Abstract
The endothelial glycocalyx is a gel-like structure that is bound to the luminal surface of the vascular endothelium. At the interface between flowing blood and endothelial cells, the glycocalyx has several functions that are critical for the maintenance of a healthy vasculature, particularly in regard to the vascular endothelium. Within the vasculature the glycocalyx modulates vascular resistance to maintain blood flow homogeneity in the microcirculation, mechanotransduces fluid shear stress to the endothelium, and buffers endothelial cells from plasma oxidants, cytokines, and circulating immune cells. In advanced age and cardiovascular disease (CVD), the glycocalyx is deteriorated. Moreover, glycocalyx deterioration may precede traditional measurements of age-related vascular dysfunction, such as impaired endothelium-dependent dilation and large artery stiffness, suggesting that a deteriorated glycocalyx could initiate age-related CVD pathology.
Introduction
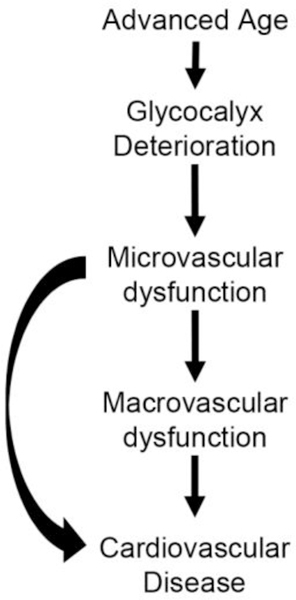
The endothelial glycocalyx is a gel-like structure that is bound to the luminal surface of the vascular endothelium, and is composed of a network of glycosaminoglycans, glycoproteins, and glycolipids with the predominant glycosaminoglycans being heparin sulfate, chondroitin sulfate, and hyaluronan [1]. Within the microvasculature the glycocalyx has several important functions including modulating vascular resistance to maintain blood flow homogeneity in the microcirculation [2,3], mechanotransducing fluid shear stress to the endothelium [4,5], modulating vascular permeability [6,7], and buffering endothelial cells from plasma oxidants [8], cytokines [9], and circulating immune cells [10,11]. These functional properties of the glycocalyx were identified using invasive surgical techniques in animal models, but recent technological advances in intravital microscopic imagery coupled with automated capture and analysis software have permitted glycocalyx properties in humans to be non-invasively imaged and rapidly evaluated in an unbiased fashion in a large number of microvessels. Subsequently, the deterioration of the glycocalyx using this technique has been identified in healthy older adults [12], several age-related chronic disease states, such as cardiovascular disease (CVD) [13], end-stage renal disease [14], and diabetes [15–17], as well as in patients with sepsis [18] and the auto-immune disease, systemic sclerosis [19,20]. Currently, there is little direct mechanistic evidence to link a deteriorated glycocalyx to age-related CVD pathology. The focus of this brief review will be to explore the working hypothesis that a deteriorated glycocalyx plays a role in pathological features that precede age-related CVD (Figure 1).
Figure 1.
Working hypothesis demonstrating how the deterioration of the glycocalyx in advanced age leads to age-related cardiovascular disease.
