Abstract
Background
Staphylococcus aureus is one of the most isolated pathogens from the airways of cystic fibrosis (CF) patients. There is a lack of information about the clonal nature of S. aureus cultured from CF patients and their impact on disease. We hypothesized that patients would differ in their clinical status depending on S. aureus clonal carriage profiles during persistence.
Methods
During a 21-months prospective observational multicenter study (Junge et al., 2016), 3893 S. aureus isolates (nose, oropharynx, and sputa) were cultured from 183 CF patients (16 German centers, 1 Austrian center) and subjected to spa-sequence typing to assess clonality. Data were associated to lung function, age, gender, and antibiotic treatment by multivariate regression analysis.
Results
Two hundred and sixty-five different spa-types were determined with eight prevalent spa-types (isolated from more than 10 patients): t084, t091, t008, t015, t002 t012, t364, and t056. We observed different carriage profiles of spa-types during the study period: patients being positive with a prevalent spa-type, only one, a dominant or related spa-type/s. Patients with more antibiotic cycles were more likely to be positive for only one spa-type (p = 0.005), while older patients were more likely to have related (p = 0.006), or dominant spa-types (p = 0.026). Two percent of isolates were identified as methicillin-resistant S. aureus (MRSA) and evidence of transmission of clones within centers was low.
Conclusion
There was a significant association of antibiotic therapy and age on S. aureus carriage profiles in CF patients indicating that antibiotic therapy prevents acquisition of new clones, while during aging of patients with persisting S. aureus, dominant clones were selected and mutations in the spa-repeat region accumulated.
Keywords: Staphylococcus aureus, cystic fibrosis, persistent infection, spa-typing, clonal lineages, airway infection
Introduction
Cystic fibrosis (CF) is a life limiting genetic disease, which especially affects the lungs of CF patients with mucus retention and chronic bacterial infection of the airways leading to decreased lung function and reduced life expectancy (Elborn, 2016). Staphylococcus aureus is one of the earliest pathogens, which can be isolated from the airways of CF infants already (Cystic Fibrosis Foundation Patient Registry, 2018; European Cystic Fibrosis Society Patient Registry, 2018). S. aureus has a clonal population structure (Lindsay et al., 2006), is equipped with many virulence factors and can persist in the airways of CF patients for extended periods (Kahl et al., 2003; Schwerdt et al., 2018).
Several studies showed that in young CF patients, there is an increased lower airway inflammation with neutrophilic inflammation and pro-inflammatory cytokines and more clinical disease in case of S. aureus cultures compared to S. aureus-negative patients (Sagel et al., 2009; Gangell et al., 2011; Wong et al., 2013). However, there is less knowledge about the impact of S. aureus in older patients. In a prospective longitudinal multicenter study including 195 patients with persistent S. aureus cultures, we recently showed that in CF patients, who were older than 6 years, independent risk factors for worse lung function were high bacterial density in oropharyngeal cultures, exacerbations, elevated IL-6 levels, the presence of S. aureus small colony variants (SCVs), and co-infection with Stenotrophomonas maltophilia (Junge et al., 2016).
To determine the clonality of S. aureus, sequencing of the variable number of tandem repeat (VNTR) region of protein A, (SpA) spa-typing, represents an elegant, easy to perform, and low cost method compared to the more sophisticated and more cost intensive whole genome sequencing (WGS) (Harmsen et al., 2003; Koreen et al., 2004; O’Hara et al., 2016). Spa-types are assigned according to the sequence of base pairs within the repeats, which mostly consist of 24 base pairs, and the numbers of repeats, which range from 1 to 27 numbers as presented on the SpaServer1. It is also possible to cluster spa-types into related clonal complexes (spa CC) with defined common ancestors depending on their repeat composition (Mellmann et al., 2007). Interestingly, during persistence of S. aureus within CF airways, it has been shown that mutations occur in this region with deletions and duplications of repeats or point-mutations within repeats, leading to different spa-types (Kahl et al., 2005), which are closely related according to spa CCs. The relatedness of such clones with different spa-types, but very similar repeat successions was verified by pulsed-field gel electrophoresis (Kahl et al., 2005), multi-locus sequence typing (MLST) (Hirschhausen et al., 2013) or WGS (Schwartbeck et al., 2016). In different studies, we now confirmed that persistent S. aureus isolates persisting in the airways of CF patients, which are assigned to the same or related spa-types, which differed in their VNTR region by various mutations as outlined above, were confirmed to belong to the same clone by WGS (Schwartbeck et al., 2016; Langhanki et al., 2018; Herzog et al., 2019). Therefore, it seems that spa-sequence typing is a suitable method to analyze the relatedness of S. aureus isolates. Also, the VNTR region has been shown to be implicated in the regulation of inflammation (Martin et al., 2009) by its ability to modulate the pro-inflammatory response of SpA (Gómez et al., 2004) depending on the number of repeats (Garofalo et al., 2012).
There is a lack of knowledge about the S. aureus clones, which reside in the airways of CF patients and their dynamics during persistence. In this study, we determined the clonality of S. aureus isolates (n = 3893), which were cultured during a prospective long-term observational multicenter study (Junge et al., 2016), by spa-typing.
We hypothesized that lung disease of patients would differ depending on the carriage profiles of S. aureus depending on the clonality of isolates within the airways during our prospective study.
