Abstract
Background.
This study investigated whether higher maternal choline levels mitigate effects of marijuana on fetal brain development. Choline transported into the amniotic fluid from the mother activates α7-nicotinic acetylcholine receptors on fetal cerebro-cortical inhibitory neurons, whose development is impeded by cannabis blockade of their cannabinoid-1(CB1) receptors.
Methods.
Marijuana use was assessed during pregnancy from women who later brought their newborns for study. Mothers were informed about choline and other nutrients, but not specifically for marijuana use. Maternal serum choline was measured at 16 weeks gestation.
Results.
Marijuana use for the first 10 weeks gestation or more by 15% of mothers decreased newborns’ inhibition of evoked potentials to repeated sounds (d’ = 0.55, p < 0.05). This effect was ameliorated if women had higher gestational choline (rs = −0.50, p = 0.011). At 3 months of age, children whose mothers continued marijuana use through their 10th gestational week or more had poorer self-regulation (d’ = −0.79, p < 0.05). This effect was also ameliorated if mothers had higher gestational choline (rs = 0.54, p = 0.013). Maternal choline levels correlated with the children’s improved duration of attention, cuddliness, and bonding with parents.
Conclusions.
Prenatal marijuana use adversely affects fetal brain development and subsequent behavioral self-regulation, a precursor to later, more serious problems in childhood. Stopping marijuana use before 10 weeks gestational age prevented these effects. Many mothers refuse to cease use because of familiarity with marijuana and belief in its safety. Higher maternal choline mitigates some of marijuana’s adverse effects on the fetus.
Keywords: Cannabis, choline, fetal development, marijuana, pregnancy, receptors nicotinic
Introduction
Despite warnings of marijuana’s dangers in pregnancy by many authorities, use has increased, especially in younger women (Brown et al., 2017; Volkow et al., 2017; American College of Obstetricians and Gynecologists, 2018). Many women are using at conception, and many continue to use as a natural remedy for morning sickness, depression, and stress and anxiety (Roberson et al., 2014; Brown et al., 2017). Intrauterine effects can include altered blood flow, neurological problems, and growth restriction (El Marroun et al., 2009, 2010; Metz et al., 2017). Late first trimester is a vulnerable exposure period when fetal brain cerebro-cortical laminae develop (Richardson et al., 1995; Huang et al., 2009). Subsequent effects occur on childhood brain development, behavior, and cognition (Fried et al., 2003; Goldschmidt et al., 2012; El Marroun et al., 2016). Today’s marijuana cigarettes with up to 10-fold more tetrahydrocannabinol (THC) than in 1990 may be more detrimental (Cabrera, 2016). No current prenatal interventions prevent THC’s effects (Calvigioni et al., 2014). The only advice is to promote ‘good parenting skills…to compensate for a less than optimal prenatal environment’ (Huizink, 2015).
Ten weeks gestation begins a critical period when cerebro-cortical laminae are forming but maternal choline levels are lowest (Huang et al., 2009; Orczyk-Pawilowicz et al., 2016). Although choline has several functions in development, the mechanism of choline’s effects on cerebro-cortical inhibition involves direct activation of α7-nicotinic cholinergic receptors responsible for maturation of inhibitory and excitatory neurotransmission (Alkondon et al., 1997; Liu et al., 2006). Null mouse mutants of its gene CHRNA7 block this effect (Stevens et al., 2014). Corroborating evidence is CHRNA7 pharmacogenomic effects in clinical trials of phosphatidylcholine supplements (Ross et al., 2013, 2016). α7-Nicotinic acetylcholine receptors are expressed in early gestation at levels nearly 10-fold higher than in newborns and adults, but they do not receive acetylcholine synapses until just before birth (Court et al., 1997; Descarries et al., 2008). Millimolar concentrations of choline in amniotic fluid are sufficient for it to be an agonist at fetal α7-nicotinic receptors (Alkondon et al., 1997; Ilcol et al., 2002). The expression of CB1 receptors on the same interneurons as CHRNA7 suggests a specific mechanism for the mitigating effects of higher choline levels on fetal brain development for mothers who use marijuana.
Newborns’ auditory P50 sensory gating paradigm assesses the development of cerebral inhibition and is thus an early biomarker of the competing effects of THC and choline on the child’s fetal interneuron development. The initial stimulus activates a response P50S1 and also activates collateral inhibitory interneurons. Strength of the inhibition is tested by the decrease in response P50S2 after a second stimulus (Adler et al., 1982). Inhibition of the P50S2 response is the critical variable that reflects the activity of the interneurons that are the site of the convergence of effects of choline and THC (Miller and Freedman, 1995). Lower P50 inhibition in newborns predicts childhood behavior problems in attention and social withdrawal associated with ADHD and other mental illnesses (Ross et al., 2016).
The hypothesis of this prospective study is that higher levels of maternal choline in early gestation might mitigate marijuana’s effects on fetal brain development as measured by the newborn’s P50 inhibition and the child’s behavior at 3 months of age. Higher choline levels improved fetal brain development and early childhood behavior in studies that found positive behavioral effects through 4 years of age (Wu et al., 2012; Ross et al., 2013, 2016; Caudill et al., 2018; Jacobson et al., 2018; Freedman et al., 2019). Healthy women and women with mental illness, infection, and alcoholism benefitted. However, no study has examined the relationship of maternal choline levels to marijuana exposure in pregnancy. A model for the use of a prenatal nutrient to protect fetal development is folic acid prevention of spina bifida, which is effective in a wide range of maternal risks (Beaudin and Stover, 2009).
Participants and methods
Maternal assessment and recruitment
Women were enrolled from a public safety-net prenatal clinic at 14–16 weeks gestation from July 2013 until July 2016. Gestational age was timed from the last menstrual period and by ultrasound. Exclusions were fetal anomaly and major maternal medical morbidity. The Colorado Multiple Institution Review Board approved the study; all participants gave informed consent. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Women were asked to participate in a prospective study of stress in pregnancy, including drug use, on their child’s development. The women were informed that drug use would be assessed by interviewers and by urine toxicology and these results were not reportable to authorities under Colorado law.
Psychiatric diagnoses were made using the Structured Clinical Interview for DSM-IV Axis I Disorders with DSM-5 criteria. Self-ratings on Center for Epidemiological Studies of Depression-R (CESD-R), State-Trait Anxiety Inventory-State Version (STAI-S), and the Perceived Stress Scale (PSS) were performed. Maternal sociodemographics and health, including infections and BMI, were assessed. Labor, delivery, and neonatal parameters were recorded from the medical record. Investigators were blinded to drug choline levels during assessments.
Assessment of maternal substance use
Mothers had structured interviews at 16 weeks gestation to assess use at conception, including substance types and frequency, and use during subsequent weeks, anchored by calendar dates and gestational milestones including pregnancy tests and prenatal visits. The interview was repeated every 6 weeks through term. Urine toxicology (Alere iCup Dx14, Waltham MA) was obtained at 16 weeks. Recent research finds that self-report and urine toxicology, although not always concordant, both indicate the timing of past use equally well (Smith et al., 2018b).
Choline measurements
Maternal plasma choline and its metabolite betaine at 16 weeks gestation were assayed by the Colorado Translational Research Center Metabolomics Laboratory using mass spectroscopy. Blood samples were obtained at least two hours after breakfast. Plasma was quickly separated by refrigerated centrifugation to prevent platelet phosphatidylcholine release.
