Abstract
This study sought to determine the incidence, predictors, and outcomes of postoperative atrial fibrillation in patients undergoing implantation of left ventricular assist devices. A retrospective analysis of all patients who underwent left ventricular assist device implantation from 2013 to 2014 was conducted. Postoperative atrial fibrillation, survival, and thrombotic complications were evaluated after surgery. A total of 47 patients (mean age 56.4±12.5 years, 33 male) were included and followed for a median of 331 days. Within 30 days of surgery, 13 (28%) patients developed postoperative atrial fibrillation at mean 7.9±8.5 days. Obstructive lung disease was a predictor of postoperative atrial fibrillation (p=0.01). Postoperative atrial fibrillation was not associated with increased mortality, length of stay, or thrombotic complication within 30 days. Postoperative atrial fibrillation was predictive of recurrent new atrial fibrillation (24% versus 5.5%) after 30 days of left ventricular assist device implantation. Also, postoperative atrial fibrillation was associated with increased risk of ischemic stroke and device thrombosis during follow up (p=0.01). These results show that unlike in other cardiac surgery, postoperative atrial fibrillation does not have a negative impact on early postoperative morbidity or mortality. However, postoperative atrial fibrillation is a predictor for future atrial fibrillation, ischemic stroke, and device thrombosis.
Keywords: Postoperative atrial fibrillation, left ventricular assist device, device thrombosis
INTRODUCTION
Atrial fibrillation (AF) is common after cardiac surgery and occurs in approximately 20 to 50 percent of patients. It is associated with increased mortality, morbidity, length of hospital stay, and costs of care.1–8 Various risk factors have been associated with the development of postoperative AF (POAF) including increasing age, male gender, and left ventricular dysfunction.1,2,5–8 While the mechanisms which facilitate POAF have not been fully elucidated, POAF has been linked to altered atrial electrical property induced by post-operative inflammation, oxidative stress, and neurohormonal activation.9–15
POAF has been primarily described after coronary artery bypass graft (CABG) or valvular surgery. However, there are no reports about the incidence of POAF after left ventricular assist devices (LVAD) implantation and its impact on overall clinical outcome. Patients who require LVAD represent a population distinct from CABG and valvular surgery groups given their advanced degree of left ventricular systolic dysfunction, the potential for postoperative right ventricular failure, and the overall requirement for anticoagulation. In addition, a history of AF is associated with thromboembolic events and worsened clinical outcome in patients undergoing LVAD.16,17 Thus, we sought to characterize the incidence, predictors and clinical impact of POAF in patients undergoing implantation of LVAD.
MATERIALS AND METHODS
Study Population
All consecutive patients who underwent LVAD implantation at the University of Chicago Medical Center between January 2013 to December 2014 were included in the study. The study was approved by the hospital Institutional Review Board. Eligible patients were aged 18 years or older without history of AF prior to LVAD implantation. These patients were accepted as de novo POAF. Patients with history of AF before LVAD surgery were excluded from the study population as AF in the postoperative period in these patients was considered a recurrence of AF in the setting of surgical stress. AF was defined based on latest American College of Cardiology, American Heart Association and Heart Rhythm Society guideline.18 All patients received continuous flow LVADs and no patients received biventricular assist devices. Patients were managed based on individual requirements regarding pump speeds, rate control, and antiarrhythmic therapy. There was no standardized use of antiarrhythmic therapy as prophylaxis against POAF. All patients were initially treated with antiplatelet therapy and warfarin with a goal international normalized ratio (INR) of 2.0 to 3.0. INR goals were individualized for patients in follow up based on subsequent bleeding or thrombotic events. Unfractionated heparin was used for inpatient bridging and low molecular weight heparin was used for outpatient bridging.
Data Collection
Electronic medical records, electrocardiograms (ECG) and cardiac implantable electrical device (CIED) interrogations were reviewed to determine incidence of arrhythmia, conduction intervals and clinical outcomes. Surface ECGs (12-lead) were analyzed for underlying rhythm and PR interval. A PR interval greater than 200 milliseconds was considered prolonged. Echocardiograms in the year prior to LVAD implantation were reviewed for left atrial volume index (LAVI).19 Ischemic stroke and device thrombosis were considered post-operative if occurring within 30 days of the LVAD.
Follow-up
Patients were followed from the time of LVAD implantation until death, transplantation, loss to follow up, or until the end of the review period in September 2015. In addition to post-operative inpatient care, patients had close monitoring in the outpatient setting at our institution.