Materials and Methods
Patients, Specimens, and Bacteria
Staphylococcus aureus isolates (n = 3963), which were collected during a prospective 21-months multicenter study from 195 CF patients from 16 CF centers in Germany and 1 center in Austria (Junge et al., 2016), were used. Inclusion criteria were persistent S. aureus cultures a year before recruitment and being older than 6 years to be able to perform lung function tests. Exclusion criteria were chronic Pseudomonas aeruginosa or Burkholderia cepacia airway cultures. Specimens from nose, throat, and sputum were sent to the central study laboratory in Münster, where microbiological cultures were performed according to the requirements for CF airway cultures (Hogardt et al., 2006). S. aureus isolates were distinguished regarding size (normal/SCV phenotype), hemolysis (no hemolysis/weak/strong), and pigmentation (gray/white/yellow) on Columbia blood agar (Becton Dickinson, Heidelberg, Germany) incubated at 37°C, and on Schaedler agar (Becton Dickinson, Heidelberg, Germany) incubated at 37°C at 5% CO2. All S. aureus isolates with different phenotypes including hemolytic, non-hemolytic isolates, different pigmented isolates, and different size of isolates (SCVs, normal) were stored at −80°C and subjected to spa-sequence typing. For this study, all isolates were included in the further analysis.
Susceptibility Testing
All S. aureus isolates were subjected to susceptibility testing. Normal isolates were tested by VITEK 2 system (bioMérieux), and SCVs by agar diffusion testing on Columbia blood agar due to the requirements of SCVs for thymidine (Hogardt et al., 2006).
Antibiotic Treatment
In case report forms (CRFs), physicians reported antibiotic treatment of patients. For this analysis, only antibiotics directed against S. aureus were evaluated: first and second generation cephalosporins, antistaphylococcal penicillins, aminoglycosides, sulfamethoxazole/trimethoprim, clindamycin, rifampin.
Spa-Typing
Spa-sequence typing was performed by amplification of the variable region of protein A by PCR with ensuing sequencing according to Harmsen et al. (2003). Spa-types were assigned according to the Ridom StaphType software (Ridom GmbH, Würzburg, Germany).
BURP
By using the Based Upon Repeat Pattern method (BURP, Ridom StaphType software, Ridom GmbH, Würzburg, Germany) (Mellmann et al., 2007), we examined the clonal relatedness of spa-types for each individual patient as well as for the entire collection of isolates within and between each center.
Whole Genome Sequence-Based Typing
To uncover the genetic relationships of the S. aureus isolates, a subset of strains (Supplementary Table S5) was compared via WGS-based typing using the Illumina MiSeq platform (Illumina Inc., San Diego, CA, United States) (Mellmann et al., 2016). After quality trimming, coding core genome regions were compared in a gene-by-gene approach (core genome multilocus sequence typing, cgMLST) using the SeqSphere+ software version 6.0.0 (Ridom GmbH, Münster, Germany) and the published S. aureus cgMLST target scheme (Leopold et al., 2014). To display the clonal relationship of genotypes, the minimum spanning tree algorithm was applied using the same software. Genotypes differing in ≤24 alleles were rated as closely related. For backwards compatibility with classical molecular typing the spa-types were extracted from the WGS data in silico.
Statistical Analysis
We used SPSS (v.25, IBM) and SAS for the statistical tests and set the local significance level at α < 0.05. We used Mann–Whitney U-tests and logistic regression to run the tests. All models were adjusted to age and gender.
In addition to spa-types, we analyzed the categories age, gender, percentage of visits with antistaphylococcal antibiotics, percentage of visits with exacerbation, and the mean lung function measured as forced expiratory volume in 1 s in percent (FEV1%) predicted. We computed the variables as follows: The percentage of visits with antibiotics in relation to all visits of each patient (AB_percentage) and the percentage of visits with exacerbations in relation to all visits of each patient. We computed the mean lung function (mean FEV1% predicted) according to Quanjer et al. (2012). For the distribution of patients into the different S. aureus profiles, spa-types of all S. aureus isolates collected at all visits of individual patients were analyzed together.
Results
One hundred and eighty-three of one hundred ninety-five recruited CF patients remained S. aureus positive throughout the study with at least 50% of cultures being culture positive for S. aureus indicating persistent infection. In 1120 of 1278 visits (range 1–18, mean seven visits per patient), S. aureus was cultured (88%) from the airway specimens. From 1929 samples, 3893 different S. aureus isolates were cultured with a mean number of isolates of 21 per patient (range of 1–83), Supplementary Table S1. There was a difference in the number of spa-types dependent on the site (p < 0.001) with the fewest number of different spa-types in sputa, followed by nose and throat (Supplementary Table S1).
Population Structure of S. aureus Isolates as Assessed by spa-Typing
The 3893 S. aureus isolates could be assigned to 265 different spa-types. In each patient an average of 3.21 spa-types (range 1–12) was observed. For 7 of the 3893 isolates, no spa-type could be determined. These isolates were defined as non-typable.
