Abstract
Purpose of review
Research findings from the fields of motor learning and exercise physiology suggest specific training parameters that can be manipulated during physical rehabilitation profoundly influence skilled task performance. This review details the rationale for some of these training variables and their application in selected intervention studies focused on improving walking function in patients post-stroke.
Recent findings
Basic and applied studies have shown that the amount, intensity and variability of specific task-practice applied during rehabilitation interventions can affect recovery of walking post-stroke. Many studies detailing the effects of conventional, therapist- and mechanically assisted interventions may incorporate some of these training parameters but minimize others, and their relative contributions may influence walking outcomes. Specific patient factors, such as the stroke acuity and degree of impairments, appear to influence the relative contributions of these training variables, and different patient subgroups may benefit from greater emphasis on specific parameters.
Summary
The present findings suggest these training parameters should be considered when evaluation or implementing physical interventions directed towards improving locomotor function post-stroke. More work is needed to understand their optimal combinations to maximize walking outcomes in patients with different levels of impairment post-stroke.
Keywords: locomotion, dose, physical therapy, walking recovery
Introduction
Recovery of independent ambulation post-stroke is a primary determinant of community participation and a major focus of rehabilitation [1], although it is not clear which interventions maximize walking outcomes. Various training strategies have demonstrated positive results, including traditional impairment-based strategies, gait training interventions, use of robotic devices, and orthotics or electrical stimulation [2]. However, the relative efficacy of many interventions is uncertain, and their adoption into clinical practice following preliminary studies occurs prior to understanding what contributed to the positive results. Subsequent larger trials often demonstrate more limited success of these same strategies, and their use may be abandoned without appreciation of why they failed. Given these ambiguous results, rehabilitation professionals lack clarity on the best strategies for locomotor recovery post-stroke.
An alternative strategy to avert this cycle is to determine how various interventions incorporate specific training parameters thought to influence locomotor function. Previous work in the fields of motor control and exercise physiology has suggested that the amount, intensity and variability of task-specific practice can strongly influence neuromuscular and cardiopulmonary changes underlying improved locomotor function [3, 4**]. Many studies incorporate some of these parameters, but limit use of others, which may influence the overall results of these interventions in different patient subgroups. This review delineates evidence underlying the potential utility of these parameters, with subsequent evaluation of how different studies apply these variables. Understanding the relative contributions of these factors and their application in specific patient populations may provide insight into the relative success or failure of published training protocols.
Training parameters that influence locomotor function
While many demographic and health-related variables contribute to walking recovery post-stroke, specific modifiable training parameters also appear to influence locomotor outcomes. Recent reports describe some of these parameters as the “dose” of an intervention [5, 6**], analogous to medication prescription, and focus on the number of sessions. While this is a critical variable, other factors likely play important roles. In the present discussion, we describe the rationale of a few of these training parameters, and data to support their use during rehabilitation interventions.
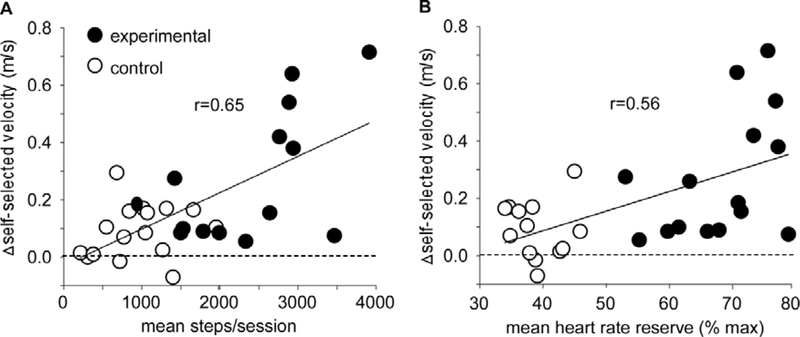
Across multiple studies, the specificity and amount of task practice appear to influence the connectivity of residual neural circuits following neurology injury to improve enhance motor recovery [7, 8]. For walking recovery, both the number of sessions and activities performed within sessions influence the amount of practice [5, 6, 9], and number of steps may be a more accurate measure of “amount” [10]. Long-standing data in animal models of spinal cord injury (SCI) suggest large amounts of stepping practice leads to greater locomotor performance as compared to little practice [11, 12] or practice of non-stepping tasks [13]. The success of many interventions utilized in patients post-stroke may be due to provision of large amounts of stepping practice, as focused stepping training can achieve 1500–4000 steps during 1-hr sessions. Recent studies further suggest these amounts are related to walking outcomes [3, 4, 10](Fig 1A).