Glycocalyx Structure and Function
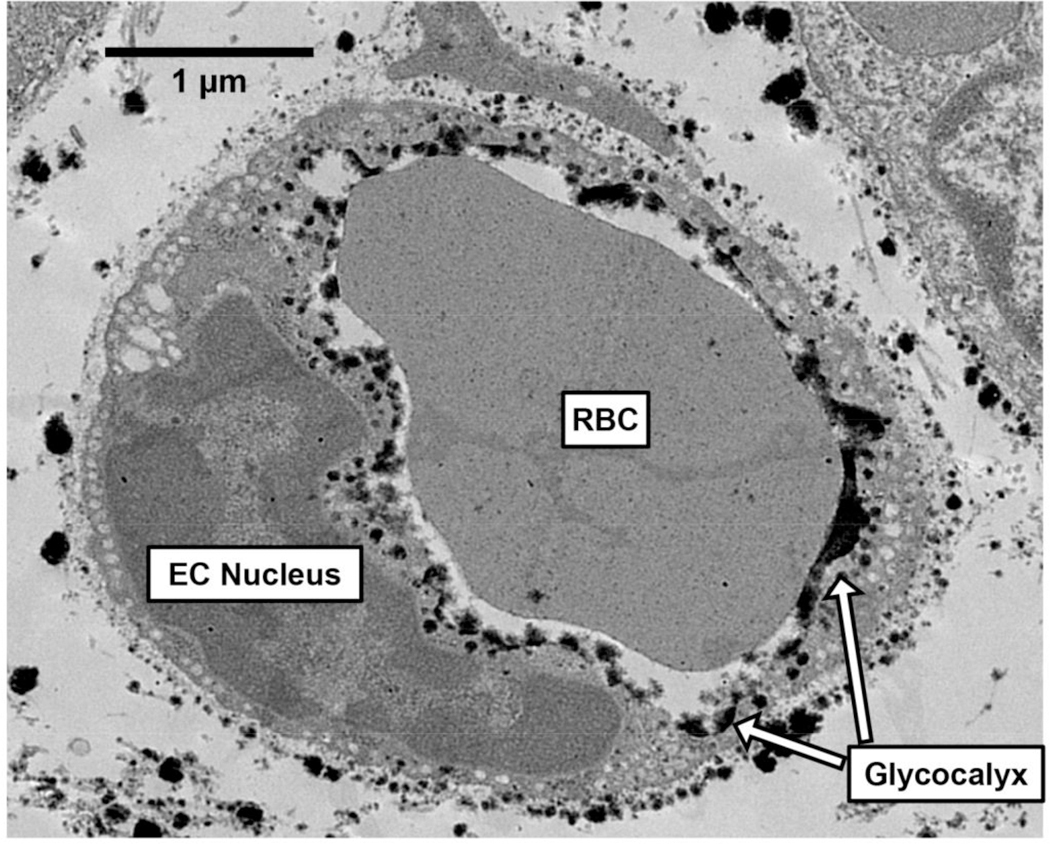
In Figure 2, we have included an electron micrograph from our laboratory that shows a mouse soleus microvessel with a glycocalyx that lines its endothelial wall. Indeed, the first visual evidence of the endothelial glycocalyx was demonstrated by Luft in 1966 using electron microscopy, in which the glycocalyx was described as an ‘endocapillary layer’ that had ‘an irregular, fluffy, indeterminate boundary’, and therefore, must be the ‘interface over which blood flows in the vascular system, its presumptive mucus-like properties very likely have an important bearing on normal vascular function, and in vascular disease as well’ [21]. Prior to the discovery of the glycocalyx, a relationship between arterial diameter and blood hematocrit (i.e., percentage of red blood cells [RBCs] per unit of blood) had been reported, indicating that with decreasing vessel diameter, blood hematocrit is reduced [22]. The Fåhræus effect can partly explain this relationship, as there is a tendency for flowing RBCs to travel in the center of a glass tube, leaving a RBC-free plasma layer near the tube wall. Thus, with any given decrease in tube diameter, the proportion of space taken up by the RBC-free plasma layer is increased, lowering tube hematocrit (i.e., the volume percentage of RBCs in a linear segment) [23]. However, tube hematocrit in isolated microvessels in vivo is lower than would be expected in glass tubes. This led Klitzman and Duling to theorize that an additional RBC-free plasma layer exists near the capillary wall that was distinct from the plasma layer that occurs as a result of the Fåhræus effect [24]. Support for the existence of this additional plasma layer came from a subsequent study that simultaneously compared capillary tube hematocrit to discharge hematocrit (i.e., volume percentage of RBC outflow from capillaries) in microvessels across a range of diameters [25]. Indeed, the discrepancy between hematocrit measurements in live microvessels vs. glass tubes can be explained by the existence of the glycocalyx, a component of the vessel that is not freely permeable to RBCs. Later studies would find that heparinase infusion, which enzymatically degrades heparin sulfates, eliminates the glycocalyx, and transiently increases tube hematocrit [26] and lowers flow resistance in mesenteric arterioles [27]. Moreover, epi-illumination, which also degrades the glycocalyx, has been shown to increase the column width of flowing RBCs in microvessels [28]. These findings provided a proof of principle that the glycocalyx has a strong influence on microvascular hemodynamics and tube hematocrit in microvessels.
Figure 2.
Electron micrograph of a mouse soleus microvessel with a glycocalyx that lines its endothelial wall. EC, endothelial cell; RBC, red blood cell.