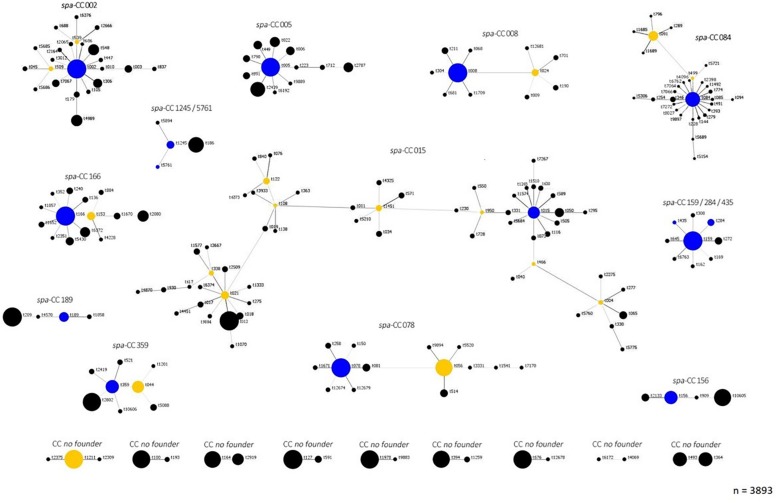
To visualize the population structure of the study S. aureus isolates, all isolates were grouped into clonal complexes by BURP analysis, which compares the base sequence of the repeat region of the individual spa-types. The population structure of all isolates revealed a highly diverse, but also clonal population structure of S. aureus with 192 of 265 spa-types (72%) belonging to 12 spa CCs with related repeat regions, while 36 were specified as singletons without relation to any other spa-type in this study (Figure 1). There were eight prevalent spa-types, which were isolated from more than 10 patients: t084, t091, t008, t015, t002, t012, t346, and t056, Table 1. Further information of the number of patients, S. aureus isolates, spa-types and spa CCs of the individual centers is given in Supplementary Table S2 and Supplementary Figures S1–S17.
FIGURE 1.
Population structure according to spa clonal complexes (CC) of S. aureus CF isolates. This figure demonstrates the clonal relatedness of all 265 spa-types of the 3893 S. aureus study across the 17 participating centers consisting of 16 German and 1 Austrian center. The analysis of the relationship of spa-types was performed based on the BURP algorithm as implemented in the Ridom StaphType software. 192 of the spa-types could be assigned to 12 CC: CC015, CC02, CC05, CC08, CC084, CC166, CC189, CC1245/5761, CC159/284/435, CC359, CC078, and CC156. The main founder spa-type is shown in blue in the middle of each CC, the sub founder marked in yellow, which leads to the following branch. All descendant spa-types are grouped around their respective founder. The size of each circle demonstrates the number of S. aureus isolates with the respective spa-type. For 19 spa-types, the BURP algorithm could determine no founder spa-type and therefore no clonal complex. These spa-types are presented as “no founder” and appear at the bottom of the figure. Thirty-six spa-types could not be related to other spa-types in our study. Therefore, BURP assigned them as singletons (dots not shown in this figure). Spa-types with four or less repeats were excluded from the analysis (n = 18, <1% of all spa-types), since no reliable information about the phylogenetic relatedness can be inferred. Singletons: t099, t185, t246, t370, t377, t488, t647, t746, t803, t1345, t1406, t1416, t1491, t1707, t2441, t2553, t2845, t3258, t5152, t5682, t5683, t5688, t5690, t5758, t5759, t6191, t6193, t6194, t6195, t6375, t7271, t9886, t9887, t9888, t9896, t12680. Excluded spa-types: t026, t103, t129, t227, t362, t390, t524, t559, t605, t693, t779, t1050, t1544, t1991, t2383, t3745, t5687, t7065.
TABLE 1.
Patients with prevalent spa-types.
| Spa-type | Spa clonal complex | Isolates (n) | Percentage of all isolates (%) | Patients (n) | Percentage of patients1 (%) | Patients with persistence2 (n) | Percentage of patients with persistence (%) | Centers3 (n) |
| t084 | CC084 | 310 | 7.8 | 36 | 19 | 18 | 50 | 16 |
| t091 | CC084 | 178 | 4.5 | 27 | 14 | 11 | 40 | 14 |
| t008 | CC008 | 138 | 3.5 | 18 | 9 | 11 | 61 | 10 |
| t015 | CC015 | 112 | 2.8 | 18 | 9 | 7 | 39 | 12 |
| t002 | CC002 | 108 | 2.8 | 16 | 9 | 7 | 44 | 9 |
| t012 | CC015 | 208 | 5.2 | 15 | 8 | 9 | 60 | 8 |
| t346 | CC084 | 72 | 1.8 | 14 | 8 | 3 | 21 | 9 |
| t056 | CC078 | 75 | 1.9 | 12 | 7 | 5 | 42 | 9 |
1Percentage of all patients with isolation of that prevalent spa-type. 2At least 50% of specimen of patients being positive for that prevalent spa-type. 3Number of centers, in which the prevalent spa-type was isolated.
Patients Distinguished According to Special Carriage Profiles of spa-Types
Our prospective longitudinal study allowed observing different dynamics of spa-types within patients as a post hoc analysis of our data. Therefore, we classified patients according to the different carriage profiles of their spa-types for the subsequent analysis. According to our definitions, the classification of patients to the different carriage profiles is not necessarily exclusive, Supplementary Table S3. We created models with logical influence structures and analyzed the relation between the different carriage profiles and the categories age, gender, percentage of visits with antibiotics, percentage of visits with exacerbation, and FEV1% predicted, Supplementary Table S3.
Carriage Profile 1 – Prevalent spa-Types
We defined a spa-type as prevalent, if the spa-type was isolated from more than 10 patients. Eight spa-types were more prevalent than all other spa-types: t084, t091, t008, t015, t002, t012, t346, and t056 (Table 1). To be assigned to this group, at least in 50% of visits, prevalent spa-types had to be present in patients’ specimens (n = 68). This group was compared to patients without persisting prevalent spa-types (n = 115).
Carriage Profile 2 – Patients With Only One spa-Type
In some patients, only S. aureus isolates belonging to one spa-type were cultured during the entire study. Another single isolate with a different spa-type in one respiratory specimen at one visit was accepted. There were 64 patients with only one spa-type compared to patients with several spa-types (n = 119).
Carriage Profile 3 – Dominant spa-Types
A dominant spa-type was defined as a spa-type, which was traceable throughout all visits of the patient with more than 50% of all isolates of this individual patient (n = 65). Other clones could occur but were not observed persistently throughout the study period. Patients with dominant spa-types were compared to patients without dominant spa-types (n = 54).