Figure 1.
Scatterplot of relations of A) amount of stepping practice (steps/session) and B) intensity of stepping practice (percentage of predicted peak HR reserve) on changes in gait speed in subacute stroke following high intensity stepping activity vs conventional (control) interventions (modified from (4)).
Providing stepping practice alone may be insufficient to maximize walking outcomes, and the intensity of locomotor activities may be an important factor. In the field of exercise physiology, the intensity of walking tasks is commonly defined as power output [i.e., workload] and manipulated by increasing walking speed [10, 14, 15] or carried loads [16]*. Relative power output is estimated using cardiopulmonary and metabolic measures, which reflect the underlying neuromuscular activity during locomotor tasks. High-intensity training is associated with greater release of modulatory [17] and trophic factors [18] that contribute to increased synaptic connectivity of active neural circuits [19],improved muscle strength [20] and greater cardiovascular capacity [15] that underlie improved locomotor performance (Fig 1B).
In addition, variability of task-specific practice, rather than practice of variable tasks, has also been shown consistently to modulate motor learning [21–23]. Although less studied during walking training, variability during locomotor tasks has been described and tested in separate ways. In animal models of SCI, the role of kinematic variability was evaluated by testing the effects of strict kinematic guidance during training as compared to providing assistance only as needed to continue stepping [24]. Separately, the effects of task variability was tested by comparing forward treadmill training to stepping training in different directions [25], or as compared to overground and stair climbing [26]. Consistent with the motor learning literature, variability of stepping practice leads to greater errors and, with the most difficult tasks, less overall amounts of practice, but results in greater locomotor recovery on retention tests. Mechanisms underlying the effects of variable training are not certain, but could involve increased recruitment of residual circuits subserving these multiple varied tasks [23, 25], resulting in greater locomotor recovery. To date, however, there are limited additional data regarding the effects of variable stepping training, although the use of these three parameters appear to modulate motor learning across multiple studies.
Interventions to improve locomotor function post-stroke
With these training parameters in mind, we now evaluate the current evidence for specific types of interventions, and how the incorporation of these parameters, or lack thereof, may influence the results of many intervention studies. In Table 1, we summarize the characteristics of various physical interventions applied to patients post-stroke, and the relative incorporation of these training parameters (categorized as low, medium, or high) as described in primary studies.
Table 1.
Incorporation of identified training parameters during specific physical interventions directed towards improved walking outcomes as described in published studies.
| specificity | amount | intensity | variability | |
|---|---|---|---|---|
| conventional training | ||||
| traditional exercises | low | low | low | low* |
| therapist-assisted training | ||||
| treadmill with BWS | medium | high | low-medium | low |
| aerobic treadmill training | medium | high | high | low |
| overground training | high | medium | low-high | medium |
| variable training | high | medium | low-high | high |
| robotic-assisted training | ||||
| exoskeletal devices | medium | high | low | low |
| elliptical devices | medium | high | low | low-medium |
| ankle devices | ||||
| ankle foot orthoses | -- | medium** | -- | -- |
| FES systems | -- | medium** | -- | -- |
Classification of low, medium, high delineate the relative contribution of each parameter during this intervention; where selected interventions vary in their application of some of these interventions (* - traditional exercise may provide practice of variable tasks but not variable stepping practice; ** -AFOs and FES systems in themselves may allow greater practice)
Conventional interventions
Observational data [10, 27] suggest interventions utilized in the clinical setting to improve locomotor function incorporate traditional strategies that address multiple impairments and functional limitations post-stroke. Interventions focusing on deficits in strength, postural stability and coordination are often combined with whole or part practice of walking and other functional tasks. As higher intensity exercises lead to short-term increases in spasticity and abnormal movement patterns [28], therapists may limit patient effort to prevent these patterns. Collectively, these studies suggest that clinical interventions result in limited amounts of practice of any single activity [27] and patients seldom reach higher exercise intensities [29–31]. As expected, small gains in walking function are often observed using these strategies [4, 10]. In meta-analyses the describe studies focused on specific impairments, such as strength or balance training [32, 33], gains in these impairments are observed, with limited walking improvements.
