Abstract
Background
Adolescents and young adults (AYA) with HIV experience poorer health outcomes compared to adults. To improve care for AYA with HIV, information about patterns of costly healthcare resource utilization is needed.
Methods
Among 13–30-year-olds in the US HIV Research Network, we stratified outpatient visits, ED visits and inpatient days/person-year (PY) by HIV acquisition model (perinatal [PHIVY], non-perinatal [NPHIVY]), age (13–17, 18–23, 24–30y), CD4 strata (<200, 200–499, ≥500cells/μL) and VL suppression (<, ≥400copies/mL[c/mL]) combined with ARV use.
Results
Among 4,450 AYA (PHIVY: 15%; NPHIVY: 85%), mean follow-up was 2.8y. Among PHIVY, most person-time (PT) was spent between ages 13–23y (13–17y: 43%; 18–23y: 45%), CD4 ≥500/μL (61%), and VL <400c/mL (69%). Among NPHIVY, most PT was spent between ages 24–30y (56%), with CD4 ≥500/μL (54%), and with VL <400c/mL (67%). PT spent while prescribed ARVs and with VL ≥400c/mL was 29% (PHIVY) and 24% (NPHIVY). For PHIVY and NPHIVY, outpatient visit rates were higher at younger ages (13–17y, 18–23y), lower CD4 (<200, 200–499/μL), and among those prescribed ARVs. Rates of ED visits and inpatient days were higher during PT spent at older ages (18–23y, 24–30y), lower CD4 (<200, 200–499/μL), and VL ≥400c/mL. Utilization was higher among PHIVY than NPHIVY (outpatient: 12.1 vs. 6.0/PY; ED: 0.4 vs. 0.3/PY; inpatient: 1.5 vs. 0.8/PY).
Conclusions
More ED visits and inpatient days were observed during time spent at older ages, lower CD4 count, and VL ≥400c/mL. Interventions to improve virologic suppression and immune response may improve outcomes, and thus decrease costly resource utilization, for AYA with HIV.
Keywords: resource utilization, antiretroviral therapy, HIV viral load, CD4 count, adolescence, youth
INTRODUCTION
The US Centers for Disease Control and Prevention (CDC) reports that 22% of new HIV diagnoses occur in adolescents and young adults (AYA) aged 13 to 24 years in the US.1 Nearly 61,000 AYA are now living with HIV in the US.2 AYA with HIV experience poorer outcomes compared to adults at every step of the HIV care continuum, from diagnosis through starting antiretroviral therapy (ART) to virologic suppression.3–5 With combination ART (cART), hospitalizations for persons with HIV of all ages have declined.6,7 Despite improving trends, AYA with HIV are hospitalized more frequently than AYA without HIV.8 AYA who have lived with HIV their entire lives (youth with perinatally-acquired HIV, PHIVY), and AYA who have newly acquired HIV (youth with non-perinatally-acquired HIV, NPHIVY), may experience HIV differently despite traversing the same, uniquely challenging developmental time period of adolescence. Data on the impact of these poorer outcomes for US AYA with HIV on healthcare resource utilization, however, are limited, and none, to our knowledge, examine the impact of mode of HIV acquisition, CD4 and viral load.8–11
Understanding healthcare resource utilization among AYA with HIV may inform policies and practices to improve outcomes among these vulnerable youth.12 Such data are also useful to inform health policy model-based economic projections,13,14 which rely on data derived from adults.15 For example, recent cost estimates to achieve presidential goals to end the HIV epidemic vary widely ($291 million to $25 billion/year).16–18 Our objectives were therefore to analyze resource utilization - including outpatient, emergency, and inpatient hospital care - among AYA with HIV in the HIV Research Network (HIVRN) by mode of HIV acquisition, age, CD4 count, viremia and antiretroviral use, as well as to describe resource utilization associated with specific AIDS-defining conditions (ADCs).
METHODS
Study population
We analyzed data from the HIVRN, a clinical cohort attending 5 pediatric and 13 adult US HIV clinics.19 Sites were hospital- (14) and community-based (4), and geographically diverse (Northeast: 8; South: 5; Midwest: 1; West: 4). The study population included participants 13–30-years-old between January 2006 to December 2015 and with ≥1 CD4 count and viral load (VL) measurement after enrollment during the study period. Demographic, laboratory and medication data were extracted from electronic databases and by chart review. Data were combined across sites at the coordinating center, Johns Hopkins University, to produce a uniform database.19 Institutional review boards at participating sites approved the study. Reported/recorded route of transmission determined categorization as youth with perinatally-acquired (PHIVY) or non-perinatally-acquired HIV (NPHIVY). Race/ethnicity categories were self-reported as mutually exclusive. Sex/gender was recorded/reported as mutually exclusive male/female/transgender. Based on guidelines and practice patterns during the study period, as in prior work, we defined cART regimens as one of two mutually exclusive types expected to be suppressive: (1) ≥3 drugs from ≥2 classes, or (2) a protease inhibitor (PI, excluding ritonavir alone) + 1 drug from another class.20–26 Although individual circumstances may justify alternative ART approaches, during the study period they were not standard of care and were not expected to suppress VL.20–26 Given variable uptake of guideline-concordant ART approaches, we also examined the impact of including ≥3 nucleos(t)ide reverse transcriptase inhibitors in the cART definition in a sensitivity analysis. A change in antiretroviral therapy (ARV) regimen was defined as a change in ≥1 medications. Loss to follow-up was defined as no data recorded for >12 months for any reason other than documented care transfer.
Outcome measures
Primary outcome measures included rates of outpatient visits, emergency department (ED) visits, and inpatient hospital days per person-year (PY). Outpatient visits included primary care (medical doctor, physician assistant or nurse practitioner in the HIV clinic), nurse and social worker visits. For specific ADCs, we assessed primary care visits, ED visits and inpatient hospital days; social work and nurse visits were not routinely linked to ADCs. Because some ADCs occur exclusively (e.g. malignant cervical dysplasia) or more frequently (e.g. Kaposi’s sarcoma) by sex, in an additional analysis, we examined resource utilization associated with ADCs by recorded sex/gender.
We assessed utilization associated with specific ADCs, as defined by the CDC classification.27 A single ADC could be associated with multiple outpatient, ED, and inpatient events; in this case, we assigned each type of utilization (outpatient visits, ED visits, and inpatient days) to the same event. For co-occurring ADCs likely to differentially impact inpatient length of stay (LOS) (e.g. pulmonary tuberculosis and cachexia), we assigned the LOS to the ADC of greater severity. For co-occurring ADCs likely to similarly impact LOS (e.g. cryptococcal meningitis and toxoplasmosis), we assigned the LOS to both diagnoses. Co-occurring ADC designations are listed in eTables 1–3.
Statistical analyses
We estimated average utilization of outpatient visits (primary care, social work and nurse visits), ED visits and inpatient hospital days per person-year stratified by mode of acquisition (PHIVY versus NPHIVY), age (13–17, 18–23, and 24–30 years), CD4 count (<200, 200–499, ≥500 cells/μL), and VL and ARV status (VL/ARV) at time of event. We defined VL/ARV status based on person-time spent in each of 3 categories: 1) suppressive ARVs: VL <400 copies/mL and prescribed any ARVs (i.e. inclusive of suppressive non-cART regimens), 2) nonsuppressive cART: VL ≥400 copies/mL and prescribed cART, and 3) no ARVs: VL ≥400 copies/mL and not prescribed any ARVs.28 Virologic suppression was defined as VL <400 copies/mL based on historic assay lower limits of detection at participating HIVRN sites. Person-time spent in CD4 count and VL/ARV strata was estimated by calculating person-time in between each change in CD4 count, VL/ARV status, and age strata. When available, the nearest measurement prior to baseline was also used. Last available CD4 count and VL were carried forward until the end of follow-up.