Carriage Profile 4 – Related spa-Types
By BURP analysis, all S. aureus isolates of patients were grouped according to the repeat sequence of their spa-types and their relatedness. To be assigned to the group of patients with related spa-types, in at least 50% of visits of these patients, isolates with related spa-types had to be present. The group of patients with related spa-types (n = 33, exemplified for six patients in Table 2, for all patients in Supplementary Table S4) was compared to patients without related spa-types (n = 86). Mutations observed in isolates with related spa-types were: deletions (n = 24), duplications (n = 14), point-mutations (n = 12), and combined mutations (n = 6, Table 2 and Supplementary Table S4).
TABLE 2.
Mutations within the VNTR region of related spa-types.
| Patient | All clones1 | Related clones2 | Non-related3 | Percentage of related clones4 (%) | Number of isolates5 | Spa-type6 | VNTR region7 | Mutations8 | Repeat9 | Nucleotide sequence of the repeat region10 |
| C1P4 | 5 | 4 | 1 | 80 | 20 | t050 | 08-16-02-16-34-34-17-34-16-34 | |||
| 1 | t295 | 08-16-02-16-34-34-17-34-34 | del | |||||||
| 2 | t008 | 11-19-12-21-17-34-24-34-22-25 | ||||||||
| 1 | t024 | 11-12-21-17-34-24-34-22-25 | del | |||||||
| C1P7 | 9 | 5 | 4 | 56 | 1 | t277 | 09-20-16-13-13-17-34-16-34 | r20 | AAAGAAGACAACAACAAACCTGGC | |
| 1 | t040 | 09-02-16-13-17-34-16-34 | del and pm | r02 | AAAGAAGACAACAAAAAACCTGGC | |||||
| 13 | t004 | 09-02-16-13-13-17-34-16-34 | pm | |||||||
| 7 | t346 | 07-23-12-34-12-12-23-02-12-23 | r12 | AAAGAAGACAACAACAAGCCTGGT | ||||||
| 1 | t2398 | 07-23-12-34-12-66-23-02-12-23 | pm | r66 | AAAGAAGACAGCAACAAGCCTGGT | |||||
| C2P6 | 3 | 2 | 1 | 67 | 4 | t084 | 07-23-12-34-34-12-12-23-02-12-23 | r12 | AAAGAAGACAACAACAAGCCTGGT | |
| 1 | t4096 | 07-23-21-12-34-34-12-12-23-02-12-23 | dupl and pm | r21 | AAAGAAGACAACAACAAGCCTGGC | |||||
| C2P9 | 7 | 3 | 4 | 43 | 26 | t084 | 07-23-12-34-34-12-12-23-02-12-23 | |||
| 1 | t346 | 07-23-12-34-12-12-23-02-12-23 | del | |||||||
| 1 | t085 | 07-23-12-34-34-12-23-02-12-23 | del | |||||||
| C3P3 | 4 | 2 | 2 | 50 | 7 | t078 | 04-21-12-41-20-17-12-12-17 | |||
| 6 | t081 | 04-21-12-41-20-17-12-17 | del | |||||||
| C3P9 | 5 | 2 | 3 | 40 | 13 | t499 | 07-23-12-12-34-12-12-23-02-12-23 | |||
| 1 | t9897 | 07-23-12-12-34-12-12-12-23-02-12-23 | dupl |
1All clones: all different spa-types isolated from the airways of this patient. 2Related clones: number of spa-types, which evolved most likely due to mutational events in the “variable number of repeat” region of spa during persistence. 3Non-related clones: number of additional clones with spa-types characterized by a non-related repeat region of spa.4Percentage of related clones: percentage of isolates with related spa-types.5Number of isolates with the respective spa-types. 6Spa-type: the different spa-types of patients with related spa-types; ancestor strains are marked in bold. 7VNTR-region: the sequence of the repeats within the VNTR-region; the mutated repeats are marked in bold in the ancestor strain. 8Mutations: the mutational event that caused the changed repeat succession: del, deletion; pm, point-mutation; dupl, duplication. 9Repeat: the number of the repeat, which shows a point-mutation, which leads to a different repeat number and to a different spa-type. 10Nucleotide sequence: the changed nucleotide sequence of the repeat caused by one point-mutation, which is marked in bold.
Multi-regression analyses of the different carriage profiles did not show any significant differences concerning gender, visits with exacerbations or lung function. Also, the carriage profiles of patients with prevalent and non-prevalent spa-types did not reveal any significant clinical differences.
Age and Antibiotic Therapy Were Associated With Carriage Profiles
The more often patients were treated with antibiotics, the higher was the probability for the patients for being positive for only one spa-type (p = 0.005). Patients with dominant or related spa-types were significantly older (p = 0.026 and p = 0.006) compared to patients with non-dominant or unrelated spa-types.
