Abstract
Objective:
To characterize differences in postoperative opioid prescribing across surgical, non-surgical, and advanced practice providers.
Background:
There is a critical need to identify best practices around perioperative opioid prescribing. To date, differences in postoperative prescribing among providers are poorly understood.
Methods:
This is a retrospective multi-center analysis of commercial insurance claims from a statewide quality collaborative. We identified 15,657 opioid-naïve patients who underwent a range of surgical procedures between January 2012 and October 2015 and filled an opioid prescription within 30 days postoperatively. Our primary outcome was total amount of opioid filled per prescription within 30 days postoperatively (in oral morphine equivalents [OME]). Hierarchical linear regression was used to determine the association between provider characteristics (specialty, advanced practice providers [nurse practitioners and physician assistants] vs. physician, and gender) and outcome while adjusting for patient factors.
Results:
Average postoperative opioid prescription amount was 326 ± 285 OME (equivalent: 65 tablets of 5 mg hydrocodone). Advanced practice providers accounted for 19% of all prescriptions, and amount per prescription was 18% larger in this group compared to physicians (315 vs. 268, P<0.001). Primary care providers accounted for 13% of all prescriptions and prescribed on average 279 OME per prescription. The amount of opioid prescribed varied by surgical specialty and ranged from 178 OME (urology) to 454 OME (neurosurgery).
Conclusions:
Advanced practice providers account for one-in-five postoperative opioid prescriptions and prescribe larger amounts per prescription relative to surgeons. Engaging all providers involved in postoperative care is necessary to understand prescribing practices, identify barriers to reducing prescribing, and tailor interventions accordingly.
Mini-Abstract:
In this statewide study, we studied differences in opioid prescribing within 30 days after surgery across different types of providers. Advanced practice providers accounted for one-in-five opioid prescriptions and prescribed larger amounts per prescription compared to physicians. Engaging all providers involved in postoperative care is necessary to understand prescribing patterns and implement best practices.
Introduction
An alarming proportion of opioids remain unused after surgery1, demanding attention to current surgical prescribing practices.2 Excess prescribing creates the potential for diversion of prescription opioids into the community, which is a major culprit in the opioid epidemic and implicated in more than half of patients who report opioid misuse.3 Additionally, persistent opioid use after surgery is common and often under-recognized, highlighting the importance of risk assessment and judicious pain management during postoperative care.4–8 Therefore, surgeons play a crucial role in this public health problem through their stewardship of opioid prescribing.
To date, there are no clear standards for estimating postoperative analgesic requirements, and no consensus guidelines for perioperative opioid prescribing. Studies have demonstrated that surgical opioid prescribing varies widely and often exceeds patient consumption.1,9,10 In attempt to reduce excess prescribing, professional organizations and policy makers have called for closer attention to pain management, including improved opioid education, stricter limits on initial opioid prescription amounts, and the mandatory use of prescription monitoring drug programs.11–13 However, to effectively target interventions and policies, it is important to understand which providers prescribe opioids after surgery. Postoperative opioid prescribing is not limited to surgeons, and understanding the stakeholders who contribute to opioid prescribing following surgery is necessary to develop best practices.
In this context, we sought to describe patterns of postoperative opioid prescribing by provider specialty and level (advanced practice provider [APP] vs. physician). Using data from a statewide collaborative quality improvement program, we examined commercial insurance claims for adults undergoing common non-emergent surgical procedures. Our primary aim was to identify the types of providers prescribing opioid analgesics during the 30-day postoperative period, and to describe provider-level differences in the amount of opioid prescribed. We hypothesized that prescribing is concentrated among surgical providers in the postoperative period, and that prescribing is lower amongst APPs compared to physicians, given the lack of surgeon education directed to postoperative opioid prescribing.
