Abstract
Non-tuberculous mycobacterial pulmonary disease is growing in incidence and prevalence. However, it is frequently overlooked as a differential diagnosis by both clinicians and radiologists alike due to its non-specific clinical features, wide spectrum of radiological findings and difficulty in isolating the causative organism. The aim of this article is to illustrate the spectrum and follow-up of the radiological findings of non-tuberculous mycobacterial pulmonary disease and the challenges involved in making a diagnosis.
Introduction: what is NTM?
The non-tuberculous mycobacteria (NTM) are classified as mycobacterial species other than Mycobacterium tuberculosis (TB) and Mycobacterium leprae (M. leprae).1 NTM are ubiquitous environmental organisms found in soil and water with over a 150 NTM species identified.2
NTM can cause lung, sinus, lymph node, central nervous system and disseminated infection.1 The NTM species commonly recognised as causing NTM-PD are Mycobacterium avium complex known as MAC (includes avium and intracellulare species), M. kansasii, M. xenopi, M. abscessus, M. fortuitium and M. chelonae.2 NTM species can be subclassified into slow or fast growers with the faster growers (M. abscessus, M. fortuitinium and M. chelonae) associated with inflammatory lung damage.1
Epidemiology and risk factors
There have been multiple studies that have reported increased prevalence of NTM-PD disease over the past four decades.1 In the UK, it has been reported that there has been an increase in NTM positive cultures from 4.0/100,000 in 2007 to 6.1/100,000 in 2012.1 Improved laboratory detection, with increased awareness may account for these findings. However, there is evidence to suggest that the increase is true and explained by changing environmental and host factors.1
Environmental factors include better survival of the organism in home water heaters, increased exposure to contaminated water and soil due to swimming, showers, hot tubs and gardening.1 Host susceptibility has also changed over time with an increased immunosuppressed population either due to increasing use of immunosuppressive therapy and HIV/AIDS.1 It has been postulated that host and population immunity to NTM has decreased due to the declining rates of TB.1 Increasing antibiotic use in chronic lung disease has also been implicated.1
Other risk factors associated with NTM-PD include chronic lung diseases such as chronic pulmonary obstructive lung disease, cystic fibrosis, low body mass index, low vitamin D, malnutrition, gastro-oesophageal reflux disease and rheumatoid arthritis.1 A well-recognised phenomenon is “Lady Windermere syndrome”, which occurs classically in tall elderly ladies with pectus excavatum and mitral valve prolapse.1 Person to person transmission of NTM-PD is unusual though has been found in patients with cystic fibrosis.1
Diagnostic criteria and clinical features
NTM-PD is notoriously difficult to diagnose due to its non-specific clinical presentation, wide spectrum of radiological findings and difficulty in isolating the causative organism.3 In addition, NTM can reside within the lung without causing any lung damage.1
Due to difficulty in diagnosis, the American Thoracic Society (ATS) and Infectious diseases society of America has released diagnostic criteria which has also been incorporated in the British Thoracic Society (BTS) guidelines. These include clinical, radiological and mycobacterial findings (Table 1).1,4
Table 1.
Clinical and microbiological diagnostic criteria for the diagnosis of NTM-PD
|
Clinical/Radiological 1a. Pulmonary symptoms 1b. Radiological—nodules, cavities and airways disease with bronchiectasis, bronchial wall thickening, mucus plugging and centrilobular nodules. 2. Appropriate exclusion of other diagnoses such as lung carcinoma and TB |
Microbiological
|
AFB, acid-fast bacteria; ATS, American Thoracic Society; NTM, non-tuberculous mycobacteria; NTM-PD, non-tuberculous mycobacterial pulmonary disease.
Adapted from BTS and ATS guidelines.1,4
Radiology
The chest radiograph is the initial investigation for diagnosing NTM-PD though it is often normal or non-diagnostic. Consequently, the British Thoracic Society guidelines advise that CT thorax should be performed in all suspected cases of NTM-PD.1 The CT examination of the thorax should be a supine inspiratory volumetric contiguous scan with high-resolution sections with an ideal slice thickness of 1 mm. The examination can be done with or without intravenous contrast enhancement depending on clinical symptoms, signs and chest radiograph findings.
