concealment.
Cooper 2006 Randomisation was accomplished with a computer‐generated list and sequentially numbered sealed, opaque envelopes.
Dubois 2006 Eligible patients were randomised in a 1:1 ratio using sealed envelopes.
Ernest 1999 Randomisation was done by the hospital chart number (odd or even).
Ernest 2001 Randomisation was done by the hospital record number (odd or even).
Foley 1990 Patients were randomly assigned to either a treatment or non‐treatment group by medical record number.
Gallagher 1985 Randomisation and allocation concealment were by computerised system.
Golub 1994 Random sequence was computer generated. Allocation concealment was by the use of sealed opaque envelopes.
Goodwin 1983 Randomisation was according to random number tables. The methods of allocation concealment were unknown.
Greenhalgh 1995 Randomisation scheme controlled by the pharmacy.
Greenough 1993 Randomised controlled trial. Allocation concealment was by the use of sealed opaque envelopes.
Grundmann 1982 The study is reported as prospectively randomised, but the methods of randomisation and allocation concealment are unknown.
Jelenko 1978 The study is reported as randomised but the method of randomisation and allocation concealment are unknown.
Kanarek 1992 Randomised controlled trial. Allocation concealment was by the use of sealed opaque envelopes.
Lowe 1977 The method of allocating random numbers is unknown. Sealed envelopes were used to ensure allocation concealment.
Lucas 1978 Allocation was based on the last digit of each patient's case number. Ninety‐four patients were randomised in total but the number of deaths was not reported in the final report. However, in a preliminary report, based on 52 of the randomised patients, deaths were reported.
Maitland 2005 Randomised control trial using a sealed card system.
Maitland 2011 Multicentre, open, randomised, controlled study. Sealed opaque envelopes were used to assist allocation concealment.
McNulty 1993 The study is reported as randomised but the method of randomisation and allocation concealment are unknown.
Nielsen 1985 This study is reported as randomised but the method of allocation concealment is not described.
Nilsson 1980 Randomised controlled trial. Allocation concealment was by the use of sealed opaque envelopes.
Oca 1999 Randomisation was done by sequentially numbered, sealed, opaque envelopes.
Randomisation was accomplished by random number tables via sealed, serially numbered envelopes.
Pockaj 1994 The study is reported as randomised but the method of randomisation and allocation concealment are unknown.
Prien 1990 The study is reported as randomised but the method of randomisation and allocation concealment are unknown.
Quinlan 2004 Randomised double blind controlled trial.
Rackow 1983 Randomisation was according to a pre‐determined randomisation schedule, but the methods and the allocation concealment are unknown.
Rubin 1997 Allocation concealment was by a sealed opaque envelope system in the hospital pharmacy.
SAFE 2004 Central randomisation accessed on the Internet through a secure website with use of a minimisation algorithm. Blinding was assured through the use of specially designed masking cartons and specially designed and manufactured administration sets. The authors report that the effectiveness of the blinding was confirmed in a formal study before the trial was initiated.
Shah 1977 Randomised controlled trial. Allocation by sealed envelope.
Skillman 1975 The study is reported as randomised but the method of randomisation and allocation concealment are unknown.
So 1997 Randomised controlled trial. Allocation concealment was by computerised system.
Tollofsrud 1995 The method of generating random numbers is unknown. Allocation concealment was by sealed opaque envelopes.
Virgilio 1979 Randomisation was determined using random number tables. Methods of allocation concealment are unknown.
Woittiez 1998 Randomised controlled trial. Allocation concealment was by the use of sealed opaque envelopes.
Wojtysiak 1992 Randomisation was determined using random number tables. Allocation concealment was inadequate.
Woods 1993 Patients with even hospital numbers were allocated to the group receiving albumin, while those with odd hospital numbers were allocated to the group not receiving supplemental albumin.
Zetterstrom 1981a Patients were randomly divided into two groups. Allocation concealment was by the use of sealed opaque envelopes.
Zetterstrom 1981b Patients were randomly divided into two groups. Allocation concealment was by the use of sealed opaque envelopes.
Effects of interventions
For hypovolaemia the pooled relative risk of death following albumin administration was 1.02 (95% confidence interval (CI) 0.92 to 1.13). This estimate was heavily influenced by the results of the SAFE trial which received 75.2% of the weight. For burns the relative risk was 2.93 (95% CI 1.28 to 6.72), and for hypoalbuminaemia the relative risk was 1.26 (95% CI 0.84 to 1.88). There was no substantial heterogeneity between the trials in the various categories (Chi2 = 26.66, df = 31, P = 0.69). The pooled relative risk of death with albumin administration was 1.05 (95% CI 0.95 to 1.16).
Discussion
Because many of the trials included in this meta‐analysis are small and many are poorly concealed, the results must be interpreted with caution. The SAFE trial, however, is a notable exception. The SAFE trial included a total of 6997 randomised participants, allocation was well concealed, the use of a minimisation algorithm helped to ensure that baseline characteristics were well balanced, vigorous attempts were made to ensure that the participating clinicians were blind to the type of fluid that was administered, and an intention‐to‐treat analysis was undertaken. The SAFE trial provided no evidence that albumin reduced mortality in patients with hypovolaemia, although the possibility of a modest benefit or harm could not be excluded.
This systematic review was first updated in November 2001. At that time, one additional trial was identified and included (Bland 1973). This trial compared albumin and dextrose infusions in new‐born infants with low cord serum protein levels who were considered to be at risk of respiratory distress. This trial meets the eligibility criteria for the review (hypoproteinaemia) but had been overlooked in the original search. However, the inclusion of this trial does not change the conclusions of the review. The latest update of this review (July 2011) includes one further recently published trial that meets the inclusion criteria (Maitland 2011); it does not change the conclusions of the review.
Summary of main results
There is no evidence that albumin reduces mortality in patients with hypovolaemia, burns or hypoproteinaemia. For patients with burns or hypoproteinaemia, there is a suggestion that albumin administration may increase mortality.
Mortality was selected as the outcome measure in this systematic review for several reasons. In the context of critical illness, death or survival is a clinically relevant outcome that is of immediate importance to patients, and data on death are reported in nearly all studies. Furthermore, one might expect that mortality data would be less prone to measurement error or biased reporting than would data on pathophysiological outcomes. The use of a pathophysiological end‐point as a surrogate for an adverse outcome assumes a direct relationship between the two, an assumption that may sometimes be inappropriate. Finally, when trials collect data on a number of physiological end‐points, there is the potential for bias due to the selective publication of end‐points showing striking treatment effects. Because we obtained mortality data for all but four of the included trials, the likelihood of bias due to selective publication of trial outcomes is minimal.
Potential biases in the review process
Although publication bias is a potent threat to the validity of systematic reviews, it is unlikely to have had an important impact in this study. There was no evidence of funnel plot asymmetry on visual inspection. In some of the trials included in this review, allocation concealment was inadequate or was unclear. As a result, it is possible that more severely ill patients were preferentially allocated to the albumin treated group, which may account for the increased mortality risk in this group. Nevertheless, when the analyses were repeated, including only those trials in which allocation concealment involved a method that would be expected to reduce the risk of foreknowledge of treatment allocation, the point estimates were little different.
Authors' conclusions
Implications for practice.
For patients with hypovolaemia there is no evidence that albumin reduces mortality when compared with cheaper alternatives such as saline. There is no evidence that albumin reduces mortality in critically ill patients with burns and hypoalbuminaemia and there is a suggestion that albumin may increase the risk of death.
Implications for research.
The possibility that there may be highly selected populations of critically ill patients in which albumin may be indicated remains open to question. However, in view of the absence of evidence of a mortality benefit from albumin and the increased cost of albumin compared to alternatives such as saline, it would seem reasonable that albumin should only be used within the context of well concealed and adequately powered randomised controlled trials.
Feedback
Human albumin solution
Summary
1. It would be helpful to state that this review was published in the BMJ in 1998, to summarise the subsequent correspondence in print and on the BMJ website, and to note the respects (if any) in which this Cochrane review differs from the BMJ publication. 2. It would be valuable to summarise the report of the Committee for Safety of Medicines (CSM) on this review in the Comments and Criticisms section, with a rejoinder by the authors. 3. Because mortality was not the primary endpoint in any of the trials reviewed, it would be useful to note the primary outcomes of each trial, under 'characteristics of included trials'. 4. It would be helpful if the number of participants in each arm of each reviewed trial appeared under 'characteristics of included trials.'