Aside from being the interface between flowing blood and endothelial cells, the glycocalyx has several functions that serve to promote a more appropriate blood flow distribution in the microcirculation that may be critical for the maintenance of a healthy vasculature (Figure 3A). In advanced age, although there is no deficit in bulk blood flow at rest or during exercise to the hindlimb musculature in rats, the distribution of blood flow to exercising muscles is markedly altered in old rats, resulting in a distributional shift in blood flow from oxidative to glycolytic muscles during submaximal endurance exercise [29]. The apparent mismatch in exercise-induced blood flow distribution has major ramifications on exercise performance, and may be the result of impaired microvascular perfusion in advanced age [30,31]. Indeed, we have observed lower markers of microvascular perfusion at rest in older mice and humans that is accompanied by a deteriorated glycocalyx [12]. The glycocalyx passively modulates resistance to blood flow in microvessels, which serves to maintain blood flow homogeneity within the microvasculature [2,3]. Without a glycocalyx, differences in microvessel diameter at bifurcations result in inconsistencies in blood flow distribution due to a greater resistance and blood viscosity in the smaller microvessel at a bifurcation. Thus, one potential explanation for alterations in blood flow hemodynamics in advanced age may be the inability to efficiently and effectively distribute blood flow within the microvasculature that result from a deteriorated glycocalyx. In addition to promoting a more appropriate blood flow distribution within the microvasculature, the glycocalyx also acts as a buffer to lessen the impact of capillary irregularities on flowing RBCs, resulting in a lower driving pressure required for RBC passage in capillaries [32]. Although the role of the glycocalyx in facilitating oxygen transfer from RBCs to target tissues is less understood, a thicker and less penetrable glycocalyx requires greater RBC deformability that could promote elongation of RBCs, increasing their velocity and longitudinal passage, which could augment oxygen exchange capacity at the associated capillaries [33]. Taken together, without the glycocalyx the microvasculature appears to lack efficiency in blood flow distribution. The result of this could lead to impaired microvascular perfusion, which may also impact some of the pathophysiological alterations in vascular function that occur in larger arteries in age-related CVD (i.e., hypertension, tissue hypoxia).
Figure 3.
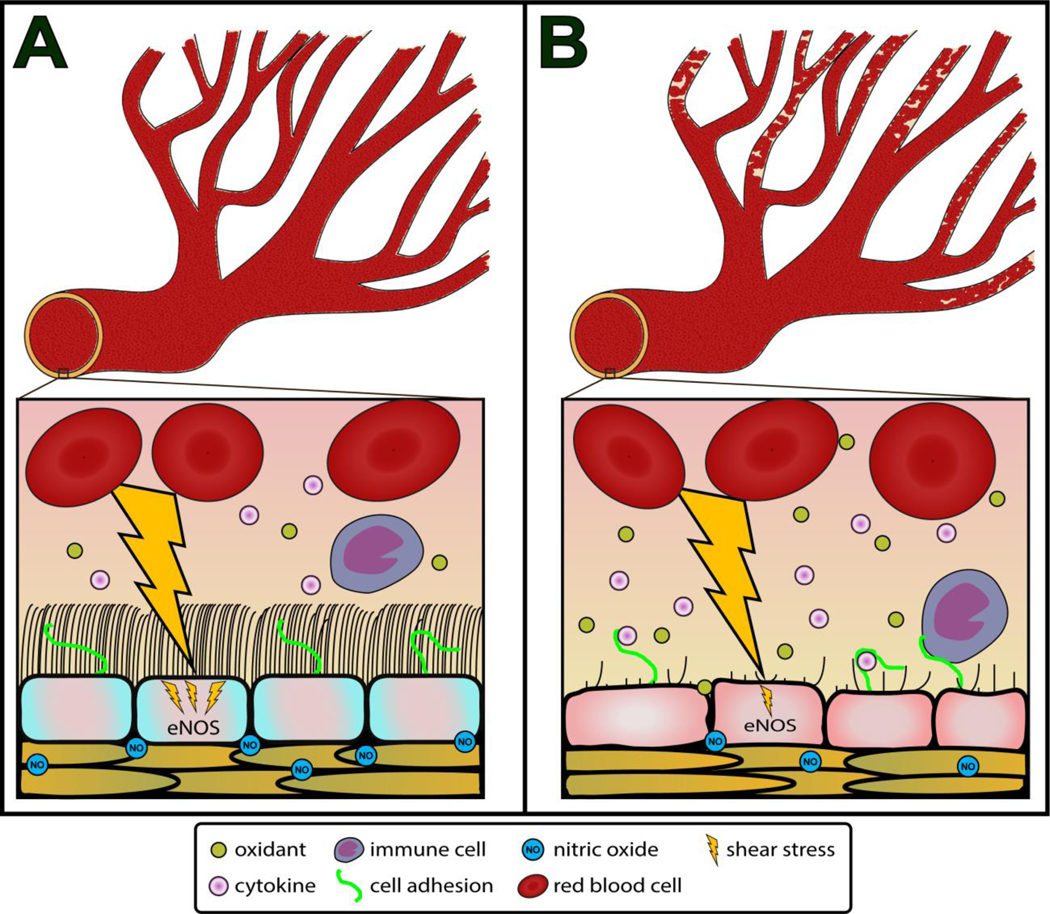
The role of glycocalyx in maintaining the blood flow distribution. Figure 3A depicts the arterial vasculature in youth, where the glycocalyx contributes to the maintenance of blood flow homogeneity, protects endothelial cells from the inflammatory cytokines, oxidants, and immune cells, and mechanotransduces shear stress to the endothelial cells stimulating nitric oxide (NO) production by endothelial NO synthase (eNOS). Figure 3B depicts the arterial vasculature in advanced age. Age-related glycocalyx deterioration results in greater blood flow heterogeneity between branch points, while impairing mechanotransduction of shear stress to the endothelium lessening NO production, and leaving the endothelium vulnerable to plasma inflammatory cytokines, oxidants, and immune cell adhesion that further reduce NO bioavailability.
Role of Glycocalyx in Aged Endothelium
