Abstract
Subcortical brain structures are integral to motion, consciousness, emotions and learning. We identified common genetic variation related to the volumes of the nucleus accumbens, amygdala, brainstem, caudate nucleus, globus pallidus, putamen and thalamus, using genome-wide association analyses in almost 40,000 individuals from CHARGE, ENIGMA and UK Biobank. We show that variability in subcortical volumes is heritable, and identify 48 significantly associated loci (40 novel at the time of analysis). Annotation of these loci by utilizing gene expression, methylation and neuropathological data identified 199 genes putatively implicated in neurodevelopment, synaptic signaling, axonal transport, apoptosis, inflammation/infection and susceptibility to neurological disorders. This set of genes is significantly enriched for Drosophila orthologs associated with neurodevelopmental phenotypes, suggesting evolutionarily conserved mechanisms. Our findings uncover novel biology and potential drug targets underlying brain development and disease.
Subcortical brain structures are essential for the control of autonomic and sensorimotor functions1,2, the modulation of processes involved in learning, memory and decision-making3,4, and in emotional reactivity5,6 and consciousness7. They often act through networks influencing input to and output from the cerebral cortex8,9. The pathology of many cognitive, psychiatric and movement disorders is restricted to, begins in or predominantly involves subcortical brain structures and related circuitries10. For instance, tau pathology has shown to manifest itself early in the brainstem of individuals with Alzheimer’s disease before spreading to cortical areas through efferent networks11. Similarly, the formation of Lewy bodies and Lewy neurites in Parkinson’s disease appears early in the lower brainstem (and olfactory structures) before affecting the substantia nigra12.
Recent investigations have identified genetic loci influencing the volumes of the putamen, caudate and pallidum, which pointed to genes controlling neurodevelopment and learning, apoptosis and the transport of metals13,14. However, a larger study combining these samples and including individuals of a broad age range across diverse studies would enable increased power to identify additional novel genetic variants contributing to variability in subcortical structures, and further improve our understanding of brain development and disease.
We sought to identify novel genetic variants influencing the volumes of seven subcortical structures (the nucleus accumbens, amygdala, caudate nucleus, putamen, globus pallidus, thalamus and brainstem (including the mesencephalon, pons and medulla oblongata)), through genome-wide association (GWA) analyses in almost 40,000 individuals from 53 study samples (Supplementary Tables 1-3) from the Cohorts of Heart and Aging Research in Genomic Epidemiology (CHARGE) consortium, the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) consortium and UK Biobank.
Results
Heritability.
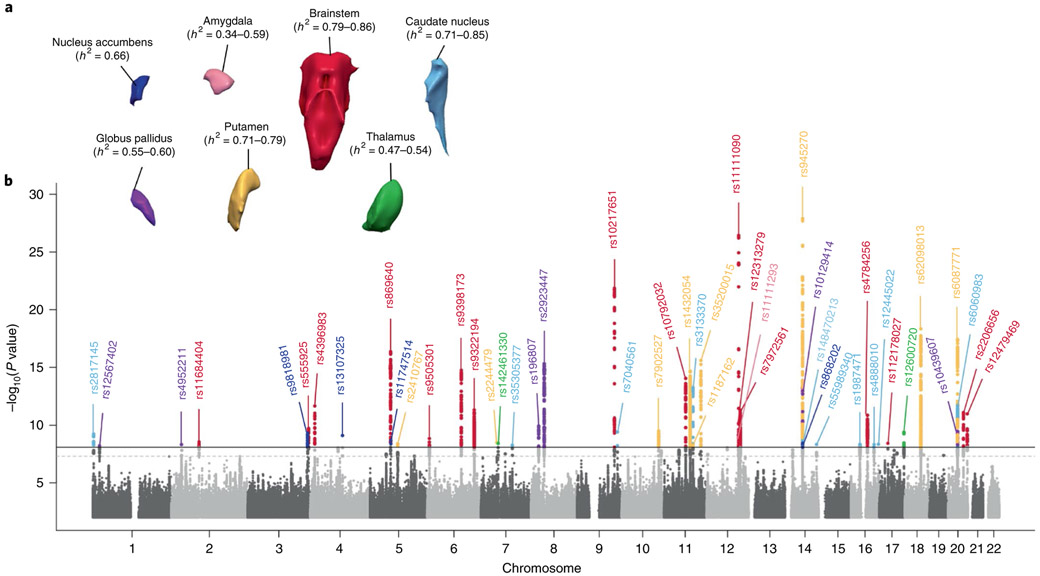
To examine the extent to which genetic variation accounts for variation in subcortical brain volumes, we estimated their heritability in two family-based cohorts: the Framingham Heart Study (FHS) and the Austrian Stroke Prevention Study Family Study (ASPS-Fam). Our analyses were in line with previous studies conducted in twins15, suggesting that variability in subcortical volumes is moderately to highly heritable. The structures with the highest heritability in the FHS and ASPS-Fam were the brainstem (ranging from 79–86%), caudate nucleus (71–85%), putamen (71–79%) and nucleus accumbens (66%), followed by the globus pallidus (55–60%), thalamus (47–54%) and amygdala (34–59%) (Fig. 1 and Supplementary Table 4). We additionally estimated single-nucleotide polymorphism (SNP)-based heritability (h2g) using genome-wide complex trait analysis (GCTA) in the Rotterdam Study, and linkage disequilibrium score regression (LDSC) in the full European sample. As expected, SNP-based heritability estimates were somewhat lower, ranging from 17% for the amygdala to 47% for the thalamus using GCTA, and ranging from 9% for the amygdala to 33% for the brainstem using LDSC. These values are consistent with heritability estimates reported by UK Biobank14.
Fig. 1 ∣. Heritability and Manhattan plot of genetic variants associated with subcortical brain volumes in the European sample.
a, Family-based heritability (h2) estimates were performed with SOLAR in the FHS (n = 895) and ASPS-Fam (n = 370). b. Combined Manhattan plot highlighting the most significant SNPs across all subcortical structures (nucleus accumbens, n = 32,562; amygdala, n = 34,431; brainstem, n = 28,809; caudate, n = 37,741; pallidum, n = 34,413; putamen, n = 37,571; thalamus, n = 34,464). Variants are colored differently for each structure as in a. Linear regression models were adjusted for sex, age, age2, total intracranial volume (CHARGE and ENIGMA) or total brain volume (UK Biobank), and population stratification. The solid horizontal line denotes genome-wide significance, as set in this study after additional Bonferroni correction for six independent traits (P < 5 × 10−8/6 = 8.3 × 10−9 for two-sided tests). The dashed horizontal line denotes the classic genome-wide threshold of P < 5 × 10−8. Individual Manhattan plots are provided in the Supplementary Note.
Genome-wide associations.
We undertook a GWA analysis on the magnetic resonance imaging (MRI)-derived volumes of subcortical structures using the 1000 Genomes Project16 reference panel (phase 1; version 3) for imputation of missing variants in CHARGE and ENIGMA. UK Biobank performed imputation of variants using the Haplotype Reference Consortium (HRC) reference panel17 (see details on image acquisition and genotyping in Supplementary Tables 5 and 6, respectively). Our sample comprised up to n = 37,741 individuals of European ancestry from 48 study samples across CHARGE, ENIGMA and UK Biobank. Additionally, we included three samples for generalization in African Americans (up to n = 769) and two for generalization in Asians (n = 341). Details on the population characteristics, definition of the outcome and genotyping are provided in Supplementary Tables 2-5. Each study examined the association between genetic variants with a minor allele frequency (MAF) of ≥1% and the volumes of subcortical structures (average volume for bilateral structures) using additive genetic models adjusted for sex, age and total intracranial volume (or total brain volume in UK Biobank), as well as age2, population structure, psychiatric diagnosis (ENIGMA cohorts), and study site when applicable. After quality control, we conducted meta-analyses per ethnicity combining all samples using sample-size-weighted fixed-effects methods in METAL18. An analysis of genetic correlations (rg) showed consistency of associations across the CHARGE and ENIGMA consortia (combined) and UK Biobank (rg > 0.94; P < 1.46 × 10−15), showing the similar genetic architecture of subcortical volumes in these two datasets.
We identified 48 independent genome-wide significant SNPs across all seven subcortical structures, 40 of which were novel at the time of analysis (Table 1). Among these, 26 SNPs were located within genes (one missense; 25 intronic) and 22 were located in intergenic regions. Most of the inflation observed in the quantile plots (Supplementary Fig. 1) was due to polygenic effects. We carried forward these 48 SNPs for in silico generalization in African American and Asian samples, and performed a combined meta-analysis of all samples (Supplementary Table 7). Of the 46 SNPs present in the generalization samples, the direction of association was the same for 13 across all ethnicities and for an additional six SNPs in either the African American or the Asian samples. In the combined meta-analysis, 43 of the 48 associations remained significant, and for 21 SNPs, the strength of association increased when all samples were combined. Although we did not find significant associations for most SNPs at the generalization sample level (probably due to their limited sample size), the sign test for the direction of effect suggested that a large proportion of the SNPs associated with subcortical volumes in the European sample were also associated in the African American and Asian samples at the polygenic level (P < 1 × 10−4; Supplementary Table 8).