Reply
1. We agree that it is important to direct the reader to other published versions of the review and will ensure that readers are alerted to the BMJ publication. We do not think it is appropriate to summarise the correspondence in response to this review, as to do so would run the risk of misrepresenting the views of the correspondents. At the time of first publication the Cochrane review was identical to the review published in the BMJ. However, the Cochrane review will be regularly updated to take account of new information from randomised controlled trials. 2. The Cochrane Database of Systematic Reviews is an international database and for this reason we believe that it would be inappropriate to give undue emphasis to the deliberations of the British Committee on Safety of Medicines (CSM). 3. Mortality was recorded in all but two of the trials included in our systematic review. However, we have no information on whether this was considered by the trialist to be the primary endpoint and would be interested to hear where the author of the comment found this information. How does the author of the comment define a primary endpoint? The concept of a primary endpoint implies a selection within the mind of the trialist of the most important endpoint. We would also ask whether it is appropriate that a process within the mind of a trialist should impact importantly on the estimation of the effect of albumin on mortality, and if so, what is the scientific basis for this. 4. We have included the number of participants in each arm of each reviewed trial in the section 'characteristics of included trials' as suggested.
Contributors
Author of comments: Dr Andrew Herxheimer Author of Responses: Ian Roberts
Human albumin solution
Summary
I gather that a further trial ‐ prompted by the review ‐ is now planned and possibly underway in Australasia. If so, I think this should be mentioned, preferably with a link to a record for the trial on the meta‐Register of Controlled Trials.
Reply
Details of this ongoing trial are now in the on‐going studies section.
Contributors
Author of comments: Ian Chalmers, UK Author of response: Ian Roberts, UK
Human albumin solution
Summary
Human albumin solution for resuscitation and volume expansion in critically ill patients
Summary of comment
1. In the hypovolaemia group, five randomised controlled trials were incorrectly included and should be deleted(1).
2. The Cochrane Albumin Review excluded or omitted extensive randomised controlled trials' evidence in the three categories of indications, namely, hypovolaemia, burns and hypoalbuminaemia(2) and this excluded and omitted evidence indicated that albumin may reduce rather than increase mortality.
(1) Horsey P Albumin and hypovolaemia ‐ is the Cochrane evidence to be trusted? Lancet 2002 359 70‐72
(2) Willkes MM and Navickis RJ Patient survival after human albumin administration: a meta‐analysis of randomised controlled trials.
Annals of Internal Medicine 2001 135 149‐164
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
We are grateful to Dr Horsey for his thoughtful comments on our systematic review of albumin administration in critically ill patients. The comments were first made as a commentary in The Lancet (2002;359:70‐72). Our response to these comments was published in the same issue (Lancet 2002:359:72‐3). We are pleased that this discussion will now be available to readers of the Cochrane Library.
Dr Horsey feels that some of the trials included under the category 'hypovolaemia' would be more appropriate in a different category. We accept that in some clinical situations hypovolaemia and hypoalbuminaemia co‐exist so that deciding which category would be most appropriate is a matter for judgement. Also, as Dr Horesy points out, the relationship between hypovolaemia and low blood pressure can be complicated, and the presence of the latter might not always signify the former. Nevertheless, our judgements about the categories were made without knowledge of the results of the trials and we are reluctant to change these post‐hoc.
We are grateful to Dr Horsey for drawing our attention to the meta‐analysis by Wilkes et al that was funded by the Plasma Protein Therapeutics Association. Because the inclusion criteria for the Cochrane Injuries Group Albumin Reviewer and the Wilkes reviews are different it does not follow that the two reviews should include the same trials.
We are pleased that our systematic review has stimulated so much interest from the intensive care community. However, it is a cause for concern that four years following the publication of our review, in which we concluded that there is no evidence that albumin administration reduced mortality in critically ill patients and a suggestion that it may increase mortality, that albumin continues to be used and promoted. Hopefully, the SAFE trial (www.safestudy.net) will provide the evidence needed to resolve this issue.
Contributors
Comment: Dr PJ Horsey
Reply: Professor Ian Roberts
What's new
| Date | Event | Description |
|---|---|---|
| 29 July 2011 | New citation required but conclusions have not changed | One new trial has been included, and the text has been amended accordingly. The conclusions remain the same. The editorial group is aware that a clinical trial by Prof. Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication point out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. One trial by Prof. Boldt is included in this review (Boldt 1993), but there were no deaths in the trial and so the data do not contribute to the results. The inclusion of the trial has no impact on the conclusions of the review. The authors of the review have changed. |
| 14 October 2008 | New search has been performed | Five new trials have been included in the review, and the analysis, results and discussion sections have been amended accordingly. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 9 June 2008 | Amended | Converted to new review format. |
| 20 August 2004 | New citation required and conclusions have changed | August 2004. One new trial has been included (SAFE 2004) with the analysis, results and discussion amended accordingly. |
| 6 February 2002 | New search has been performed | February 2002. An updated search for new trials was done in September 2002. One trial was found meeting the inclusion criteria but did not record data on mortality (Ernest 2001). Since the review was first published several new randomised control trials have been initiated. Details of these trials are given in the ongoing trials section. |
| 19 November 1999 | Feedback has been incorporated | Feedback added. Response to feedback added. |
Notes
Please note that this review was also published in the BMJ 1998;317:235‐40 (CIGAR 1998).
August 2011: The editorial group is aware that a clinical trial by Prof. Joachim Boldt has been found to have been fabricated (Boldt 2009). As the editors who revealed this fabrication point out (Reinhart 2011; Shafer 2011), this casts some doubt on the veracity of other studies by the same author. One trial by Prof. Boldt is included in this review (Boldt 1993), but there were no deaths in the trial and so the data do not contribute to the results. The inclusion of the trial has no impact on the conclusions of the review.
Acknowledgements
We thank the Intensive Care National Audit & Research Centre in London for help with identifying trials for this review and for their extensive handsearching activities. We are grateful to AJ Woittiez for providing unpublished trial data from the trial that was registered in the Medical Editors' Trial Amnesty. We thank Elizabeth Bryant, Information Officer at Centeon Limited, and Martin O'Fobve at Bio Products Limited, for searching their databases for albumin trials. We thank Anne Greenough for re‐examining individual patient records in order to provide data on mortality. We are also grateful to Peter Sandercock for his assistance in the editorial process. Thanks also to Carol Lefebvre of the UK Cochrane Centre, for the original search strategy and for conducting the original database searches. Subsequent searches were conducted by the Injuries Group Trials Search Co‐ordinator.
2011 update: The authors wish to thank Alain Li Wan Po and Leah Li for their contribution to the original version of the review and updates to 2011.
Appendices
Appendix 1. Search strategy
PubMed [www.ncbi.nlm.nih.gov/sites/entrez/] (searched 10 June 2011 limit: last 60 days) Cochrane Injuries Group Specialised Register (searched 31 May 2011) 1.fluid* and (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*) 2.rehydrate* and (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*) 3.volum* and (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*) 4.1 or 2 or 3 5.Albumin* 6.4 and 5 Cochrane Central Register of Controlled Trials 2011, Issue 2 (The Cochrane Library) #1MeSH descriptor Fluid Therapy explode all trees #2fluid* #3((volume or rehydrat*) near3 (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*)) #4(#1 OR #2 OR #3) #5MeSH descriptor Albumins explode all trees #6MeSH descriptor Serum Albumin explode all trees #7albumin* #8(#5 OR #6 OR #7) #9(#4 AND #8) #10(#9), from 2008 to 2011
MEDLINE (Ovid) 1948 to Week 3 May 2011 1.exp Fluid Therapy/ 2.fluid*.ti,ab. 3.((volume or rehydrat*) adj3 (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*)).ti,ab. 4.1 or 2 or 3 5.exp Albumins/ 6.exp Serum Albumin/ 7.albumin*.mp. 8.5 or 6 or 7 9.4 and 8 10.randomi?ed.ab,ti. 11.randomized controlled trial.pt. 12.controlled clinical trial.pt. 13.placebo.ab. 14.clinical trials as topic.sh. 15.randomly.ab. 16.trial.ti. 17.10 or 11 or 12 or 13 or 14 or 15 or 16 18.(animals not (humans and animals)).sh. 19.17 not 18 20.9 and 19 21.(2008* or 2009* or 2010* or 2011*).ed. 22.20 and 21 EMBASE (Ovid) 1980 to Week 21 2011 1.albumin*.ab,ti. 2.exp human serum albumin/ 3.exp recombinant human serum albumin/ 4.exp serum albumin/ 5.1 or 2 or 3 or 4 6.exp fluid resuscitation/ 7.((volume or rehydrat*) adj3 (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*)).ti,ab. 8.fluid*.ti,ab. 9.6 or 7 or 8 10.5 and 9 11.exp Randomized Controlled Trial/ 12.exp controlled clinical trial/ 13.randomi?ed.ab,ti. 14.placebo.ab. 15.*Clinical Trial/ 16.randomly.ab. 17.trial.ti. 18.11 or 12 or 13 or 14 or 15 or 16 or 17 19.exp animal/ not (exp human/ and exp animal/) 20.18 not 19 21.10 and 20 22.(2008* or 2009* or 2010* or 2011*).em. 23.21 and 22 CINAHL (EBSCO) 1982 to May 2011 S1(MH "Fluid Therapy+") S2Fluid* S3(volume or rehydrat*) and (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*) S4S1 or S2 or S3 S5albumin* S6(MH "Albumins+") S7(MH "Serum Albumin") S8S5 or S6 or S7 S9(MH "Clinical Trials+") S10 (MH "Randomized Controlled Trials") S11 S9 or S10 S12 S4 and S8 and S12 S13 S12 (2008‐11) ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) 1970 to May 2011, ISI Web of Science: Conference Proceedings Citation Index‐ Science (CPCI‐S) 1990 to May 2011 1.Topic=((volume or rehydrat*) same (solution* or replac* or therap* or substitut* or restorat* or resuscitat* or rehydrat*)) OR Topic=(Fluid*) 2.Topic=(albumin*) 3.1 and 2 4.Topic=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) 5.Topic=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial) OR Topic=(controlled clinical trial OR controlled trial OR clinical trial OR placebo) 6.4 or 5 7.Topic=(human*) 8.6 and 7 9.3 and 8
Data and analyses
Comparison 1. Supplemental albumin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 deaths | 38 | 10842 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.95, 1.16] |
| 1.1 hypovolaemia | 22 | 9880 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.92, 1.13] |
| 1.2 burns | 4 | 205 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.28, 6.72] |
| 1.3 hypoalbuminaemia | 12 | 757 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.84, 1.88] |