Whole Genome Sequence-Based Typing
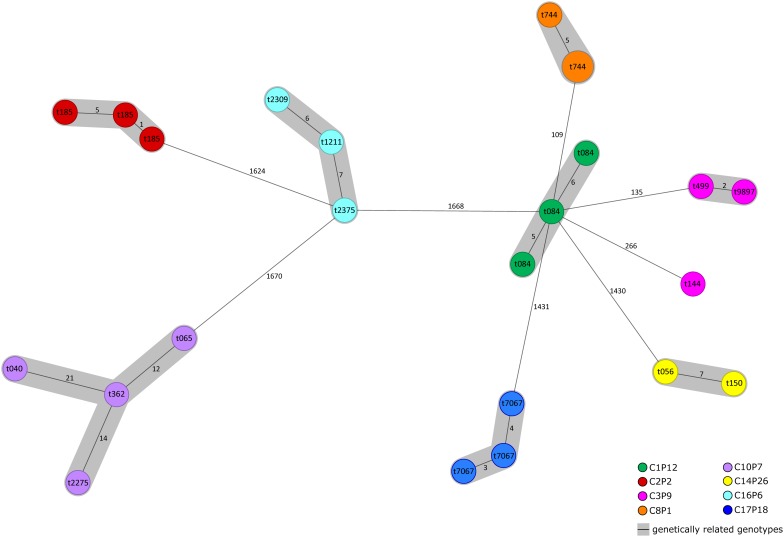
In total, 24 S. aureus strains were chosen from patients with different carriage profiles(Supplementary Table S5) for WGS-based analysis to determine their genetical relatedness. In silico extraction of spa-types resulted in the same spa-type as ascertained via classical spa-typing, except in one case, in which no spa-type could be detected via WGS. Minimum spanning tree analysis revealed eight clusters of genetical related isolates and one singleton (Figure 2). Each cluster contained S. aureus strains derived from only one patient, thereby confirming previous spa-typing analysis results. Only one isolate, categorized as related clone via classical spa-typing (t144, C3P9), was detected to be non-related to other isolates derived from the same patient, indicating either larger evolutionary events or co-infection with different clones of related spa-types.
FIGURE 2.
Minimum spanning tree of S. aureus isolates illustrating their genotypic relationship. 24 S. aureus strains are displayed representing different carriage profiles based on 1861 cgMLST target genes, pairwise ignoring missing values. Size of circles correlates with the number of identical genotypes. Different colors of circles indicate patients, from which S. aureus strains were chosen. Spa-types are given within different circles. Numbers on connecting lines indicate allele differences, gray shading indicates a close genetic similarity between different genotypes.
Susceptibility of S. aureus Isolates
The susceptibility testing of all isolates revealed low resistance rates for antistaphylococcal antibiotics (in percentage of tested isolates) with the following resistance rates: penicillin 74%, oxacillin (MRSA) 2%, erythromycin 27%, clindamycin 22%, gentamicin 8%, levofloxacin 3%, trimethoprim/sulfamethoxazole 9%, rifampin < 1%, vancomycin 0%, linezolid 0%, fusidic acid 1%, and fosfomycin 1%.
Transmission of S. aureus
Spa-typing of all isolates allowed to observe, if transmission of S. aureus clones occurred within CF centers. If spa-types were cultured from at least three patients within one center, there could be a possible event of transmission, Supplementary Table S6. However, most of these spa-types belonged to prevalent clones (20 of 25 possible events). Therefore, the culture of these spa-types from several patients could be just due to the higher prevalence of such clones in the community. However, without knowledge about other epidemiological data, it is difficult to evaluate transmission. Also, in most patients the possible transmitted spa-types were not persistently cultured (Supplementary Table S4).
Discussion
Staphylococcus aureus is one of the earliest and one of the most prevalent pathogens isolated from the airways of CF patients (Cystic Fibrosis Foundation Patient Registry, 2018; European Cystic Fibrosis Society Patient Registry, 2018), which persists for several years or even decades in spite of anti-staphylococcal therapy (Kahl et al., 1998, 2003; Andersen et al., 2014; Schwerdt et al., 2018). Nevertheless, there are only few studies, which evaluate the impact of S. aureus clonal lineages on lung disease in older CF patients (Wong et al., 2013; Junge et al., 2016).
Therefore, data from our earlier study (Junge et al., 2016) and from the analysis of spa-sequence typing of the collected isolates from this study are of interest to the CF community to shed more light on the behavior and impact of S. aureus and special S. aureus carriage profiles (prevalent, single, dominant, or related spa-types) during persistence in CF. Of importance is also that our study was conducted in an area, where CF patients are not treated with continuous anti-staphylococcal therapy as performed in the United Kingdom (Littlewood et al., 2009) or Australia (Bell and Robinson, 2008), but patients were rather treated, if S. aureus was cultured from the respiratory specimens or if symptoms occurred with S. aureus positive airway cultures.
Here, we determined the molecular clonality of almost 4000 S. aureus isolates from 183 CF patients during a 21-months study. There are some important findings of our study with the determination of S. aureus clonality during a long-term period in a large number of CF patients. Interestingly, there was not a special S. aureus spa-type, which we identified to be associated with a more severe lung disease during our study. Therefore, all S. aureus clones are able to cause lung disease and a more severe course of the CF disease may dependent on other most likely host related factors. Similar results have been shown recently in the study by Grundmann et al. (2010) who investigated the population structure of invasive S. aureus isolates. The study of invasive S. aureus revealed that all-cause mortality of S. aureus invasive infection was independent of spa-types indicating that there was no spa-type that stood out with respect to hypervirulence.
In our study, there were eight S. aureus spa-types that were isolated from more than 10 patients (prevalent clones). Interestingly, seven of the eight prevalent spa-types were not also the most prevalent spa-types of S. aureus isolates from studies of healthy nasal carriers in Germany [t084, t091, t008, t015 t012, t056, and t346; Holtfreter et al. (2016)], but all of our prevalent spa-types also belonged to the 20 most prevalent spa-types of invasive S. aureus isolates from a recent European study (Grundmann et al., 2010) indicating that most S. aureus isolates from CF patients originate from common clones present in the community setting and are not acquired during hospital contacts or stays. Furthermore, such prevalent S. aureus strains do not only belong to carriage strains (Holtfreter et al., 2016), but also to S. aureus strains that can cause severe life threatening infection (Grundmann et al., 2010). Also, in comparison to the study from Garbacz et al. (2018), in which 215 S. aureus isolates from 107 CF patients from Poland were characterized by spa-typing, four of our eight prevalent spa-types were also part of the five most common spa-types of their study (t015, t084, t091, and t002). Therefore, our findings are representative for S. aureus isolates cultured also from the airways of CF patients from other countries.
