Abstract
Objective:
To compare the diagnostic performance of breast MRI with abbreviated protocol (AB-MRI) and full ddiagnostic protocol (FDP-MRI) for surveillance of females with a personal history of breast cancer
Methods:
In this retrospective study, we analyzed the outcomes of total 1312 post-operative screening breast MRI matched from 1045 AB-MRI and 677 FDP-MRI, which had histologic confirmation for suspicious MRI findings or 1 year negative follow-up images. This study was approved by the institutional review board and informed patient consent was waved. AB-MRI consists of T2 weighted scanning and dynamic contrast-enhanced imaging including one pre-contrast and two post-contrast scans. We compared the diagnostic performance for recurrent breast cancer in terms of sensitivity, specificity, positive-predictive value, negative-predictive value, and accuracy and area under the curve between the screening AB-MRI and FDP-MRI.
Results:
Overall, 13 recurrent tumors among 1312 post-operative cases screened with breast MRI (1.0%) were detected including 8 invasive cancer, 2 cases of in situ cancer, and 3 cases of metastatic lymph nodes. The sensitivity and negative predictive value were 70 vs 100 and 99.5% vs 100% in AB-MRI and FDP-MRI. Specificity, positive predictive value, accuracy, and area under the curve of AB-MRI and FDP-MRI were 98.0% vs 96.9%, 35.0% vs 23.1%, 97.6% vs 97.0%, and 0.840 vs 0.985, respectively.
Conclusion:
The performance of AB-MRI was comparable to that of FDP-MRI in detecting recurrent breast cancer and decreased false positive cases.
Advances in knowledge:
AB-MRI provides a reliable alternative with similar diagnostic performance and shorter MRI acquisition time.
Introduction
Females with a personal history of breast cancer have increased risk of cancer recurrence,1,2 and early detection of secondary breast cancer in the asymptomatic phase can improve survival by 27–47% compared with the detection in the symptomatic phase.3 Several studies have reported that supplemental screening with breast MRI is also very valuable in females with personal history of breast cancer, with high diagnostic performance and acceptable positive biopsy results.4–6 The addition of MRI to screening mammography not only increased cancer detection rate, but also improved the detection of early-stage and biologically aggressive breast cancers.5,6
However, screening breast MRI is not widely used in practice compared with its proven positive results.5,7 First, MRI is still very expensive to perform and may be associated with long acquisition and reading times. Second, MRI showed lower specificity and higher false positive rate than conventional screening mammography.8 In a recent study, abbreviated protocol of breast MRI (AB-MRI) has been introduced by Kuhl et al,9 and is expected to decrease the scan time and reading time without compromising sensitivity or specificity compared with the conventional full diagnostic protocol of MRI (FDP-MRI). The study suggested that the sensitivity and specificity of AB-MRI was comparable to FDP-MRI. Many studies investigating AB-MRI were published after Kuhl et al.5,10–18 However, all these studies compared AB-MRI and FDP-MRI after creating AB-MRI via selection of specific sequences derived from single MRI, which was obtained using FDP-MRI. The performance of AB-MRI was evaluated by independently reviewing the created AB-MRI and FDP-MRI images. Therefore, the results of screening breast MRI using AB-MRI were estimated under simulated conditions. Many studies compared the MR images of AB-MRI and FDP-MRI in a few selected cases with proven pathology enriched with cancers or only in biopsy-proven breast cancer cases.10,11,13–16,18
There were few reports about comparison of diagnostic performance between AB-MRI and FDP-MRI with large patients’ number or matched cohort study. Thus, we hypothesized that a matched-cohort analysis may be able to control nonequivalent clinical-pathologic variables affecting diagnostic performance outcomes of AB-MRI. Therefore, the purpose of our study was to investigate the real performance of screening breast AB-MRI by scanning only abbreviated protocol in patients with a personal history of breast cancer, and compared with that of screening breast FDP-MRI.
Methods and materials
Subjects
This retrospective study was approved by the institutional review board and informed patient consent was waved. Screening breast MRI in patients with a personal history of breast cancer using AB-MRI was initiated in September 2015, and 1255 patients with a personal history of breast cancer underwent screening breast MRIs with AB-MRI between September 2015 and December 2016 in Samsung Medical Center. Before that period, FDP-MRI was used for screening the patients with a personal history of breast cancer. Screening breast MRI was not routinely performed for all patients after breast cancer surgery but for some pre-menopausal females with dense breast tissue or patients who were diagnosed by age 50. In the patients who had screening breast MRI after surgery, annual MRI was performed alone between the scheduled annual mammography with ultrasound alternatively per 6 months for 2–5 years.