1.1. Analysis.
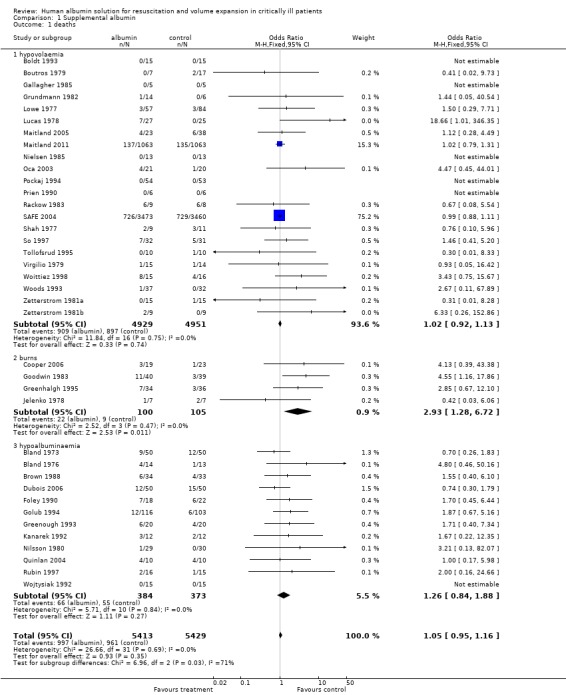
Comparison 1 Supplemental albumin, Outcome 1 deaths.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bland 1973.
| Methods | Randomised controlled trial. Therapy cards were randomised in pairs matched for weight. Method of allocation concealment not fully described. | |
| Participants | Newborn infants considered at high risk for developing respiratory distress. Those with a cord serum protein level less than 4.6g/100ml and at least one of the following; birthweight less than 2500g, gestational age less than 37 weeks, arterial pH less than 7.25. | |
| Interventions | 1) Intervention (n=50) received 8ml/kg 25% salt poor albumin. 2) Control group (n=50) received 8ml/kg 5% dextrose in water. | |
| Outcomes | Deaths reported within 28 days. | |
| Notes | ||
Bland 1976.
| Methods | Randomised controlled trial. Method of allocation concealment not fully described. | |
| Participants | Premature infants (less than 37 weeks gestation), with hypoproteinaemia (cord serum total protein of 4.6g/100ml or less). | |
| Interventions | 1) Intervention group (n=14) received 8ml/kg salt‐poor albumin. 2) Comparison group (n=13) received 8ml/kg glucose in water. | |
| Outcomes | Deaths reported. | |
| Notes | Length of follow‐up unspecified. | |
Boldt 1993.
| Methods | Randomised controlled trial. Method of allocation concealment not described in published report. Authors were contacted and confirmed the use of sealed opaque envelopes. | |
| Participants | Men undergoing elective aortocoronary bypass grafting, who had a pulmonary capillary wedge pressure of less than 5mmHg after induction of anaesthesia. | |
| Interventions | 1) Intervention (n=15): Albumin 5%. 2) Control (n=15): No additional volume. | |
| Outcomes | Deaths not reported. Authors were contacted and confirmed that there were no deaths in the albumin nor the control group. | |
| Notes | Follow‐up to 1 day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Boutros 1979.
| Methods | Randomised controlled trial. Method of allocation concealment not fully described. | |
| Participants | Participants were undergoing major operative procedures on the abdominal aorta. | |
| Interventions | 1) Intervention group (n=7) received albumin in 5% dextrose. 2) Control group (n=17) received 5% dextrose in lactated Ringers and 5% dextrose in 0.45 NaCl. Allocated fluids were used on admission to ICU, following surgery. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to 48 hours after the end of the operation. | |
Brown 1988.
| Methods | Randomised controlled trial. Patients entered into the study were assigned to one of two treatment groups by a table of random numbers. Method of allocation concealment not described. Author contacted ‐ no allocation concealment. | |
| Participants | All patients who required central TPN and had hypoalbuminaemia (serum albumin concentration below 3.0g/dl). Patients who were thermally injured, had nephrotic syndrome or required protein restriction were excluded. | |
| Interventions | 1) The intervention group received central TPN plus normal serum albumin (n=33). 2) The control group (n=34) received central TPN alone. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
Cooper 2006.
| Methods | Randomised controlled trial, not blinded. | |
| Participants | Eligible adults over 15 years old suffering from thermal injury not more than 12 hours before enrolment. | |
| Interventions | 1) Intervention (n=19) received 5% albumin plus Ringer's lactate. 2) Control (n=23) received Ringer's lactate. | |
| Outcomes | Multiple Organ Dysfunction Score (MODS) and deaths reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Patients were allocated to study groups with stratified randomization with a computer‐generated randomization list and sequentially numbered sealed, opaque envelopes. Randomization was stratified by center..." p. 81 |
Dubois 2006.
| Methods | Prospective, controlled, randomized, open, single centre study. | |
| Participants | All patients admitted to the hospital ICU with serum albumin less than or equal to 30g/L/. Exclusion criteria were expected length of stay <72 hrs., life expectancy <3 months or a do not resuscitate order, albumin administration in the preceding 24 hrs. or evidence of fluid overload. | |
| Interventions | 1) Intervention (n=50) received 300 mL of 20% albumin solution on day 1 and 200 mL on subsequent days when serum albumin concentration was lower than 31 g/L. 2) Control (n=50) received Ringer's Lactate. | |
| Outcomes | Effect of albumin on organ function as assessed by a delta Sequential Organ Failure Assessment score. Deaths not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Eligible patients were randomized in a 1:1 ratio using sealed envelopes. When a patient was assigned to one group, he or she remained in that group whether or not he or she received the planned treatment." |
Ernest 1999.
| Methods | Randomised controlled trial, not blinded. | |
| Participants | 18 septic, critically ill patients where a fluid infusion was clinically indicated. | |
| Interventions | 1) 5% albumin (n=9). 2) normal saline (n=9). | |
| Outcomes | Information on death not collected. | |
| Notes | Follow up for about an hour after infusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
Ernest 2001.
| Methods | Randomised controlled trial, not blinded. | |
| Participants | 40 postoperative cardiac surgical patients. | |
| Interventions | 1) 5% albumin (n=23). 2) normal saline (n=17). | |
| Outcomes | Information on death not collected. | |
| Notes | Follow up for 40 minutes after infusion. Trial conducted in 1992. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
Foley 1990.
| Methods | Patients were randomly assigned to either a treatment or non‐treatment group by medical record number. | |
| Participants | Hypoalbuminaemic (serum albumin <25g/L) critically ill patients. Potential subjects with Child's class C cirrhosis were excluded. | |
| Interventions | 1) The treatment group (n=18) received 25‐50g per day of 25% albumin in addition to full nutritional support with parenteral nutrition. Albumin administration was continued daily until serum albumin levels exceeded 25 g/L after which patients received additional albumin as needed to keep the albumin level at 25 g/L or higher. 2) The non treatment group (n=22) received no exogenous concentrated albumin. | |
| Outcomes | Deaths reported. | |
| Notes | Follow up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
Gallagher 1985.
| Methods | Randomised controlled trial. Method of allocation concealment not described. Author contacted ‐ allocation concealment by computerised system ‐ patient details were entered before treatment assignment was revealed. | |
| Participants | Patients after coronary artery bypass graft surgery. | |
| Interventions | 1) Treatment group received 5% albumin (n=5). 2) The control group received lactated Ringers (n=5). | |
| Outcomes | Deaths were not reported. Author contacted and confirmed that there were no deaths in either group. | |
| Notes | Follow‐up to 1 day. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Golub 1994.