Table 1 ∣.
Genome-wide association results for subcortical brain volumes in Europeans from the CHARGE and ENIGMA consortia and UK Biobank
| SNP | Chromosome | Position | Function | A1/A2 | A1 frequency | Weight (SNP n) | Z score | P valuea | Directionb | I2c |
|---|---|---|---|---|---|---|---|---|---|---|
| Nucleus accumbens (n = 32,562) | ||||||||||
| rs9818981d | 3 | 190,602,087 | Intergenic | A/G | 0.09 | 32,282 | −6.23 | 4.70 × 10−10 | −−− | 63.2 |
| rs13107325 | 4 | 103,188,709 | Missense | T/C | 0.06 | 32,283 | 6.15 | 7.74 × 10−10 | +++ | 76.2 |
| rs11747514d | 5 | 65,839,259 | Intronic | T/G | 0.22 | 32,562 | −5.99 | 2.11 × 10−9 | −−− | 0 |
| rs868202d | 14 | 56,195,762 | Intergenic | T/C | 0.56 | 32,562 | 5.90 | 3.55 × 10−9 | +++ | 0 |
| Amygdala (n = 34,431) | ||||||||||
| rs11111293d | 12 | 102,921,296 | Intergenic | T/C | 0.78 | 34,313 | 6.25 | 4.16 × 10−10 | +++ | 0 |
| Brainstem (n = 28,809) | ||||||||||
| rs11111090 | 12 | 102,326,461 | Intergenic | A/C | 0.52 | 28,809 | 10.79 | 3.70 × 10−27 | +++ | 0 |
| rs10217651d | 9 | 118,923,652 | Intronic | A/G | 0.39 | 28,809 | 9.78 | 1.40 × 10−22 | +++ | 0 |
| rs869640d | 5 | 65,015,128 | Intronic | A/C | 0.72 | 28,809 | −8.40 | 4.36 × 10−17 | −−− | 9.5 |
| rs9398173d | 6 | 109,000,316 | Intronic | T/C | 0.33 | 28,809 | −7.95 | 1.80 × 10−15 | −−− | 19.0 |
| rs10792032d | 11 | 68,984,602 | Intergenic | A/G | 0.49 | 28,648 | 7.75 | 9.08 × 10−15 | +++ | 39.4 |
| rs4396983d | 4 | 15,132,604 | Intergenic | A/G | 0.44 | 28,809 | −7.02 | 2.27 × 10−12 | −−− | 73.6 |
| rs9322194d | 6 | 149,920,249 | Intronic | T/C | 0.34 | 28,156 | 6.91 | 4.94 × 10−12 | +++ | 0 |
| rs7972561d | 12 | 107,139,983 | Intronic | A/T | 0.33 | 28,809 | 6.90 | 5.05 × 10−12 | +++ | 0 |
| rs2206656d | 20 | 49,130,119 | Intronic | C/G | 0.61 | 28,809 | 6.83 | 8.26 × 10−12 | +++ | 0 |
| rs12479469d | 20 | 61,145,196 | Intergenic | A/G | 0.33 | 25,822 | −6.80 | 1.08 × 10−11 | −−− | 65.6 |
| rs4784256d | 16 | 52,814,559 | Intergenic | A/G | 0.40 | 28,809 | 6.76 | 1.41 × 10−11 | +++ | 0 |
| rs555925d | 3 | 193,544,359 | intergenic | T/G | 0.41 | 27,934 | 6.37 | 1.88 × 10−10 | +++ | 62.9 |
| rs12313279d | 12 | 102,846,504 | Intronic | A/G | 0.29 | 28,809 | 6.21 | 5.39 × 10−10 | +++ | 24.9 |
| rs9505301d | 6 | 7,887,131 | Intronic | A/G | 0.89 | 28,691 | −6.05 | 1.41 × 10−9 | −−− | 43.2 |
| rs11684404d | 2 | 88,924,622 | Intronic | T/C | 0.66 | 28,809 | −5.95 | 2.73 × 10−9 | −−− | 0 |
| rs112178027d | 17 | 27,564,013 | Intergenic | T/C | 0.17 | 28,809 | −5.90 | 3.67 × 10−9 | −−− | 0 |
| Caudate nucleus (n = 37,741) | ||||||||||
| rs3133370 | 11 | 92,026,446 | Intergenic | T/C | 0.67 | 37,741 | 7.52 | 5.59 × 10−14 | +++ | 44.9 |
| rs6060983d | 20 | 30,420,924 | Intronic | T/C | 0.70 | 37,741 | 7.04 | 1.95 × 10−12 | +++ | 0 |
| rs7040561d | 9 | 128,528,978 | Intronic | A/T | 0.85 | 34,049 | −6.26 | 3.84 × 10−10 | −−− | 0 |
| rs2817145d | 1 | 3,133,422 | Intronic | A/T | 0.19 | 35,598 | 6.20 | 5.71 × 10−10 | +++ | 65.3 |
| rs148470213d | 14 | 56,193,700 | Intergenic | T/C | 0.54 | 29,429 | 6.18 | 6.48 × 10−10 | ++? | 0 |
| rs1987471d | 16 | 28,825,866 | Intergenic | T/G | 0.63 | 37,741 | 5.87 | 4.40 × 10−9 | +++ | 0 |
| rs12445022d | 16 | 87,575,332 | Intergenic | A/G | 0.33 | 37,741 | 5.87 | 4.45 × 10−9 | +++ | 0 |
| rs55989340d | 14 | 100,635,222 | Intergenic | A/G | 0.74 | 37,741 | −5.86 | 4.62 × 10−9 | −−− | 52.0 |
| rs4888010d | 16 | 73,895,046 | Intergenic | A/G | 0.47 | 37,741 | 5.86 | 4.67 × 10−9 | +++ | 74.9 |
| rs35305377d | 7 | 99,938,955 | Intronic | A/G | 0.55 | 33,429 | −5.84 | 5.36 × 10−9 | −−− | 47.8 |
| Globus pallidus (n = 34,413) | ||||||||||
| rs2923447 | 8 | 42,439,848 | Intergenic | T/G | 0.59 | 34,413 | 8.11 | 4.88 × 10−16 | +++ | 34.0 |
| rs10129414d | 14 | 56,193,272 | Intergenic | A/G | 0.44 | 34,413 | −7.53 | 5.11 × 10−14 | −−− | 0 |
| rs196807d | 8 | 24,682,649 | Intergenic | A/G | 0.18 | 34,295 | 6.44 | 1.17 × 10−10 | +++ | 21.1 |
| rs10439607d | 20 | 30,258,541 | Intronic | A/G | 0.30 | 34,413 | −6.28 | 3.35 × 10−10 | −−− | 0 |
| rs4952211d | 2 | 32,611,512 | Intronic | T/C | 0.43 | 34,252 | −5.86 | 4.72 × 10−9 | −−− | 61.9 |
| rs12567402d | 1 | 21,870,213 | Intronic | T/C | 0.33 | 34,214 | 5.81 | 6.17 × 10−9 | +++ | 0 |
| Putamen (n = 37,571) | ||||||||||
| rs945270 | 14 | 56,200,473 | Intergenic | C/G | 0.58 | 37,571 | 15.03 | 5.02 × 10−51 | +++ | 57.3 |
| rs62098013 | 18 | 50,863,861 | Intronic | A/G | 0.38 | 37,571 | 8.92 | 4.59 × 10−19 | +++ | 33.9 |
| rs6087771 | 20 | 30,306,724 | Intronic | T/C | 0.71 | 36,291 | 8.69 | 3.75 × 10−18 | +++ | 7.5 |
| rs35200015d | 11 | 117,383,215 | Intronic | A/G | 0.19 | 37,571 | −8.19 | 2.51 × 10−16 | −−− | 0 |
| rs1432054 | 11 | 83,260,225 | Intronic | A/G | 0.64 | 37,571 | −7.94 | 2.10 × 10−15 | −−− | 0 |
| rs7902527d | 10 | 118,715,399 | Intronic | A/G | 0.24 | 37,108 | 6.29 | 3.13 × 10−10 | +++ | 0 |
| rs2244479d | 7 | 50,738,987 | Intronic | T/C | 0.65 | 36,291 | −5.92 | 3.17 × 10−9 | −−− | 32.1 |
| rs2410767d | 5 | 87,705,268 | Intronic | C/G | 0.78 | 37,571 | 5.88 | 3.99 × 10−9 | +++ | 0 |
| rs1187162d | 11 | 92,011,126 | Intergenic | T/C | 0.42 | 37,571 | 5.84 | 5.14 × 10−9 | +++ | 0 |
| Thalamus (n = 34,464) | ||||||||||
| rs12600720d | 17 | 78,448,640 | Intronic | C/G | 0.69 | 33,023 | 6.25 | 4.06 × 10−10 | +++ | 0 |
| rs142461330d | 7 | 55,012,097 | Intergenic | T/C | 0.92 | 34,185 | −5.90 | 3.69 × 10−9 | −−− | 0 |
Linear regression models are adjusted for sex, age, age2, total intracranial volume (CHARGE and ENIGMA) or total brain volume (UK Biobank), and population stratification.