Therapist assisted stepping training
Clearly, more practice can be achieved when interventions focus directly on stepping activities. A number of therapist-assisted stepping (locomotor or gait) training protocols have been evaluated, although many tested interventions vary in the amount, intensity and variability of stepping practice provided. Some of the initial studies that provided focused stepping practice utilized therapist-assisted treadmill training with body weight support (BWS). These studies were modelled from investigations of animal models of SCI [12], with attention towards ensuring afferent-related stepping feedback during training [34]. When applied to patients post-stroke, therapists provided practice on a motorized treadmill using BWS and physical assistance to approximate normal stepping kinematics [35], including weight-shifting for limb loading and facilitation of limb advancement in an effort to provide these afferent cues. Early treadmill studies with BWS generated substantial enthusiasm with greater gains as compared to training without BWS [36], overground training [37], or no interventions [38], particularly in patients with limited function. Meta-analyses combining multiple treadmill training approaches suggest gains are observed primarily for ambulatory patients [39], although well-controlled studies involving non-ambulatory patients comparing treadmill vs overground training decreases the time to achieve independent walking [40, 41].
While much attention has been directed towards normalizing kinematics during training, the positive outcomes of these trials could also be explained by provision of greater amounts of stepping practice. Previous and recent studies suggest a consistent relation between the amount of stepping practice and improvements in walking outcomes post-stroke [4, 10]. Provision of BWS over a treadmill may allow greater amounts of stepping practice at potentially higher speeds. The importance of normalizing kinematics remains unclear however, as facilitation strategies results in similar gains as compared as assist-as-needed strategies [42].
The efficacy of treadmill training with BWS was questioned by the multicenter LEAPS trial (Locomotor Experience Applied Post-Stroke) [43], in which walking gains were similar following stepping training primarily on a treadmill as compared to a strength and balance interventions. While the amount of practice provided in each training group is debated [44], one potential explanation for these results is the low cardiovascular intensities achieved during stepping practice. Despite training at higher speeds, restriction of heart rates [HR] achieved resulted in average mid-training values that can be lower than those observed during walking at self-selected speeds [16]. Conversely, previous data suggest aerobic treadmill training with goals to achieve 60–85% of maximum HR results in consistent changes in gait endurance [45], even when stepping amounts are controlled [16, 46]. Importantly, selected measures of gait quality (i.e., symmetry) are improved and not worsened with high vs lower intensity training [4, 47, 48]**. Certainly treadmill training with BWS can target higher intensities [49], although explicit focus on achieving aerobic training zones may be necessary.
Despite some positive findings of aerobic treadmill training, changes in overground gait speed and community mobility are sometimes small and often not greater than gains observed with non-specific or lower-intensity training [10, 15]. A hypothesis that may account for this finding is that training on a treadmill may entrain walking patterns, and may not be as specific to the variable demands of overground walking [50]. A recent study in ambulatory chronic stroke survivors suggests greater gait improvements with overground vs treadmill training when training intensity is controlled [51]*. These data conflict directly with results in non-ambulatory stroke survivors [40], which may be due to the ease of providing stepping practice on a treadmill in a more dependent patients. These data highlight the potential importance of selecting different training parameters in patients with different levels of impairment.
An alternative to overground or treadmill training is the use of variable stepping training, which involves practice of multiple stepping tasks, including walking over variables distances, in different directions, and around or over obstacles including curbs/stairs [3, 4, 52]. A common goal of variable stepping paradigms is to provide stepping practice that is more specific to the demands of community mobility [44], although animal studies suggest gains in standard walking assessments as well [24–26]. Application of variable stepping may not be utilized clinically, however, possibly due in part to the notion that increasing errors during practice does not focus on normalizing gait kinematics, although recent studies suggest variable, high intensity training results in large changes in function and gait quality in subacute and chronic stroke survivors [3, 4], even as compared to conventional strategies [4]. Conversely, a recent study evaluating the effects of variable overground training to treadmill training without focusing on intensity revealed no differences in recovery between these strategies, however [44], and more studies are needed to understand its impact when applied singly or with other training parameters.