Stable isotope standards for betaine (N,N,N-trimethylglycine, cat no D-3352) and choline (cat no D-2464) were purchased from CDN Isotopes. Serum samples were thawed on ice, then 20 μL was extracted with 480 μL of ice-cold extraction buffer (5:3:2 MeOH: MeCN:H2O) containing 0.1 μM each of N,N,N-trimethylglycine-D9 (betaine) and [1,1,2,2-D4]choline. Extraction was performed by vigorous agitation at 4° C for 30 min followed by centrifugation at 12 000 rpm, 4° C for 10 min. A 100 μL aliquot of supernatant was transferred to a glass vial, dried under N2 flow, and resuspended in an equal volume of water containing 0.1% (v/v) formic acid. Aqueous extracts were analyzed by ultra-high pressure liquid chromatography-mass spectrometry (UHPLC-MS) on a Thermo Vanquish UHPLC (San Jose, CA) coupled to a Thermo Q Exactive mass spectrometer (Bremen, Germany) via positive electrospray ionization. Solvents were water (phase A) and acetonitrile (phase B) supplemented with formic acid (0.1%) and the flow rate was 0.25 mL/min. Metabolites were separated using a Kinetex C18 (Phenomenex, Torrance, CA) column (2.1 × 150 mm, 1.7 μm) with a 6 min gradient of 0–2 min 2% B; 2–2.5 min increase to 25% B; 2.5–4 min hold at 25% B; 4– 4.01 min decrease to 2% B; and 4.01–6 min hold at 2% B. The Q Exactive mass spectrometer was operated in a full scan mode over the range of 65–950 m/z. Samples were randomized and a quality control sample was injected every 10 runs. The coefficient of variation was <10%. Data analysis was performed using Maven Metabolomic Analysis and Visualization Engine (Princeton University) following file conversion by MassMatrix (Case Western Reserve University). Absolute concentrations were obtained using the equation: [light choline] = (peakarealight/peakareaheavy) [heavy choline] *DF, where DF = dilution factor, in this case, 25 (i.e. 20 μ of serum in a total 500 μ volume).
Mothers received information on diets higher in choline, but dietary intake was not estimated because of the low relationship of self-reported intake to maternal choline levels in pregnant women, r = 0.2 (Wu et al., 2012). The placental choline transporter CLT1 produces amniotic fluid levels approximately twice maternal plasma levels (Ilcol et al., 2003; Baumgartner et al., 2015). Uptake is proportional to maternal plasma concentration, which suggests that higher peak levels may be important determinants of amniotic fluid levels (Iwao et al., 2016). Maternal levels obtained in non-fasting conditions, as in the present study, can be elevated, but only after high-choline meals that exceed the recommended daily intake (Zeisel et al., 1980; Holm et al., 2003; Abratte et al., 2009). Prenatal vitamin use did not affect choline levels. Only choline activates α7-nicotinic receptors (Alkondon et al., 1997). We found no effects of its metabolite betaine.
Neonatal physiological recording of cerebral inhibition
Newborns were studied at 1 month (44 weeks) after birth adjusted for gestational age. Vertex electroencephalogram, electro-oculogram, submental electromyogram, and respiration were continuously recorded while infants napped (Kisley et al., 2003; Hunter et al., 2011). Recording of the cerebral auditory evoked potential P50, a positive EEG wave 50 ms post-stimulus, occurred in the second active sleep episode, the developmental precursor of REM sleep, identified by low-voltage desynchronized vertex activity with the absence of K-complexes, change in respiration, and large eye movements with submental atonia (Anders et al., 1971). The second active sleep episode was reached ~45 min after sleep onset. In adults, P50 inhibition in REM and waking are equivalent (Griffith and Freedman, 1995).
Two identical auditory stimuli are delivered 500 ms apart to elicit P50S1 and P50S2. P50 inhibition is often assessed as amplitude ratios P50S2/P50S1 or (P50S1—P50S2)/P50S1 (Adler et al., 1982). However, the skew inherent in ratios limits their power for correlation with risk factors. P50S2 amplitude, covaried for P50S1, which is normally distributed, has therefore also been used (Smith et al., 1994). Lower P50S2 amplitudes indicate increased inhibition. The assumption is that P50S1 variance is small, compared to P50S2 variance. In 151 newborns, effect sizes for P50S1 differences between newborns whose mothers had no known risk v. women with depression or schizophrenia ranged from 0–0.16. Effect sizes for a decrease in P50S2 amplitude were 0.21–0.50 (Hunter et al., 2011). The effect of maternal schizotypy on newborn P50 inhibition has been replicated by another group, who also found increased P50S2 amplitudes (Smith et al., 2018a). Intraclass correlation between two newborn recordings 1 week apart is rICC = 0.84. Other technical aspects of recordings have been published (Hunter et al., 2008; 2015).
Childhood behavioral assessments
Parents completed the Infant Behavior Questionnaire-Revised Short Form (IBQ-R) when the infant was 3 months of age (Gartstein and Rothbart, 2003; Putnam et al., 2014). The Parental Distress Subscale of the Parenting Stress Index was also completed as a possible covariate for parental bias (Abidin, 2012). The 91-item IBQ-R Short Form, commonly used to study behavior in children at this age, rates 14 aspects of child behavior, which the IBQ-R developers clustered into 3 indices by factor analysis. Surgency summarizes the child’s level of activity and positive effect. Negativity summarizes fearfulness and anxiety. Regulation summarizes duration of attention, responsiveness to parents, and enjoyment of quiet play. Two components of Regulation are also in Surgency, smiling and soothability, and the two indices are highly correlated in the present sample (r = 0.51, p < 0.001). Covariation with Surgency isolates elements of Regulation that are more specific to the early development of attention and less attributable to the child’s general psychomotor activation. A similar covariance between Regulation and Surgency (0.80) has been documented by another group, who have also proposed revisions to the factor structure (Bosquet-Enlow et al., 2016).
Statistical analyses
Neonatal P50S2 inhibition and childhood IBQ-R Regulation were the two principal outcomes, based on a previous work that found P50 inhibition was a biomarker of choline’s effect and that regulatory behaviors were the most affected outcome (Ross et al., 2016). Kolmogorov–Smirnov tests for each outcome did not find a significant deviation from normal distributions. One-way ANOVAs with Tukey’s post-hoc contrasts or χ2 with Fisher’s exact test analyzed differences between the four marijuana exposure categories. Generalized linear models analyzed the four maternal marijuana-use groups as a categorical effect and the choline level as a continuous effect, with infant sex as a covariate. Factor analysis of the other maternal sociodemographic, gestational, labor and delivery, and neonatal covariates by principal components analysis with Varimax rotation and Kaiser normalization identified three factors termed Socio-economic, Neonatal Status, and Maternal Health (online Supplementary Table A1).
Choline’s effect size on P50 inhibition in a previous study was Cohen’s d’ = 0.7 (Cheatham et al., 2012). We expected 20% of the women would have adequate choline levels and 30% attrition (Cheatham et al., 2012; Ross et al., 2016). Therefore, we enrolled 200 women to have power 1-β > 0.95, α = 0.05, 1-tail to observe an overall choline effect. For the women who continued marijuana usage 10 weeks or later, the post hoc power for the observed effect of marijuana was for P50 inhibition d’ = 0.55, 1-β = 0.79, 1-tail α = 0.05 and for IBQ-R Regulation d’ = 0.79, 1-β; = 0.93, 1-tail α = 0.05; the power for the observed effect for the maternal choline level was for P50 inhibition ∣ρ∣ = 0.40, 1-β = 0.68, 1-tail α = 0.05 and for IBQ-R Regulation ∣ρ∣ = 0.53, 1-β = 0.85, 1-tail α = 0.05.