We selected 1045 patients with post-operative screening MRIs out of 1255 patients with screening MRIs using AB-MRI, in patients with histologically confirmed lesions or 1 year negative follow-up images. Potential control group were identified among patients with post-operative screening MRIs using FDP-MRI, also histologically confirmed for suspicious lesions or 1 year negative follow-up images from the MRI database: a list of patients who performed MRI examinations including study date, patients’ age and sex, purpose of examinations, applied MRI protocol and machine, referring physician, and results of MRI interpretations, and the name of interpreting radiologist, between April 2008 and September 2015.
We compared the patient’s characteristics of AB-MRI and FDP-MRI groups in terms of mean age at MRI, interval between the breast cancer operation and screening MRI examination, Breast Imaging Reporting and Data System (BI-RADS) category of the screening MRI examination, status of antihormonal treatment, and the stage of the operated cancer. Because the mean age (50.4 ± 9.1 vs 45.6 ± 6.8 years) and the stage of the operated breast cancer varied significantly between the AB-MRI and FDP-MRI groups (p < 0.001, p < 0.05), we performed case matching for objective comparison in statistical analysis, using two variables including patients’ age and stage of the operated breast cancer in the two groups. Finally, 656 matched pairs from AB-MRI and FDP-MRI groups were included for analysis of diagnostic performance of screening breast MRI in both AB-MRI and FDP-MRI groups. Characteristics of 1312 cases from AB-MRI and FDP-MRI after matching the statistically different parameters are summarized in Table 1.
Table 1.
Characteristics of 1312 screening breast MRI
| AB-MRI (n = 656) | FDP-MRI (n = 656) | p-value | |
|---|---|---|---|
| Age at MRI mean (SD)a, y | 46.20 (6.91) | 45.73 (6.81) | 0.211 |
| OP-MR interval (months, range)b | 31.9 (5 ~ 180) | 33.4 (5 ~ 161) | 0.550 |
| MR BI-RADSc | 0.658 | ||
| Positive | 20 | 26 | |
| Negative | 636 | 630 | |
| Antihormonal treatmentd (%) | 546 (83.3%) | 518 (79.0%) | 0.130 |
| Previous cancer stagee (%) | |||
| 0 | 78 (11.9) | 67 (10.2) | 0.705 |
| 1 | 325 (49.5) | 325 (49.5) | |
| 2 | 192 (29.3) | 206 (31.4) | |
| 3 | 61 (9.3) | 58 (8.8) |
ABP, abbreviated protocol;BI-RADS, Breast Imaging Reporting and Data System; FDP, full diagnostic protocol; OP, operation; SD, standard deviation.
a and b: t-test, c–e: χ2 test.
Breast MRI protocol
The protocols of full diagnostic and abbreviated sequences of breast MRI are compared in Table 2.
Table 2.
Protocol of DCE-MRI vs AB-MRI
| FDP-MRI (Full diagnostic protocol) | AB-MRI (Abbreviated protocol) | ||||
|---|---|---|---|---|---|
| Scanning (23 min) | Series of images | Plane | Scanning (9 min) | Series of images | Plane |
| T1 without fat suppression (3 min) | T1 without fat suppression | Axial | T2 without fat suppression (5 min) | T2 without fat suppression | Axial |
| T2 with fat suppression (5 min) |
T2 with fat suppression | Axial | T1 contrast-enhanced dynamic 3D with fat suppression (3 ~ 4 min) Pre-contrast, Post-contrast first, Post-contrast second |
3D dynamic CE images (three phases) |
Axial |
| DWI (3 min) | DWI, ADC map | Axial | |||
| T1 contrast-enhanced dynamic 3D with fat suppression (7 ~ 8 min) Pre-contrast, Post-contrast first, Post-contrast second, Post-contrast third, Post-contrast fourth, Post-contrast fifth, Post-contrast sixth |
3D Dynamic CE images (seven phases) |
Axial | |||
| Standard Subtraction (first – pre, second – pre) |
Axial | Standard subtraction (first – pre, second – pre) |
Axial | ||
| Reversed Subtraction (first – sixth, second – sixth) |
Axial | Reversed subtraction (first – second) |
Axial | ||
| MIP, both breasts | Axial, Sagittal |
MIP, both breasts | Axial, Sagittal |
||
| MPR (second post CE) both | Sagittal | ||||
| Delayed T1 FS CE (axilla) (3 ~ 4 min) |
T1 with fat suppression | Axial | |||
AB-MRI, abbreviated protocol of breast MRI; ADC, apparent diffusion coefficient; CE, contrast-enhanced;3D, three-dimensional; 3D, three-dimensional; DWI, diffusion-weighted images;FDP-MRI, full diagnostic protocol MRI; FS, fat suppression; MIP, maximum intensity projection; MPR, multiplanar reconstruction.
Protocol of FDP-MRI vs AB-MRI.