The endothelium is a dynamic tissue that performs many vital functions throughout the arterial tree, such as releasing molecules that regulate a properly functioning vascular network, exchanging fluid and molecules between the blood and surrounding tissues, participating and facilitating the immune response, and controlling vascular resistance in response to changes in blood flow through the regulation of arterial tone in resistance vessels [34,35]. Advancing age is the strongest independent predictor of endothelial dysfunction [36], and the age-related decline in endothelial function in both the microcirculation and larger arteries contributes to a host of pathophysiological changes, including augmented large and resistance arterial tone, induction of greater oscillatory shear stress, and large artery stiffening [37,38]. Our laboratory has extensively studied the effects of aging on vascular physiology [39], and has observed a dysfunctional endothelium that is accompanied by a deteriorated glycocalyx in several animal models of aging and age-related diseases (unpublished observations). Because the glycocalyx resides on the luminal surface of the endothelial cell membrane, it is the interface between endothelial cells and flowing blood, stimulating the release of nitric oxide from endothelial cells via mechanotransduction of fluid shear stress to the endothelium [4,5]. Indeed, the glycocalyx is the endothelium’s ‘shear sensor’, as endothelial cells in culture proliferate and align to the direction of laminar flow when a glycocalyx is present, but do not proliferate and align after heparinise-induced glycocalyx degradation [5]. Hyaluronan also has a critical role in mechanotransduction of shear stress to the endothelium, as laminar shear stress stimulates the integration of hyaluronan into the glycocalyx [40], and hyaluronan integration into the glycocalyx augments nitric oxide release in response to increased shear stress [41]. These findings indicate that increases in shear stress are critical to glycocalyx synthesis, which then allows endothelial cells to rapidly respond to changes in shear stress. Recently, we reported that aged arteries have a reduction in arterial HAS2, the enzyme responsible for the synthesis of high molecular weight hyaluronan [12]. Thus, it is plausible that age-related changes in endothelial function may result from an arterial HAS2 deficiency-mediated lack of high molecular weight hyaluronan that impairs glycocalyx synthesis. Further study is warranted in this area, particularly to better define the timeline of these pathophysiological changes as they occur across the lifespan.
The vascular endothelium is particularly vulnerable to chronic elevations in oxidative stress [42], and prevention of oxidative stress-induced endothelial dysfunction can be achieved by an intact glycocalyx [8] (Figure 3B). Arterial oxidants can induce endothelial dysfunction by reducing nitric oxide bioavailability via uncoupling of nitric oxide synthase within endothelial cells [43]. Although the glycocalyx can act as a physical barrier to plasma oxidants, it also can quench plasma oxidants via glycocalyx bound superoxide dismutase [8]. Thus, an intact glycocalyx may augment endothelial function by alleviating the plasma oxidant-mediated suppression of nitric oxide bioavailability in endothelial cells. It is unknown whether a degraded glycocalyx precedes or is a consequence of age-related CVD. To the best of our knowledge no data exist that support the idea that a degraded glycocalyx precedes age-related vascular endothelial dysfunction, although accumulated vascular function data from large cross-sectional studies of healthy humans indicate that small artery endothelial dysfunction is initiated nearly two decades before larger conduit artery endothelium dysfunction is present [39]. Our laboratory has observed a reduction in endothelial glycocalyx thickness in mice by 12 months of age (unpublished observations), corresponding to early middle-age in humans. Therefore, it is possible that microvascular dysfunction, as a consequence of a degraded glycocalyx in early middle-age, initiates a gradual decline in endothelial function that progresses from small to large arteries over a period of decades.
