Abstract
BACKGROUND
Clinical benefits of plasma as an adjunct for treatment of hemorrhagic shock (HS) have been well established. However, its use is not without risk. Little is understood regarding the clinical implications of plasma variability. We hypothesized there to be interdonor variability in plasma that would impact endothelial and organ function post injury.
METHODS
Pulmonary endothelial cells (EC) were incubated with plasma from 24 random donors and transendothelial electrical resistance (TEER) was measured. Plasma units with a more or less protective effect on reducing EC permeability were selected for testing in vivo. Syndecan-1 and cytokines were measured. Mice underwent laparotomy then HS followed by resuscitation with the selected plasma units and were compared to mice receiving no resuscitation and shams. Lung tissue was sectioned and stained for myeloperoxidase, pulmonary syndecan-1 and scored for lung histopathologic injury.
RESULTS
Plasma from 24 donors revealed variability in the reversal of EC monolayer hyperpermeability; TEER for the more protective plasma was significantly higher than for the less protective plasma (0.801±0.022 vs. 0.744±0.035; p=.002). Syndecan-1 was also markedly increased in the less protective compared to the more protective plasma (38427±1257 vs. 231±172pg/mL, p<.001), while cytokines varied. In vivo, the more protective plasma mitigated lung histopathologic injury compared to the less protective plasma (1.56±0.27 vs. 2.33±0.47, respectively, p=.005). Similarly, myeloperoxidase was significantly reduced in the more protective compared to the less protective plasma group (2.590±0.559 vs. 6.045±1.885; p=0.02). Lastly, pulmonary syndecan-1 immunostaining was significantly increased in the more protective compared to the less protective plasma group (20.909±8.202 vs. 9.325±3.412; p=0.018).
CONCLUSIONS
These data demonstrate significant interdonor variability in plasma that can adversely influence the protective effects of plasma-based resuscitation on HS-induced lung injury. This may have important implications for patient safety and clinical outcomes.
Keywords: Hemorrhagic shock, trauma, resuscitation, syndecan-1
BACKGROUND
Hemorrhagic shock is a leading cause of death in both military and civilian trauma(1,2). Early and empiric use of fresh frozen plasma (FFP) in bleeding trauma patients has led to a decrease in early deaths(3–5). Additionally, a recent large randomized clinical trial has shown a mortality benefit with transfusion of FFP in the pre-hospital environment for patients in hemorrhagic shock(6). Early plasma-based resuscitative strategies have decreased mortality and the benefit of plasma appears to extend beyond its ability to correct trauma-induced coagulopathy and provide hemorrhage control and is thought to involve additional protective effects to a dysfunctional endothelium. This has been termed the endotheliopathy of trauma (EoT) and leads to coagulopathy, inflammation, degradation of the endothelial barrier, tissue edema, and end organ injury(7).
Previous investigations have demonstrated that resuscitation with plasma versus lactated ringers provides benefits in both in vitro and in vivo models of hemorrhagic shock by reducing injury, inflammation and permeability(8–10). Laboratory data have implicated circulating syndecan-1 as being a key surrogate marker for endothelial injury and compromise of the endothelial glycocalyx(11,12). Syndecan-1 is a heparan sulfate cell surface proteoglycan that forms the structural backbone of the endothelial glycocalyx(11,13). Hemorrhagic shock leads to shedding of its intraluminal ectodomain. Shed ectodomains are associated with enhanced shock, inflammation, and endothelial damage and independently predict mortality in injured patients(11,12,14). The early use of plasma restores the endothelial glycocalyx after hemorrhage and reduces circulating syndecan-1(13,15).
The use of plasma, however, is not without risk. Complications such as transfusion-transmitted infectious diseases and volume overload may be associated with plasma administration(16,17). To ensure equivalent protection to all plasma recipients, we asked the question, are all donor units of plasma created equal? We therefore hypothesized there to be interdonor variability in plasma that would impact physiologic organ (pulmonary) function in recipients of plasma following hemorrhagic shock.
METHODS
In vitro:
Donor plasma
Plasma was obtained from healthy donors thru the Bonfils/Vitalant Blood Bank Research Donor Program, Denver, Colorado. Per standard blood bank procedures, plasma was frozen and stored at −20°C within eight hours until ready for testing. Once thawed, it was used within 30 minutes. Ages of donors ranged from 16 to 81 years old. Seven were female and seventeen were male. Eight donors each were from blood groups A, B and O.
Endothelial cell permeability
Human lung microvascular endothelial cells (HLMVEC; PromoCell) were grown to confluence in endothelial basic medium-2 (EBM-2; Lonza) supplemented with 10% fetal bovine serum (FBS) and growth factors(9).
Cell barrier function was assessed by measuring transendothelial electrical resistance (TEER) in real-time using the electric cell-substrate impedance sensing (ECIS) system (ECIS 1600, Applied BioPhysics, Troy, NY) as we have described(10). A change in TEER across the cell monolayer indicated increased or decreased paracellular permeability. HLMVECs were grown to confluence on L-cysteine reduced, fibronectin-coated electrodes and serum starved for one hour before treatment. At time zero, 5% donor plasma was added to the experimental wells. Monolayer resistance was recorded at 4 kHz over eight-minute intervals for 2 hours. TEER measurement was replicated 4–8 times with each plasma donor unit. For a period of 1 hour after the plasma addition period, changes in TEER from each well’s baseline (ratio of post- to pre-treatment) were computed, averaged for each group (with average control changes subtracted), and expressed as the area under the curve for 1 hour.
