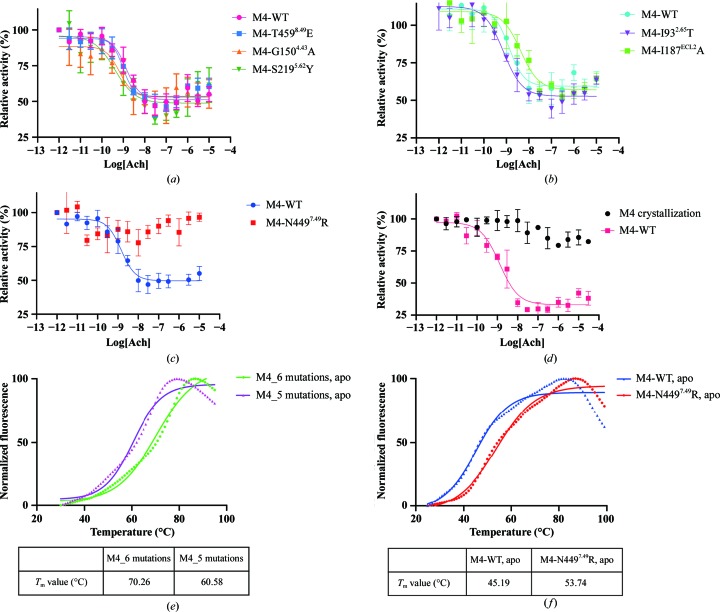
Figure 1.
Pharmacological characterization and thermostability assay of mutants of M4. (a, b) Dose–response studies of agonist Ach activity for each mutant compared with wild-type M4 (M4-WT). The EC50 values (mean ± SEM) of Ach are 1.059 ± 0.1489, 1.036 ± 0.139, 1.777 ± 0.6448, 2.314 ± 1.012, 0.9343 ± 0.1192 and 6.357 ± 1.262 nM for the M4-WT, T4598.49E, G1504.43A, S2195.62Y, I932.65T and I187ECL2A constructs, respectively. (c, d) Gi activation assays of M4 with the key point mutation N4497.49R and M4 with six mutations as a function of Ach compared with that of M4-WT. (e) Thermostability assay of the crystallization construct with six mutations (M4_6 mutations) and a construct with the other five mutations apart from N4497.49R (M4_5 mutations), where the fusion protein used in the constructs is PGS. (f) Thermostability assay of M4-N4497.49R and M4-WT with the fusion protein modified T4 lysozyme. The N4497.49R mutant showed an increase in the melting temperature (T m) by about 8.1 ± 1.1°C.