FDP-MRI
The post-operative MRIs with FDP-MRI were performed using 1.5 T Achieva scanner (Philips Medical Systems, Best, The Netherlands) (n = 255) and 3.0 T Achieva scanner (Philips Medical Systems, Best, The Netherlands) (n = 401) with a dedicated bilateral phased-array breast coil, with the patient in the prone position. The MRI protocol consisted of axial turbo spine-echo T2- and fat-suppressed T2 weighted sequences, and a three-dimensional dynamic contrast-enhanced sequence. Axial dynamic contrast-enhanced images were obtained with one pre-contrast and six post-contrast dynamic series. A 0.1 mmol/kg bolus injection of gadobutrol (Gadovist; Bayer Healthcare, Berlin, Germany) was carried out via an antecubital vein, followed by a 20 ml saline flush. After contrast administration, images were acquired from 30 s, six times per every 60 s, with gradient echo sequence (eTHRIVE) and the acquisition time for one scanning was about 60 s. The parameters on 1.5 T scanner were as follows: repetition time/echo time (ms), 6.5/2.5; 1.5 mm sections without gap; flip angle, 12°; matrix size, 376 × 374; and field of view, 32 × 32 cm. Images with the 3.0 T scanner were obtained under the following parameters: repetition time/echo time (ms), 4.6/2.3; 1.5 mm sections with no gap; flip angle, 24°; matrix size, 512 × 512; and field of view, 32 × 32 cm. The time for scanning ranged from 25 to 27 min. After image acquisition, standard subtraction images (pre-contrast images were subtracted from the early post-contrast images) and reversed subtraction images (the last post-contrast images were subtracted from the early post-contrast images) were obtained automatically on a pixel-by-pixel basis. Reformatted bilateral sagittal images and reformatted three-dimensional maximum intensity projection (MIP) images were also obtained.
AB-MRI
The AB-MRIs were also performed using both 1.5 T Achieva scanner (Philips Medical Systems, Best, The Netherlands) (n = 208) and 3.0 T Achieva scanner (Philips Medical Systems, Best, The Netherlands) (n = 448). AB-MRI consists of axial turbo spin echo T2 weighted imaging and axial dynamic contrast imaging of pre-contrast and two post-contrast sequences. Post-contrast enhanced images were obtained twice from 30 s after contrast injection and the acquisition time for one scanning was about 60 s. Standard subtraction images and reversed subtraction images were generated from pre- and two post-contrast images. Additionally, axial and sagittal MIP images were processed. The total scan time ranged from 10 to 11 min.
Breast MRI analysis
We analyzed the radiologic reports of screening breast MRI from the MRI database. All radiologic reports of AB-MRI and FDP-MRI were made by same radiologist: one of five breast-specialized radiologists with 10–25 years of experience in breast imaging, according to the American College of Radiology BI-RADS. Both breasts, both axillae and internal mammary areas were evaluated and reported. We included BI-RADS category four or five results in positive results and performed MR-guided biopsy or second look ultrasound with or without ultrasound-guided biopsy. If the lesion was too small to do biopsy, or when the patients refused biopsy, follow-up MRIs were performed. BI-RADS category 1, 2 and 3 assessments were included in negative MRI results and follow-up MRI or routine post-operative follow-up including mammography and ultrasound with or without MRI were conducted.
Statistical analysis
We compared the diagnostic performance of MRI for detection of recurrent breast cancer in terms of sensitivity, specificity, positive-predictive value (PPV), negative-predictive value (NPV), and accuracy and area under the curve (AUC) between the AB-MRI and FDP-MRI. We analyzed the statistical differences of patient’s ages, the interval between operation and MRI examination and cancer stage between the two groups of AB-MRI and FDP-MRI using t-test and χ2 test. We selected matched cases from AB-MRI and FDP-MRI groups using propensity score matching (caliper = 0.5), and compared the diagnostic performance between the two matched groups of breast MRIs. All statistical analyses were performed using software (SAS, v. 9.0.0 v. 9.0.0, SAS Institute Inc., Cary, NC). A p-value of less than 0.05 was considered statistically significant.
Results
Outcomes of screening MRI
Overall, 13 recurrent tumors were detected among 1312 post-operative screening breast MRI using AB-MRI and FDP-MRI (13/1312, 1.0%). Eight cases were diagnosed as invasive cancer; two cases were in situ cancer and three cases were metastatic lymph nodes. One invasive ductal carcinoma (IDC) was detected in contralateral breast and others were detected in ipsilateral breast and internal mammary area. According to the BI-RADS classification, category 1, 2, and 3 were considered as negative results and category 4, 5 were considered as positive results, and followed by histological confirmation or further imaging evaluation. In the patients with negative MRI results, follow-up MRI was performed after 1 year (69%), however, some of them had only follow-up mammography, ultrasound, and clinical records. In the AB-MRI group, 636 MRIs (97.0%) were assessed as negative results and 20 MRIs (3.0%) represented positive results. Among the 20 positive MR results in AB-MRI group, 13 were histologically confirmed. 7 out of 13 histologically confirmed lesions were malignant (Figure 1), and underwent completion mastectomy, adjuvant chemotherapy, or chemotherapy with radiation therapy.