P values are two tailed. Significance was set at P < 8.3 × 10−9 after additional Bonferroni correction for six independent traits (5 × 10−8/6).
Direction of association, ordered as CHARGE, ENIGMA, and UK Biobank.
Heterogeneity as estimated proportion of total variance.
Novel SNPs. A1, coded allele; A2, non-coded allele.
To functionally annotate the 48 SNPs identified in the European sample, we used Locus Zoom19, investigated expression quantitative trait loci (eQTLs) and methylation QTLs (meQTLs) in postmortem brains from the Religious Order Study and the Rush Memory and Aging Project (ROSMAP), and queried cis- and trans-eQTL datasets in brain and non-brain tissues for the top 48 SNPs or their proxies (linkage disequilibrium r2 > 0.8), using the European population reference (Supplementary Tables 9-12). Lead variants and their proxies were annotated to genes based on the combination of physical proximity, eQTLs and meQTLs, which in some instances assigned more than one gene to a single SNP. Most of our index SNPs had genes assigned based on more than one functional source. This strategy allowed us to identify 199 putatively associated genes (Supplementary Table 13). More details are provided in the Supplementary Note.
Associations with cognition and neuropathology.
Although individual SNPs were not related to neuropathological traits or cognitive function in ROSMAP (Supplementary Table 14), we found that the cortical messenger RNA expression of 12 of our putatively associated genes was associated with neuropathological alterations typically observed in Alzheimer’s disease (Supplementary Table 15). These included β-amyloid load/the presence of neuritic plaques (APOBR, FAM65C, KTN1, NUPR1 and OPA1) and tau density/neurofibrillary tangles (FAM65C, MEPCE, OPA1 and STAT1). Many of these genes—together with ANKRD42, BCL2L1, RAET1G, SGTB and ZCCHC14—were also related to cognitive function.
Phenotypic and genetic correlations.
We explored both phenotypic (Supplementary Table 16) and genetic (Supplementary Table 17) correlations among subcortical volumes. We also investigated genetic correlations of subcortical volumes with traits previously examined in the CHARGE and ENIGMA consortia, including MRI-defined brain volumes20–22, stroke subtypes23, anthropometric traits24, general cognitive function25, Alzheimer’s disease26, Parkinson’s disease27, bipolar disorder and schizophrenia28, and attention deficit/hyperactivity disorder (ADHD)29. We observed strong phenotypic and genetic overlap among most subcortical structures using LDSC methods, consistent with our finding that many of the loci identified have pleiotropic effects on the volumes of several subcortical structures.
As expected, we found strong genetic correlations among the nuclei composing the striatum—particularly between the nucleus accumbens and the caudate nucleus (P = 9.83 × 10−19) and between the nucleus accumbens and the putamen (P = 1.02 × 10−17). The genetic architecture of thalamic volume highly overlapped with that of most subcortical volumes, except for the caudate nucleus. In contrast, there were no significant genetic correlations for the volume of the brainstem with that of most structures, with the exception of very strong correlations with volumes of the thalamus (P = 1.56 × 10−22) and the globus pallidus (P = 1.52 × 10−21). Individual-level analyses using GCTA in the Rotterdam Study (n = 3,486) showed similar correlations despite the smaller sample.
We also observed strong genetic correlations for hippocampal volumes with amygdalar and thalamic volumes. Height correlated with thalamic volumes, and the volume of the brainstem was inversely correlated with ADHD. Notably, caudate nucleus volumes correlated with white matter hyperintensity burden.
Cross-species analysis.
To investigate for potential evolutionarily conserved requirements of our gene set in neurodevelopment, neuronal maintenance or both, we examined the available genetic and phenotypic data from the fruit fly Drosophila melanogaster. Importantly, compared with mammalian models, the fly genome has been more comprehensively interrogated for roles in the nervous system. We found that a large proportion of candidate genes for human subcortical volumes are strongly conserved in the Drosophila genome (59%), and many of these genes appear to have conserved nervous system requirements (Supplementary Table 18). To examine whether this degree of conservation was greater than that expected by chance, we leveraged systematic, standardized phenotype data based on FlyBase annotations using controlled vocabulary terms. Indeed, 22% of the conserved fly homologs are documented to cause ‘neuroanatomy-defective’ phenotypes in flies, representing a significant (P = 7.3 × 10−4), nearly twofold enrichment compared with 12.9% representing all Drosophila genes associated with such phenotypes (Supplementary Table 19).
Partitioning heritability.
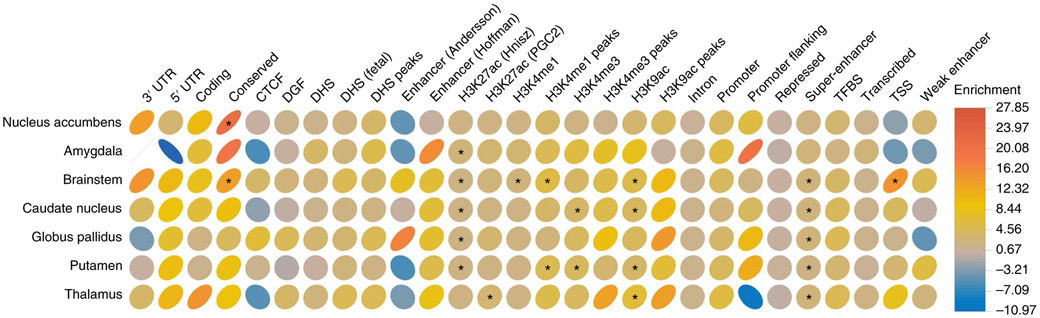
We further investigated enrichment for functional categories of the genome using stratified LDSC methods30 (Fig. 2). Super-enhancers were significantly enriched in most subcortical structures, with 17% of SNPs explaining 43% of SNP heritability in the brainstem, 39% in the caudate, 44% in the pallidum, 37% in the putamen and 38% in the thalamus. Similarly, strong enrichment was observed for regular enhancers (H3K27ac annotations from Hnisz et al.31) in several subcortical structures, explaining over 60% of their SNP heritability. Conserved regions were enriched in the nucleus accumbens and the brainstem, with 2.6% of SNPs explaining 53 and 35% of their SNP heritability, respectively. Finally, only the brainstem showed enrichment for transcription start sites, with 1.8% of SNPs explaining 26% of this structure SNP heritability. The full results are presented in Supplementary Table 20.
Fig. 2 ∣. Partitioning heritability by functional annotation categories.
Analyses performed in the European sample (nucleus accumbens, n = 32,562; amygdala, n = 34,431; brainstem, n = 28,809; caudate, n = 37,741; pallidum, n = 34,413; putamen, n = 37,571; thalamus, n = 34,464). Plotted ellipses represent enrichment (proportion of h2g explained/proportion of SNPs in a given functional category) for subcortical structures (y axis) across 28 functional categories (x axis). The color bar indicates the magnitude and direction of enrichment. Starred pairs denote significant over-representation after Bonferroni correction for 168 tests (28 annotation categories and six independent traits; P < 3 × 10−4). CTCF, CCCTC-binding factor; DGF, digital genomic footprint; DHS, DNase I hypersensitivity site; PGC2, Psychiatric Genomics Consortium; TFBS, transcription-factor-binding sites; TSS, transcription start site; UTR, untranslated region. Sources represented on the x axis are described in ref. 30.
Protein–protein interactions.