| Methods | Computer randomisation ‐ method of allocation concealment not described. Author contacted and confirmed that allocation concealment was by the use of sealed opaque envelopes. | |
| Participants | Patients in the surgical intensive care unit of a community hospital with circulating albumin concentrations of <3.0g/dL. | |
| Interventions | 1) The treatment group (n=116) received 37.5g/day of albumin until the circulating albumin concentration increased to 3.0g/dL. 2) The control group received no supplemental albumin. Both groups received standard nutritional support. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Goodwin 1983.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | 79 thermally injured patients. No other inclusion criteria were reported. All of the participants were previously healthy young adults. | |
| Interventions | 1) The treatment group (n=40) group received 2.5% albumin in Ringer's lactate. 2) The control group (n=39) Ringers lactate. Allocated fluid was used throughout resuscitation. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
Greenhalgh 1995.
| Methods | Method of random allocation not described. Author contacted and confirmed the use of a randomisation scheme controlled by the pharmacy. | |
| Participants | Patients aged 18 years or younger with acute burns. | |
| Interventions | 1) High albumin group (n=34): Patients were supplemented with human albumin to maintain serum levels between 2.5 and 3.5g/dL. Albumin was supplied as a continuous drip of 25% human albumin at a rate of 3‐10mL/hour. Supplementation was discontinued if serum albumin levels remained >2.5 g/dL without supplementation or if intravenous support was discontinued. 2) Low albumin group (n=36): Patients were not given albumin supplementation unless levels dropped <1.5 g/dL. During burn shock, patients were allowed to receive albumin if they had levels <2.0 g/dL and were receiving >4 mL/Kg/% burn fluid resuscitation. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Greenough 1993.
| Methods | Randomised controlled trial. Allocation concealment by sealed opaque envelopes. | |
| Participants | Infants between 24 and 34 weeks gestational age, who were ventilator dependent, and had a serum albumin level of less than or equal to 30g/l. | |
| Interventions | 1)Intervention group (n=20) received 5ml/kg 20% salt‐poor human albumin. 2) Control group (n=20) received 5ml/kg of the infant's maintenance fluids. | |
| Outcomes | Deaths were not reported. Author contacted and provided data on deaths. | |
| Notes | Follow‐up to 24 hours after infusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Grundmann 1982.
| Methods | Randomised controlled trial. Method of allocation concealment not fully described. | |
| Participants | Participants were undergoing partial gastrectomy. The average age was 50 years (range 19‐84). | |
| Interventions | 1) Intervention group (n=14) group received human albumin. 2) Control group (n=6) details of crystalloid were not reported. Allocated fluid was continued for 4 days after operation. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
Jelenko 1978.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | Participants had burns covering more than 20% of body surface. | |
| Interventions | 1) Intervention group (n=7) received albumin in hypertonic saline (240MeQ Na+, 120 MeQ Chloride, 120 MeQ lactate, 3.5 torr/liter); 2) Control group (n=7) received hypertonic saline (240MeQ Na+, 120 MeQ Chloride, 120 MeQ lactate). Allocated fluid was used to the end of resuscitation. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to 5 days. | |
Kanarek 1992.
| Methods | Randomised controlled trial. Allocation concealment by sealed opaque envelopes. | |
| Participants | Sick premature newborn infants whose serum albumin was less than 3g/dL. | |
| Interventions | 1) Intervention group (n=12) received TPN with added albumin. 2) Control group (n=12) received no added albumin. | |
| Outcomes | Deaths reported. | |
| Notes | Length of follow‐up unspecified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Lowe 1977.
| Methods | Randomised controlled trial. The solutions were randomised by opening a sealed envelope containing a card denoting the appropriate fluid. | |
| Participants | Participants were undergoing emergency laparotomy for acute abdominal trauma. | |
| Interventions | 1) Intervention group (n=57) received 50g albumin in 200ml in Ringers lactate; 2) Crystalloid group (n=84) received Ringer's lactate. Allocated fluid was used throughout the pre‐ and intra‐operative period. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to 5 days post‐operatively. Data on the 30 participants with chest injuries who were left out of the Lowe 1977 report, but included in Moss 1981, have been included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Lucas 1978.
| Methods | Randomised controlled trial. Randomisation was based on the last digit of each patient's case number. | |
| Participants | 52 seriously injured patients. | |
| Interventions | 1) Intervention group (n=27) received supplemental salt‐poor albumin totaling a maximum of 150g during operation and 150g per day over the next five days. 2) Control group (n=25) received standard resuscitation regimen but no supplemental albumin. | |
| Outcomes | Deaths reported. | |
| Notes | In the final report of 94 randomised patients deaths were not reported. However, in this preliminary report of 52 injured patients deaths were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
Maitland 2005.
| Methods | Unblinded, randomised, controlled trial. | |
| Participants | Kenyan children older than 2 months with symptomatic sever malarial anaemia (haemoglobin less than 5g/dl). | |
| Interventions | 1) Intervention (n=23) received 4.5% albumin. 2) Intervention (n=20) received 0.9% saline. 3) Control (n=18) received only maintenance (rescue emergency intervention when required). | |
| Outcomes | Change in perfusion rates. Deaths not reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "On admission to the HDU, children were randomly assigned to PTM with one of the three treatments using sealed card system. The sealed cards were available 24h/day; either the admitting doctor or nurse assigned the therapy at admission based on laboratory results or, in an emergency, on clinical suspicion." p. 128. |
Maitland 2011.
| Methods | Multicentre open, randomised, controlled study | |
| Participants | Children (n=3170) aged between 60 days and twelve years of age, with severe febrile illness, randomly assigned within two strata (stratum A was children with severe febrile illness and impaired perfusion but without severe hypotension ‐ stratum B was children with severe hypotension). | |
| Interventions | Children were randomly allocated to rapid volume replacement over the course of 1 hour with either: 1) 20 ml 5% Human Albumin solution per kg body weight (n=1063) 2) 20 ml 0.9% Saline solution per kg body weight (n=1063) | |
| Outcomes | Mortality at 4 weeks after randomisation | |
| Notes | Children (n=1044) assigned to no treatment were not included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Trial numbers kept inside opaque, sealed envelopes. Opened in numerical order by clinician. |
McNulty 1993.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | 28 Patients following elective cardiopulmonary bypass. | |
| Interventions | 1) Intervention group (n=14) received 5% albumin. 2) Control group (n=14) received isotonic crystalloid. | |
| Outcomes | Deaths not reported. | |
| Notes | Length of follow‐up unspecified. | |
Myburgh 2007.
| Methods | A double blind, randomised, controlled SAFE 2004 trial. "Randomization was stratified by a diagnosis of trauma (defined as injury to the body caused by mechanical forces, excluding burns)." p. 875. | |
| Participants | 460 patients with traumatic brain injuries. | |
| Interventions | 1) Intervention (n=231) received 4% albumin. 2) Control (n=229) received normal saline. | |
| Outcomes | Mortality rate at 28 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Randomization was carried out centrally with the use of a minimization algorithm, and the service was accessed on the Internet through a secure Web site." SAFE 2004 p. 2248 |
Nielsen 1985.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | Patients admitted for reconstructive surgery of the abdominal aorta. Twenty six patients were randomised. | |
| Interventions | 1) Intervention (n=13): 80 g of albumin administered in units of 100 ml 20% human serum albumin on the day of the operation and 20 g albumin daily on the following three postoperative days. 2) Control group (n=13): no supplemental albumin. | |
| Outcomes | Deaths not reported. Author when contacted confirmed that there were no deaths in either group. | |
| Notes | Follow‐up 4 days. | |
Nilsson 1980.
| Methods | Randomised controlled trial. Allocation concealment by sealed opaque envelopes. | |
| Participants | Patients with colorectal cancer undergoing elective surgery with resection of the tumour and primary anastomosis. | |
| Interventions | 1) Intervention group (n=29) received 20‐25g per day of albumin (as 5% albumin or 20% albumin) for three days, starting on the day after the operation. 2) Control group (n=30) received no albumin. | |
| Outcomes | Deaths reported. | |
| Notes | Follow up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Oca 1999.
| Methods | Randomised controlled trial. Allocation concealment was by the use of sealed opaque sequentially numbered envelopes. Information obtained on contact with the author. | |
| Participants | 24 neonates being treated for hypotension. Hypotension was defined as an oscilometric mean arterial blood pressure <30 mmHg for at least 30 minutes. Exclusion criteria consisted of proven sepsis, life‐threatening congenital abnormalities, congenital hear disease, unresolved thoracic air leak, insulin‐requiring maternal diabetes mellitus or treatment with high‐frequency ventilation. | |
| Interventions | 1) 5% albumin (n=11). 2) normal saline (n=13). | |
| Outcomes | Mean arterial blood pressure. | |
| Notes | Follow‐up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Oca 2003.