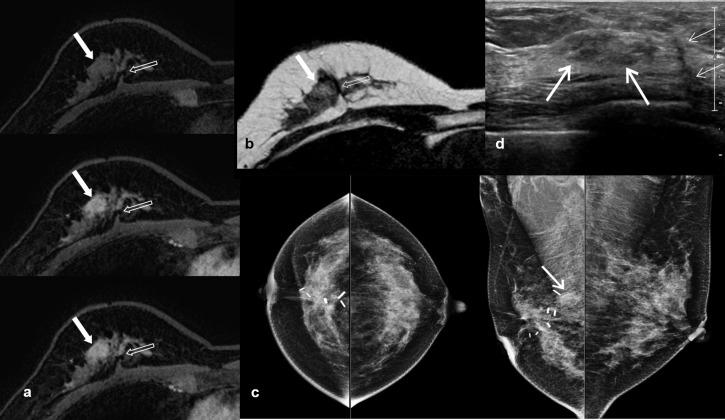
Figure 1.
A case of recurrent breast cancer using post-operative screening MRI with AB-MRI. A 50-year-old female, who had right breast conserving surgery for ductal carcinoma in situ 3 years ago, underwent post-operative screening MRI. Dynamic contrast-enhanced images (a) consisted of pre-, and two post-contrast sequences and axial T2 weighted images without fat suppression (b) demonstrated 1.4 cm irregular mass (arrow) adjacent to the post-operative scar (opened arrow). Mammography (c) revealed asymmetry corresponding to the lesions on screening MRI only in the right MLO view (arrow). The targeted breast ultrasound (d) showed an area of parenchymal heterogeneity (arrows) in the right breast, and the lateral side-of scar (thin arrows). Ultrasound-guided core needle biopsy of this lesion showed recurrent ductal carcinoma in situ. AB-MRI, abbreviated protocol of breast MRI; MLO, mediolateral oblique.
Seven patients with positive MRI results were followed up without biopsy. Three had corresponding benign-looking lesions on ultrsound and mammography, and showed no interval change on follow up MRI (n = 2) or ultrasound (n = 1) after 1 year. Four lesions showed no corresponding lesions on both spot compression and magnification view of mammography and targeted ultrasound, and MR-guided biopsies were recommended. However, they refused MR-guided biopsy and follow-up MR images after 1 year showed no change (n = 2), decreased (n = 1), or disappeared (n = 1) lesions with negative mammography and ultrasound. All those seven lesions were considered as false-positive lesions.
Among the 636 patients with negative MR results in AB-MRI group, recurrences were diagnosed in 3 (0.5%) patients before the next screening MRI and were considered as false-negative cases. One patient had interval palpable cancer in contralateral breast 7 months after the negative screening MRI, and the other two cancers were detected by alternative screening mammography (n = 1) (Figure 2) and screening ultrasound (n = 1) 6 months after the negative screening MRI. Two IDCs were minimal cancers (0.7 cm) with negative lymph node. Palpable interval cancer was high grade and screening mammography detected cancer was intermediate grade. One ultrasound-detected lesion was metastatic lymph node in ipsilateral axilla without breast lesion.
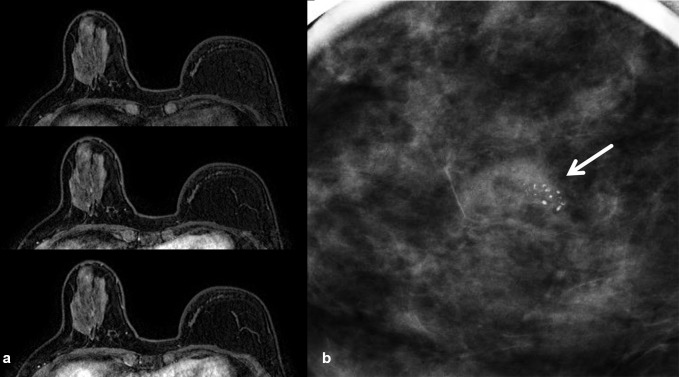
Figure 2.
A case of contralateral recurrent breast cancer which was not apparent on AB-MRI. A 53-year-old female, who had left total mastectomy with flap reconstruction for IDC 1.5 years ago, underwent post-operative screening MRI. Dynamic contrast-enhanced images (a) consisted of pre-, and two post-contrast sequences showed no abnormal enhancement in right breast. Screening mammography 6 months after the AB-MRI (b) showed grouped coarse heterogeneous microcalcifications (arrow) in right upper outer breast. Stereotactic vacuum-assisted biopsy was performed and the lesion was confirmed as ductal carcinoma in situ. Following surgery revealed intermediate grade invasive ductal carcinoma. AB-MRI, abbreviated protocol of breast MRI; IDC, invasive ductal carcinoma.
