Abstract
Dual Energy X-ray Absorptiometry (DXA) is a vital tool for assessing bone health in patients at risk for fragility fractures. In pediatric patients, this technology is used in conjunction with clinical fracture history to diagnosis osteoporosis and monitor treatment response. Childhood and adolescence is characterized by linear growth and bone mass accrual; thus there are important differences in the interpretation of bone measurements obtained by DXA in these young patients. This review aims to explore the current indications for DXA use and interpretation of DXA in the pediatric age group using currently available reference databases. Limitations of DXA in pediatric patients, specifically in children with short stature, will be explored. We will review several pathophysiologic mechanisms that may lead to low bone density in children, discussing representative diseases and the recommendations for monitoring bone health with DXA in these conditions. Finally, we will highlight new methods by which DXA imaging can gather additional information on bone health in children and may improve our ability to predict fractures and osteoporosis.
Keywords: Dual Energy X-ray Absorptiometry, Child, Pediatrics
1. Introduction
Over the last 30 years, Dual Energy X-ray Absorptiometry (DXA) has emerged as a valuable tool for evaluating bone health and fracture risk in adults. For pediatric patients, the utility of this measurement technique has increased substantially over the last 10–15 years as age appropriate reference data from healthy children have become available [1–9]. There has also been a significant increase in the published literature on the natural history of DXA derived bone mineral density (BMD) in a variety of pediatric chronic diseases. In conjunction with fracture history, DXA results may be used to make a diagnosis of osteoporosis in children and adolescents [10].
This review aims to explore the current indications for DXA use, interpretation of DXA in the pediatric age group using current reference databases, the utility of DXA for prediction of both fractures and osteoporosis risk, and future directions for this field.
2. DXA use in pediatric patients
2.1. DXA measures
DXA measurements generate information about bone mass (bone mineral content, BMC, measured in grams) and the projected area of bone (measured in cm2). This information is then used to calculate an areal BMD (aBMD, g/cm2). DXA scans are unable to measure bone depth. Therefore, aBMD estimates can be falsely low in short patients with smaller bones or falsely elevated in tall patients with larger bones. How to account for this important factor in children who are growing at varying rates will be discussed in more detail later in this review. Debate continues over which DXA outcome is optimal to understand the bone health of pediatric patients. Both BMC and aBMD have been associated with fracture risk [11]. However, BMC is not influenced by size as significantly as BMD and some experts thus consider this measure to be more reliable in pediatric patients [4]. On the other hand, data from the Bone Mineral Density in Childhood Study found aBMD to be more precise across all pediatric age ranges and reference data for aBMD are most accessible for clinicians at the present time [12].
2.2. Scan sites
The resolution capabilities of DXA allow only total aBMD to be measured. Estimates of compartmental (trabecular vs. cortical) aBMD are inferred by measuring specific sites. The lumbar spine is comprised of primarily trabecular bone. Therefore, aBMD measures obtained at this site provide information on the trabecular compartment, whereas measurements of primarily cortical sites (1/3 distal radius, femoral neck, or whole body) are used to make inferences about cortical bone density. In children, the recommended scanning sites are the anterior-posterior lumbar spine and the total body less head (TBLH) [10]. The TBLH is derived from the whole body scan and as >80% of the skeleton is comprised of cortical bone, the TBLH skeletal site provides an assessment of the cortical compartment. Exclusion of the head for skeletal outcomes is recommended for several reasons [13]. First, the skull comprises a relatively large portion of the skeleton. Second, bone mineralization at the skull is not affected by nutritional or environmental factors such as weight-bearing activity that impact BMD throughout the rest of the body. Finally, skull fractures do not represent true osteoporotic fractures. Thus, the recommendation is to not include this region in BMD measurements [10]. Reference ranges for the TBLH are available for children and adolescents ages 5–20 years [6]. In addition, this skeletal site provides additional information on body composition with measures of lean mass and fat mass that may be helpful in evaluating children with chronic conditions. Reference ranges are available for the lumbar spine [8,14,15]. Both of these sites are highly reproducible in children with co-efficients of variation similar to that in adults [12].
2.3. Other skeletal sites
In adults, the femoral neck (FN) is a recommended scanning site. In growing children, concerns arise regarding the reproducibility of DXA results at any region of the hip due to variations in the development of skeletal landmarks which lead to difficulty in ensuring proper positioning [16]. However, in later adolescence, this issue may not be as significant and this skeletal site may be particularly useful in older children who are likely to continue to have bone mineralization deficits into adulthood [12]. The 1/3 distal radius is a site of primarily cortical bone whereas the ultradistal radius is primarily trabecular bone. DXA scans at these sites have been used in certain pediatric populations when TBLH and lumbar spine are not feasible, for example in children with spinal rods or severe obesity. Unfortunately, scans at this site have the poorest precision compared to other measurement sites and reference data are limited [6,8]. Finally, DXA scans of the lateral distal femur (LDF) have been helpful in evaluating aBMD of non-ambulatory patients [17,18]. Results for this site are generated using DXA software for the distal radius, thus additional technologist training is required [19]. References ranges are also available only for Hologic scanners [20,21].
2.4. Reliability and validity
As with any test, a BMD result is only as good as the scanner from which it was generated. It is important that the clinician be familiar with the precision or reproducibility of the BMD results generated for their particular DXA scanner. Precision errors of the machine are reported to be <1% [22]. However, additional error can be introduced with variation in patient positioning and motion artifacts [23,24]. Technologists should receive periodic reviews to ensure standardization of scan acquisition and analysis technique. Importantly, clinicians need to have a good understanding of the least significant change (LSC) value for their machine. This value represents the precision error for the measurement and is especially important in the interpretation of serial scans. As discussed earlier, children are growing and acquiring new bone. Therefore, BMD is expected to change over time. Whether the change represents a true increase or decrease in BMD, or is considered to be clinically relevant, depends on the interpretation of the measure in light of the machine’s LSC.
2.5. Frequency of scanning
While radiation exposure from a DXA scan is minimal (0.1–6 μSv; less than a typical chest X-ray), the risk is not negligible especially if scans are obtained too frequently. The recommended minimum interval between any DXA scans should be 6–12 months [25].
3. Use of DXA in the healthy pediatric population
DXA is the most commonly used densitometric technique for children throughout the world [26] and is the only recommended modality for clinical assessment of bone density. When ordering a DXA scan for a child or adolescent, the clinician must consider many factors including fracture history, family history, associated risk factors, and how results would influence patient care [27]. Guidelines have been set forth by the Pediatric Position Development Conference (PDC) of the International Society for Clinical Densitometry (ISCD). However, many of these recommendations are based on expert consensus as there is limited scientific evidence to help guide clinicians in the use of DXA in pediatric populations [10].