To our knowledge there are no recognised guidelines as to how patients with confirmed NTM-PD should be followed up with imaging with decisions often made due to worsening clinical symptoms and signs or chest radiograph changes.1
There are three recognised radiological presentations of NTM-PD: fibrocavitatory, nodular-bronchiectatic and hypersensitivity pneumonitis often described as “hot tub lung”.
Fibrocavitatory disease
Fibrocavitatory NTM-PD is often seen in patients with pre-existing lung disease and commonly seen in elderly white males.5 It is characterised by cavities with an upper lobe predominance (Figure 1). The cavities can be small and thin walled with associated centrilobular nodules due to endobronchial involvement.5,6 Pleural thickening can also be a feature.5,6 It is often difficult to differentiate the fibrocaviatory form from TB or malignancy based on radiology alone (Figure 1).7 Fibrocavitatory disease is associated with an increased finding of aspergilloma.8
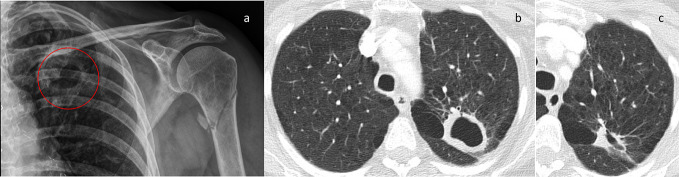
Figure 1.
Representative images of a patient with fibrocavitatory NTM-PD due to M. kansasii. (a) A cavity (circled) was incidentally found in the left upper lobe of the lung on a shoulder radiograph of a patient who presented with a traumatic fracture of their left humerus. (b) Axial CT image of the chest demonstrates a thick walled cavity in the left upper lobe with background emphysematous disease. The differential diagnosis and clinical concern was of a cavitating squamous cell primary lung carcinoma as the patient had a smoking history. M. kansasii was grown post-bronchoscopic lavage. (c) Axial CT image of the chest from the same patient after appropriate treatment demonstrates an interval reduction in size of the cavity.
Nodular-bronchiectatic
Nodular-bronchectatic NTM-PD is often seen in patients with no known pre-existing lung disease.5 It is characterised by bronchiectasis, bronchial wall thickening with mucus plugging, multiple nodules (including centrilobular tree-in-bud nodularity) and occasionally focal consolidation5,6 (Figure 2). The nodular-bronchiectatic form can be subdivided into two subgroups of mild cylindrical bronchiectasis with nodules and extensive more severe bronchiectasis of the varicose and cystic type with tree-in-bud centrilobular nodules.9
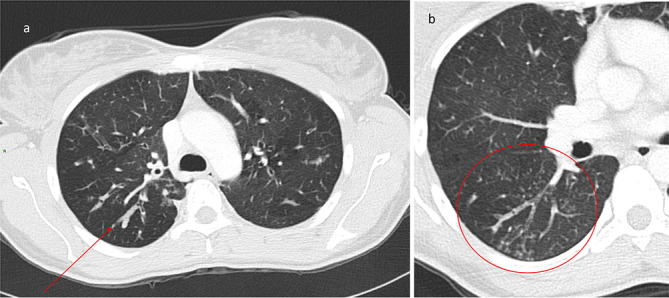
Figure 2.
