Abstract
Tumour metastasis, the movement of tumour cells from a primary site to progressively colonize distant organs, is a major contributor to the deaths of cancer patients. Therapeutic goals are the prevention of an initial metastasis in high-risk patients, shrinkage of established lesions and prevention of additional metastases in patients with limited disease. Instead of being autonomous, tumour cells engage in bidirectional interactions with metastatic microenvironments to alter antitumour immunity, the extracellular milieu, genomic stability, survival signalling, chemotherapeutic resistance and proliferative cycles. Can targeting of these interactions significantly improve patient outcomes? In this Review preclinical research, combination therapies and clinical trial designs are re-examined.
Metastases, or the consequences of their treatment, are the greatest contributors to deaths from cancer. Clinical metastatic disease results from several selective forces. Pathways that fuel initial tumorigenesis, described as the ‘trunk’ of a cancer evolutionary tree, can also endow tumour cells with metastatic properties and de novo drug resistance. Two types of ‘limb’ pathway emerge from the tree trunk: events that induce acquired resistance to therapy and pathways that induce or accelerate metastasis to distant organs1. Cancer therapy has largely concentrated on druggable targets in the trunk tumorigenesis pathways, such as receptor tyrosine kinases, and uses sequential and combination therapies to minimize drug resistance. Metastasis-related limbs of the cancer evolutionary tree lag far behind in terms of identifying and drugging targets, validating their efficacy in rationally designed clinical trials and incorporating these therapies into the standard of care (SOC). Many of the metastasis-directed therapies under development are cytostatic, not cytotoxic, in preclinical experiments, making their clinical validation problematic. Overt scepticism exists in the pharmaceutical industry and some academic quarters about the concept of drugging metastasis. This Review challenges this notion with the hypothesis that our emerging understanding of metastasis, in particular the last step, metastatic colonization, will identify druggable pathways that will enhance the efficacy of current treatments.
What is metastasis? Genomically, analyses of matched sets of a patient’s primary tumour and distant metastasis reveal mutations common to both and, almost universally, mutations that are distinct to a metastasis. Functionally, tumour cells begin metastasis by invasion of the tissue surrounding the primary tumour. Tumour cells enter the bloodstream, either directly or via the lymphatics system; traversal of the bloodstream most frequently ends in arrest at the first capillary bed encountered. Tumour cells then extravasate the bloodstream to land on ‘foreign soil’. Paget2, 100 years ago, described metastasis in botanical terms as the interaction of ‘seeds’ (tumour cells) and ‘congenial soil’ (the metastatic microenvironment). How a foreign tissue becomes congenial contributes to metastatic colonization, that is, the progressive outgrowth of tumour cells at the distant site. The meta-static soil can be altered by bone marrow-derived cells before tumour cell arrival, termed the premetastatic niche. Eventually the cellular composition, immune status, blood supply, extracellular matrix (ECM) and virtually every other aspect of the metastatic site can be altered to favour colonization.
Besides the component pathways, other attributes of metastasis are important considerations in its therapeutic targeting. Multiple mechanistic pathways can mediate each of the requisite steps of metastasis. Like the repertoire of receptor tyrosine kinases, in which inhibition of one pathway can be overcome by activation of another kinase or a downstream mutation, inhibition of one metastasis pathway may be insufficient. Metastasis can pause part way — a state known as dormancy3. Do dormant metastatic cells require distinct therapeutic agents? Pathways mediating metastasis can be operative in multiple organs or they can be more site specific. Site-specific metastasis trials are becoming more common for bone and brain lesions. The metastatic process may begin early or late in primary tumour formation4, and may require a brief period or decades to complete. These factors may influence patient selection and trial design. So when, in the patient’s clinical course, do we most effectively halt metastasis? The fuel for metastasis may be genomic instability in all of its forms: metastases stand at the end of a progressive loss of the checks on normal chromosome stability, DNA repair and regulated gene expression. Genomic instability can be found in a metastatically competent subclone of a primary tumour and/or can appear in the metastatic lesions5–9. Genomic instability is hypothesized to create many cellular pheno types, any one of which may have all the necessary properties to complete the metastatic process. Can therapeutic targeting of processes that control genomic stability improve outcome? Few of the tumour cells that originally invade the surrounding tissue of the primary tumour complete the metastatic process10; however, those that do go on to kill the patient. Can we identify the meta-statically competent tumour cells or their products in the circulation as biomarkers or end points for earlier intervention? These and other complexities of metastasis must be thoughtfully confronted to produce successful drugs.
This Review identifies functional pathways of metastasis that are potentially efficacious for the prevention and treatment of metastases. It discusses the preclinical credentials that are required of lead antimetastatic agents. Finally, it looks into how we demonstrate an antimeta-static outcome in the clinic within reasonable time, patient and funding limits and how these drugs could be incorporated into the existing SOC.
Where are we?
Patient survival.
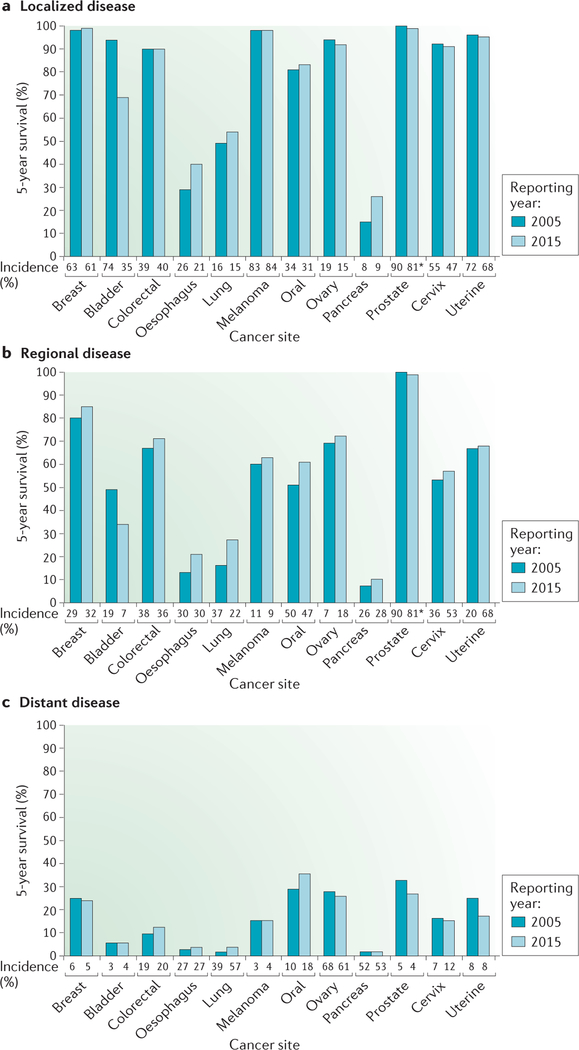
For the overwhelming majority of cancer patients, a diagnosis of metastatic disease indicates a terminal illness. Although cancer death rates have declined, do patients with metastatic disease share equally in the improvements? Cancer incidence and 5-year survival data11,12 provide a broad impression (FIG. 1). Patients initially diagnosed with localized disease often experience excellent 5-year survival (FIG. 1a). Those with regional isease at diagnosis (for example, patients with invasion of cancer to the regional lymph nodes) have lower survival overall, but, excluding patients with bladder or prostate cancer, patients often have survival gains between the 2005 and 2015 reporting periods (FIG. 1b). Only 4 of the 12 cancer sites assessed (colorectal, oesophageal, lung and oral) were associated with gains in the survival of patients with distant metastatic disease at diagnosis, and only 1 site demonstrated a survival gain of more than 3% (FIG. 1c). Alarmingly, the 5-year survival of several types of cancer (including ovarian, prostate and uterine cancer) decreased between the two reporting periods. These trends could be debated because newer immunotherapy and molecular advances were not incorporated. Recent immuno therapies have extended survival in melanoma13, and new androgen receptor inhibitors have improved the survival of patients with metastatic prostate cancer14. However, independent analyses of survival in the metastatic setting for specific cancers paint a similarly dismal picture. Over a 30-year period, results of randomized clinical trials failed to show sustained evidence of increased survival of patients with metastatic breast cancer15. Modest gain or no gain in survival was reported for metastatic gastric and pancreatic cancers16,17. These reports also include data for patients who were diagnosed with non-metastatic disease and then became metastatic, in contrast to the more limited data from the Surveillance, Epidemiology and End Results (SEER) programme of the US National Cancer Institute. Current approaches to metastatic disease are not improving satisfactorily.
Figure 1 |. Few improvements in 5-year survival for cancer patients initially diagnosed with metastatic disease.
The percentage of patients surviving for 5 years is plotted based on their initial disease staging of localized (organ confined), regional (invasion to lymph nodes) or distant (metastases detected by imaging) using the US National Cancer Institute Surveillance, Epidemiology and End Results (SEER) registries11,12. Data covering 1995–2000 and 2004–2010 were reported in 2005 and 2015, respectively, to determine where improvements were attained. With few exceptions, 5-year survival after a diagnosis of localized disease was excellent; where it was low in 2005, gains were observed in 2015. Regional disease survival rates fluctuated by cancer type, but the majority saw increased survival in the later reporting period. Patients with metastatic disease at diagnosis had lower overall 5-year survival rates, with fewer than 20% of patients surviving after 5 years for half of the cancer sites. The increase in survival between the 2005 and 2015 reporting periods was under 3% in three of the four cancer types for which increased survival was seen. For each type, stage categories may not total 100% because of insufficient information for all cases. Beneath each plot is the incidence of each stage at diagnosis for the reporting period. *Localized and regional data were combined.
Metastasis as an uninvited aspect of traditional drug development.
Most of the cancer drugs approved by the US Food and Drug Administration (FDA) or other regulatory agencies were preclinically validated as anti-tumorigenic and were initially tested in clinical trials that enrolled patients with metastatic disease. These trials recruit patients with measurable metastatic disease and ask whether a treatment will shrink established lesions (responses) or extend patient overall survival (OS) or progression-free survival (PFS). Success in the metastatic setting often sets in motion the next hypothesis: that the drug will be effective in preventing metastasis. Many of these drugs were not initially tested in metastatic pre-clinical models. It was assumed either that drugs that target tumour growth would also target metastasis, or that interrupting distinct metastatic limbs of the cancer evolutionary tree would not be necessary in the face of overwhelming growth inhibition. In the setting of adjuvant trials, patients with evidence of aggressive disease but no identifiable distant metastases are treated to prevent metastatic colonization, with disease recurrence, OS and PFS as end points. These trials assume that cancer is a systemic disease. The adverse effect profile is very important, as the patient population is healthier than patients with metastatic disease.