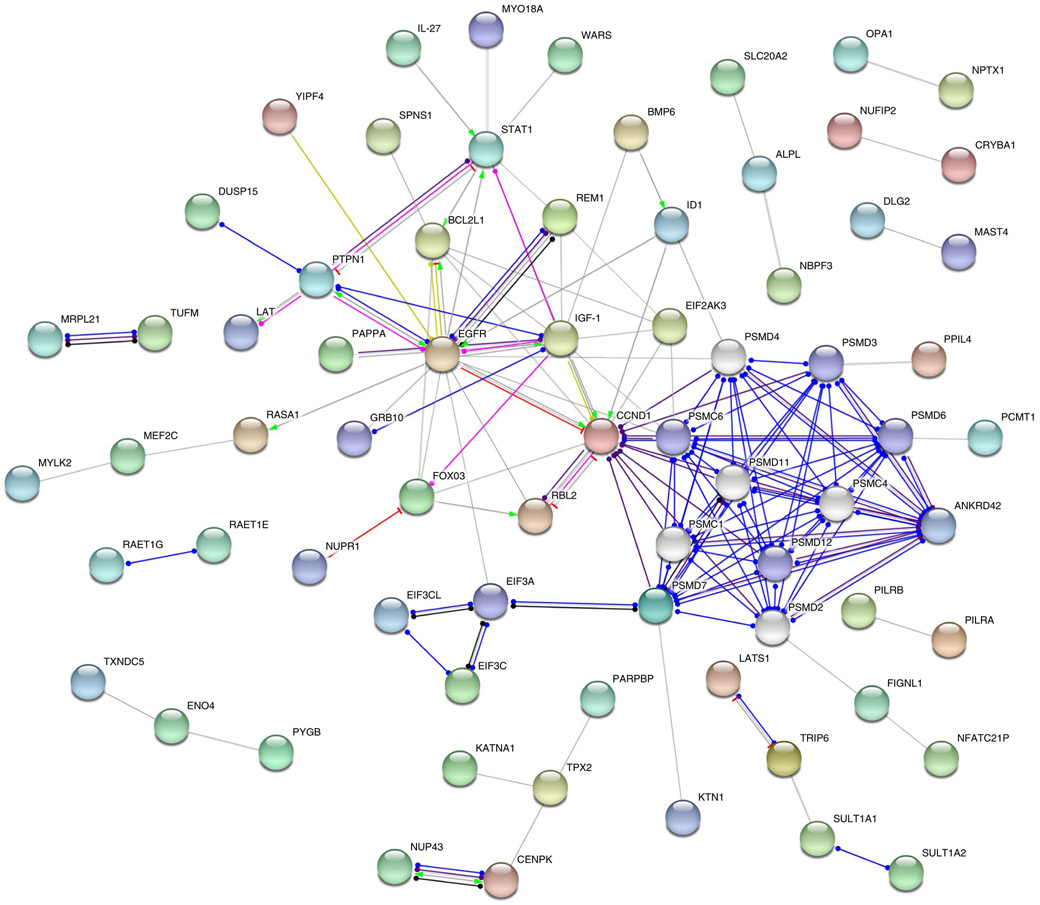
To explore potential functional relationships between proteins encoded by our set of genes, we conducted protein–protein interaction analyses in STRING32. Our results showed enrichment of genes involved in brain-specific pathways (that is, regulation of neuronal death and neuronal apoptosis), as well as immune-related (that is, antigen processing and Epstein–Barr virus infection) and housekeeping processes (that is, proteasome, cell differentiation and signaling). Figure 3 shows the protein network, and the detailed pathways are presented in Supplementary Table 21.
Fig. 3 ∣. Protein–protein interaction network of 148 genes enriched for common variants influencing the volume of subcortical structures.
The arrowheads represent protein–protein associations, where the edge color indicates the predicted mode of action (bright green, activation; pink, post-translational modification; red, inhibition; dark blue, binding, purple, catalysis; light blue, phenotype; black, reaction; yellow, transcriptional regulation) and the arrowhead shape represents the predicted action effects (pointed arrow, positive; flat arrow, negative; oval arrow, unspecified). Colored nodes represent the queried proteins and first shell of interactors (five maximum), whereas white nodes represent the second shell of interactors (five maximum).
Discussion
We undertook a large GWA meta-analysis of variants associated with MRI-derived volumes of the nucleus accumbens, amygdala, brainstem, caudate nucleus, globus pallidus, putamen and thalamus, including almost 40,000 individuals from 53 study samples worldwide. Our analyses identified a set of 199 candidate genes influencing the volume of these subcortical brain structures, most of which have relevant roles in the nervous system.
Our results show wide overlap of genetic variants determining the volume of subcortical structures, as elucidated from genetic correlations and individual look-ups among structures. We found that 26 candidate genes may influence more than one structure. For instance, significant SNPs near KTN1 are also associated with the volume of the nucleus accumbens, caudate nucleus and globus pallidus, suggesting that this genomic region may have an important role in determining multiple subcortical brain volumes during development. Furthermore, 14 of the candidate genes were associated with the caudate, globus pallidus and putamen, supporting the shared genetic architecture of the functionally defined corpus striatum.
We identified genes implicated in neurodevelopment. We confirm that the 11q14.3 genomic region near the FAT3 gene, which was previously associated with the caudate nucleus13, additionally associated with the putamen in our analysis. This gene encodes a conserved cellular adhesion molecule implicated in neuronal morphogenesis and cell migration, based on mouse genetic studies33. SNPs near PBX3 were associated with caudate volume. PBX3 is robustly expressed in the developing caudate nucleus of the non-human primate Macaca fuscata, consistent with a role in striatal neurogenesis34.
We found several genes involved in insulin/insulin-like growth factor 1 (IGF-1) signaling, including IGF1, PAPPA, GRB10, SH2B1 and TXNDC5, across the amygdala, brainstem, caudate and putamen. PAPPA encodes a secreted metalloproteinase that cleaves IGF-binding proteins, thereby releasing bound IGF. Although IGF may be beneficial in early- and midlife, its effects may be detrimental during aging. Studies of pregnancy-associated plasma protein A similarly support antagonistic pleiotropy. Low circulating pregnancy-associated plasma protein A levels are a marker for adverse outcomes in human embryonic development35, but in later life, higher levels have been associated with acute coronary syndromes and heart failure36,37. Furthermore, Grb10 and SH2B1 act as regulators of insulin/IGF-1 signaling through their SH2 domains38. Finally, TXNDC5 has been suggested to increase IGF-1 activity by inhibiting the expression of IGF-binding protein 1 in the context of rheumatoid arthritis39.
Additional genes related to neurodevelopment include PTPN1 (brainstem), ALPL and NBPF3 (both related to the globus pallidus) and SLC20A2 (nucleus accumbens). In studies of both human and mouse embryonic stem cells, PTPN1 was implicated as a critical regulator of neural differentiation40. In addition, PTPN1 encodes a target for the transcriptional regulator encoded by MECP2, which causes the neurodevelopmental disorder Rett syndrome, and inhibition of PTPB1 is being explored as a therapeutic strategy in mouse Rett models41. ALPL mediates neuronal differentiation early during development and postnatal synaptogenesis in transgenic mouse models42. ALPL may also help propagate the neurotoxicity induced by tau43, and its activity increases in Alzheimer’s disease44 and cognitive impairment45. NBPF3 belongs to the neuroblastoma breakpoint family, which encodes domains of the autism- and schizophrenia-related DUF1220 protein46. SLC20A2, related to the globus pallidus and the thalamus, encodes an inorganic phosphate transporter for which more than 40 mutations have been described in association with familial idiopathic basal ganglia calcification (Fahr’s syndrome)47,48. It is interesting to note that the other three solute carrier genes were identified in this GWA (SLC12A9, SLC25A29 and SLC39A8), suggesting that the molecular transport of metals, amino acids and other solutes across the cellular membrane could play an important role in the development of subcortical brain structures.
Several genes were related to synaptic signaling pathways. We found a SNP in NPTX1 related to the thalamus, a gene expressed in the nervous system. The encoded protein restricts synapse plasticity49 and induces β-amyloid neurodegeneration in human and mouse brain tissues50. Additionally, we identified an intronic SNP in SGTB for the brainstem, which was an eQTL for the expression of SGTB in the dorsolateral prefrontal cortex (DLPFC). Experimental rat models showed that βSGT, which is highly expressed in the brain, forms a complex with the cysteine string protein and heat-shock protein cognate complex (CSP/Hsc70) to function as a chaperone guiding the refolding of misfolded proteins near synaptic vesicles51. Other experimental studies in Caenorhabditis elegans, showed that genetic manipulation of the ortholog sgt-1 suppresses toxicity associated with expression of the human β-amyloid peptide52. Other genes involved in synaptic signaling are CHPT1 (brainstem), which is involved in phosphatidylcholine metabolism in the brain, KATNA1 (brainstem), a conserved regulator of neuronal process formation, outgrowth and synaptogenesis53,54, and DLG2 (putamen), encoding an evolutionarily conserved scaffolding protein involved in glutamatergic-mediated synaptic signaling and cell polarity55 that has been associated with schizophrenia56, cognitive impairment57 and Parkinson’s disease58.
Another set of SNPs point to genes involved in autophagy and apoptotic processes, such as DRAM1 and FOXO3, both of which are related to brainstem volumes. DRAM1 encodes a lysosomal membrane protein involved in activating TP53-mediated autophagy and apoptosis59, and mouse models mimicking cerebral ischemia and reperfusion have found that inhibiting the expression of DRAM1 worsens cell injury60. The top SNP was also associated with a CpG site proximate to active transcription start sites upstream of DRAM1 in several mature brain tissues. FOXO3 has recently been identified as pivotal in an astrocyte network conserved across humans and mice involved in stress, sleep and Huntington’s disease61, and has been related to longevity62. In Drosophila, a FOXO3 ortholog regulates dendrite number and length in the peripheral nervous system63, and in the zebrafish Danio rerio, Foxo3a knockdown led to apoptosis and mispatterning of the embryonic central nervous system64. Additional genes involved in apoptotic processes are BCL2L1 (globus pallidus and putamen), BIRC6 (globus pallidus) and OPA1 (brainstem).