In FDP-MRI group, 630 MRIs (96.0%) were negative and 26 MRIs (4.0%) were positive. Among 26 cases with positive MR results, 6 out of 18 histologically confirmed lesions were recurrent cancers. One patient with metastatic internal mammary lymph received palliative radiation and chemotherapy. The other patients received completion total mastectomy, contralateral breast conserving surgery and radiation therapy, with or without adjuvant chemotherapy.
Eight cases were followed up without biopsy. Two of them were not reproduced by the scanning for localization at MR-guided biopsy. Five cases were followed up with MRI. Four lesions that were disappeared or decreased on follow-up MRI, were downgraded to BI-RADS 1 or 2. One lesion showed no change on follow-up MRI and downgraded to BI-RADS 3 after 1 year. Three lesions that showed typical benign findings on targeted ultrasound and mammography were considered as benign and followed up with only mammography and ultrasound. The results of screening breast MRI and histologic or follow-up imaging results are summarized in Table 3.
Table 3.
Outcomes of screening breast MRI
| Results of MRI | Mode of confirmation | Method of biopsy | Results of biopsy | ||
|---|---|---|---|---|---|
| AB-MRI (n = 656) | Positive (20) | Biopsy or FNA (13) | Ultrasound-guided CNB (11) | Malignant (5) | IDC (3) ILC (1) DCIS (1) |
| Benign (6) | Fat necrosis (2) Stromal fibrosis (1) Usual ductal epithelial hyperplasia (1) Intrauctal papilloma (1) Fibroadenoma (1) |
||||
| Ultrasound-guided FNA (2) | Malignant (2) | Metastatic carcinoma (internal mammary LN) | |||
| Follow up (7) | Benign (7) | ||||
| Negative (636) | Biopsy or FNA (3) | Ultrasound-guided CNB (2) | Malignant (2) | IDC | |
| Ultrasound-guided FNA (1) | Malignant (1) | Metastatic carcinoma (axillary LN) | |||
| Follow up (633) | Negative results of follow up images after 1 year (633) | ||||
| FDP-MRI (n = 656) | Positive (26) | Biopsy or FNA (18) | Ultrasound-guided CNB (6) | Malignant (3) | IDC (1) Metaplastic carcinoma (1) DCIS (1) |
| Benign (3) | Stromal fibrosis (2) Nonspecific benign (1) |
||||
| Ultrasound-guided FNA (1) | Malignant (1) | Metastatic carcinoma (internal mammary LN) | |||
| MR-guided VAB (9) | Malignant (1) | IDC | |||
| Benign (8) | Sclerosing adenosis (1) Stromal fibrosis (1) Atypical ductal hyperplasia (1) Fibrocystic change (1) Chronic inflammation (1) Fibroadenomatous mastopathy (1) Non-specific benign (2) |
||||
| Ex after MR-guided localization (2) | Malignant (1) | Invasive cribriform carcinoma | |||
| Benign (1) | Atypical ductal hyperplasia | ||||
| Follow up (8) | Benign (8) | ||||
| Negative (630) | Follow up (630) | Negative results of follow up images after 1 year (630) | |||
CNB, core needle biopsy; FNA, fine needle aspiration; VAB, vacuum-assisted biopsy; Ex, excisional biopsy; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; DCIS, ductal carcinoma in situ; LN, lymph node.
Diagnostic performance: AB-MRI vs FDP-MRI
Diagnostic performances of matched 656 cases are listed in Table 4. Sensitivity was better in the FDP group (70.0% vs 100%), while PPV was higher in the AB-MRI group than the FDP-MRI group (35.0% vs 23.1%) suggesting a decreased number of false-positive cases with AB-MRI (Figure 3). Specificity and accuracy were slightly better in AB-MRI group. However, all the results showed no statistical significance.
Table 4.
Diagnostic performance of 1312 screening MRI with 1-year follow-up
| Index | AB-MRI (n = 656) | FDP-MRI (n = 656) | p-value | |
|---|---|---|---|---|
| Original | Bonferroni corrected | |||
| Sensitivity | 70.0% (7/10) | 100% (6/6) | 0.4083 | 1 |
| Specificity | 98.0% (633/646) | 96.9% (630/650) | 0.2983 | 1 |
| PPV | 35.0% (7/20) | 23.1% (6/26) | 0.5755 | 1 |
| NPV | 99.5% (633/636) | 100% (630/630) | 0.2510 | 1 |
| Accuracy | 97.6% (640/656) | 97.0% (636/656) | 0.6122 | 1 |
| AUC | 0.840 | 0.985 | >0.999 | |
AB-MRI, abbreviated protocol of breast MRI; AUC, area under the curve;FDP-MRI, full diagnostic protocol-MRI; NPV, negative predictive value;PPV, Positive predictive value.
