Abstract
Objectives:
To evaluate the role of stereotactic body radiotherapy (SBRT) as a local ablative treatment (LAT) in oligometastatic pancreatic cancer.
Methods:
Patients affected by histologically confirmed stage IV pancreatic adenocarcinoma were included in this analysis. Endpoints are local control (LC), progression-free survival (PFS), and overall survival (OS).
Results:
From 2013 to 2017, a total of 41 patients were treated with SBRT on 64 metastases. Most common sites of disease were lung (29.3%) and liver (56.1%). LC at 1 and 2 years were 88.9% (95% CI 73.2–98.6) and 73.9% (95% CI 50–87.5), respectively. Median LC was 39.9 months (95% CI 23.3—not reached).
PFS rates at 1 and 2 years were 21.9% (95% CI 10.8–35.4) and 10.9% (95% CI 3.4–23.4), respectively. Median PFS was 5.4 months (95%CI 3.1–11.3).
OS rates at 1 and 2 years were 79.9% (95% CI 63.7–89.4) and 46.7% (95% CI 29.6–62.2). Median OS was 23 months (95%CI 14.1–31.8).
Conclusions:
Our results, although based on a retrospective analysis of a small number of patients, show that patients with oligometastatic pancreatic cancer may benefit from local treatment with SBRT. Larger studies are warranted to confirm these results.
Advances in knowledge:
Selected patients affected by oligometastatic pancreatic adenocarcinoma can benefit from local ablative approaches, like SBRT
Introduction
Despite the significant progress in early diagnosis and the therapeutic improvements over the last decades, the prognosis of pancreatic cancer patients remains dismal. Many patients present with metastatic disease at the time of diagnosis or early develop distant metastases.
As a result of the poor prognosis, the number of therapeutic strategies investigated to extend survival is increasing.
Historically, the standard treatment for metastatic pancreatic tumor was systemic chemotherapy based on gemcitabine with a median overall survival (OS) of about 7 months.1 More aggressive regimen with FOLFIRINOX (folinic acid- fluorouracil-irinotecan-oxaliplatin) or nab-paclitaxel–gemcitabine improved outcomes with a median OS of 11.1 and 8.5 months, respectively.2,3
Oligometastatic pancreatic cancer with a durable stable disease may represent a favorable clinical scenario, opening a crucial prospective for the integration of ablative local therapies in the therapeutic pathway of these patients.4,5
The use of stereotactive body radiotherapy (SBRT) was investigated in different settings of primary and oligometastatic disease with encouraging results, utilizing either a single dose or a small number of fractions.6
In the last years, the integration of SBRT and systemic therapies provided promising results in the treatment of locally advanced unresectable pancreatic cancer.7–9
On the other hand, SBRT approach has been shown to be an effective treatment for inoperable liver and lung metastases,10,11 particularly in terms of local control (LC).
Different from conventional radiotherapy, SBRT entails precise delivery of high dose in few fractions, with a complete tumor ablation and maximal normal tissue sparing.12
Considering the non-invasiveness, the safety, and the efficacy, SBRT represents an ablative local therapy that provides an additional tool in the multimodal treatment of oligometastatic pancreatic cancer.
Aim of current study was to evaluate efficacy of SBRT in these selected setting of patients.
Methods and materials
Study population
In our analysis, we included patients with histologically confirmed diagnosis of pancreatic adenocarcinoma, who developed metachronous or synchronous metastases. We selected patients with a performance status of 0–2, treatment-naïve or previously treated with chemotherapy. Systemic treatment was allowed during and after SBRT. We excluded metastases already locally treated. A maximum of five metastases in up to two sites was allowed. Patients were categorized as oligometastatic de novo if they did not previously receive any kind of active therapy, apart primary treatment if oligometastases were metachronous. Patients were considered as “induced oligometastatic” if they had received systemic or other local ablative therapies in their history for metastatic disease. Finally, we also included in this analysis oligoprogressive patients (up to three progressing metastatic sites with all other sites stable or responding to a previous or concurrent medical therapy). Staging was performed with CT, MRI, and/or PET scan according to physician choice. All cases were discussed at the multidisciplinary tumor board and the local ethic committee approved the analysis. The study was conducted in accordance with Good Clinical Practice guidelines, the ethical principles of the Declaration of Helsinki and local regulations. All patients signed informed consent.