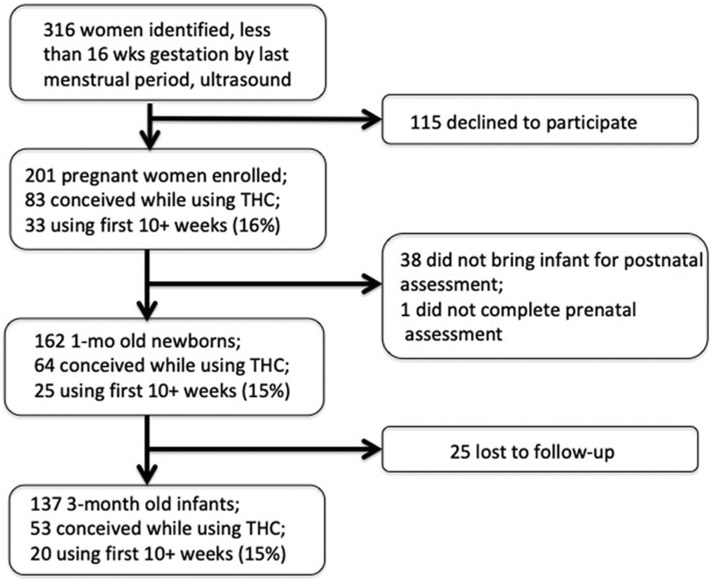
Attrition from prenatal enrollment to postnatal assessments is inevitable. Most were due to the mother’s leaving the area to return to her own mother’s house to raise her child. The percentage of women at each time point who used marijuana for at least 10 weeks gestation was no different, indicating no differential dropout based on marijuana use (Fig. 1).
Fig. 1.
Enrollment of subjects from initial prenatal visit through the child’s first year. The proportion of mothers who were using marijuana at 10 weeks gestation did not change at each stage.
Results
Marijuana use during gestation
Of the 201 women who enrolled in the trial, 162 women brought their newborns for P50 evoked potential recording at 1 month of age, adjusted for gestational age at birth; 98 (60%) did not use marijuana, 26 (16%) reported using it only at the time of conception, 13 (8%) continued during the first weeks of gestation but had discontinued by 10 weeks gestation, and 25 (15%) continued use from conception to 10 weeks gestation, and then with variable frequency until term. The four categories of mothers based on their gestation marijuana use – none, conception, 4 weeks gestation, and 10 weeks or more – were selected because each successive time period had a significantly different proportion of mothers using marijuana compared to the previous period (online Supplementary Fig. A1). Most women smoked marijuana 1–3 times per week.
All women were informed by both their treating clinicians and research personnel about the risks to the fetus of substance use, including marijuana. Mothers who continued to use marijuana during pregnancy were using it at conception. The most common reasons for continuing marijuana were the mothers’ belief that it was safer and more effective than pharmaceuticals for morning sickness, depression, and stress.
Urine toxicology was assessed at 14–16 weeks gestation for 91 mothers, of whom 12 were positive for marijuana. Urine samples were not collected from the other mothers because of a protocol error. Over half the mothers who said they used marijuana tested negative, a rate found in other studies (El Marroun et al., 2010, 2011). Only 1 mother who denied marijuana use tested positive.
Women who used marijuana were younger and less educated (Table 1). Differences in depression, anxiety and stress were expected based on the mothers’ self-medication of these symptoms with marijuana. Smoking and other drug use were rare and not related to marijuana use. Alcohol use was related to marijuana use with 22 (88%) women reporting marijuana use also using alcohol at 10 weeks gestation. BMI was increased in mothers who used marijuana. Preterm labor was more common and vaginal birth less common among marijuana users. Neonatal complications were not different in those who used marijuana.
Table 1.
Maternal demographics, gestation, labor, and delivery
| No Marijuana N = 98 |
Marijuana only at conception N = 26 |
Marijuana <10 weeks gestation N = 13 |
Marijuana ⩾10 weeks gestation N = 25 |
ANOVA or χ2 p |
|
|---|---|---|---|---|---|
| Race and ethnicity: | |||||
| European N (%) | 84 (86%) | 19 (73%) | 8 (62%) | 21 (84%) | 0.12 |
| African N (%) | 5 (5%) | 2 (8%) | 2 (15%) | 1 (4%) | 0.5 |
| Native American N (%) | 5 (5%) | 2 (8%) | 2 (15%) | 2 (8%) | 0.6 |
| Mixed N (%) | 4 (4%) | 3 (12%) | 1 (8%) | 1 (4%) | 0.5 |
| Hispanic N (%) | 48 (49%) | 18 (69%) | 7 (54%) | 14 (54%) | 0.3 |
| Maternal parameters | |||||
| Maternal age years | 30.9 ± 5.8 | 27.9 ± 5.7 | 26.9 ± 5.9 | 29.0 ± 6.1 | 0.02 |
| Education years | 14.2 ± 3.1 | 12.4 ± 2.5a | 11.9 ± 3.6 | 13.3 ± 4.3 | 0.009 |
| Mental disorders: | |||||
| Bipolar mood disorder N (%) | 0 | 3 (12%) | 1 (8%) | 3 (12%) | 0.005 |
| Major depressive disorder N (%) | 0 | 9 (35%)b | 4 (31%)b | 11 (42%)b | 0.009 |
| Anxiety disorder N (%) | 0 | 1(4%) | 4 (31%)b | 2 (8%) | <0.001 |
| Schizophrenia spectrum disorders N (%) | 0 | 1 (4%) | 0 | 1 (4%) | 0.2 |
| Alcohol use disorder N (%) | 0 | 1 (4%) | 2 (15%)b | 0 | 0.001 |
| Substance use during pregnancy: | |||||
| Alcohol trimester 1 N (%) | 0 (0%) | 17 (65%)b | 12 (92%)b | 24 (96%)b | <0.001 |
| Alcohol trimester 2 N (%) | 0 (0%) | 0 (0%) | 0 (0%) | 22 (88%)b | <0.001 |
| Alcohol trimester 3 N (%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (48%)b | <0.001 |
| Cocaine N (%) | 0 | 2 (8%) | 2 (15%) | 0 | 0.02 |
| Opioid N (%) | 0 | 1 (4%) | 0 | 0 | 0.2 |
| Tobacco N (%) | 6 (6%) | 2 (8%) | 2 (15%) | 2 (8%) | 0.3 |
| Marijuana use after pregnancy: | |||||
| 1 month N (%) | 3 (3%) | 1 (4%) | 0 | 2 (8%) | 0.6 |
| 1 year N (%) | 5 (5%) | 1 (4%) | 0 | 3 (12%) | 0.4 |
| Obstetrical history | |||||
| Gravidity N | 3.2 ± 1.9 | 2.6 ± 1.6 | 3.1 ± 2.3 | 2.8 ± 1.5 | 0.5 |
| Term delivery N | 1.3 ± 1.4 | 1.0 ± 1.2 | 1.4 ± 1.3 | 1.0 ± 1.0 | 0.6 |