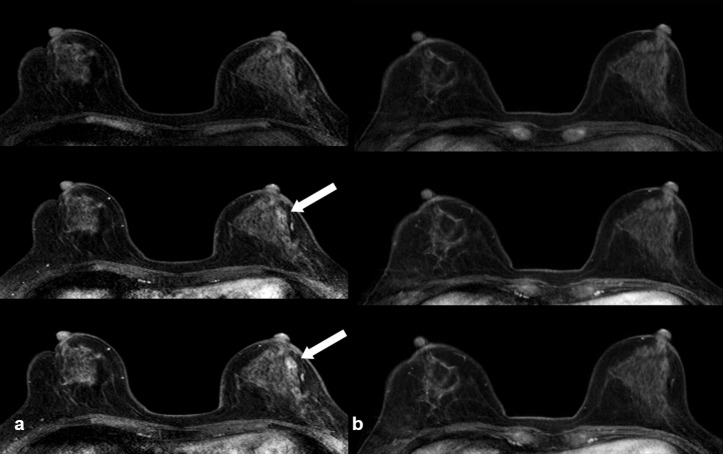
Figure 3.
A case of benign mass using FDP-MRI but not apparent under AB-MRI. A 55-year-old female underwent bilateral breast-conserving surgery for IDC. Post-operative screening MRI using FDP-MRI 2 years after left breast conserving surgery showed a 1 cm-size benign mass in the left mid-outer breast (arrow), especially well demarcated in the delayed image (pre-, first- and sixth post-contrast images) (a). Follow-up MRI using AB-MRI was performed after a year, and benign mass on previous MRI was not seen on AB-MRI (b). AB-MRI, abbreviated protocol of breast MRI; FDP-MRI, full diagnostic protocol-MRI; IDC, invasive ductal carcinoma.
Discussion
The diagnostic performance of AB-MRI was comparable to that of FDP-MRI in our study. Even though, the overall sensitivity of AB-MRI was low to 70% (78% for in-breast recurrence), only one IDC was palpable true interval cancer and the other two lesions were asymptomatic small breast lesion and axillary lymph node that were detected on the alternative screening mammography and ultrasound after 6 months.
Small number of overall recurrence (16/1312) also affected decreased sensitivity. NPV of ABP was as high as in FDP (99.5% vs 100%). Furthermore, the specificity, PPV, and accuracy of AB-MRI was slightly higher than in FDP-MRI (98.0 vs 96.9%, 35.0 vs 23.1%, 97.6 vs 97.0%, respectively), which indicated that false-positive findings were less frequent with the AB-MRI. Considering the perspective of high false-positive rate of MRI, Wernli et al19 recently reported that breast MRI leads higher biopsy rate compared with mammography in female with a personal history of breast cancer. In our study, AB-MRI showed increased PPV and low false-positive rate without compromising the other diagnostic performance parameters, which suggested the possible increase in diagnostic yield of invasive cancer by AB-MRI via reduction in the major drawbacks of MRI such as low specificity and high false-positive findings leading to unnecessary short-term follow-up or biopsy.
Breast MRI is a well-recognized diagnostic modality with high sensitivity for the detection of breast cancer, and MRI differentiated recurrent tumors from morphological distortion and contour deformity of breast parenchyma due to previous surgery, compared with conventional mammography or ultrasound in many studies8,20 reviewed 35 published studies involving breast MRI for the detection of recurrent tumor compared with conventional modalities, and reported that breast MRI showed high sensitivity (75–100%) for detection of recurrent cancer in females treated with breast conserving surgery and radiation therapy or total mastectomy. Cho et al5 showed that addition of breast MRI to routine mammography in patients who had breast conserving treatment increased sensitivity and the detection rate of recurrent cancer, compared with mammography alone. They reported that all detected cancers were clinically negative and most of them were Stage 0 or 1 (13/17, 76.5%).
Although the potential of breast MRI as a screening tool is well established, its use in the surveillance of females with a personal history of breast cancer was disputed. Until the recent release of American College of Radiology guideline for breast MRI screening,21 screening breast MRI in a female with a personal history of breast cancer was considered to have insufficient evidence to recommend for or against MRI screening. Now, the most recent recommendations for annual screening breast MRI included females with BRCA gene mutation carriers and their untested first-degree relatives, females who received radiation therapy to the chest between ages of 10 and 30 years, females with calculated lifetime risk of 20% or more, females with personal histories of breast cancer and dense breast tissue or those diagnosed before age 50. MRI also should be considered for females with atypical ductal hyperplasia, atypical lobular hyperplasia, or lobular carcinoma in situ, especially if other risk factors are present.
However, the high cost and long scanning time of MRI are important limitations of screening MRI. AB-MRI is expected to provide a cost-effective solution, if confirmed by future trials. The simplified breast MRI using AB-MRI decreases both scan time and reading time. In the study by Kuhl et al,9 scan time decreased from 17 to 3 min, and reading time decreased to 28 s. Other subsequent studies also reported decreased scan times (4.4 ~ 15 min) and interpretation time (44 s ~ 1.55 min).12,15 Decreased reading time also contributes to the use of screening breast MRI by decreasing the work load of radiologists. In our study, scan time decreased from 27 to 11 min. Nevertheless, our AB-MRI comprised two phases of post-contrast enhanced imaging and our T2 weighted images covered high axillary areas for detection of recurrent lymph nodes.