Techniques of radiotherapy
In all patients, we delineated the clinical target volume (CTV) with a simulation CT imaging, using a coregistration with MRI scan or PET scan in selected cases. Gross tumor volume (GTV) was equal to CTV and an additional 5 to 10 mm margin was added to CTV to design the planning target volume (PTV). In order to avoid reducing the internal organ movement, we used abdominal compression in case of liver lesions, and we obtained a simulation with a 4D-CT scan in case of moving targets (e.g., lung or liver lesions).
Response assessment
Response to therapy was assessed 3 months after the end of the SBRT, then every 3 months for the first year and every 6 months from the second year. The regular follow-up assessment consisted of clinical evaluation and an imaging exam (CT, MRI, or PET) according to physician preference. Tumor response was graded according to European Organization for Research and Treatment of Cancer Response Evaluation Criteria In Solid Tumors (EORTC-RECIST) criteria v. 1.16. We used PET Response Criteria in Solid Tumors (PERCIST) to assess metabolic response in patients who underwent PET scan for restaging.
Statistical analysis
This was a retrospective single-center analysis. As endpoints, we selected LC, progression-free survival (PFS), and OS. LC was defined as the time from the beginning of SBRT to the progression of treated metastases or last follow-up. PFS was calculated from the beginning of SBRT to the progression of in-field or out-field metastases, while OS was defined as the time from the beginning of SBRT to either death or last follow-up.
We used the log-rank test to perform the univariate analysis and Cox proportional hazards regression to estimate hazard ratios (HR); we ran a multivariable stepwise cox regression analysis with a significance level of p < 0.05. Statistical calculations were performed using STATA, v. 13.
Results
From 2013 to 2017, a total of 41 patients were treated with SBRT on 64 metastases. Median age was 66.3 (range 43.5–80.8) and median time from diagnosis of primary tumor to metastatic setting was 17.2 months (range 0–54.2). Most patients (33, 80.5%) underwent surgical removal of the primary tumor, eight of them also received adjuvant chemotherapy and radiotherapy, while 17 received only adjuvant chemotherapy. Performance status was 1 and 2 in 19 (46.3%) and 2 (4.9%) patients, respectively. Majority of patients were treated on de novo appearance of metastases (46.3%), followed by induced oligometastatic state (41.5%) and oligoprogressive state (12.2%). Only seven (17.1%) patients were naïve to systemic therapy at time of SBRT and 25 (61%) patients were treated on one single lesion. Most common sites of disease were lung (29.3%) and liver (56.1%). Five (12.2%) patients had extra target lesions not treated with SBRT. Median biologically effective dose (BED) was 105.6 (57.6–262.5).
Tables 1–3 summarize patients and treatment characteristics.
Table 1.
Patients’ characteristics
| Number (%) | |
|---|---|
| Sex | |
| 0 | 22 (53.6) |
| 1 | 19 (46.3) |
| Age, median (range) | 66.3 (43.5–80.8) years |
| Time to metastatic status | 17.2 (0–54.2) months |
| Age at metastatic status | 66.9 (44.9–81.9) years |
| Performance status | |
| 0 | 20 (48.8) |
| 1 | 19 (46.3) |
| 2 | 2 (4.9) |
| Comorbidities | |
| No | 6 (14.6) |
| Yes | 35 (85.4) |
| Time from mets to SBRT, median (range) | 8.7 (0–44) months |
| Ca 19.9, median (range) | 53 (10–3453) |
| Missing data: | 21 pts |
| Type oligometastatis status | |
| De novo | 19 (46. 3) |
| Induced | 17 (41.5) |
| Oligoprogression | 5 (12.2) |
| Timing of metastases | |
| Synchronous | 2 (4.9%) |
| Metachronous | 39 (95.1%) |
Table 2.