| Preterm delivery (<37 weeks) N | 0.2 ± 0.7 | 0.2 ± 0.5 | 0.2 ± 0.6 | 0.1 ± 0.3 | 0.9 |
| Miscarriage, ectopic, aborted N | 0.7 ± 1.1 | 0.5 ± 0.8 | 0.5 ± 1.2 | 0.7 ± 0.8 | 0.7 |
| Living children N | 1.4 ± 1.4 | 1.1 ± 1.2 | 1.5 ± 1.4 | 1.2 ± 1.1 | 0.6 |
| Self-ratings 16 weeks gestation: | |||||
| CESD-R | 11.0 ± 7.3 | 17.7 ± 11.0a | 18.1 ± 7.4a | 21.0 ± 11.0a | <0.001 |
| STA-I | 32.9 ± 8.7 | 40.8 ± 10.3a | 38.4 ± 9.4 | 40.1 ± 25.1 | 0.001 |
| PSS | 21.0 ± 11.0 | 26.0 ± 7.5a | 26.2 ± 7.7 | 28.5 ± 8.7a | <0.001 |
| Other maternal parameters: | |||||
| Pre-pregnancy BMI | 26.4 ± 5.6 | 30.6 ± 6.7a | 27.7 ± 9.3 | 27.7 ± 9.3 | 0.04 |
| Common respiratory, urinary, or vaginal infection in first 16 weeks gestation N (%) | 31 (32%) | 12 (47%) | 6 (46%) | 17 (64%)b | 0.01 |
| Prenatal vitamins with folic acid N (%) | 91 (93%) | 19 (73%)b | 12 (92%) | 22 (88%) | 0.04 |
| Labor and delivery: | |||||
| Hypertension N (%) | 12 (12%) | 0 | 0 | 0 | 0.04 |
| Pre-eclampsia N (%) | 5 (5%) | 0 | 0 | 0 | 0.3 |
| Gestational diabetes N (%) | 9 (9%) | 0 | 0 | 0 | 0.1 |
| Preterm labor N (%) | 8 (8%) | 2 (8%) | 1 (8%) | 8 (32%)b | 0.01 |
| Induced or no labor N (%) | 26 (26%) | 2 (8%) | 2 (15%) | 10 (40%) | 0.05 |
| Vaginal birth N (%) | 75 (77%) | 23 (88%) | 9 (70%) | 13 (52%)b | 0.02 |
| Meconium stained amniotic fluid N (%) | 31(32%) | 2 (8%) | 2 (15%) | 4 (16%) | 0.004 |
| Newborn: | |||||
| Sex female N (%) | 49 (51%) | 11 (42%) | 7 (54%) | 10 (40%) | 0.7 |
| Gestational age at birth weeks | 38.7 ± 2.8 | 39.4 ± 1.4 | 38.9 ± 1.4 | 39.1 ± 1.9 | 0.6 |
| Premature (<37 weeks) N (%) | 9 (9%) | 1 (4%) | 0 | 1 (4%) | 0.5 |
| Birth weight g | 3134 ± 655 | 3224 ± 465 | 3113 ± 524 | 3216 ± 607 | 0.9 |
| Birth weight range g | 695–4275 | 2120–4238 | 1915–3825 | 1790–4280 | |
| Small for gestational age <10%ile N (%) | 4 (4%) | 2 (8%) | 1 (8%) | 4 (16%) | 0.2 |
| Large for gestational age >90%ile N (%) | 9 (9%) | 2 (8%) | 1 (8%) | 3 (12%) | 0.9 |
| Head circumference cm | 34.5 ± 2.8 | 34.2 ± 1.4 | 34.6 ± 1.3 | 34.6 ± 1.7 | 0.9 |
| Length cm | 48.9 ± 5.4 | 49.7 ± 2.7 | 49.0 ± 3.8 | 49.8 ± 2.9 | 0.8 |
| APGAR 5 min | 8.74 ± 0.76 | 8.96 ± 0.34 | 8.62 ± 0.77 | 8.81 ± 0.40 | 0.9 |
| Jaundice N (%) | 44 (45%) | 11 (42%) | 4 (31%) | (8) 32% | 0.6 |
| Days in NICU | 1.98 ± 7.48 | 0.12 ± 0.59 | 0 | 0.96 ± 4.90 | 0.7 |
| Formula feeding only N (%) | 8 (8%) | 2 (8%) | 0 | 2 (8%) | 0.8 |
| Parenting Stress Index Parental Distress Scale 3 months postnatal | 27.0 ± 7.4 | 25.9 ± 12.0 | 23.3 ± 7.3 | 25.6 ± 6.8 | 0.8 |
Different from mothers who did not use marijuana, Tukey p<0.05.
Different from mothers who did not use marijuana, Fisher’s exact test p<0.05.
Levels of choline and its metabolite betaine were not different in women who used marijuana, compared to those who did not (Table 2). Nor were they affected by the mother’s mental or medical status or socio-demographic differences or prenatal vitamin use. Choline levels were not related to any labor, delivery, or neonatal issues.
Table 2.
Maternal metabolomics and newborn and child outcomes
| Maternal levels | No Marijuana |
Marijuana only at conception |
Marijuana <10 weeks |
Marijuana ⩾10 weeks |
ANOVA p |
|---|---|---|---|---|---|
| Maternal metabolomics at 16 weeks gestation | |||||
| N = 98 | N = 26 | N = 13 | N = 25 | ||
| Choline μM | 6.44 ± 1.89 | 7.90 ± 2.50 | 7.60 ± 2.00 | 6.45 ± 1.81 | 0.8 |
| Betaine μM | 11.7 ± 3.6 | 10.5 ± 3.8 | 9.5 ± 3.2 | 11.4 ± 3.9 | 0.7 |
| Newborn electrophysiology at 1-month post gestation age | |||||
| N = 98 | N = 26 | N = 13 | N = 25 | ||
| P50S1 μV | 1.64 ± 0.80 | 1.78 ± 0.89 | 1.84 ± 0.92 | 1.78 ± 0.95 | 0.7 |
| P50S2 μV | 0.71 ± 0.56 | 0.93 ± 0.62 | 0.95 ± 0.38 | 1.11 ± 0.86a | 0.02 |
| Child IBQ-R ratings of behavior at 3 months of age | |||||
| N = 84 | N = 21 | N = 12 | N = 20 | ||
| Surgency | 4.26 ± 1.17 | 4.30 ± 1.12 | 4.31 ± 1.10 | 4.04 ± 0.94 | 0.7 |
| Negativity | 3.08 ± 0.86 | 2.92 ± 0.75 | 3.63 ± 1.20 | 3.09 ± 1.07 | 0.3 |
| Regulation | 5.25 ± 0.66 | 5.23 ± 0.73 | 5.23 ± 0.65 | 5.18 ± 0.76 | 1.0 |
| Regulation adjusted for Surgency | 4.47 ± 0.55 | 4.58 ± 0.50 | 4.59 ± 0.77 | 3.97 ± 0.70a | 0.002 |
Different from children of mothers who did not use marijuana, Tukey p<0.05
Gestational development of cerebral inhibition
Gestational development of central nervous system inhibition was assessed at 44 weeks gestational age, 1 month after full term birth and later for premature births, as the P50S2 evoked potential amplitude to the second of two paired auditory stimuli (S1, S2). P50S2 amplitude was significantly different between the children of the different groups of marijuana users (F3,158 = 3.65, p = 0.024; Table 2). Newborns of mothers who used marijuana at 10 weeks gestation or longer had less inhibition with greater P50S2 amplitudes, 1.11 ± 0.86 μV, than newborns whose mothers did not use 0.71 ± 0.56 μV, Tukey’s p < 0.05, d’ = 0.55.