In children and adolescents without chronic disease, indications for obtaining a DXA scan to assess bone density include a clinically significant fracture history or apparent “osteopenia” on a standard radiograph. A clinically significant fracture history was defined by the PDC to include two or more fractures of long bones before ten years of age, or three or more long-bone fractures before 19 years of age [25]. These criteria do not include stress fractures, which occur as overuse injuries. Consideration of the mechanism of injury and level of trauma should be made for each fracture incident, as high-energy trauma fractures (e.g., road traffic accidents, falls from >3 m, blunt trauma, sports-related injuries) should be excluded when considering fracture history. With a wide degree of variation and complexity in mechanisms of falls, blunt trauma, and twists, clinical judgment is needed to discern which fractures can be excluded, conservatively, including questionable fractures. While consensus from the PDC agreed that a non-traumatic vertebral compression fracture was sufficient to make the diagnosis of osteoporosis in children regardless of aBMD Z-score, some clinicians obtain a DXA scan in this clinical scenario as a baseline for future comparison.
There is not sufficient evidence to recommend DXA measurements for pediatric patients who have recurrent fractures of the digits or phalanges, those receiving depot medroxyprogesterone acetate [28] or anticonvulsants [29], or those with low BMD (known by radiograph or other incidental finding), but no history of significant fracture. However, in conjunction with other risk factors such as a family history of hip fractures, or poor nutrition affecting bone, the clinical decision can change.
In otherwise healthy children, obtaining a DXA scan must be carried out with the goal to reduce future fracture risk by identifying patients who would benefit from interventional therapies and treatments. Case control studies have found that children with forearm fractures had lower aBMD compared to those who did not fracture and odds of fracture increased 28–41% for every 1 standard deviation (SD) decrease in BMD as derived by DXA [30]. Data on the utility of DXA measures to predict incident fractures in otherwise healthy children are limited. One such prospective study found an 89% increased risk of fracture for every 1 SD decrease in BMC [11]. Both BMC and aBMD measured at the total body and lumbar spine were significantly associated with increased risk for an upper extremity fracture (hazard ratios: 1.53–2.47) [31]. While any fracture may have clinical significance, these studies were primarily interested in the most common fracture of childhood, the forearm fracture. The ability of DXA scans in otherwise healthy children to predict the occurrence of osteoporotic fractures (low trauma, fragility fractures or vertebral compression fractures) in adulthood is unknown and likely will remain so due to feasibility issues in conducting such a study.
4. Indications for DXA in pediatric patients with chronic diseases
Apart from healthy children and adolescents with significant fracture histories, DXA has an important role in monitoring and managing treatment of children and adolescents with chronic disease. Recommendations for pediatric use of DXA have been published by numerous academic organizations for various chronic diseases. We aim to summarize those findings below.
Chronic diseases can affect bone mineralization by impairing bone formation, increasing bone resorption, or both [32–34] In addition, many diseases are associated with impaired linear growth, delayed puberty, and/or poor nutrition. In assessing the bone health of children with chronic disease, it is important to consider the underlying disease state. Herein, we discuss several chronic medical conditions known to have an impact on bone health categorized by prominent mechanism: primary bone disease, inflammatory diseases, glucocorticoid exposure, hormone deficiency, inadequate nutrition or nutrient malabsorption, and immobility. Fig. 1 describes the many pediatric chronic diseases that have been found to affect bone health and this patient group continues to expand. Therefore, this report includes a selection of commonly encountered diseases where DXA may aid in the management and treatment of the pediatric patient. Recommendations for DXA use in each category, as well as specific recommendations for selected diseases within categories, will be discussed. As this report is not exhaustive, clinicians are encouraged to use judgment for their individual pediatric patients.
Fig. 1.
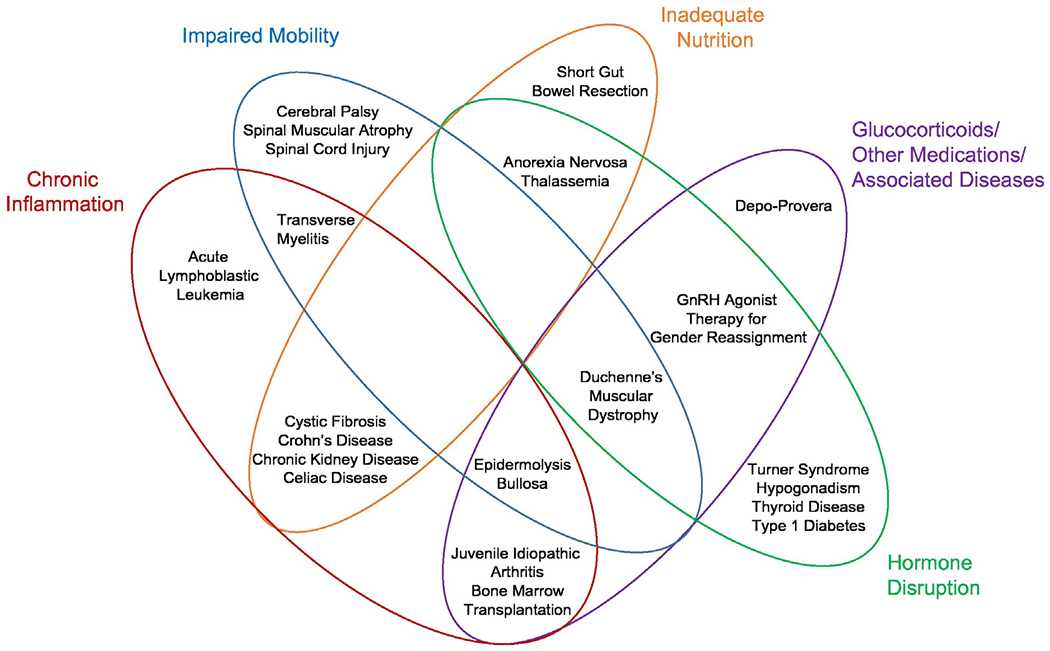
The multifactorial causes of impaired bone mineralization are shown which may occur in a variety of pediatric chronic illnesses. This figure is not meant to be exhaustive, but to encourage clinicians to think of overlapping mechanisms impacting bone formation and resorption during childhood and adolescence. Key pathophysiologic mechanisms are highlighted.
For all children or adolescents with primary or secondary bone disease (or those at risk), DXA scans should be obtained only when the results will influence the management of the patient or the use of available interventions [25]. When no intervention is available, or a given intervention would be implemented regardless of DXA results, DXA measurements are not recommended.