Previous treatments characteristics
| Primary treatment | |
| Radiotherapy | 3 (7.3) |
| Chemotherapy | 4 (9.7) |
| Surgery | 8 (19.5) |
| RT-CHT | 2 (4.9) |
| MT +surgery | 16 (39) |
| Surgery +RT-CHT | 8 (19.5) |
| Previous local tx | |
| No | 24 (58.5) |
| Yes | 17 (41.5) |
| Previous chemotherapy | |
| No | 7 (17.1) |
| one line | 16 (39) |
| two lines | 12 (29.3) |
| three or more lines | 6 (14.6) |
| Chemotherapy before SBRT | |
| Gem +Nabpaclitaxel | 10 (24.4) |
| FOLFOX/XELOX | 6 (14.6) |
| FOLFIRINOX | 6 (14.6) |
| Gemcitabine | 20 (48.8) |
| 5FU / Capecitabine | 2 (4.9) |
| GEMOX | 3 (7.3) |
| Cis +Gem | 3 (7.3) |
| Irinotecan | 2 (4.9) |
| Chemotherapy during SBRT | |
| Gemcitabine | 2 (4.9) |
| Chemotherapy after SBRT | |
| Gem +Nabpaclitaxel | 1 (2.4) |
| Gemcitabine | 2 (4.9) |
| 5FU / Capecitabine | 1 (2.4) |
| Irinotecan | 4 (9.7) |
Table 3.
SBRT characteristics
| Number of treated lesions, median (range) | 1 (1–4) |
| 1 | 25 (61) |
| 2 | 10 (24.4) |
| 3 | 5 (12.2) |
| 4 | 1 (2.4) |
| Site of treated metastases | |
| Lung | 12 (29.3) |
| Liver | 23 (56.1) |
| Lymph node | 5 (12.2) |
| Lung and liver | 1 (2.44) |
| Extra target lesions | |
| No | 36 (87.8) |
| Yes | 5 (12.2) |
| BED, median (range) | 105.6 (57.6–262.5) |
| Ablative dose | |
| No | 11 (26.8) |
| Yes | 30 (73.2) |
| Systemic therapy during SBRT | |
| No | 39 (95.1) |
| Yes | 2 (4.9) |
| SBRT dose | |
| Lung: | |
| 32 Gy 4 fx | 1 (2.4) |
| 48 Gy 4 fx | 11 (26.8) |
| 60 Gy 8 fx | 1 (2.4) |
| Liver: | |
| 45 Gy 6 fx | 3 (7.3) |
| 48 Gy 3 fx | 1 (2.4) |
| 48 Gy 6 fx | 2 (4.9) |
| 54 Gy 3 fx | 1 (2.4) |
| 54 Gy 6 fx | 3 (7.3) |
| 60 Gy 3 fx | 5 (12.2) |
| 60 Gy 6 fx | 3 (7.3) |
| 63 Gy 6 fx | 1 (2.4) |
| 67.5 Gy 3 fx | 2 (4.9) |
| 75 Gy 3 fx | 3 (7.3) |
| Lymph node: | |
| 36 Gy 6 fx | 1 (2.4) |
| 45 Gy 6 fx | 3 (7.3) |
| 48 Gy 4 fx | 1 (2.4) |
| Systemic therapy after SBRT | |
| No | 32 (78.1) |
| Yes | 9 (21.9) |
Median follow-up was 23 months (95% CI 14.1–31.8). Best local response was classified as complete response in 12 (29.4%) patients, partial response in 19 (46.3%) patients, stable disease in nine (21.9%) patients and progressive disease in one (2.4%) patient (Table 4). LC at 1 and 2 years were 88.9% (95%CI 73.2–98.6) and 73.9% (95%CI 50–87.5), respectively (Figure 1). Median LC was 39.9 months (95% CI 23.3–not reached). At univariate analysis, none of the analyzed factor impacted on LC.