The plasma units with greater and lesser effects on endothelial cell permeability were then selected for use in our in vivo model and are indicated as the more protective (highest TEER, lowest permeability) or less protective plasma (lowest TEER, highest permeability). The two units selected were also both male with the same ABO blood type.
Syndecan-1 levels of donor plasma
Levels of human syndecan-1 were measured in donor units of plasma using enzyme-linked immunosorbent assay (ELISA) according to manufacturer’s instructions (BosterBio, Pleasanton, CA). Samples were repeated in triplicate.
Cytokine and chemokine profile of donor plasma
Human 20-plex kits were purchased from R&D Systems and used according to the manufacturer’s protocol with modifications as described below. In summary, plasma samples were thawed, vortexed and centrifuged at 16,000 x g for four minutes at 4 °C prior to use. Samples were then diluted 1:2 in appropriate diluent, mixed with magnetic beads coated with capture antibodies in a 96-well plate and incubated for two hours at room temperature on a horizontal orbital microplate shaker at 800 rpm. A 7-point standard curve was used. Plates were washed three times with wash buffer then incubated with biotinylated detection antibodies for one hour at room temperature on an orbital shaker. Three additional washes were performed followed by incubation with streptavidin-PE for 30 minutes at room temperature on an orbital shaker. A final three washes were performed before magnetic beads were resuspended in wash buffer and immediately read on a MAGPIX instrument. xPONENT 4.2 software (Luminex Corp.) was used for data acquisition on the MAGPIX reader. Samples were measured in triplicate.
In vivo:
Mouse model of hemorrhagic shock
All procedures performed were approved by the University of Maryland School of Medicine Animal Welfare Committees. The experiments were conducted in compliance with the National Institutes of Health guidelines on the use of laboratory animals. All animals were housed at constant room temperature with a 12:12-h light-dark cycle with access to food and water ad libitum. Male C57BL/6J mice were used at 8 to 10 weeks of age and weighing approximately 25 grams. Our established model of trauma-hemorrhagic shock was utilized(10,18). Under isoflurane anesthesia, a midline laparotomy incision was made, the intestines inspected and then the incision was closed. The femoral artery was cannulated for continuous hemodynamic monitoring, blood withdrawal and resuscitation. Mean arterial blood pressure (MAP) was continuously recorded via the femoral arterial line. After a 10-minute period of equilibration, mice were bled to a mean arterial pressure (MAP) of 35±5 mmHg, which was maintained for 90 minutes. Shams underwent anesthesia and placement of catheters but were not subjected to laparotomy or hemorrhagic shock. Shock animals were resuscitated with the selected plasma donor units at 1x shed blood volume and compared with animals that underwent no resuscitation. Hemodynamics were tracked for 30 minutes after resuscitation, then catheters were removed and the animals were allowed to recover from anesthesia. Three hours after the end of shock, animals were sacrificed by exsanguination under isoflurane anesthesia and lungs were harvested for further analysis. This was chosen based on our previous investigation showing pulmonary protection and partial restitution of pulmonary syndecan-1 by plasma at this time point(15).
Lung histopathologic injury
The left lung was embedded in optimal cutting temperature compound (OCT) at the time of harvest and stored at −80°C. Lung tissue was sectioned and stained with hematoxylin and eosin (H&E) and scored on a 3-point scale for alveolar thickness, capillary congestion, and cellularity as described by Hart et al. and as we have reported(9,19,20). The overall lung injury score was calculated by averaging the three parameters.
Lung inflammation
Lung inflammation was assessed by myeloperoxidase (MPO) immunostaining as an indicator of neutrophil influx. Lung tissue was sectioned and then incubated with MPO primary antibody (rabbit polyclonal antibody; Abcam, Cambridge, MA) followed by incubation with secondary antibody (goat anti-rabbit Texas Red, Life Technologies, Eugene, OR). Random images were taken from each lung section with a fluorescent microscope (Nikon Eclipse E800) at 200X magnification and immunofluorescence quantified using Image J software. Results are reported as relative fluorescence units (RFU).
Pulmonary syndecan-1 immunostaining
To detect pulmonary syndecan-1, lungs were sectioned and then incubated with syndecan-1 primary antibody (mouse monoclonal antibody; Santa Cruz Biotechnology) followed by incubation with secondary antibody (goat anti-mouse Texas Red; Life Technologies). Random images were taken from each lung section with a fluorescent microscope at 200X and quantified using Image J software. Results are reported as RFU.
Statistical Analysis
Data were analyzed by one-way analysis of variance (ANOVA) with Bonferroni correction. Adjustment for different variances was made using Welch robust test of equality of means and the Welch significance is reported where available; p values < 0.05 were considered significant. Data are expressed as mean ± standard deviation (SD). In vitro data were repeated in triplicates unless otherwise noted; for in vivo experiments n=6–7/group. Sample size was based on our previous study using the same mouse model of hemorrhagic shock(13).
RESULTS
In the initial preliminary set of experiments, we tested plasma from ten different healthy male plasma donors and found significant variability in the ability of plasma from different donors to reduce endothelial cell hyperpermeability (Fig. 1 supplementary). Endothelial cells incubated with donor FFP33 demonstrated significantly less permeability (more protective donor) compared to cells incubated with donor FFP30 (less protective donor). Based on this in vitro permeability data, these two donor units of plasma were tested for in vivo pulmonary protection in our mouse model of hemorrhagic shock. As shown in Supplementary Figure 2A and B, there were significant differences in pulmonary histopathology and inflammation between donors when mice were subjected to hemorrhagic shock, with the more protective donor plasma demonstrating less injury and inflammation. Based on these data, we next performed a validation and more in depth analysis to confirm these preliminary findings.