We classified the S. aureus clones into four different S. aureus carriage groups according to the profiles of the cultivation of S. aureus spa-types throughout the study period in this long-term observational study as a post hoc analysis. Such grouping of patients according to S. aureus carriage profiles was used to compare the patients in regard to demographic and clinical findings.
The more patients were treated with antibiotics, the higher was the probability to culture only one spa-type (p = 0.005). The fact that antibiotic treatment affected the number of different clones, indicates that CF patients are highly susceptible for the acquisition of new S. aureus strains, if not treated with antibiotics. Such new incoming strains will be on the one hand in competition with residing strains, on the other hand, resident strains can acquire new genetic information by horizontal gene transfer (Quanjer et al., 2012) as shown in an earlier study by Langhanki et al. (2018). In consequence, the acquisition of genes could lead to an optimized gene pool, which could facilitate persistence.
Another significant finding of our study was that older patients were more likely to be culture positive for related spa-types, which share the overall composition of the repeats of the VNTR region of spa, but which are characterized by mutations in this region consisting of deletions of repeats, duplications, or point-mutations within repeats, all of which are leading to different spa-types. The occurrence of related clones in CF patients has been shown earlier by our group in different studies (Kahl et al., 2003; Hirschhausen et al., 2013; Schwartbeck et al., 2016). To confirm the relatedness of clones not only by spa-typing, we also performed WGS of a number of isolates from different patients, Supplementary Table S5. Importantly, all isolates sequenced by WGS confirmed our spa-typing results except genome sequencing of S. aureus isolates from patient C3P9, of which two isolates were closely related but a third isolate differed by more than 200 bp indicating that either larger evolutionary events or co-infection with different clones of related spa-types occurred.
Interestingly, most mutations that occurred in the VNTR region were due to deletions of repeats, which is in line with Garofalo et al. (2012), who showed that there was an inverse correlation of the length of repeats and the length of S. aureus infection in CF patients and patients with chronic osteomyelitis. It has been shown that the VNTR region modulates the inflammatory response induced by protein A (Martin et al., 2009). Therefore, by deleting repeats during microevolution of the VNTR region, the pro-inflammatory response induced by protein A is decreased with less recruitment of neutrophils thereby facilitating S. aureus persistence in the hostile niche of CF airways.
There are some limitations of our study: with our 21-months study, we only got a short glimpse into the clonal behavior of S. aureus during persistence within the airways. Therefore, our data should be validated by long-term studies since in many patients, S. aureus persist for many years or even decades (Kahl et al., 1998; Hirschhausen et al., 2013; Andersen et al., 2014; Schwerdt et al., 2018). Another disadvantage was, that we only included patients, who were older than 6 years and who were already colonized or infected by S. aureus persistently. It would be also interesting to follow infants after neonatal screening to observe early S. aureus dynamics in CF patients.
Conclusion
The molecular analysis of S. aureus during our prospective longitudinal observational study showed that transmission of clones within centers and antibiotic resistance rates of S. aureus were low. Furthermore, our study revealed that antibiotic therapy had a strong impact on S. aureus carriage profiles that were cultured from the airways. Patients that were more often treated were more likely to be positive for only one S. aureus clone indicating that antibiotic therapy prevented acquisition of other S. aureus clones thereby minimizing horizontal gene transfer by other new incoming clones. Furthermore, age had an impact not only on the culture of related but also on the culture of dominant clones. This indicates that during S. aureus persistence mutations in the VNTR region of spa are accumulating, especially such mutations, which cause a less pro-inflammatory response by protein A, and that clones, which are optimized for persistence in the airways, are being selected.
Members of the Staphylococcal Cf Study Group
Department of Paediatric Pulmonology and Neonatology, Medizinische Hochschule Hannover, Hannover, Germany: Sibylle Junge. Clinical Research Group, Department of Paediatric Pulmonology and Neonatology, Medizinische Hochschule Hannover, Hannover, Germany: Burkhard Tümmler. CF-Center Innsbruck, Department of Paediatrics, University Hospital, Austria: Helmut Ellermunter. Department of Paediatrics, University Hospital Münster, Münster, Germany: Angelika Dübbers. Department of Paediatrics, Clemenshospital Münster, Münster, Germany: Peter Küster. Ruhr University Paediatric Clinic at St Josef Hospital, Bochum, Germany: Manfred Ballmann and Cordula Koerner-Rettberg. Department of Paediatrics, University Hospital Essen, Essen, Germany: Jörg Große-Onnebrink. Paediatricians “Kinderärztliche Ambulanz” Hamburg, Germany: Eberhardt Heuer and Wolfgang Sextro. CF Center, Department of Paediatrics, University Clinics Jena, Jena, Germany; Pediatric Pulmonology/Cystic Fibrosis, Brandenburg Medical School (MHB) University. Brandenburg an der Havel, Germany: Jochen G. Mainz. Department of Paediatrics, University Clinics Dresden, Dresden, Germany: Jutta Hammermann. Department of Pediatrics, University Clinics Tübingen, Tübingen, Germany: Ute Graepler-Mainka. Department of Paediatric Pulmonology and Immunology, Charité – Universitätsmedizin Berlin, Campus Virchow Klinikum, Berlin, Germany: Doris Staab. University Hospital Halle, Halle, Germany: Bettina Wollschläger. Children’s Hospital Osnabrück, Osnabrück, Germany: Rüdiger Szczepanski. Department of Paediatrics, University of Düsseldorf, Düsseldorf, Germany: Antje Schuster. Park Schönefeld Clinics Kassel, Germany: Friedrich-Karl Tegtmeyer. Ruhrlandklinik Essen, Germany: Sivagurunathan Sutharsan. University Clinics Leipzig, Germany: Alexandra Wald.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethik-Kommission der Ärztekammer Westfalen-Lippe und des Universitätsklinikum Münster (2007-496-f-S). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin and by the participating patients.