Methods
Study Population
We analyzed a dataset of commercial insurance claims from the Michigan Value Collaborative, the data collection methods of which are described previously.14,15 This collaborative quality improvement program aggregates preferred provider organization insurance claims from Blue Cross Blue Shield of Michigan—a plan covering roughly 50% of Michigan’s commercially insured individuals—as part of an effort to improve the value of healthcare in Michigan. The MVC deidentifies and securely stores these claims. Using claims data, we identified surgical episodes of care and the associated postoperative opioid fills. We included adult patients who underwent the following procedures between January 1, 2012 and October 31, 2015: general surgery (open or laparoscopic appendectomy, open or laparoscopic cholecystectomy, Roux-en-Y gastric bypass, sleeve gastrectomy, open or laparoscopic non-cancer colectomy), oncologic surgery (open or thorascopic lung cancer resection, prostatectomy, colorectal cancer resection, esophagectomy, pancreatectomy, gastrectomy), cardiac and vascular surgery (coronary artery bypass graft, carotid endarterectomy, valve surgery), joint and spine surgery (knee replacement, hip replacement, spine surgery), and gynecologic surgery (hysterectomy). We only included patients who required an inpatient stay. This study was approved by the University of Michigan Institutional Review Board.
We included medical and pharmacy claims from 1 year before surgery (to confirm preoperative opioid-naïve status) to 30 days after surgery. We limited our analysis to patients who were opioid-naïve in the year before surgery to minimize variation in opioid use attributable to opioid tolerance. As previously defined, patients were considered opioid-naïve if they did not fill an opioid prescription between 365 and 31 days prior to surgery.4 To minimize the potential impact of outlier cases, we excluded patients with postoperative length of hospital stay greater than 30 days. Lastly, we excluded patients with additional procedure codes for anesthesia within 30 days of the index procedure.
Provider Characteristics
We analyzed the association between provider specialty, type (physician vs. APP), and gender on postoperative prescription amount. APPs included nurse practitioners and physician assistants. Unique providers were identified via National Provider Identifier (NPI) codes attributed to each pharmaceutical claim. Each NPI was then merged with the publicly available National Plan and Provider Enumeration System16, which contains provider self-reported specialty, type, and gender. We grouped surgical specialties into the following categories: general surgery, urology, cardiothoracic and vascular surgery, orthopedic surgery, neurosurgery, oral and maxillofacial/otolaryngology/plastic surgery, and gynecology. We grouped non-surgical specialties into the following categories: primary care, pain medicine, emergency medicine, and other non-surgical specialties.
Outcome
Our primary outcome was the amount of opioid prescribed per prescription among prescriptions written within 30 days after surgery. We identified postoperative opioid prescriptions from pharmacy claims using National Drug Codes linked to generic drug names. For standardization, opioid dosage in milligrams was converted to oral morphine equivalents (OME) using standard published conversions factors.17,18 The OME dosage for each prescription was multiplied by the total quantity filled to compute our outcome of individual prescriptions amount (OME per prescription).
Analysis
Because we were interested in describing provider characteristics associated with the amount of opioid prescribed per prescription, the unit of analysis for this study was individual prescription. Therefore, for patients with multiple prescriptions, each prescription was considered a unique observation. Prescription amount was log-transformed to ensure normally distributed residuals in the linear regression model. We used a multivariable hierarchical linear regression model to estimate the association of provider factors with individual prescription amount while adjusting for patient factors. This model had three levels: individual prescription, patient factors, and provider factors. This allowed adjustment for clustering of prescriptions within patients (i.e. accounting for patients with multiple prescriptions) and patients within providers.
To risk-adjust for patient factors, we included patient age, gender, and comorbidities in the model. We also adjusted for hospital LOS, as duration of hospital course may impact amount of outpatient opioids prescribed. Because provider specialty was included in the model and determines surgical procedure performed (for example, no general surgeons performed arthroplasty), we did not additionally include surgical procedure as a patient-level covariate in our primary model, as there was significant collinearity between the two variables. However, as a sensitivity analysis, we ran a model adjusting for surgical procedure without including provider specialty to compare findings between this model and the primary model. Comorbidities were identified from claims over the preoperative year using the Centers for Medicare and Medicaid Services (CMS) Hierarchical Condition Categories.19,20 This is an established methodology used by CMS for risk adjustment, including 79 comorbid conditions with associated ICD-9 codes. For parsimony, we used stepwise selection to identify comorbidities that were associated with outcome (P<0.10), and this subset of comorbidities was entered into the final model. To describe the comorbidity profiles of the study cohort, these 79 comorbidities were categorized by organ system. To determine if the effect of APP status on outcome was dependent upon provider specialty, we tested for an interaction between the two variables. Two-sided tests with significance levels of α=0.05 were used for significance testing. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and Stata version 14.2 (StataCorp LLC, College Station, TX).