When estimating person-time for outpatient, ED, and inpatient utilization rates, we excluded person-time when patients had VL <400 copies/mL and were not prescribed ARVs, as well as when patients had VL ≥400 copies/mL while being prescribed an ARV regimen other than cART. To describe resource utilization associated with ADCs, all person-time was included.
We report crude outpatient, ED, and inpatient utilization rates and 95% confidence intervals; rates simultaneously stratifying by all variables (mode of acquisition, age, CD4 count, and VL/ARV status) are reported in the supplemental appendix.
RESULTS
Study population
Among 4,540 participants, there were 12,641 person-years of active outpatient care (Table 1). We excluded 1,568 person-years (12%) from the person-time distribution (Tables 2 and 3) and outpatient, ED and inpatient rates analyses (Figures 1a–c and eTables 4–9). We excluded 126 person-years (1.0%) while participants had VL ≥400 copies/mL and were on a regimen other than cART; 122 person-years (1.0%) while participants had VL <400 copies/mL while off ARVs, 465 person-years (3.7%) while participants had VL <400 copies/mL and were missing ARV data, and 854 person-years (6.8%) while participants had VL ≥400 copies/mL and were missing ARV data, from a total of 1,222 participants. To assess resource utilization associated with individual ADCs, all 12,641 person-years were analyzed (Figure 2).
Table 1.
Characteristics of HIVRN participants
| Perinatally HIV-infected | Non-perinatally HIV-infected | Total population | ||||
|---|---|---|---|---|---|---|
| N (% of total) | 695 | (15) | 3,845 | (85) | 4,540 | (100) |
| Demographic characteristics | ||||||
| Age at baseline, years, mean (SD) | 16.9 | (3.7) | 22.3 | (2.4) | 21.4 | (3.3) |
| Gender, n (%) | ||||||
| Female | 361 | (52) | 899 | (23) | 1,260 | (28) |
| Male | 331 | (48) | 2,887 | (75) | 3,218 | (71) |
| Transgender | 3 | (0) | 55 | (1) | 58 | (1) |
| Unknown | - | - | 4 | (0) | 4 | (0) |
| Year of birth, median (range) | 1992 | (1982,2002) | 1988 | (1982,2002) | 1989 | (1982,2002) |
| Race | ||||||
| Black / African-American, n (%) | 440 | (63) | 2,434 | (63) | 2,874 | (63) |
| American Indian / Aleutian / Eskimo | -- | 9 | (0) | 9 | (0) | |
| Asian / Pacific Islander | 2 | (0) | 54 | (1) | 56 | (1) |
| White / other, n (%) | 113 | (16) | 627 | (16) | 740 | (16) |
| Hispanic ethnicity, n (%) | 138 | (20) | 661 | (17) | 799 | (18) |
| Not reported, n (%) | 2 | (0) | 60 | (2) | 62 | (1) |
| Baseline clinical characteristics | ||||||
| CD4 cell count strata, mean (SD) | ||||||
| <200/μL | 93 | (13) | 506 | (13) | 599 | (13) |
| 200–499/μL | 200 | (29) | 1,904 | (50) | 2,104 | (46) |
| ≥500/μL | 402 | (58) | 1,435 | (37) | 1,837 | (41) |
| Viral load <400 copies/mL at baseline, no. (%) | 404 | (58) | 1,033 | (27) | 1,437 | (32) |
| Prescribed cARTa | 377 | (54) | 973 | (25) | 1,350 | (30) |
| Prescribed ARVs but not cART | 18 | (3) | 25 | (1) | 43 | (1) |
| No ARVs | 2 | (0) | 29 | (1) | 31 | (1) |
| Missing ARV data | 7 | (1) | 6 | (0) | 13 | (0) |
| Viral load ≥400 copies/mL at baseline, no. (%) | 291 | (45) | 2,812 | 73 | 3,076 | (68) |
| Prescribed cART | 241 | (35) | 2,080 | (54) | 2,321 | (51) |
| Prescribed ARVs but not cART | 23 | (3) | 50 | (1) | 73 | (2) |
| No ARVs | 24 | (4) | 658 | (17) | 682 | (15) |
| Missing ARV data | 3 | (0) | 24 | (1) | 27 | (1) |
| CD4 cell count tests per person per year during follow-up, mean (SD) | 3.3 | (1.7) | 2.4 | (1.2) | 2.5 | (1.3) |
| Viral load tests per person per year during follow-up, mean (SD) | 3.4 | (1.9) | 2.4 | (1.2) | 2.6 | (1.4) |
| Total ARV regimens per person during follow up, mean (SD)a | 2.8 | (2.2) | 1.5 | (1.5) | 1.7 | (1.7) |
| Years of follow-up, mean (SD) | 4.2 | (3.1) | 2.5 | (2.3) | 2.8 | (2.5) |
| Cumulative loss to follow-up, no. (%)b | 52 | (8) | 753 | (20) | 805 | (18) |
| Return to study after loss to follow-up, no. (%) | 29 | (56) | 407 | (54) | 436 | (54) |
| Death during study, no. (%) | 6 | (1) | 11 | (0) | 17 | (0) |
Data are presented as number (%) or mean (SD).
Regimen change was defined as a change in any single drug.
Loss to follow-up was defined no recorded data in the database for >12m, for any reason other than documented care transfer.
ARV, antiretroviral; cART, combination antiretroviral therapy
Table 2.
Distribution of person-time stratified by mode of infection, age, CD4 count and viral load / antiretroviral status
| PHIVY | NPHIVY | |||||||
|---|---|---|---|---|---|---|---|---|
| Distribution of person-time during follow-up | No. % of Participants, (N=692) (%)a | Person-time, years (%)b | No. % of Participants, (N=3,835) (%)a | Person-time, years (%)b | ||||
| Age | ||||||||
| 13–17y | 415 | (60) | 1,157 | (43) | 140 | (4) | 101 | (1) |
| 18–23y | 518 | (75) | 1,220 | (45) | 2,917 | (76) | 3,617 | (43) |
| 24–30y | 173 | (25) | 316 | (12) | 2,239 | (58) | 4,662 | (56) |
| CD4 cell count | ||||||||
| ≥500/μL | 519 | (75) | 1,633 | (61) | 2,614 | (68) | 4,539 | (54) |
| 200 to 500/μL | 415 | (60) | 753 | (28) | 2,616 | (68) | 3,112 | (37) |
| <200/μL | 186 | (27) | 308 | (11) | 717 | (19) | 729 | (9) |
| VL/ARV status | ||||||||
| Suppressive ARV Therapy | 603 | (87) | 1,861 | (69) | 2,714 | (71) | 5,580 | (67) |
| Nonsuppressive cART | 434 | (63) | 792 | (29) | 2,444 | (64) | 1,999 | (24) |
| No ARV Therapy | 25 | (4) | 41 | (2) | 686 | (18) | 800 | (10) |
Number of participants contributing person-time towards a given stratum.