Robotic-assisted stepping training
The development and clinical use of robotic devices that facilitate locomotor training has evolved over 2 decades, with initial efforts to minimize therapist’s burden to provide stepping assistance. The present discussion focuses on devices that facilitate limb advancement, as no data suggest superiority of robotic vs non-motorized devices to provide BWS. Most systems are classified as exoskeletal devices, which are secured to patients’ limbs and allow stepping within specified kinematic trajectories, or elliptical devices which use end-point control to facilitate practice. The Lokomat exoskeleton [53] and elliptical GaitTrainer [54] have garnered the most attention, although many available devices now provide advanced features such as compliant assistance [55] and visual feedback [56], with newer exoskeletons allowing stepping overground [57]. The clear value of these systems is to provide greater amounts of stepping practice, and recent meta-analyses in non-ambulatory patients demonstrate improve independent walking compared to conventional interventions [58]. Conversely, other measures such as gait speed do not change significantly, particularly in chronic stroke survivors, with greater changes following elliptical vs exoskeletal training [59].
While these data are promising, the findings are hampered by comparisons of robotic training that focuses on stepping practice, to conventional interventions that limit stepping activities. When robotic training protocols are compared to strategies that prioritize stepping in patients with subacute or chronic stroke, negligible differences are observed [60, 61] or non-robotic interventions demonstrate superiority [48, 62]. Potential short-comings of robotic training are likely the intensity and variability of practice these systems provide. Robotic-assisted walking often results in lower muscular and metabolic costs as compared to providing assistance only as needed [63, 64]. Kinematic and task variability is also limited during robotic assisted training, possibly more so during exoskeletal training [59, 63], and may minimize patients’ ability to explore strategies to relearn locomotor tasks. Most overground exoskeletal training strategies face similar limitations, and many may provide less stepping practice than treadmill-based systems. Indeed, recent analyses of the effects of overground exoskeletal systems indicate negligible differences in walking as compared to conventional strategies [57].
Electrical and mechanical ankle devices
Strategies to improve ankle function has been a research priority for decades given its importance during normal locomotor function, and the loss of dorsi- and plantarflexor function post-stroke. Passive mechanical bracing using an ankle foot orthosis (AFO) is the easiest and most often performed strategy to offset dorsiflexion weakness or ankle instability. These devices have been shown to elicit immediate and sustained increases in walking speed [65] likely due to the ability to ambulate to a greater extent without risk of injury or falls. Alternative strategies include use of functional electrical stimulation (FES) to enhance dorsiflexion [66–68], with some interest in plantarflexor FES to promote greater propulsive forces [69].
When the comparative efficacy of these different strategies is evaluated, however, small differences in functional outcomes are observed. Three recent multicenter trials have compared the effects of AFOs or dorsiflexion FES on immediate or long-term effects of walking function without accompanying training [66–68], resulting in no difference in walking gains. A recent study comparing the effect of FES during faster walking training as compared to fast or slow training without FES demonstrated very little changes in walking distance between fast training groups, but with a potential benefit on cost of walking [69]. A potential explanation for the negligible differences is that the stepping activities provided during these studies were similar. Importantly, however, there appears to be a subset of limited community ambulators that respond to FES systems, indicating that single intervention strategies may not be appropriate for all participants [70].
Conclusion
The present review suggests that identification of how various rehabilitation interventions incorporate specific training parameters may provide insight into their potential utility. Strategies that prioritize stepping training appear to be superior to non-stepping strategies, and practice at higher intensites with use of orthotics may facilitate gains in walking. The larger amounts of stepping practice using mechanically-assisted devices may be helpful in more impaired individuals, although the optimal assistance is not clear. If allowing or increasing variability and achieving high intensity can enhance recovery over practicing normal kinematics, perhaps less therapist or robotic assistance may be needed. Accordingly, while “dose” can be characterized by number of therapy sessions, what is actually done in those sessions (i.e., how much, how intense, how difficult) may be more important, and can elucidate the strengths and weaknesses of many interventions. These concepts, while relatively simple, are complicated by the notion that specific strategies may be important early during stroke recovery, whereas others may need to be prioritized in the later stages. Identifying the contributions of these parameters single or in combination in patients with varying levels of impairment may lead to more efficient training interventions to minimize walking disability.
Key Points.
The amount, intensity and variability of specific task practice are well-known training parameters that influence neural, muscular and cardiopulmonary function during skilled learning and locomotor recovery following neurology injury.