4.1. Primary bone disease
In pediatric primary bone diseases, of which osteogenesis imperfecta (OI) is the most common and extensively studied, DXA scans are utilized for monitoring disease progression and the effect of treatment. A baseline DXA scan is recommended at the start of any treatment, and then periodically for follow-up [35]. In patients with OI, those with Type I OI are expected to benefit the most from use of DXA as it is informative in making the diagnosis, as well as aiding in treatment and management decisions [36].
4.2. Inflammatory diseases (examples: inflammatory bowel disease, rheumatoid arthritis, cystic fibrosis)
Chronic inflammation is associated with bone disease as the inflammatory cascade (e.g., interleukin-6 or IL-6, tumor necrosis factor-α) leads to osteoclastogenesis and increased bone resorption via activation of RANK ligand [37]. Low aBMD has been reported in numerous chronic inflammatory conditions including inflammatory bowel disease (IBD) and rheumatoid arthritis. At the time of diagnosis, pediatric patients with inflammatory bowel disease (IBD) had lower aBMD Z-scores and the risk for low aBMD was positively associated with BMI [38,39] and negatively with IL-6 activity [39]. Treatment of IBD results in increased bone formation. However, these patients often fail to achieve peak bone mass during puberty [40]. Low aBMD at the lumbar spine was associated with low body mass index (BMI) and increased cumulative glucocorticoid exposure, important confounding factors to consider [39, 41 ]. Vertebral compression fractures have been reported in pediatric patients with IBD even before treatment [39,41,42]; however, these studies came from an era before biologic and immune modulating therapies were first-line. Other studies suggest that patients with IBD are not at increased risk for fracture [43,44]. There are no known studies that associate aBMD with fracture risk in pediatric IBD patients. Expert consensus guidelines recommend obtaining DXA bone density measures at presentation in pediatric patients with IBD and continued monitoring via repeat scans every 1 to 2 years [45]. Treatment of IBD with biologics that target the inflammatory cascade show promising effects on bone turnover and bone density [46]. As these therapeutics become the mainstay of treatment for inflammatory conditions, it may be that patients are less likely to suffer impaired bone mineralization as a sequela of their underlying disease.
4.3. Glucocorticoid use
Glucocorticoids are used to treat many chronic diseases, including IBD, juvenile idiopathic arthritis, cystic fibrosis, asthma, and Duchenne muscular dystrophy (DMD) [47]. Long-term glucocorticoid use has been associated with low BMD, as well as increased fracture risk in adults [48]. Studies in children are limited, but one study found children exposed to more than four rounds of glucocorticoid therapy had an increased risk of fracture [49]. Vertebral fractures occur in pediatric patients receiving higher doses (>0.5 mg/kg/day) of glucocorticoids at a prevalence rate of about 6% [50]. Spine aBMD is significantly lower in children on chronic glucocorticoid therapy compared to healthy children, but the confounding effects of underlying disease are unknown [51].
Specific recommendations for DXA monitoring in pediatric patients receiving glucocorticoid treatment varies by underlying condition and duration of use. Liu et al. recommend obtaining baseline DXA measures for children who will be receiving > 3 months of glucocorticoid therapy [52]. Follow-up screening is recommended for 6 to 12 months after initiation of therapy. The American College of Rheumatology has recommended monitoring of all children and adolescents receiving glucocorticoids for a duration of 3 months or longer [53]. Interpretation of DXA results in children on chronic glucocorticoids is challenging as these agents are known to also impair linear growth and delay puberty. The considerations in interpretation of these results will be discussed in a later section.
4.4. Hormone deficiencies secondary to oncology treatments
The Children’s Oncology Group recommends baseline aBMD screening by DXA by 18 years of age for childhood cancer survivors, or 2 years after the completion of therapy for patients who received treatments known to affect BMD [54]. These treatments included methotrexate, glucocorticoids, or hematopoetic cell transplantation [55]. No follow-up scans are necessary if bone density is found to be normal, unless there are other clinical indications such as a non-traumatic fracture [56]. Cranial radiation exceeding 24 Gy/year is known to have deleterious effects on bone, and aBMD is lower in childhood cancer survivors compared to healthy controls [57,58]. However, this amount of cranial radiation is known to increase the risk of pituitary hormonal deficiency, including growth hormone and gonadotropin deficiency. Few studies have been able to examine the effects of radiation alone on bone.
In the setting of childhood cancer, the etiology of low aBMD is multifactorial and includes consideration of treatment exposures, nutritional status, limited physical activity, hormone disruption, and chronic inflammation [59,60]. As new treatments for childhood cancer are frequently being introduced, we encourage clinicians to consider the implications of each treatment on bone health. DXA screening for pediatric patients on these or other agents with unknown effects on bone may be warranted.
4.5. Malnutrition & malabsorption
Malnutrition can be a consequence of many chronic conditions in pediatric patients. Malnutrition does not simply mean inadequate total caloric intake for energy demands or being underweight, but also applies to insufficient intake of calcium, vitamin D and/or protein. Anorexia nervosa, an eating disorder with a high prevalence in adolescents, is an example of malnutrition in the absence of other disease, with features that may also apply to other conditions, especially those diseases that are accompanied by malnutrition. Low aBMD has been found in patients with anorexia nervosa, [61,62], but only one study has correlated aBMD with fractures [63]. The American College of Radiology currently recommends DXA use in girls and adolescents with anorexia nervosa or other eating disorders [64]. Females with a restrictive eating disorder and a history of hypothalamic amenorrhea are at increased risk for low bone density as low lean body mass was a significant predictor of bone loss, independent of the effects of estrogen [65]. In the context of other diseases, malnutrition can affect both males and females and should be considered among other risk factors as an indication for DXA screening measurements of bone density.
Malabsorption of nutrients is associated with IBD, chronic kidney disease, cystic fibrosis, and other conditions, often in combination with other risk factors, such as glucocorticoid use. In short bowel syndrome, a model of malabsorption alone, decreased bone mineral content and low bone mass are often seen [66].
4.6. Immobility
Weight-bearing physical activity is vital for bone formation [67,68], and conversely, inactivity can lead to weakened bones and low BMD [69]. In children and adolescents with paralysis, muscular dystrophy, or cerebral palsy among other disease associated with impaired mobility, this is of particular concern. Many of these diseases include concurrent risk factors for bone health, such as malnutrition and growth hormone deficiency, and all risk factors must be evaluated together.