Participants may contribute person-time to more than one stratum.
For subjects while aged 13 to 17, 18 to 23, and 24 to 30 years: the median frequency of CD4 cell count measurements during follow-up was 3.8, 2.7, and 2.2 per year, respectively; the median frequency of HIV RNA measurements during follow-up was 4.1, 2.7, and 2.2 per year, respectively.
ARV, antiretroviral; cART, combination antiretroviral therapy; VL, viral load; NPHIVY, non-perinatally acquired HIV; PHIVY; perinatally acquired HIV
Table 3.
Distribution of CD4 cell count and viral load / antiretroviral status by age and mode of infection
| Person-time, years (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHIV | NPHIVY | |||||||||||
| Variable | 13–17y | 18–23y | 24–30y | 13–17y | 18–23y | 24–30y | ||||||
| CD4 cell count | ||||||||||||
| ≥500/μL | 866 | (75) | 639 | (52) | 128 | (40) | 60 | (60) | 1,916 | (53) | 2,563 | (55) |
| 200 to 500/μL | 248 | (21) | 395 | (32) | 110 | (35) | 38 | (38) | 1,418 | (39) | 1,656 | (36) |
| <200/μL | 44 | (4) | 186 | (15) | 78 | (25) | 2 | (2) | 283 | (8) | 443 | (10) |
| VL/ARV status | ||||||||||||
| Suppressive ARV Therapy | 921 | (80) | 756 | (62) | 184 | (58) | 54 | (53) | 2,211 | (61) | 3,316 | (71) |
| Nonsuppressive cART | 220 | (19) | 441 | (36) | 132 | (42) | 22 | (22) | 896 | (25) | 1,081 | (23) |