Results
Our study cohort included 15,657 patients who filled prescriptions in the 30-day period following surgery. Table 1 demonstrates the patient characteristics of this cohort. The majority of patients were female (61%), and the average age was 49.4 ± 11.2. The most common procedures were general surgical (34%), joint and spine (35%), and gynecologic (20%) surgical procedures. The most common comorbidities were diabetes (13%), cardiovascular disease (11%), and morbid obesity (10%).
Table 1:
Patient Characteristics
| Patient Characteristic | No. (%) or mean ± standard deviation |
|---|---|
| Total No. Patients | 15,657 |
| Gender | |
| Male | 6,144 (39.2%) |
| Female | 9,513 (60.8%) |
| Age | 49.4 ± 11.2 |
| Type of Surgery | |
| General | 5,362 (34.2%) |
| Joint and spine | 5,543 (35.4%) |
| Oncologic | 824 (5.3%) |
| Cardiothoracic/Vascular | 758 (4.8%) |
| Gynecologic | 3,170 (20.2%) |
| Comorbidity | |
| Cardiovascular disease | 1,723 (11.0%) |
| Pulmonary | 767 (4.9%) |
| Diabetes | 2,066 (13.2%) |
| Kidney disease | 133 (0.8%) |
| Liver disease | 92 (0.6%) |
| Other gastrointestinal | 500 (3.2%) |
| Coagulopathy | 562 (3.6%) |
| Morbid obesity | 1,624 (10.4%) |
| Musculoskeletal | 636 (4.1%) |
| Cancer | 1,548 (9.9%) |
| Psychiatric | 591 (3.8%) |
We analyzed a total of 21,413 prescriptions written by 4,923 providers (Table 2). We found that 19% of all opioid prescriptions were written by APPs, and 81% were written by physicians. Primary care providers accounted for 13% of all postoperative opioid prescriptions, and 0.8% were written by emergency providers. Orthopedic and general surgical providers each accounted for 28% of all prescriptions, reflective of the sample’s procedural case mix. Male providers accounted for 72% of all opioid prescriptions. The average individual prescription amount in the postoperative 30-day period was 326 ± 285 OME (equivalent to 65 tablets of 5 mg hydrocodone).
Table 2:
Provider characteristics
| Provider characteristics | No. (%) of all prescriptions |
|---|---|
| Total No. | 21,413 |
| Gender | |
| Male | 15,466 (72.2%) |
| Female | 5,947 (27.8%) |
| Provider Level | |
| Physician | 17,375 (81.1%) |
| Advanced practice provider | 4,038 (18.9%) |
| Provider Specialty | |
| General surgery | 5,922 (27.7%) |
| Orthopedic Surgery | 6,058 (28.3%) |
| Gynecology | 3,204 (15.0%) |
| Neurosurgery | 1,136 (5.3%) |
| Cardiothoracic/Vascular | 804 (3.8%) |
| Oral surgery, Otolaryngology, Plastic Surgery | 41 (0.2%) |
| Urology | 500 (2.3%) |
| Primary Care | 2,777 (13.0%) |
| Pain Medicine | 58 (0.3%) |
| Emergency | 175 (0.8%) |
| Other | 738 (3.4%) |
Table 3 illustrates the association of provider characteristics with postoperative prescription amount, adjusted for patient factors and accounting for clustering within provider and hospital. The primary outcome from this multi-level linear regression model was log-transformed OMEs per prescription, and the estimate describes the multiplicative increase in OMEs associated with a unit increase in the respective variable. APPs prescribed 18% more OME per prescription on average compared to physicians (P<0.001), and male providers prescribed 10% more OME per prescription on average compared to female providers (P<0.001). The adjusted mean individual prescription amount is shown in Figure 1 by provider specialty. There was 2.6-fold variation in prescription amount across surgical specialty; urology prescribed the least amount of OME per prescription (178 [95% C.I. 161, 196]), whereas orthopedic surgery (414 [95% C.I. 398, 432]) and neurosurgery (454 [95% C.I. 420, 492]) prescribed the most. Among non-surgical specialties, primary care providers prescribed an average of 279 OME per prescription (95% C.I. 270, 289) and emergency providers prescribed an average of 163 OME per prescription (95% C.I. 148, 180).