IN VITRO
Endothelial cell permeability differs between plasma donors
Plasma from 24 donors was assessed for its ability to reduce EC monolayer permeability using the ECIS assay and TEER was measured as an indicator of permeability. A higher resistance indicated a greater decrease in paracellular permeability of the endothelial monolayers. There was variability across donors in the reversal of endothelial cell hyperpermeability as shown in Figure 1A. A plasma donor with a higher protective capability on EC permeability (Donor 10, male age 18) and lower protective capability on EC permeability (Donor 18, male age 71) were selected for further study. These donors were chosen based on their respective permeability changes and the fact that they were both male, as most blood centers no longer accept female plasma. There was a significant difference in TEER between the more protective (0.801±0.022) and less protective (0.744±0.035; Welch p=.002) donors that were selected (Figure 1B). Donors ranged in age from 16 to 81 years of age. Twelve donors from ages 16 to 21 had a significantly higher mean TEER than the twelve donors ages 70–81 (0.784±0.012 vs. 0.769±0.011, respectively; Welch p=.004). Mean TEER was similar when plasma samples were stratified by sex or blood type. Mean TEER for male donors was similar to mean TEER for female donors (0.773±0.014 vs. 0.783±0.011, respectively; Welch p=0.094). Mean TEER was also similar when donors were stratified into blood groups A, B and O (0.78±0.013 vs. 0.775±0.017 vs. 0.773±0.008, respectively; Welch p=0.510).
Figure 1: Endothelial cell permeability differs between plasma donors.
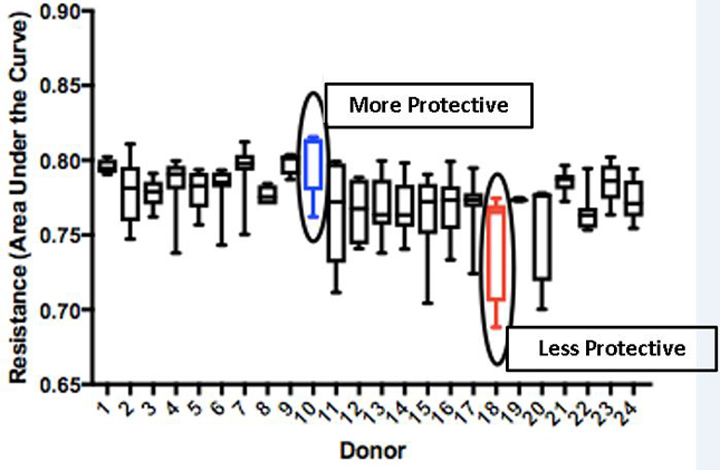
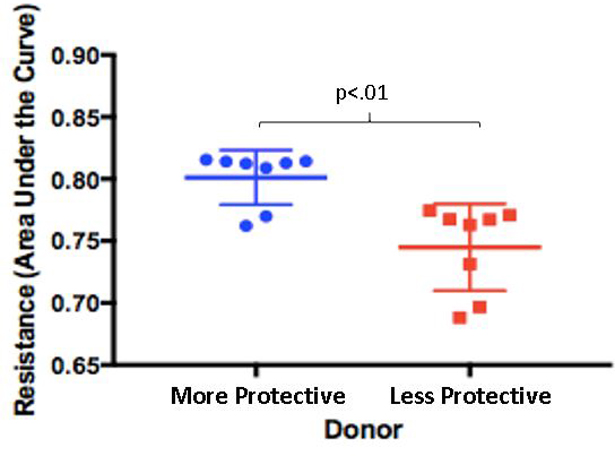
A. HLMVECs were treated with 5% donor plasma from 24 donors. TEER was measured as an indicator of permeability (with higher TEER indicating lower permeability and lower TEER indicating higher permeability). B. There was a significant difference in TEER between the more protective and less protective donors (p<.01). These units were selected for use in the in vivo experiments.
Similarly, syndecan-1 in these donor plasma samples differed, with a significantly higher level in the less protective plasma than in the more protective plasma (38427±1257pg/mL vs. 231±172pg/mL, Welch p<.001).
Cytokine and chemokine profile differs between donors
Levels of twenty different analytes were measured and compared between the more protective and less protective plasma donors. Twelve of the analytes were either undetectable or below the interpretable limits of the assay (TNFα, IL-6, IL-1 β, IFNɣ, CCL4, IL-1α, IL-4, IL-17, IL-2, GM-CSF, IL-5 and G-CSF). Measurements of the eight detectable analytes are shown in Table 1. Levels of IL-8 and IL-10 were not significantly different between the two plasma donors. MCP-1 and IL1-Ra were significantly increased in the less protective plasma while VEGF-A, FGF basic, CXCL5 and TPO were all increased in the more protective plasma.