| No ARV Therapy | 17 | (2) | 23 | (2) | 1 | (0) | 25 | (25) | 510 | (14) | 265 | (6) |
ARV, antiretroviral; cART, combination antiretroviral therapy; VL, viral load; NPHIVY, non-perinatally acquired HIV; PHIVY; perinatally acquired HIV
Figure 1a-c.
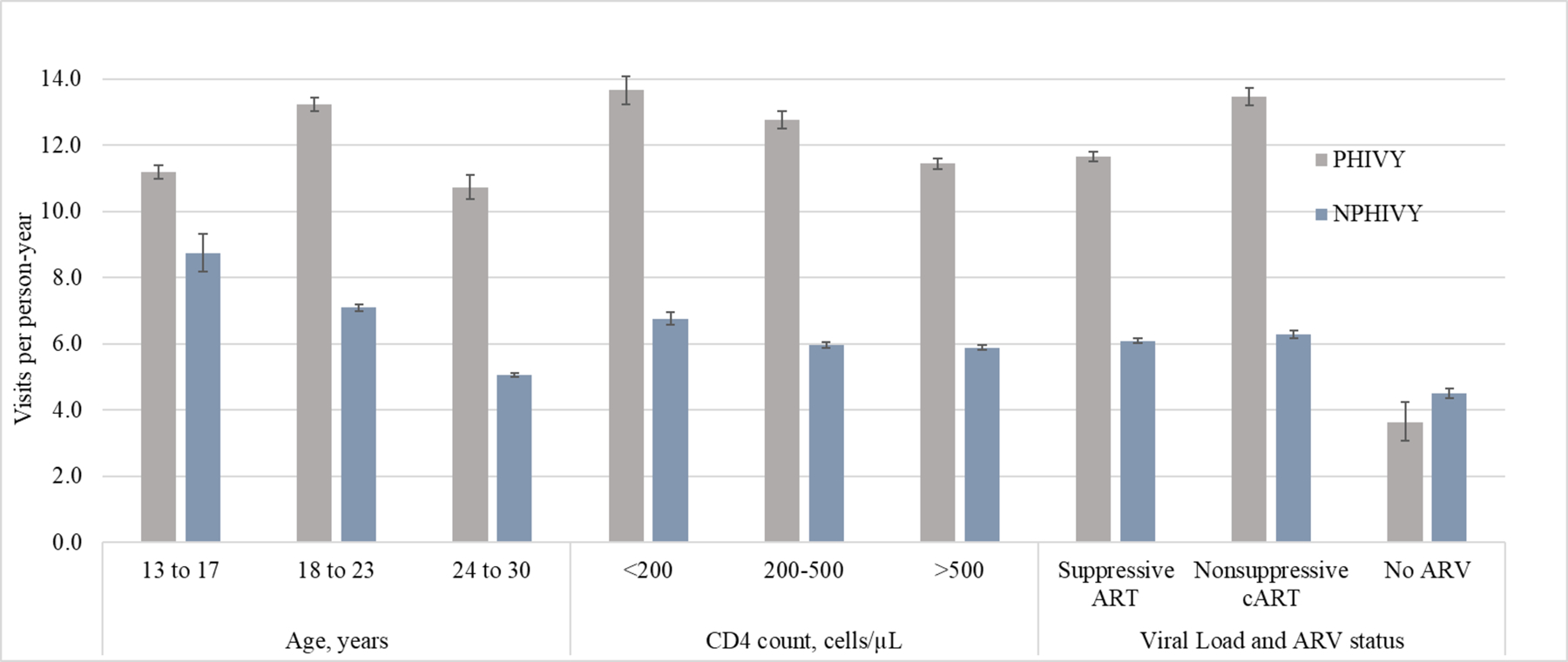
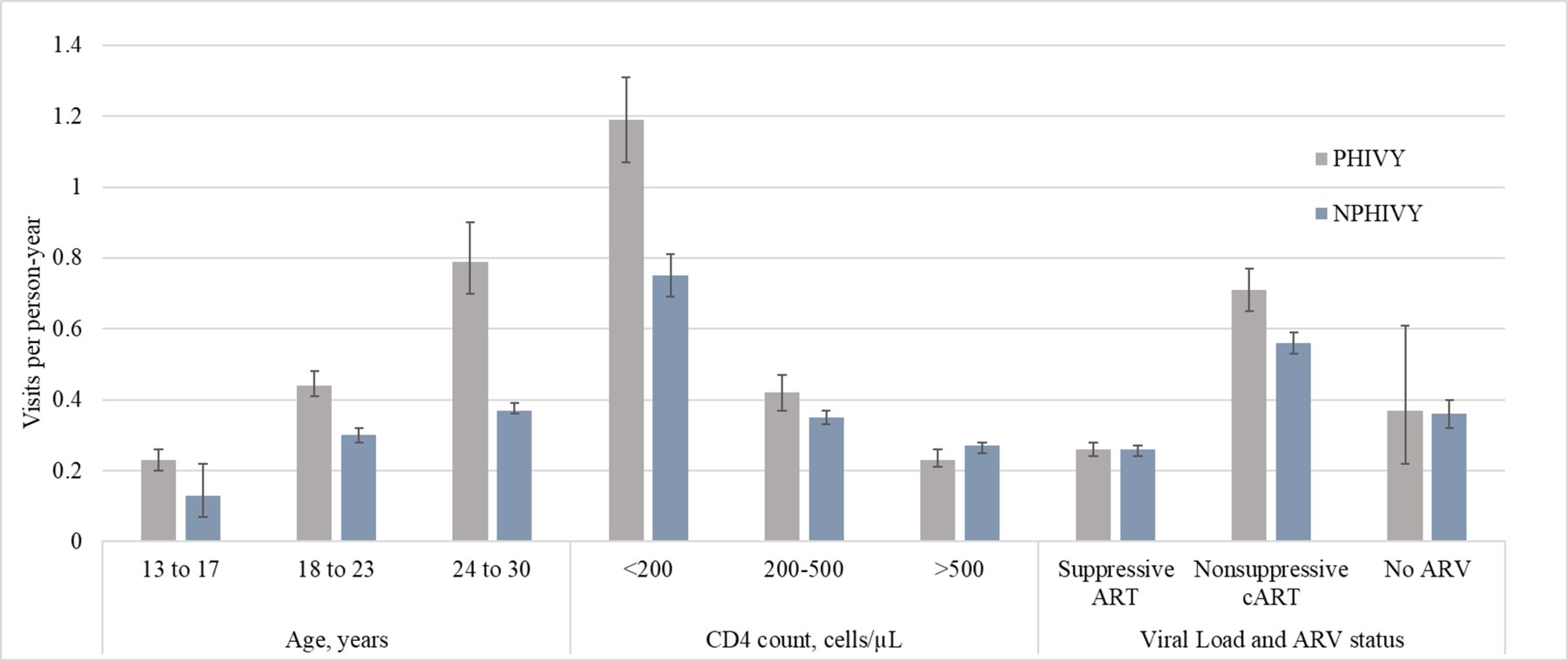
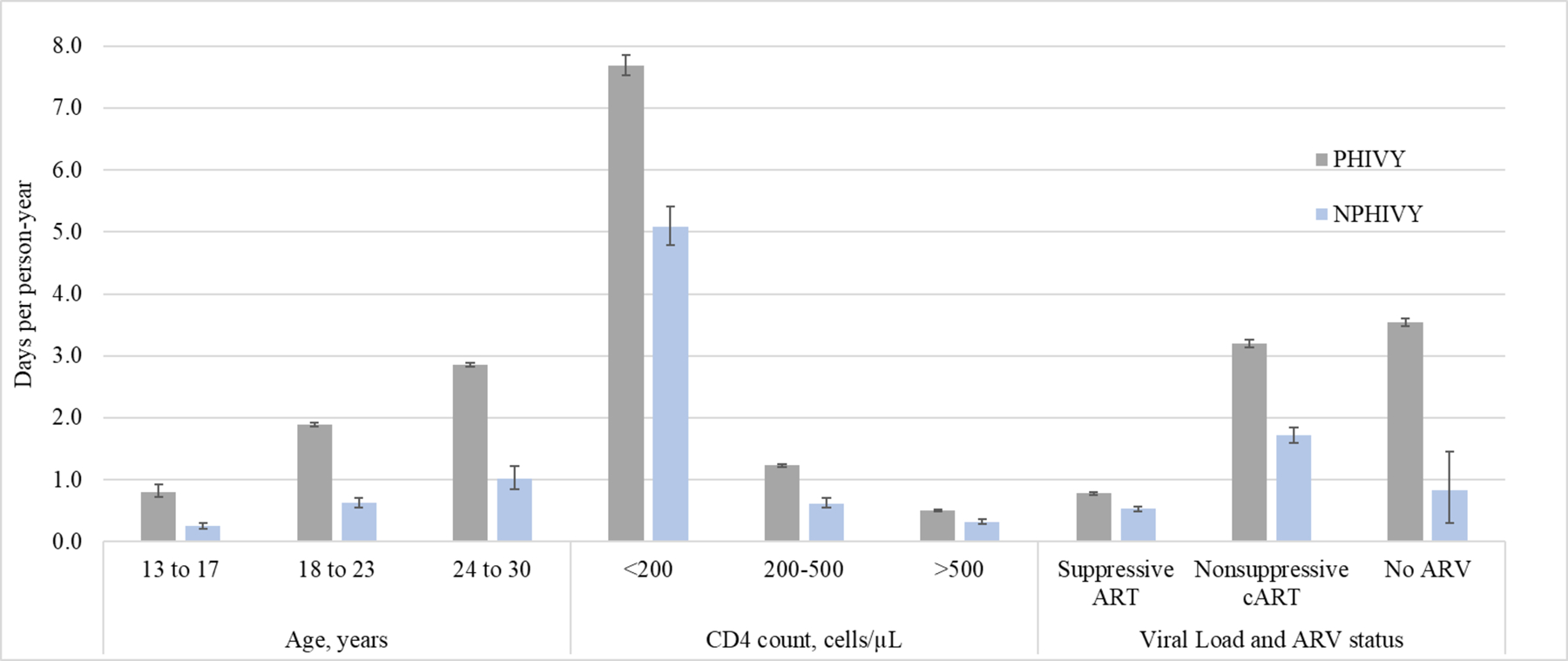
Outpatient visits, emergency medical care visits, and inpatient days per person-year
VL: viral load; ARV: antiretroviral
Error bars indicate Poisson 95% confidence intervals.
Figure 2.
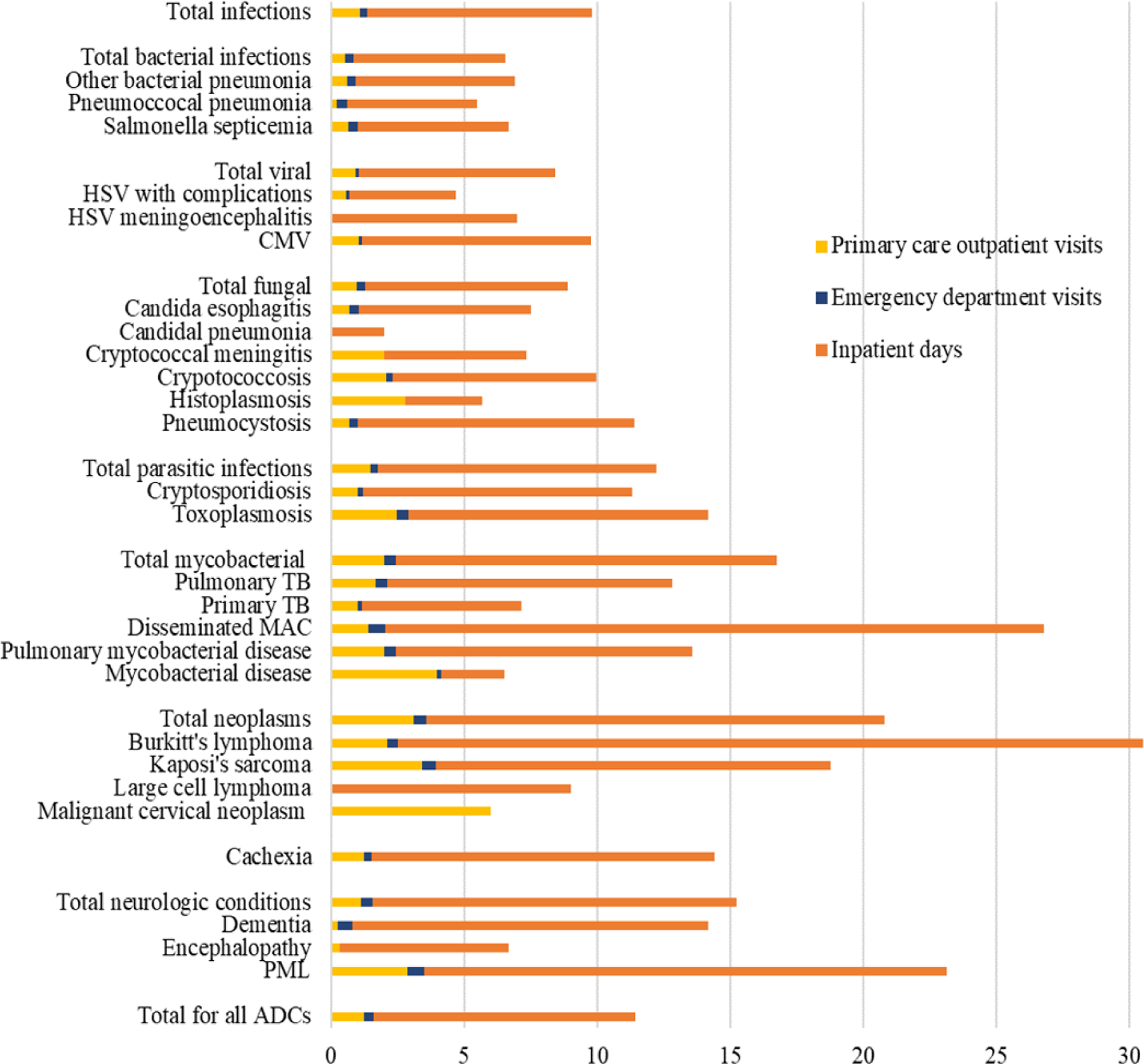
Primary care outpatient visits, emergency department visits and inpatient days per AIDS-defining condition
For the category Total for all ADCs, all AIDS-defining conditions (ADCs) are averaged. For the category Total infections all individual infections are averaged. Total bacterial infections, total viral infections, total fungal infections and total mycobacterial infections are comprised of individal bacterial, viral, fungal and mycobacterial infections, respectively. Mycobacterial disease may comprise either tuberculosis or non-tuberculous mycobacteria and thus is distinguished from, for example, disseminated Mycobacterium avium complex (MAC).