Statistical Analysis
Baseline continuous and categorical variables were compared between patients with and without POAF using two sample t-testing and Fischer’s exact test respectively. Potential predictors for the development of POAF were investigated by univariate and multivariate logistic regression. Outcomes at 30 days for length of stay, incidence of thromboembolic events and mortality were evaluated by t-test and Kaplan-Meier analysis. Long term survival and thromboembolic event rates were estimated by Kaplan-Meier and Cox-regression analysis. Right sided censoring was used in patients who were transplanted or expired. Multivariate models are presented in the form of point estimates of the hazard ratios, with 95 percent confidence intervals (CI). Continuous data are presented as means ± standard deviation, and n represents the number of patients with specified categorical covariate data.
RESULTS
Patient Characteristics
Of the patients undergoing LVAD implantation from January 2013 to December 2014, a total of 47 patients had no prior history of AF and were included in the study population. A total of 55 patients underwent LVAD implantation in this time period but were excluded because of a prior history of AF. In this excluded population 42% of patients had AF in the postoperative period. Among these patients, mean age was 56.4 ± 12.4 years, 70% were male, 38% had ischemic cardiomyopathy and 67% received LVAD as destination therapy. The median duration of follow up was 331 days (Range 12–970 days). Detailed baseline characteristics for the total population and subgroups based on the development of POAF are given in Table 1.
Table 1.
Baseline characteristics of the patient cohort
| Characteristic | Study Cohort (n=47) | Patients with POAF (n=13) | Patients without POAF (n=34) | p-value for POAF vs. no POAF |
|---|---|---|---|---|
| Male, n (%) | 33 (70%) | 9 (69%) | 25 (74%) | 0.65 |
| Age, mean years | 56.4 ± 12.4 | 62.7 ± 8.3 | 54.8 ± 13.1 | 0.11 |
| Left ventricular ejection fraction, % | 20.1 ± 5.8 | 19.6 ± 4.5 | 20.6 ± 6.0 | 0.26 |
| LA volume index >40 mL/m2, % (n) | 70% (18 of 26) | 71% (5 of 7) | 68% (13 of 19) | 1.0 |
| PR interval >200 msec, % | 13% (6 of 46) | 17% (2 of 12) | 12% (4 of 34) | 1.0 |
| Ischemic cardiomyopathy, n (%) | 18 (38%) | 5 (38%) | 13 (38%) | 1.0 |
| Valvular heart disease, n (%) | 31 (66%) | 8 (62%) | 23 (68%) | 0.96 |
| Hypertension, n (%) | 38 (81%) | 11 (92%) | 27 (79%) | 1.0 |
| Diabetes, n (%) | 24 (41%) | 6 (46%) | 18 (53%) | 0.93 |
| Thyroid disease, n (%) | 9 (19%) | 4 (31%) | 5 (15%) | 0.40 |
| Obstructive sleep apnea, n (%) | 15 (32%) | 4 (31%) | 11 (32%) | 1.0 |
| COPD, n (%) | 11 (23%) | 6 (46 %) | 5 (15%) | 0.01 |
| CABG, n (%) | 9 (19%) | 3 (23%) | 6 (18%) | 0.99 |
| Baseline creatinine, (mg/dL) | 1.5 ± 0.8 | 1.7 ± 1.0 | 1.4 ± 0.6 | 0.31 |
| Baseline albumin, (g/dL) | 3.4 ± 0.4 | 3.4 ± 0.5 | 3.5 ± 0.4 | 0.50 |
Abbreviations: CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; LA, left atria; POAF, postoperative atrial fibrillation;
Incidence POAF
POAF developed in 13 patients (28%) on a median of 6 days after LVAD implantation (mean 7.9±8.5 days). POAF was paroxysmal in 11 of 13 patients with no intervention in 4 of the 11 patients with resolution. Amiodarone was used in 6 of the 11 and direct current cardioversion in 2 of the 11 patients. AF remained persistent in 1 patient who did not have intervention and in 1 patient who was treated with both amiodarone and cardioversion. POAF developed similarly in 27% of men and 29% of women. Patients with POAF were older than no POAF (mean age 62.7±8 versus 54.8±13 years) though this was not statistically significant. The cardiopulmonary bypass time was not significantly different in patients with and without POAF (165.4±49.4 min versus 135.7±47.2 min, respectively, p=0.08) and the mean duration was 143.9±49 min in our total cohort. Overall, other comorbidities were comparable in patients with and without POAF with the exception of chronic obstructive pulmonary disease (COPD, p=0.01) as in Table 1.