Identifying how specific training studies directed towards improving walking post-stroke incorporate these parameters may provide insight into the relative success of many interventions.
A priority for future studies is to understand how the incorporation of these parameters applied singly or in combination may vary to maximize recovery during the different stages of stroke rehabilitation.
Acknowledgements
We would like to thank Carey Holleran and Patrick Hennessy for their initial discussions related to this manuscript.
Financial support and sponsorship:
The present work is supported by NIDILRR H133B031127, NIH – R01-NS079751 and a Department of Defense Spinal Cord Injury Clinical Trials Award (SC001265).
Footnotes
Conflicts of interest:
The authors report no conflicts of interest.
References
- 1.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: how important and obtainable is it and what measures appear predictive? Archives of physical medicine and rehabilitation. 2004;85(2):234–9. [DOI] [PubMed] [Google Scholar]
- 2.Bogey RA, Hornby TG. Gait training strategies utilized in poststroke rehabilitation: Are we really making a difference? Topics in stroke rehabilitation. 2007;14(6):1–8. [DOI] [PubMed] [Google Scholar]
- 3.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilitation and neural repair. 2014;28(7):643–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Camardo J, et al. Variable Intensive Early Walking Poststroke (VIEWS): A Randomized Controlled Trial. Neurorehabilitation and neural repair. 2015. in press.** Two recent studies that incorporate high intensity stepping practice in variable contexts and its influence on walking speed, distance and gait symmetry in chronic and subacute stroke and as compared to conventional strategy matched to an equivalent number of training sessions.
- 5.Lang CE, Lohse KR, Birkenmeier RL. Dose and timing in neurorehabilitation: prescribing motor therapy after stroke. Current opinion in neurology. 2015;28(6):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke; a journal of cerebral circulation. 2014;45(7):2053–8.** Recent summary of the potential cumulative effects of dose of interventions, defined as number of training sessions, and functional outcomes during stroke rehabilitation.
- 7.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10(12):861–72. [DOI] [PubMed] [Google Scholar]
- 8.Edgerton V, Tillakaratne N, Bigbee A, de Leon R, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145–67. [DOI] [PubMed] [Google Scholar]
- 9.Veerbeek JM, Koolstra M, Ket JC, van Wegen EE, Kwakkel G. Effects of augmented exercise therapy on outcome of gait and gait-related activities in the first 6 months after stroke: a meta-analysis. Stroke; a journal of cerebral circulation. 2011;42(11):3311–5. [DOI] [PubMed] [Google Scholar]
- 10.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke; a journal of cerebral circulation. 2010;41(1):129–35. [DOI] [PubMed] [Google Scholar]
- 11.Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, De Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. Journal of neurotrauma. 2007;24(6):1000–12. [DOI] [PubMed] [Google Scholar]
- 12.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. Journal of neurophysiology. 1998;79(3):1329–40. [DOI] [PubMed] [Google Scholar]
- 13.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. Journal of neurophysiology. 1998;80(1):83–91. [DOI] [PubMed] [Google Scholar]
- 14.Globas C, Becker C, Cerny J, Lam JM, Lindemann U, Forrester LW, et al. Chronic stroke survivors benefit from high-intensity aerobic treadmill exercise: a randomized control trial. Neurorehabilitation and neural repair. 2012;26(1):85–95. [DOI] [PubMed] [Google Scholar]
- 15.Macko RF, Ivey FM, Forrester LW, Hanley D, Sorkin JD, Katzel LI, et al. Treadmill exercise rehabilitation improves ambulatory function and cardiovascular fitness in patients with chronic stroke: a randomized, controlled trial. Stroke; a journal of cerebral circulation. 2005;36(10):2206–11. [DOI] [PubMed] [Google Scholar]
- 16.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. Journal of neurologic physical therapy : JNPT. 2015;39(2):95–102.* Recent report of improvements in gait endurance following high vs low intensity training during treadmill and overground walking with matched number of repetitions.