AIDS: Acquired Immunodeficiency Syndrome; CMV: Cytomegalovirus; HSV: Herpes simplex virus; PML: Progressive multifocal leukoencephalopathy; MAC: Mycobacterium avium complex, TB: Tuberculosis
Table 1 reports baseline and follow-up characteristics. Overall, 15% were PHIVY and 28% were female. Among NPHIVY, the most frequently recorded HIV acquisition risk factor was male who has sex with males (63%). Race/ethnicity were recorded as 63% Black, 16% White/other, 18% Hispanic, and 1% not reported. Baseline CD4 strata was <200 cells/μL in 13%; 200–499 cells/μL in 46%; and ≥500 cells/μL in 41%. At baseline, 81% were prescribed cART and 32% had VL <400 copies/mL. Among those with VL ≥400 copies/mL at baseline, 2% were prescribed ARVs but not cART, 15% were prescribed no ARVs, and 1% were missing ARV data. During the study period, on average, 2.5 CD4 count and 2.6 VL measurements were recorded per person per year and patients were prescribed an average of 1.7 ART regimens. Mean follow-up was 2.8 person-years, and cumulative loss to follow-up was 18%. Of those lost to follow-up, 54% returned during the study period. There were 17 deaths.
Person-time distribution
Among PHIVY, 43% of person-time was spent from 13–17 years, 45% from 18–23 years, and 12% from 24–30 years (Table 2). Among NPHIVY, 1% of person-time was spent from 13–17 years, 43% from 18–23 years, and 56% from 24–30 years. Among both PHIVY and NPHIVY, most person-time was spent with CD4 count ≥500 cells/μL (61% and 54%, respectively). Both PHIVY and NPHIVY spent most person-time on suppressive ARVs (69% and 66%), with 29% and 24% of person-time on nonsuppressive cART, and 2% and 10% of person-time off ART. Of PHIVY and NPHIVY, 87% and 71% of participants ever had VL <400 copies/mL during the study period. In a sensitivity analysis of person-time distribution, given variable uptake of guideline-concordant ART approaches, we included ≥3 nucleos(t)ide reverse transcriptase inhibitors in the definition of cART expected to be suppressive; this comprised <1% of overall person-time.
Among PHIVY, person-time spent at CD4 ≥500 cells/μL was lower at older ages (13–17y: 75%; 18–23y: 52%; 24–30y: 40%, Table 3); among NPHIVY, there was no difference by age (13–17y: 60%; 18–23y: 53%; 24–30y: 55%). Among PHIVY, person-time spent on suppressive ARVs was lower at older ages (13–17y: 80%; 18–23y: 62%; 24–30y: 58%); in contrast, among NPHIVY, person-time spent on suppressive ARVs was higher at older ages (13–17y: 53%; 18–23y: 61%; 24–30y: 71%).
Age-, CD4-, and VL and ARV-stratified outpatient visits
The proportion of individuals having any outpatient visit was 97% (PHIVY) and 98% (NPHIVY). Overall for PHIVY and NPHIVY, there were 12.1 and 6.0 outpatient (including primary care, social work and nurse) visits/PY respectively. Among PHIVY, the overall outpatient visit rate was highest in the 18–23y group: 13.2/PY (95% CI 13.0–13.5) vs. 13–17y: 11.2 (95% CI 11.0–11.4) and 24–30y: 10.7 (95% CI 10.4–11.1, Figure 1a, left section). Of outpatient visits among PHIVY, primary care visits were higher at younger ages (eFigure 1); social work visits were greatest in the 18–23y age group (eFigure 2); and nurse visits were similar by age (eFigure 3). Among NPHIVY, overall outpatient visit rates were lower at older ages; 13–17y: 8.7/PY (95% CI 8.2–9.3); 18–23y: 7.1/PY (95% CI 7.0–7.2); 24–30y: 5.1/PY (95% CI 5.0–5.1). For both PHIVY and NPHIVY, rates of overall outpatient visits (Figure 1a, middle section) and primary care outpatient visits (eFigure 1) were higher during person-time spent at lower CD4 counts. Considering VL/ARV status, for both NPHIVY and PHIVY, overall outpatient visit rates were highest during person-time spent on nonsuppressive cART, whereas the lowest outpatient visit rates were during person-time spent not prescribed ART (Figure 1a, right section). Incorporating all age, CD4, and VL/ARV strata variables, compared to NPHIVY, PHIVY had similar rates of primary care outpatient visits (eTable 5), and generally higher rates of social work and nurse visits in the suppressive ARV and non-suppressive cART strata (eTables 6 and 7).
Age-, CD4-, and VL and ARV-stratified ED visits
The proportion of individuals having any ED visit was 37% (PHIVY) and 26% (NPHIVY). Overall, for PHIVY and NPHIVY, there were 0.4 and 0.3 visits/PY, respectively. Among PHIVY and NPHIVY, the lowest rates of ED visits were during person-time spent at ages 13–17y (0.2/PY and 0.1/PY, respectively), CD4 ≥500 cells/μL (0.2/PY and 0.3/PY) and on suppressive ART (0.3/PY and 0.3/PY, Figure 1b). Among PHIVY and NPHIVY, rates of ED visits increased during person-time spent at older ages or lower CD4 counts. Among PHIVY and NPHIVY, when considering VL/ARV status only, the highest rates of ED visits occurred during person-time spent on nonsuppressive cART (PHIVY: 0.7/PY [95% CI 0.7–0.8]; NPHIVY: 0.6/PY [95% CI 0.5–0.6]). After stratifying by all age, CD4, and VL/ARV strata variables, among patients on non-suppressive cART, the highest rates of ED visits occurred during person-time with CD4 count <200 cells/μL (eTable 8).