Where metastasis preclinical data have been reported in the traditional drug development process, they have often muddied the waters. Different androgen deprivation therapies exerted disparate effects on invasion and metastasis in prostate cancer by engaging distinct metastasis-associated signalling pathways18. Several approved drugs, including mutant BRAF inhibitors19, paclitaxel20, cisplatin21, anti-androgens22, everolimus23 and sunitinib24, have stimulated metastasis in preclinical models. These stimulatory effects may result from systemic toxic effects, initiating wound healing-type recovery that is laced with growth factors used by metastasizing tumour cells25. Preclinical metastasis data are often directed at identifying resistance mechanisms and potential rational treatment combinations26,27.
Although progress in the adjuvant setting, and to a lesser extent the metastatic setting, is undeniable, would the identification of therapies that halt metastasis improve outcomes for patients? Would we select different lead agents or combinations by incorporating preclinical data on metastatic progression?
A metastasis drug development report card.
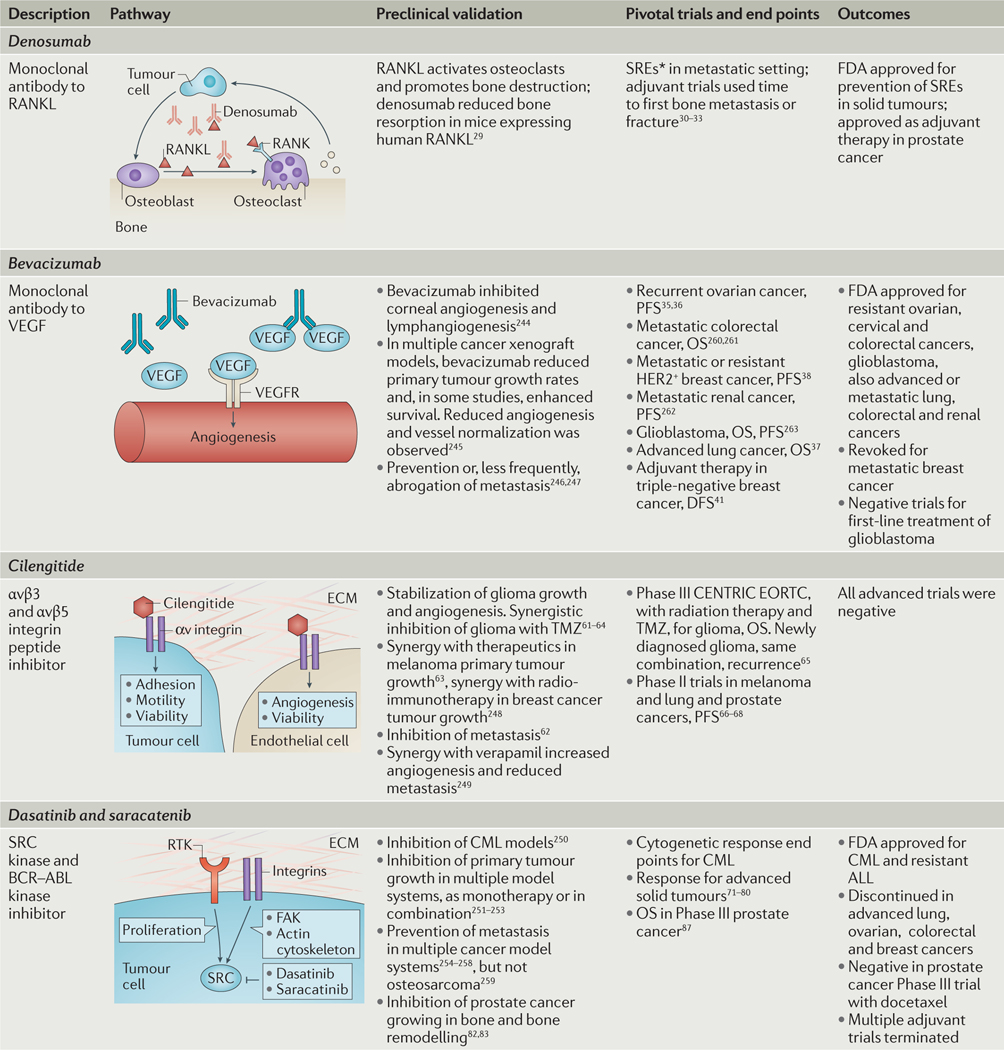
Given the potential contributions of metastasis to drug development and patient outcomes, how have the several recent attempts to incorporate metastasis pathways and end points into drug development fared? TABLE 1 summarizes the preclinical and clinical experience for four potential antimetastatic drugs. Denosumab, a humanized monoclonal antibody that binds receptor activator of NF-κB ligand (RANKL; also known as TNFSF11), interrupts the ‘vicious cycle’ of bone metastasis colonization. In the vicious cycle, tumour cells arriving in the bone produce factors that activate bone-forming osteoblasts to produce RANKL, which in turn activates bone-destroying osteoclasts to degrade bone. As it is destroyed, the bone matrix releases bound factors such as transforming growth factor-β (TGFβ), activating tumour cells and reinitiating the cycle28. Preclinical data demonstrated that denosumab hit its intended target in healthy mice29. In the metastatic setting, trials of denosumab enrolled patients with bone metastases and used an unusual metastasis-relevant end point, a skeletal-related event (SRE). This is a deleterious event such as a bone fracture from expansion of an existing metastasis or a new metastasis. Significant reductions in SREs for denosumab compared with SOC were observed for both breast and prostate cancer30,31. No difference was observed in the traditional OS end point. Denosumab was then tested in adjuvant trials and was shown to delay initial bone metastases in patients with castration-resistant prostate cancer or patients with postmenopausal breast cancer on aromatase-inhibitor therapy32,33.
Table 1 |.
Preclinical and clinical history of four metastasis-directed drug development efforts
ALL, acute lymphoblastic leukaemia; CML, chronic myelogenous leukaemia; DFS, disease-free survival; ECM, extracellular matrix; FAK, focal adhesion kinase; FDA, US Food and Drug Administration; OS, overall survival; PFS, progression-free survival; RANK, receptor activator of NF-κB; RANKL, RANK ligand; RTK, receptor tyrosine kinase; TMZ, temozolomide; VEGF, vascular endothelial growth factor.
Skeletal-related event (SRE) captures the deleterious effects of new lesions and progression of existing lesions to cause patient morbidity.
The responses to bevacizumab, a humanized antibody to vascular endothelial growth factor (VEGF) — which is important in angiogenesis — have been mixed. Angiogenesis is hypothesized to contribute to metastatic colonization by providing new capillaries to deliver oxygen and nutrients34. VEGF is a growth and permeability factor for capillary endothelial cells. Preclinically, bevacizumab was initially tested on corneal angiogenesis, multiple primary tumours and, occasionally, metastasis models. Trials were conducted in the initial or refractory (after progression on other therapy) metastatic settings with survival end points. Bevacizumab is approved by the FDA for several cancers (FDA approval for bevacizumab), although the absolute increases in survival have been nominal in ovarian35,36 and refractory non-small-cell lung cancer37. Conditional approval was revoked in breast cancer38 and other cancers were negative for survival end points39,40. Adjuvant trials were negative in triple-negative breast cancer41 and colorectal cancer42,43. The lack of adjuvant efficacy in breast cancer contrasts with an increase in pathological complete response (pCR), or the complete disappearance of a primary tumour, in a neoadjuvant trial (treatment before surgery) using a similar patient population44,45.
What is behind these mixed results? Preclinical data indicate that bevacizumab can initiate a vascular remodelling response, to normalize vessels and render them resistant to the drug46; bevacizumab also stimulates compensatory pathways, increasing tumour cell motility and invasion47,48. Other anti-angiogenic regimens shrink primary tumours but enhance metastasis in response to the resultant hypoxia24,49–51. Bevacizumab seems to have been a good prospect for targeting tumours but was it an optimal one for targeting metastases? Additional contributing factors include the existence of other functional pathways that regulate angiogenesis, for instance, the angiopoietin 2 (ANGPT2) pathway involved in vessel stabilization52. It will be of interest to determine whether targeting multiple aspects of angiogenesis provides a more substantial survival advantage. Also, the requirement for angiogenesis in metastasis varies53,54. In some models angiogenesis is unnecessary — co-option of the existing vasculature is sufficient for metastasis, or tumour cells induce a vascular network55–57.
Cilengitide, a cyclic peptide inhibitor of αvβ3 and αvβ5 integrins, has been considered as a prospective candidate for metastasis therapeutics. Integrins are receptors that consist of one each of several possible α and β subunits and they mediate the adhesion of tumour cells to the ECM to affect angiogenesis, viability, invasion and colonization58–60. Preclinical work demonstrated prevention of metastasis by cilengitide as monotherapy61,62 or in combination with other agents63, as well as prevention of glioma growth and invasion64. Clinical development proceeded through Phase III trials in glioma and were negative for an OS end point65. As glioma progression does not involve distant metastasis, these trials could potentially be dismissed. However, Phase II trials were conducted in patients with bone metastatic prostate cancer, metastatic melanoma and advanced non-small-cell lung cancer without showing significant clinical activity66–68. Failure here may be due to simple drug development principles rather than an absence of a role of these inte-grins in the metastasis pathway, as the compound had a very short half-life in vivo.