Author Contributions
CW analyzed the data and wrote the manuscript with the help of BK. DG analyzed the data. SK and AM were responsible for whole genome sequencing of S. aureus isolates, analysis of the sequencing data, and construction of the Figure 2. SH performed susceptibility analyses. NB and CH performed spa-typing. SJ, BT, HE, AD, PK, MB, CK-R, JG-O, EH, WS, JM, JH, UG-M, DS, BW, RS, AS, F-KT, SS, and AW provided patient specimens and CRFs. GP contributed to the study design. BK initiated and was responsible for the study and its design. All authors read and accepted the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling Editor declared a shared affiliation, though no other collaboration, with one of the authors JM at the time of review.
Funding. This project was supported by a grant from the Mukoviszidose e.V. (S05/07), Germany, and partly by grants of the Interdisciplinary Center for Clinical Research (IZKF Münster; Kah2/016/16) and the Transregional Collaborative Research Center 34 (C7) to BK.
Contributor Information
Staphylococcal CF Study Group:
Sibylle Junge., Burkhard Tümmler., Helmut Ellermunter, Angelika Dübbers, Peter Küster, Manfred Ballmann, Cordula Koerner-Rettberg, Jörg Große-Onnebrink, Eberhardt Heuer, Wolfgang Sextro, Jochen G, Jutta Hammermann, Ute Graepler-Mainka, Doris Staab, Bettina Wollschläger, Antje Schuster, Friedrich-Karl Tegtmeyer, Sivagurunathan Sutharsan, and Alexandra Wald
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2020.00230/full#supplementary-material
References
- Andersen C., Kahl B. C., Olesen H. V., Jensen-Fangel S., Nørskov-Lauritsen N. (2014). Intravenous antibiotics given for 2 weeks do not eradicate persistent Staphylococcus aureus clones in cystic fibrosis patients. Clin. Microbiol. Infect. 20 O285–O291. 10.1111/1469-0691.12406 [DOI] [PubMed] [Google Scholar]
- Bell S. C., Robinson P. J. (2008). Cystic Fibrosis Standards of Care, Australia. Sydney, NSW: Cystic Fibrosis Australia. [Google Scholar]
- Cystic Fibrosis Foundation Patient Registry (2018). Cystic Fibrosis Foundation Patient Registry. Annual Data Report Bethesda, MD: Cystic Fibrosis Foundation. [Google Scholar]
- Elborn J. S. (2016). Cystic fibrosis. Lancet 388 2519–2531. 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- European Cystic Fibrosis Society Patient Registry (2018). European Cystic Fibrosis Society Patient Registry. Annual Data Report 2016 Karup: European Cystic Fibrosis Society. [Google Scholar]
- Gangell C., Gard S., Douglas T., Park J., De Klerk N., Keil T., et al. (2011). Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin. Infect. Dis. 53 425–432. 10.1093/cid/cir399 [DOI] [PubMed] [Google Scholar]
- Garbacz K., Piechowicz L., Podkowik M., Bania J. (2018). Emergence and spread of worldwide Staphylococcus aureus clones among cystic fibrosis patients. Infect. Drug Resist. 11 247–255. 10.2147/IDR.S153427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo A., Giai C., Lattar S., Gardella N., Mollerach M., Kahl B. C., et al. (2012). The length of the Staphylococcus aureus protein A polymorphic region regulates inflammation: impact on acute and chronic infection. J. Infect. Dis. 206 81–90. 10.1093/infdis/jis311 [DOI] [PubMed] [Google Scholar]
- Gómez M. I., Lee A., Reddy B., Muir A., Soong G., Pitt A., et al. (2004). Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10 842–848. 10.1038/nm1079 [DOI] [PubMed] [Google Scholar]
- Grundmann H., Aanensen D. M., van den Wijngaard C. C., Spratt B. G., Harmsen D., Friedrich A. W., et al. (2010). Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 7:e1000215. 10.1371/journal.pmed.1000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen D., Claus H., Witte W., Rothgänger J., Claus H., Turnwald D., et al. (2003). Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41 5442–5448. 10.1128/jcm.41.12.5442-5448.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog S., Dach F., de Buhr N., Niemann S., Schlagowski J., Chaves-Moreno D., et al. (2019). High nuclease activity of long persisting Staphylococcus aureus isolates within the airways of cystic fibrosis patients protects against NET-mediated killing. Front. Immunol. 10:2552. 10.3389/fimmu.2019.02552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhausen N., Block D., Bianconi I., Bragonzi A., Birtel J., Lee J. C., et al. (2013). Extended Staphylococcus aureus persistence in cystic fibrosis is associated with bacterial adaptation. Int. J. Med. Microbiol. 303 685–692. 10.1016/J.IJMM.2013.09.012 [DOI] [PubMed] [Google Scholar]
- Hogardt M., Haeußler S., Balke B., Kahl B. C., Schmoldt S., Leitritz L., et al. (2006). MIQ 24: Atemwegsinfektionen bei Mukoviszidose: Qualitätsstandards in der Mikrobiologisch-Infektiologischen Diagnostik. Munich: Urban & Fischer. [Google Scholar]
- Holtfreter S., Grumann D., Balau V., Barwich A., Kolata J., Goehler A., et al. (2016). Molecular epidemiology of Staphylococcus aureus in the general population in Northeast Germany: results of the study of health in Pomerania (SHIP-TREND-0). J. Clin. Microbiol. 54 2774–2785. 10.1128/JCM.00312-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge S., Görlich D., den Reijer M., Wiedemann B., Tümmler B., Ellemunter H., et al. (2016). Factors associated with worse lung function in cystic fibrosis patients with persistent Staphylococcus aureus. PLoS One 11:e0166220. 10.1371/journal.pone.0166220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl B., Herrmann M., Schulze Everding A., Koch H. G., Becker K., Harms E., et al. (1998). Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J. Infect. Dis. 177 1023–1029. 10.1086/515238 [DOI] [PubMed] [Google Scholar]
