Abstract
Background:
This randomized trial studied performance of Option B+ in Mozambique and evaluated an enhanced retention package in public clinics.
Setting:
The study was conducted at 6 clinics in Manica and Sofala Provinces in central Mozambique.
Methods:
Seven hundred sixty-one pregnant women tested HIV+, immediately initiated antiretroviral (ARV) therapy, and were followed to track retention at 6 clinics from May 2014 to May 2015. Clinics were randomly allocated within a stepped-wedge fashion to intervention and control periods. The intervention included (1) workflow modifications and (2) active patient tracking. Retention was defined as percentage of patients returning for 30-, 60-, and 90-day medication refills within 25–35 days of previous refills.
Results:
During control periods, 52.3% of women returned for 30-day refills vs. 70.8% in intervention periods [odds ratio (OR): 1.80; 95% confidence interval (CI): 1.05 to 3.08]. At 60 days, 46.1% control vs. 57.9% intervention were retained (OR: 1.82; CI: 1.06 to 3.11), and at 90 days, 38.3% control vs. 41.0% intervention (OR: 1.04; CI: 0.60 to 1.82). In prespecified subanalyses, birth before pickups was strongly associated with failure—women giving birth before ARV pickup were 33.3 times (CI: 4.4 to 250.3), 7.5 times (CI: 3.6 to 15.9), and 3.7 times (CI: 2.2 to 6.0) as likely to not return for ARV pickups at 30, 60, and 90 days, respectively.
Conclusions:
The intervention was effective at 30 and 60 days, but not at 90 days. Combined 90-day retention (40%) and adherence (22.5%) were low. Efforts to improve retention are particularly important for women giving birth before ARV refills.
Keywords: Mozambique, Option B+ retention, ART adherence, stepped wedge, implementation science
INTRODUCTION
Lost to follow-up (LTFU) among HIV-positive pregnant and breastfeeding women has been a persistent obstacle to the prevention of maternal-to-child HIV transmission and sustained maternal HIV care in sub-Saharan Africa.1–10 In 2012/13, the World Health Organization endorsed the Option B+ strategy, in which lifelong antiretroviral (ARV) therapy (ART) is offered to all HIV-positive pregnant and postpartum women at the time of diagnosis in antenatal care (ANC) or the breastfeeding period, regardless of CD4 count.11,12 This new approach was intended to streamline the care cascade to increase ART uptake,6,12,13 but its implementation has been marked by continued high rates of LTFU in sub-Saharan Africa,14–19 particularly in the first 3 months after treatment initiation.20–23 Recent findings from national and provincial data in Mozambique suggest similar LTFU patterns.24,25
A number of factors have been identified that contribute to poor ART retention among pregnant women in low-resource settings, such as inadequate counseling, long wait times, stigma, fear of disclosure to male partners, and concerns with side effects.13,14,18,26–28 These data and experiences have underscored the pressing need to develop and test interventions that can improve early ART retention and adherence.17
By 2009 in Mozambique, less than 25% of eligible mothers started ART, and an estimated 29% of infants born to HIV-positive women acquired HIV.29,30 Mozambique’s 11.1% HIV prevalence30 and under-5 mortality rate of 97 per 1000 live births were among the highest in Africa.31 Ministry of Health (MOH) adopted Option B+ and began rollout of services in July 2013.32 Preliminary data from the early rollout demonstrated that poor early ART retention has continued to challenge the health system in Mozambique.33,34 Recent analysis of 2013 data revealed a 38% 6-month LTFU for Option B+ enrollees,33 whereas a Sofala Province study using 2016 data show 44% LTFU at 10 weeks postpartum and low viral suppression.25
The implementation study described here sought to improve early Option B+ retention in large public clinics in a high prevalence region of Mozambique using a combination intervention that is feasible in a context of significant resource constraints. The study was designed to develop, pilot, and evaluate the impact of an innovative health facility-based intervention using more aggressive patient tracking and follow-up after ART initiation (The study is registered at ClinicalTrials.gov ).
METHODS
Setting
The intervention was conducted in Manica and Sofala Provinces in central Mozambique, where HIV prevalence is higher than the national rates—15.6% and 17.8%, respectively for women, 14.8% and 12.6%, respectively for men,35 and an estimated 18% among pregnant women in 200936 in both provinces. Under-5 mortality is estimated at 107 per 1000 live births in Manica and 83 in Sofala,37 and pediatric HIV infection contributed to 16% of child mortality.38 In 2010, approximately 178,000 women attended a first ANC visit in both provinces, and 164,000 were tested for HIV; 5829 (6% of ANC first visits) tested positive in Manica and 9364 (12%) in Sofala.39
The B+ rollout began in Manica and Sofala in July 2013 to high volume sites with adult ART.32,34 By 2014, 62 of 97 health facilities in Manica and 70 of 156 facilities in Sofala were implementing B+.32 Across both provinces, over 90% of pregnant women make at least 1 ANC visit. However, nearly 90% of pregnant women in Sofala Province present late to their first visit in their second or third trimester. By 2014, 97% of those with first ANC consults were also tested for HIV.32 Over 90% of those testing positive in both provinces also initiated ART in the new Option B+ strategy.40
In 2013, this intervention study was initiated at 6 of the highest volume primary-level clinics in Manica and Sofala.41 The team conducted formative research at these 6 facilities to inform the design of the facility-level intervention, followed by a second phase in which the intervention was implemented and evaluated.34,42 Because the health system has major workforce and resource limitations, formative research centered on identifying resources already available to design an intervention that, if successful, could be scaled up within these constraints. Health workers were invited to participate in the design of the intervention to ensure feasibility and to solicit their participation.