Other genes have been implicated in axonal transport. We confirm the association between the 13q22 locus near KTN1 with putamen volume13, and expand by showing that this region is also associated with the nucleus accumbens, caudate and the globus pallidus. The most significant SNP (rs945270) is a robust eQTL for KTN1 in peripheral blood cells. This gene encodes a kinesin-binding protein involved in the transport of cellular components along microtubules65, and impairment of these molecular motors has been increasingly recognized in neurological diseases with a subcortical component66. The 5q12 locus upstream from MAST4 was associated with nucleus accumbens volume. MAST4 encodes a member of the microtubule-associated serine/threonine kinases. This gene has been associated with hippocampal volumes20 and juvenile myoclonic epilepsy67, and it appears to be differentially expressed in the prefrontal cortex of atypical cases of frontotemporal lobar degeneration68. In Drosophila, the knockdown of a conserved MAST4 homolog enhanced the neurotoxicity of human tau69, which aggregates to form neurofibrillary tangle pathology in Alzheimer’s disease. Furthermore, we identified SNPs near NEFL and NEFM (globus pallidus), where the top SNP was an eQTL for these genes in subcortical brain tissue and esophagus mucosa. NEFL encodes the light chain, and NEFM the medium chain of the neurofilament. The proteins encoded by these genes determine neuronal caliber and conduction velocity70. Mutations in NEFL and NEFM genes have been related to neuropsychiatric disorders, and both proteins encoded by these genes are increasingly recognized as powerful biomarkers of neurodegeneration71.
Finally, several of our candidate genes are also involved in inflammation, immunity and infection (ANKRD42, DEFB124, IL27, NLRC4, PILRA/B, TRIM23 and TRIM4), in line with the protein–protein interaction analysis highlighting the Kyoto Encyclopedia of Genes and Genomes–Epstein–Barr virus infection pathway. This suggests that immune-related processes may be an important determinant influencing subcortical volumes, as has been shown by other GWA studies of neurologic traits72,73.
Overall, the loci identified by our study pinpoint candidate genes not only associated with human subcortical brain volumes, but also reported to disrupt invertebrate neuroanatomy when manipulated in Drosophila and many other animal models. Thus, our results are in line with the knowledge that the genomic architecture of central nervous system development has been strongly conserved during evolution. Partitioning heritability results suggest the nucleus accumbens and brainstem are particularly enriched in conserved regions.
One of the main limitations of our study was the small size of our generalization samples, which limits the generalizability of our results to non-European ethnicities. However, our analyses suggest significant concordance for the direction of effect across all ethnicities at the polygenic level. We hope diverse samples become increasingly available to further confirm our findings and make new discoveries. Additionally, we have focused on the discovery of common and less frequent variants. Further efforts to also reveal rare variants and epigenetic signatures associated with subcortical structures will provide an even more refined understanding of the underlying mechanisms involved.
In conclusion, we describe multiple genes associated with the volumes of MRI-derived subcortical structures in a large sample, leveraging diverse bioinformatics resources to validate and followup our findings. Our analyses indicate that the variability of evolutionarily old subcortical volumes of humans is moderately to strongly heritable, and that their genetic variation is also strongly conserved across different species. The majority of the variants identified in this analysis point to genes involved in neurodevelopment, regulation of neuronal apoptotic processes, synaptic signaling, axonal transport, inflammation/immunity and susceptibility to neurological disorders. We show that the genetic architecture of subcortical volumes overlaps with that of anthropometric measures and neuropsychiatric disorders. In summary, our findings expand the current understanding of the genetic variation related to subcortical structures, which can help in the identification of novel biological pathways of relevance to human brain development and disease.
Methods
Study population.
The present effort included 53 study samples from the CHARGE consortium74, ENIGMA consortium75 and UK Biobank76. Briefly, the CHARGE consortium is a collaboration of predominantly population-based cohort studies investigating the genomics of age-related complex diseases, including those of the brain (https://depts.washington.edu/chargeco/wiki/). The ENIGMA consortium brings together various studies, approximately 75% of which are population based, with the remainder using case control designs for various neuropsychiatric or neurodegenerative diseases (http://enigma.ini.usc.edu/). UK Biobank is a large-scale prospective epidemiological study of over 500,000 individuals aged 40–69 years from the United Kingdom, which was established to investigate the genetic and non-genetic determinants of middle- and old-age diseases (https://www.ukbiobank.ac.uk/).
Our sample consisted of up to n = 37,741 individuals of European ancestry. We additionally included three generalization samples of African Americans (up to n = 769) and two generalization samples of Asians (n = 341). All participants provided written informed consent and the investigators on the participating studies obtained approval from their institutional review board or equivalent organization. The institutional review boards of Boston University and the University of Southern California, as well as the local ethics board of Erasmus University Medical Center approved this study.
Exclusion criteria comprised prevalent dementia or stroke at the time of the MRI scan and, when available, the presence of large brain infarcts or other neurological pathologies seen during MRI that could substantially influence the measurement of brain volumes (for example, brain tumor or trauma). Individual studies applied the exclusion criteria before analysis.
Definition of phenotypes.
Our study investigated the volumes of seven subcortical structures: the nucleus accumbens, amygdala, brainstem, caudate nucleus, globus pallidus, putamen and thalamus. These phenotypes were defined as the mean volume (in cm3) of the left and right hemispheres, with the exception of the brainstem, for which the total volume (in cm3) was used. Each study contributed MRI data obtained using diverse scanners, field strengths and acquisition protocols. The estimations of volumes for the seven subcortical brain structures and total intracranial volume were generated following freely available and inhouse segmentation methods that were previously described and validated. The summary statistics for subcortical brain volumes in CHARGE study samples are presented in Supplementary Table 3. The study-specific MRI protocols and software are described in Supplementary Table 5. We recently published results describing the genetic variation associated with hippocampal volumes20; therefore, we have not included the hippocampus in this report.
Genotyping.
Genotyping was performed using a variety of commercial arrays across the participating studies. Study samples and genetic variants underwent similar quality control procedures based on the genetic homogeneity, call rate, MAF and Hardy–Weinberg equilibrium. Good-quality variants were used as input for imputation to the 1000 Genomes Project (phase 1; version 3) reference panel16, or the HRC (version 1.1)17 in UK Biobank, using validated software packages. A detailed description of the genotyping and quality control carried out by each study is described in Supplementary Table 6.
Heritability.
Heritability of subcortical brain volumes was estimated in the FHS77 and ASPS-Fam78—two population-based cohorts with family structure. We used SOLAR79 to determine the ratio of the genetic variance to the phenotypic variance, including variance component models that were adjusted for age, sex and total intracranial volume, as well as age2 and principal components if required, in the same way as described for the GWA analysis. We also estimated the variance of subcortical structures explained by SNPs in a sample of n = 3,486 unrelated participants from the Rotterdam Study using GCTA80, and additionally in the full European sample using LDSC methods81. Supplementary Table 4 provides family- and SNP-based heritabilities for subcortical structures.
Genome-wide associations and meta-analysis.
For CHARGE and ENIGMA, each study undertook a GWA analysis on the volumes of seven MRI subcortical brain structures (or those that were available to each study), according to a common predefined analysis plan. Studies including unrelated participants performed linear regression analyses, whereas those including related participants conducted linear mixed models to account for familial relationships. Models assumed additive genetic effects and were adjusted for age, sex, total intracranial volume and, if applicable, age2, principal components to account for population stratification, psychiatric diagnosis (ENIGMA cohorts), and study site. Individual studies shared summary statistics to a centralized, secured computing space. Analysis in the UK Biobank sample followed a similar approach in n = 8,312 unrelated participants, although the genetic data used for these analyses used only those variants imputed using the HRC17 reference panel. As the data released by UK Biobank did not include total intracranial volume, linear regression models in this sample were adjusted for age, age2, sex, total brain volume and principal components. We used LDSC methods81 to investigate the genetic correlations for all subcortical structures between the CHARGE and ENIGMA consortia combined and UK Biobank. There was no evidence suggesting differences in the genetic architecture of both samples.
Before meta-analysis, we performed quality control on the summary statistics from each study sample by using a series of quality checks implemented in EasyQC82. Filters were set to remove SNPs with poor imputation (R2 < 0.5), rare SNPs (MAF < 0.1%) or SNPs with an effective allele count (2 × MAF × study sample size × imputation quality) of <20. Finally, we only considered variants present in at least 70% of the total European sample for each structure.