Table 1:
Cytokine and Chemokine profile of plasma donors
| Less Protective Plasma | More Protective Plasma | p-value (Welch) | |
|---|---|---|---|
| IL-8 | 3.172±1.374 | 3.972±0.164 | 0.420 |
| IL-10 | 7.166±0.31 | 26.572±31.122 | 0.393 |
| MCP-1 | 153.971±3.924 | 121.309±4.525 | 0.001 |
| VEGF-A | 16.648±2.796 | 54.022±0.732 | 0.001 |
| IL-1Ra | 401.450±94.873 | 127.245±1.43 | 0.038 |
| FGF basic | 3.717±0 | 4.266±0.138 | N/A |
| CXCL5 | 66.776±2.992 | 922.711±22.004 | <0.001 |
| TPO | 202.781±0 | 358.564±0 | N/A |
Multiplex assay was used to measure twenty analytes in two donor plasmas. Eight analytes were detectable by this assay. Samples were measured in triplicate and data is reported as mean±SD. Abbreviations: IL=interleukin, MCP=monocyte chemoattractant protein, VEGF=vascular endothelial growth factor, IL-1Ra=interleukin-1 receptor antagonist, FGF=fibroblast growth factor, CXCL5=C-X-C motif ligand 5, TPO=thrombopoeitin.
IN VIVO
No difference in MAP post hemorrhagic shock between donors
Resuscitation with the either the more protective (Donor 10) or less protective plasma (Donor 18) was associated with a significantly higher MAP post hemorrhagic shock compared to mice receiving no resuscitation, however there was no significant difference in the post-resuscitation MAP between the more protective and the less protective plasma groups (Figure 2).
Figure 2: Mean Arterial Pressure (MAP).
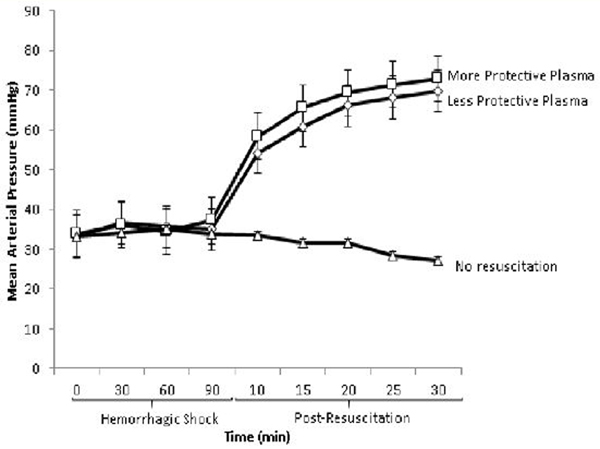
MAP was recorded during the 90 minutes of hemorrhagic shock and for the first 30 minutes of resuscitation. MAP was similar between groups during hemorrhagic shock. Resuscitation with either the more protective or less protective plasma was associated with a significantly higher MAP post hemorrhagic shock compared to mice receiving no resuscitation (p<.05 by two-way ANOVA with Bonferroni post hoc, n=6–7 per group), however there was no significant difference in the post-resuscitation MAP between the more protective and the less protective plasma groups.
Pulmonary injury and inflammation were reduced by the more protective donor plasma
Mice resuscitated with the more protective plasma from Donor 10 demonstrated improved lung injury scores compared with mice resuscitated with the less protective plasma from Donor 18. Despite similar post-resuscitation MAPs, the more protective plasma mitigated lung histopathologic injury compared to the less protective plasma (1.56±0.27 vs. 2.33±0.47, respectively for Donor 10 vs. Donor 18, p=0.005). Mice resuscitated with the less protective plasma from Donor 18 were comparable to mice undergoing no resuscitation (2.33±0.47 vs. 2.39±0.71, p=.869) (Figure 3).
Figure 3: Lung histopathologic injury was reduced by the protective donor plasma.
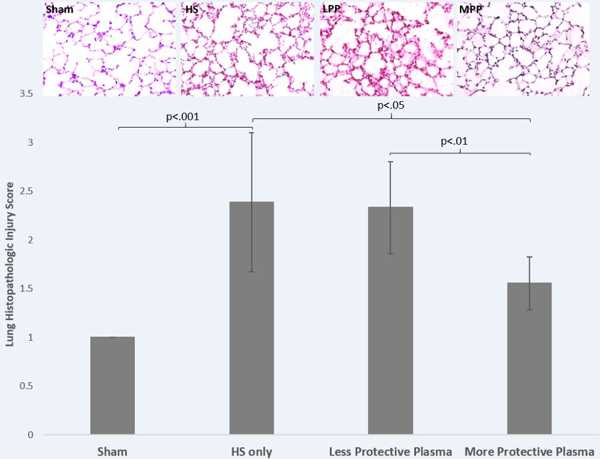
Mice underwent 90 minutes of hemorrhagic shock followed by resuscitation with 1x shed blood of more protective plasma or less protective plasma and compared to mice with no resuscitation (HS only) or sham. Shown are representative images and the corresponding lung injury scores. Data is reported as mean ± SD with n=6–7/group and was analyzed by one-way ANOVA with Bonferroni post hoc. Abbreviations: HS=hemorrhagic shock only/no resuscitation, MPP=more protective plasma, LPP=less protective plasma.
Lung MPO, an indicator of neutrophil influx was significantly different between treatment groups (Welch p=0.001). MPO was increased in the less protective plasma group compared to the more protective plasma group (6.045±1.885 vs. 2.590±0.559, p=0.02)(Figure 4). Similar to lung injury, MPO immunostaining was comparable between less protective plasma and no resuscitation (6.045±1.885 vs. 7.024±3.101, p=0.78).
Figure 4: Pulmonary inflammation was reduced by protective donor plasma.