Cerebral palsy (CP), a group of permanent movement disorders of varying degrees, highlights the effect of limited mobility in pediatric patients. BMD is frequently low in these children [70] and correlates with ambulatory status [71]. Fracture risk is higher among children with CP, specifically at the distal femur [72]. DXA scans of the distal femur have been shown to predict fracture risk in children with CP and are thus recommended for monitoring bone health in these patients [18,73], particularly in patients undergoing any intervention for improvement of BMD, such as weight-bearing activities or bisphosphonate treatment.
In summary, there are several important mechanisms by which chronic disease in childhood can have significant bone health effects. We have highlighted categories of diseases where DXA measurements of bone density and body composition may be indicated. While DXA monitoring may be useful to identify which children with a given chronic disease are at risk for poor bone health and fracture, clinicians need to be cognizant of the limitations to DXA use in these populations including improper positioning due to contractures or invalid results due to instrumentation (e.g., spinal rods, feeding tubes). In addition, while radiation exposure from a DXA scan is low, these children often undergo many other imaging procedures in the course of their disease thus one must be aware of the potential cumulative radiation exposure. Using these examples as guidelines, and with good clinical judgment, clinicians can assess the mechanism of a disease to determine whether a patient’s bone health is at risk, and ordering DXA scans is indicated.
5. Interpretation of pediatric DXA scans
5.1. Nomenclature for DXA reports
Interpreting DXA scans in children and adolescents is a complex process [74]. Children are growing and thus mineral is being accrued as the bones increase in length and width. The bone map that is generated at the time of a DXA appointment is constantly enlarging and changing shape, consistent with normal growth and development. As a result, however, the data can be more difficult to interpret, compared to similar measures in adults. BMD increases well into the second and even third decade of life when peak bone mass is achieved [75]. In adults, a T-score is reported which compares the patient’s aBMD to that of healthy young adults ages 20–30 years, representing a time when peak bone mass is reached. In children, it is inappropriate to use T-scores as peak bone mass has yet to occur, even though scanners often generate them. Instead, a comparison to an age, sex, and race matched reference range or Z-score is recommended. Through the work of the Bone Mineral Density in Childhood Study and others, these references ranges have been developed [1,3,5–8]. Due to differences in scanning protocols and varying projectional errors between machine manufacturers, crosscalibrations to allow conversion of DXA data obtained from one machine manufacturer to another has not been successful [9]. Therefore, the current recommendations are to obtain longitudinal scans on the same type of DXA machine and follow the manufacturers algorithm for calibration with software updates.
When the child’s aBMD Z-score is ≤−2.0, the appropriate terminology is “low bone mass or bone mineral density for age”. As discussed previously, DXA Z-scores alone cannot be used to diagnosis osteoporosis in children and thus this terminology should not appear in the pediatric report without clear evidence of skeletal fragility in the child’s history. Similarly, the term “osteopenia” is commonly used in adults to describe an aBMD T-score between −1.0 and −2.5. However, it is never appropriate to use this term to describe a pediatric bone density [10].
5.2. Adjustments for stature
The effects of linear growth and pubertal development on the skeleton must be considered when evaluating BMD. Many chronic diseases or the treatments for these diseases result in poor growth and delayed puberty. When DXA scans are ordered in children with these conditions, the standard Z-score only considers the age and sex of the child. Because BMC and aBMD are highly influenced by bone size, short children may appear to have low aBMD Z-scores if an additional adjustment for their height or pubertal status is not made [76,77]. Table 1 summarizes the many proposed methods to correct for height or pubertal status [9, 78–84]. No method adequately addresses all the potential concerns; no single adjustment technique is recommended. Instead, it is important that clinicians are aware of the need to make an adjustment in certain clinical scenarios and choose the method that best addresses a given patient’s need. Regardless of method selected, clinicians need to be aware that none of these methods have been validated in terms of incident fracture prediction. However, lumbar spine BMAD was associated with an increased odds of vertebral fractures (odds ratio 9.3, CI: 5.3–14.9) whereas TBLH-BMC adjusted for both height and lean tissue mass was associated with an increased odds of long bone fractures (odds ratio 6.5, CI: 4.1–10.2) [79].
Table 1.
Body size adjustment techniques.
| Method | Technique | Population | Advantages | Limitations |
|---|---|---|---|---|
| Bone mineral apparent density (BMAD) | An assumed depth is included in the equation, converting a two dimensional projected area into a volume of space | • Calculation is simple and applicable in clinical practice • Correlates with volumetric BMD measures from QCT • Correlates with forearm fracture risk [78] • Predictive of vertebral fracture [79] |
• Does not apply to the TBLS site • Assumes discrete shape (cube or cylinder) of the vertebra that may not be anatomically correct |
|
| Carter Method [80] | BMAD = BMC / Ap3/2 | Women Ages 17–40 years | ||
| Kroger Method [81] | BMAD = BMD × {4 / (π × width of LS) | Children Ages 6–19 years | ||
| Height for age Z-score adjustment (HAZ) [82] | Model correlates child’s height standard deviation | Children Ages 7–17 years | • Analysis tool available for public use (https://bmdcs.nichd.nih.gov) • Approach was least biased as compared to other size adjustment techniques |
• Necessitates reliable height measurement • Reference ranges calculated from children of normal stature, thus extrapolation of data occurs in extremely short children • Hologic system only • Effect of pubertal timing not considered |
| Bone age adjustment | Skeletal maturity is assessed via bone age which is then used as the age-matched comparison | • Bone age is associated with pubertal maturation | • Bone age measures are subjective • No reference range data • Children with genetic short stature syndromes often have normal bone ages |
|
| Height age adjustment | Age at which child’s height is at the 50th percentile is used as the age-matched comparison | • Clinically easy | • Does not take into account pubertal maturation | |
| Body composition adjustment [79,83,84] | Lean muscle mass is critical for bone formation (Mechanostat Theory). After adjustment for age, additional adjustments are made after considering: 1. Is lean muscle mass appropriate for height? (LBM for height) 2. Is BMD appropriate for lean muscle mass? (BMC for LBM) |
Patient samples Ages 3–30 years [83] Ages 4–20 years [84] |
• Many chronic diseases also cause sarcopenia in addition to poor linear growth. This method allows further delineation of deficits in BMD as primarily due to height (bones short for age but LBM normal) or thin bones (lean muscle mass low for height) or both (BMD low for height and LBM). | • Adjustment for LBM may affect BMD, there is no evidence that fracture risk is affected in adults [84] |
6. Other assessments with DXA imaging: the future of DXA in pediatrics
6.1. Vertebral fracture assessment
Non-traumatic vertebral compression fractures are sufficient to make the diagnosis of osteoporosis in children [85] and are not uncommon in certain disease populations. The gold standard for the diagnosis of these fractures is a spinal radiograph; however, vertebral fracture assessment (VFA) is a method where a DXA scan can be used to identify moderate (grade 2) and severe (grade 3) compression fractures with high sensitivity in adults [86]. In pediatrics, there have been few studies validating VFA to diagnosis VCFs. Early studies were disappointing as the resolution of older DXA machines were inadequate to visualize clearly the vertebrae of children. However, newer literature has been promising. Kyriakou et al. found VFA to be highly reproducible and accurate for detecting compression fractures in children with a variety of primary and secondary bone diseases, as well as have a high positive predictive value (90%) and high negative predictive value (95%) [87]. VFA exposes the patient to less radiation than the standard radiograph and can be done at the same time as a routine DXA measure (i.e., aBMD measure of the anteroposterior spine), decreasing risk and saving time, important factors when considering a screening measure in pediatrics. However, VFA is not without limitations. Assessment of vertebral bodies in the mid-thoracic region is still suboptimal in all age groups and while scan quality has improved, readability is limited in younger children. Further studies are needed to validate the findings of this group and also determine the utility ofVFA for detecting incident fractures in at risk pediatric populations.