Fixed-effects meta-analyses weighting for sample size was performed using METAL18, given that not all samples used the same methods for acquisition and post-processing of brain images. We used the LDSC intercept to correct for population stratification and cryptic relatedness81. Quantile and Manhattan plots are presented for each subcortical structure in Supplementary Fig. 1. To correct for multiple comparisons across our seven traits, we calculated the Pearson’s correlation among subcortical structures, adjusting for age, sex and intracranial volume in n = 4,459 participants from the Rotterdam Study. After 1,000 permutations, the resulting number of independent traits was six, leading to the definition of a significant threshold as P < (5 × 10−8/6) = 8.3 × 10−9. To select our top independent SNPs in the European meta-analysis, we ran a multi-SNP-based conditional and joint association analysis (GCTA-COJO)80 using n = 6,921 participants from the Rotterdam Study as the reference sample. In secondary analyses, we looked for associations of our index SNPs (the most significant variant in each locus) with the other six subcortical structures.
We conducted separate meta-analyses by ancestry, and further performed a combined meta-analysis including all samples. Forest plots were created to explore the contribution of participating studies to each of the significant SNPs (Supplementary Fig. 4). To assess signal overlap with African American and Asian samples, we first clumped variants with P < 1 × 10−4 in the European sample, and then ran binomial sign tests for the correlation of the direction of association across ethnic groups.
Functional annotations.
We used Locus Zoom19, based on the hg19 UCSC Genome Browser assembly, for the visualization of the nearest genes within a ±500-kilobase genomic region. We also investigated cis (1-megabase) eQTLs and meQTLs for our index SNPs in postmortem brains from ROSMAP. In ROSMAP, the DLPFC was selected for initial multi-omics data generation, as it is relevant to multiple common neuropathologies and cognitive phenotypes in the aging population83. RNA was extracted from the gray matter of DLPFC, and next-generation RNA sequencing was done on the Illumina HiSeq for samples with an RNA integrity score of >5 and a quantity threshold of >5 μg, as previously described83,84. We quantile-normalized the fragments per kilobase of transcript per million fragments mapped, correcting for batch effect with Combat84,85. These adjusted fragments per kilobase of transcript per million fragments mapped values were used for analysis. A subset of n = 407 participants had quality-controlled RNA sequencing data and were included in the eQTL analysis.
DNA methylation levels from the gray matter of the DLPFC were measured using the Illumina HumanMethylation450 BeadChip, and the measurements underwent quality control processing as previously described (that is, detection P < 0.01 for all samples)83, yielding n = 708 participants with 415,848 discrete CpG dinucleotide sites with methylation measurement. Any missing methylation levels from any of quality-controlled CpG dinucleotide sites were imputed using a k-nearest neighbor algorithm for k = 100 (ref. 83). A subset of n = 488 participants in our study had quality-controlled genome-wide methylation data and were included in the cis-meQTL analysis. Finally, the associations between our index SNPs and CpG sites were plotted along Roadmap Epigenomics chromatin states for ten brain tissues86.
We further queried cis- and trans-eQTLs in non-brain and brain tissues from additional eQTL repositories87. We searched for proxies to our index SNPs with linkage disequilibrium r2 > 0.8, using the European population reference in rAggr (1,000 G; phase 1; March 2012), then queried index and proxy SNPs against eQTLs from diverse databases88. Blood cell-related eQTL studies included: fresh lymphocytes and leukocytes; leukocyte samples in individuals with celiac disease; whole blood samples; lymphoblastoid cell lines (LCLs) derived from asthmatic children; HapMap LCLs from three populations; a separate study on HapMap Utah Residents with Northern and Western European Ancestry (CEU) LCLs; LCL population samples; neutrophils; CD19+ B cells; primary phytohemagglutinin-stimulated T cells; CD4+ T cells; peripheral blood monocytes; long non-coding RNAs in CD14+ monocytes purified from white blood cells and CD14+ monocytes before and after stimulation with lipopolysaccharide or interferon-γ; CD11+ dendritic cells before and after Mycobacterium tuberculosis infection; a separate study of dendritic cells before or after stimulation with lipopolysaccharide, influenza or interferon-β; micro-RNA QTLs, DNase I QTLs, histone acetylation QTLs and ribosomal occupancy QTLs queried for LCLs; and splicing QTLs and micro-RNA QTLs queried in whole blood. Non-blood cell tissue eQTL searches included: omental and subcutaneous adipose; visceral fat stomach; endometrial carcinomas; ER+ and ER− breast cancer tumor cells; liver; osteoblasts; intestine; normal and cancerous colon; skeletal muscle; breast tissue (normal and cancerous); lung; skin; primary fibroblasts; sputum; pancreatic islet cells; prostate; rectal mucosa; and arterial wall and heart tissue from left ventricles and left and right atria. Micro-RNA QTLs were also queried for gluteal and abdominal adipose and liver. MeQTLs were queried in pancreatic islet cells. Further messenger RNA and micro-RNA QTLs were queried from ER+ invasive breast cancer samples, as well as colon, kidney renal clear, lung and prostate adenocarcinoma samples. Brain eQTL studies included: brain cortex; cerebellar cortex; cerebellum; frontal cortex; gliomas; hippocampus; inferior olivary nucleus (from medulla); intralobular white matter; occipital cortex; parietal lobe; pons; prefrontal cortex; putamen (at the level of the anterior commissure); substantia nigra; temporal cortex; thalamus; and visual cortex. eQTL data were integrated from online sources, including ScanDB89, the GTEx Portal90 and the Pritchard Lab91. Cerebellum, parietal lobe and liver eQTL data were downloaded from ScanDB. Cis-eQTLs were limited to those with P < 1.0 × 10−6 and trans-eQTLs were limited to those with P < 5.0 × 10−8. The results for GTEx Analysis version 6 for 48 tissues were downloaded from the GTEx Portal (https://www.gtexportal.org). For all gene-level eQTLs, if at least one SNP passed the tissue-specific empirical threshold in GTEx, the best SNP for that eQTL was always retained.
Associations of cognition and neuropathology phenotypes with gene expression in the brain.
We further related cognitive function and neuropathological findings to the expression of the 199 genes influencing subcortical volumes in 508 brains from the ROSMAP samples.
Briefly, brain autopsies were performed as previously described, and each brain was inspected for common pathologies relating to loss of cognition in aging populations92,93. In this report, we included: neurofibrillary tangles; neuritic plaques; β-amyloid load; tau density; hippocampal sclerosis; Lewy bodies; and neuronal loss in substantia nigra. Neurofibrillary tangles and neuritic plaques were visualized by modified Bielschowsky silver stain, then counted and scaled in five brain regions: mid-frontal; temporal; inferior parietal; entorhinal cortex; and hippocampus CA1. Composite scores for each of these three pathology types were derived by scaling the counts within each of the five regions and taking the square root of the average of the regional scaled values to account for their positively skewed distribution92-94. β-amyloid load and tau tangle density were measured by immunohistochemistry and square root transformed as previously described95. Lewy bodies were identified using immunohistochemistry, and were further dichotomized as present or absent based on the recommendations of the Report of the Consortium on DLB International Workshop96. Hippocampal sclerosis was recorded as either present or absent, as evaluated by hematoxylin and eosin staining. Nigral neuronal loss was assessed in the substantia nigra in the mid- to rostral midbrain near or at the exit of the third nerve using hematoxylin and eosin staining and 6-μm sections and a semiquantitative scale (0–3)97.
Global cognition was computed as a composite score of 19 (Religious Order Study) and 17 (Rush Memory and Aging Project) cognitive tests performed during annual evaluations, including five cognitive domains: episodic memory; semantic memory; working memory; perceptual speed; and visuospatial ability92,93. From these scores, we created normalized summary measures to limit the influence of outliers. We used global cognition proximate to death to derive cognitive reserve. Separately, the residual slope of global cognitive change and the residual slopes of cognitive change in the five cognitive domains were derived through general linear mixed models, controlling for age at enrollment, sex and education.
Phenotypic and genetic correlations.
We estimated the Pearson’s partial phenotypic correlations among the volumes of subcortical structures in 894 participants from the FHS. Similarly to the GWA, these analyses were corrected for the effects of sex, age, age2, total intracranial volume and principal component 1.
Genetic correlation analyses were performed using LDSC methods81. The GWA meta-analysis results for the seven subcortical brain structures were correlated with each other’s, as well as with published GWA studies on the following traits: hippocampal volume20; intracranial volume21; white matter hyperintensities22; stroke subtypes23; adult height and body mass index24; fat-free mass and whole-body water mass98; Alzheimer’s disease26; Parkinson’s disease27; general cognitive function25; bipolar disorder and schizophrenia28; and ADHD29.
Look-up of functional orthologs in D. melanogaster.