Table 3:
Factors associated with the amount of postoperative opioid filled in the 30 days following surgery.
| Estimate | 95% Confidence Interval | P-value | |
|---|---|---|---|
| Provider Characteristics | |||
| Advanced practice provider (ref: Physician) | 1.18 | (1.12, 1.23) | <0.001 |
| Specialty (reference: General Surgery) | |||
| Orthopedic Surgery | 2.04 | (1.93, 2.16) | <0.001 |
| Gynecology | 0.96 | (0.91, 1.02) | 0.223 |
| Neurosurgery | 2.24 | (2.05, 2.45) | <0.001 |
| Cardiothoracic and vascular | 1.15 | (1.04, 1.26) | 0.004 |
| OMFS Otolaryngology Plastic Surgery | 0.97 | (0.77, 1.23) | 0.829 |
| Urology | 0.88 | (0.79, 0.97) | 0.014 |
| Primary Care | 1.37 | (1.30, 1.45) | <0.001 |
| Pain Medicine | 1.50 | (1.21, 1.86) | <0.001 |
| Emergency | 0.80 | (0.72, 0.89) | <0.001 |
| Other non-surgical | 1.17 | (1.11, 1.23) | <0.001 |
| Male Provider (ref: Female prescriber) | 1.10 | (1.06, 1.14) | <0.001 |
| Patient Characteristics | |||
| Age | 1.001 | (1.000, 1.002) | <0.001 |
| Male | 1.05 | (1.04, 1.07) | <0.001 |
| Hospital length of stay | 1.02 | (1.02, 1.02) | <0.001 |
| Comorbidity | |||
| Rheumatoid Arthritis and Inflammatory Connective Tissue Disease | 1.05 | (1.01, 1.08) | 0.008 |
| Spinal Cord Disorders/Injuries | 1.12 | (1.04, 1.21) | 0.002 |
| Bone Joint. Muscle Infections/Necrosis | 1.08 | (1.01, 1.16) | 0.029 |
| Intestinal Obstruction/Perforation | 0.98 | (0.94, 1.02) | 0.358 |
| Aspiration and Specified Bacterial Pneumonias | 1.14 | (1.00, 1.30) | 0.042 |
| Morbid Obesity | 1.05 | (1.03, 1.07) | <0.001 |
| Ischemic or Unspecified Stroke | 0.86 | (0.78, 0.94) | 0.001 |
| Major Depressive. Bipolar, and Paranoid Disorders | 1.05 | (1.02, 1.09) | 0.003 |
| Multiple Sclerosis | 1.12 | (1.02, 1.23) | 0.020 |
| Lymphoma and Other Cancers | 1.12 | (1.05, 1.20) | 0.001 |
| Coagulation Defects and Other Specified Hematological Disorders | 1.02 | (0.99, 1.06) | 0.150 |
Outcome is log-transformed OME per prescription. The estimates are interpreted as the multiplicative increase in OME per prescription for each one-unit increase in the independent variable. OMFS = oral and maxillofacial surgery.
Figure 1: Adjusted mean opioid prescription amount, by provider specialty.