- 17.Jacobs BL, Fornal CA. Serotonin and motor activity. Curr Opin Neurobiol. 1997;7(6):820–5. [DOI] [PubMed] [Google Scholar]
- 18.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiology of learning and memory. 2007;87(4):597–609. [DOI] [PubMed] [Google Scholar]
- 19.Luft AR, Macko RF, Forrester LW, Villagra F, Ivey F, Sorkin JD, et al. Treadmill exercise activates subcortical neural networks and improves walking after stroke: a randomized controlled trial. Stroke; a journal of cerebral circulation. 2008;39(12):3341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straube DD, Holleran CL, Kinnaird CR, Leddy AL, Hennessy PW, Hornby TG. Effects of dynamic stepping training on nonlocomotor tasks in individuals poststroke. Physical therapy. 2014;94(7):921–33. [DOI] [PubMed] [Google Scholar]
- 21.Shea CH, Kohl RM Specificity and variabilitiy of practice. Res Q Exerc Sport. 1990;61:169–77. [DOI] [PubMed] [Google Scholar]
- 22.Shea CH, Morgan RL. Contextual interference effects of the acquisition, retention, and transfer of a motor skill.. J Exp Psychol: Hum Learn Mem. 1979;5:179–87. [Google Scholar]
- 23.Kantak SS, Sullivan KJ, Fisher BE, Knowlton BJ, Winstein CJ. Neural substrates of motor memory consolidation depend on practice structure. Nat Neurosci. 2010;13(8):923–5. [DOI] [PubMed] [Google Scholar]
- 24.Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(41):10564–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah PK, Gerasimenko Y, Shyu A, Lavrov I, Zhong H, Roy RR, et al. Variability in step training enhances locomotor recovery after a spinal cord injury. The European journal of neuroscience. 2012;36(1):2054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336(6085):1182–5. [DOI] [PubMed] [Google Scholar]
- 27.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Archives of physical medicine and rehabilitation. 2009;90(10):1692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain : a journal of neurology. 2007;130(Pt 1):159–69. [DOI] [PubMed] [Google Scholar]
- 29.Kuys S, Brauer S, Ada L. Routine physiotherapy does not induce a cardiorespiratory training effect post-stroke, regardless of walking ability. Physiotherapy research international : the journal for researchers and clinicians in physical therapy. 2006;11(4):219–27. [DOI] [PubMed] [Google Scholar]
- 30.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: is the intensity adequate to induce a training effect? Archives of physical medicine and rehabilitation. 2002;83(10):1378–83. [DOI] [PubMed] [Google Scholar]
- 31.Prajapati SK, Mansfield A, Gage WH, Brooks D, McIlroy WE. Cardiovascular responses associated with daily walking in subacute stroke. Stroke research and treatment. 2013;2013:612458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van de Port IG, Wood-Dauphinee S, Lindeman E, Kwakkel G. Effects of exercise training programs on walking competency after stroke: a systematic review. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2007;86(11):935–51. [DOI] [PubMed] [Google Scholar]
- 33.Ding M Tai Chi for stroke rehabilitation: a focused review. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 2012;91(12):1091–6. [DOI] [PubMed] [Google Scholar]
- 34.Behrman A, Harkema S. Locomotor training after human spinal cord injury: a series of case studies. Physical therapy. 2000;80(7):688–700. [PubMed] [Google Scholar]
- 35.Hesse S, Konrad M, Uhlenbrock D. Treadmill walking with partial body weight support versus floor walking in hemiparetic subjects. Archives of physical medicine and rehabilitation. 1999;80(4):421–7. [DOI] [PubMed] [Google Scholar]
- 36.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke; a journal of cerebral circulation. 1998;29(6):1122–8. [DOI] [PubMed] [Google Scholar]
- 37.Kosak MC, Reding MJ. Comparison of partial body weight-supported treadmill gait training versus aggressive bracing assisted walking post stroke. Neurorehabilitation and neural repair. 2000;14(1):13–9. [DOI] [PubMed] [Google Scholar]