Another set of disappointments were the SRC inhibitors dasatinib and saracatinib. Dasatinib inhibits the BCR–ABL fusion protein underlying chronic myelogenous leukaemia (CML) and the SRC non-receptor kinase. Saracatinib is an independent inhibitor of the same targets. SRC is phosphorylated downstream of multiple receptors, including those for adhesion and cytokines, as well as receptor tyrosine kinases and G-protein-coupled receptors. SRC signalling is best described in tumour motility and invasion, in which activated SRC forms a complex with focal adhesion kinase (FAK, also known as PTK2), forming focal adhesions, lamellipodia and stress fibres, and causing contraction of the actin cytoskeleton; roles in angiogenesis, proliferation and survival are also documented. SRC activation stimulated metastasis in multiple model systems69. Metastasis was significantly prevented when dasatinib or saracatinib was given early and continuously in model systems, including pancreatic, thyroid, prostate, urothelial, ovarian and gastric cancers, melanoma, multiple myeloma and fibrosarcoma, and synergized with several other drugs. Regression of lesions was infrequently demonstrated. In breast cancer, primary tumours regressed using a dasatinib combination with rapamycin, an mTOR inhibitor. In this same model, dasatinib reduced the number of lung metastases, but no additive or synergistic effect was observed with the dasatinib plus rapamycin combination70.
On the basis of this substantial preclinical evidence, SRC inhibition was anticipated to be a block-buster antimetastatic agent. Dasatinib is approved by the FDA for treatment of CML and relapsed acute lympho blastic leukaemia based on its BCR–ABL kinase inhibitory activity (FDA approval for dasatinib), but its clinical activity in metastatic disease has been nothing but disastrous. Saracatinib development was discontinued by its manufacturer. The overwhelming majority of the trials conducted were in the metastatic setting with response and PFS as end points, and both drugs sometimes resulted in long-term stable disease. As mono-therapy, saracatinib and dasatinib were both negative in trials of hormone receptor-negative breast cancer71,72, hormone receptor-positive or HER2 (also known as ERBB2)-positive advanced breast cancer73, recurrent or persistent ovarian cancer74, refractory colorectal cancer75, advanced melanoma76,77, extensive stage small-cell lung cancer78, recurrent or metastatic head and neck cancer79 and metastatic or locally advanced gastric cancer80. Combination trials in similar settings were negative, as was a trial using a gene signature of SRC activation to personalize trial enrolment81. Bone metastasis in castration-resistant prostate cancer provides another example: in mice, saracatinib in combination with docetaxel inhibited bone turnover, prevented bone metastasis82 and inhibited growth of tumour implanted into bone83. In patients with refractory disease, dasatinib monotherapy was negative for a response end point84; when administered to chemotherapy-naive men, dasatinib produced stable disease and reductions in bone turnover markers in urine85,86. The combination of dasatinib and docetaxel was negative for an OS end point87.
These data actually raise a wealth of potential reasons why SRC inhibition may still be a good anti-metastatic agent. First, the overwhelming majority of the preclinical data indicated a prevention of metastasis, not a shrinkage of overt lesions. This would be tested in an adjuvant trial. The trial end points may be wrong: for the prostate cancer bone metastasis trials, the SRE end point that was successfully applied to the denosumab trials was not used. The patient populations used may have been inappropriate for the trial: the role of SRC inhibition in chemoresistant disease was not established preclinically, but this patient population was frequently enrolled; the prostate cancer trials using earlier, chemotherapy-naive patients were the most promising. Second, drug combination studies in general may be problematic. Preclinical studies are often conducted using a low dose of both drugs to see a statistical interaction. In the clinic, both drugs are used at or near the maximum tolerated dose (MTD). Is this the same? Third, standard drug development features of the SRC inhibitors may have been important; substantial Grade 3 and some Grade 4 adverse reactions occurred. Would the side effect profile preclude an adjuvant trial in healthier patients?
In summary, denosumab indicates that metastasis can be drugged. Attributes of this effort included pre-clinical experiments conducted in the target organ of metastasis and a clinical trial design based on a relevant end point caused directly by the metastatic pathway. Limitations identified in other drug development efforts include an inadequate understanding of the molecular pathway in metastatic colonization, poor drug characteristics, overinterpretation of early-phase trial data, a preclinical focus on effects on the primary tumour and the wrong trial design.
New targets
Which part of the metastatic cascade?
Many pathways have been validated to facilitate or interrupt meta-stasis, but have yet to be drugged. The most promising candidate pathways will not only be functionally validated but will be open to intervention in many patients after diagnosis.
The entire metastatic process represents a potential therapeutic target for patients with localized disease. However, localized disease represents a minority of certain cancers at diagnosis (localized disease is particularly rare for patients with cancer of the ovary, pancreas, oral cavity, lung, oesophagus and colorectum; FIG. 1) and is therefore of limited applicability. For regional disease, the likelihood of distant metastasis formation increases, as reflected in survival rates. Regional disease includes more than a quarter of all diagnosed breast, colorectal, lung, pancreatic, cervical, oral and oesophageal cancers (FIG. 1b). At this stage, tumour cells have probably spread systemically and are sitting dormant in distant organs or beginning to colonize, but are too small to be detected by imaging. Adjuvant systemic therapy is administered but unfortunately does not adequately control progression. What remains as an open therapeutic window is the metastatic colonization process. Could interruption of mechanistic pathways mediating metastatic colonization supplement standard adjuvant regimens to prevent further progression and improve survival? For patients with limited, treatable metastatic disease, interruption of the metastatic colonization by other tumour cells in the distant organs could prevent the outgrowth of additional metastases.
Seeding.
Many mechanistic pathways are involved in the initial invasion of tumour cells from the primary site: travel through the circulation, arrest either at the next capillary bed or in a site-specific manner, and extravasation. These include: tumour–tumour and tumour–ECM adhesion molecules; diverse proteases; plasticity programmes such as the epithelial–mesenchymal transition (EMT) and stem or tumour-initiating cell pathways that are fuelled by EMT; anoikis; and adhesion to the vascular endothelium. As noted above, most cancer patients at the time of diagnosis may have already completed these processes, leaving them unavailable for therapeutic intervention. An exception would be the reseeding of metastases from established metastases. Clinical evidence for this is emerging from DNA sequencing studies88, but additional human and preclinical mechanistic data are needed to support translational efforts.
Dormancy.
For breast and prostate cancers, metastatic colonization is delayed by years or decades in a proportion of patients, a process termed dormancy. Dormancy may be a key therapeutic window by which to target metastatic colonization. Clinically, dormancy is defined as an unusually long time between removal of the primary tumour and subsequent relapse in a patient who has been clinically disease free. In preclinical models, tumour cells disseminate but do not steadily form overt metastases. Tumour cells can enter dormancy nestled in their eventual metastatic site; alternatively, tumour cells are found in bone marrow, with prognostic relevance89. Bone marrow may constitute a reservoir for dormant tumour cells that can eventually mobilize and colonize elsewhere.
Dormancy can be achieved by many means — for example, an exit from the cell cycle by tumour cells, balanced proliferation and apoptosis signalling, or host responses such as angiogenesis or immune activation. In immune dormancy, immunoediting may occur, whereby tumour cells expressing strong neoantigens are eliminated by the immune system. Dormant residual tumour cells expressing relatively weak antigens remain, to escape if further evolution blunts immune control90. In this scenario, it could be hypothesized that dormant tumour cells expressing relatively weak antigens may be refractory to immunotherapies upon relapse.
A lack of preclinical model systems that adddress the complexity of dormancy has precluded widespread research. In the past, many poorly metastatic cell lines were established and compared with related, more aggressive cell lines91. With more modern testing, it is possible that they may provide additional dormancy model systems. Even with a model in hand, experiments require relatively long times and the end points are difficult, that is, identification and characterization of single tumour cells in distant sites. Areas of research interest include the identification of niches that promote dormancy92,93, dor-mancy as a p38 (also known as MAPK14)-driven stress response94,95, the relationship of stem cell pathways and dormancy96, tumour cell adhesion molecules97 and ECM cues for reactivation of cell growth98.
To illustrate the potential clinical relevance of cell cycle exit in dormancy, a hormone receptor-negative breast cancer preclinical model was used. Mice were injected with either a steadily metastasizing or a dormant cell line; they were randomized to vehicle or the chemo-therapeutic agent doxorubicin. The progressing cell line produced abundant metastases, which were reduced by drug treatment. The dormant cell line produced few metastases, which were unaffected by the drug99. The dormant tumour cells were insensitive to traditional antiproliferative drugs.
Potential translational approaches to dormancy include targeting signalling pathways that maintain the dormant state95, synthetic lethal combinations to kill G0 tumour cells, monoclonal antibody targeting of single tumour cells and extension of the length of maintenance anti-hormonal treatments100. As an example, a small-molecule inhibitor of the tumour cell lysophosphatidic acid receptor 1 (LPAR1) pathway not only prevented overt metastasis formation in models of breast cancer, but shifted the majority of the remaining disseminated tumour cells to the G0 resting state and activated p38 stress signalling95. This type of inhibitor may stand as a candidate dormancy-inducing agent. One of the simplest ways to test this hypothesis clinically may be to enroll patients with breast cancer who are at highest risk of metastasis, such as patients with remaining primary tumours after neoadjuvant chemotherapy and surgery, patients with multiple positive lymph nodes or patients with chest wall recurrences. After randomization to the intervention or placebo, the primary end point would then be time to the development of a distant metastasis. Further validation of potential biomarkers of residual disease, such as circulating mutant DNA may provide a secondary readout.
Metastatic colonization.
Metastatic colonization remains the optimal window for therapeutic development in prevention of metastasis in the adjuvant setting, and prevention of the development of additional lesions in the limited metastatic setting. So, what is metastatic colonization, and why is it not just like primary tumour growth? Metastatic colonization fuses pathways and alterations found in the primary mass, early events in metastasis and events important to outgrowth in a foreign location. Genomic alterations provide examples: each tumour has oncogenic mutations; they represent the trunk of the cancer evolutionary tree. The prevalence and expression patterns of these mutations, for instance, in TP53 (REF. 101), KRAS101, ESR1 (which encodes oestrogen receptor-α; ERα)102,103, MYC104 and BRAF105,106, may be further exacerbated in metastases. Many of the pathways affected by these truncal mutations have been demonstrated to affect metastatic ability107–111, although a causal role in post-extravasation colonization has not been established.