Data collected during the formative period revealed generally high but heterogeneous LTFU at 30 and 90 days after ART initiation; 30-day retention rates varied from 27% to 70% and 90-day retention ranged from 5% to 32%.34 This variation was not associated with patient volume differences or staffing patterns but may have resulted from variability in initial training quality. Data gathered from June 2014 through August 2015 indicated an average of 329 new ANC enrollees per month (range 173–525), an average of 33 HIV-positive women identified per month (age range 19–49), and an average of 31 women per site who initiated ART within 7 days of testing. Interviews and observations during the formative research period indicated that B+ training quality varied, there was minimal workflow modification, and no systematic B+ patient tracking had been implemented.43,44
At each site, HIV testing and ART initiation and management (TDF + 3 TC + EFV, single daily fixed-dose combination) had been integrated into the first ANC visit with the B+ rollout. The standard of care (SOC) at each site included maternal child health (MCH) nurses trained to initiate and manage ART through the postpartum period. A “Mother-to-mother” (in Portuguese maes para maes or MpM) peer counseling program existed at each site but relied on non-governmental organization partner funding. There was no systematic patient tracking system at any site, no regular chart review, and MCH nurse tasks were not clearly defined. Community health workers, called activistas, were inconsistently engaged at each facility. They provided some active follow-up for patient peer-counseling on side effects and ART and made home visits to defaulters when possible.34 Some activistas reported that they had begun using texting on their own initiative to communicate with some patients.
Intervention Components
Using the formative research findings, an intervention was designed to include 2 core components as further detailed in the published protocol.42 These components included enhancement of some existing activities in the SOC while adding several new activities as defined below:
Component 1
A model for workflow modification of the existing SOC was developed to support the new adherence and retention package described in component 2 (below). The new model
Defined specific new tasks for each MCH nurse to optimize patient flow and coordinate patient follow-up;
Allocated additional activistas to each site (if gaps existed) and defined new tasks to support calling/texting patients, and conduct home visits; and
Established new supportive supervision processes using continuous quality improvement principles.
Component 2
An adherence and retention package included new activities and tools added to the B+ SOC at each site. The package
Established monthly clinical chart reviews by clinic directors and MCH nurses;
Established Adherence Committees (AC) consisting of MCH nurses and Community Health Workers who met weekly to coordinate patient follow-up strategies including home visits, counseling, and texting/phone call reminders with consenting patients. Patient filing systems were improved to track files. Regardless of texting/call consent, activistas would conduct home visits for defaulters. The study provided an SMS texting/phone call protocol and tracking spreadsheet tool with predefined messages for reminders before 30-day visits, and follow-up messages to defaulters (5 days after a missed appointment). Patients could choose to receive phone calls instead of texts. Activistas were provided cell phones and credit;
Provided an enhanced counseling package with improved messaging for counseling sessions in the first 30 days (ART initiation, 7 days, 3 weeks and at the first 30-day refill appointment), and subsequent 60- and 90-day visits. Content focused on side effects, patient navigation through care, and male involvement. Similar messages were provided to MPM groups;
Established supportive supervision including (1) periodic refresher Option B+ training provided for staff; (2) data review in AC to improve patient flow, (3) a B+ checklist for the facility clinical director to ensure quality in patient tracking registries and follow-up, and (4) a B+ checklist for MCH nurse supervision of activistas.
Clinics were randomly allocated within a stepped-wedge design to intervention and control periods. Before randomization, the 6 sites were stratified by province (3 in each), and 1 site from each province was randomly selected to initiate the intervention at each of 3 stepped time points.42 Before initiation of the intervention, health workers at each site participated in an in-depth training with the research team. Each step was separated by 3 months to allow an adequate number of people to be tested and initiate ART in each site (1.5 or 1 month testing plus 14 days) and outcomes to be measured before the subsequent step (1.5, ie, 45 days post-ART initiation). To be eligible, women had to be newly diagnosed with HIV in their first ANC visit and have initiated ART within 14 days of the HIV test in the first ANC visit.
Outcomes—Variable Definitions
The intervention focused primarily on retention-in-care as the key outcome. Given the narrow definition of retention based on 30-day pharmacy refills, retention and adherence were closely linked outcomes. ARV adherence was also estimated to assess the effect of improved retention on adherence. Data were derived from routine health facility registries and forms. Following the existing SOC, mothers who tested HIV positive were entered into an ART registry, an ART patient paper chart was opened, and a pharmacy form called an FILA (in Portuguese, a Ficha Individual de Levantamento de ARV, or individual ARV pickup record) was used to record pharmacy refills (TDF + 3 TC + EFV, single daily fixed-dose combination).34 Research teams extracted data from these data sources each month and consolidated them into a single Microsoft Access database. The patients were then identified using a new study-specific identification number for analysis. Trained team members extracted data and compared it across data sources. Data collection was repeated if inconsistencies were identified.
As Rollins et al (2014) have argued there is no standard definition of ART “retention-in-care.”16 Clinic attendance after 12 months is often equated with full retention, whereas inconsistent attendance is infrequently measured thus failing to capture the early more fine-grained patterns of attendance, care, and refills.16 Therefore, outcomes for this study were measured at 30, 60, and 90-day refill periods. Retention-in-care was defined as the percentage of HIV+ pregnant women who returned for their first (30-day), second (60-day), and third (90-day) ART medication refills within 25–35 days of their previous refill. To better capture the effect of the intervention’s patient follow-up activities after each pickup period, “retention” at each step was not conditional on whether the previous pickup had been “on time.” For example, if a patient made their first 30-day refill pickup at 36 days, the second refill at the “60-day” pickup would begin counting 30 days from the previous 36-day refill date. We also measured the proportion of whoever returned for ARV refills over 90 days, and the proportion who returned within 25–35 days for each sequential visit. Related ARV adherence measures calculated using pharmacy refills and days covered are described in Figure 2.
FIGURE 2.