6.2. Trabecular bone score
While the ISCD criteria for diagnosing osteoporosis in pediatric patients requires a low BMD Z-score in the setting of long bone fragility fractures, it is recognized that many patients with fragility fractures have BMD measures >−2.0. Thus, additional factors, such as microarchitecture and bone morphology influence fracture risk. Trabecular bone score (TBS) is a method that uses the lumbar spine DXA image gray-scale pixel variations to generate an estimate of trabecular microarchitecture. TBS is associated with vertebral and nonvertebral fractures [88] and is useful in fracture risk assessment of adults with secondary osteoporosis [89,90]. Published TBS data in children are limited. In healthy adolescents, TBS was positively correlated with age and spinal aBMD in females, whereas in males, spinal BMC, lean mass and fat mass were the independent predictors ofTBS in a multivariate analysis [91]. One study of adolescent girls with anorexia nervosa found spinal TBS to highly correlated with aBMD Z-scores at several skeletal sites, as well as trabecular BMD and the stress strain index obtained by pQCT of the distal tibia [92]. The major limitation ofTBS use in pediatrics is the lack of available reference data and until those data exist, the additional value TBS provides to traditional DXA measures is unknown.
7. Conclusions
DXA imaging has provided pediatric bone experts with an excellent tool to assess bone health in children. While there are limitations to the use of this modality in young patients, significant advancements over the last several decades have improved the utility of DXA measures to guide clinical care for children with disorders of bone mineralization. DXA assessments will certainly continue to play a significant role in future research aimed at understanding bone development and their impact on ultimate fracture risk, both during childhood and into the adult years.
Footnotes
Conflicts of interest
None.
References
- [1].Southard RN, Morris JD, Mahan JD, Hayes JR, Torch MA, Sommer A, et al. , Bone mass in healthy children: measurement with quantitative DXA, Radiology 179 (3) (1991) 735–738. [DOI] [PubMed] [Google Scholar]
- [2].Kroger H, Kotaniemi A, Kroger L, Alhava E, Development of bone mass and bone density of the spine and femoral neck—a prospective study of 65 children and adolescents, Bone Miner. 23 (3) (1993) 171–182. [DOI] [PubMed] [Google Scholar]
- [3].Faulkner RA, Bailey DA, Drinkwater DT, McKay HA, Arnold C, Wilkinson AA, Bone densitometry in Canadian children 8–17 years of age, Calcif. Tissue Int 59 (5) (1996) 344–351. [DOI] [PubMed] [Google Scholar]
- [4].Ellis KJ, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, et al. , Z score prediction model for assessment of bone mineral content in pediatric diseases, J. Bone Miner. Res 16 (9) (2001) 1658–1664. [DOI] [PubMed] [Google Scholar]
- [5].Arabi A, Nabulsi M, Maalouf J, Choucair M, Khalife H, Vieth R, et al. , Bone mineral density by age, gender, pubertal stages, and socioeconomic status in healthy Lebanese children and adolescents, Bone 35 (5) (2004) 1169–1179. [DOI] [PubMed] [Google Scholar]
- [6].Kalkwarf HJ, Zemel BS, Gilsanz V, Lappe JM, Horlick M, Oberfield S, et al. , The bone mineral density in childhood study: bonemineral content and density according to age, sex, and race, J. Clin. Endocrinol. Metab 92 (6) (2007) 2087–2099. [DOI] [PubMed] [Google Scholar]
- [7].Ward KA, Ashby RL, Roberts SA, Adams JE, Zulf Mughal MUK, Reference data for the Hologic QDR Discovery dual-energy X ray absorptiometry scanner in healthy children and young adults aged 6–17 years, Arch. Dis. Child 92 (1) (2007) 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. , Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study, J. Clin. Endocrinol. Metab 96 (10) (2011) 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Crabtree NJ, Shaw NJ, Bishop NJ, Adams JE, Mughal MZ, Arundel P, et al. , Amalgamated reference data for size-adjusted bone densitometry measurements in 3598 children and young adults-the ALPHABET Study, J. Bone Miner. Res. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Crabtree NJ, Arabi A, Bachrach LK, Fewtrell M, Fuleihan GE-H, Kecskemethy HH, et al. , Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions, J. Clin. Densitom 17 (2) (2014) 225–242. [DOI] [PubMed] [Google Scholar]
- [11].Clark EM, Ness AR, Bishop NJ, Tobias JH, Association between bone mass and fractures in children: a prospective cohort study, J. Bone Miner. Res 21 (9) (2006) 1489–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shepherd JA, Wang L, Fan B, Gilsanz V, Kalkwarf HJ, Lappe J, et al. , Optimal monitoring time interval between DXA measures in children, J. Bone Miner. Res 26 (11) (2011) 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Taylor A, Konrad PT, Norman ME, Harcke HT, Total body bone mineral density in young children: influence of head bone mineral density, J. Bone Miner. Res 12 (4) (1997) 652–655. [DOI] [PubMed] [Google Scholar]
- [14].Kelly J, Damron T, Grant W, Anker C, Holdridge S, Shaw S, et al. , Cross-sectional study of bone mineral density in adult survivors of solid pediatric cancers, J. Pediatr. Hematol. Oncol 27 (5) (2005) 248–253. [DOI] [PubMed] [Google Scholar]