In other cases, new genetic alterations, not seen in the matched primary tumour, are observed. These alterations are considered limbs on the phylogenetic tree, as they occur late in the evolution of the disease. In a landmark study of prostate cancer progression, amplification or mutation of both mismatch repair and DNA double strand break repair genes was a hallmark only of the metastases9. The data suggest that dual inhibition of DNA repair pathways, if achievable without synergistic toxicities, may be lethal to metastases. In a study of matched primary tumours and brain metastases, 53% of the brain metastases harboured clinically actionable mutations not seen in the patient’s primary tumour112. These data suggest that therapies directed at truncal mutations may be efficacious in distinct clinical settings based on expression patterns in metastasis. Epigenetic changes are likely to outnumber genetic alterations and may change during the colonization process.
Metastatic colonization also mechanistically involves tumour signalling pathways regulating diverse cellular functions (BOX 1). In addition to these cell-intrinsic pathways, metastatic colonization represents the interaction between the tumour cell and its foreign microenvironment. FIGURE 2 outlines interactions between tumour cells and aspects of their new environment, including the premetastatic niche, the ECM, nonspecific immunity (also known as innate immunity), adaptive immunity, and angio-genesis. In general, tumour cells do not land in a foreign site and colonize it as it is; instead, they extensively modify the environment, recruiting bone marrow-derived cells and immune cells and activating wound response programmes in the tissue. Of the metastasis translational targets currently under development, TGFβ, a secreted cytokine, provides an example of the potential and possible pitfalls in targeting colonization (BOX 2). Each of these pathways may hold added attraction as potential therapeutic targets, in that inhibition of one protein often inhibits myriad downstream interactions and phenotypes.
Box 1 |. Potential tumour cell targets in metastatic colonization.
In addition to mutational events, tumour cells alter multiple signalling pathways in order to colonize a foreign organ. Many of the functionally validated signalling pathways in metastatic colonization focus on end points other than proliferation.
Metastatic colonization involves the maintenance or enhancement of signalling pathways mediating tumour cell viability and resistance to death. Protection from apoptosis in metastatic colonization is afforded by autocrine interleukin-6 (IL-6)–signal transducer and activator of transcription 3 (STAT3) signalling, which reduces caspase 3 activation162, overexpression of the CUB domain-containing protein 1 (CDCP1)-mediated anti-apoptotic pathway163 and overexpression of the B-cell lymphoma 2-like (BCL-XL) anti-apoptotic protein156. A role for DNA repair in overcoming DNA damage from reactive oxygen species, leading to loss of viability, has been documented for brain metastasis of breast cancer164.
The role of autophagy has been debated in cancer, but multiple reports link autophagy with increased metastatic colonization as an adaptive survival mechanism165,166. The autophagic response in colonization is negatively regulated by the NMYC downstream-regulated gene 1 (NDRG1) metastasis suppressor167.
Multiple genes involved in metastatic dissemination have been shown to have roles in metastatic colonization, using haematogenous metastasis assays. These include SRC168, diverse proteases, transforming growth factor-β (TGFβ)169,170, RHO family members171, β-catenin172 and cell adhesion molecules. Transcriptional programmes induced by SNAIL (also known as SNAI1)173, inhibitor of DNA binding 1 (ID1)174 and GATA binding protein 3 (GATA3)175 are also operative in metastatic colonization.
Non-coding RNAs such as microRNAs (miRNAs)176–178 and long non-coding RNAs179 regulate complex gene expression patterns to effect colonization.
Pathways operative in generating tumour-initiating cells functionally promote metastatic colonization180–182.
Protein expression patterns are often distinct in metastases, including the downregulation of metastasis suppressor genes183. Metastasis suppressors inhibit many steps in colonization including tumour cell transcriptional programmes184,185, survival after arrival in a distant organ186,187, stress-induced autophagy167, vasoconstriction188 and cell cycle progression189.
Metastatic tumour cell phenotypes are plastic. Tumour cells may undergo an epithelial–mesenchymal transition (EMT) to invade, which is reversed (mesenchymal–epithelial transition, MET) in colonization. It has been reported that layered onto this plasticity is transdifferentiation of tumour cells to myofibroblasts in the microenvironment. The discovery of overarching cellular programmes controlling this plasticity may represent another therapeutic opportunity in metastasis.
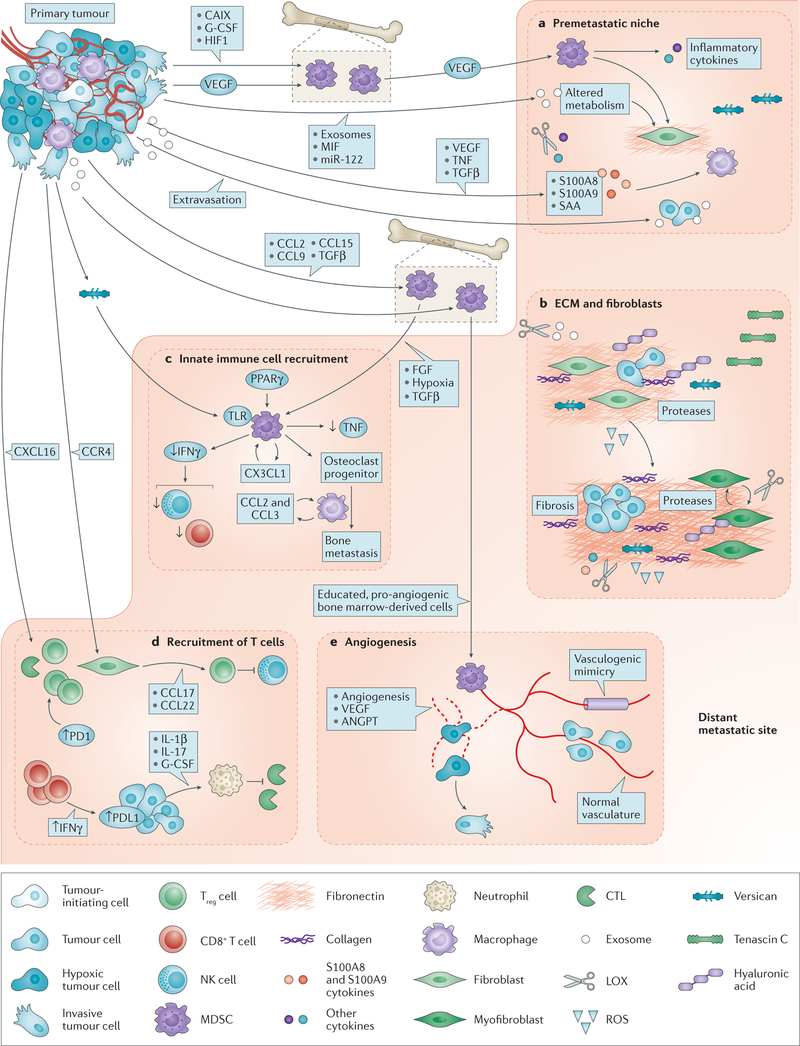
Figure 2. Functional interactions between tumour cells and the metastatic microenvironment in colonization.
a | Premetastatic niche. Primary tumour cells upregulate vascular endothelial growth factor (VEGF), causing VEGF receptor-positive (VEGFR+) haematopoietic bone marrow cells (also called myeloid-derived suppressor cells (MDSCs)) to migrate to the lung, upregulating fibronectin deposition in the extracellular matrix (ECM) by resident fibroblasts and producing inflammatory cytokines. Disseminated tumour cells then home to these locations for preferential colonization206,207. A premetastatic niche is also formed by tumour secretion of VEGF, tumour necrosis factor (TNF) or transforming growth factor-β (TGFβ), stimulating lung tissue to produce S100A8 and S100A9 chemokines, which serve as chemoattractants for alveolar and peritoneal macrophages and tumour cells208,209. Primary tumour hypoxic conditions favour formation of a premetastatic niche by producing lysyl oxidase (LOX) to alter the microenvironment210, carbonic anhydrase (CAIX) to mobilize MDSCs211, and suppression of natural killer (NK) cell activation212. Exosomes produced by the primary tumour educate MDSCs and alter the premetastatic microenvironment ECM and metabolism directly130,131,213. b | ECM and fibroblasts. Colonizing tumour cells functionally interact with altered levels of hyaluronic acid, fibronectin, tenascin C and collagens in the ECM. The ECM is remodelled by various proteases produced by tumour cells and the activated microenvironment, with downstream effects on adhesion and tumour viability123. Integrins, receptors for ECM components, mediate many interactions between tumour cells and the altered ECM to effect colonization58. Fibroblasts in the microenvironment are activated by tumour cells or their secreted factors; comigration of primary tumour fibroblasts with tumour cells to metastatic sites also occurs in model systems214. Activated fibroblasts contribute to multiple aspects of colonization, including angiogenesis, inflammation, immunity and tumour growth potential. Fibrosis is an out-of-control activation of myofibroblasts to produce higher amounts of ECM. When fibrosis is induced by drugs or radiation treatment, experimental metastases are elevated215. c | Innate immunity. Bone marrow-derived myeloid cells, which are macrophage-like, are stimulated to migrate to sites of metastasis by TGFβ, which promotes metastatic colonization by diminishing arginase, reactive oxygen species (ROS) and interferon-γ (IFNγ) production, leading to decreased T cell-dependent antitumour immunity198. Other tumour-derived factors118,216, ECM components217 and hypoxia mobilize myeloid cells. Their activity is regulated by growth factors218, toll-like receptor (TLR)217 and peroxisome proliferator-activated receptor-γ (PPARγ)219 signalling; a chemokine (C-X3-C motif) ligand 1 (CX3CL1) loop promotes their viability115. Myeloid cells also transdifferentiate into metastasis effector cells220. Macrophages from host tissue and circulation also facilitate colonization through chemokine cascades114. Reciprocal signalling between macrophages and tumour cells enhances the viability of both cell populations115,221. NK cells also join the site of colonization and provide innate immune functions. d | T cell-mediated immunity. Influx and activation of tumour infiltrating lymphocytes (TILs) is mediated by chemokines such as C-X-C motif chemokine ligand 16 (CXCL16)117. Programmed cell death protein 1 (PD1) immune checkpoint expression is increased on CD8+ TILs in the metastatic microenvironment222. In turn, CD8+ TILs secrete IFNγ to upregulate PD1 ligand 1 (PDL1) on metastatic tumour cells223,224. The PD1–PDL1 pathway inactivates the cytotoxic T-lymphocyte (CTL) effector arm. Tumour cells halt immune responses in several ways. They produce interleukins (ILs), leading to the production of granulocyte-colony stimulating factor (G-CSF), mobilization of neutrophils and inactivation of CTLs225. Tumour chemokine networks can recruit T regulatory (Treg) cells, shutting down NK cell activity226. A balance of tumour zinc finger E-box binding homeobox 1 (ZEB1) and microRNA miR-200 regulates PDL1 expression227. e | Vascular system. Angiogenesis is stimulated by hypoxia. Endothelial cells proliferate, migrate and encircle to form capillaries. The process is facilitated by MDSCs, which are ‘educated’ by tumour-derived exosomes131. Other pathways such as angiopoietin (ANGPT) signalling, stabilize vessels52. Residual hypoxia stimulates tumour invasion24,49–51. Other sources of blood supply include co-option of the existing vasculature and vasculogenic mimicry, which is the formation of blood-conducting tubes by tumour cells55–57,228. CCL, chemokine (C-C motif) ligand; CCR, chemokine (C-C motif) receptor; MIF, macrophage migration inhibitory factor; SAA, serum amyloid A.