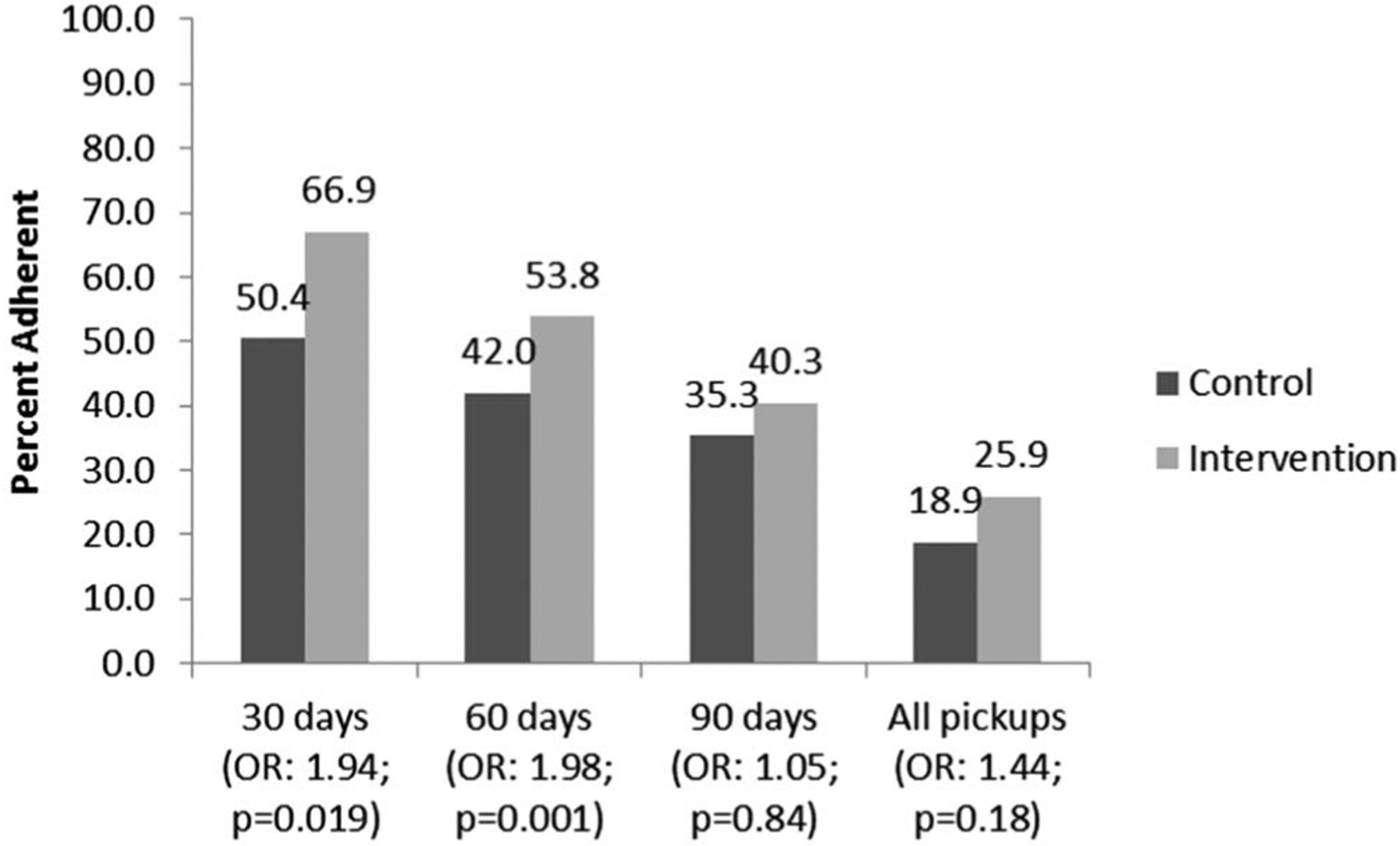
Percentage of HIV+ pregnant women who achieved $90% adherence based on pharmacy refills for their first (30-day), second (60-day), and third (90-day) ARV therapy (ART) medication refills, Sofala and Manica Provinces, Mozambique. *Adherence was assessed by pharmacy refill data and calculated as the total number of days of ARV medication dispensed within the period between the date of the initial pharmacy fill and the date of each subsequent refill (excluding those medications received on the latest refill date) divided by the number of days between these 2 dates. Pharmacy refill estimations of adherence correlate with self-reported adherence measures51–53 and with clinical outcomes such as viral load suppression and the development of ARV resistance.51,54 Our adherence measure was dichotomized into those with optimal adherence (≥90%) vs. those with suboptimal adherence (<90%). A 90% cutoff was chosen because this level of adherence is likely adequate to achieve viral suppression and avoid the development of resistance.55,56 Adherence at each step was not conditional on previous pickup to better capture the effect of the intervention patient follow-up activities after each pickup period, especially for defaulters. However, overall adherence at 90 days was also measured.
Statistical Approach
We used generalized linear mixed models with a logit link function, as all outcome variables were binary.45 We included a linear fixed effect for time, or each “step” in the stepped-wedge, and a binary fixed intervention effect which was our main outcome of interest. Separate models were used for each outcome. We accounted for clustering using clinic-level random intercepts, with a clinic by intervention interaction allowing the treatment effect to vary by cluster. We adjusted for calendar time by including a linear term for stepped-wedge “steps” in the model (0, 1, 2, and 3). For prespecified birth subanalyses, we included a binary fixed effect of birth before scheduled ARV refill, and a birth by intervention interaction. All analyses used an alpha value of 0.05 and 2-tailed tests. Analyses were conducted using Stata 14.
Process Evaluation
To track fidelity to the core components, the research team conducted weekly visits for the first 6 weeks to “fine tune” the intervention, monthly visits to collect routine data, and quarterly visits to conduct focus group discussions with health workers and HIV+ mothers in peer groups to assess intervention strengths and challenges. The team collected key outcomes data from registries and patient files per the stepped-wedge schedule.
Ethical Approval
The study protocol was approved by the Mozambique MOH National Health Bioethics Committee and the University of Washington Institutional Review Board.