| Methods | Randomised, unblinded, controlled trial. | |
| Participants | Newborn infants who were <24 hours old and were admitted to the ICU to receive one of two solutions for volume expansion. 41 infants were included in the trial. | |
| Interventions | 1) Intervention (n=21) received 4% albumin. 2) Control (n=20) received normal saline. | |
| Outcomes | Magnitude of change in mean arterial blood pressure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Randomization was accomplished by random number tables via sealed, serially numbered envelopes" p. 474 |
Pockaj 1994.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | Participants required fluid resuscitation as a result of vascular leak syndrome associated with Interleukin‐2 therapy for metastatic cancer. | |
| Interventions | 1) Intervention group (n=54) received 5% albumin n 154meq/L NaCl; 2) Control group (n=53) received 0.9% normal saline with 154Meq/L NaCl. | |
| Outcomes | Deaths reported. | |
| Notes | Length of follow‐up unspecified. | |
Prien 1990.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | Patients undergoing hemipancreato‐duodenectomy (Whipple's operation). | |
| Interventions | 1) Intervention group (n=6) received 20% human albumin to maintain central venous pressure at the pre‐operative level. 2) Control group (n=6) received Ringer's lactate. | |
| Outcomes | Deaths reported. | |
| Notes | Length of follow‐up unspecified. | |
Quinlan 2004.
| Methods | Prospective, randomised, placebo‐controlled study. | |
| Participants | Patients meeting the American European Consensus criteria for acute lung injury. | |
| Interventions | 1) Intervention (n=10) received albumin (25g of a 25% solution) every 8 hrs for a total of nine doses. 2) Control (n=10) received normal saline administered in identical fashion and volume. | |
| Outcomes | Deaths noted. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomized to receive 25g of human albumin every 8 hrs or a placebo (normal saline, administered in a double‐blind fashion and targeted to normalization of serum total protein." p. 755 ‐ 756. |
Rackow 1983.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | Participants were above 18 years of age, and had any one of the following pre‐determined indicators of shock: systolic blood pressure of 90mmHg or less, a cardiac index of less than 2.2L./min.m2, a serum arterial lactate greater than 18mg/dl and WP less than 15mmHg. | |
| Interventions | 1) Intervention group ( n= 9) received 5% albumin. 2) Control group (n=8) received 0.9% NaCl. Allocated fluid was given as needed until the end of resuscitation. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
Rubin 1997.
| Methods | Patients were randomised using "a closed envelope system in the pharmacy". | |
| Participants | Patients with hypoalbuminaemia (<2.5g/dL) who required TPN for at least six days, were not pregnant or under age, and did not have metastatic cancer, cirrhosis, or nephrotic syndrome. | |
| Interventions | 1) Intervention group (n=16) 25g on normal serum albumin. 2) Control group (n=15) 100 mL of normal saline placebo over a 1 hour period daily. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
SAFE 2004.
| Methods | Central randomisation accessed on the Internet through a secure website with use of a minimisation algorithm. Blinding was assured through the use of specially designed masking cartons and specially designed and manufactured administration sets. The authors report that the effectiveness of the blinding was confirmed in a formal study before the trial was initiated. | |
| Participants | Patients 18 years of age or older admitted to ICU who the treating clinician judged to require fluid administration to maintain or increase intravascular volume, with this decision supported by the fulfilment of at least one objective criterion. Patients admitted after cardiac surgery, after liver transplantation, or for the treatment of burns were excluded. | |
| Interventions | 1) 4% Albumin or
2) Normal saline The allocated study treatment was used for all fluid resuscitation in the ICU until death or discharge or until 28 days after randomisation. The treating clinicians determined the amount and rate of fluid administration according to each patient's clinical status and response to treatment. |
|
| Outcomes | Deaths reported. | |
| Notes | 28 day mortality. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | "Randomization was carried out centrally with the use of a minimization algorithm and the service was accessed on the Internet through a secure Web site." p. 2248. |
Shah 1977.
| Methods | Randomised controlled trial, allocation by sealed envelope. | |
| Participants | Patients with severe, multiple trauma and a systolic blood pressure of less than 90mmHg. All patients were adults and both sexes were included. | |
| Interventions | 1) Intervention group (n=9) 5% salt‐poor albumin in Ringers lactate. 2) Control group (n=11) Ringer's lactate for resuscitation, volume infused guided by physiological parameters. | |
| Outcomes | Death reported. | |
| Notes | Length of follow‐up not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Skillman 1975.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | Participants were undergoing elective abdominal reconstructive surgery. | |
| Interventions | 1) Intervention group received 25% concentrated salt‐poor albumin and 5% albumin in saline. 2) Control group received Ringer's lactate with 5% dextrose. Allocated fluid was given intra‐operatively. All patients received crystalloids only for pre‐loading before surgery. | |
| Outcomes | Deaths were not reported. Author could not be contacted. | |
| Notes | Follow‐up to 1 day. | |
So 1997.
| Methods | Randomised controlled trial. Method of allocation concealment not described. Author contacted and confirmed that allocation concealment was by computer randomisation. Details of patient were entered before group allocation revealed. | |
| Participants | Pre‐term infants weighing 540 to 1959g at birth, with gestational ages of 23 to 34 weeks, who developed hypotension within the first two hours of life. | |
| Interventions | 1) Intervention group (n=32) were given 5% albumin at a dose of 10mg/Kg by slow intravenous infusion over 30 minutes. 2) Control group (n=31) were given 0.9%NaCl at a dose of 10mg/kg by slow intravenous infusion over 30 minutes. | |
| Outcomes | Deaths reported. | |
| Notes | Follow up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Tollofsrud 1995.
| Methods | Randomised controlled trial. Allocation concealment by sealed opaque envelopes. | |
| Participants | Patients undergoing elective coronary artery bypass surgery. Patients with left ventricular ejection fraction less than 40%, valvular heart disease, ventricular aneurysm, arrhythmia, diabetes mellitus, renal failure or lung disease were excluded. | |
| Interventions | 1) Intervention group (n=10) received albumin 40mg/ml whenever fluid was required to stabilise haemodynamics. 2) Control group (n=10) received Ringers acetate. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to 48 hours. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Virgilio 1979.
| Methods | Randomised controlled trial. Method of allocation concealment not described. | |
| Participants | Participants were undergoing abdominal aortic surgery. | |
| Interventions | 1) Intervention group (n=15) received 5% albumin in Ringer's lactate 2) Control group (n=14) received Ringers lactate. Allocated fluid was used during operation for maintenance of pre‐defined physiological parameters, and the resuscitation was continued with the allocated fluid until the day following the operation. This was followed by 5% dextrose in half‐normal saline, with potassium chloride as needed. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up 2 and a half weeks. | |
Woittiez 1998.
| Methods | Randomised controlled trial. Allocation concealment by sealed opaque envelopes. | |
| Participants | Post‐operative intensive care patients. | |
| Interventions | 1) Intervention group (n=15) received 20% albumin. 2) Control group (n=16) received 0.9% NaCl. | |
| Outcomes | Unpublished data on deaths were provided by the trialist. | |
| Notes | Length of follow‐up unspecified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Wojtysiak 1992.
| Methods | Randomised controlled trial. Table of random numbers was used to generate the random sequence. Method of allocation concealment not described in published report. The author was contacted and indicated that there was inadequate allocation concealment. | |
| Participants | Patients between the ages of 18 and 75 years who were to receive parenteral nutrition and had a serum albumin concentration <3.0 g/dL. Patients were excluded if they had renal impairment, liver impairment or were haemodynamically unstable. | |
| Interventions | 1) Intervention group (n= 15) had 25g of human albumin added to each litre of parenteral nutrition. 2) Control group (n=15) had no supplemental albumin. | |
| Outcomes | Deaths not reported in published report. Author when contacted confirmed that there were no deaths in either group. | |
| Notes | Follow‐up to 5 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
Woods 1993.
| Methods | Randomised controlled trial. Patients with even hospital numbers were randomised to the group receiving albumin while those patients with odd hospital numbers were randomised to the group not receiving supplemental albumin. | |
| Participants | Patients undergoing surgery for abdominal aortic aneurysm, aortoiliac or aortofemoral bypass. | |
| Interventions | 1) Intervention group (n=37): albumin was replaced to a level greater than or equal to 3.5 g/dL. 2) Control group (n=32): received no supplemental albumin. | |
| Outcomes | Deaths reported. | |
| Notes | Follow‐up to discharge. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
Zetterstrom 1981a.
| Methods | The patients were randomly divided into two groups. The method of allocation concealment is not described. Author was contacted and confirmed the use of sealed opaque envelopes. | |
| Participants | Adult patients undergoing elective major abdominal surgery. | |
| Interventions | 1) Intervention group (n=15) 2) Control group (n=15) A similar schedule of fluid therapy and blood replacement was followed in the intervention and control groups. However, the albumin group received a 20% solution of human albumin intravenously according to the following scheme: At the end of the operation: 100ml. Postoperatively on the day of the operation: 200‐300 ml. First day after the operation: 200 ml. Following 3 days 100 ml each day. | |
| Outcomes | Deaths reported. | |
| Notes | Length of follow‐up unspecified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Zetterstrom 1981b.