Box 2 |. Transforming growth factor-β as a metastatic colonization target.
Transforming growth factor-β (TGFβ) is a secreted protein that controls the proliferation and differentiation of cells. It binds to a receptor complex that recruits and phosphorylates SMAD family proteins. The SMAD proteins enter the nucleus and act as transcription factors. Other SMADs and regulatory proteins can block the pathway190.
Both small-molecule inhibitors of, and antibodies to, TGFβ have been developed, and they prevented metastasis in several model systems191,192. Side effects measured in long-term experiments seemed nominal. One of the important advantages of TGFβ as a metastatic colonization target is that it affects multiple pathways, including extracellular matrix (ECM) remodelling193, tumour–microenvironment interactions194–196, transcriptional programmes197, immunity198, angiogenesis193,199 and tumour cell viability196,199 (FIG. 2a,c).
A potential problem is the switch in TGFβ function from a tumour suppressor in normal cells to a metastasis stimulator in aggressive cancer cells. The switch mechanism is complex and incompletely understood in mice but involves distinct intracellular signalling194,200,201 and tumour–microenvironment interactions that favour myeloid-derived suppressor cell (MDSC)-mediated inflammation198. To the extent that metastases in humans have ‘switched’ their signalling profile, which is incompletely known, it can be hypothesized that TGFβ targeting will be an effective antimetastatic colonization approach. A recent study identified a gene signature based on the eukaryotic translation initiation factor (eIF) family of transcription factors as distinguishing suppression versus promotion of tumour progression202; this and other pathways can be tested for prediction of patient responses to TGFβ inhibitors and, if positive, used to enroll patients.
Other issues in drug development for this pathway include the number of specific TGFβ family members that are functionally involved in metastatic progression, and rational combinations with standard of care therapy. Early-stage trials of drugs that target TGFβ in patients with advanced cancer are under way with end points of safety, survival, response rate, immune function and serum markers of progression. These end points do not yet test metastasis prevention, which is an end point used in preclinical studies of these drugs203.
Chemokines.
A family of diverse chemokines regulates immune cell migration in inflammation and homeostasis by interacting with G-protein-coupled receptors (FIG. 2c,d). Chemokines such as chemokine (C-X-C motif) ligand 12 (CXCL12), interacting with chemokine (C-X-C motif) receptor 4 (CXCR4), are widely expressed and associated with multi-organ metastasis; other chemo kines have been proposed to mediate organ-specific colonization, such as chemokine (C-C motif) receptor 9 (CCR9) stimulation of melanoma metastasis to the intestine113. Chemokines contribute to metastatic colonization by promoting the infiltration and survival of macrophages114,115 and regulation of T cell-mediated antitumour immune responses116. Other chemokines are inhibitory. CXCL16 expression by colon cancer cells inhibited metastasis by attracting natural killer (NK) cells and CD8+ T cells that express CXCR6 (REF. 117).
Preclinical metastasis prevention experiments include anti-chemokine (C-C motif) ligand 2 (CCL2) for liver metastases118 and anti-CXCL12 for prevention of non-small-cell lung cancer metastasis119. An anti-sense strategy for CCL17 silenced genes in T regulatory (Treg) cells and blocked lung metastasis in a breast cancer model116. AMD3100 is a small-molecule inhibitor of CXCR4, which has FDA approval for stem cell mobilization (FDA approval for plerixafor), and has preclinical antimetastatic activity in ovarian cancer120. This drug is in widespread clinical testing in haematological tumours; an early-stage trial in metastatic pancreatic, ovarian and colorectal cancers is currently recruiting121.
An issue in the development of drugs that target chemokines is the existence of multiple family members with potentially overlapping functions. For instance, melanoma metastasis to the brain upregulated the expression of CXCL10, CCL4 and CCL17 in cerebrospinal fluid122. It will be of interest to determine how the immune functions of chemokine targeting interact with immune checkpoint therapy.
Lysyl oxidase.
Lysyl oxidase (LOX) is a secreted enzyme, induced by hypoxia, that crosslinks collagens and elastin in the ECM. Its mechanism of action in metastatic colonization includes mobilization of bone marrow cells in the premetastatic niche and alterations in the ECM, which are thought to alter integrin engagement and survival signalling (FIG. 2a,b). An antibody to LOX, as well as β-aminopropionitrile (BAPN), a small-molecule inhibitor of the LOX family, prevented the formation of breast cancer metastases123. Interestingly, both agents stabilized metastatic burden when administered at progressively later times in an experimental metastasis assay.
LOX-like 2 (LOXL2), is a relative of LOX that binds the E47 transcription factor and alters the expression of fibronectin and cytokines in the lung to affect bone marrow recruitment124. An antibody to LOXL2, simtuzumab, was tested in a randomized Phase II trial in combination with chemotherapy in refractory metastatic colorectal cancer. Using a PFS end point, simtuzumab did not improve outcome beyond chemotherapy125. This trial suffers from issues that affected the SRC inhibitors: lack of an adjuvant trial to match preclinical data that demonstrated metastasis prevention, and reliance on synergy with chemotherapy. The LOXL family has also been associated with both suppression of tumorigenesis and stimulation of metastasis126, and patient selection may be a key issue for this and other trials.
Exosomes.
Exosomes are small, cell-derived vesicles that promote cell–cell communication. Besides their biological roles in metastasis, they may constitute a techno logical marvel. Exosomes have roles in the premetastatic niche, angiogenesis, immune function and tumour cell communication with the microenvironment (FIG. 2a,e). Exosome cargoes are heterogeneous, both metastasis suppressive127–129 and metastasis accelerating130–133. Clearly, additional work is needed in the field to sort out the complexity of exosome cargoes as well as the conditions facilitating their release and uptake. Detection of exosomes or their cargo may provide a liquid biopsy of minimal residual disease to enable earlier interventions in trials.
Exosomes also hold translational importance as an engineering platform. Although the strategy is in its infancy, exogenous small interfering RNAs (siRNAs) have been engineered into exosomes and delivered in vivo134, suggesting that metastasis-suppressive cargoes could eventually be delivered to target cells. Several exploratory clinical studies have opened to evaluate exosomes from cancer patients135,136, potentially activate the immune system137,138 and predict response to therapy139. In situations in which metastasis-promoting or immune-suppressive exosomes dominate, an exosome-removing trap using affinity plasmapheresis has been developed140.
Site-specific metastases.
Layered onto general pathways mediating metastatic colonization are functional interactions between tumour cells and aspects of the micro-environment that are either unique or selective. As an example, the vicious cycle of osteoclastic (bone-degrading) bone metastases involves RANKL, which has been successfully targeted by denosumab (TABLE 1). The good news is that many additional factors functionally interact to regulate bone metastasis formation and stand as prospective therapeutic targets to further improve patient outcomes. These include adhesive events (met adherin (MTDH; also known as LYRIC))141, interleukin-11 (IL-11), hypoxia and chemokines. A pathway for delayed formation of osteolytic bone metastases centres on tumour cell SRC providing survival signalling against the apoptotic signals induced by CXCL12 and tumour necrosis factor-related apop tosis-inducing ligand (TRAIL; also known as TNFSF10) in the bone microenvironment142.
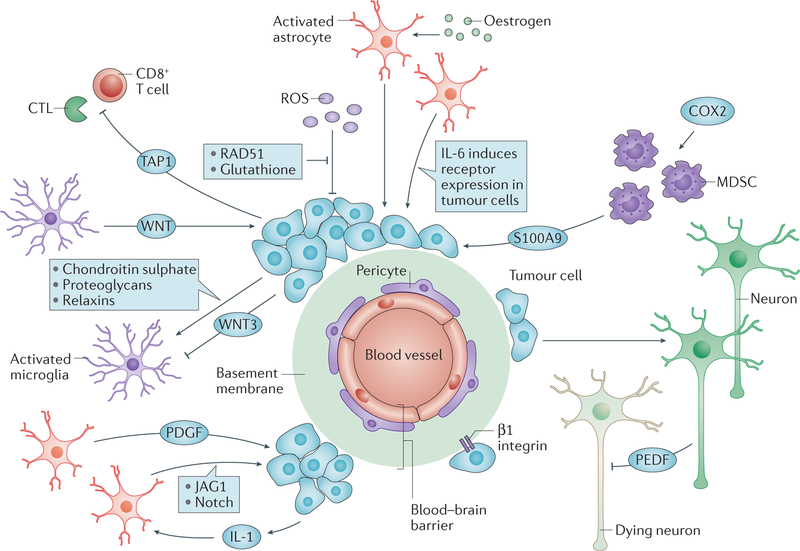
A series of tumour–microenvironment functional interactions is also emerging for brain metastases, which are common in lung and breast cancers, and in melanoma143 (FIG. 3). Some of these pathways overlap with those involved in metastatic colonization in other tissues, whereas other pathways are unique to the site. Reciprocal interactions have been functionally demonstrated between tumour cells and activated astrocytes and microglia that are recruited as a neuroinflammatory response to the presence of tumour cells144. Translational development of these metastasis pathways will also have to surmount the partially intact blood–brain barrier, making drug delivery inefficient145.