The diagnostic performance of AB-MRI as a screening tool was excellent in several reader studies with 89 ~ 100% sensitivity without decreasing the specificity compared with the FDP-MRI.9,10,13,17 However, there are a few possible limitations associated with AB-MRI. Because of the lack of delayed dynamic contrast images, interpretation or identification of lesions may be a challenge. A few lesions apparent only on delayed contrast-enhanced images may be missed. Most of the benign lesions with mild and delayed enhancement or in situ carcinoma with subtle enhancement on the delayed phase may be affected. In some cases of breast cancer, the detailed characterization of lesion was insufficient if only the main invasive area was enhanced in the early phase of contrast enhancement.
In our study, false-negative cases on AB-MRI were consisted of one interval palpable contralateral breast recurrence, one contralateral breast recurrence visualized only on follow-up mammography as suspicious microcalcifications and one ipsilateral axillary lymph node metastasis detected only on follow-up ultrasound. After retrospective image review, we concluded that they were not apparently visualized on AB-MRI performed before 6 and 7 months before the detection of recurrence. They could be possibly visualized on FDP-MRI in delayed phase but it wasn’t certain considering calcifications often not showed abnormal enhancement on MRI examination.22 Mover, considering the primary role of screening MRI is to detect cancer, additional scanning after diagnosis of breast cancer may be needed. Furthermore, our goal was to screen for invasive cancer using the screening breast MRI rather than in situ carcinoma, and most of the malignant lesions that show mild enhancement only on delayed phase were low grade and/or in situ carcinoma.
Our study has some limitations. First, we obtained screening breast MRI using FDP-MRI and AB-MRI in different periods. We used FDP-MRI in breast MRI for post-operative surveillance before September 2015, and subsequently obtained screening breast MRI using AB-MRI. We reviewed and analyzed the radiological report of screening breast MRI and medical records of the patients instead of reviewing MR images retrospectively. Because the results of screening breast MRI in post-operative patients are affected by the experience of interpreting radiologists, the different periods of breast MRI acquisition might affect the study results including low false-positive results of AB-MRI. Second, we did not match previous treatments including operation type (breast conserving surgery or mastectomy), chemotherapy, and radiation therapy between AB-MRI and FDP-MRI groups. However, the post-operative screening MRI was usually recommended for the pre-menopausal females with breast conserving surgery and dense breasts. We also did not match the background parenchymal enhancement of screening MRI, because radiologic reports before 2015 did not have the description of background parenchymal enhancement in many cases. Third, all patients were not followed up with screening MRI after the first screening MRI, some patients were followed up with only mammography and ultrasound after 1 year especially when the previous screening MRI was negative. Another limitation is that the total number of recurrence is relatively small (16/1312) to conclude any statistically significant difference between AB-MRI and FDP-MRI. However, our cancer detection rete of 10/1000 was similar to those (10/1000 ~ 15/1000) of the previous studies13,16 and there surely was a trend of higher PPV of AB-MRI than FDP-MRI. Finally, we analyzed the diagnostic performance based on the presence of recurrent tumor after 1 year follow-up of clinical record and images. All patients with negative MRI results had follow-up images although some of them underwent only follow-up mammography and ultrasound with clinical examination.
In conclusion, the performance of AB-MRI was comparable to that of FDP-MRI in detecting recurrent breast cancer and decreased false-positive cases. AB-MRI provides a reliable alternative with similar diagnostic performance and shorter MRI acquisition time in the MRI surveillance of females with a personal history of breast cancer.
Contributor Information
Ko Woon Park, Email: kowoon.park@samsung.com.
Sol Bee Han, Email: justinapark@hanmail.com.
Boo-Kyung Han, Email: bookyung.han@samsung.com.
Eun Sook Ko, Email: es.ko@samsung.com.
Ji Soo Choi, Email: jisoo.choi@samsung.com.
Sun Jung Rhee, Email: sj.rhee@samsung.com.
Eun Young Ko, Email: claudel@skku.edu.