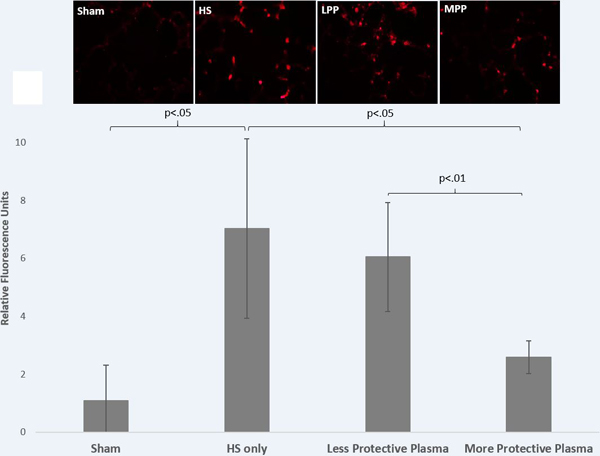
Mice underwent 90 minutes of hemorrhagic shock followed by resuscitation with 1x shed blood of the more protective plasma or the less protective plasma and compared to mice with no resuscitation (HS only) or sham. Myeloperoxidase immunostaining was assessed as an indicator of neutrophil infiltration. Shown are representative images and the corresponding quantitation. Data is reported as mean ± SD with n=6–7/group and was analyzed by one-way ANOVA with Bonferroni post hoc. Abbreviations: HS=hemorrhagic shock only/no resuscitation, MPP=more protective plasma, LPP=less protective plasma.
Pulmonary syndecan-1 immunostaining was increased by the protective donor plasma
Pulmonary syndecan-1 immunostaining was also significantly different between treatment groups (Welch p=0.002). Pulmonary syndecan-1 was significantly increased in the more protective plasma group compared to the less protective plasma group (20.909±8.202 vs. 9.325±3.412, p=0.018) and to the no resuscitation group (9.964±1.635, p=0.034) while the less protective plasma and no resuscitation groups were comparable (9.325±3.412 vs. 9.964±1.635, p=0.9) (Figure 5).
Figure 5: Pulmonary syndecan-1 immunostaining was increased by the protective donor plasma.
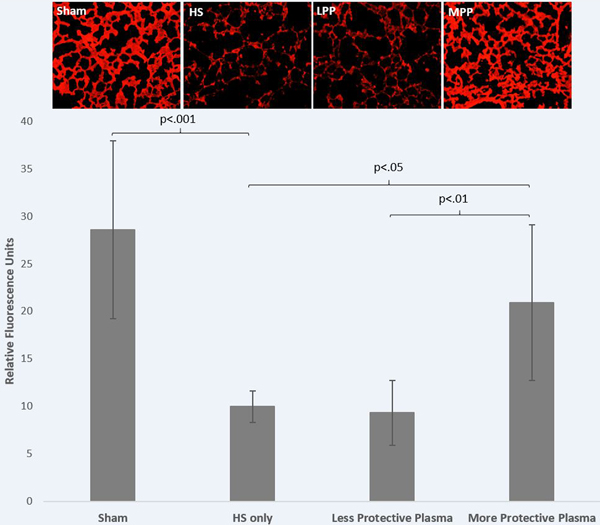
Mice underwent 90 minutes of hemorrhagic shock followed by resuscitation with 1x shed blood of the more protective plasma or the less protective plasma and compared to mice with no resuscitation (HS only) or sham. Pulmonary syndecan-1 immunostaining was assessed. Shown are representative images and the corresponding quantitation. Data is reported as mean±SD with n=6–7/group and was analyzed by one-way ANOVA with Bonferroni post hoc. Abbreviations: HS=hemorrhagic shock only/no resuscitation, MPP=more protective plasma, LPP=less protective plasma.
DISCUSSION
We have previously shown that plasma has potent protective effects on endothelial cell function and vascular barrier integrity in vitro and in vivo following hemorrhagic shock (9,15,21). We now hypothesized there to be interdonor variability among plasma that may modulate these protective effects. We have indeed demonstrated that significant interdonor variability exists in plasma donor units that affects organ specific (lung) functions both in vitro and in vivo. Despite similar mean arterial blood pressure in the early post resuscitation period, interdonor plasma variability was associated with differences in lung histopathologic injury, inflammation and pulmonary syndecan-1 immunostaining. In fact, the less protective plasma (as defined by in vitro testing) was not significantly different than mice subjected to hemorrhagic shock alone with no resuscitation.
Very little is currently understood regarding plasma variability. Reports have indicated that variability may stem from the method used to prepare plasma (e.g., fresh frozen; liquid; solvent detergent-treated; and spray dried, solvent detergent-treated)(22). Evidence in the literature suggests the differences in plasma effects and potency may be dependent on soluble factors present within. Other groups have shown that levels of transforming growth factor beta (TGF-β) and adiponectin in plasma may lead to functional differences in plasma efficacy(23,24). In addition, our novel data suggest that interdonor variability exists between donor units of FFP, a consideration that has not yet been studied. Only one report that we are aware of has described interdonor variability in FFP, which noted only in passing the existence of variations in the levels of tumor necrosis factor alpha (TNFα) and interleukin (IL)-10 (25). Our data did not demonstrate a difference in IL-10 levels between the more protective plasma and less protective plasma and TNFα was unmeasurable in both donor plasmas. Our less protective plasma donor (aged) demonstrated significantly higher levels of monocyte chemotactic protein-1 (MCP-1) and interleukin-1 receptor antagonist (IL-1Ra). MCP-1 is a potent chemotactic factor for monocytes/macrophages to sites of inflammation while IL-1Ra exerts its anti-inflammatory activity by blocking IL-1. In the setting of trauma, MCP-1 has been shown to be positively correlated with development of nosocomial infection, trauma-associated sepsis, multiple organ dysfunction (MOD) and increased ICU length of stay(26,27). IL-1Ra has also been shown to be elevated in burn patients who develop sepsis and trauma patients who develop MOD (28,29).