Predictors of POAF
Based on the current literature, the following variables were considered as potential predictors of development of post-operative AF: demographic features (age and sex), the presence of heart disease (ischemic heart disease, valvular heart disease, LAVI, conduction intervals and left ventricular dysfunction), associated clinical conditions (history of CABG, diabetes mellitus, hypertension, COPD, thyroid disease, renal insufficiency, hypoalbuminemia, and obstructive sleep apnea). Univariate analyses showed that COPD was the only predictor for the development of POAF after LVAD implantation (OR 5.0 (1.2–22.4), p=0.03). This association remained significant when including all other potential predictors in a multivariate model.
Short-term impact of POAF
Clinical outcomes in first 30 days in patients with and without POAF are summarized in Table 2. In both Kaplan-Meier and Cox proportional hazards models which adjusted for clinical covariates, POAF was not associated increased length of stay, 30-day ischemic stroke or device thrombosis, or 30-day mortality. Thus, POAF has no significant negative impact early clinical outcome.
Table 2.
Postoperative outcomes in first 30 days in patients with and without postoperative atrial fibrillation
| Outcome | Patients with POAF (n=13) | Patients without POAF (n=34) |
|---|---|---|
| Length of stay, (days) | 19.1 ± 11.4 | 21.1 ±13.8 |
| Ischemic stroke, n (%) | 1, 7.7% | 1, 2.9% |
| Device thrombosis, n (%) | 0, 0% | 0, 0% |
| Mortality, n (%) | 1, 7.7% | 1, 2.9% |
Abbreviations: POAF, postoperative atrial fibrillation
Long-term outcome of POAF
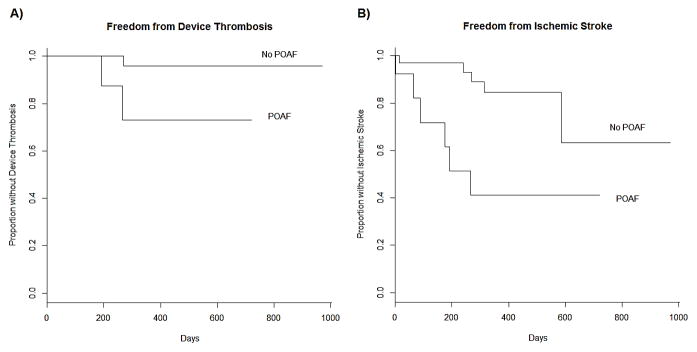
Similar to short-term outcomes, POAF was not associated with increased long-term mortality. In 76% of patients who developed POAF, there was no recurrence of AF during follow up. However, POAF was predictive of the development of future AF during the long-term follow up with 24% of patients with POAF developing AF as compared to 5.5% in patients without POAF. Further, POAF was associated with both an increased long-term risk of device thrombosis (p=0.02) and ischemic stroke (p=0.02) in Kaplan-Meier analyses (Figure 1). In a Cox proportional hazards model adjusting for age, gender, history of hypertension, and diabetes mellitus, POAF remained a significant predictor for thromboembolic event (either ischemic stroke or device thrombosis) as summarized in Table 3 (p=0.01).
Figure 1.
Postoperative atrial fibrillation is associated with an increased rate of device thrombosis (A) and ischemic stroke (B) after left ventricular assist device implantation. Abbreviations: POAF, postoperative atrial fibrillation.
Table 3.
Predictors of thromboembolic events after left ventricular assist device placement
| Baseline Characteristic | Odds ratio (95% CI) | p-value |
|---|---|---|
| Postoperative atrial fibrillation | 5.53(1.40–21.73) | 0.01 |
| Increasing Age | 1.01(0.94–1.07) | 0.88 |
| Male Gender | 1.73 (0.41–7.34) | 0.46 |
| Diabetes Mellitus | 2.66 (0.66–10.77) | 0.17 |
| Hypertension | 1.16 (0.22–5.99) | 0.86 |
DISCUSSION
This study demonstrates that POAF is a common complication in patients receiving LVAD. POAF following LVAD implantation was not associated with the adverse postoperative outcomes related to length of stay, postoperative stroke, or mortality as observed in other cardiac surgery populations. However, POAF was a significant risk factor for the development of future AF, ischemic stroke, and device thrombosis in the long term after LVAD implantation. Thus, the impact of POAF on overall clinical outcome following LVAD is different from other cardiac surgery populations given their comorbidities, distinct hemodynamics, and requirement for anticoagulation.