Representative images demonstrating nodular-bronchiectatic NTM-PD. The organism isolated was MAC. (a) Axial CT image of the chest at the level of the inferior pulmonary veins which shows bronchiectasis in the middle lobe with partial collapse (thick arrow) and tree-in-bud nodularity of the posterior segment of the right lower lobe (thin arrow). (b) Axial CT image of the chest in another patient demonstrates bronchiectasis in the right lower lobe with associated nodularity. In addition, there is also the ancillary signs of an air-fluid level within the oesphagus suggesting possible gastrooesphageal reflux (arrow) and chest wall deformity. MAC, Mycobacterium avium complex
Solitary lobar involvement can occur with the middle lobe followed by the right upper lobe most commonly affected.10
Peripheral bronchial mucus plugging is also a feature of nodular-bronchiectatic NTM-PD compared with patients with bronchiectasis who are NTM negative.8 The mucus plugging is often seen as subsegmental bronchial occlusion containing air bubbles or lucencies and in our experience raises the possibility of unsuspected NTM infection (Figure 3).
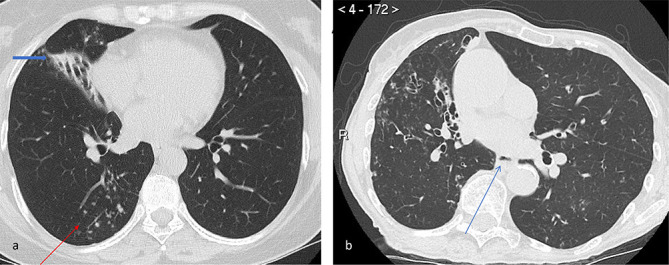
Figure 3.
Axial CT images from a patient with variant cystic fibrosis who cultured both MAC and abscessus. (a) There is mucous plugging in the posterior segment of the right upper lobe with associated thickened and dilated bronchi (arrow). Note the presence of air trapping throughout the lungs. (b) A further axial slice from the same patient of the apical segment of the right lower lobe. Mucous plugging with air bubbles in the mucus can be clearly seen (circled). There is also associated centrilobular nodularity. MAC, Mycobacterium avium complex.
There is a subgroup of predominantly elderly females with no pre-existing disease who culture MAC or occasionally M. kansasii where the findings are confined to the middle lobe or lingula.1,5 This has been termed “Lady Windermere syndrome” and is thought to be secondary to cough suppression.5
It is recognised that NTM in its nodular form can present as a solitary nodule or mass often as an incidental finding mimicking malignancy or a tuberculoma.11 These are often avid on CT-positron emission tomography imaging requiring biopsy or excision to confirm the diagnosis.11
Hypersensitivity syndrome—“hot-tub lung”
This presentation occurs secondary to a hypersensitivity pneumonitis to MAC following inhalation of aerosolised contaminated water often found in those patients using a hot-tub.12 The chest radiograph can be normal, but can show, diffuse fine nodular and reticulonodular opacities and areas of consolidation. CT of the thorax shows ground-glass opacification, centrilobular nodules and air trapping similar to the CT findings in subacute hypersensivity pneumonitis.12 Treatment commonly involves removal from the contaminated water.12
Ancillary findings
Additional findings can be observed in patients who present with the fibrocavitatory and nodular-bronchiectatic forms of NTM-PD, which may help the radiologist raise the possibility of NTM-PD. These include decreased body fat and muscle (sarcopaenia) , chest wall deformities, dilated oesophagus which may contain an air-fluid level and hiatus hernia associated with gastro-oesophageal reflux disease5,6 (Figure 2) .
Special groups
The immunosuppressed population should be considered separately as their radiological presentation can differ due to altered immune status.
Within the HIV/AIDS population, MAC tends to be a more common organism and often coexists with other opportunistic organisms. As a result, it is difficult to ascertain how much of the findings are due to MAC alone. However, in studies focussing on this population, mediastinal lymphadenopathy which may be necrotic13 seems to be the most common manifestation, with airspace opacities, miliary nodules, and pleural effusion reported infrequently.5 Normal chest radiographic findings are not infrequent.5
In the non-HIV immunocompromised population, a recent case–control study demonstrated that the non-HIV immunosuppressed population demonstrated more ill-defined and larger nodules which can be more than 2 cm in size with additional cavitation more commonly seen when compared with immunocompetent NTM-PD patients.14
Imaging prognostic factors9
It is often difficult to predict outcomes of patients with NTM-PD as the clinical course is variable. There are three recognised distinct disease patterns of progression identified on CT:
Fibrocavitary disease: severe cavitation, consolidation and extensive bronchiectasis.