Age-, CD4-, and VL and ARV-stratified inpatient hospital days
The proportion of individuals having any hospitalizations was 26% (PHIVY) and 13% (NPHIVY). Overall, PHIVY and NPHIVY experienced 1.5 and 0.8 inpatient days/PY, respectively. Average LOS was 6.2 days (PHIVY) and 5.9 days (NPHIVY). Among PHIVY and NPHIVY, the oldest youth (25–30y) spent the most days inpatient (2.9 [95% CI 2.7–3.1] and 1.0 days [95% CI 1.0–1.1], respectively), compared to other ages (13–17y: 0.8 [95% CI 0.8–0.9] and 0.3 days [95% CI 0.2–0.4]; 18–24y: 1.9 [95% CI 1.8–2.0] and 0.6 days [95% CI 0.6–0.7]). Among PHIVY and NPHIVY, the fewest inpatient days occurred during person-time spent with CD4 ≥500 cells/μL (0.5 days [95% CI 0.5–0.6] and 0.3 days [95% CI 0.3–0.3], respectively) or suppressive cART (0.8 days [95% CI 0.7–0.8] and 0.5 days [95% CI 0.5–0.6]). After stratifying by all age, CD4, and VL/ARV strata variables, PHIVY compared to NPHIVY generally had higher or similar rates of inpatient stays during person-time spent at age 18–23 years, and at most CD4 counts in the suppressive ARV therapy and nonsuppressive cART categories (eTable 9).
AIDS-defining conditions and mortality
The proportion of individuals experiencing any ADC was 11% (PHIVY) and 7% (NPHIVY). The proportion of females, males and transgender individuals experiencing any ADC was 8%, 6%, and 5% respectively. Overall, the rate of any ADC (first diagnosis or recurrent) was 4.5/100PY (including and excluding malignant cervical neoplasm). On average, there were 1.3 primary care outpatient visits, 0.3 ED visits and 9.8 inpatient days per ADC (Figure 2). Candida esophagitis (17%), pneumocystis (11%) and cytomegaloviral disease (9%) were the most frequently occurring ADCs. The ADCs with the greatest utilization per event for primary care outpatient visits were malignant cervical neoplasm (6.0 visits/diagnosis), mycobacterial disease (4.0 visits/diagnosis) and Kaposi’s sarcoma (3.4 visits/diagnosis); for ED visits: disseminated mycobacterium avium complex (MAC; 0.6 visits/diagnosis) and progressive multifocal leukoencephalopathy (0.6 visits/diagnosis); and, for inpatient days: Burkitt’s lymphoma (28.0 days/diagnosis), disseminated MAC (24.7 days/diagnosis) and progressive multifocal leukoencephalopathy (19.6 days/diagnosis, Figure 2). ADC-associated resource utilization by sex/gender is reported in eFigures 4–6. The mortality rate was 0.2/100PY for PHIVY and 0.1/100PY for NPHIVY.
DISCUSSION
We described outpatient visits, ED visits, and inpatient days among AYA with HIV ages 13–30 in the HIVRN according to mode of HIV acquisition, and time-updated age, CD4 count, and VL/ARV status. We also assessed outpatient visits, ED visits and inpatient hospital days associated with specific ADCs. This analysis had three key findings.
First, among both PHIVY and NPHIVY, we found that inpatient and ED care resource utilization increased and primary care outpatient utilization decreased with person-time spent at older ages, at lower CD4 counts and with unsuppressed VL. We account for the potential confounding of the younger cohort being predominantly PHIVY by assessing person-time and stratifying simultaneously by mode of transmission, age, CD4 and VL/ARV status. These findings expand on those of a previous study which found higher rates of hospitalizations among 17–24-year-old compared to 5–16-year-old PHIVY in the HIVRN.8 Our findings are also consistent with Medicaid data associating poor adherence with higher total hospital days.29 The finding of declining outpatient utilization as age increases is consistent with national trends for adolescents without HIV. Age, rather than a change in virologic suppression, appears to be driving declining outpatient utilization in NPHIVY, who spent a larger fraction of person-time virologically suppressed at older ages; this likely reflects a shift from regular outpatient engagement to symptom/event-driven care in acute care settings.30 Few studies examine trends in national utilization rates across the adolescent and young adult age spectrum;31 one Type I diabetes study found that outpatient visits declined and emergency care visits increased with age.32 These data underscore the importance of improving access to lower-acuity care and preventive services for AYA with HIV as for other chronic conditions.33
Second, we observed that overall PHIVY had higher rates of overall utilization compared to NPHIVY. After accounting for the greater time spent at lower CD4 count, at VL ≥400 copies/mL, and younger age among PHIVY (eTables 4–9), the observation of greater utilization among PHIVY versus NPHIVY generally persisted for social work and nurse visits as well as for hospitalizations in the 18–23-year-old age group. While distinguishing between outcomes among PHIVY and NPHIVY is critical to improving HIV-related health outcomes, data with this degree of granularity are not often reported, likely in part due to small numbers of PHIVY in the US and, particularly in international settings, difficulty identifying the route of infection for patients diagnosed in adolescence.34,35 Higher rates of viremia and advanced immunosuppression have been previously reported in older PHIVY compared to younger PHIVY.28,36 We found that both PHIVY and NPHIVY in HIVRN experienced increased ED visits and inpatient hospital days with older age, lower CD4 count and unsuppressed viral load. However, we also observed differences between PHIVY and NPHIVY. Social work visits increased during ages 18–23 years for PHIVY, which may reflect the substantial challenges PHIVY face related to chronic illness as they transition from long-term pediatric to adult providers and navigate emergence into early adulthood.37 For NPHIVY, conversely, social work visits declined by age strata, along with primary care outpatient visits. Higher resource utilization rates among PHIVY compared to NPHIVY in certain categories may reflect the longer duration of HIV illness, the persistence of childhood care engagement patterns, or sex differences (Female PHIVY vs. NHIPVY: 52% vs. 23%), in particular related to reproductive healthcare.38,39
Third, while ADCs were infrequent, they contributed substantially to resource utilization. Similar to adults, candidiasis and pneumocystis were the most common ADCs.40 The average LOS for any ADC was 11.7 days. The overall average LOS for any hospitalization was 6.2 (PHIVY) and 5.9 (NPHIVY) days. While comparisons are limited due to differences in calendar year and age groupings, our reported LOS are similar to national data. Nationally, mean LOS declined (6.8 versus 6.1 days) for HIV-related hospital stays from 2006 to 2013; however, cost per stay increased over the same period ($12,589 to $13,300, inflation-adjusted).40 Previous studies among AYA have reported higher rates of ADCs with poorer HIV disease control.28,41 In addition to advancing interventions to improve HIV management for AYA and thus avert ADCs, vaccine-preventable or -mitigatable conditions such as pneumococcal pneumonia and malignant cervical neoplasm present opportunities for optimizing care.