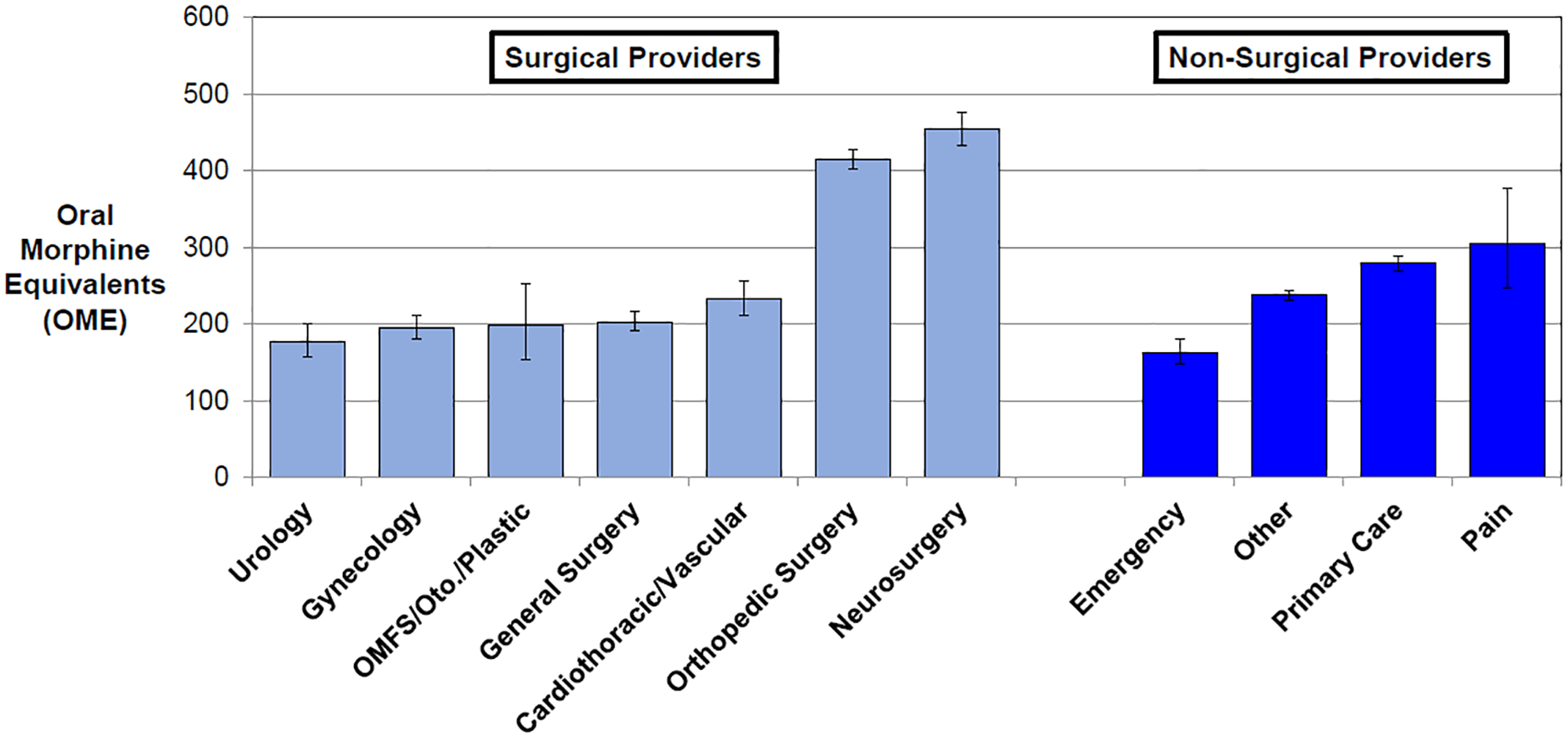
The figure shows adjusted oral morphine equivalents (OME) per individual opioid prescription within 30-days postoperatively. Surgical specialties are represented by light blue and non-surgical specialties are dark blue. Error bars represent the 95% confidence intervals. OMFS=Oral and Maxillofacial Surgery. Oto=Otolaryngology.
Figure 2 shows adjusted mean individual prescription amount over 30-days postoperatively among APPs compared to physicians, across specialty. Overall, average postoperative prescription amount by APPs was 315 OME (95% C.I. 303, 328), compared to 268 OME (95% C.I. 263, 273) prescribed by physicians (P<0.001). This trend in APPs prescribing higher OME than physicians persisted across all specialties. As a sensitivity analysis, when adjusting for surgical procedure type, without accounting for provider specialty, APPs prescribed 5% more OME per prescription on average compared to physicians (P=0.010).
Figure 2: Adjusted mean opioid prescription amount, by advanced practice provider vs. physician.

The figure shows adjusted oral morphine equivalents (OME) per individual opioid prescription within 30-days postoperatively. Advanced practice providers within each specialty are represented by checkered bars, and physicians are represented by solid bars. Error bars represent the 95% confidence intervals. OMFS=Oral and Maxillofacial Surgery. Oto=Otolaryngology.