Figure 3 |. A wealth of mechanistic translational targets in metastasis to the brain.
In addition to the basic pathways of colonization, bidirectional interactions between tumour cells and the microenvironments of specific tissues foster metastatic colonization. In the brain, tumour cells breach the blood–brain barrier to extravasate using tissue-nonspecific and tissue-specific adhesion molecules and proteases228,229. Tumour cells then adhere to the vascular basement membrane via β1 integrins230. Activated astrocytes congregate around the developing metastasis and are stimulated by tumour-derived cytokines231. The astrocytes produce growth factors that are stimulatory for the tumour cells231,232, and elevate tumour cell expression of receptors for cytokines in the microenvironment233. Systemic hormones such as oestrogen can further activate astrocytes to stimulate colonization234. Activated microglia, resident macrophage-like cells, also surround a developing metastasis. Their activation is determined by a balance of tumour cell stimulatory and inhibitory factors235–238. In turn, they activate tumour cells via the WNT pathway. The brain is infiltrated by myeloid-derived suppressor cells (MDSCs) in a premetastatic phase239, fuelled by cyclooxygenase 2 (COX2) and T cells. Tumours shut down T cell responses using ATP binding cassette transporter 1 (TAP1; also known as ABCB2)240. The brain microenvironment mounts a protective reaction by the secretion of reactive oxygen species (ROS)241. Overexpression of DNA double strand break repair genes by tumour cells attenuates the ROS-induced DNA damage164 and overexpression of serpins inactivates death signals from the microenvironment242. Neuronal cell death is a consequence of metastasis formation, but is attenuated by tumour-derived pigment-epithelial-derived factor (PEDF)243. It is likely that many of the mediators of brain metastatic colonization are active in other anatomical locations as well, via a different set of host microenvironmental cells, suggesting that any therapeutics that are developed may be more broadly applicable. CTL, cytotoxic T lymphocyte; IL-6, interleukin 6; JAG1, Jagged 1; PDGF, platelet-derived growth factor.
Treating established metastases
Shrinking an established metastasis represents a higher bar than preventing the formation of an overt lesion. Drugs must kill tumour cells rather than just being cyto-static. Overt metastases contain millions of tumour cells. They often have a tortuous vasculature and elevated hydrostatic pressure, causing poor drug penetration. Other than the limited efficacy of cytotoxic drugs to produce responses and, at best, marginally enhance survival, what approaches hold promise?
Two general approaches are under development for treating established metastases. BOX 3 details the programmed cell death protein 1 (PD1; also known as PDCD1) and cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) immune checkpoint inhibitor approaches. In general, these inhibitors are producing clinical responses and long-term PFS improvements in a subpopulation of patients with metastatic melanoma, lung cancer or renal cancer146–149. These cancers rank high on a graph of tumour neoantigen levels90, suggesting that other, less antigenic, cancer types may not be immediately as responsive.
Box 3 |. Immune therapy approaches for established metastatic disease.
Most translational metastasis research focuses on prevention, that is, mice are treated early and continuously with an investigational agent. Established metastases are often treated with radiation therapy and chemotherapy, but additional, efficacious, less toxic, avenues are needed. Two immune checkpoints have begun to turn the tide on immune therapy for patients with established metastatic disease.
Adaptive responses to tumours involve antigen–major histocompatibility complex (MHC) protein binding to the T cell receptor. This is coordinated with a co-stimulatory interaction of the surface glycoprotein CD28 that is expressed on the T cell with a B7 receptor on the antigen-presenting cell. Cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) outcompetes CD28 for binding to B7 to reduce immune activation. Ipilimumab, a monoclonal antibody that blocks CTLA4, preclinically enhanced the effector T cell subpopulation while downregulating a suppressor T cell subpopulation146,149. Clinically, ipilimumab extended overall survival (OS) in resistant metastatic melanoma, as monotherapy or in combination with a vaccine, leading to US Food and Drug Administration (FDA) approval (FDA approval for ipilimumab). Hallmarks of its activity were an initial flare in lesion size before gradual shrinkage and long-term responses in the metastases of a minority of patients.
Programmed cell death protein 1 (PD1, which is encoded by PDCD1) is a member of the CD28 co-stimulatory receptor family found on activated T cells, B cells and myeloid cells. Its upregulation on T cells after chronic antigen presentation is a marker of T cell exhaustion. PD1 ligand 1 (PDL1), is expressed by many tumour cells to dampen immune responses. In initial preclinical experiments animals were treated with PD1 antibodies or tumour cells were injected into Pdcd1–/– mice, which stabilized disease, both as monotherapy and in combination with other immunotherapy147,148. Both
pembrolizumab and nivolumab, monoclonal antibodies that block PD1, show responses and long-term progression-free survival (PFS) in a proportion of patients with metastatic lung cancer or melanoma (FDA approval for pembrolizumab and nivolumab). Interestingly, PD-1 blockade was also highly effective in mismatch repair-deficient tumours, for instance, a subset of colorectal carcinomas204.
The combination of anti-PD1 and anti-CTLA4 therapy was superior to either therapy alone in untreated metastatic melanoma205.
The second approach to shrinking established metastases is cytotoxic. Use of α-particle-emitting radionuclides, alone or linked to monoclonal antibodies, constitutes an avenue of some promise. These radionuclides offer the advantages of high energy to kill tumour cells, and low penetration, which minimizes damage to surrounding normal tissue. In a Phase III trial, radium-223 increased the survival of patients with metastatic prostate cancer150. Most of the other α-emitting radionuclide approaches attach the radionuclide to a monoclonal antibody that binds tumour tissue to a far greater extent than normal tissue, so that its applicability is limited only by the abundance and specificity of the antigens. Preclinical experiments in which FDA-approved monoclonal antibodies were stably linked to α-emitters demonstrated regressions in metastatic peritoneal cancer151. Similarly, addition of toxic drugs to an anti-HER2 monoclonal antibody enhanced response rates in breast cancer metastases152.
With this abundance of worthwhile leads for preventing and treating metastases, momentum has built for translational progress. The next steps to consider are establishment of adequate preclinical credentials and clinical testing of potential metastasis-directed therapies.
Formulae for greater success
Preclinical experiments.
Mouse experimentation for preclinical therapeutic development has been justifiably criticized for poor reproducibility. Many types of animal model exist, including xenografts, syngeneic models, genetically engineered mouse (GEM) models and patient-derived xenografts (PDXs); each has strengths and weaknesses153. Traditional xenograft experiments involve injection of tumour cells subcutaneously or ortho-topically into the tissue of origin. Mice bearing xenograft tumours are dosed with an investigational compound and the size of primary tumour growth is measured over time. Retardation of primary tumour growth is considered a success, whereas in the clinic, tumours growing at different rates are still growing and are considered progressive disease, potentially with increased patient PFS. In primary tumour xenograft models, the tumour cells have not interacted with the metastatic microenvironments at all, and this preclinical experimental design would only be relevant for targeting initial oncogenic trunk mutations and any effects they may have on metastasis.
Metastasis preclinical models are absolutely necessary for the development of metastasis-related therapeutics. Orthotopic injection of tumour cells to form a primary tumour and then seed metastases (spontaneous metastasis) is the gold standard. The full metastatic process is modelled in this manner. Similar experiments using PDXs eliminate the potential biases by culture of cell lines on plastic, and have been reported to recapitulate the sites of metastasis and response to therapy observed in the patients from which they are derived154. Some GEM models produce metastases. Weaknesses of spontaneous metastasis models include a paucity of metastases, usually producing ‘yes or no’ animal data, rendering small but potentially significant differences hard to validate, and requiring relatively long times for metastasis development.
Injection of tumour cells directly into the circulation can mimic aspects of metastatic colonization (experimental metastasis). Tumour cells are injected into the tail vein, portal vein or left cardiac ventricle to colonize the lungs, liver, and bone and brain, respectively. Tumour cells can also be injected into a body cavity, such as the peritoneum. In experimental metastasis models, a bolus of tumour cells is injected rather than a constant stream of disseminating cells from a primary tumour as would happen in the natural situation, and the premetastatic niche has not preformed. However, for drug development, these assays measure most of the process of metastatic colonization, and provide good quantification in a reasonable time frame.
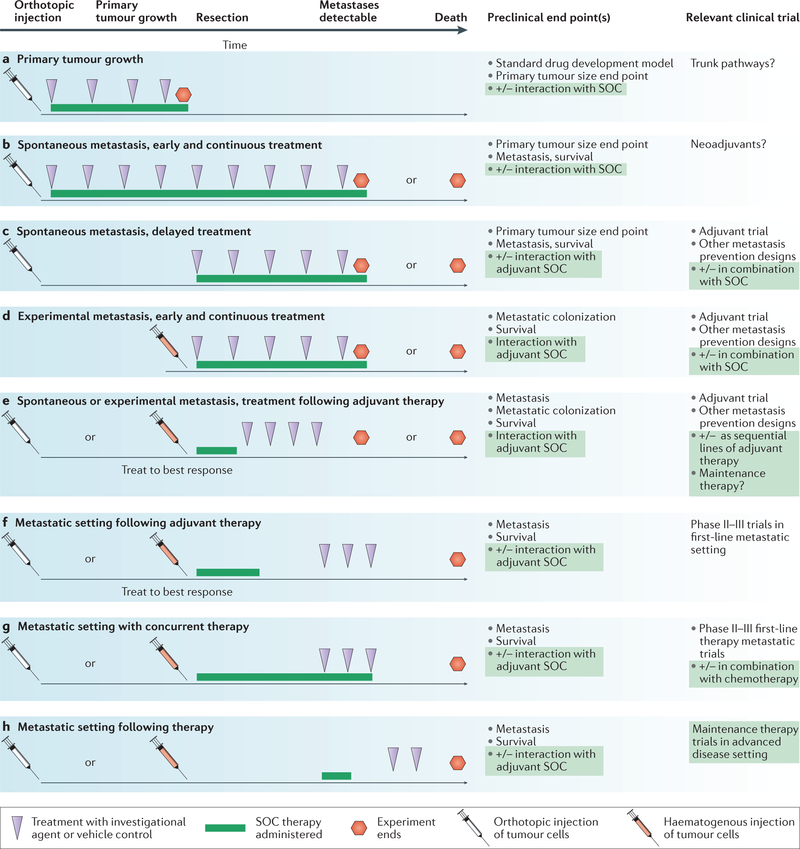
Beyond these generalities several considerations are important. FIGURE 4 shows potential designs of preclinical models for the development of metastatic therapies. Most commonly, the designs in FIG. 4b,d are reported, using either spontaneous or experimental metastasis assays and delivering the investigational compound from the beginning of the experiment onwards. However, the adjuvant setting is best modelled by administration of the investigational compound after primary tumour removal (FIG. 4c) or, potentially, treatment delayed until after tumour cells have extravasated (FIG. 4d).