- [15].Kalkwarf HJ, Zemel BS, Yolton K, Heubi JE, Bone mineral content and density of the lumbar spine of infants and toddlers: influence of age, sex, race, growth, and human milk feeding, J. Bone Miner. Res 28 (1) (2013) 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McKay HA, Petit MA, Bailey DA, Wallace WM, Schutz RW, Khan KM, Analysis of proximal femur DXA scans in growing children: comparisons of different protocols for cross-sectional 8-month and 7-year longitudinal data, J. Bone Miner. Res 15 (6) (2000) 1181–1188. [DOI] [PubMed] [Google Scholar]
- [17].Khoury DJ, Szalay EA, Bone mineral density correlation with fractures in nonambulatory pediatric patients, J. Pediatr. Orthop 27 (5) (2007) 562–566. [DOI] [PubMed] [Google Scholar]
- [18].Henderson RC, Berglund LM, May R, Zemel BS, Grossberg RI, Johnson J, et al. , The relationship between fractures and DXA measures of BMD in the distal femur of children and adolescents with cerebral palsy or muscular dystrophy, J. Bone Miner. Res 25 (3) (2010) 520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mueske NM, Chan LS, Wren TA, Reliability of lateral distal femur dual-energy X-ray absorptiometry measures, J. Clin. Densitom 17 (4) (2014) 522–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Henderson RC, Lark RK, Newman JE, Kecskemthy H, Fung EB, Renner JB, et al. , Pediatric reference data for dual X-ray absorptiometric measures of normal bone density in the distal femur, AJR Am. J. Roentgenol 178 (2) (2002) 439–443. [DOI] [PubMed] [Google Scholar]
- [21].Zemel BS, Stallings VA, Leonard MB, Paulhamus DR, Kecskemethy HH, Harcke HT, et al. , Revised pediatric reference data for the lateral distal femur measured by Hologic Discovery/Delphi dual-energy X-ray absorptiometry, J. Clin. Densitom 12 (2) (2009) 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Binkovitz LA, Henwood MJ, Pediatric DXA: technique and interpretation, Pediatr. Radiol 37 (1) (2007) 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koo WW, Walters J, Bush AJ, Technical considerations of dual-energy X-ray absorptiometry-based bone mineral measurements for pediatric studies, J. Bone Miner. Res 10 (12) (1995) 1998–2004. [DOI] [PubMed] [Google Scholar]
- [24].Margulies L, Horlick M, Thornton JC, Wang J, Ioannidou E, Heymsfield SB, Reproducibility of pediatric whole body bone and body composition measures by dual-energy X-ray absorptiometry using the GE Lunar Prodigy, J. Clin. Densitom 8 (3) (2005) 298–304. [DOI] [PubMed] [Google Scholar]
- [25].Gordon CM,Leonard MB, Zemel BS, 2013 Pediatric Position Development Conference: executive summary and reflections, J. Clin. Densitom 17 (2) (2014) 219–224. [DOI] [PubMed] [Google Scholar]
- [26].Bachrach LK, Osteoporosis and measurement of bone mass in children and adolescents, Endocrinol. Metab. Clin. N. Am 34 (3) (2005) 521–535. [DOI] [PubMed] [Google Scholar]
- [27].Bachrach LK, Sills IN, Bone densitometry in children and adolescents, Pediatrics 127 (1) (2011) 189–194. [DOI] [PubMed] [Google Scholar]
- [28].Depot medroxyprogesterone acetate and bone effects. Committee Opinion No. 602. American College of Obstetricians and Gynecologists, Obstet. Gynecol 123 (2014) 1398–1402. [DOI] [PubMed] [Google Scholar]
- [29].Petty SJ, O’Brien T, Wark J, Anti-epilepticmedication and bone health, Osteoporos. Int 18 (2) (2007) 129–142. [DOI] [PubMed] [Google Scholar]
- [30].Kalkwarf HJ, Laor T, Bean JA, Fracture risk in children with a forearm injury is associated with volumetric bone density and cortical area (by peripheral QCT) and areal bone density (by DXA), Osteoporos. Int 22 (2) (2011) 607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flynn J, Foley S, Jones G, Can BMD assessed by DXA at age 8 predict fracture risk in boys and girls during puberty?: an eight-year prospective study, J. Bone Miner. Res 22 (9) (2007) 1463–1467. [DOI] [PubMed] [Google Scholar]
- [32].Halton JM, Atkinson SA, Fraher L, Webber C, Gill GJ, Dawson S, et al. , Altered mineral metabolism and bonemass in children during treatment for acute lymphoblastic leukemia, J. Bone Miner. Res 11 (11) (1996) 1774–1783. [DOI] [PubMed] [Google Scholar]
- [33].Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, et al. , Physiologic regulators of bone turnover in young women with anorexia nervosa, J. Pediatr 141 (1) (2002) 64–70. [DOI] [PubMed] [Google Scholar]
- [34].Tuchman S, Thayu M, Shults J, Zemel BS, Burnham JM, Leonard MB, Interpretation of biomarkers of bone metabolism in children: impact of growth velocity and body size in healthy children and chronic disease, J. Pediatr 153 (4) (2008) 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bianchi ML, Leonard MB, Bechtold S, Högler W, Mughal MZ, Schönau E, et al. , Bone health in children and adolescents with chronic diseases that may affect the skeleton: the 2013 ISCD Pediatric Official Positions, J. Clin. Densitom 17 (2) (2014) 281–294. [DOI] [PubMed] [Google Scholar]
- [36].Zionts L, Nash J, Rude R, Ross T, Stott N, Bone mineral density in children with mild osteogenesis imperfecta, Bone & Joint Journal 77 (1) (1995) 143–147. [PubMed] [Google Scholar]
- [37].Turk N, Cukovic-Cavka S, Korsic M, Turk Z, Vucelic B, Proinflammatory cytokines and receptor activator of nuclear factor kappaB-ligand/osteoprotegerin associated with bone deterioration in patients with Crohn’s disease, Eur. J. Gastroenterol. Hepatol 21 (2) (2009) 159–166. [DOI] [PubMed] [Google Scholar]
- [38].Lopes LH, Sdepanian VL, Szejnfeld VL, de Morais MB, Fagundes-Neto U, Risk factors for low bone mineral density in children and adolescents with inflammatory bowel disease, Dig. Dis. Sci 53 (10) (2008) 2746–2753. [DOI] [PubMed] [Google Scholar]