In addition to elevations in plasma oxidants, advanced age is also associated with chronic, low grade inflammation, characterized by increases in pro-inflammatory cytokines and immune cell adhesion [44,45] that are also deleterious to the vascular endothelium [9]. Similar to plasma oxidants, pro-inflammatory cytokines and immune cells can be buffered from coming into contact with cell adhesion sites on endothelial cells by the endothelial glycocalyx [9–11]. Although acute TNF-α infusion degrades the glycocalyx, it is likely that the glycocalyx can, at least partially, protect endothelial cells from TNF-α, as well as other pro-inflammatory cytokines [46]. When the glycocalyx is deteriorated via pro-inflammatory cytokines the endothelium becomes a more hospitable site for immune cell adhesions and infiltration [46], which itself promotes further inflammation. On its own, both plasma oxidants and pro-inflammatory cytokines can induce endothelial dysfunction, but in advanced age, increases in plasma oxidants and pro-inflammatory cytokines occur simultaneously, acting in a vicious cycle to negatively impact the aged vasculature [47].
Conclusions
In this short review, we have provided evidence to support our working hypothesis that age-related glycocalyx deterioration is an initiating factor in vascular dysfunction that occurs in advanced age and age-related CVD. A healthy glycocalyx promotes a more efficient microvasculature that is characterized by a more appropriate blood flow distribution and microvascular perfusion that prevents many of the upstream pathophysiological alterations that occur in age-related CVD. In addition to microvascular perfusion, there is evidence that the glycocalyx is also critical for a properly functioning vascular endothelium. Foremost, the glycocalyx facilitates the ability of the endothelium to sense and respond to changes in shear stress by releasing nitric oxide. An intact glycocalyx also protects the endothelium from plasma oxidants and pro-inflammatory cytokines that would otherwise bombard endothelial cells, leading to a reduction in endothelial cell nitric oxide bioavailability. We, and others, have shown that the glycocalyx is deteriorated in advanced age and in a multitude of age-related CVD states. Moreover, we have observed a deteriorated glycocalyx in as early as middle age (unpublished observations), indicating that glycocalyx deterioration may precede age-related impairments in vascular endothelial function, as endothelial dysfunction tends to occur later in this timeline. Further study is warranted to explore this working hypothesis that a degraded glycocalyx is one of the earlier consequences of vascular aging that then initiates vascular dysfunction and ultimately results in age-related CVD. If so, it is likely that the glycocalyx could be a novel therapeutic target to ameliorate age-related vascular dysfunction. One potential strategy is to administer glycocalyx precursors via dietary supplementation, which has been shown to restore the glycocalyx in patients with diabetes [17]. However, it remains to be seen whether dietary supplementation with glycocalyx precursors and similar strategies that can restore the glycocalyx are also capable of ameliorating arterial dysfunction.
Highlights.
The endothelial glycocalyx is a gel-like structure that lines the endothelium.
In advanced age and cardiovascular disease (CVD), the glycocalyx is deteriorated.
An intact glycocalyx is critical to a properly functioning endothelium.
Glycocalyx deterioration may initiate age-related CVD pathology.
Acknowledgements
This work was supported by the National Institutes of Health [R01 AG050238, R44 AG053131, K02 AG045339, K99 AT010017].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG: The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007, 454:345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pries AR, Secomb TW: Microvascular blood viscosity in vivo and the endothelial surface layer. Am J Physiol Heart Circ Physiol 2005, 289:H2657–2664. [DOI] [PubMed] [Google Scholar]
- 3.McClatchey PM, Schafer M, Hunter KS, Reusch JE: The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am J Physiol Heart Circ Physiol 2016, 311:H168–176.**This study found that the endothelial glycocalyx promotes a more homogenous blood flow throughout the microcirculation by passively modulating flow resistance at microvessel bifurcations.