Figure 4 |. Meaningful incorporation of preclinical metastasis models into drug development.
Potential experimental designs for an antimetastatic investigational agent are mapped along a timeline. Tumour cell injection can either be orthotopic, into the tissue of origin (white needles) or haematogenous for experimental metastasis (red needles). The investigational agent (purple arrowheads) can be delivered for all or part of the assay; optimally it should reflect the oral or intravenous dosing to be used in the clinic. Standard of care (SOC) therapy (green line), at a clinically achievable dose, can be added before, concurrent with or after the investigational agent and can use agents approved in the adjuvant or metastatic setting. a | Standard drug development uses primary tumour growth as an end point, which completely neglects the metastatic process. b–e | Model systems with different degrees of applicability to adjuvant setting trials preventing metastatic colonization. f–h | Model systems with different applicabilities to metastatic setting trials. End points include the number and size of metastases by histology and imaging, pharmacodynamic markers of drug activity or tumour biology, and survival.
Additional parameters can be considered using osteolytic bone metastasis (TABLE 1) as an example. Bone metastases occur predominantly in ER+ breast cancer and this subtype of cell line should be used in the preclinical model. ER+ breast cancer cells can form bone metastases following left cardiac ventricle injection155. The investi gational compound can be started after tumour cell injection in a prevention scenario, or after the development of lesions in a metastatic setting. Incorporation of SOC therapy is an often overlooked but important variable. The SOC could include an anti-hormonal treatment and denosumab. The relationship of SOC and investigational agent dosing must be decided — will the investigational agent be dosed with denosumab, or following progression on denosumab? Most of the proposed osteolytic bone metastasis investigational agents have not demonstrated interaction with, or superiority to, denosumab, which constitutes incomplete preclinical validation.
Traditional drug development parameters also matter. Was a clinically achievable dose and schedule of compounds used? Was the route of administration comparable to what will be used in the clinic, typically oral or intravenous? Did the compound hit its target? Can these data be used to identify and test biomarkers or pharmaco dynamic markers for prediction of clinical responses?
For outcomes, imaging must be used in conjunction with histopathology. Has the model just produced micrometastases? These lesions could persist without complications in the human, or could progressively grow, so their importance is uncertain. How are micrometastases and macrometastases delineated? Data from more than one metastasis model system, and often more than one target organ are needed; examples exist in which metastasis rates vary in different organs156. In addition to metastasis counts, survival end points can be used.
The next generation of models will evaluate clonal mutational dynamics, in both treatment-naive and treatment-resistant settings157. Deep sequencing will also be valuable in identifying tumour neoantigens as candidates and biomarkers of immunotherapy90. More models must be developed to reflect the heterogeneity of human disease. These improved models must be tied to expanded data on the transcriptional and genomic diversity in human metastases.
Embracing combination therapies.
Given the extraordinary genomic and phenotypic instability of metastatic tumour cells, it is almost inconceivable that a single metastasis-directed monotherapy will cure the majority of patients. Human immunodeficiency virus (HIV) is another deadly therapeutic target that, like metastatic cancer, easily mutates. Initial efforts with monotherapies produced some responses but they lacked durability. Recognizing that a dire public health emergency required concerted efforts, two and eventually three drugs produced by different companies were combined into an effective, durable therapy that minimized the development of resistance158. Other important aspects of this effort that are salient to metastasis research include combining distinct classes of drugs, and having a test for minimal residual disease to monitor efficacy. Along with the success of the original 3-drug combination, 25 drugs in 6 classes have received FDA approval, providing back-up regimens.
Clinical trials for metastasis-directed therapies.
It is hypothesized that interruption of metastatic pathways will significantly augment the benefits of current cancer therapies. Because these pathways are functional in most cancer types, drug development and successful clinical testing offers the possibility of a blockbuster drug that is applicable to many patients159. Most preclinical metastasis therapy experiments point to a significant delay in the development of metastases rather than a shrinkage of established metastatic lesions. This end point should be beneficial to the clinical prevention of an initial metas tasis, or the prevention of additional metastases in patients with limited, treated, metastatic disease, in adjuvant setting trials.
Many antimetastatic therapies under development interrupt colonization pathways rather than kill a proliferating tumour cell. They are cytostatic, not cytotoxic. Such agents will shrink an established lesion only if they coincidentally synergize with chemotherapy or radiation therapy. These facts lead to the disturbing conclusion that antimetastatic therapies will not produce traditional responses (complete response (CR) or partial response (PR)) in metastatic setting trials — stable disease would be the best expected result of a cytostatic agent. It is likely that many compounds with metastasis-preventive activity have failed in traditional clinical trials and are lost.
Designs that are germane to the preclinical end point of prevention or delay of metastasis include trials in the adjuvant setting. Adjuvant trials typically deliver systemic therapy following initial surgery to patients with colon, lung, pancreatic, breast, prostate and other cancers. The hypothesis is that tumour cells have already escaped the primary mass and are residing in distant locations as occult tumour cells or micrometastases and so adjuvant therapy should prevent their outgrowth. Primary end points include PFS and OS. There are substantial problems implementing adjuvant trials for potential metastasis-preventive therapies: adjuvant trials typically require large patient populations, take a relatively long time to mature and incur high costs. Thus they are considered only when there is a wealth of positive earlier clinical data. This is a problem if a cyto-static metastasis-preventive therapy has not produced responses in traditional Phase II trials of patients with metastatic disease.
Several alternative trial designs could measure metastasis prevention. Smaller trials using super-high-risk patients such as those with multiple positive lymph nodes could be useful for a signal of antimetastatic activity160. Metastatic disease would develop more rapidly and in a higher percentage of patients, minimizing the time needed and the size of the cohort.
For some cancers initial metastatic lesions are successfully treated, but the patient remains at an unacceptably high risk for the development of additional metastases. These patients can be randomized to a metastasis-preventive agent or placebo in a randomized Phase II secondary metastasis prevention trial. Examples include post-curative surgery for liver metastases161, and post-neurosurgery or stereotactic radiotherapy for brain metastases160. The primary end point would be time to a new metastasis. Alternative end points such as circulating tumour cells or circulating tumour DNA can be investigated. If eventually validated, it is possible that these easily accessed biopsy methods could serve as earlier end points to hasten clinical trials.
In lung cancer, advanced disease is treated with chemo-therapy, resulting in clinical benefit that is often short term in duration. Phase III randomized maintenance therapy trials have been extensively conducted. Patients with advanced cancer received standard initial chemotherapy and were then randomized to a long course of investigational therapy (chemotherapy or truncal mutation inhibitor therapy) or placebo until progression or unacceptable toxicity occurred; end points were PFS or OS. Such designs could be adapted to include a metastasis-preventive agent, to halt further colonization or to induce dormancy. Similar trial designs of long-term treatment with a low dose of drug have been proposed to induce metastatic dormancy in genitourinary cancers, called metronomic therapy3.
Conclusion
Interruption of metastasis pathways holds preclinical and clinical promise for cancer patients with, or at risk of, metastatic disease. Multiple agents have been demonstrated to prevent or shrink metastases and serve as targets for therapeutic development. Lessons learned from HIV therapeutic development may prove useful, that is, to combine multiple classes of therapy, treat early and have multiple secondary options.
It is the responsibility of researchers to provide pre-clinical model data that adequately validate a potential antimetastasis therapeutic agent. Furthermore, there should be a responsibility to prioritize and fund grants to perform this work. Given the hesitancy of the pharmaceutical industry, academic and government organizations will need to fund alternative metastasis prevention trials until a sign of success emerges.
DATABASES
SEER: http://seer.cancer.gov/
FURTHER INFORMATION
FDA approval for bevacizumab: http://www.cancer.gov/about-cancer/treatment/drugs/fda-bevacizumab
FDA approval for dasatinib: http://www.cancer.gov/about-cancer/treatment/drugs/fda-dasatinib
FDA approval for ipilimumab: http://www.cancer.gov/about-cancer/treatment/drugs/fda-ipilimumab
FDA approval for nivolumab: http://www.cancer.gov/about-cancer/treatment/drugs/nivolumab
FDA approval for pembrolizumab: http://www.cancer.gov/about-cancer/treatment/drugs/fda-pembrolizumab
FDA approval for plerixafor: http://www.cancer.gov/about-cancer/treatment/drugs/fda-plerixafor
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Glossary
- Standard of care
(SOC). Also known as best practice, treatment for each type and stage of cancer that is accepted in general practice by health care professionals, and iterated in guidelines such as those of the US National Comprehensive Cancer Network.
- Metastatic colonization
The progressive growth of a lesion in a foreign location.
- Invasion
Cancer cells traverse normal tissues in groups or as single cells, using reversible adhesion, proteolytic destruction and motility.
- Genomic instability
A state of high frequency of mutations in a cell, including nucleic acid sequences, chromosomal rearrangements and aneuploidy.
- Localized disease
In the clinic, disease that is limited to the tissue or organ in which it began.
- Regional disease
Cancer that has grown beyond the original tumour and spread to nearby lymph nodes or tissues.
- Overall survival
(OS). The length of time, either from disease diagnosis or the beginning of treatment, until death.
- Progression-free survival
(PFS). A metric of patient response to therapy, measured from the time of treatment initiation or clinical trial enrolment until either detectable lesions increase, based on standard measurement criteria, or the patient dies.
- Adjuvant trials
Clinical trials to test whether an additional treatment after primary therapy will lower the risk of cancer recurrence.