The more protective plasma donor (young) had higher levels of vascular endothelial growth factor A (VEGF-A), fibroblast growth factor (FGF) basic, CXCL5 and thrombopoietin (TPO). The role of VEGF-A is complex and not well understood. VEGF-A is a known inducer of endothelial cell permeability in vitro and circulating VEGF is increased in patients after burn and trauma, however severe complications after these insults are associated with lesser increases in serum VEGF(30). FGF basic is a heparan sulfate growth factor expressed in a number of cell types including endothelial cells. It has been shown to potentiate VE-cadherin stability in vitro and is upregulated in the lung following intestinal ischemia and reperfusion in vivo, suggesting that higher levels in donor plasma may confer protection to endothelial cells after hemorrhagic shock (31,32). CXCL5 is a chemotactic factor that is a member of the CXC subfamily of chemokines. It is expressed in alveolar epithelial cells and has been shown to recruit neutrophils during lipopolysaccharide induced lung inflammation in mice (33). TPO is the principal hematopoietic cytokine that stimulates thrombopoiesis. Increased TPO levels may enhance platelet activation during burn injury and sepsis and contribute to increased platelet volume and count after acute blood loss (34,35). While these cytokines and chemokines are altered in patients with trauma, burns, and sepsis, the plasma used in the current experiments was from healthy donors meeting standard criteria for blood donation. Extrapolating the relationships and functions of these factors from studies of critically ill patients may not be appropriate. Based on these results, it is likely that the increased efficacy of the more protective plasma is conferred by factors not measured in this study or complex feedback and feed-forward relationships between cytokines that are beyond the scope of these experiments.
There were also differences in syndecan-1 between donors. We hypothesize that the less protective plasma donor may possess varying degrees of stress and/or inflammation associated with circulating pro-inflammatory factors, which may be reflected by circulating syndecan-1. In fact, elevations in circulating MCP-1 (as seen in the less protective plasma) are associated with diabetes, Alzheimer’s disease and obesity as well as a number of other inflammatory conditions (36–38). Older age, obesity, and atherosclerosis are all known pro-inflammatory conditions that could be responsible for the observed results(39–42). Our data in fact suggest that age may be an important factor as donors in the more protective plasma group were young (ages 18 and 19) while donors in the less protective plasma group were older (ages 65 and 71).
The clinical implications of the current results are unknown. Given that several large randomized clinical trials have shown the beneficial effects of plasma, it seems likely that the majority of plasma conveys protective effects to patients in hemorrhagic shock(3,5,6,43). The benefit of plasma in such studies is primarily attributable to its hemostatic property, which is unlikely to differ significantly between donors. The pulmonary effects of interdonor variability in patients are less clear since the exact mechanism of action of plasma on these endpoints has yet to be defined. Based on our findings in vitro, most donor units demonstrated comparable changes in permeability. Thus, although only a small percentage of plasma units would likely be less protective, this may have important functional and safety implications for the recipient. Development of a high throughput screening method could potentially allow blood banks to screen plasma units to identify potentially less protective donor units, thereby increasing the safety profile and improving the risk/benefit ratio of plasma transfusion for patients. The current study demonstrated significant differences between the more protective and less protective units in the levels of circulating syndecan-1 as well as other cytokines. Syndecan-1 or other factors that modulate inflammation may be potential biomarkers with which to identify less protective plasma units. The mechanism for the identified variability warrants further study.
There are several limitations to the current study. We have demonstrated interdonor variability in vitro and in vivo in a limited number of donor plasma units. However, results of our two independent sets of experiments with different donors showed similar results. We also used human plasma in a murine model of hemorrhagic shock. The use of human plasma in mice allows us to study the clinical product used in humans, which we believe to be of important translational benefit, although xeno-incompatibility is a possible confounder. However, we have previously shown a lack of species-specific differences in pulmonary indices following hemorrhagic shock(20). Additionally, while our model of trauma/hemorrhagic shock does result in coagulopathy(44), we did not assess interdonor variability in correcting coagulopathy in the current study and instead focused on endothelial function and inflammation. Lastly, we only examined effects of plasma in lungs, as the lungs are the most frequently injured organ after trauma(45). Effects of variability in other organs are not known.
In conclusion, donor variability among plasma units was demonstrated in an in vitro assay and this variability was then further validated in an in vivo model of trauma and hemorrhagic shock. We have demonstrated that variability among plasma donors affects its ability to modulate lung histopathologic injury, inflammation and pulmonary syndecan-1 expression. Elevated levels of syndecan-1 in donor plasma may allow us to identify those units of plasma that may be less protective to patients undergoing resuscitation. This variability may have important implications for patient safety and clinical outcomes.