- 38.Hesse S, Bertelt C, Jahnke MT, Schaffrin A, Baake P, Malezic M, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke; a journal of cerebral circulation. 1995;26(6):976–81. [DOI] [PubMed] [Google Scholar]
- 39.Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. The Cochrane database of systematic reviews. 2014;1:CD002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ada L, Dean CM, Morris ME, Simpson JM, Katrak P. Randomized trial of treadmill walking with body weight support to establish walking in subacute stroke: the MOBILISE trial. Stroke; a journal of cerebral circulation. 2010;41(6):1237–42. [DOI] [PubMed] [Google Scholar]
- 41.Dean CM, Ada L, Bampton J, Morris ME, Katrak PH, Potts S. Treadmill walking with body weight support in subacute non-ambulatory stroke improves walking capacity more than overground walking: a randomised trial. Journal of physiotherapy. 2010;56(2):97–103. [DOI] [PubMed] [Google Scholar]
- 42.Yagura H, Hatakenaka M, Miyai I. Does therapeutic facilitation add to locomotor outcome of body weight--supported treadmill training in nonambulatory patients with stroke? A randomized controlled trial. Archives of physical medicine and rehabilitation. 2006;87(4):529–35. [DOI] [PubMed] [Google Scholar]
- 43.Duncan PW, Sullivan KJ, Behrman AL, Azen SP, Wu SS, Nadeau SE, et al. Body-weight-supported treadmill rehabilitation after stroke. The New England journal of medicine. 2011;364(21):2026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DePaul VG, Wishart LR, Richardson J, Thabane L, Ma J, Lee TD. Varied Overground Walking Training Versus Body-Weight-Supported Treadmill Training in Adults Within 1 Year of Stroke: A Randomized Controlled Trial. Neurorehabilitation and neural repair. 2014. [DOI] [PubMed] [Google Scholar]
- 45.Pang MY, Charlesworth SA, Lau RW, Chung RC. Using aerobic exercise to improve health outcomes and quality of life in stroke: evidence-based exercise prescription recommendations. Cerebrovascular diseases. 2013;35(1):7–22. [DOI] [PubMed] [Google Scholar]
- 46.Ivey FM, Stookey AD, Hafer-Macko CE, Ryan AS, Macko RF. Higher Treadmill Training Intensity to Address Functional Aerobic Impairment after Stroke. J Stroke Cerebrovasc Dis. 2015;24(11):2539–46.* This study reported improvements in peak aerobic capacity following high vs low intensity training when matched for total distance and time.
- 47.Kuys SS, Brauer SG, Ada L. Higher-intensity treadmill walking during rehabilitation after stroke is feasible and not detrimental to walking pattern or quality: a pilot randomized trial. Clinical rehabilitation. 2010. [DOI] [PubMed] [Google Scholar]
- 48.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke; a journal of cerebral circulation. 2008;39(6):1786–92. [DOI] [PubMed] [Google Scholar]
- 49.Mackay-Lyons M, McDonald A, Matheson J, Eskes G, Klus MA. Dual effects of body-weight supported treadmill training on cardiovascular fitness and walking ability early after stroke: a randomized controlled trial. Neurorehabilitation and neural repair. 2013;27(7):644–53. [DOI] [PubMed] [Google Scholar]
- 50.Hollman JH, Watkins MK, Imhoff AC, Braun CE, Akervik KA, Ness DK. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait & posture. 2016;43:204–9. [DOI] [PubMed] [Google Scholar]
- 51.Combs-Miller SA, Kalpathi Parameswaran A, Colburn D, Ertel T, Harmeyer A, Tucker L, et al. Body weight-supported treadmill training vs. overground walking training for persons with chronic stroke: a pilot randomized controlled trial. Clinical rehabilitation. 2014;28(9):873–84.* This is a recent study evaluating the effects of overground vs treadmill training with attempts to match for intensity in ambulatory individuals with stroke, demonstrating higher increases in walking speeds with overground training.