Nodular-bronchiectatic: mild bronchiectasis and nodules.
Nodular-bronchiectatic: extensive bronchiectasis and high prevalence of tree-in-bud opacification.
The highest mortality is seen in Group 1 (Figure 4). These patients are often older, have a higher c-reactive protein and platelet count with lower serum albumin and body mass index. Patients who exhibit mild bronchiectasis with nodules often exhibit a more indolent course (Figure 5). Disease progression in nodular-bronchiectatic NTM has been shown to be related to extent of disease.10
Figure 4.
Patients with severe cavitation, consolidation or extensive bronchiectasis often have the worst prognosis. A series of axial CT images of the chest from July 2016 to Sept 2017 of a patient with M. abscessus infection demonstrating progression and regression of multiple lung cavities over time who presented with life-threatening haemoptysis. The last CT image showing a large left upper lobe cavity with central opacification due to haemorrhage.
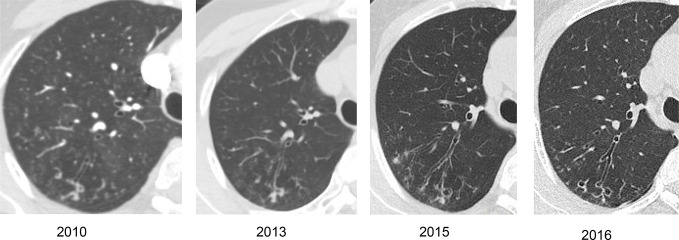
Figure 5.
Serial axial CT images of the chest of a female patient with no existing lung disease from whom MAC was repeatedly isolated. Over time there has been slow progression of bronchiectasis and nodularity in the posterior segment of the right upper lobe. MAC, Mycobacterium avium complex.
Current issues
While the incidence of TB has been decreasing, the incidence and prevalence of NTM-PD has been increasing.1 This is associated with increased morbidity and mortality and increased costs to healthcare system.1 One study has found that the cost of NTM-PD was four-fold higher than those for matched controls with hospitalisation comprising 63% of the cost.15
Radiology plays a pivotal role in the diagnosis and follow-up of these patients. As NTM-PD presents with non-specific symptoms, it is important for radiologists to be aware of the characteristic imaging findings within the lungs and associated ancillary findings so that they can raise NTM-PD as a possible differential diagnosis.
It can be impossible to diagnose NTM-PD solely on imaging findings alone, as it can mimic TB and malignancy,7 particularly the fibrocavitatory form. There has been one study that has suggested that the cavities in NTM-PD are more thin-walled.16 However, a definitive diagnosis cannot be made based on this observation alone. Within the immunosuppressed population the radiological findings are more varied and this should be considered when interpreting the imaging of this group of patients.
Finally, radiology can aid clinicians in helping predict disease progression as the clinical course can be so variable. Patients who exhibit severe cavitation, consolidation and extensive bronchiectasis have the worst prognosis.9
Conclusion
NTM-PD is a difficult to diagnose and manage. Radiologists can aid with early diagnosis and disease monitoring. It is important to be aware of the characteristic imaging findings. Multidisciplinary team working is vital with close discussion with clinicians and microbiologists to reach the final diagnosis.
Contributor Information
Besma Musaddaq, Email: besma.musaddaq@nhs.net.
Joanne R Cleverley, Email: joanne.cleverley@nhs.net.