Supplementary Material
Acknowledgments
This work was presented at the 49th Annual Meeting of the Western Trauma Association, March 03–08, 2019 in Snowmass, Colorado
This manuscript was funded in part by the National Institutes of Health grant RO1GM107482 and Department of Defense grant W81XWH-17–2-0054
Footnotes
Study type: Basic science
Level of Evidence: not applicable
The authors have no conflicts of interest
REFERENCES
- 1.Kauvar DS, Lefering R, Wade CE. Impact of Hemorrhage on Trauma Outcome: An Overview of Epidemiology, Clinical Presentations, and Therapeutic Considerations. J Trauma Inj Infect Crit Care. 2006. June;60(Supplement):S3–11. [DOI] [PubMed] [Google Scholar]
- 2.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011). J Trauma Acute Care Surg. 2012. December;73(6):S431–7. [DOI] [PubMed] [Google Scholar]
- 3.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008. September;248(3):447–58. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, Tilley BC, Baraniuk S, Fox EE, Wade CE, Podbielski JM, del Junco DJ, Brasel KJ, Bulger EM, Callcut RA, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015. February 3;313(5):471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) Study. JAMA Surg. 2013. February 1;148(2):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, Adams PW, Daley BJ, Miller RS, Harbrecht BG, et al. Prehospital Plasma during Air Medical Transport in Trauma Patients at Risk for Hemorrhagic Shock. N Engl J Med. 2018. July 26;379(4):315–26. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, Butler FK, Cap AP, Cohen MJ, Davenport R, et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: definitions, current practice, and knowledge gaps. Shock. 2014. May;41 Suppl 1(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wataha K, Menge T, Deng X, Shah A, Bode A, Holcomb JB, Potter D, Kozar R, Spinella PC, Pati S. Spray-dried plasma and fresh frozen plasma modulate permeability and inflammation in vitro in vascular endothelial cells. Transfusion. 2013. January;53 Suppl 1(Suppl 1):80S–90S. [DOI] [PubMed] [Google Scholar]
- 9.Pati S, Peng Z, Wataha K, Miyazawa B, Potter DR, Kozar RA. Lyophilized plasma attenuates vascular permeability, inflammation and lung injury in hemorrhagic shock Raju R, editor. PLoS One. 2018. February 2;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potter DR, Baimukanova G, Keating SM, Deng X, Chu JA, Gibb SL, Peng Z, Muench MO, Fomin ME, Spinella PC, et al. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma Acute Care Surg. 2015. June;78:S7–17. [DOI] [PubMed] [Google Scholar]
- 11.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang W, Zaske AM, Menge T, Kozar RA. Modulation of Syndecan-1 Shedding after Hemorrhagic Shock and Resuscitation McNeil P, editor. PLoS One 2011. August 19;6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A High Admission Syndecan-1 Level, A Marker of Endothelial Glycocalyx Degradation, Is Associated With Inflammation, Protein C Depletion, Fibrinolysis, and Increased Mortality in Trauma Patients. Ann Surg. 2011. August;254(2):194–200. [DOI] [PubMed] [Google Scholar]
- 13.Wu F, Peng Z, Park PW, Kozar RA. Loss of Syndecan-1 Abrogates the Pulmonary Protective Phenotype Induced by Plasma After Hemorrhagic Shock. Shock. 2017. September;48(3):340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, Baer LA, Tomasek JS, Henriksen HH, Stensballe J, Cotton BA, Holcomb JB, Johansson PI, et al. Syndecan-1: A Quantitative Marker for the Endotheliopathy of Trauma. J Am Coll Surg. 2017. September;225(3):419–27. [DOI] [PubMed] [Google Scholar]
- 15.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma Restoration of Endothelial Glycocalyx in a Rodent Model of Hemorrhagic Shock. Anesth Analg. 2011. June;112(6):1289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. NIH Public Access; 2012. May;52 Suppl 1(Suppl 1):65S–79S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saadah NH, van Hout FMA, Schipperus MR, le Cessie S, Middelburg RA, Wiersum-Osselton JC, van der Bom JG. Comparing transfusion reaction rates for various plasma types: a systematic review and meta-analysis/regression. Transfusion. 2017. September;57(9):2104–14. [DOI] [PubMed] [Google Scholar]
- 18.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal Ischemia-Reperfusion Injury Is Lectin Complement Pathway Dependent without Involving C1q. J Immunol. 2005. May 15;174(10):6373–80. [DOI] [PubMed] [Google Scholar]
- 20.Peng Z, Pati S, Fontaine MJ, Hall K, Herrera A V., Kozar RA. Lack of species-specific difference in pulmonary function when using mouse versus human plasma in a mouse model of hemorrhagic shock. J Trauma Acute Care Surg. 2016. November;81(5):S171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pati S, Matijevic N, Doursout M-F, Ko T, Cao Y, Deng X, Kozar RA, Hartwell E, Conyers J, Holcomb JB. Protective Effects of Fresh Frozen Plasma on Vascular Endothelial Permeability, Coagulation, and Resuscitation After Hemorrhagic Shock Are Time Dependent and Diminish Between Days 0 and 5 After Thaw. J Trauma Inj Infect Crit Care. 2010. July;69(Supplement):S55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinella PC, Frazier E, Pidcoke HF, Dietzen DJ, Pati S, Gorkun O, Aden JK, Norris PJ, Cap AP. All plasma products are not created equal. J Trauma Acute Care Surg. 2015. June;78(6):S18–25. [DOI] [PubMed] [Google Scholar]