Table 4.
SBRT and patients outcome
| Best local response | |
| CR | 12 (29.4) |
| PR | 19 (46.3) |
| SD | 9 (21.9) |
| PD | 1 (2.4) |
| Local progression | |
| No | 33 (80.5) |
| Yes | 8 (19.5) |
| Distant progression | |
| No | 6 (14.6) |
| Yes | 35 (85.4) |
| Last status | |
| Ned | 3 (7.32) |
| Alive with metastases | 11 (26.8) |
| Death of disease | 25 (60.1) |
| Death other causes | 2 (4.9) |
Figure 1.
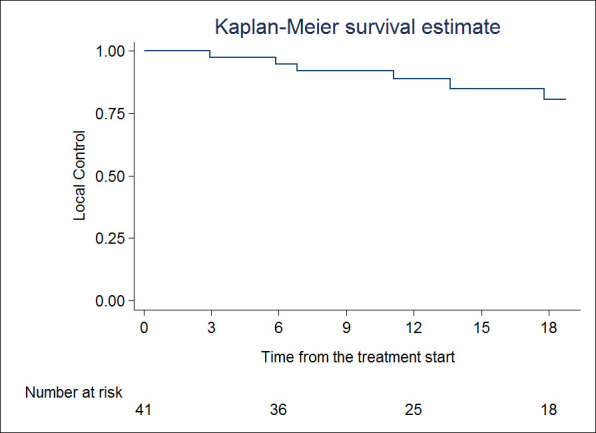
Local control
Distant progression rates at 1 and 2 years were 25.6% (95%CI 13.4–39.8) and 13.2% (95%CI 4–27.1); median distant PFS was 5.8 months (95% CI 3.2–14.7). At univariate analysis for distant PFS, sex (HR 2.32, 95% CI 1.12–4.78;p = 0.047) and the presence of extra target disease (HR 2.75, 95% CI 1.01–7.34; p = 0.047) were statistically significant. At multivariable analysis for distant PFS, sex (HR 3.21, 95% CI 1.44–7.13;p = 0.004) and the presence of extra target disease (HR 5.04, 95% CI 1.65–15.3; p = 0.004) continued to be significant.
PFS rates at 1 and 2 years were 21.9% (95% CI 10.8–35.4) and 10.9% (95% CI 3.4–23.4), respectively (Figure 2). Median PFS was 5.4 months (95% CI 3.1–11.3). For PFS, sex (HR 2.50, 95% CI 1.20–5.20; p = 0.014), time to metastases (HR 0.96, 95% CI 0.94–0.99; p = 0.034), extra target disease (HR 3.60, 95% CI 1.32–9.81; p = 0.012), BED (HR 1.00, 95% CI 1.00–1.01; p = 0.033) were impacting. At multivariable analysis, sex (HR 4.59, 95% CI 1.90–11; p = 0.001), time to metastases (HR 0.96, 95% CI 0.93–0.99; p = 0.031) and extra target disease (HR 7.36, 95% CI 2.24–24.15; p = 0.001) were statistically significantly associated to PFS.
Figure 2.