REFERENCES
- 1.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-Year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347: 1233–41. doi: 10.1056/NEJMoa022152 [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-Year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 2002; 347: 1227–32. doi: 10.1056/NEJMoa020989 [DOI] [PubMed] [Google Scholar]
- 3.Houssami N, Ciatto S, Martinelli F, Bonardi R, Duffy SW. Early detection of second breast cancers improves prognosis in breast cancer survivors. Ann Oncol 2009; 20: 1505–10. doi: 10.1093/annonc/mdp037 [DOI] [PubMed] [Google Scholar]
- 4.Arazi-Kleinman T, Skair-Levy M, Slonimsky E, Maly B, Uziely B, Libson E, et al. Journal Club: is screening MRI indicated for women with a personal history of breast cancer? analysis based on biopsy results. AJR Am J Roentgenol 2013; 201: 919–27. doi: 10.2214/AJR.11.8450 [DOI] [PubMed] [Google Scholar]
- 5.Cho N, Han W, Han B-K, Bae MS, Ko ES, Nam SJ, et al. Breast cancer screening with mammography plus ultrasonography or magnetic resonance imaging in women 50 years or younger at diagnosis and treated with breast conservation therapy. JAMA Oncol 2017; 3: 1495–502. doi: 10.1001/jamaoncol.2017.1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schacht DV, Yamaguchi K, Lai J, Kulkarni K, Sennett CA, Abe H. Importance of a personal history of breast cancer as a risk factor for the development of subsequent breast cancer: results from screening breast MRI. AJR Am J Roentgenol 2014; 202: 289–92. doi: 10.2214/AJR.13.11553 [DOI] [PubMed] [Google Scholar]
- 7.Brennan S, Liberman L, Dershaw DD, Morris E. Breast MRI screening of women with a personal history of breast cancer. AJR Am J Roentgenol 2010; 195: 510–6. doi: 10.2214/AJR.09.3573 [DOI] [PubMed] [Google Scholar]
- 8.Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012; 307: 1394–404. doi: 10.1001/jama.2012.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers R-D, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and Maximum-Intensity Projection—A novel approach to breast cancer screening with MRI. JCO 2014; 32: 2304–10. doi: 10.1200/JCO.2013.52.5386 [DOI] [PubMed] [Google Scholar]
- 10.Chen S-Q, Huang M, Shen Y-Y, Liu C-L, Xu C-X. Abbreviated MRI protocols for detecting breast cancer in women with dense breasts. Korean J Radiol 2017; 18: 470–5. doi: 10.3348/kjr.2017.18.3.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol 2015; 22: 1157–62. doi: 10.1016/j.acra.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 12.Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol 2016; 13: 374–80. doi: 10.1016/j.jacr.2015.08.015 [DOI] [PubMed] [Google Scholar]
- 13.Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol 2016; 85: 815–23. doi: 10.1016/j.ejrad.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 14.Machida Y, Shimauchi A, Kanemaki Y, Igarashi T, Harada M, Fukuma E. Feasibility and potential limitations of abbreviated breast MRI: an observer study using an enriched cohort. Breast Cancer 2017; 24: 411–9. doi: 10.1007/s12282-016-0718-z [DOI] [PubMed] [Google Scholar]
- 15.Mango VL, Morris EA, David Dershaw D, Abramson A, Fry C, Moskowitz CS, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol 2015; 84: 65–70. doi: 10.1016/j.ejrad.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 16.Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated combined Mr protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer 2016; 16: 207–11. doi: 10.1016/j.clbc.2016.02.008 [DOI] [PubMed] [Google Scholar]
- 17.Petrillo A, Fusco R, Sansone M, Cerbone M, Filice S, Porto A, et al. Abbreviated breast dynamic contrast-enhanced MR imaging for lesion detection and characterization: the experience of an Italian oncologic center. Breast Cancer Res Treat 2017; 164: 401–10. doi: 10.1007/s10549-017-4264-y [DOI] [PubMed] [Google Scholar]
- 18.Strahle DA, Pathak DR, Sierra A, Saha S, Strahle C, Devisetty K. Systematic development of an abbreviated protocol for screening breast magnetic resonance imaging. Breast Cancer Res Treat 2017; 162: 283–95. doi: 10.1007/s10549-017-4112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wernli KJ, Ichikawa L, Kerlikowske K, Buist DSM, Brandzel SD, Bush M, et al. Surveillance breast MRI and mammography: comparison in women with a personal history of breast cancer. Radiology 2019; 292: 311–8. doi: 10.1148/radiol.2019182475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn EM, Coveney AP, Redmond HP. Use of magnetic resonance imaging in detection of breast cancer recurrence: a systematic review. Ann Surg Oncol 2012; 19: 3035–41. doi: 10.1245/s10434-012-2341-3 [DOI] [PubMed] [Google Scholar]
- 21.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J Am Coll Radiol 2018; 15(3 Pt A): 408–14. doi: 10.1016/j.jacr.2017.11.034 [DOI] [PubMed] [Google Scholar]
- 22.Bazzocchi M, Zuiani C, Panizza P, Del Frate C, Soldano F, Isola M, et al. Contrast-Enhanced breast MRI in patients with suspicious microcalcifications on mammography: results of a multicenter trial. AJR Am J Roentgenol 2006; 186: 1723–32. doi: 10.2214/AJR.04.1898 [DOI] [PubMed] [Google Scholar]