- [39].Sylvester FA, Wyzga N, Hyams JS, Davis PM, Lerer T, Vance K, et al. , Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease, Inflamm. Bowel Dis 13 (1) (2007) 42–50. [DOI] [PubMed] [Google Scholar]
- [40].Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Makitie O, Compromised peak bone mass in patients with inflammatory bowel disease—a prospective study, J. Pediatr 164 (6) (2014) 1436–1443.e1. [DOI] [PubMed] [Google Scholar]
- [41].Laakso S, Valta H, Verkasalo M, Toiviainen-Salo S, Viljakainen H, Makitie O, Impaired bone health in inflammatory bowel disease: a case-control study in 80 pediatric patients, Calcif. Tissue Int 91 (2) (2012) 121–130. [DOI] [PubMed] [Google Scholar]
- [42].Wong SC, Catto-Smith AG, Zacharin M, Pathological fractures in paediatric patients with inflammatory bowel disease, Eur. J. Pediatr 173 (2) (2014) 141–151. [DOI] [PubMed] [Google Scholar]
- [43].Persad R, Jaffer I, Issenman RM, The prevalence of long bone fractures in pediatric inflammatory bowel disease, J. Pediatr. Gastroenterol. Nutr 43 (5) (2006) 597–602. [DOI] [PubMed] [Google Scholar]
- [44].Kappelman MD, Galanko JA, Porter CQ, Sandler RS, Risk of diagnosed fractures in children with inflammatory bowel diseases, Inflamm. Bowel Dis 17 (5) (2011) 1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pappa H,Thayu M, Sylvester F, Leonard M, Zemel B, Gordon C, A clinical report on skeletal health of children and adolescents with inflammatory bowel disease, J. Pediatr. Gastroenterol. Nutr 53 (1) (2011) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Griffin LM,Thayu M, Baldassano RN, DeBoer MD, Zemel BS, Denburg MR, et al. , Improvements in bone density and structure during anti-TNF-alpha therapy in pediatric Crohn’s disease, J. Clin. Endocrinol. Metab 100 (7) (2015) 2630–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ward LM, Osteoporosis due to glucocorticoid use in children with chronic illness, Horm. Res. Paediatr 64 (5) (2005) 209–221. [DOI] [PubMed] [Google Scholar]
- [48].Sambrook PN, Corticosteroid osteoporosis: practical implications of recent trials, J. Bone Miner. Res 15 (9) (2000) 1645–1649. [DOI] [PubMed] [Google Scholar]
- [49].Van Staa T, Cooper C, Leufkens H, Bishop N, Children and the risk of fractures caused by oral corticosteroids, J. Bone Miner. Res 18 (5) (2003) 913–918. [DOI] [PubMed] [Google Scholar]
- [50].LeBlanc C, Ma J,Taljaard M, Roth J, Scuccimarri R, Miettunen P, et al. , Incident vertebral fractures and risk factors in the first three years following glucocorticoid initiation among pediatric patients with rheumatic disorders, J. BoneMiner. Res 30 (9) (2015) 1667–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Hansen KE, Kleker B, Safdar N, Bartels CM (Eds.),A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children, Semin. Arthritis Rheum 44 (1) (2014) 47–54 (Elsevier). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu D, Ahmet A, Ward L, Krishnamoorthy P, Mandelcorn ED, Leigh R, et al. , A practical guide to themonitoring andmanagement of the complications of systemic corticosteroid therapy, Allergy, Asthma Clin. Immunol 9 (1) (2013) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Buehring B, Viswanathan R, Binkley N,Busse W, Glucocorticoid-induced osteoporosis: an update on effects and management, J. Allergy Clin. Immunol 132 (5) (2013) 1019–1030. [DOI] [PubMed] [Google Scholar]
- [54].Children’s Oncology Group, Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, Version 3.0Available on-line: www.survivorshipguidelines.org.
- [55].Wasilewski-Masker K, Kaste SC, Hudson MM, Esiashvili N, Mattano LA, Meacham LR, Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature, Pediatrics 121 (3) (2008) e705–e713. [DOI] [PubMed] [Google Scholar]
- [56].Dickerman JD, The late effects of childhood cancer therapy, Pediatrics 119 (3) (2007) 554–568. [DOI] [PubMed] [Google Scholar]
- [57].Aisenberg J, Hsieh K, Kalaitzoglou G, Whittam E, Heller G, Schneider R, et al. , Bone mineral density in young adult survivors of childhood cancer, J. Pediatr. Hematol. Oncol 20 (3) (1998) 241–245. [DOI] [PubMed] [Google Scholar]
- [58].Gurney J, Kaste S,Liu W, Srivastava D, Chemaitilly W, Ness K, et al. , Bonemineral density among long-term survivors of childhood acute lymphoblastic leukemia: results from the St. Jude Lifetime Cohort Study, Pediatr. Blood Cancer 61 (7) (2014) 1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hesseling PB, Hough SF, Nel ED, van Riet FA, Beneke T, Wessels G, Bonemineral density in long-term survivors of childhood cancer, Int. J. Cancer 78 (s 11) (1998) 44–47. [PubMed] [Google Scholar]
- [60].Mulder JE, Bilezikian JP, Bone density in survivors of childhood cancer, J. Clin. Densitom 7 (4) (2004) 432–442. [DOI] [PubMed] [Google Scholar]
- [61].Binkovitz LA, Sparke P, Henwood MJ, Pediatric DXA: clinical applications, Pediatr. Radiol 37 (7) (2007) 625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Misra M, Klibanski A, Bone health in anorexia nervosa, Curr. Opin. Endocrinol. Diabetes Obes 18 (6) (2011) 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].DiVasta AD, Feldman HA, Gordon CM, Vertebral fracture assessment in adolescents and young women with anorexia nervosa: a case series, J. Clin. Densitom 17 (1) (2014) 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Radiology ACo, ACR–SPR–SSR Practice Parameter for the Performance of Dual-energy X-ray Absoprtiometry (DXA), 2013.