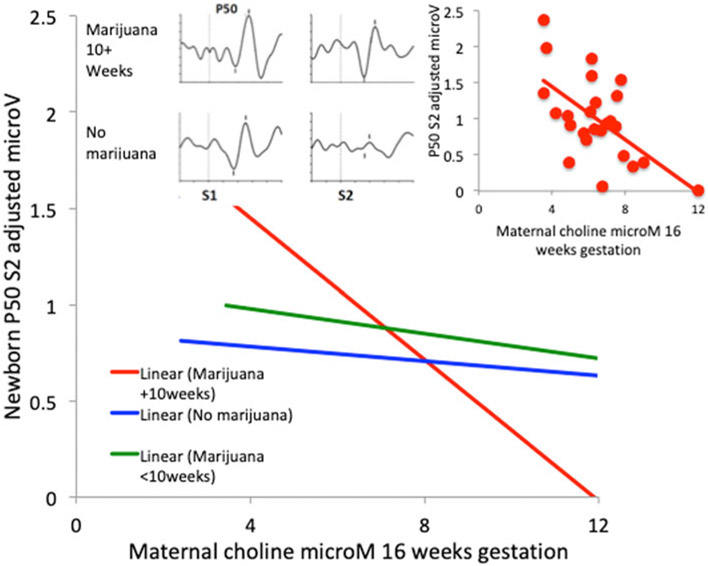
Higher maternal choline levels decreased newborn P50S2 amplitude for all women β = −0.49 [95% CI −0.77 to −0.20] p = 0.001. Effects of marijuana use and choline level on P50 inhibition had significant interaction (Wald , p = 0.027; Fig. 2, online Supplementary Table A2). Effects of the socio-economic, neonatal status, and maternal health factors were not significant (online Supplementary Tables A1, A2). The effect of the choline level was greatest for newborns whose mothers used marijuana at 10 weeks of gestation or longer (β = −0.40 [95% CI −0.75 to −0.01], p = 0.034; rs = −0.50, p = 0.011, Fig. 2 inset). Effects of choline were not significant in mothers who did not use marijuana during gestation [β = −0.10 (95% CI −0.23 to 0.03) p > 0.1]; their P50 inhibition was already at the level observed in healthy women previously (Hunter et al., 2011).
Fig. 2.
Effects of gestational exposure to marijuana and maternal choline levels on newborn cerebral auditory evoked potential (P50) inhibition. Newborns whose mothers used at conception only are grouped with those who used <10 weeks gestation, because there was little difference in the outcome. Inset (left): Newborn P50S1 and P50S2 auditory evoked responses: Mother used marijuana through week 16, P50S2 amplitude 2.97 μV, choline level 3.54 μM; Mother did not use marijuana, P50S2 amplitude 0.05 μV, choline level 8.81 μM. Vertical axes −2.5 to 2.5 μV; horizontal axes −50 to 125 ms. Inset (right): Individual subject data for mothers who used marijuana 10+ weeks, rs = −0.50, p = 0.011.
Child sex and maternal BMI and alcohol use did not affect the newborns’ P50 inhibition (online Supplementary Tables A3-A5). Six women used marijuana during lactation, and eight were using at 1 year (Table 1). Their newborns’ outcomes were similar to others in their gestational use groups.
Infant behavior at 3 months of age
At 3 months of age, IBQ-R ratings of Regulation, adjusted for Surgency, were significantly different between the children of the different maternal groups of marijuana users (F3,132 = 5.21, p = 0.002). Children of mothers who used marijuana at 10 weeks gestation or longer had lower ratings, 3.97 ± 0.70, than the children of mothers who did not use, 4.47 ± 0.55, Tukey’s p < 0.05, d’ = 0.79. Children of mothers who stopped using at conception or before 10 weeks had intermediate values (Table 2).
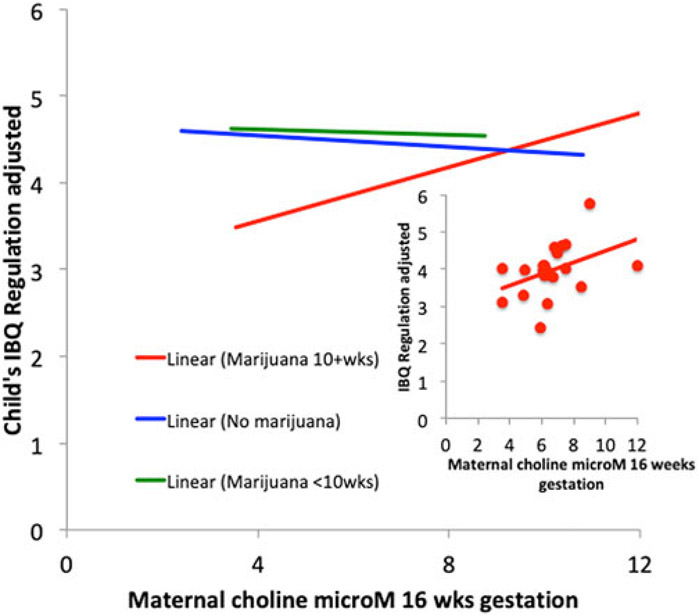
Higher maternal choline levels increased Regulation in 3-month-olds for all women β = 0.17 [95% CI 0.02–0.32] p = 0.022. Effects of marijuana use and choline level on Regulation had significant interaction (Wald , p = 0.022; Fig. 3, online Supplementary Table A6). The effect of the choline level was greatest for children whose mothers used marijuana at 10 weeks of gestation or longer (β = 0.53 [95% CI 0.08–0.98] p = 0.02; rs = 0.54, p = 0.013). Duration of attention (β = 0.69 [95% CI 0.04–1.35] p = 0.04), enjoyment of play with toys (β = 0.59 [95% CI 0.15–1.03] p = 0.01), and cuddliness and bonding with parents (β = 0.54 [95% CI 0.01–1.08] p = 0.03) were the most affected symptom ratings; all were in the Regulation index.
Fig. 3.
Effects of gestational exposure to marijuana and maternal choline levels on the 3-month-old child’s self-regulation behavior. Inset: Subject data for mothers who used marijuana 10+ weeks, rs = 0.54, p = 0.013.
Higher P50S2 amplitude reduced IBQ-R Regulation β = −0.421, but the effect was not significant. The Parental Distress subscale of the Parenting Stress Index was not different between the marijuana-using and non-using groups and had no effect on IBQ-R ratings.
Comment
Effects of maternal marijuana use on inhibition of the newborn’s P50 auditory evoked potential provide evidence soon after birth of adverse effects on fetal brain development, as suspected from studies of older children and adolescents exposed to marijuana prenatally (Fried and Watkinson, 2001; Goldschmidt et al., 2012). This study is the first to detect central nervous system effects in newborns before most mothers have resumed postnatal marijuana use, and it identifies a vulnerable gestational period for effects of marijuana on fetal brain development. Mothers reported relatively moderate levels of marijuana use and most stopped marijuana before delivery, but use for the first 10 gestational weeks was sufficient to adversely affect fetal brain development. By 3 months of age, adverse effects on the child’s self-regulation appeared. Higher maternal choline levels mitigated these effects.
α7-Nicotinic acetylcholine receptors are expressed on the same hippocampal inhibitory interneurons as Cannabinoid 1 (CB1) receptors (Morales et al., 2008). Animal models demonstrate that prenatal THC interferes with endogenous cannabinoid signaling through CB1 receptors that normally promote the development of these interneurons by transactivating the TrkB receptor, whereas choline activation of α7-receptors promotes induction of the potassium membrane pumps that support their inhibitory neurotransmission (Liu et al., 2006; de Salas-Quiroga et al., 2015). Cholinergic activation enhances and THC decreases long-term potentiation, a mechanism of learning and memory (de Salas-Quiroga et al., 2015; Freund et al., 2016). Impairment of interneuron development by prenatal marijuana diminishes normal social and executive behavior (Vargish et al., 2017). In humans CB1 receptors and mRNA are already expressed at the earliest time observed, 14–20 weeks of gestation, especially in the developing limbic system (Biegon and Kerman, 2001; Mato et al., 2003; Wang et al., 2003). CHRNA7 mRNA and α7-nicotinic receptors are also expressed beginning at 8–9 weeks for gestation, including in the limbic system (Court et al., 1997; Agulhon et al., 1999; Birnbaum et al., 2014; Kunii et al., 2015). During this gestational period interneurons begin their development by migrating from ganglionic eminence and expressing GAD; by 20 weeks they are synthesizing synaptophysin and the mature chloride pump KCC2 that will generate the chloride gradient (Vanhatalo et al., 2005; Bayatti et al., 2008; Zecevic et al., 2011).