- 4.Pries AR, Secomb TW, Gaehtgens P: The endothelial surface layer. Pflugers Arch 2000, 440:653–666. [DOI] [PubMed] [Google Scholar]
- 5.Yao Y, Rabodzey A, Dewey CF: Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol 2007, 293:H1023-H1030. [DOI] [PubMed] [Google Scholar]
- 6.van den Berg BM, Vink H, Spaan JA: The endothelial glycocalyx protects against myocardial edema. Circ Res 2003, 92:592–594. [DOI] [PubMed] [Google Scholar]
- 7.Adamson RH: Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol 1990, 428:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Bolli R, Qiu Y, Tang X-L, Murphree SS, French BA: Gene therapy with extracellular superoxide dismutase attenuates myocardial stunning in conscious rabbits. Circulation 1998, 98:1438–1448. [DOI] [PubMed] [Google Scholar]
- 9.Mulivor AW, Lipowsky HH: Inflammation- and ischemia-induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol 2004, 286:H1672–1680. [DOI] [PubMed] [Google Scholar]
- 10.Vink H, Constantinescu AA, Spaan JA: Oxidized lipoproteins degrade the endothelial surface layer : implications for platelet-endothelial cell adhesion. Circulation 2000, 101:1500–1502. [DOI] [PubMed] [Google Scholar]
- 11.Mulivor AW, Lipowsky HH: Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol 2002, 283:H1282–1291. [DOI] [PubMed] [Google Scholar]
- 12.Machin DR, Bloom SI, Campbell RA, Phuong TTT, Gates PE, Lesniewski LA, Rondina MT,Donato AJ: Advanced age results in a diminished endothelial glycocalyx. Am J Physiol Heart Circ Physiol 2018, 315:H531–H539.**This study observed that in advanced age in multiple tissues of mice and humans that there is age-related glycocalyx deterioration.
- 13.Martens RJ, Vink H, van Oostenbrugge RJ, Staals J: Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis 2013, 35:451–454. [DOI] [PubMed] [Google Scholar]
- 14.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H: Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol 2012, 23:1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, Holleman F, Diamant M,Heine RJ, Hoekstra JB, et al. : Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006, 55:1127–1132. [DOI] [PubMed] [Google Scholar]
- 16.Groen BB, Hamer HM, Snijders T, van Kranenburg J, Frijns D, Vink H, van Loon LJ: Skeletal muscle capillary density and microvascular function are compromised with aging and type 2 diabetes. J Appl Physiol 2014, 116:998–1005. [DOI] [PubMed] [Google Scholar]
- 17.Broekhuizen L, Lemkes B, Mooij HL, Meuwese MC, Verberne H, Holleman F, Schlingemann RO, Nieuwdorp M, Stroes ES, Vink H: Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010, 53:2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donati A, Damiani E, Domizi R, Romano R, Adrario E, Pelaia P, Ince C, Singer M: Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc Res 2013, 90:86–89. [DOI] [PubMed] [Google Scholar]
- 19.Machin DR, Gates PE, Vink H, Frech TM, Donato AJ: Automated Measurement of Microvascular Function Reveals Dysfunction in Systemic Sclerosis: A Cross-sectional Study. J Rheumatol 2017, 44:1603–1611.**This study identified an deteriorated glycocalyx in patients with systemic sclerosis using both automated and manual measurement techniques, and showed a strong relation between these measurements.