Discussion
In this statewide insurance claims-based analysis, opioid prescribing following surgery varied by provider specialty and type. APPs contributed one-in-five postoperative opioid prescriptions, and prescribed significantly larger amounts per prescription compared to physicians, independent of specialty. Primary care providers accounted for 13% of all prescriptions and prescribed relatively large amounts of opioid per prescription. The amount of opioid prescribed per prescription varied across surgical specialty, and the average OME per prescription was nearly twice as high for patients receiving prescriptions from orthopedic and neurosurgical providers compared to other surgical specialties. This likely reflects expected differences in pain and analgesic needs attributable to specialty-specific differences in patients, procedures, and recovery needs. Nevertheless, these findings highlight important differences across providers that can inform policy development as well as strategic efforts to promote safe opioid prescribing.
Prior studies have documented wide variation of postoperative opioid prescribing within and between specific procedures9,10, across hospitals10, and across geographical regions.21,22 These studies, however, did not evaluate provider-level differences in opioid prescribing. Our findings demonstrate a wide range of postoperative prescription amounts across providers and specialties. Orthopedic and neurosurgical providers prescribed high amounts, consistent with prior work showing higher OME prescribed after arthroplasty and spine surgery compared to general surgery.10 As aforementioned, this variability by surgical specialty may in part be appropriate due to patient and procedural differences, and may not necessarily reflect overprescribing. However, these findings show that differences in opioid prescribing exist across surgical specialties, and one-size-fits all policy approaches to reducing opioid prescribing may not be feasible. For example, current legislative efforts in many states focus on broad prescribing limitations, such as limiting new opioid prescriptions to 7 days supply12,13 or 200 OME total23. Although such policies may be effective in some prescribing settings, our results suggest that surgical specialties and their respective patient populations will be differentially affected by such policies. Provider input will be key to developing policies that balance safer opioid prescribing with effective pain management.
Our findings are in accordance with a recent single-center study of surgical prescribing patterns which demonstrated higher prescribing among APPs compared to surgeons.24 In the emergency setting, however, studies have shown conflicting results. One multi-center study found that APPs were less likely to prescribe an opioid for musculoskeletal pain25, whereas a larger but single-center study reported the opposite finding (for a broader range of diagnoses).26 Because APPs are commonly utilized to improve physician productivity and access to care27, they may be the recipients of postoperative opioid refill requests and be hesitant to prescribe smaller amounts. Alternatively, APPs may have different perceptions and attitudes around pain management that lead to higher prescribing. Nurses are more likely to rate a patient’s pain higher and to select opioid analgesia28,29, and this may translate to nurse practitioner prescribing practices. There is a clear need for further study, and engaging APPs will be key when developing policies and interventions for safe opioid prescribing. Additionally, prescribing differs by provider gender, as male providers prescribed higher amounts of opioid compared to female providers, even after accounting for differences in gender by specialty and level. The reason for this discrepancy is unclear, and prior literature on the topic is mixed.30,31 There may be differences in how men and women establish treatment alliances with their patients or perceive patients’ pain levels and response to analgesics. However, future research is needed to explore the connection between provider gender and analgesia practices in the surgical setting.
Postoperative opioid prescribing is not limited to surgeons. Primary care providers prescribed 13% of all opioids in the 30-days after surgery in our study of previously opioid-naïve patients. It is therefore vital for surgeons to coordinate postoperative care with our primary care colleagues. For opioid-naïve patients, the surgeon should assume sole responsibility for the patient’s immediate postoperative pain management. To avoid overlapping opioid prescriptions, a marker of inappropriate prescribing32, surgeons should query Prescription Drug Monitoring Program (PDMP) reports.33–35 By alerting providers to uncoordinated prescribing, PDMPs have been shown to reduce prescribing amounts and reduce multiple visits to providers and pharmacies.36 Additionally, new-persistent opioid use is a real risk after surgery.4,6–8 When patients have persistent postoperative pain, transferring pain management responsibility to a primary care physician or pain specialist should be done with a clearly communicated transition of care.