RESULTS
A total of 761 pregnant women tested HIV+, initiated ART, and were followed to track retention and adherence outcomes across 6 clinics in Sofala and Manica Provinces, Mozambique, from May 2014 to August 2015. Baseline cohort data were collected in May 2014 and the intervention initiated in the first 2 sites in October 2014 (Table 1). Based on stepped-wedge intervention initiation, of the 761 pregnant women testing HIV+, 390 (51.2%) tested HIV+ during the intervention period and 371 (48.8%) tested HIV+ during the control period. Overall, women had a mean age of 24.9 years, mean gestational age of 21.6 weeks at first ANC visit, mean CD4 count of 470.1, and parity of 1.6 births at study entry (Table 2). Individual demographic characteristics were well balanced between women testing HIV+ in the intervention versus control periods (Table 2).
TABLE 1.
Stepped-Wedge Initiation and Number Enrolled Over Time
| Health | Intervention | |||
|---|---|---|---|---|
| Unit | 0 | 1 | Total | Date Initiated |
| Clinic 1 | 18 | 104 | 122 | October 27, 2014 |
| Clinic 2 | 19 | 113 | 132 | October 27, 2014 |
| Clinic 3 | 52 | 55 | 107 | January 27, 2015 |
| Clinic 4 | 26 | 48 | 74 | January 27, 2015 |
| Clinic 5 | 162 | 39 | 201 | April 27, 2015 |
| Clinic 6 | 94 | 31 | 125 | April 27, 2015 |
| Total | 371 | 390 | 761 | |
TABLE 2.
Demographics of 716 Women Engaging in Option B+ Among Intervention Versus Control Periods, May 2014–May 2015, Sofala, Mozambique
| Characteristic | Total (N, %) | Intervention (n, %) | Control (n, %) |
|---|---|---|---|
| Total | 761 (100) | 390 (51.2) | 371 (48.8) |
| Mean (SD) age among those with complete data | 24.9 (5.3) | 24.8 (5.3) | 25.1 (5.4) |
| Age <18 | 34 (4.5) | 21 (5.4) | 13 (3.5) |
| Age 18–24 | 424 (55.7) | 217 (55.6) | 207 (55.8) |
| Age 25–29 | 146 (19.2) | 77 (19.7) | 69 (18.6) |
| Age 30–34 | 112 (14.7) | 51 (13.1) | 61 (16.4) |
| Age 35+ | 42 (5.5) | 22 (5.6) | 20 (5.4) |
| Missing | 3 (0.39) | 2 (0.51) | 1 (0.27) |
| Mean (SD) gestational age at first ANC among those with complete data | 21.6 (5.9) | 22.0 (5.9) | 21.3 (5.8) |
| Gestational age <16 | 80 (10.5) | 38 (9.7) | 42 (11.3) |
| Gestational age 16–20 | 179 (23.5) | 91 (23.3) | 88 (23.7) |
| Gestational age 21–25 | 136 (17.9) | 66 (16.9) | 70 (18.9) |
| Gestational age 26–30 | 125 (16.4) | 65 (16.7) | 60 (16.2) |
| Gestational age >30 | 32 (4.2) | 20 (5.1) | 12 (3.2) |
| Missing | 209 (27.5) | 110 (28.2) | 99 (26.7) |
| Mean (SD) CD4 result among those with complete data | 470.1 (312.0) | 460.9 (361.0) | 480.0 (249.1) |
| CD4 <200 | 44 (5.8) | 23 (5.9) | 21 (5.7) |
| CD4 200–299 | 77 (10.1) | 47 (12.1) | 30 (8.1) |
| CD4 300–399 | 85 (11.2) | 45 (11.5) | 40 (10.8) |
| CD4 400–499 | 79 (10.4) | 38 (9.7) | 41 (11.1) |
| CD4 ≥500 | 172 (22.6) | 84 (21.5) | 88 (23.7) |
| Missing | 304 (39.9) | 153 (39.2) | 151 (40.7) |
| Mean (SD) of parity among those with complete data | 1.6 (1.5) | 1.7 (1.6) | 1.5 (1.5) |
| Parity of 0 | 203 (26.7) | 96 (24.6) | 107 (28.8) |
| Parity of 1 | 154 (20.2) | 81 (20.8) | 73 (19.7) |
| Parity of 2 | 138 (18.1) | 71 (18.2) | 67 (18.1) |
| Parity of 3+ | 168 (22.1) | 101 (25.9) | 67 (18.1) |
| Missing | 98 (12.9) | 41 (10.5) | 57 (15.4) |
Retention at 30, 60, and 90 Days
During control periods, 52.3% of women returned within 5 days before or after their scheduled 30-day ARV pickup (first refill), compared with 70.8% of women in intervention periods [odds ratio (OR): 1.80; 95% confidence interval (CI): 1.05 to 3.08]. This difference was 46.1% control vs. 57.9% intervention at 60 days (second refill); (OR: 1.82; CI: 1.06 to 3.11), and 38.3% control vs. 41.0% intervention at 90 days (third refill); (OR: 1.04; CI: 0.60 to 1.82); (Fig. 1). The intracluster correlation coefficients for 30, 60, and 90 days were as follows: 0.041 (CI: 0.0097 to 0.15); 0.0078 (CI: 0.00018 to 0.26); and 0.0067 (CI: 0.00021 to 0.17), respectively.
FIGURE 1.