In this cohort, the incidence and postoperative time of onset of POAF was comparable to that reported in other populations undergoing coronary artery bypass grafting or valvular surgery.1,2,5–7 This similarity indicates shared mechanisms of POAF in the LVAD population such as post-operative inflammation, oxidative stress, and neurohormonal activation.20 Although there was a trend for a longer cardiopulmonary bypass time in patients with POAF, it was not a predictor for POAF in our cohort. As in other cardiothoracic surgery populations, COPD was a significant risk factor for POAF in patients receiving LVAD.1,5,7,21 COPD has also been identified as an independent risk factor for the development of AF in non-surgical populations as well.22,23 This association is partially mediated by right atrial dilation and elevated right sided filling pressures.22,23 This effect is likely amplified in patients receiving LVAD with increased right ventricular preload and leftward bowing of the septum.24 Conversely atrial tachyarrhythmia can impair right ventricular function in LVAD patients though this was not examined in this study.25
Because this population had mechanical circulatory support, the hemodynamic impact of POAF is likely minimized after the surgery and therefore length of stay and mortality outcomes were not affected. This is consistent with literature demonstrating that only persistent AF is associated with worsened long-term mortality in LVAD patients.17
In patients with POAF after LVAD, there was an increased risk of recurrence of AF and thromboembolic complications in long-term follow up. This finding is consistent with investigations of other populations with secondary AF due to surgery, infection, or myocardial infarction.26–29 This suggests that even with hemodynamic support, the atria of these patients remain significantly diseased and pathologically remodeled. In addition, because patients in this cohort were uniformly anticoagulated unless contraindicated due to bleeding complication, patients with POAF or AF and LVAD may benefit from more aggressive anticoagulation. Indeed, while the inherent thrombogenicity of the LVAD is the most significant factor for thromboembolic events, AF prior to LVAD has been associated with increased thromboembolic events and thromboembolic events at higher levels of anticoagulation.16,17
The present study is limited by its retrospective nature and is a single-center cohort. Given the size of the population, it may be underpowered to fully assess all risk factors for the POAF and may not be able to detect more subtle impacts of POAF on morbidity and mortality. Specifically, pump speed differences between the POAF and no POAF groups may not be represented in this data.30 These results may also not apply to the broader population of end stage heart failure patients who received different circulatory assist devices or had different anticoagulation strategies.
In summary, the incidence of POAF is high in patients receiving a LVAD. Although it is well tolerated in the short and long term, POAF predisposes patients with LVADs for a higher risk of recurrent AF and future thromboembolic events. Awareness of the frequent occurrence of POAF and its complications in patients with LVAD may help to provide better clinical outcomes through earlier detection and treatment in this complex patient population. A larger prospective study to determine further predictors of POAF and the efficacy of alternative long-term anticoagulation strategies in patients with LVAD and AF is warranted.
Acknowledgments
Funding: Cevher Ozcan, MD is supported by the National Institutes of Health (NHLBI Grant # 1K08HL117082-01A1).
Abbreviations
- AF
atrial fibrillation
- POAF
postoperative atrial fibrillation
- CABG
coronary artery bypass grafting
- LVAD
left ventricular assist device
- ECG
electrocardiogram
- CIED
cardiac implantable electronic device
- COPD
chronic obstructive pulmonary disease
Footnotes
Conflicts of Interest: N. Uriel receives consulting fees and research grant support from St. Jude Medical and Medtronic. V Jeevanandam receives consulting fees from St. Jude, Medtronic, and Reliant Heart.