For the cross-species assessment of gene–phenotype relationships in Drosophila, we relied on a similar analytic approach as in previous work99. Human genes were mapped to corresponding Drosophila orthologs using the Drosophila Integrated Ortholog Prediction Tool (https://www.flyrnai.org/diopt)100, which incorporates 14 distinct algorithms to define orthology. Fly gene orthologs were defined based on a Drosophila Integrated Ortholog Prediction Tool score of ≥2, indicating that at least two algorithms were in agreement on the pairing. When more than one of the fly ortholog was predicted, all such genes meeting this threshold were included in our analyses. This resulted in a gene set consisting of 168 Drosophila homologs of human candidate genes at subcortical volume susceptibility loci. The resulting 37 genes associated with neuroanatomy-defective phenotypes in Drosophila (22%) were annotated based on the controlled vocabulary terms implemented in FlyBase (http://flybase.org/)101. Genes causing neuroanatomy-defective phenotypes in Drosophila include both loss-of-function and gain-of-function genetic manipulations of fly gene homologs. Loss-of-function studies included both classical mutant alleles (for example, point mutations, gene deletions or transposon insertions) or gene knockdown using RNA interference transgenic strains. Gain-of-function experiments were based on tissue-specific overexpression of the fly gene orthologs. The hypergeometric overlap test was used to assess for enrichment of neuroanatomy-defective phenotypes among the conserved gene set.
Protein–protein interactions and network analysis.
We used the human STRING database resource (string-db.org)32 for the exploration of direct (physical) and indirect (functional) protein–protein interactions based on the gene set derived from the GWA results and functional annotations (Supplementary Table 13). The input parameters included a medium-confidence interaction score (0.4) with first and second shells of a maximum of five interactors. Finally, we generated a protein–protein interaction network based on known and predicted interactions.
Partitioning heritability.
Partitioned heritability was estimated with stratified LDSC methods30. This method partitions SNP heritability using GWA study summary results and accounting by linkage disequilibrium. We used the meta-analysis results from the European sample to partition SNPs by 28 functional categories, including: coding; intron; promoter; 3′5′ untranslated region; digital genomic footprint; transcription factor binding site; chromHMM and Segway annotations for six cell lines; DNase I hypersensitivity sites; H3K4me1, H3K4me3 and H3K9ac marks; two sets of H3K27ac marks; super-enhancers; conserved regions in mammals; and FANTOM5 enhancers. Significance was set at P < (0.05/(28 × 6)) = 3 × 10−4.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The genome-wide summary statistics that support the findings of this study are available from the CHARGE dbGaP (accession code: phs000930) and ENIGMA (http://enigma.ini.usc.edu/research/download-enigma-gwas-results) websites.
Supplementary Material
Acknowledgements
We thank all of the study participants for contributing to this research. Full acknowledgements and grant support details are provided in the Supplementary Note.
Footnotes
Online content
Any methods, additional references, Nature Research reporting summaries, source data, statements of code and data availability and associated accession codes are available at https://doi.org/10.1038/s41588-019-0511-y.
Competing interests
D.P.H. is currently an employee at Genentech. D.J. has received travel and speaker’s honoraria from Janssen–Cilag, as well as research funding from DFG. R.L.B. is a consultant for Pfizer and Roche. P.A. is a scientific adviser for Genoscreen. T.Y.W. is a consultant and advisory board member for Allergan, Bayer, Boehringer–Ingelheim, Genentech, Merck, Novartis, Oxurion (formerly ThromboGenics) and Roche, and is a co-founder of Plano and EyRiS. A.M.M. has received grant support from Eli Lilly, Janssen, Pfizer and the Sackler Trust. B.M.P. serves on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson. A.M.-L. is a member of the advisory board for the Lundbeck International Neuroscience Foundation and Brainsway, a member of the editorial board for the American Association for the Advancement of Science and Elsevier, a faculty member of the Lundbeck International Neuroscience Foundation and a consultant for Boehringer Ingelheim. W.J.N. is the founder and scientific lead of Quantib BV, in addition to being a shareholder. M.M.N. is a shareholder of Life & Brain, receives a salary from Life & Brain, has received support from Shire for attending conferences and has received financial remuneration from the Lundbeck Foundation, Robert Bosch Foundation and Deutsches Ärzteblatt for participation in scientific advisory boards. B.F. has received educational speaking fees from Shire and Medice. H.J.G. has received travel grants and speaker’s honoraria from Fresenius Medical Care, Neuraxpharm and Janssen–Cilag, as well as research funding from Fresenius Medical Care.
Supplementary information is available for this paper at https://doi.org/10.1038/s41588-019-0511-y.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marsden CD The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology 32, 514–539 (1982). [DOI] [PubMed] [Google Scholar]
- 2.Yin HH & Knowlton BJ The role of the basal ganglia in habit formation. Nat. Rev. Neurosci 7, 464–476 (2006). [DOI] [PubMed] [Google Scholar]
- 3.McDonald AJ & Mott DD Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. J. Neurosci. Res 95, 797–820 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hikosaka O, Kim HF, Yasuda M & Yamamoto S Basal ganglia circuits for reward value-guided behavior. Annu. Rev. Neurosci 37, 289–306 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salzman CD & Fusi S Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu. Rev. Neurosci 33, 173–202 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floresco SB The nucleus accumbens: an interface between cognition, emotion, and action. Annu. Rev. Psychol 66, 25–52 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Fabbro F, Aglioti SM, Bergamasco M, Clarici A & Panksepp J Evolutionary aspects of self- and world consciousness in vertebrates. Front. Hum. Neurosci 9, 157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander GE, DeLong MR & Strick PL Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci 9, 357–381 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Jahanshahi M, Obeso I, Rothwell JC & Obeso JA A fronto–striato–subthalamic–pallidal network for goal-directed and habitual inhibition. Nat. Rev. Neurosci 16, 719–732 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Shepherd GM Corticostriatal connectivity and its role in disease. Nat. Rev. Neurosci 14, 278–291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stratmann K et al. Precortical phase of Alzheimer’s disease (AD)-related Tau cytoskeletal pathology. Brain Pathol. 26, 371–386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Tredici K, Rub U, De Vos RA, Bohl JR & Braak H Where does Parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol 61, 413–426 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Hibar DP et al. Common genetic variants influence human subcortical brain structures. Nature 520, 224–229 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott LT et al. Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562, 210–216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renteria ME et al. Genetic architecture of subcortical brain regions: common and region-specific genetic contributions. Genes Brain Behav. 13, 821–830 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke L et al. The 1000 Genomes Project: data management and community access. Nat. Methods 9, 459–462 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarthy S et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet 48, 1279–1283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willer CJ, Li Y & Abecasis GR METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruim RJ et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26, 2336–2337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibar DP et al. Novel genetic loci associated with hippocampal volume. Nat. Commun 8, 13624 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams HH et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat. Neurosci 19, 1569–1582 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verhaaren BF et al. Multiethnic genome-wide association study of cerebral white matter hyperintensities on MRI. Circ. Cardiovasc. Genet 8, 398–409 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malik R et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet 50, 524–537 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yengo L et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum. Mol. Genet 27, 3641–3649 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies G et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat. Commun 9, 2098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkle BW et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet 51, 414–430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon-Sanchez J et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet 41, 1308–1312 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bipolar Disorder and Schizophrenia Working Group of the Psychiatric Genomics Consortium. Genomic dissection of bipolar disorder and schizophrenia, including 28 subphenotypes. Cell 173, 1705–1715.e16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demontis D et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet 51, 63–75 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finucane HK et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet 47, 1228–1235 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hnisz D et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szklarczyk D et al. STRINGv10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deans MR et al. Control of neuronal morphology by the atypical cadherin Fat3. Neuron 71, 820–832 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K et al. Expression of FOXP2 in the developing monkey forebrain: comparison with the expression of the genes FOXP1, PBX3, and MEIS2. J. Comp. Neurol 509, 180–189 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Kjaer-Sorensen K et al. Pregnancy-associated plasma protein A (PAPP-A) modulates the early developmental rate in zebrafish independently of its proteolytic activity. J. Biol. Chem 288, 9982–9992 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayes-Genis A et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N. Engl. J. Med 345, 1022–1029 (2001). [DOI] [PubMed] [Google Scholar]