This analysis has several limitations. First, our results are limited to patients engaged in care, and out-of-care person-time utilization patterns are unknown. Second, data-capture is limited to HIVRN sites and some visits (e.g. mental health, substance use) may occur at outside sites; however, a state-wide insurance claims study at an HIVRN site demonstrated that 91% of hospital admissions occurred at the same hospital, suggesting that any underestimation of these utilization rates due to care received elsewhere may be modest.42 Third, by design, we did not analyze whether hospital admissions may have been preceded by outpatient and ED visits, which is how charges are often captured. Fourth, given small numbers of events and the limitations of the database, we did not assess for differences by calendar year, insurance type, or by care site (e.g. pediatric vs. adult centers);40,43 a previous analysis comparing care at pediatric and adult HIVRN sites found no differences in ART initiation rates, but did find higher rates of ART discontinuation at adult sites.44
In conclusion, AYA with HIV had higher resource utilization with more ED visits and inpatient days during time spent at older ages, lower CD4 counts, or unsuppressed VL. While ADCs were rare, associated resource utilization was substantial. Interventions to improve outpatient care engagement and durable virologic suppression as AYA with HIV age may improve outcomes for this growing population as they transition to adulthood.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the participants and study staff in the HIVRN study as well as the sponsoring agencies. We also thank Ms. Julia Foote for assistance in preparing the manuscript for publication.
Sources of Support:
The HIV Research Network is supported by the Agency for Healthcare Research and Quality (HHSA290201100007C), the Health Resources and Services Administration (HHSH250201600009C), the National Institutes of Health (U01 DA036935, P30 AI094189, UL1TR000427), and the Clinical Investigation and Biostatistics Core of the UC San Diego Center for AIDS Research (P30 AI036214).
This research received funding from the following funding sources, which had no role in this research: the Eunice Kennedy Shriver National Institute for Child Health and Human Development (K08HD094638 to AMN; R01 HD079214 to ALC and KP); the Eleanor and Miles Shore Scholars in Medicine Fellowship (to AMN); the Harvard University Center for AIDS Research (P30AI060354 to AMN, MH, FL, RAP); the International Maternal Pediatric AIDS Clinical Trials Network Early Investigator Award (UM1AI068632 to AMN); the National Institute of Allergy and Infectious Diseases (NIAID T32 AI007433 to AMN); the Charles Hood Foundation Child Health Research Award (ALC, AMN, KP); and the Johns Hopkins Center for AIDS Research (P30A1094189 to ALA).
The conclusions and opinions expressed in this article are those of the authors. No official endorsement by the Department of Health and Human Services, the National Institutes of Health, or the Agency for Healthcare Research and Quality or other funders is intended or should be inferred.
LIST OF ABBREVIATIONS
- ADC
AIDS-defining condition
- AIDS
Acquired Immunodeficiency Syndrome
- ARV
Antiretroviral medication
- AYA
Adolescents and young adults
- cART
combination antiretroviral therapy
- CI
Confidence interval
- ED
Emergency department
- HSV
Herpes simplex virus
- HIV
Human immunodeficiency virus
- RNA
Ribonucleic acid
- MAC
Mycobacterium avium complex
- PHIVY
Youth with perinatally acquired HIV
- NPHIVY
Youth with non-perinatally acquired HIV
- PI
Protease Inhibitor
- PML
Progressive multifocal leukoencephalopathy
- TB
Tuberculosis
- VL
viral load
Footnotes
Meeting Presentations: The findings in this manuscript were presented as a poster presentation at the 2019 International Workshop on HIV Pediatrics (Mexico City, Mexico; July 19–20, 2019). A poster presentation is also planned for IDWeek (Washington, D.C.; October 2–6, 2019).
Competing interests
The authors have no conflicts of interest or financial disclosures.
Participating Sites
Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.)
Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.)
Drexel University, Philadelphia, Pennsylvania (Amy Baranoski, M.D., Sara Allen, C.R.N.P.)
Fenway Health, Boston, Massachusetts (Stephen Boswell, M.D., Kenneth Mayer, M.D.)
Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., Richard Moore, M.D., Allison Agwu M.D.)
Montefiore Medical Group, Bronx, New York (Robert Beil, M.D.)
Montefiore Medical Center, Bronx, New York (Uriel Felsen, M.D.)
Mount Sinai St. Luke’s and Mount Sinai West, New York, New York (Judith Aberg, M.D., Antonio Urbina, M.D.)
Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.)
Parkland Health and Hospital System, Dallas, Texas (Ank Nijhawan, M.D., Muhammad Akbar, M.D.)
St. Jude’s Children’s Research Hospital and University of Tennessee, Memphis,
Tennessee (Aditya Gaur, M.D.)
Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.)
Trillium Health, Rochester, New York (William Valenti, M.D.)
University of California, San Diego, California (W. Christopher Mathews, M.D.)
University of Wisconsin-Madison, Madison, Wisconsin (Ryan Westergaard, M.D.)
Sponsoring Agencies
Agency for Healthcare Research and Quality, Rockville, Maryland (Fred Hellinger, Ph.D., John Fleishman, Ph.D.)
Health Resources and Services Administration, Rockville, Maryland (Robert Mills, Ph.D., Faye Malitz, M.S.)
Data Coordinating Center
Johns Hopkins University (Richard Moore, M.D., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., Cindy Voss, M.A., Charles Collins, M.P.H., Rebeca Diaz-Reyes, M.S.P.H.)
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2014; vol.26 http://www.cdc.gov/hiv/library/reports/surveillance/. Published November 2015. Accessed November 13, 2019. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data - United States and 6 dependent areas, 2014. HIV Surveillance Supplemental Report 2016;21(No. 4). http://www.cdc.gov/hiv/library/reports/surveillance/. Published July 2016. Accessed November 13, 2019. [Google Scholar]
- 3.Hall HI, Frazier EL, Rhodes P, et al. Differences in HIV care and treatment among subpopulations in the United States. JAMA Intern Med. Jul 22 2013;173(14):1337–1344. [DOI] [PubMed] [Google Scholar]
- 4.Yehia BR, Rebeiro P, Althoff KN, et al. Impact of age on retention in care and viral suppression. J Acquir Immune Defic Syndr. Apr 1 2015;68(4):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDs. Mar 2014;28(3):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry SA, Fleishman JA, Moore RD, Gebo KA, Network HIVR. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001–2008. J Acquir Immune Defic Syndr. Apr 1 2012;59(4):368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994–2005. AIDS. Jul 11 2008;22(11):1345–1354. [DOI] [PubMed] [Google Scholar]
- 8.Berry SA, Gebo KA, Rutstein RM, et al. Trends in hospitalizations among children and young adults with perinatally acquired HIV. Pediatr Infect Dis J. May 2014;33(5):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munakata J, Benner JS, Becker S, Dezii CM, Hazard EH, Tierce JC. Clinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virus. Med Care. Oct 2006;44(10):893–899. [DOI] [PubMed] [Google Scholar]
- 10.Rutstein RM, Gebo KA, Siberry GK, et al. Hospital and outpatient health services utilization among HIV-infected children in care 2000–2001. Medical care. Sep 2005;43(9 Suppl):III31–39. [DOI] [PubMed] [Google Scholar]
- 11.Wilson LS, Basu R, Christenson M, et al. Pediatric HIV costs across three treatment eras from 1986 to 2007. Pediatrics. September 2010;126(3):e541–549. [DOI] [PubMed] [Google Scholar]
- 12.Rotheram-Borus MJ, Lee SJ, Swendeman D. Getting to zero HIV among youth: Moving beyond medical sites. JAMA Pediatr Oct 15 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. Nov 2006;44(11):990–997. [DOI] [PubMed] [Google Scholar]
- 14.Schackman BR, Fleishman JA, Su AE, et al. The lifetime medical cost savings from preventing HIV in the United States. Med Care. Apr 2015;53(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neilan AM, Dunville R, Ocfemia MCB, et al. The optimal age for screening adolescents and young adults without identified risk factors for HIV. J Adolesc Health. Jan 2018;62(1):22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borre ED, Hyle EP, Paltiel AD, et al. The clinical and economic impact of attaining national HIV/AIDS strategy treatment targets in the United States. J Infect Dis. Oct 17 2017;216(7):798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Health & Human Services. Statement on FY2020 Budget Proposal for Ending the HIV Epidemic in America. Published March 11, 2019. https://www.hhs.gov/about/news/2019/03/11/statement-on-fy2020-budget-proposal-for-ending-the-hiv-epidemic-in-america.html. Accessed November 13, 2019. [Google Scholar]
- 18.McNeil DG. Trump’s proposed budget undermines his H.I.V. plan, experts say. New York Times. March 12, 2019. www.nytimes.com. [Google Scholar]
- 19.Gebo KA, Moore RD, Fleishman JA. The HIV Research Network: a unique opportunity for real time clinical utilization analysis in HIV. Hopkins HIV Rep. Nov 2003;15(6):5–6. [PubMed] [Google Scholar]
- 20.Panel on antiretroviral therapy and medical management of HIV-infected children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. 2006, 2008, 2009, 2012. https://aidsinfo.nih.gov/guidelines/archive/pediatric-guidelines/. Accessed November 13, 2019. Accessed January 13, 2017.