P50 inhibition has the advantage as a biomarker that it can be assessed near birth when the parents’ effect on the child’s development is still minimal and it is directly dependent on the development of the interneurons that co-expression CB1 and CHRNA7 (Miller and Freedman, 1995). However, a limitation is that P50 inhibition is also multi-determined. In addition to the influence of CB1 and α7-nicotinic receptors, the inhibitory effect on limbic pyramidal neurons is dependent on GABAA and GABAB presynaptic and postsynaptic receptors, feed-forward activation of the interneurons by NMDA-type glutamate receptors, presynaptic release of acetylcholine modulated by 5HT3 receptors, and inactivation of the inhibition by α1-noradrenergic receptors (Freedman, 2014). Other genes involved are DISC1, ERB4, GRID2, GRM3, and GRIK4 (Greenwood et al., 2011).
A second limitation of this observational study is that the effects of marijuana and choline cannot be rigorously isolated from other environmental and genetic influences on fetal development. Factor analysis of socio-economic, maternal health, and neonatal status parameters was used to construct summary covariates of these many possible influences. None of the three factors had significant effects on marijuana–choline interaction. Marijuana usage, despite its adverse effects on the offspring’s later cognitive and behavioral development, had few effects on general fetal growth in this and other samples (El Marroun et al., 2009). Prenatal vitamins with folate were strongly advised; lower usage has been associated with marijuana use, but was not generally found here (Knight et al., 1994). Depression was common in all women who used marijuana, but only women who continued marijuana for 10 gestational weeks or more had affected offspring. Obesity and alcohol use were often comorbid with marijuana use, but they had no significant effect when assessed with marijuana in joint analyses of outcome. The younger age of mothers who use marijuana is consistent with population-wide studies (Brown et al., 2017). Cigarette smoking has been comorbid with marijuana use in other samples, but not in the present one. Parental ratings of the child’s behavior might be influenced by the parent’s postnatal marijuana use. However, few mothers used marijuana immediately postpartum, and effects on ratings were not significant.
P50 inhibition was selected as a biomarker because, in addition to its mechanistic significance, the loss of inhibition predicts early childhood problems in attention and social function Ross et al., 2016. These early childhood behaviors, including self-regulation and problems with attention and social withdrawal, are recognized as a pre-disposing factor for psychosis, substance abuse, and depression (Rutter, Kim-Cohen, and Maughan, 2006; Pine and Fox, 2015; Kertz et al., 2016; Olson et al., 2017). Self-regulation has been shown to be a mediating factor between prenatal maternal substance abuse and the child’s later psychopathology (Lin et al., 2018). Lower IBQ-R Regulation is specifically associated with decreased reading readiness at age 4 years and decreased conscientiousness, organization, and increased distractibility at age 9 years (Gartstein et al., 2016; Slobodskaya and Kozlova, 2016). As the child matures, Regulation positively modulates both Surgency and Negativity to help the child meet social expectations (Ahadi et al., 1993). Positive effects of higher prenatal maternal choline persist for at least 7 years (Boeke et al., 2013). Thus, the children in this study are likely to be influenced by the effect of their mothers’ marijuana and choline for much of their development. Longer-term behavioral and cognitive consequences of marijuana and choline in pregnancy unfold over decades as the infant matures (Fried et al., 2003; Goldschmidt et al., 2012).
Improved maternal prenatal nutrition to promote fetal development to prevent adverse effects on the child’s mental function and mental health is now recognized as a global public health priority (Stephenson et al., 2018). Specifically for prenatal choline, the American Medical Association now recommends ‘evidence-based amounts of choline in all prenatal vitamins’ (American Medical Association, 2017). Prenatal vitamins currently contain as little as 10–50 mg choline. Phosphatidylcholine supplements up to 6300 mg (equivalent to 900 mg choline) raise maternal choline to the highest levels in this study without serious adverse effects (Cheatham et al., 2012; Food and Drug Administration, 2016; Ross et al., 2016). This intervention assures maternal choline intake above the FDA minimum recommended intake (550 mg) and is less than half the maximum amount considered safe (3000–3500 mg) (Hoffman et al., 2019). Ideally, expectant parents will heed warnings about the adverse effects of prenatal marijuana use on their child, but, regardless of the parents’ decision, clinicians have a dual obligation to respect their autonomy and to provide the best possible health care for the mother and fetus (American College of Obstetricians and Gynecologists, 2016). Care would appear from this study to include enhancing the mother’s choline level to protect the fetus’s brain development.
Supplementary Material
Acknowledgements
Randal G. Ross (MD), who died during the course of this study, was responsible for its initial conceptualization and design.
Financial support. The study was supported by the Institute for Children’s Mental Disorders; The Anschutz Foundation; National Institutes of Health NIH/NCATS grant number UL1 TR001082 (all authors); NICHD grant number K12HD001271-11 (Dr Hoffman).
Footnotes
Data. Data from the study are available by request.
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S003329171900179X.
Conflict of interest. The authors report no conflict of interest.
References
- Abidin RR (2012) Parenting Stress Index, 3rd Edn. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Abratte CM, Wang W, Li R, Axume J, Moriarty DJ and Caudill MA (2009) Choline status is not a reliable indicator of moderate changes in dietary choline consumption in premenopausal women. The Journal of Nutritional Biochemistry 20, 62–69. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC and Freedman R (1982) Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry 17, 639–654. [PubMed] [Google Scholar]
- Agulhon C, Abitbol M, Bertrand D and Malafosse A (1999) Localization of mRNA for CHRNA7 in human fetal brain. Neuroreport 10, 2223–2227. [PubMed] [Google Scholar]
- Ahadi SA, Rothbart MK and Ye R (1993) Children’s temperament in the US and China: similarities and differences. European Journal of Personality 7, 359–378. [Google Scholar]
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A and Albuquerque EX (1997) Choline is a selective agonist at alpha7 nicotinic acetylcholine receptors in rat brain neurons. European Journal of Neuroscience 9, 2734–2742. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists (2016) Refusal of Medically Recommended Treatment During Pregnancy. Committee Opinion 664. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Ethics/Refusal-of-Medically-Recommended-Treatment-During-Pregnancy?IsMobileSet=false (Accessed 12 May 2019).
- American College of Obstetricians and Gynecologists (2018) Committee Opinion 722: Marijuana use during pregnancy and lactation. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Marijuana-Use-During-Pregnancy-and-Lactation, October 2017 (Accessed 13 June 2018).
- American Medical Association (2017) Proceedings of the 2017 Annual Meeting House of Delegates. Downloaded from (https://www.ama-assn.org/about/proceedings-2017-annual-meeting-house-delegates). 27 November 2017.