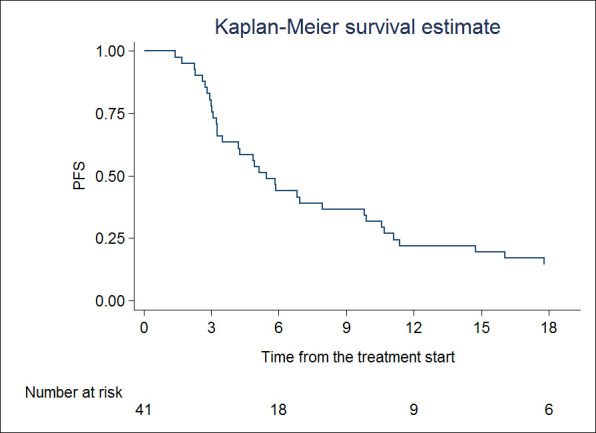
Progression-free survival
OS at 1 and 2 years were 79.9% (95% CI 63.7–89.4) and 46.7% (95% CI 29.6–62.2), respectively (Figure 3). In terms of OS, univariate analysis showed that time to metastases (HR 0.95, 95% CI 0.91–0.99; p = 0.036) and BED (HR 1.00, 95% CI 1.00–1.01; p = 0.017) were statistically significant. No factor was significant at multivariate analysis.
Figure 3.
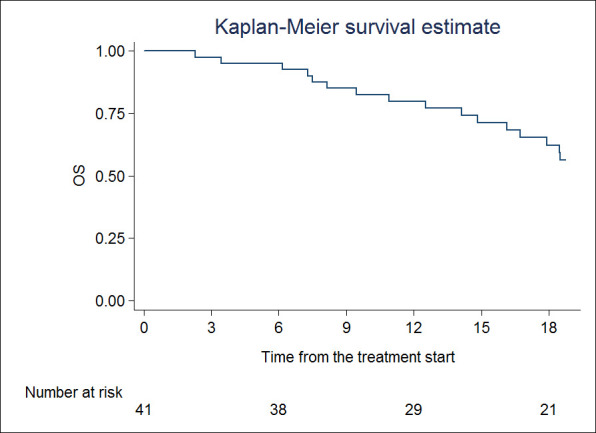
Overall survival
Discussion
We reported a single institution retrospective experience on the use of SBRT for oligometastatic pancreatic cancer. Despite the raising interest toward the use of local ablative therapies for oligometastases, patients affected by pancreatic cancer are poorly represented in the main published series. However, our analysis shows that an oligometastatic state could exist also in this setting of patients. We observed a median OS of 23 months, OS at 1 and 2 years were 79.9% (95% CI 63.7–89.4) and 46.7% (95% CI 29.6–62.2), respectively. PFS rates at 1 and 2 years were 21.9% (95% CI 10.8–35.4) and 10.9% (95% CI 3.4–23.4). Median PFS was 5.4 months.
These results are worse if compared with those obtained in a larger series of patients with oligometastatic disease from any primary (median OS 34 months, median PFS 8.7 months).13 However, considering the dismal prognosis of pancreatic carcinoma when compared with almost all other solid tumors, these survival rates still support the existence of a limited setting of oligometastatic patients. Moreover, compared with results obtainable with standard chemotherapies for metastatic pancreatic adenocarcinoma, our results seem to be very encouraging.1,2
The key question also is how we can predict the prognosis and distinguish a real oligometastatic patient (with an indolent disease, taking advantage from local ablative therapies) from a false oligometastatic patient, in which the radiologically evident disease is just the tip of the iceberg.
There are few available data in literature about clinical factors able to identify oligometastatic patients. Performance status, multiple metastases, large metastases (>3 cm), metachronous metastases, and pre-SBRT chemotherapy were found as poor prognostic factors by Fode et al.14 In a similar series, De Vin et al highlighted a relationship between histology, disease-free interval (DFI), site of metastases, and gender, with survival.15 A recursive partitioning analysis (RPA)16 based on clinical parameters (primary histology, DFI, number of metastases, age and metastatic site) allowed the discrimination of five different prognostic classes for OS. In our previous experience on oligometastatic patients from different histologies, site of metastases, primary histology, age, local response of the irradiated lesion(s), and presence of extra target disease were all factors related with survival.13
The main limitation of these studies is the heterogeneity of analyzed patients, including different primary tumors, histologies, metastatic sites, etc. Therefore, they can give just some indications, not easily applicable in specific disease settings, like pancreatic adenocarcinoma. Indeed, pancreatic cancer is usually not represented in these large database analyses, probably included in the miscellaneous histologies, usually indicated as “others.”