This study has several limitations. This study population represents commercially insured patients from a single state, and the findings therefore may not be generalizable across all patients and states. In particular, laws regarding opioid prescribing rights of APPs may differ by state. Nevertheless, our data represents a diverse range of hospitals and covers a large proportion of Michigan. Additionally, claims data allows identification of opioid prescriptions that were filled, but it is not possible to identify number of pills consumed from these data; therefore, we cannot truly identify excess prescribing. Indications for opioid use is also unknown with these data. By limiting our analysis to opioid-naïve patients, however, it is likely that the majority of opioid prescriptions in the 30-days after surgery were for postoperative pain. We were also unable to identify resident status and training level among the physician prescribers, which could have a substantial impact on prescribing practice. Regarding risk adjustment, when quantifying the effect of APP on prescription amount, we were unable to simultaneously adjust for both surgical procedure and provider specialty; however, in our sensitivity analysis adjusting for surgical procedure, APP remained significantly associated with higher prescribing, though with a lower effect size. Thus, the effect of APP on prescription amount may range from 5% higher OME to 18% higher OME. Lastly, we did not account for timing of individual prescriptions within the 30-day postoperative period. It is possible that subsequent prescriptions for persistent pain are larger than initial prescriptions, which could account for primary care providers prescribing larger amounts than most surgical specialties.
In conclusion, amount of opioid prescribed per prescription is high in the 30 days after surgery and varies by provider specialty and type. APPs of all specialties account for a substantial proportion of postoperative opioid prescriptions and provide larger amounts compared to surgeons. Primary care physicians also provide opioid prescriptions within the postoperative period. All providers involved in the postoperative care of surgical patients can play an important role in developing best practices for responsible and safe postoperative opioid prescribing. Engaging each of these provider groups will be critical to understand existing prescribing practices and to tailor opioid prescribing policies and interventions accordingly.
Acknowledgments
Financial Support: Supported by the National Research Service Award postdoctoral fellowship (No. 5T32 CA009672-23) (J.S.-J.L.), the National Institute on Drug Abuse (Research Project Grant No. R01 DA042859) (C.M.B., M.J.E., and J.F.W.), and the Michigan Department of Health and Human Services (C.M.B., M.J.E., and J.F.W.).
Footnotes
Conflicts of interest: C.M.B. reports a patent for Peripheral Perineural Dexmedetomidine licensed to the University of Michigan and is a consultant with Tonix. C.M.B. has received research funding from Neuros Medical.
Publisher's Disclaimer: Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Michigan Department of Health and Human Services. Support for the Michigan Value Collaborative is provided by Blue Cross Blue Shield of Michigan as part of the Blue Cross Blue Shield of Michigan Value Partnerships program; however, the opinions, beliefs and viewpoints expressed by the authors do not necessarily reflect those of Blue Cross Blue Shield of Michigan or any of its employees.
References
- 1.Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152(11):1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Ann Surg. 2017;265(4):728–730. [DOI] [PubMed] [Google Scholar]
- 3.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM. Prescription opioid use, misuse, and use disorders in U.S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med. 2017;167(5):293–301. [DOI] [PubMed] [Google Scholar]
- 4.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in us adults. JAMA Surg. 2017:152(6):e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun EC, Darnall B, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176(9):1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ. 2014;348:g1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soneji N, Clarke HA, Ko DT, Wijeysundera DN. Risks of developing persistent opioid use after major surgery. JAMA Surg. 2016;151(11):1083–1084. [DOI] [PubMed] [Google Scholar]
- 8.Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425–430. [DOI] [PubMed] [Google Scholar]
- 9.Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265(4):709–714. [DOI] [PubMed] [Google Scholar]
- 10.Thiels CA, Anderson SS, Ubl DS, et al. Wide variation and overprescription of opioids after elective surgery. Ann Surg. 2017;266(4):564–573. [DOI] [PubMed] [Google Scholar]
- 11.Meara E, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375(1):44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Commonwealth of Massachusetts. An Act relative to substance use, treatment, education and prevention. 2016;House, No. 4056. Available at: http://www.massmed.org/advocacy/key-issues/opioid-abuse/conference-committee-report--an-act-relative-to-substance-use,-treatment,-education-and-prevention-(pdf)/.
- 13.Scutti S, Kounang N. CVS will limit opioid prescriptions to 7 days. CNN. http://www.cnn.com/2017/09/22/health/cvs-prescription-restrictions-opioids-bn/index.html. Published September 22, 2017. Accessed October 28, 2017. [Google Scholar]
- 14.Herrel LA, Syrjamaki JD, Linsell SM, Miller DC, Dupree JM. Identifying drivers of episode cost variation with radical prostatectomy. Urology. 2016;97:105–110. [DOI] [PubMed] [Google Scholar]
- 15.Ellimoottil CE, Syrjamaki JD, Voit B, Guduguntla V, Miller DC, Dupree JM. Validation of a claims-based algorithm to characterize episodes-of-care. Am J Manag Care. 2017;23(11):e382–e386. [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Data dissemination: announcing changes to the National Plan and Provider Enumeration System (NPPES) downloadable file. https://www.cms.gov/Regulations-and-Guidance/Administrative-Simplification/NationalProvIdentStand/DataDissemination.html. Updated August 4, 2016. Accessed October 18, 2017.