- Anders T, Emde R and Parmelee A (1971) A Manual of Standardized Terminology, Techniques and Criteria for Scoring of States of Sleep and Wakefulness in Newborn Infants. Los Angeles: UCLA Brain Information Service, NINDS Neurological Information Network. [Google Scholar]
- Baumgartner HK, Trinder KM, Galimanis CE, Post A, Phang T, Ross RG and Winn VD (2015) Characterization of choline transporters in the human placenta over gestation. Placenta. 36, 1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JFH, Lindsay L and Clowry GJ (2008) A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cerebral Cortex 18, 1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin AE and Stover PJ (2009) Insights into metabolic mechanisms underlying folate-responsive neural tube defects: a minireview. Birth Defects Research Part A: Clinical and Molecular Teratology 85, 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegon A and Kerman IA (2001) Autoradiographic study of pre- and postnatal distribution of cannabinoid receptors in human brain. Neuroimage 14, 1463–1468. [DOI] [PubMed] [Google Scholar]
- Birnbaum R, Jaffe AE, Hyde TM, Kleinman JE and Weinberger DR (2014) Prenatal expression patterns of genes associated with neuropsychiatric disorders. American Journal of Psychiatry 171, 758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E and Oken E (2013) Choline intake during pregnancy and child cognition at age 7 years. American Journal of Epidemiology 177, 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet-Enlow M, White MT, Hails K, Cabrera I and Wright RJ (2016) The infant behavior questionnaire-revised: factor structure in a culturally and sociodemographically diverse sample in the United States. Infant Behavior and Development. 43, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM and Hasin DS (2017) Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002–2014. Journal of the American Medical Association 317, 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera A (2016) Colorado marijuana’s potency getting ‘higher’. Cable Network News, https://www.cnn.com/2016/10/21/health/colorado-mariju-nana-potency-above-national-average/index.html (Accessed 14 July 2018).
- Calvigioni D, Hurd YL, Harkany T and Keimpema E (2014) Neuronal substrates and functional consequences of prenatal cannabis exposure. European Child & Adolescent Psychiatry 23, 931–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudill MA, Strupp BJ, Muscalu L, Nevins JEH and Canfield RL (2018) Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. The FASEB Journal 32, 2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheatham CL, Goldman BD, Fischer LM, da Costa K-AA, Reznick JS and Zeisel SH (2012) Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. The American Journal of Clinical Nutrition 96, 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court JA, Lloyd S, Johnson M, Griffiths M, Birdsall NJM, Piggott MA, Oakley AE, Ince PG, Perry EK and Perry RH (1997) Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Developmental Brain Research 101, 93–105. [DOI] [PubMed] [Google Scholar]
- de Salas-Quiroga A, Díaz-Alonso J, García-Rincón D, Remmers F, Vega D, Gómez-Cañas M, Lutz B, Guzman M and Galve-Roperh I (2015) Prenatal exposure to cannabinoids evokes long-lasting functional alterations by targeting CB 1 receptors on developing cortical neurons. Proceedings of the National Academy of Sciences 112, 13693–13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descarries L, Aznavour N and Hamel E (2008) The acetylcholine innervation of cerebral cortex: new data on its normal development and its fate in the hAPP(SW,IND) mouse model of Alzheimer’s disease. Journal of Neural Transmission 112, 149–162. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Steegers EAP, Jaddoe VWV, Hofman A, Verhulst FC, van den Brink W and Huizink AC (2009) Intrauterine cannabis exposure affects fetal growth trajectories: the generation R study. Journal of the American Academy of Child and Adolescent Psychiatry 48, 1173–1181. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Steegers EAP, Roos-Hesselink JW, Jaddoe VWV, Hofman A, Verhulst FC, van den Brink W and Huizink AC (2010) A prospective study on intrauterine cannabis exposure and fetal blood flow. Early Human Development 86, 231–236. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Jaddoe VWV, Hofman A, Verhulst FC, Van Den Brink W, van den Brink W and Huizink AC (2011) Agreement between maternal cannabis use during pregnancy according to self-report and urinalysis in a population-based cohort: the generation R study. European Addiction Research 17, 37–43. [DOI] [PubMed] [Google Scholar]
- El Marroun H, Tiemeier H, Franken IH, Jaddoe VW, van der Lugt A, Verhulst FC, Lahey BB and White T (2016) Prenatal cannabis and tobacco exposure in relation to brain morphology: a prospective neuroimaging study in young children. Biological Psychiatry 79, 971–979. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration (2016) Food Labeling: Revision of the Nutrition and Supplement Facts Labels. Federal Register, May 27, 903–904. [PubMed] [Google Scholar]
- Freedman R (2014) Alpha7-nicotinic receptor agonists for cognitive enhancement in schizophrenia. Annual Review of Medicine 65, 245–261. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hunter SK, Law AJ, Wagner BD, D’Allesandro A, Christians U, Noonan KQ, Wywra A and Hoffman MC (2019) Higher gestational choline levels in maternal infection are protective for infant brain development. Journal of Pediatrics 208, 198–206e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund RK, Graw S, Choo KS, Stevens KE, Leonard S and Dell’Acqua ML (2016) Genetic knockout of the α7 nicotinic acetylcholine receptor gene alters hippocampal long-term potentiation in a background strain-dependent manner. Neuroscience Letters 627, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA and Watkinson B (2001) Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology 23, 421–430. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B and Gray R (2003) Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and Teratology. 25, 427–436. [DOI] [PubMed] [Google Scholar]
- Gartstein MA and Rothbart MK (2003) Studying infant temperament via the revised infant behavior questionnaire. Infant Behavior and Development 26, 64–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Putnam S and Kliewer R (2016) Do infant temperament characteristics predict core academic abilities in preschool-aged children? Learning and Individual Differences. NIH Public Access 45, 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L, Richardson GA, Willford JA, Severtson SG and Day NL (2012) School achievement in 14-year-old youths prenatally exposed to marijuana. Neurotoxicology and Teratology 34, 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gu RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R and Braff DL (2011) Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the consortium on the genetics of schizophrenia. American Journal of Psychiatry 168, 930–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JM and Freedman R (1995) Normalization of the auditory P50 gating deficit of schizophrenic patients after non-REM but not REM sleep. Psychiatry Research 56, 271–278. [DOI] [PubMed] [Google Scholar]
- Hoffman MC, Olincy A, D’Alessandro A, Reisz JA, Hansen KC, Hunter SK, Freedman R and Ross RG (2019) Effects of phosphatidylcholine and betaine supplements on women’s serum choline. Journal of Nutrition & Intermediary Metabolism 16, 100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm PI, Ueland PM, Kvalheim G and Lien EA (2003) Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography-tandem mass spectrometry. Clinical Chemistry 49, 286–294. [DOI] [PubMed] [Google Scholar]
- Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, Miller MI and Mori S (2009) Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. Journal of Neuroscience 29, 4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink AC (2015) Prenatal maternal substance use and offspring outcomes: overview of recent findings and possible interventions. European Psychologist 20, 90–101. [Google Scholar]
- Hunter SK, Corral N, Ponicsan H and Ross RG (2008) Reliability of P50 auditory sensory gating measures in infants during active sleep. Neuroreport 19, 79–82. [DOI] [PubMed] [Google Scholar]