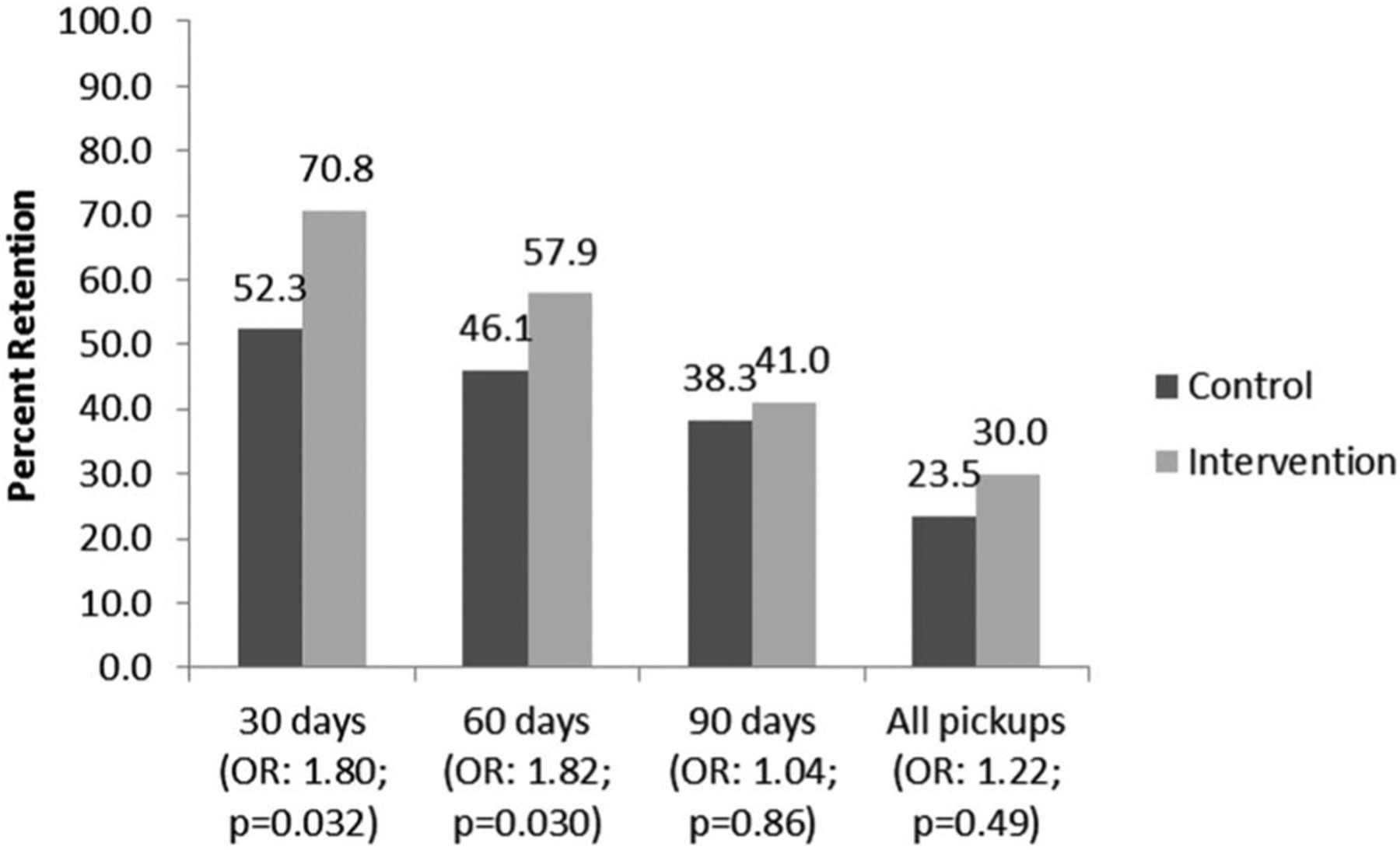
Percentage of HIV+ pregnant women who returned for their first (30-day), second (60-day), and third (90-day) ARV therapy (ART) medication refills within 25–35 days of previous refill, Sofala and Manica Provinces, Mozambique.
Combining these 3 visits together, 23.5% of women in control periods and 30.0% of women in intervention periods returned within 5 days before or after the appointment date of all 3 medication pickups (OR: 1.22; CI: 0.68 to 2.20). In addition, 72.2% of women testing HIV+ during control periods and 85.9% of women in intervention periods ever returned after initiating ART (OR: 2.48; CI: 1.33 to 4.62). Estimated medication adherence at 30, 60, and 90 days based on pharmacy refill data is presented in Figure 2.
Prespecified Intervention Subanalyses
In prespecified subanalyses, birth before scheduled pickups was strongly associated with LTFU—women giving birth before their ARV pickup were 33.3 times (CI: 4.4 to 250.3), 7.5 times (CI: 3.6 to 15.9), and 3.7 times (CI: 2.2 to 6.0) as likely to not return for ARV pickups at 30, 60, and 90 days, respectively. (The total proportion of women delivering by 30, 60, and 90 days is indicated in Table 3, column 2). The intervention showed a trend toward higher effect sizes for retaining women who gave birth before ARV pickups at 30 days (Interaction OR: 5.0; CI: 0.52 to 47.5) and 60 days (interaction OR: 2.4, CI: 0.96 to 6.2), but not 90 days (Interaction OR: 0.97, CI: 0.49 to 1.9); (Table 3).
TABLE 3.