References
- 1.Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Ann Thorac Surg. 1993;56:539–549. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 2.Aranki SF, Shaw DP, Adams DH. Predictors of Atrial Fibrillation After Coronary Artery Surgery: Current Trends and Impact on Hospital Resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 3.Echahidi N, Pibarot P, O’Hara G, Mathieu P. Mechanisms, prevention, and treatment of atrial fibrillation after cardiac surgery. J Am Coll Cardiol. 2008;51:793–801. doi: 10.1016/j.jacc.2007.10.043. [DOI] [PubMed] [Google Scholar]
- 4.Jongnarangsin K, Oral H. Postoperative Atrial Fibrillation. Cardiol Clin. 2009;27:69–78. doi: 10.1016/j.ccl.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Mathew JP, Fontes ML, Tudor IC. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 6.Mathew JP, Parks R, Savino JS. Atrial Fibrillation Following Coronary Artery Bypass Graft Surgery. JAMA. 1996;276:300–3006. [PubMed] [Google Scholar]
- 7.Almassi GH, Schowalter T, Nicolosi AC. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–511. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villareal RP, Hariharan R, Liu BC. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Ishii Y, Schuessler RB, Gaynor SL. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111:2881–2888. doi: 10.1161/CIRCULATIONAHA.104.475194. [DOI] [PubMed] [Google Scholar]
- 10.Kim YM, Kattach H, Ratnatunga C, Pillai R, Channon KM, Casadei B. Association of Atrial Nicotinamide Adenine Dinucleotide Phosphate Oxidase Activity With the Development of Atrial Fibrillation After Cardiac Surgery. J Am Coll Cardiol. 2008;51:68–74. doi: 10.1016/j.jacc.2007.07.085. [DOI] [PubMed] [Google Scholar]
- 11.Mayr M, Yusuf S, Weir G. Combined Metabolomic and Proteomic Analysis of Human Atrial Fibrillation. J Am Coll Cardiol. 2008;51:585–594. doi: 10.1016/j.jacc.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 12.Bruins P, te Velthuis H, Yazdanbakhsh AP. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 13.Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am J Cardiol. 2004;93:1176–1178. doi: 10.1016/j.amjcard.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 14.Hogue CW, Domitrovich PP, Stein PK. RR interval dynamics before atrial fibrillation in patients after coronary artery bypass graft surgery. Circulation. 1998;98:429–434. doi: 10.1161/01.cir.98.5.429. [DOI] [PubMed] [Google Scholar]
- 15.Kalman JM, Munawar M, Howes LG. Atrial fibrillation after coronary artery bypass grafting is associated with sympathetic activation. Ann Thorac Surg. 1995;60:1709–1715. doi: 10.1016/0003-4975(95)00718-0. [DOI] [PubMed] [Google Scholar]
- 16.Stulak JM, Deo S, Schirger J. Preoperative atrial fibrillation increases risk of thromboembolic events after left ventricular assist device implantation. Ann Thorac Surg. 2013;96:2161–2167. doi: 10.1016/j.athoracsur.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez AD, Calenda B, Gandhi PU, Nair AP, Anyanwu AC, Pinney SP. Clinical impact of atrial fibrillation in patients with the HeartMate II left ventricular assist device. J Am Coll Cardiol. 2014;64:1883–1890. doi: 10.1016/j.jacc.2014.07.989. [DOI] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Alpert JS. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Hear J - Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 20.Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061–1073. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 21.Mason DP, Marsh DH, Alster JM. Atrial Fibrillation After Lung Transplantation: Timing, Risk Factors, and Treatment. Ann Thorac Surg. 2007;84:1878–1884. doi: 10.1016/j.athoracsur.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 23.Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012–1016. doi: 10.1183/09031936.03.00051502. [DOI] [PubMed] [Google Scholar]
- 24.Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant. 2015;34:1123–1130. doi: 10.1016/j.healun.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Hottigoudar RU, Deam AG, Birks EJ, McCants KC, Slaughter MS, Gopinathannair R. Catheter ablation of atrial flutter in patients with left ventricular assist device improves symptoms of right heart failure. Congest Heart Fail. 2013;19:165–171. doi: 10.1111/chf.12034. [DOI] [PubMed] [Google Scholar]
- 26.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term Outcomes Following Development of New-Onset Atrial Fibrillation During Sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwich P, Buth KJ, Légaré J-F. New Onset Postoperative Atrial Fibrillation is Associated with a Long-Term Risk for Stroke and Death Following Cardiac Surgery. J Card Surg. 2013;28:8–13. doi: 10.1111/jocs.12033. [DOI] [PubMed] [Google Scholar]
- 28.Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative Atrial Fibrillation and the Long-term Risk of Ischemic Stroke. JAMA. 2014;312:616–622. doi: 10.1001/jama.2014.9143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lubitz SA, Yin X, Rienstra M. Long-Term Outcomes of Secondary Atrial Fibrillation in the Community: The Framingham Heart Study. Circulation. 2015;131:1648–1655. doi: 10.1161/CIRCULATIONAHA.114.014058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maltais S, Killic A, Nathan S. PREVENtion of HeartMate II pump Thrombosis through clinical management: the PREVENT multi-center study. J Heart Lung Transplant. 2017;36:1–12. doi: 10.1016/j.healun.2016.10.001. [DOI] [PubMed] [Google Scholar]