- Hunter SK, Kisley MA, McCarthy L, Freedman R and Ross RG (2011) Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophrenia Bulletin 37, 1200–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Gillow SJ and Ross RG (2015) Stability of P50 auditory sensory gating during sleep from infancy to 4 years of age. Brain Cognition 94, 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilcol YO, Uncu G and Ulus IH (2002) Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery, and in newborns. Archives of Physiology and Biochemistry 110, 393–399. [DOI] [PubMed] [Google Scholar]
- Ilcol YO, Yilmaz Z and Ulus IH (2003) Serum free and phospholipid-bound choline decrease and surgery and methylprednisolone administration in dogs. Neuroscience Letters 339, 195–198. [DOI] [PubMed] [Google Scholar]
- Iwao B, Yara M, Hara N, Kawai Y, Yamanaka T, Nishihara H, Inoue T and Inazu M (2016) Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochemistry International 93, 40–50. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, Lewis CE, Dodge NC, Hoyme HE, Zeisel SH, Meintjes EM, Duggan CP and Jacobson JL (2018) Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: a randomized, double-blind, placebo-controlled clinical trial. Alcoholism: Clinical and Experimental Research 42, 1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertz SJ, Belden AC, Tillman R and Luby J (2016) Cognitive control deficits in shifting and inhibition in preschool age children are associated with increased depression and anxiety over 7.5 years of development. Journal of Abnormal Child Psychology 44, 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisley MA, Polk SD, Ross RG, Levisohn PM and Freedman R (2003) Early postnatal development of sensory gating. Neuroreport 14, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EM, James H, Edwards CH, Spurlock BG, Oyemade UJ, Johnson AA, West WL, Cole OJ, Westney LS and Westney OE (1994) Relationships of serum illicit drug concentrations during pregnancy to maternal nutritional status. Journal of Nutrition 124, 973S–980S. [DOI] [PubMed] [Google Scholar]
- Kunii Y, Zhang W, Xu Q, Hyde TM, McFadden W, Shin JH, Deep-Soboslay A, Ye T, Li C, Kleinman JE, Wang KH and Lipska BK (2015) CHRNA7 and CHRFAM7AmRNAs: co-localized and their expression levels altered in the postmortem dorsolateral prefrontal cortex in major psychiatric disorders. American Journal of Psychiatry 172, 1122–1130. [DOI] [PubMed] [Google Scholar]
- Lin B, Ostlund BD, Conradt E, Lagasse LL and Lester BM (2018) Testing the programming of temperament and psychopathology in two independent samples of children with prenatal substance exposure. Development & Psychopathology 30, 1023–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Neff RA and Berg DK (2006) Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 314, 1610–1613. [DOI] [PubMed] [Google Scholar]
- Mato S, Del Olmo E and Pazos A (2003) Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. European Journal of Neuroscience 17, 1747–1754. [DOI] [PubMed] [Google Scholar]
- Metz TD, Allshouse AA, Hogue CJ, Goldenberg RL, Dudley DJ, Varner MW, Conway DL, Saade GR and Sliver RM (2017) Maternal marijuana use, adverse pregnancy outcomes, and neonatal morbidity. American Journal of Obstetrics and Gynecology 217, 478.el–478.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL and Freedman R (1995) The activity of hippocampal interneurons and pyramidal cells during the response of the hippocampus to repeated auditory stimuli. Neuroscience 69, 371–381. [DOI] [PubMed] [Google Scholar]
- Morales M, Hein K and Vogel Z (2008) Hippocampal interneurons co-express transcripts encoding the α7 nicotinic receptor subunit and the cannabinoid receptor 1. Neuroscience 152, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SL, Choe DE and Sameroff AJ (2017) Trajectories of child externalizing problems between ages 3 and 10 years: contributions of children’s early effortful control, theory of mind, and parenting experiences. Development & Psychopathology 29, 1333–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orczyk-Pawilowicz M, Jawien E, Deja S, Hirnle L, Zabek A and Mlynarz P (2016) Metabolomics of human amniotic fluid and maternal plasma during normal pregnancy. PLOS ONE 11, e0152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS and Fox NA (2015) Childhood antecedents and risk for adult mental disorders. Annual Review of Psychology 66, 459–485. [DOI] [PubMed] [Google Scholar]
- Putnam SP, Helbig AL, Gartstein MA, Rothbart MK and Leerkes E (2014) Development and assessment of short and very short forms of the infant behavior questionnaire-revised. Journal of Personality Assessment 96, 445–458. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Day NL and Goldschmidt L (1995) Prenatal alcohol, marijuana, and tobacco use: infant mental and motor development. Neurotoxicology and Teratology 17, 479–487. [DOI] [PubMed] [Google Scholar]
- Roberson EK, Patrick WK and Hurwitz EL (2014) Marijuana use and maternal experiences of severe nausea during pregnancy in Hawai’i. Hawai’i Journal of Medicine and Public Health 73, 283–287. [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, Leonard S, Stevens KE and Freedman R (2013) Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. American Journal of Psychiatry 170, 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RG, Hunter SK, Hoffman MC, McCarthy L, Chambers BM, Law AJ, Leonard S, Zerbe GO and Freedman R (2016) Perinatal phosphatidylcholine supplementation and early childhood behavior problems: evidence for CHRNA7 moderation. American Journal of Psychiatry 173, 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Kim-Cohen J and Maughan B (2006) Continuities and discontinuities in psychopathology between childhood and adult life. Journal of Child Psychology and Psychiatry 47, 276–295. [DOI] [PubMed] [Google Scholar]
- Slobodskaya HR and Kozlova EA (2016) Early temperament as a predictor of later personality. Personality and Individual Differences 99, 127–132. [Google Scholar]
- Smith DA, Boutros NN and Schwarzkopf SB (1994) Reliability of P50 auditory event-related potential indices of sensory gating. Psychophysiology 31, 495–502. [DOI] [PubMed] [Google Scholar]
- Smith E, Crawford T, Thomas M and Reid V (2018a). Schizotypy and sensory gating: a 6-month-old EEG study. Schizophrenia Bulletin 44, S301–S302. [Google Scholar]
- Smith MJ, Alden EC, Herrold AA, Roberts A, Stern D, Jones J, Barnes A, O’Connor KP, Huestis MA and Breiter HC (2018b) Recent self-reported cannabis use is associated with the biometrics of delta-9-tetrahydrocannabinol. Journal of Studies on Alcohol and Drugs 79, 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J, Heslehurst N, Hall J, Schoemaker DAJM, Hutchinson J, Cade JE, Barrett G, Crozier SR, Barker M, Kumaran K, Yajnik CS, Baird J and Mishra GD (2018) Before the beginning: nutrition and lifestyle in the preconception period and its importance for future health. The Lancet 391, 1830–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Choo KS, Stitzel JA, Marks MJ and Adams CE (2014) Long-term improvements in sensory inhibition with gestational choline supplementation linked to α7 nicotinic receptors through studies in Chrna7 null mutation mice. Brain Research 1552, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo S, Palvas JM, Andersson S, Rivera C, Voipio J and Kaila K (2005) Slow endogenous activity transients and developmental expression KCC2 in the immature human cortex. European Journal of Neuroscience 22, 2799–2804. [DOI] [PubMed] [Google Scholar]
- Vargish GA, Pelkey KA, Yuan X, Chittajallu R, Collins D, Fang C and McBain CJ (2017) Persistent inhibitory circuit defects and disrupted social behaviour following in utero exogenous cannabinoid exposure. Molecular Psychiatry 22, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Compton WM and Wargo EM (2017) The risks of marijuana use during pregnancy. Journal of American Medical Association 317, 129–130. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Keller E and Hurd YL (2003) Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694. [DOI] [PubMed] [Google Scholar]
- Wu BTF, Dyer RA, King DJJ, Richardson KJ and Innis SM (2012) Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE 7, e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic N, Hu F and Jakovcevski I (2011) Interneurons in the developing human neocortex. Developmental Neurobiology 71, 18–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Growden JH, Wurtman RJ, Magil SG and Logue M (1980) Normal plasma choline responses to ingested lecitin. Neurology 30, 1226–1229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.