To the best of our knowledge, the present work is the first aimed at identifying prognostic factors that could predict PFS, OS or LC in oligometastatic pancreatic cancer.
From our data, the presence of extra target disease (i.e., disease controlled by systemic therapy and not directly irradiated) is a predictor of shorter PFS. From this observation, we could derive two possible conclusions. The first message is that, if technically feasible, all visible disease should be irradiated with ablative purposes. Our data are consistent with those reported by Xu et al in oligoprogressive non-small cell lung cancer, where the authors showed that treating just a part of the macroscopic disease with local ablative therapies was less beneficial than treating all visible disease.17
Another prognostic factor, predicting both PFS and OS in our patients was the DFI from primary diagnosis to metastases occurrence. Similar observations were reported by Wong and Hong in their studies.16,18 While Wong suggested DFI as a continuous variable as in our experience, Hong et al used a cut-off of 75 months to separate patients in five different prognostic classes.
Interestingly, also the dose of RT was correlated with OS in our experience. The delivery of a really ablative dose is necessary to have a real impact on the patients’ survival.
Also, Salama et al in a dose escalation trial showed that higher doses are related with higher LC rates.19 Although with a lower median BED, also Hong et al correlated BED with OS. Indeed, they found that patients treated with BED of >75 had a 3-year OS of 61% compared to 43% (95% CI 34±54%) for those treated with BED <75. Also, PFS and LC were correlated with BED, differently from our results.
Clinical parameters are the easiest to use in the clinical practice as predictive tools for survival, however they are just a surrogate of a different biology of the disease. Therefore, circulating biomarkers, like microRNAs or circulating tumor cells, could give a more realistic picture of the disease behavior. For example, analyzing three different (miR-23b, miR-449a, and miR-449b) researchers were able to identify two different groups of oligometastatic patients with a different prognosis.18 Similar analysis could be helpful also in identifying oligometastatic pancreatic patients.
Considering a recent study by the University of Texas MD Anderson Cancer Center, in which the genetic mutation/deletion status of the SMAD4 gene was correlated with patterns of recurrence in patients with metastatic disease,20 this gene could represent a good starting point also in oligometastatic patients.
The implementation of this line of research and the identification of tumor-specific biomarker predictors for local and distant recurrence could guide the patient selection and the choice of therapies toward the integration of systemic and local therapies, such as SBRT.
Conclusion
Our results, although based on a retrospective analysis of a small number of patients, show that patients with oligometastatic pancreatic cancer may benefit from local treatment with SBRT.
Further investigations are required for a better definition of a real oligometastatic state and for a better integration of local and systemic therapies.
Footnotes
Patient consent: Informed consent was obtained from all individual participants included in the study.
Ethics approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Contributor Information
Marta Scorsetti, Email: marta.scorsetti@hunimed.eu.
Tiziana Comito, Email: tiziana.comito@humanitas.it.
Davide Franceschini, Email: davide.franceschini@humanitas.it.
Ciro Franzese, Email: ciro.franzese@humanitas.it.
Maria Giuseppina Prete, Email: maria.prete@humanitas.it.
Antonio D'Alessio, Email: antonio.dalessio@humanitas.it.
Silvia Bozzarelli, Email: silvia.bozzarelli@humanitas.it.
Lorenza Rimassa, Email: lorenza.rimassa@humanitas.it.
Armando Santoro, Email: armando.santoro@humanitas.it.