| Methods | Patients were randomly divided into two groups. Method of allocation concealment was not described. Author was contacted and confirmed the use of sealed opaque envelopes. | |
| Participants | Patients undergoing elective reconstruction of the abdominal aorta. | |
| Interventions | 1) Intervention group (n=9) 2) Control group (n=9) Postoperatively, the aim of fluid administration was to keep the pulmonary arterial occlusion pressure equal to the preoperative level. When lower values were recorded, the patients in the control group were given a balanced electrolyte solution of the Ringer type, whereas the albumin patients received a 5% solution of human albumin. | |
| Outcomes | Deaths reported. | |
| Notes | Length of follow‐up unspecified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Artru 1989 | Intervention to control intracranial pressure not directed at fluid resuscitation. |
| Bellomo 2006 | Trial was subset of SAFE 2004 trial, thus offering no new data. |
| Brehme 1993 | Intervention directed at haemodilution, not at volume replacement. |
| Carlon 1979 | Randomised controlled trial of pre‐operative volume expansion during anaesthesia. |
| Fiorica 1991 | Not a randomised trial. The first 18 patients received standard maintenance crystalloid solution. The next 10 consecutive patients received 100g of a concentrated 25% albumin solution. |
| Goslinga 1992 | Intervention directed at haemodilution, not volume replacement. |
| Grundmann 1985 | This was a randomised controlled trial of 220 patients, 106 were given albumin when their colloid osmotic pressure (COP) fell below 24 cm water and 114 were given albumin when their COP fell below 29 cm water. Patients were not randomised to albumin or no albumin, nor were they randomised to supplemental albumin versus normal amounts of albumin, rather, this was a trial of different criteria for albumin supplementation. It is unlikely therefore that the two arms of the trial were comparable and hence the trial is excluded. |
| Grundmann 1986 | This was a randomised controlled trial to examine whether postoperative human albumin supply is justified in intensive care patients in the case that the colloid osmotic pressure decreases below 26 centimetres of water. The therapy group received human albumin only if the colloid osmotic pressure dropped below 26 cm water. The control group also received albumin but only for resuscitation of cardiac output and central venous pressure. The trial was excluded because both intervention and control groups received albumin. |
| Hauser 1980 | Cross‐over trial. |
| Lagonidis 1995 | Intervention was pre‐loading for coronary artery bypass surgery. |
| Lennihan 2000 | Participants had suffered subarachnoid hemorrhage and therefore did not meet the inclusion criteria. |
| Magder 1999 | Participants were stable patients following cardiopulmonary bypass surgery and therefore did not meet the inclusion criteria. |
| Maitland 2005a | Trial was subset of SAFE 2004 trial, thus offering no new data. |
| Martin 1999 | Intervention involved comparison of albumin with furosemide verus placebo therefore did not meet the inclusion criteria. |
| Metildi 1984 | Participants were admissions to an intensive care and a trauma unit with adult respiratory distress syndrome and established pulmonary failure. Included both trauma and non‐trauma patients and therefore did not meet the inclusion criteria for the review. |
| Steinberg 1989 | Cross‐over trial. |
| Tomita 1994 | Randomised controlled trial of normal versus high oncotic pressure following head injury. Patients were not randomised to albumin or no albumin. Albumin and furosemide were used together to achieve high oncotic pressure. |
Contributions of authors
For the original version of the review; Phil Alderson (UK Cochrane Centre) searched The Cochrane Central Register of Controlled Trials for relevant trials, extracted the data from the trials, and commented on the paper; Frances Bunn (London School of Hygiene & Tropical Medicine) searched the Cochrane Injuries Group Specialised Register for relevant trials, obtained copies of relevant papers, wrote to authors for further information on allocation concealment, and commented on the paper; Carol Lefebvre (UK Cochrane Centre) designed the original search strategies for The Cochrane Central Register of Controlled Trials and EMBASE, and searched these two databases for relevant trials; Leah Li (Institute of Child Health) did the funnel plot and the regression test of funnel plot asymmetry; Alain Li Wan Po (Centre for Evidence‐Based Pharmacotherapy, University of Nottingham) helped to write the paper; Ian Roberts (London School of Hygiene & Tropical Medicine) designed the protocol, extracted data from the trials, contacted authors for unpublished data, and wrote the paper; Gillian Schierhout proposed the study hypothesis, and conducted preliminary searches of MEDLINE, EMBASE, and BIDS Index to Scientific and Technical Proceedings. For the 2008 update, Mia Pearson and Ian Roberts screened the search results, extracted data from the trials and updated the analysis, results and discussion sections of the review. For the 2011 update, Karen Blackhall updated the search for studies. Ian Roberts extracted data from the additional trial (Maitland 2011) and updated the analysis with Karen Blackhall.
Sources of support
Internal sources
Institute of Child Health, University College London, UK.
External sources
NHS Research and Development, UK.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bland 1973 {published data only}
- Bland RD, Clarke TL, Harden LB, Meyer JL, Ries JP, Madden WA, et al. Early albumin infusion to infants at risk for respiratory distress. Archives of Disease in Childhood 1973;48:800‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bland 1976 {published data only}
- Bland RD, Clarke TL, Harden LB. Rapid infusion of sodium bicarbonate and albumin into high‐risk premature infants soon after birth: A controlled, prospective trial. American Journal of Obstetrics and Gynecology 1976;124:263‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 1993 {published data only}
- Boldt J, Knothe C, Zickmann B, Andres P, Dapper F, Hempelmann G. Influence of different intravascular volume therapies on platelet function in patients undergoing cardiopulmonary bypass. Anesthesia and Analgesia 1993;76:1185‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boutros 1979 {published data only}
- Boutros AR, Ruess R, Olson L, Hoyt JL, Baker WH. Comparison of hemodynamic, pulmonary, and renal effects of use of three types of fluids after major surgical procedures on the abdominal aorta. Critical Care Medicine 1979;7(1):9‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brown 1988 {published data only}
- Brown RO, Bradley JE, Bekemeyer WB, Luther RW. Effect of albumin supplementation during parenteral nutrition on hospital morbidity. Critical Care Medicine 1988;16:1177‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cooper 2006 {published data only}
- Cooper AB, Cohn SM, Zhang HBS, Hanna K, Stewart TE, Slutsky AS. Five percent albumin for adult burn shock resuscitation: Lack of effect on daily multiple organ dysfunction score. Transfusion 2006;46(1):80‐9. [DOI] [PubMed] [Google Scholar]
Dubois 2006 {published data only}
- Dubois M J, Orellana‐Jimenez C, Melot C, Backer D, Berre J, Leeman M, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study.[see comment]. Critical Care Medicine 2006;34(10):2536‐40. [DOI] [PubMed] [Google Scholar]
Ernest 1999 {published data only}
- Ernest D, Belzberg AS, Dodek PM. Distribution of normal saline and 5% albumin infusions in septic patients. Critical Care Medicine 1999;27(1):46‐50. [DOI] [PubMed] [Google Scholar]
Ernest 2001 {published data only}
- Ernest D, Belzberg AS, Dodek PM. Distribution of normal saline and 5% albumin infusions in cardiac surgical patients. Critical Care Medicine 2001;29(19):2299‐302. [DOI] [PubMed] [Google Scholar]
Foley 1990 {published data only}
- Foley EF, Borlase BC, Dzik WH, Bistrian BR, Benotti PN. Albumin supplementation in the critically ill: a prospective randomised trial. Archives of Surgery 1990;125:739‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gallagher 1985 {published data only}
- Gallagher JD, Moore RA, Kerns D, Jose AB, Botros SB, Flicker S, et al. Effects of colloid or crystalloid administration on pulmonary extravascular water in the postoperative period after coronary artery bypass grafting. Anesthesia and Analgesia 1985;64:753‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Golub 1994 {published data only}
- Golub R, Sorrento JJ Jr, Cantu R Jr, Nierman DM, Moideen A, Stein HD. Efficacy of albumin supplementation in the surgical intensive care unit: a prospective, randomized study. Critical Care Medicine 1994;22(4):613‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goodwin 1983 {published data only}
- Goodwin CW, Dorethy J, Lam V, Pruitt BA Jr. Randomized trial of efficacy of crystalloid and colloid resuscitation on hemodynamic response and lung water following thermal injury. Annals of Surgery 1983;197(5):520‐31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenhalgh 1995 {published data only}
- Greenhalgh DG, Housinger TA, Kagan RJ, et al. Maintenance of serum albumin levels in pediatric burn patients: a prospective, randomized trial. Journal of Trauma 1995;39(1):67‐73; discussion 73‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Greenough 1993 {published and unpublished data}
- Greenough A, Emery E, Hird MF, Gamsu HR. Randomised controlled trial of albumin infusion in ill preterm infants. European Journal of Pediatrics 1993;152:157‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grundmann 1982 {published data only}