REFERENCES
- 1.Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017; 72(Suppl 2): ii1–64. doi: 10.1136/thoraxjnl-2017-210927 [DOI] [PubMed] [Google Scholar]
- 2.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 2014; 6: 210–20. doi: 10.3978/j.issn.2072-1439.2013.12.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryu YJ, Koh W-J, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis 2016; 79: 74–84. doi: 10.4046/trd.2016.79.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175: 367–416. doi: 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 5.Martinez S, McAdams HP, Batchu CS. The many faces of pulmonary nontuberculous mycobacterial infection. AJR Am J Roentgenol 2007; 189: 177–86. doi: 10.2214/AJR.07.2074 [DOI] [PubMed] [Google Scholar]
- 6.Erasmus JJ, McAdams HP, Farrell MA, Patz EF. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. Radiographics 1999; 19: 1487–505. doi: 10.1148/radiographics.19.6.g99no101487 [DOI] [PubMed] [Google Scholar]
- 7.Matveychuk A, Fuks L, Priess R, Hahim I, Shitrit D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Respir Med 2012; 106: 1472–7. doi: 10.1016/j.rmed.2012.06.023 [DOI] [PubMed] [Google Scholar]
- 8.Fowler SJ, French J, Screaton NJ, Foweraker J, Condliffe A, Haworth CS, et al. Nontuberculous mycobacteria in bronchiectasis: prevalence and patient characteristics. Eur Respir J 2006; 28: 1204–10. doi: 10.1183/09031936.06.00149805 [DOI] [PubMed] [Google Scholar]
- 9.Cowman SA, Jacob J, Obaidee S, Andres Floto R, Wilson R, Haworth CS, et al. Latent class analysis to define radiological subgroups in pulmonary nontuberculous mycobacterial disease. BMC Pulm Med 2018; 18: 145. doi: 10.1186/s12890-018-0675-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi Y, Lee KS, Kim SK, Koh WJ. Unilateral lung involvement of nodular Bronchiectatic Mycobacterium avium complex pulmonary diseases: proportion and evolution on serial CT studies. AJR Am J Roentgenol 2019; 212: 1010–17. doi: 10.2214/AJR.18.20589 [DOI] [PubMed] [Google Scholar]
- 11.Hong SJ, Kim TJ, Lee J-H, Park J-S. Nontuberculous mycobacterial pulmonary disease mimicking lung cancer. Medicine 2016; 95: e3978. doi: 10.1097/MD.0000000000003978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartman TE, Jensen E, Tazelaar HD, Hanak V, Ryu JH. CT findings of granulomatous pneumonitis secondary to Mycobacterium avium-intracellulare inhalation: "hot tub lung". AJR Am J Roentgenol 2007; 188: 1050–3. doi: 10.2214/AJR.06.0546 [DOI] [PubMed] [Google Scholar]
- 13.Fraser RS, Müller NL, Colman NC, Paré PD. The pulmonary manifestations of human immunodeficiency virus infection : Fraser R. S, Müller N. L, Colman N. C, Paré P. D, Fraser and Paré’s Diagnosis of Diseases of the Chest: 4th edition, 4-Volume Set. Philadelphia, Pa: Saunders; 1999. 1654–5. [Google Scholar]
- 14.Lee Y, Song JW, Chae EJ, Lee HJ, Lee CW, Do KH, et al. CT findings of pulmonary non-tuberculous mycobacterial infection in non-AIDS immunocompromised patients: a case-controlled comparison with immunocompetent patients. Br J Radiol 2013; 86: 20120209. doi: 10.1259/bjr.20120209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diel R, Jacob J, Lampenius N, Loebinger M, Nienhaus A, Rabe KF, et al. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J 2017; 49: 1602109. doi: 10.1183/13993003.02109-2016 [DOI] [PubMed] [Google Scholar]
- 16.Kim C, Park SH, Oh SY, Kim SS, Jo KW, Shim TS, et al. Comparison of chest CT findings in nontuberculous mycobacterial diseases vs. Mycobacterium tuberculosis lung disease in HIV-negative patients with cavities. PLoS One 2017; 12: e0174240. doi: 10.1371/journal.pone.0174240 [DOI] [PMC free article] [PubMed] [Google Scholar]