REFERENCES
- 1.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691: 1691e1703–703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817: 1817e1825–25. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–703. doi: 10.1056/NEJMoa1304369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era of cancer therapy. Jpn J Clin Oncol 2010; 40: 107e111 10.1093/jjco/hyp167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellman S, Weichselbaum RR, Oligometastases WRR. Oligometastases. J Clin Oncol 1995; 13: 8: 8e10–10. doi: 10.1200/JCO.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 6.Alongi F, Arcangeli S, Filippi AR, Ricardi U, Scorsetti M. Review and uses of stereotactic body radiation therapy for Oligometastases. Oncologist 2012; 17: 1100–7. doi: 10.1634/theoncologist.2012-0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comito T, Cozzi L, Clerici E, Franzese C, Tozzi A, Iftode C, et al. Can stereotactic body radiation therapy be a viable and efficient therapeutic option for unresectable locally advanced pancreatic adenocarcinoma? results of a phase 2 study. Technol Cancer Res Treat 2017; 16: 295–301. doi: 10.1177/1533034616650778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015; 121: 1128–37. doi: 10.1002/cncr.29161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comito T, Cozzi L, Zerbi A, Franzese C, Clerici E, Tozzi A, et al. Clinical results of stereotactic body radiotherapy (SBRT) in the treatment of isolated local recurrence of pancreatic cancer after R0 surgery: a retrospective study. Eur J Surg Oncol 2017; 43: 735–42. doi: 10.1016/j.ejso.2016.12.012 [DOI] [PubMed] [Google Scholar]
- 10.Scorsetti M, Arcangeli S, Tozzi A, Comito T, Alongi F, Navarria P, et al. Is stereotactic body radiation therapy an attractive option for unresectable liver metastases? A preliminary report from a phase 2 trial. Int J Radiat Oncol Biol Phys 2013; 86: 336–42. doi: 10.1016/j.ijrobp.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 11.Comito T, Cozzi L, Clerici E, Campisi MC, Liardo RLE, Navarria P, et al. Stereotactic ablative radiotherapy (SABR) in inoperable oligometastatic disease from colorectal cancer: a safe and effective approach. BMC Cancer 2014; 14: 619. doi: 10.1186/1471-2407-14-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavanagh B, Timmerman R. Stereotactic irradiation of tumors outside the central nervous system : In Perez and Brady’s Principles and Practice of Radiation Oncology. 5th edition Baltimore, MD: Lippincott, Williams, and Wilkin; 2007. 389–90. [Google Scholar]
- 13.XXXX.
- 14.Fode MM, Høyer M. Survival and prognostic factors in 321 patients treated with stereotactic body radiotherapy for oligo-metastases. Radiother Oncol 2015; 114: 155–60. doi: 10.1016/j.radonc.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 15.de Vin T, Engels B, Gevaert T, Storme G, De Ridder M, et al. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol 2014. doi: 10.1371/journal.pone.0195149 [DOI] [PubMed] [Google Scholar]
- 16.Hong JC, Ayala-Peacock DN, Lee J, Blackstock AW, Okunieff P, Sung MW, et al. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: a multi-institutional pooled analysis. PLoS One 2018; 13: e0195149. doi: 10.1371/journal.pone.0195149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q, Zhou F, Liu H, Jiang T, Li X, Xu Y, et al. Consolidative local ablative therapy improves the survival of patients with synchronous oligometastatic NSCLC harboring EGFR activating mutation treated with first-line EGFR-TKIs. J Thorac Oncol 2018; 13: 1383–92. doi: 10.1016/j.jtho.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 18.Wong AC, Watson SP, Pitroda SP, Son CH, Das LC, Stack ME, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer 2016; 122: 2242–50. doi: 10.1002/cncr.30058 [DOI] [PubMed] [Google Scholar]
- 19.Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 2012; 118: 2962–70. doi: 10.1002/cncr.26611 [DOI] [PubMed] [Google Scholar]
- 20.Herman JM, Jabbour SK, Lin SH, Deek MP, Hsu CC, Fishman EK, et al. Smad4 loss correlates with higher rates of local and distant failure in pancreatic adenocarcinoma patients receiving adjuvant chemoradiation. Pancreas 2018; 47: 208–12. doi: 10.1097/MPA.0000000000000985 [DOI] [PMC free article] [PubMed] [Google Scholar]