- Grundmann R, Meyer H. The significance of colloid osmotic pressure measurement after crystalloid and colloid infusions. Intensive Care Medicine 1982;8:179‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jelenko 1978 {published data only}
- Jelenko C 3rd. Fluid therapy and the HALFD method. Journal of Trauma 1979;19 Suppl(11):866‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Jelenko C 3rd, Solenberger RI, Wheeler ML, Callaway BD. Shock and resuscitation. III. Accurate refractometric COP determinations in hypovolemia treated with HALFD. Journal of the American College of Emergency Physicians 1979;8(7):253‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jelenko C 3rd, Wheeler ML, Callaway BD, Divilio LT, Bucklen KR, Holdredge TD. Shock and resuscitation. II: volume repletion with minimal edema using the "HALFD" (Hypertonic Albuminated Fluid Demand) regimen. Journal of the American College of Emergency Physicians 1978;7(9):326‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jelenko C 3rd, Williams JB, Wheeler ML, et al. Studies in shock and resuscitation, I: use of a hypertonic, albumin‐containing, fluid demand regimen (HALFD) in resuscitation. Critical Care Medicine 1979;7(4):157‐67. [MEDLINE: ] [PubMed] [Google Scholar]
Kanarek 1992 {published data only}
- Kanarek KS, Williams PR, Blair C. Concurrent administration of albumin with total parenteral nutrition in sick newborn infants. Journal of Parenteral and Enteral Nutrition 1992;16:49‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lowe 1977 {published data only}
- Lowe RJ, Moss GS, Jilek J, Levine HD. Crystalloid versus colloid in the etiology of pulmonary failure after trauma ‐ a randomized trial in man. Critical Care Medicine 1979;7(3):107‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lowe RJ, Moss GS, Jilek J, Levine HD. Crystalloid vs colloid in the etiology of pulmonary failure after trauma: a randomized trial in man. Surgery 1977;1(6):676‐83. [MEDLINE: ] [PubMed] [Google Scholar]
- Moss GS, Lowe RJ, Jilek J, Levine HD. Colloid or crystalloid in the resuscitation of hemorrhagic shock: a controlled clinical trial. Surgery 1981;89(4):434‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Lucas 1978 {published data only}
- Clift DR, Lucas CE, Ledgerwood AM, Sardesai V, Kithier K, Grabow D. The effect of albumin resuscitation for schock on the immune response to tetanus toxoid. Journal of Surgical Research 1982;32:449‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Johnson SD, Lucas CE, Gerrick SJ, Ledgerwood AM, Higgins. Altered coagulation after albumin supplements for treatment of oligaemic shock. Archives of Surgery 1979;114:379‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lucas CE, Bouwman DL, Ledgerwood AM, Higgins R. Differential serum protein changes following supplemental albumin resuscitation for hypovolaemic shock. Journal of Trauma 1980;20(1):47‐51. [MEDLINE: ] [PubMed] [Google Scholar]
- Lucas CE, Weaver D, Higgins RF, Ledgerwood AM, Johnson SD, Bouwman DL. Effects of albumin versus non‐albumin resuscitation on plasma volume and renal excretory function. Journal of Trauma 1978;18:565‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Weaver DW, Ledgerwood AM, Lucas CE, Higgins R, Bouwman DL, Johnson SD. Pulmonary effects of albumin resuscitation for severe hypovolaemic shock. Archives of Surgery 1978;113:387‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maitland 2005 {published data only}
- Maitland K, Pamba A, English M, Peshu N, Levin M, Marsh K, et al. Pre‐transfusion management of children with severe malarial anaemia: a randomised controlled trial of intravascular volume expansion. British Journal of Haematology 2005;128(3):393‐400. [DOI] [PubMed] [Google Scholar]
Maitland 2011 {published data only}
- Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot‐Olupot P, Akech SO, et al. Mortality after fluid bolus in African children with severe infection. New England Journal of Medicine 2011:(Electronic). 0028‐4793 (Linking). [DOI] [PubMed] [Google Scholar]
McNulty 1993 {published data only}
- McNulty SE, Sharkey SJ, Asam B, Lee JH. Evaluation of STAT‐CRIT Hematocrit Determination in comparison to Coulter and Centrifuge: the effects of isotonic hemodilution and albumin administration. Anesthesia and Analgesia 1993;76:830‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Myburgh 2007 {published data only}
- Myburgh J, Cooper J, Finfer S, Bellomo R, Norton R, Bishop N, et al. Saline or Albumin for Fluid Resuscitation in Patients With Traumatic Brain Injury. New England Journal of Medicine 2007;357(9):874‐84. [DOI] [PubMed] [Google Scholar]
Nielsen 1985 {published data only}
- Nielsen OM, Engell HC. Effects of maintaining normal plasma colloid osmotic pressure on renal function and excretion of sodium and water after major surgery: a randomised study. Danish Medical Bulletin 1985;32:182‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Nielsen OM, Engell HC. Extracellular fluid volume and distribution in relation to changes in plasma colloid osmotic pressure after major surgery. A randomised study. Acta Chirurgica Scandinavica 1985;151:221‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Nielsen OM, Thunedborg P, Jorgensen K. Albumin administration and acute phase proteins in abdominal vascular surgery: a randomised study. Danish Medical Bulletin 1989;36:496‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Nilsson 1980 {published data only}
- Nilson E, Lamke O, Liljedahl SO, Elfstrom K. Is albumin therapy worthwhile in surgery for colorectal cancer?. Acta Chirurgica Scandinavica 1980;146:619‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Oca 1999 {published data only}
Oca 2003 {published data only}
- Oca MJ, Nelson M, Donn SM. Randomized trial of normal saline versus 5% albumin for the treatment of neonatal hypotension. Journal of Perinatology 2003;23(6):473‐6. [DOI] [PubMed] [Google Scholar]
Pockaj 1994 {published data only}
- Pockaj BA, Yang JC, Lotze MT, et al. A prospective randomized trial evaluating colloid versus crystalloid resuscitation in the treatment of the vascular leak syndrome associated with interleukin‐2 therapy. Journal of Immunotherapy 1994;15(1):22‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prien 1990 {published data only}
- Prien T, Backhaus N, Pelster F, Pircher W, Bunte H, Lawin P. Effect of intraoperative fluid administration and colloid osmotic pressure on the formation of intestinal edema during gastrointestinal surgery. Journal of Clinical Anesthesia 1990;2:317‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Quinlan 2004 {published data only}
- Quinlan GJ, Mumby S, Martin GS, Bernard GR, Gutteridge JM, Evans TW. Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Critical Care Medicine 2004;32(3):755‐9. [DOI] [PubMed] [Google Scholar]
Rackow 1983 {published data only}
- Rackow EC, Falk JL, Fein IA, et al. Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch, and saline solutions in patients with hypovolemic and septic shock. Critical Care Medicine 1983;11(11):839‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Rubin 1997 {published data only}
- Rubin H, Carlson S, DeMeo M, Ganger D, Craig R. Randomized, double‐blind study of intravenous human albumin in hypoalbuminemic patients receiving total parenteral nutrition. Critical Care Medicine 1997;25:249‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
SAFE 2004 {published data only}
- The SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. New England Journal of Medicine 2004;350:2247‐56. [DOI] [PubMed] [Google Scholar]
Shah 1977 {published data only}
- Shah DM, Broner BD, Dutton RE, Newell JC, Powers SR. Cardiac output and pulmonary wedge pressure. Use for evaluation of fluid replacement in trauma patients. Archives of Surgery 1977;112:1161‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Skillman 1975 {published data only}
- Skillman JJ, Restall DS, Salzman EW. Randomized trial of albumin vs. electrolyte solutions during abdominal aortic operations. Surgery 1975;78(3):291‐303. [MEDLINE: ] [PubMed] [Google Scholar]
So 1997 {published data only}
- So KW, Fok TF, Ng PC, Wong WW, Cheung KL. Randomised controlled trial of colloid or crystalloid in hypotensive preterm infants. Archives of Diseases of Childhood 1997;76:F43‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tollofsrud 1995 {published data only}
- Svennevig JL, Tollofsrud S, Kongsgaard U, Noddeland H, Mohr B, Ozer M, Mollnes TE. Complement activation during and after open‐heart surgery is only marginally affected by the choice of fluid for volume replacement. Perfusion 1996;11:326‐32. [DOI] [PubMed] [Google Scholar]
- Tollofsrud S, Svennevig JL, Breivik H, et al. Fluid balance and pulmonary functions during and after coronary artery bypass surgery: Ringer’s acetate compared with dextran, polygeline, or albumin. Acta Anaesthsiologica Scandinavica 1995;39:671‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Virgilio 1979 {published data only}
- Virgilio RW, Rice CL, Smith DE, et al. Crystalloid vs. colloid resuscitation: is one better? A randomized clinical study. Surgery 1979;85(2):129‐39. [MEDLINE: ] [PubMed] [Google Scholar]
Woittiez 1998 {unpublished data only}
- Timmer B, Hondebrink Y, Oude Nijhuis J, Woittiez AJJ. Restoration of colloid osmotic pressure in hypoalbuminaemic patients. Netherlands Journal of Medicine 1998;52:A42. [Google Scholar]
- Woittiez AJ. Restoration of colloid osmotic pressure in post operative intensive care patients. A randomised placebo controlled trial with albumin 20% and hydroxy‐ethyl starch. In: Medical Editors' Trial Amnesty. In: The Cochrane Controlled Trials Register In: The Cochrane Library, Issue 2, 1998. Oxford: Update Software.