- Kahl B. C., Duebbers A., Lubritz G., Haeberle J., Koch H. G., Ritzerfeld B., et al. (2003). Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J. Clin. Microbiol. 41 4424–4427. 10.1128/JCM.41.9.4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl B. C., Mellmann A., Deiwick S., Peters G., Harmsen D. (2005). Variation of the polymorphic region X of the protein A gene during persistent airway infection of cystic fibrosis patients reflects two independent mechanisms of genetic change in Staphylococcus aureus. J. Clin. Microbiol. 43 502–505. 10.1128/JCM.43.1.502-505.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreen L., Graviss S. V., Ramaswamy E. A., Naidich S., Musser J. M., Kreiswirth B. N. (2004). spa typing method for discriminating among Staphylococcus aureus isolates: implications for use of a single marker to detect genetic micro- and macrovariation. J. Clin. Microbiol. 42 792–799. 10.1128/JCM.42.2.792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhanki L., Berger P., Treffon J., Catania F., Kahl B. C., Mellmann A. (2018). In vivo competition and horizontal gene transfer among distinct Staphylococcus aureus lineages as major drivers for adaptational changes during long-term persistence in humans. BMC Microbiol. 18:152. 10.1186/s12866-018-1308-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold S. R., Goering R. V., Witten A., Harmsen D., Mellmann A. (2014). Bacterial whole-genome sequencing revisited: portable, scalable, and standardized analysis for typing and detection of virulence and antibiotic resistance genes. J. Clin. Microbiol. 52 2365–2370. 10.1128/JCM.00262-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay J. A., Moore C. E., Day N. P., Peacock S. J., Witney A. A., Stabler R. A., et al. (2006). Microarrays reveal that each of the ten dominant lineages of Staphylococcus aureus has a unique combination of surface-associated and regulatory genes. J. Bacteriol. 188 669–676. 10.1128/JB.188.2.669-676.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood J. M., Bevan A., Connett G., Conway S., Govan J., Hodson M. (2009). Antibiotic Treatment for Cystic Fibrosis: Report of the UK Cystic Fibrosis Trust Antibiotic Group. Kent: Cystic Fibrosis Trust. [Google Scholar]
- Martin F. J., Gomez M. I., Wetzel D. M., Memmi G., O’Seaghdha M., Soong G., et al. (2009). Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J. Clin. Invest. 119 1931–1939. 10.1172/JCI35879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellmann A., Bletz S., Böking T., Kipp F., Becker K., Schultes A., et al. (2016). Real-time genome sequencing of resistant bacteria provides precision infection control in an institutional setting. J. Clin. Microbiol. 54 2874–2881. 10.1128/JCM.00790-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellmann A., Weniger T., Berssenbrügge C., Rothgänger J., Sammeth M., Stoye J., et al. (2007). Based Upon Repeat Pattern (BURP): an algorithm to characterize the long-term evolution of Staphylococcus aureus populations based on spa polymorphisms. BMC Microbiol. 7:98. 10.1186/1471-2180-7-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara F. P., Suaya J. A., Ray G. T., Baxter R., Brown M. L., Mera R. M., et al. (2016). Spa typing and multilocus sequence typing show comparable performance in a macroepidemiologic study of Staphylococcus aureus in the United States. Microb. Drug Resist. 22 88–96. 10.1089/mdr.2014.0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quanjer P. H., Stanojevic S., Cole T. J., Baur X., Hall G. L., Culver B. H., et al. (2012). Multi-ethnic reference values for spirometry for the 3–95 year age range: the global lung function 2012 equations. Eur. Respir. J. 40 1324–1343. 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagel S. D., Gibson R. L., Emerson J., McNamara S., Burns J. L., Wagener J. S., et al. (2009). Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J. Pediatr. 154 183–188. 10.1016/j.jpeds.2008.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartbeck B., Birtel J., Treffon J., Langhanki L., Mellmann A., Kale D., et al. (2016). Dynamic in vivo mutations within the ica operon during persistence of Staphylococcus aureus in the airways of cystic fibrosis patients. PLoS Pathog. 12:e1006024. 10.1371/journal.ppat.1006024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdt M., Neumann C., Schwartbeck B., Kampmeier S., Herzog S., Görlich D., et al. (2018). Staphylococcus aureus in the airways of cystic fibrosis patients – a retrospective long-term study. Int. J. Med. Microbiol. 308 631–639. 10.1016/j.ijmm.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Wong J. K., Ranganathan S. C., Hart E. (2013). Staphylococcus aureus in early cystic fibrosis lung disease. Pediatr. Pulmonol. 48 1151–1159. 10.1002/ppul.22863 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.