- [65].Grinspoon S, Miller K, Coyle C, Krempin J, Armstrong C, Pitts S, et al. , Severity of osteopenia in estrogen-deficient women with anorexia nervosa and hypothalamic amenorrhea 1, The Journal of Clinical Endocrinology & Metabolism 84 (6) (1999) 2049–2055. [DOI] [PubMed] [Google Scholar]
- [66].Ament ME, Bone mineral content in patients with short bowel syndrome: the impact of parenteral nutrition, J. Pediatr 132 (3) (1998) 386–388. [DOI] [PubMed] [Google Scholar]
- [67].Behringer M, Gruetzner S, McCourt M, Mester J, Effects of weight-bearing activities on bone mineral content and density in children and adolescents: a meta-analysis, J. Bone Miner. Res 29 (2) (2014) 467–478. [DOI] [PubMed] [Google Scholar]
- [68].Rautava E, Lehtonen-Veromaa M, Kautiainen H, Kajander S, Heinonen OJ, Viikari J, et al. , The reduction of physical activity reflects on the bone mass among young females: a follow-up study of 142 adolescent girls, Osteoporos. Int 18 (7) (2007) 915–922. [DOI] [PubMed] [Google Scholar]
- [69].Zerwekh JE, Ruml LA, Gottschalk F, Pak CY, The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects, J. Bone Miner. Res 13 (10) (1998) 1594–1601. [DOI] [PubMed] [Google Scholar]
- [70].Henderson RC, Lark RK, Gurka MJ, Worley G, Fung EB, Conaway M, et al. , Bone density and metabolism in children and adolescents with moderate to severe cerebral palsy, Pediatrics 110 (1 Pt 1) (2002) e5. [DOI] [PubMed] [Google Scholar]
- [71].Finbraten AK, Syversen U, Skranes J, Andersen GL, Stevenson RD, Vik T, Bone mineral density and vitamin D status in ambulatory and non-ambulatory children with cerebral palsy, Osteoporos. Int 26 (1) (2015) 141–150. [DOI] [PubMed] [Google Scholar]
- [72].Stevenson RD, Conaway M, Barrington JW, Cuthill SL, Worley G, Henderson RC, Fracture rate in children with cerebral palsy, Pediatr. Rehabil 9 (4) (2006) 396–403. [DOI] [PubMed] [Google Scholar]
- [73].Harcke HT, Taylor A, Bachrach S, Miller F, Henderson RC, Lateral femoral scan: an alternative method for assessing bone mineral density in children with cerebral palsy, Pediatr. Radiol 28 (4) (1998) 241–246. [DOI] [PubMed] [Google Scholar]
- [74].Bachrach LK, Assessing bone health in children: who to test and what does it mean? Pediatr Endocrinol Rev. 2 (3) (2001) 332–336. [PubMed] [Google Scholar]
- [75].Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, et al. , Peak bone mass, Osteoporos. Int 11 (12) (2000) 985–1009. [DOI] [PubMed] [Google Scholar]
- [76].Fewtrell MS, British P, Adolescent Bone G, Bone densitometry in children assessed by dual X ray absorptiometry: uses and pitfalls, Arch. Dis. Child 88 (9) (2003) 795–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gafni RI, Baron J, Overdiagnosis of osteoporosis in children due to misinterpretation of dual-energy x-ray absorptiometry (DEXA), J. Pediatr 144 (2) (2004) 253–257. [DOI] [PubMed] [Google Scholar]
- [78].Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM, More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures, J. Bone Miner. Res 15 (10) (2000) 2011–2018. [DOI] [PubMed] [Google Scholar]
- [79].Crabtree NJ,Hogler W, Cooper MS, Shaw NJ, Diagnostic evaluation of bone densitometric size adjustment techniques in children with and without low trauma fractures, Osteoporos. Int 24 (7) (2013) 2015–2024. [DOI] [PubMed] [Google Scholar]
- [80].Carter DR, Bouxsein ML, Marcus R, New approaches for interpreting projected bone densitometry data, J. Bone Miner. Res 7 (2) (1992) 137–145. [DOI] [PubMed] [Google Scholar]
- [81].Kroger H, Kotaniemi A, Vainio P, Alhava E, Bone densitometry of the spine and femur in children by dual-energy X-ray absorptiometry, Bone Miner. 17 (1) (1992) 75–85. [DOI] [PubMed] [Google Scholar]
- [82].Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. , Height adjustment in assessing dual energy X-ray absorptiometry measurements of bone mass and density in children, J. Clin. Endocrinol. Metab 95 (3) (2010) 1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Högler W, Briody J, Woodhead HJ, Chan A, Cowell CT, Importance of leanmass in the interpretation of total body densitometry in children and adolescents, J. Pediatr 143 (1) (2003) 81–88. [DOI] [PubMed] [Google Scholar]
- [84].Leslie WD, Orwoll ES, Nielson CM, Morin SN, Majumdar SR, Johansson H, et al. , Estimated lean mass and fat mass differentially affect femoral bone density and strength index but are not FRAX independent risk factors for fracture, J. Bone Miner. Res 29 (11) (2014) 2511–2519. [DOI] [PubMed] [Google Scholar]
- [85].Bishop N, Arundel P, Clark E, Dimitri P, Farr J, Jones G, et al. , Fracture prediction and the definition of osteoporosis in children and adolescents: the ISCD 2013 Pediatric Official Positions, J. Clin. Densitom 17 (2) (2014) 275–280. [DOI] [PubMed] [Google Scholar]
- [86].Rosen HN, Vokes TJ, Malabanan AO, Deal CL, Alele JD, Olenginski TP, et al. , The Official Positions of the International Society for Clinical Densitometry: vertebral fracture assessment, J. Clin. Densitom 16 (4) (2013) 482–488. [DOI] [PubMed] [Google Scholar]
- [87].Kyriakou A, Shepherd S, Mason A, Faisal Ahmed S, A critical appraisal of vertebral fracture assessment in paediatrics, Bone 81 (2015) 255–259. [DOI] [PubMed] [Google Scholar]
- [88].Pothuaud L, Barthe N, Krieg MA, Mehsen N, Carceller P, Hans D, Evaluation of the potential use of trabecular bone score to complement bone mineral density in the diagnosis of osteoporosis: a preliminary spine BMD-matched, case-control study, J. Clin. Densitom 12 (2) (2009) 170–176. [DOI] [PubMed] [Google Scholar]
- [89].Breban S, Briot K, Kolta S, Paternotte S, Ghazi M, Fechtenbaum J, et al. , Identification of rheumatoid arthritis patients with vertebral fractures using bone mineral density and trabecular bone score, J. Clin. Densitom 15 (3) (2012) 260–266. [DOI] [PubMed] [Google Scholar]
- [90].Leslie WD, Aubry-Rozier B, Lamy O, Hans D, TBS (trabecular bone score) and diabetes-related fracture risk, J. Clin. Endocrinol. Metab 98 (2) (2013) 602–609. [DOI] [PubMed] [Google Scholar]
- [91].Shawwa K, Arabi A,Nabulsi M, Maalouf J, Salamoun M, Choucair M, et al. , Predictors of trabecular bone score in school children, Osteoporos. Int 27 (2) (2016) 703–710. [DOI] [PubMed] [Google Scholar]
- [92].Donaldson AA, Feldman HA, O’Donnell JM, Gopalakrishnan G, Gordon CM, Spinal bone texture assessed by trabecular bone score in adolescent girls with anorexia nervosa, J. Clin. Endocrinol. Metab 100 (9) (2015) 3436–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]