- 20.Miranda S, Armengol G, Le Besnerais M, Levesque H, Benhamou Y: New insights into systemic sclerosis related microcirculatory dysfunction by assessment of sublingual micr\ocirculation and vascular glycocalyx layer. Results from a preliminary study. Microvasc Res 2015, 99:72–77. [DOI] [PubMed] [Google Scholar]
- 21.Luft JH: Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc 1966, 25:1773–1783. [PubMed] [Google Scholar]
- 22.Gibson JG, Seligman AM, Peacock WC, Aub JC, Fine J, Evans RD: The Distribution of Red Cells and Plasma in Large and Minute Vessels of the Normal Dog, Determined by Radioactive Isotopes of Iron and Iodine. J Clin Invest 1946, 25:848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fåhraeus R: The suspension stability of the blood. Physiological Reviews 1929, 9:241–274. [Google Scholar]
- 24.Klitzman B, Duling BR: Microvascular hematocrit and red cell flow in resting and contracting striated muscle. Am J Physiol 1979, 237:H481–490. [DOI] [PubMed] [Google Scholar]
- 25.Desjardins C, Duling BR: Microvessel hematocrit: measurement and implications for capillary oxygen transport. Am J Physiol 1987, 252:H494–503. [DOI] [PubMed] [Google Scholar]
- 26.Desjardins C, Duling BR: Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am J Physiol 1990, 258:H647–654. [DOI] [PubMed] [Google Scholar]
- 27.Pries AR, Secomb TW, Jacobs H, Sperandio M, Osterloh K, Gaehtgens P: Microvascular blood flow resistance: role of endothelial surface layer. Am J Physiol 1997, 273:H2272–2279. [DOI] [PubMed] [Google Scholar]
- 28.Vink H, Duling BR: Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res 1996, 79:581–589. [DOI] [PubMed] [Google Scholar]
- 29.Musch TI, Eklund KE, Hageman KS, Poole DC: Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol (1985) 2004, 96:81–88. [DOI] [PubMed] [Google Scholar]
- 30.Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC: Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 2005, 146:259–268. [DOI] [PubMed] [Google Scholar]
- 31.Russell JA, Kindig CA, Behnke BJ, Poole DC, Musch TI: Effects of aging on capillary geometry and hemodynamics in rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 2003, 285:H251–258. [DOI] [PubMed] [Google Scholar]
- 32.Secomb TW, Hsu R, Pries AR: Blood flow and red blood cell deformation in nonuniform capillaries: effects of the endothelial surface layer. Microcirculation 2002, 9:189–196. [DOI] [PubMed] [Google Scholar]
- 33.Lanotte L, Tomaiuolo G, Misbah C, Bureau L, Guido S: Red blood cell dynamics in polymer brush-coated microcapillaries: A model of endothelial glycocalyx in vitro. Biomicrofluidics 2014, 8:014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R B I F: Vascular endothelium and blood flow. Handb. Exp. Pharmacol. 2006, 176:43. [DOI] [PubMed] [Google Scholar]
- 35.Pober JS, Min W, Bradley JR: Mechanisms of Endothelial Dysfunction, Injury, and Death. Annual Review of Pathology: Mechanisms of Disease 2009, 4:71–95. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF Jr, Keyes MJ, Levy D,Vasan RS, Benjamin EJ: Local shear stress and brachial artery flow-mediated diation: the Framigham Heart Study. Hypertension 2004, 44:134–139. [DOI] [PubMed] [Google Scholar]
- 37.Lakatta EG, Levy D: Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 2003, 107:139–146. [DOI] [PubMed] [Google Scholar]
- 38.Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG: Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation 1993, 88:1456–1462. [DOI] [PubMed] [Google Scholar]
- 39.Donato AJ, Machin DR, Lesniewski LA: Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res 2018, 123:825–848.**This study observed that in advanced age in multiple tissues of mice and humans that there is age-related glycocalyx deterioration.
- 40.Gouverneur M, Spaan JA, Pannekoek H, Fontijn RD, Vink H: Fluid shear stress stimulates incorporation of hyaluronan into endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 2006, 290:H458–452. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki S, Vink H, Hiramatsu O, Kajita T, Shigeto F, Spaan JA, Kajiya F: Role of hyaluronic acid glycosaminoglycans in shear-induced endothelium-derived nitric oxide release. Am J Physiol Heart Circ Physiol 2003, 285:H722–726. [DOI] [PubMed] [Google Scholar]
- 42.Eskurza I, Monahan KD, Robinson JA, Seals DR: Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J. Physiol. 2004, 556:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison DG: Endothelial function and oxidant stress. Clin Cardiol 1997, 20:II-11–17. [PubMed] [Google Scholar]
- 44.Donato AJ, Black AD, Jablonski KL, Gano LB, Seals DR: Aging is associated with greater nuclear NFkappaB, reduced IkappaBalpha, and increased expression of proinflammatory cytokines in vascular endothelial cells of healthy humans. Aging Cell 2008, 7:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR: Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res 2007, 100:1659–1666. [DOI] [PubMed] [Google Scholar]
- 46.Henry CB, Duling BR: TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am J Physiol Heart Circ Physiol 2000, 279:H2815–2823. [DOI] [PubMed] [Google Scholar]
- 47.Manea A, Manea SA, Gafencu AV, Raicu M: Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch Physiol Biochem 2007, 113:163–172. [DOI] [PubMed] [Google Scholar]