Prespecific Subanalyses of Intervention Effect Comparing Women Who Gave Birth Before Scheduled Refill With Women Who had Not Given Birth Before Scheduled Refills, May 2014–May 2015, Sofala, Mozambique*
| Characteristic | Total, N (%) | Intervention, n (%) | Control, n (%) | OR (95% CI) | P |
|---|---|---|---|---|---|
| Total | 761 (100) | 390 (51.2) | 371 (48.8) | ||
| Missing birth information | 28 (3.7) | 9 (2.3) | 19 (5.1) | ||
| Women who gave birth before first ARV refill (30 d) | |||||
| Total, (% of overall column total) | 46 (6.0) | 20 (5.1) | 26 (7.0) | ||
| Returned | 7 (15.2) | 6 (30.0) | 1 (3.8) | 2.23 (−0.05 to 4.5) | 0.055 |
| Did not return | 39 (84.8) | 14 (70.0) | 25 (96.2) | 1 (reference) | |
| Women who did not give birth before first ARV refill (30 d) | |||||
| Total, (% of overall column total) | 687 (90.3) | 361 (92.6) | 326 (87.9) | ||
| Returned | 451 (65.6) | 267 (74.0) | 184 (56.4) | 1.87 (1.05 to 3.28) | 0.031 |
| Did not return | 236 (34.4) | 94 (24.0) | 142 (43.6) | 1 (reference) Interaction P: 0.161 |
|
| Women who gave birth before second ARV refill (60 d) | |||||
| Total, (% of overall column total) | 129 (17.0) | 65 (16.7) | 64 (17.3) | ||
| Returned | 33 (25.6) | 24 (36.9) | 9 (14.1) | 4.72 (1.73 to 12.9) | 0.002 |
| Did not return | 96 (74.4) | 41 (63.1) | 55 (85.9) | 1 (reference) | |
| Women who did not give birth before second ARV refill (60 d) | |||||
| Total, (% of overall column total) | 604 (79.4) | 316 (81.0) | 288 (77.6) | ||
| Returned | 354 (58.6) | 199 (63.0) | 155 (53.8) | 1.93 (1.09 to 3.44) | 0.025 |
| Did not return | 250 (41.4) | 117 (37.0) | 133 (46.2) | 1 (reference) Interaction P: 0.062 |
|
| Women who gave birth before third refill (90 d) | |||||
| Total, (% of overall column total) | 275 (36.1) | 144 (36.9) | 131 (35.3) | ||
| Returned | 62 (22.5) | 34 (23.6) | 28 (21.4) | 1.07 (0.49 to 2.34) | 0.86 |
| Did not return | 213 (77.5) | 110 (76.4) | 103 (78.6) | 1 (reference) | |
| Women who did not give birth before third refill (90 d) | |||||
| Total, (% of overall column total) | 458 (60.2) | 237 (60.8) | 221 (59.6) | ||
| Returned | 235 (51.3) | 125 (52.7) | 110 (49.8) | 1.11 (0.60 to 2.05) | 0.75 |
| Did not return | 223 (48.7) | 112 (47.3) | 111 (50.2) | 1 (reference) Interaction P: 0.929 |
|
Numbers giving birth before each subsequent refill are cumulative totals.
Process Evaluation Data
Adherence committee activities including systematic patient tracking by activistas coordinated with MCH nurses, SMS/phone messaging, home visits to defaulters, enhanced counseling, and MpM support group activities were conducted with strong fidelity to the intervention. In total, 49% of mothers in the intervention periods consented to receive calls/SMS texting (191/390). The regular use of supportive supervision checklists was verified. Data and interviews did not indicate a significant decline in fidelity to core components over time at any of the sites. In focus group discussions, activistas and MCH nurses stated that they believed the intervention components were valuable, feasible, and effective, but high patient loads continued to be a challenge and wait times too long. The chart review process frequently did not occur across all sites (less than 50% of the time) because of lack of availability of senior staff. There were no reported drug stock outs. Although male involvement was promoted through counseling and invites, routine facility data showed little improvement.
DISCUSSION
The intervention was effective at significantly increasing retention at 30 and 60 days post-ART initiation for Option B+ mothers, but not at 90 days. Intervention effect at 90 days may be biased toward a null finding as the intervention was initiated before the third (90-day) ARV pickup for 149 women (40.0% of control women). Thus, these control women may have experienced some benefit from the intervention for their 90-day ARV pickup. Loss of intervention fidelity was not detected in the process evaluation data. The intervention had a significant impact on the proportion of mothers who ever returned after receiving their first ARVs. However, 14.1% of women in the intervention group and 22.6% of controls, a combined 18.3%, never returned after starting ART. Retention at 90 days under Option B+ was very low for both intervention and control—a combined 40% returned within 5 days of their 90-day ARV pickup date. Only 26.8% of women returned within 25–35 days from the previous refill for all 3 refill dates. These findings highlight the ongoing challenges in Mozambique to improve ART retention. The intervention’s impact on adherence was similarly significant, but the overall adherence results remain troubling. Only a combined 22.5% of mothers were optimally adherent after 90 days, raising additional concerns about viral suppression and vertical transmission.
Targeted efforts to increase retention are particularly important for women giving birth before their first few ARV pickups. Our data show that for both intervention and control groups, there were much sharper drop-offs in retention among these women compared with those who had not yet given birth. There were no changes in visit dates postpartum, but a range of other factors could influence LTFU (eg, challenges from caring for a new baby). Postpartum retention is an urgent challenge requiring additional research and innovative strategies.
The study strengths include use of a narrow definition of retention focusing on each 30-day refill pickup to provide a more detailed view of early retention patterns. The stepped-wedge design provided rigorous and robust test of the intervention, and a detailed process evaluation was conducted to assess fidelity to core components. The intervention was designed with substantial input from health workers and mothers to ensure feasibility. Limitations of the study include lack of viral load measures to evaluate impact on vertical transmission, an adherence measure that relies on pharmacy refills leading to possible adherence overestimation, and lack of longer term retention data at 6 or 12 months to better compare with other studies. The study could not identify patients who were LTFU because of death or migration to different health facilities. The multipronged combination intervention makes it difficult to disaggregate the impact of each element of the approach.
The success of the intervention shows that low-cost inputs including systematic active patient tracking, community health workers (activistas), texting/calling, and home visits that make adjustments to existing resources can improve retention. However, 49% consent rate for texting/phone calls suggests that cell phone use is only 1 element of the intervention that likely contributed to improvements. These findings are similar to recent published studies from Africa that report success with similar combination interventions. In Zambia, lay counselors and home visit follow-up reduced 6-month LTFU from 24.7% to 14.7%.46 In Uganda, Zimbabwe, and Ethiopia, 6-month retention rates ranged from about 80%–90% and were achieved with lay counselors and active follow-up in communities.19,27,47 In Kenya, texting was shown to improve postpartum clinic attendance with 19.6% of intervention women vs. 11.8% of women attending in the control group.48 A recent systematic review of 34 prevention of maternal-to-child HIV transmission intervention studies concluded that mobile phone-based reminders showed the most promising results.49
Despite improved patient flow, waiting times remained long, and consultation times brief, which has been reflected in other studies in this setting.50 Although innovative interventions can make meaningful improvement, our data strongly indicate that significant workforce expansion is needed at these sites. However, the results suggest that the major components of the intervention could still be feasibly scaled up to sites throughout Mozambique, but more research and innovation are needed to improve longer term retention and to reduce LTFU among postpartum mothers.