- 37.Funayama A et al. Serum pregnancy-associated plasma protein A in patients with heart failure. J. Card. Fail 17, 819–826 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Desbuquois B, Carre N & Burnol AF Regulation of insulin and type 1 insulin-like growth factor signaling and action by the Grb10/14 and SH2B1/B2 adaptor proteins. FEBS J. 280, 794–816 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Li J et al. TXNDC5 contributes to rheumatoid arthritis by down-regulating IGFBP1 expression. Clin. Exp. Immunol 192, 82–94 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matulka K et al. PTP1B is an effector of activin signaling and regulates neural specification of embryonic stem cells. Cell Stem Cell 13, 706–719 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Krishnan N et al. PTP1B inhibition suggests a therapeutic strategy for Rett syndrome. J. Clin. Invest 125, 3163–3177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sebastian-Serrano A et al. Tissue-nonspecific alkaline phosphatase regulates purinergic transmission in the central nervous system during development and disease. Comput. Struct. Biotechnol. J 13, 95–100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diaz-Hernandez M et al. Tissue-nonspecific alkaline phosphatase promotes the neurotoxicity effect of extracellular tau. J. Biol. Chem 285, 32539–32548 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vardy ER, Kellett KA, Cocklin SL & Hooper NM Alkaline phosphatase is increased in both brain and plasma in Alzheimer’s disease. Neurodegener. Dis 9, 31–37 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Kellett KA, Williams J, Vardy ER, Smith AD & Hooper NM Plasma alkaline phosphatase is elevated in Alzheimer’s disease and inversely correlates with cognitive function. Int. J. Mol. Epidemiol. Genet 2, 114–121 (2011). [PMC free article] [PubMed] [Google Scholar]
- 46.Searles Quick VB, Davis JM, Olincy A & Sikela JM DUF1220 copy number is associated with schizophrenia risk and severity: implications for understanding autism and schizophrenia as related diseases. Transl. Psychiatry 5, e697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu SC et al. Mutations in SLC20A2 are a major cause of familial idiopathic basal ganglia calcification. Neurogenetics 14, 11–22 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taglia I, Bonifati V, Mignarri A, Dotti MT & Federico A Primary familial brain calcification: update on molecular genetics. Neurol. Sci 36, 787–794 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Figueiro-Silva J et al. Neuronal pentraxin 1 negatively regulates excitatory synapse density and synaptic plasticity. J. Neurosci 35, 5504–5521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abad MA, Enguita M, DeGregorio-Rocasolano N, Ferrer I & Trullas R Neuronal pentraxin 1 contributes to the neuronal damage evoked by amyloid-β and is overexpressed in dystrophic neurites in Alzheimer’s brain. J. Neurosci 26, 12735–12747 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobaben S, Varoqueaux F, Brose N, Stahl B & Meyer G A brain-specific isoform of small glutamine-rich tetratricopeptide repeat-containing protein binds to Hsc70 and the cysteine string protein. J. Biol. Chem 278, 38376–38383 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Fonte V et al. Interaction of intracellular β amyloid peptide with chaperone proteins. Proc. Natl Acad. Sci. USA 99, 9439–9444 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao CX et al. Microtubule-severing protein katanin regulates neuromuscular junction development and dendritic elaboration in Drosophila. Development 141, 1064–1074 (2014). [DOI] [PubMed] [Google Scholar]
- 54.Yu W et al. The microtubule-severing proteins spastin and katanin participate differently in the formation of axonal branches. Mol. Biol. Cell 19, 1485–1498 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, Shang Y & Zhang M Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat. Rev. Neurosci 17, 209–223 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Ingason A et al. Expression analysis in a rat psychosis model identifies novel candidate genes validated in a large case-control sample of schizophrenia. Transl. Psychiatry 5, e656 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nithianantharajah J et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat. Neurosci 16, 16–24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nalls MA et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet 46, 989–993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guan JJ et al. DRAM1 regulates apoptosis through increasing protein levels and lysosomal localization of BAX. Cell Death Dis. 6, e1624 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu M, Jiang Y, Feng Q, Ouyang Y & Gan J DRAM1 protects neuroblastoma cells from oxygen-glucose deprivation/reperfusion-induced injury via autophagy. Int. J. Mol. Sci 15, 19253–19264 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scarpa JR et al. Systems genetic analyses highlight a TGFβ-FOXO3 dependent striatal astrocyte network conserved across species and associated with stress, sleep, and Huntington’s disease. PLoS Genet. 12, e1006137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donlon TA et al. FOXO3 longevity interactome on chromosome 6. Aging Cell 16, 1016–1025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sears JC & Broihier HT FoxO regulates microtubule dynamics and polarity to promote dendrite branching in Drosophila sensory neurons. Dev. Biol 418, 40–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng K et al. Knockdown of FoxO3a induces increased neuronal apoptosis during embryonic development in zebrafish. Neurosci. Lett 484, 98–103 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Santama N, Er CP, Ong LL & Yu H Distribution and functions of kinectin isoforms. J. Cell Sci 117, 4537–4549 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Liu XA, Rizzo V & Puthanveettil SV Pathologies of axonal transport in neurodegenerative diseases. Transl. Neurosci 3, 355–372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Consortium, E. et al. Genome-wide association analysis of genetic generalized epilepsies implicates susceptibility loci at 1q43, 2p16.1, 2q22.3 and 17q21.32. Hum. Mol. Genet 21, 5359–5372 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Martins-de-Souza D et al. Proteomic analysis identifies dysfunction in cellular transport, energy, and protein metabolism in different brain regions of atypical frontotemporal lobar degeneration. J. Proteome Res 11, 2533–2543 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Shulman JM et al. Functional screening in Drosophila identifies Alzheimer’s disease susceptibility genes and implicates Tau-mediated mechanisms. Hum. Mol. Genet 23, 870–877 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friede RL & Samorajski T Axon caliber related to neurofilaments and microtubules in sciatic nerve fibers of rats and mice. Anat. Rec 167, 379–387 (1970). [DOI] [PubMed] [Google Scholar]
- 71.Yuan A, Rao MV, Veeranna & Nixon, R. A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol 9, a018309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bis JC et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-associated variants involved in immune response and transcriptional regulation. Mol Psychiatry 10.1038/s41380-018-0112-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marioni RE et al. GWAS on family history of Alzheimer’s disease. Transl. Psychiatry 8, 99 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Psaty BM et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ. Cardiovasc. Genet 2, 73–80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thompson PM et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8, 153–182 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sudlow C et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsao CW & Vasan RS Cohort Profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int. J. Epidemiol 44, 1800–1813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schmidt R et al. Assessment of cerebrovascular risk profiles in healthy persons: definition of research goals and the Austrian Stroke Prevention Study (ASPS). Neuroepidemiology 13, 308–313 (1994). [DOI] [PubMed] [Google Scholar]
- 79.Almasy L & Blangero J Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet 62, 1198–1211 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang J, Lee SH, Goddard ME & Visscher PM GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet 88, 76–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bulik-Sullivan BK et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet 47, 291–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Winkler TW et al. Quality control and conduct of genome-wide association meta-analyses. Nat. Protoc 9, 1192–1212 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bennett DA, Yu L & De Jager PL Building a pipeline to discover and validate novel therapeutic targets and lead compounds for Alzheimer’s disease. Biochem. Pharm 88, 617–630 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan G et al. CD33 modulates TREM2: convergence of Alzheimer loci. Nat. Neurosci 18, 1556–1558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Johnson WE, Li C & Rabinovic A Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics 8, 118–127 (2007). [DOI] [PubMed] [Google Scholar]
- 86.Roadmap Epigenomics Association et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eicher JD et al. GRASPv2.0: an update on the Genome-Wide Repository of Associations between SNPs and phenotypes. Nucleic Acids Res. 43, D799–D804 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang X et al. Synthesis of 53 tissue and cell line expression QTL datasets reveals master eQTLs. BMC Genomics 15, 532 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang W et al. SCAN database: facilitating integrative analyses of cytosine modification and expression QTL. Database 2015, bav025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Consortium, G. T. The Genotype-Tissue Expression (GTEx) project. Nat. Genet 45, 580–585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Veyrieras JB et al. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 4, e1000214 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bennett DA et al. Overview and findings from the rush Memory and Aging Project. Curr. Alzheimer Res 9, 646–663 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennett DA, Schneider JA, Arvanitakis Z & Wilson RS Overview and findings from the religious orders study. Curr. Alzheimer Res. 9, 628–645 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Replogle JM et al. A TREM1 variant alters the accumulation of Alzheimer-related amyloid pathology. Ann. Neurol 77, 469–477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barnes LL, Schneider JA, Boyle PA, Bienias JL & Bennett DA Memory complaints are related to Alzheimer disease pathology in older persons. Neurology 67, 1581–1585 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McKeith IG et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Neurology 47, 1113–1124 (1996). [DOI] [PubMed] [Google Scholar]
- 97.Schneider JA et al. Substantia nigra tangles are related to gait impairment in older persons. Ann. Neurol 59, 166–173 (2006). [DOI] [PubMed] [Google Scholar]
- 98.Zheng J et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wangler MF, Hu Y & Shulman JM Drosophila and genome-wide association studies: a review and resource for the functional dissection of human complex traits. Dis. Model Mech 10, 77–88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu Y et al. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinformatics 12, 357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marygold SJ, Crosby MA, Goodman JL & FlyBase C Using FlyBase, a database of Drosophila genes and genomes. Methods Mol. Biol 1478, 1–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The genome-wide summary statistics that support the findings of this study are available from the CHARGE dbGaP (accession code: phs000930) and ENIGMA (http://enigma.ini.usc.edu/research/download-enigma-gwas-results) websites.