- 17.Logan J, Liu Y, Paulozzi L, Zhang K, Jones C. Opioid prescribing in emergency departments: the prevalence of potentially inappropriate prescribing and misuse. Med Care. 2013;51(8):646–653. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–141. [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Medicare and Medicaid Services. Risk Adjustment. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors.html.Updated January 3, 2017. Accessed October 18, 2017.
- 21.Paulozzi LJ, Mack KA, Hockenberry JM. Variation among states in prescribing of opioid pain relievers and benzodiazepines--United States, 2012. J Safety Res. 2014;51:125–129. [DOI] [PubMed] [Google Scholar]
- 22.Guy GP Jr., Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minnesota Department of Human Services. Opioid prescribing work group: acute pain prescribing recommendations (draft). https://mn.gov/dhs/assets/draft-acute-pain-recommendations_tcm1053-281603.pdf. Updated August 23, 2016. Accessed October 28, 2017.
- 24.Blay E Jr., Nooromid MJ, Bilimoria KY, et al. Variation in post-discharge opioid prescriptions among members of a surgical team. Am J Surg. 2017. Nov 1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas SH, Mumma S, Satterwhite A, et al. Variation between physicians and mid-level providers in opioid treatment for musculoskeletal pain in the emergency department. J Emerg Med. 2015;49(4):415–423. [DOI] [PubMed] [Google Scholar]
- 26.Beaudoin FL, Janicki A, Zhai W, Choo EK. Trends in opioid prescribing before and after implementation of an emergency department opioid prescribing policy. Am J Emerg Med. 2018. February;36(2):329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moote M, Krsek C, Kleinpell R, Todd B. Physician assistant and nurse practitioner utilization in academic medical centers. Am J Med Qual. 2011;26(6):452–460. [DOI] [PubMed] [Google Scholar]
- 28.Phelan SM, Hardeman RR. Health professionals’ pain management decisions are influenced by their role (nurse or physician) and by patient gender, age and ethnicity. Evid Based Nurs. 2015;18(2):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wandner LD, Heft MW, Lok BC, et al. The impact of patients’ gender, race, and age on health care professionals’ pain management decisions: an online survey using virtual human technology. Int J Nurs Stud. 2014;51(5):726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deepmala D, Franz L, Aponte C, Agrawal M, Jiang W. Identification of provider characteristics influencing prescription of analgesics: a systematic literature review. Pain Pract. 2013;13(6):504–513. [DOI] [PubMed] [Google Scholar]
- 31.Hampton SB, Cavalier J, Langford R. The influence of race and gender on pain management: a systematic literature review. Pain Manag Nurs. 2015;16(6):968–977. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care. 2013;19(8):648–665. [PubMed] [Google Scholar]
- 33.Greenwood-Ericksen MB, Poon SJ, Nelson LS, Weiner SG, Schuur JD. Best practices for prescription drug monitoring programs in the emergency department setting: results of an expert panel. Ann Emerg Med. 2016;67(6):755–764.e4. [DOI] [PubMed] [Google Scholar]
- 34.Manasco AT, Griggs C, Leeds R, et al. Characteristics of state prescription drug monitoring programs: a state-by-state survey. Pharmacoepidemiol Drug Saf. 2016;25(7):847–851. [DOI] [PubMed] [Google Scholar]
- 35.Ferries EA, Gilson AM, Aparasu RR, Chen H, Johnson ML, Fleming ML. The prevalence of and factors associated With receiving concurrent controlled substance prescriptions. Subst Use Misuse. 2017;52(12):1639–1645. [DOI] [PubMed] [Google Scholar]
- 36.Young LD, Kreiner PW, Panas L. Unsolicited reporting to prescribers of opioid analgesics by a state prescription drug monitoring program: an observational study with matched comparison group. Pain Med. 2017. Apr 4. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