ACKNOWLEDGMENTS
The authors thank the staff of the Beira Operations Research Center (CIOB), the Provincial Health Directorates of Manica and Sofala Provinces, the health personnel of the 6 health facilities in the study, and the staff of Health Alliance International.
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R01 HD074557) and the University of Washington Center for AIDS Research, including support from NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK of the National Institutes of Health under award number AI027757.
Footnotes
The conclusions are those of the authors and do not necessarily represent the official position of the NIH.
Presented at the 21st International Aids Conference; Poster Session; July 20, 2016; Durban, South Africa.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems’ performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:e45–e48. [DOI] [PubMed] [Google Scholar]
- 2.Braun M, Kabue MM, McCollum ED, et al. Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson L, Grant AD, Lewis J, et al. Linking women who test HIV-positive in pregnancy-related services to HIV care and treatment services in Kenya: a mixed methods prospective cohort study. PLoS One. 2014;9: e89764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wettstein C, Mugglin C, Egger M, et al. Missed opportunities to prevent mother-to-child-transmission: systematic review and meta-analysis. AIDS. 2012;26:2361–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson L, Lewis J, Grant AD, et al. Patient attrition between diagnosis with HIV in pregnancy-related services and long-term HIV care and treatment services in Kenya: a retrospective study. J Acquir Immune Defic Syndr. 2012;60:e90–e97. [DOI] [PubMed] [Google Scholar]
- 6.Gamell A, Letang E, Jullu B, et al. Uptake of guidelines on prevention of mother-to-child transmission of HIV in rural Tanzania: time for change. Swiss Med Wkly. 2013;143:w13775. [DOI] [PubMed] [Google Scholar]
- 7.Landes M, van Lettow M, Bedell R, et al. Mortality and health outcomes in HIV-infected and HIV-uninfected mothers at 18–20 months postpartum in Zomba District, Malawi. PLoS One. 2012;7:e44396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Lettow M, Bedell R, Landes M, et al. Uptake and outcomes of a prevention-of mother-to-child transmission (PMTCT) program in Zomba district, Malawi. BMC Public Health. 2011;11:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNAIDS. UNAIDS Report on the Global AIDS Epidemic Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- 10.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012;26:2039–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Programmatic Update: WHO HIV/AIDS Programme Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 12.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 13.Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS. 2013; 8:474–489. [DOI] [PubMed] [Google Scholar]
- 14.Clouse K, Schwartz S, Van Rie A, et al. “What they wanted was to give birth; nothing else”: barriers to retention in option B+ HIV care among postpartum women in South Africa. J Acquir Immune Defic Syndr. 2014; 67:e12–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clouse K, Pettifor A, Shearer K, et al. Loss to follow-up before and after delivery among women testing HIV positive during pregnancy in Johannesburg, South Africa. Trop Med Int Health. 2013;18:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollins NC, Becquet R, Orne-Gliemann J, et al. Defining and analyzing retention-in-care among pregnant and breastfeeding HIV-infected women: unpacking the data to interpret and improve PMTCT outcomes. J Acquir Immune Defic Syndr. 2014;67(suppl 2):S150–S156. [DOI] [PubMed] [Google Scholar]
- 17.Shaffer N, Abrams EJ, Becquet R. Option B+ for prevention of mother-to-child transmission of HIV in resource-constrained settings: great promise but some early caution. AIDS. 2014;28:599–601. [DOI] [PubMed] [Google Scholar]
- 18.Helova A, Akama E, Bukusi EA, et al. Health facility challenges to the provision of Option B+ in western Kenya: a qualitative study. Health Policy Plann. 2017;32:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitiku I, Arefayne M, Mesfin Y, et al. Factors associated with loss to follow-up among women in Option B+ PMTCT programme in northeast Ethiopia: a retrospective cohort study. J Int AIDS Soc. 2016;19:20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (“Option B+”) in Malawi. AIDS. 2014;28:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease C, Prevention. Impact of an innovative approach to prevent mother-to-child transmission of HIV–Malawi, July 2011-September 2012. MMWR Morb Mortal Wkly Rep. 2013;62:148–151. [PMC free article] [PubMed] [Google Scholar]
- 22.(CDC) CfDCaP. Impact of an innovative approach to prevent mother-to-child transmission of HIV–Malawi, 2011–2012. MMWR Morb Mortal Wkly Rep. 2013;62:148–151. [PMC free article] [PubMed] [Google Scholar]
- 23.Haas AD, Tenthani L, Msukwa MT, et al. Retention in care during the first 3 years of antiretroviral therapy for women in Malawi’s option B+ programme: an observational cohort study. Lancet HIV. 2016;3:e175–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Llenas-García J, Wikman-Jorgensen P, Hobbins M, et al. Retention in care of HIV-infected pregnant and lactating women starting art under Option B+ in rural Mozambique. Trop Med Int Health. 2016;21:1003–1012. [DOI] [PubMed] [Google Scholar]
- 25.Asbjornsdottir KRA, Rustagi A, Coutinho J, et al. Low Retention in Option B+ Care Among Women in Mozambique. Women and HIV Intercfar Joint Symposium on HIV Research in Women. Birmingham, Alabama: Centers for AIDS Research; 2016. [Google Scholar]
- 26.Matheson R, Moses-Burton S, Hsieh AC, et al. Fundamental concerns of women living with HIV around the implementation of Option B. J Int AIDS Soc. 2015;18(6 suppl 5):20286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kieffer MP, Mattingly M, Giphart A, et al. Lessons learned from early implementation of option B+: the elizabeth glaser pediatric AIDS foundation experience in 11 African countries. J Acquir Immune Defic Syndr. 2014;67(suppl 4):S188–S194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourlay A, Birdthistle I, Mburu G, et al. Barriers and facilitating factors to the uptake of antiretroviral drugs for prevention of mother-to-child transmission of HIV in sub-Saharan Africa: a systematic review. J Int AIDS Soc. 2013;16:18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CNCS. UNGASS: United Nations General Assembly Special Session on HIV and AIDS, Progress Report, 2008–2009 Mozambique. Maputo, Mozambique: National AIDS Council; 2010. [Google Scholar]