- 52.Ada L, Dean CM, Hall JM, Bampton J, Crompton S. A treadmill and overground walking program improves walking in persons residing in the community after stroke: a placebo-controlled, randomized trial. Archives of physical medicine and rehabilitation. 2003;84(10):1486–91. [DOI] [PubMed] [Google Scholar]
- 53.Colombo G, Wirz M, Dietz V. Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal cord. 2001;39(5):252–5. [DOI] [PubMed] [Google Scholar]
- 54.Hesse S, Uhlenbrock D, Werner C, Bardeleben A. A mechanized gait trainer for restoring gait in nonambulatory subjects. Archives of physical medicine and rehabilitation. 2000;81(9):1158–61. [DOI] [PubMed] [Google Scholar]
- 55.Krishnan C, Kotsapouikis D, Dhaher YY, Rymer WZ. Reducing robotic guidance during robot-assisted gait training improves gait function: a case report on a stroke survivor. Archives of physical medicine and rehabilitation. 2013;94(6):1202–6. [DOI] [PubMed] [Google Scholar]
- 56.Stoller O, Schindelholz M, Bichsel L, Schuster C, de Bie RA, de Bruin ED, et al. Feedback-controlled robotics-assisted treadmill exercise to assess and influence aerobic capacity early after stroke: a proof-of-concept study. Disabil Rehabil Assist Technol. 2014;9(4):271–8. [DOI] [PubMed] [Google Scholar]
- 57.Louie DR, Eng JJ. Powered robotic exoskeletons in post-stroke rehabilitation of gait: a scoping review. Journal of neuroengineering and rehabilitation. 2016;13(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. The Cochrane database of systematic reviews. 2013(7):CD006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mehrholz J, Pohl M. Electromechanical-assisted gait training after stroke: a systematic review comparing end-effector and exoskeleton devices. Journal of rehabilitation medicine. 2012;44(3):193–9. [DOI] [PubMed] [Google Scholar]
- 60.Werner C, Bardeleben A, Mauritz KH, Kirker S, Hesse S. Treadmill training with partial body weight support and physiotherapy in stroke patients: a preliminary comparison. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2002;9(6):639–44. [DOI] [PubMed] [Google Scholar]
- 61.Tong RK, Ng MF, Li LS. Effectiveness of gait training using an electromechanical gait trainer, with and without functional electric stimulation, in subacute stroke: a randomized controlled trial. Archives of physical medicine and rehabilitation. 2006;87(10):1298–304. [DOI] [PubMed] [Google Scholar]
- 62.Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabilitation and neural repair. 2009;23(1):5–13. [DOI] [PubMed] [Google Scholar]
- 63.Hornby TG, Kinnaird CR, Holleran CL, Rafferty MR, Rodriguez KS, Cain JB. Kinematic, muscular, and metabolic responses during exoskeletal-, elliptical-, or therapist-assisted stepping in people with incomplete spinal cord injury. Physical therapy. 2012;92(10):1278–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Israel JF, Campbell DD, Kahn JH, Hornby TG. Metabolic costs and muscle activity patterns during robotic- and therapist-assisted treadmill walking in individuals with incomplete spinal cord injury. Physical therapy. 2006;86(11):1466–78. [DOI] [PubMed] [Google Scholar]
- 65.Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation. 2016;47(6):e98–e169. [DOI] [PubMed] [Google Scholar]
- 66.Kluding PM, Dunning K, O’Dell MW, Wu SS, Ginosian J, Feld J, et al. Foot drop stimulation versus ankle foot orthosis after stroke: 30-week outcomes. Stroke; a journal of cerebral circulation. 2013;44(6):1660–9. [DOI] [PubMed] [Google Scholar]
- 67.Bethoux F, Rogers HL, Nolan KJ, Abrams GM, Annaswamy TM, Brandstater M, et al. The effects of peroneal nerve functional electrical stimulation versus ankle-foot orthosis in patients with chronic stroke: a randomized controlled trial. Neurorehabilitation and neural repair. 2014;28(7):688–97. [DOI] [PubMed] [Google Scholar]
- 68.Everaert DG, Stein RB, Abrams GM, Dromerick AW, Francisco GE, Hafner BJ, et al. Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: a multicenter randomized controlled trial. Neurorehabilitation and neural repair. 2013;27(7):579–91. [DOI] [PubMed] [Google Scholar]
- 69.Awad LN, Reisman DS, Pohlig RT, Binder-Macleod SA. Reducing The Cost of Transport and Increasing Walking Distance After Stroke: A Randomized Controlled Trial on Fast Locomotor Training Combined With Functional Electrical Stimulation. Neurorehabilitation and neural repair. 2016;30(7):661–70.** Recent report describing the effects of fast treadmill training combined with dorsi-and plantarflexor FES as compared to fast or slow training without FES revealed no differences in walking distance with possible improvements in metabolic costs of walking.
- 70.O’Dell MW, Dunning K, Kluding P, Wu SS, Feld J, Ginosian J, et al. Response and prediction of improvement in gait speed from functional electrical stimulation in persons with poststroke drop foot. PM & R : the journal of injury, function, and rehabilitation. 2014;6(7):587–601; quiz [DOI] [PubMed] [Google Scholar]