- 21.Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. January 29, 2008; 1–128. a pediatric HIV infection. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed November 13, 2019. [Google Scholar]
- 22.Van Dyke RB, Patel K, Siberry GK, et al. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr. Jun 1 2011;57(2):165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis. Jul 2014;14(7):572–580. [DOI] [PubMed] [Google Scholar]
- 24.Mondi A, Fabbiani M, Ciccarelli N, et al. Efficacy and safety of treatment simplification to atazanavir/ritonavir + lamivudine in HIV-infected patients with virological suppression: 144 week follow-up of the AtLaS pilot study. J Antimicrob Chemother. 2015;70(6):1843–1849. [DOI] [PubMed] [Google Scholar]
- 25.Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis. July 2015;15(7):785–792. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Molina JA, Rubio R, Rivero A, et al. Simplification to dual therapy (atazanavir/ritonavir + lamivudine) versus standard triple therapy (atazanavir/ritonavir + two nucleos(t)ides) in virologically stable patients on antiretroviral therapy: 96 week results from an open-label, non-inferiority, randomized clinical trial (SALT study). J Antimicrob Chemother. January 2017;72(1):246–253. [DOI] [PubMed] [Google Scholar]
- 27.Castro K, Ward J, Slutsker L, Buehler J, Jaffe HW, Berkelman R. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults.2019(June 14). [PubMed] [Google Scholar]
- 28.Neilan AM, Karalius B, Patel K, et al. Association of risk of viremia, immunosuppression, serious clinical events, and mortality with increasing age in perinatally Human Immunodeficiency Virus-infected youth. JAMA Pediatr. March 27 2017;171(5):450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunn K, Lafeuille MH, Jiao X, et al. Risk factors, health care resource utilization, and costs associated with nonadherence to antiretrovirals in medicaid-insured patients with HIV. J Manag Care Spec Pharm. Oct 2018;24(10):1040–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black LI, Nugent CN, Vahratian A. Access and utilization of selected preventive health services among adolescents aged 10–17 NCHS data brief, no 246. Hyattsville, MD: National Center for Health Statistics; 2016. www.cdc.gov/nchs. Accessed June 14, 2019. [PubMed] [Google Scholar]
- 31.Silber JH, Gleeson SP, Zhao H. The influence of chronic disease on resource utilization in common acute pediatric conditions. Financial concerns for children’s hospitals. Arch Pediatr Adolesc Med. Feb 1999;153(2):169–179. [DOI] [PubMed] [Google Scholar]
- 32.Garvey K, Wharam J, Zhang F, LeCates R, Laffel L, Finklestein J. Health-Care utilization across the Pediatric-to-Adult transition in a national cohort of patient with Type 1 Diabetes (T1D) outcomes in US adolescent with Type 1 Diabetes. American Diabetes Assocation 77th Scientific session. www.professional.diabetes.org/webcast. Accessed November 13, 2019. [Google Scholar]
- 33.Lawrence RS, Gootman JA, Sim LJ editors. Adolescent health services: Missing opportunitites. Committee on Adolescent Health Care Services and Models of Care for Treatment, Prevention, and Healthy Development National Research Council and Institute of Medicine. The National Academies Press; 2009. www.nap.edu. Accessed November 13, 2019. [Google Scholar]
- 34.Slogrove AL, Sohn AH. The global epidemiology of adolescents living with HIV: time for more granular data to improve adolescent health outcomes. Curr Opin HIV AIDS. May 2018;13(3):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel K, Seage GR, Burchett SK, Hazra R, Van Dyke RB, Pediatric HIVACS. Disparities in HIV viral suppression among adolescents and young adults by perinatal infection. Am J Public Health. Jul 2019;109(7):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agwu AL, Fleishman JA, Rutstein R, Korthuis PT, Gebo K. Changes in advanced immunosuppression and detectable HIV viremia among perinatally HIV-infected youth in the multisite United States HIV Research Network. J Pediatric Infect Dis Soc. Sep 2013;2(3):215–223. [DOI] [PubMed] [Google Scholar]
- 37.Kowalska JD, Popielska J, Wroblewska A, Firlag-Burkacka E, Horban A, Marczynska M. Both improvement and worsening of adherence to antiretroviral treatment can be expected while transitioning HIV-positive adolescents to adult health care. Infect Dis (Lond). Mar 11 2019:1–4. [DOI] [PubMed] [Google Scholar]
- 38.Bui AL, Dieleman JL, Hamavid H, et al. Spending on children’s personal health care in the United States, 1996–2013. JAMA Pediatr. Feb 1 2017;171(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agwu AL, Jang SS, Korthuis PT, Araneta MR, Gebo KA. Pregnancy incidence and outcomes in vertically and behaviorally HIV-infected youth. JAMA. Feb 2 2011;305(5):468–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heslin KC, Elixhauser A. HIV hospital stays in the United States, 2006–2013 HCUP Statistical Brief #206. June 2016. Agency for Healthcare Research and Quality, Rockville, MD: http://www.hcup-us.ahrq.gov. Accessed November 13, 2019. [PubMed] [Google Scholar]
- 41.Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA Jul 19 2006;296(3):292–300. [DOI] [PubMed] [Google Scholar]
- 42.Berry SA, Fleishman JA, Yehia BR, et al. Thirty-day hospital readmission rate among adults living with HIV. AIDS Aug 24 2013;27(13):2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nathan PC, Bremner KE, Liu N, et al. Resource utilization and costs in adolescents treated for cancer in pediatric vs adult institutions. J Natl Cancer Inst. Jul 19 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agwu AL, Siberry GK, Ellen J, et al. Predictors of highly active antiretroviral therapy utilization for behaviorally HIV-1-infected youth: impact of adult versus pediatric clinical care site. J Adolesc Health. May 2012;50(5):471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