- 30.UNAIDS. Global Report: Report on the Global AIDS Epidemic 2013. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- 31.MISAU. Moçambique Inquérito Demográfico de Saúde 2011. Calverton, MD: ICF International/MEASURE DHS program; 2011. [Google Scholar]
- 32.MISAU. Relatorio Anual 2014: Relatorio Anual da Actividades Relacionadas ao HIV/SIDA. Maputo, Mozambique: Mozambique Ministry of Health. [Google Scholar]
- 33.Auld AF, Shiraishi RW, Couto A, et al. A decade of antiretroviral therapy scale-up in Mozambique: evaluation of outcome trends and new models of service delivery among more than 300,000 patients enrolled during 2004—2013. J Acquir Immune Defic Syndr. 2016;73:e11–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Napua M, Pfeiffer JT, Chale F, et al. Option B+ in Mozambique: formative research findings for the design of a facility-level clustered randomized controlled trial to improve ART retention in antenatal care. J Acquir Immune Defic Syndr. 2016;72(suppl 2):S181–S188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozambique Ministry of Health. 2009 National Survey on Prevalence, Behavioral Risks and Information about HIV and AIDS in Mozambique (INSIDA). Maputo, Mozambique: Mozambique Ministry of Health; 2009. [Google Scholar]
- 36.Mozambique Ministry of Health. PMTCT 2009 Programme Report. Maputo, Mozambique: Mozambique Ministry of Health; 2009. [Google Scholar]
- 37.Fernandes QF, Wagenaar BH, Anselmi L, et al. Effects of health-system strengthening on under-5, infant, and neonatal mortality: 11-year provincial-level time-series analyses in Mozambique. Lancet Glob Health. 2014;2:e468–e477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mozambique National Institute of Statistics, U.S. Census Bureau, MEASURE Evaluation, U.S. Centers for Disease Control and Prevention. Mortality in Mozambique: Results from a 2007–2008 Post-Census Mortality Survey. Chapel Hill, NC: MEASURE Evaluation; 2012. [Google Scholar]
- 39.MISAU. National Department of Medical Assistance, National Data Report 2010 Maputo, Mozambique: Antiretroviral Treatment; 2011. [Google Scholar]
- 40.CNCS. Resposta Global à SIDA; Relatório do Progresso, 2016, Mozambique Maputo, Mozambique: National AIDS Council; 2016. [Google Scholar]
- 41.Pfeiffer J, Chapman R. Early ART Initiation Among HIV-Positive Pregnant Women in Central Mozambique Grant No. 1R01HD074557–01. Washington, DC: National Institute of Child Health and development; 2012. [Google Scholar]
- 42.Cowan JF, Micek M, Cowan JF, et al. Early ART initiation among HIV-positive pregnant women in central Mozambique: a stepped wedge randomized controlled trial of an optimized Option B+ approach. Implement Sci. 2015;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeiffer J, Napua M. Chapman R, et al. Opton B+ in Mozambique: Challenges to Retention in Care. Poster Presented at 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Vancouver, CA: International AIDS Society; 2015. [Google Scholar]
- 44.Napúa MJ. Manuel L. Vieira G, et al. Factors contributing to loss to follow-up among HIV-exposed infants in Manica and Sofala provinces—Central Mozambique. 21st International AIDS Conference 2016. July 20, 2016; Durban, South Africa. [Google Scholar]
- 45.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007;28:182–191. [DOI] [PubMed] [Google Scholar]
- 46.Herlihy JM, Hamomba L, Bonawitz R, et al. Implementation and operational research: integration of PMTCT and antenatal services improves combination antiretroviral therapy uptake for HIV-positive pregnant women in southern Zambia: a prototype for option B+? J Acquir Immune Defic Syndr. 2015;70:e123–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dzangare J, Takarinda KC, Harries AD, et al. HIV testing uptake and retention in care of HIV-infected pregnant and breastfeeding women initiated on “Option B+”in rural Zimbabwe. Trop Med Int Health. 2016; 21:202–209. [DOI] [PubMed] [Google Scholar]
- 48.Odeny TA, Bukusi EA, Cohen CR, et al. Texting improves testing: a randomized trial of two-way SMS to increase postpartum prevention of mother-to-child transmission retention and infant HIV testing. AIDS (London, England) 2014;28:2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ambia J, Mandala J. A systematic review of interventions to improve prevention of mother-to-child HIV transmission service delivery and promote retention. J Int AIDS Soc. 2016;19:20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagenaar BH, Gimbel S, Hoek R, et al. Wait and consult times for primary healthcare services in central Mozambique: a time-motion study. Glob Health Action. 2016;9:31980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fairley C, Permana A, Read T. Long-term utility of measuring adherence by self-report compared with pharmacy record in a routine clinic setting. HIV Med. 2005;6:366–369. [DOI] [PubMed] [Google Scholar]
- 52.Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001; 28:47–58. [DOI] [PubMed] [Google Scholar]
- 53.Walsh JC, Horne R, Dalton M, et al. Reasons for non-adherence to antiretroviral therapy: patients’ perspectives provide evidence of multiple causes. AIDS Care. 2001;13:709–720. [DOI] [PubMed] [Google Scholar]
- 54.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–347. [DOI] [PubMed] [Google Scholar]
- 55.Weiser SD, Guzman D, Riley ED, et al. Higher rates of viral suppression with nonnucleoside reverse transcriptase inhibitors compared to single protease inhibitors are not explained by better adherence. HIV Clin Trials. 2004;5:278–287. [DOI] [PubMed] [Google Scholar]
- 56.Bangsberg DR, Moss AR, Deeks SG. Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother. 2004; 53:696–699. [DOI] [PubMed] [Google Scholar]