Wojtysiak 1992 {published data only}
- Binkley JF, Brown RO, Wojtysiak SL, Powers DA, Kudsk KA. Effects of human albumin administration on visceral protein markers in patients receiving parenteral nutrition. Clinical Pharmacy 1993;12:377‐9. [PubMed] [Google Scholar]
- Wojtysiak SL, Brown RO, Roberson D, Powers DA, Kudsk KA. Effect of hypoalbuminaemia and parenteral nutrition on free water excretion and electrolyte‐free water resorption. Critical Care Medicine 1992;20:164‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woods 1993 {published data only}
- Woods MS, Kelley H. Oncotic pressure, albumin and ileus: the effect of albumin replacement on postoperative ileus. The American Surgeon 1993;59:758‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Zetterstrom 1981a {published data only}
- Zetterstrom H, Hedstrand U. Albumin treatment following major surgery. I. Effects on plasma oncotic pressure, renal function and peripheral oedema. Acta Anaesthsiologica Scandinavica 1981;25:125‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zetterstrom 1981b {published data only}
- Zetterstrom H. Albumin treatment following major surgery. II. Effects on postoperative lung function and circulatory adaptation. Acta Anaesthsiologica Scandinavica 1981;25:133‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Artru 1989 {published data only}
- Artru F, Philippon B, Flachaire E, et al. A controlled study of Dextran 40: effect on cerebral blood flow and metabolic rates in acute head trauma. Intensive Care Medicine 1989;15(8):499‐504. [DOI] [PubMed] [Google Scholar]
Bellomo 2006 {published data only}
- Bellomo R, Morimatsu H, French C, Cole L, Story D, Uchino S, et al. The effects of saline or albumin resuscitation on acid‐base status and serum electrolytes. Critical Care Medicine 2006;34(12):2891‐7. [DOI] [PubMed] [Google Scholar]
Brehme 1993 {published data only}
- Brehme S, Keysser G, Turowski A, Schmidt HH. Hemorheologic effects of hydroxyethyl starch 200/0.5, dextran 40, oxypolygelatine and full electrolyte solution over 48 hours. Zeitschrift fur die gesamte innere Medizinund ihre Grenzgebiete 1993;48(10):506‐10. [PubMed] [Google Scholar]
Carlon 1979 {published data only}
- Carlon GC, Kahn RC, Bertoni G, Campfield PB, Howland WS, Goldiner PL. Rapid volume expansion in patients with interstitial lung diseases. Anesthesia and Analgesia 1979;58:13‐8. [DOI] [PubMed] [Google Scholar]
Fiorica 1991 {published data only}
- Fiorica JV, Roberts WS, Hoffman MS, Barton DP, Finan MA, Lyman G, Cavanagh D. Concentrated albumin infusion as an aid to postoperative recovery after pelvic exenteration. Gynecologic Oncology 1991;43:265‐9. [DOI] [PubMed] [Google Scholar]
Goslinga 1992 {published data only}
- Goslinga H, Eijzenbach V, Heuvelmans JH, et al. Custom‐tailored hemodilution with albumin and crystalloids in acute ischemic stroke. Stroke 1992;23(2):181‐8. [DOI] [PubMed] [Google Scholar]
- Goslinga H, Eijzenbach V, Heuvelmans JH, Nes JC, Kurk RM, Bezemer PD. Individualized hemodilution in acute brain infarct using a 20% albumin solution and physiological saline solution. Ned Nederlands Tijdschrift voor Geneeskunde 1992;136(49):2422‐8. [PubMed] [Google Scholar]
- Goslinga H, Heuvelmans JH, Schmid Schonbein H. Hemodilution and rehydration in acute ischemic stroke. A preliminary report on the Amsterdam Stroke Study. Acta Medica Austriaca 1991;18 Suppl 1:41‐4. [PubMed] [Google Scholar]
Grundmann 1985 {published data only}
- Grundmann R, Heistermann S. Postoperative albumin infusion therapy based on colloid osmotic pressure. Archives of Surgery 1985;120:911‐5. [DOI] [PubMed] [Google Scholar]
Grundmann 1986 {published data only}
- Grundmann R, Lehndorff C. Indications for postoperative human albumin therapy in the intensive care unit: a prospective randomised study. Langenbecks Archiv fur Chirurgie 1986;367:235‐46. [DOI] [PubMed] [Google Scholar]
Hauser 1980 {published data only}
- Hauser CJ, Shoemaker WC, Turpin I, Goldberg SJ. Oxygen transport respons to colloids and crystalloids in critically ill surgical patients. Surgery 1980;150(6):811‐6. [PubMed] [Google Scholar]
Lagonidis 1995 {published data only}
- Lagonidis D, Magder S. Acute volume loading with colloid vs. crystalloid after coronary artery bypass. Intensive Care Medicine 1992;18 Suppl 2:225. [Google Scholar]
Lennihan 2000 {published data only}
- Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage. Stroke 2000;31(2):383‐91. [DOI] [PubMed] [Google Scholar]
Magder 1999 {published data only}
- Magder S, Lagonidis D. Effectiveness of albumin versus normal saline as a test of volume responsiveness in post‐cardiac surgery patients. Journal of Critical Care 1999;14(4):164‐71. [DOI] [PubMed] [Google Scholar]
Maitland 2005a {published data only}
- Maitland K, Pamba A, English M, Peshu N, Marsh K, Newton C, et al. Randomized trial of volume expansion with albumin or saline in children with severe malaria: Preliminary evidence of albumin benefit. Clinical Infectious Diseases 2005;40(4):538‐45. [DOI] [PubMed] [Google Scholar]
Martin 1999 {published data only}
- Martin GS, Mangialardi RJ, Wheeler AP, Berhard GR. Albumin and diuertics in acute lung injury/acute respiratory distress syndrome. American Journal of Respiratory Critical Care Medicine 1999;159(3):A376. [Google Scholar]
Metildi 1984 {published data only}
- Metildi LA, Shackford SR, Virgilio RW, Peters RM. Crystalloid versus colloid in fluid resuscitation of patients with severe pulmonary insufficiency. Surgical Gynecology and Obstetrics 1984;158(3):207‐12. [PubMed] [Google Scholar]
Steinberg 1989 {published data only}
- Steinberg B, Kochs E, Bause H, Schulte am Esch J. Effects of low molecular weight hydroxyethyl starch (HES 40) in comparison with Ringer solution on oxygen tension in skeletal muscles of infected patients. Anasthesie Intensivtherapie Notfallmedizin 1989;24(6):377‐81. [PubMed] [Google Scholar]
Tomita 1994 {published data only}
- Tomita H, Ito U, Tone O, Masaoka H, Tominaga B. High colloid oncotic therapy for contusional brain edema. Acta Neurochirurgica 1994;Suppl:547‐9. [DOI] [PubMed] [Google Scholar]
Additional references
ABPI 1998
- ABPI Compendium of data sheets and summaries of produce characteristics 1998‐99. Association of the British Pharmaceutical Industry, London 1998. [Google Scholar]
Boldt 2009
- Boldt J, Suttner S, Brosch C, Lehmann A, Roehm K, Mengitsu A. Cardiopulminary bypass priming using a high dose of a balanced hydroxyethyl starch versus an albumin‐based priming strategy. Anesthesia & Analgesia 2009;109:1752‐62. [DOI] [PubMed] [Google Scholar]
Goldwasser 1997
- Goldwasser P, Feldman J. Association of serum albumin and mortality risk. Journal of Clinical Epidemiology 1997;50(6):693‐703. [DOI] [PubMed] [Google Scholar]
McClelland 1990
- McClelland DB. Human albumin solutions. BMJ 1990;300:35‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinhart 2011
- Reinhart K, Takala J. Hydroxyethyl Starches: What Do We Still Know?. Anesthesia and Analgesia 2011;112(3):507‐11. [DOI] [PubMed] [Google Scholar]
Shafer 2011
- Shafer SL. Shadow of Doubt. Anesthesia and Analgesia 2011;112(3):498‐500. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
CIGAR 1998
- The Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review of randomised controlled trials. BMJ 1998;317:235‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]