- 23.Deng X, Cao Y, Huby MP, Duan C, Baer L, Peng Z, Kozar RA, Doursout M-F, Holcomb JB, Wade CE, et al. Adiponectin in Fresh Frozen Plasma Contributes to Restoration of Vascular Barrier Function After Hemorrhagic Shock. Shock. 2016. January;45(1):50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan C, Cao Y, Deng X, Wang W, Yang W, Liu X, Chen Z, Pati S, Kozar RA, Gonzalez EA, et al. Increased Transforming Growth Factor β Contributes to Deterioration of Refrigerated Fresh Frozen Plasma’s Effects In Vitro on Endothelial Cells. Shock. 2011. July;36(1):54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theusinger OM, Baulig W, Seifert B, Emmert MY, Spahn DR, Asmis LM. Relative concentrations of haemostatic factors and cytokines in solvent/detergent-treated and fresh-frozen plasma. Br J Anaesth. 2011. April;106(4):505–11. [DOI] [PubMed] [Google Scholar]
- 26.Lamparello AJ, Namas RA, Abdul-Malak O, Vodovotz Y, Billiar TR. Young and Aged Blunt Trauma Patients Display Major Differences in Circulating Inflammatory Mediator Profiles after Severe Injury. J Am Coll Surg. 2019. February;228(2):148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Liu Q, Liu T, Zheng Q, Xu X, Liu X, Gao W, Li Z, Bai X. Early plasma monocyte chemoattractant protein 1 predicts the development of sepsis in trauma patients. Medicine (Baltimore). 2018. April;97(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaski Greg E., Metzger Cameron, Tyler McCarroll Robert Wessel, Adler Jeremy, Cutshall Andrew, Brown Krista, MS Yoram Vodovotz, Billiar Timothy R., Todd O. McKinley M. Early Immunologic Response in Multiply Injured Patients With Orthopaedic Injuries Is Associated With Organ Dysfunction. J Orthop Trauma. 2019;33(5):220–8. [DOI] [PubMed] [Google Scholar]
- 29.Endo S, Inada K, Yamada Y, Kasai T, Takakuwa T, Nakae H, Kamei Y, Shimamura T, Suzuki T, Taniguchi S, et al. Plasma levels of interleukin-1 receptor antagonist (IL-1ra) and severity of illness in patients with burns. J Med. 1996;27(1–2):57–71. [PubMed] [Google Scholar]
- 30.Grad S, Ertel W, Keel M, Infanger M, Vonderschmitt DJ, Maly FE. Strongly Enhanced Serum Levels of Vascular Endothelial Growth Factor (VEGF) after Poly-trauma and Burn. Clin Chem Lab Med. 1998. January 1;36(6). [DOI] [PubMed] [Google Scholar]
- 31.Hatanaka K, Lanahan AA, Murakami M, Simons M. Fibroblast Growth Factor Signaling Potentiates VE-Cadherin Stability at Adherens Junctions by Regulating SHP2 Ushio-Fukai M, editor. PLoS One. 2012. May 22;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu XB. Effect of intestinal ischemia-reperfusion on expressions of endogenous basic fibroblast growth factor and transforming growth factor β in lung and its relation with lung repair. World J Gastroenterol. 2000;6(3):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiedermann FJ, Mayr AJ, Kaneider NC, Fuchs D, Mutz NJ, Schobersberger W. Alveolar Granulocyte Colony-Stimulating Factor and α-Chemokines in Relation to Serum Levels, Pulmonary Neutrophilia, and Severity of Lung Injury in ARDS. Chest. 2004. January;125(1):212–9. [DOI] [PubMed] [Google Scholar]
- 34.Hobisch-Hagen P, Jelkmann W, Mayr A, Wiedermann F, Fries D, Herold M, Klingler A, Schobersberger W. Low platelet count and elevated serum thrombopoietin after severe trauma. Eur J Haematol. 2000. March;64(3):157–63. [DOI] [PubMed] [Google Scholar]
- 35.Lupia E, Bosco O, Mariano F, Dondi AE, Goffi A, Spatola T, Cuccurullo A, Tizzani P, Brondino G, Stella M, et al. Elevated thrombopoietin in plasma of burned patients without and with sepsis enhances platelet activation. J Thromb Haemost. 2009. June;7(6):1000–8. [DOI] [PubMed] [Google Scholar]
- 36.Lee W-J, Liao Y- C, Wang Y-F, Lin I-F, Wang S- J, Fuh J- L. Plasma MCP-1 and Cognitive Decline in Patients with Alzheimer’s Disease and Mild Cognitive Impairment: A Two-year Follow-up Study. Sci Rep. 2018. December 19;8(1):1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim C-S, Park H- S, Kawada T, Kim J- H, Lim D, Hubbard NE, Kwon B- S, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes. 2006. September 14;30(9):1347–55. [DOI] [PubMed] [Google Scholar]
- 38.Zineh I, Beitelshees AL, Silverstein JH, Haller MJ. Serum Monocyte Chemoattractant Protein-1 Concentrations Associate With Diabetes Status but Not Arterial Stiffness in Children With Type 1 Diabetes. Diabetes Care. 2009. March 1;32(3):465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front Immunol. 2018. April 9;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trevethan Cravioto S. Inflammation in atherosclerosis. Arch Cardiol Mex. 2012. September;73 Suppl 1(9):S141–5. [PubMed] [Google Scholar]
- 41.Rocha VZ, Libby P. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol. 2009. June 28;6(6):399–409. [DOI] [PubMed] [Google Scholar]
- 42.Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995. May 1;91(9):2488–96. [DOI] [PubMed] [Google Scholar]
- 43.Baraniuk S, Tilley BC, Del Junco DJ, Fox EE, Van Belle G, Wade CE, Podbielski JM, Beeler AM, Hess JR, Bulger EM, et al. Pragmatic Randomized Optimal Platelet and Plasma Ratios (PROPPR) Trial: Design, rationale and implementation. Injury. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, Frith D, Pittet J-F, Cohen MJ. Increase in Activated Protein C Mediates Acute Traumatic Coagulopathy in Mice. Shock. 2009. December;32(6):659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, Maier R V., Burlew CC. Temporal trends of postinjury multiple-organ failure: Still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76(3):582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