- Pathological complete response
(pCR). The absence of residual invasive tumour cells by microscopic examination of resected tissue after neoadjuvant therapy.
- Neoadjuvant trial
In cancer, a trial testing a potential therapy before the ‘definitive’ treatment, such as primary tumour surgery.
- Half-life
In pharmacology, the time it takes for a compound to fall to one-half of its initial steady-state level.
- G-protein-coupled receptors
A family of integral membrane receptors that sense extracellular signals and activate intracellular signalling by binding to G proteins.
- Focal adhesion kinase
(FAK). Cytosolic non-receptor protein kinase typically linking extracellular matrix with the actin network, regulating cell adhesion, viability and spreading.
- Stable disease
A metric of patient response to therapy, in which measurable lesions are neither increasing nor decreasing based on standard measurement criteria.
- Maximum tolerated dose
(MTD). The highest dose of a drug or treatment that does not cause unacceptable side effects.
- Extravasation
In metastasis, the movement of tumour cells out of the circulatory system into surrounding tissues.
- Stem or tumour-initiating cell
A cell found within a cancer that is tumorigenic and can differentiate into one of several cell types found within the tumour.
- Anoikis
A form of programmed cell death induced by anchorage-dependent cells detaching from an extracellular matrix.
- Neoantigens
Peptides absent from the normal genome, caused by somatic mutations.
- Myofibroblasts
Cells with attributes of fibroblasts and smooth muscle cells that are activated to participate in wound repair.
- Mismatch repair
A form of DNA repair that corrects erroneous misincorporation of bases during replication, and other insertions, deletions and DNA damage.
- Double strand break repair
Repair of hazardous lesions in which both strands of DNA are broken, by non-homologous end joining or homologous recombination repair.
- Nonspecific immunity
Also called innate immunity, host responses to pathogens or tumour cells that do not provide long-term memory or protection.
- Adaptive immunity
Part of the immune system by which memory is acquired after an initial response to a specific antigen.
- Natural killer (NK) cells
Lymphocytes that are cytotoxic for virally infected or tumour cells, without the need for major histocompatibility complex (MHC) and T cell receptor signalling.
- Minimal residual disease
In leukaemia, a low level of tumour cells or their products in patients apparently treated successfully, detectable only with molecular markers.
- Astrocytes
Star-shaped cells in the brain and spinal cord that maintain the blood–brain barrier, provide nutrients, maintain ion balance and assist in injury repair.
- Microglia
Resident macrophage-like cells of the brain and spinal cord.
- Neuroinflammatory response
Inflammation of the central nervous system characterized by activation of endothelial and glial cells, cytokines and oedema.
- α-particle
A particle for radiation therapy consisting of a helium nucleus with high energy and low penetrance.
- Xenografts
In cancer, cells or tissues transplanted from one species to another, often human cancer cells into immunodeficient mice.
- Genetically engineered mouse (GEM) models
Mouse models in which the genome has been altered, including transgenes and targeted mutations (knockouts or knockins).
- Patient-derived xenografts
(PDXs). Patient tumour tissues implanted directly into immunodeficient mice.
- Micrometastases
Metastatic lesions that are too small for conventional detection.
Footnotes
Competing interests statement
The authors declare competing interests: see Web version for details.
References
- 1.Brosnan JA & Iacobuzio-Donahue CA A new branch on the tree: next-generation sequencing in the study of cancer evolution. Semin. Cell Dev. Biol 23, 237–242 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paget S The distribution of secondary growths in cancer of the breast. Lancet 1, 99–101 (1889). [PubMed] [Google Scholar]; The origin of the seed and soil hypothesis of metastasis.
- 3.Hensel JA, Flaig TW & Theodorescu D Clinical opportunities and challenges in targeting tumour dormancy. Nat. Rev. Clin. Oncol 10, 41–51 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Husemann Y et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Bojovic B & Crowe DL Dysfunctional telomeres promote genomic instability and metastasis in the absence of telomerase activity in oncogene induced mammary cancer. Mol. Carcinogen 52, 103–117 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Vermaat JS et al. Primary colorectal cancers and their subsequent hepatic metastases are genetically different: implications for selection of patients for targeted treatment. Clin. Cancer Res 18, 688–699 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Yachida S et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1126 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roschke A et al. Chromosomal instability is associated with higher expression of genes implicated in epithelial-mesenchymal transition, cancer invasiveness, and metastasis and with lower expression of genes involved in cell cycle checkpoints, DNA repair, and chromatin maintenance. Neoplasia 10, 1222–1230 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong MKH et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat. Commun 6, 6605 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that prostate cancer metastases harbour actionable mutations not found in matched primary tumours.
- 10.Luzzi KJ et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol 153, 865–873 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD & Jemal A Cancer statistics, 2015. CA Cancer J. Clin 65, 5–29 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Jemal A et al. Cancer statistics, 2005. CA Cancer J. Clin 55, 10–30 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Hodi FS et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reported the improved OS of patients with metastatic melanoma using an immune checkpoint inhibitor.
- 14.Scher HI et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med 367, 1187–1197 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Tevaarwerk AJ et al. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer 119, 1140–1148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernards N et al. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann. Oncol 24, 3056–3060 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Worni M et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the Surveillance, Epidemiology, and End Results registry from 1988 to 2008. Pancreas 42, 1157–1163 (2013). [DOI] [PubMed] [Google Scholar]
- 18.Lin TH et al. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/enzalutamide versus anti-androgen receptor ASC-J9 (R) lead to promotion versus suppression of prostate cancer metastasis. J. Biol. Chem 288, 19359–19369 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez-Laorden B et al. BRAF inhibitors induce metastasis in RAS mutant or inhibitor-resistant melanoma cells by reactivating MEK and ERK signaling. Sci. Signal 7, ra30 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Volk-Draper L et al. Paclitaxel therapy promotes breast cancer metastasis in a TLR4-dependent manner. Cancer Res 74, 5421–5434 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunjal PM et al. Evidence for induction of a tumor metastasis-receptive microenvironment for ovarian cancer cells in bone marrow and other organs as an unwanted and underestimated side effect of chemotherapy/radiotherapy. J. Ovarian Res 8, 20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin TH et al. Anti-androgen receptor ASC-J9 versus anti-androgens MDV3100 (enzalutamide) or casodex (bicalutamide) leads to opposite effects on prostate cancer metastasis via differential modulation of macrophage infiltration and STAT3-CCL2 signaling. Cell Death Dis 4, e764 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pool SE et al. mTOR Inhibitor RAD001 promotes metastasis in a rat model of pancreatic neuroendocrine cancer. Cancer Res 73, 12–18 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Guerin E, Man S, Xu P & Kerbel RSA Model of postsurgical advanced metastatic breast cancer more accurately replicates the clinical efficacy of antiangiogenic drugs. Cancer Res 73, 2743–2748 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratajczak MZ, Jadczyk T, Schneider G, Kakar SS & Kucia M Induction of a tumor-metastasis-receptive microenvironment as an unwanted and underestimated side effect of treatment by chemotherapy or radiotherapy. J. Ovarian Res 6, 95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLeskey SW et al. Fibroblast growth factor-IV transfection of MCF-7 cells produces cell-lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude-mice. Cancer Res 53, 2168–2177 (1993). [PubMed] [Google Scholar]
- 27.Onoda JM, Jacobs JR, Taylor JD, Sloane BF & Honn KV Cisplatin and nifedipine: synergistic cytotoxicity against murine solid tumors and their metastases. Cancer Lett 30, 181–188 (1986). [DOI] [PubMed] [Google Scholar]
- 28.Mundy G Metastasis to the bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Kostenuik PJ et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J. Bone Miner. Res 24, 182–195 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Fizazi K et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377, 813–822 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stopeck AT et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J. Clin. Oncol 28, 5132–5139 (2010). [DOI] [PubMed] [Google Scholar]; Phase III trial of denosumab to improve the time until an SRE in bone metastatic breast cancer.
- 32.Smith MR et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379, 39–46 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; Phase III trial demonstrating improvement of bone metastasis-free survival in prostate cancer.
- 33.Gnant M et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 386, 433–443 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Folkman J Role of angiogenesis in tumor growth and metastasis. Semin. Oncol 29, 15–18 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Aghajanian C et al. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol 139, 10–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perren TJ et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med 365, 2484–2496 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Herbst RS et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 377, 1846–1854 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sledge GW Anti-vascular endothelial growth factor therapy in breast cancer: game over? J. Clin. Oncol 33, 133–135 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Kim KB et al. BEAM: a randomized phase II study evaluating the activity of bevacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated advanced melanoma. J. Clin. Oncol 30, 34–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kindler HL et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol 28, 3617–3622 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron D et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol 14, 933–942 (2013). [DOI] [PubMed] [Google Scholar]
- 42.de Gramont A et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 13, 1225–1233 (2012). [DOI] [PubMed] [Google Scholar]
- 43.Allegra CJ et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J. Clin. Oncol 29, 11–16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Minckwitz G et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N. Engl. J. Med 366, 299–309 (2012). [DOI] [PubMed] [Google Scholar]
- 45.Bear HD et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N. Engl. J. Med 366, 310–320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weisshardt P et al. Tumor vessel stabilization and remodeling by anti-angiogenic therapy with bevacizumab. Histochem. Cell Biol 137, 391–401 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Fan F et al. Chronic exposure of colorectal cancer cells to bevacizumab promotes compensatory pathways that mediate tumour cell migration. Br. J. Cancer 104, 1270–1277 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Groot JF et al. Tumor invasion after treatment of glioblastoma with bevacizumab: radiographic and pathologic correlation in humans and mice. Neuro-Oncol. 12, 233–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yin T et al. Antiangiogenic therapy using sunitinib combined with rapamycin retards tumor growth but promotes metastasis. Transl Oncol 7, 221–229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebos J et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell 15, 232–239 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paez-Ribes M et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that VEGF inhibitors inhibited tumour growth but accelerated progression in mouse model systems.
- 52.Mazzieri R et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19, 512–526 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Vermeulen PB et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol 195, 336–342 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Stessels F et al. Breast adenocarcinoma liver metastases, in contrast to colorectal cancer liver metastases, display a non-angiogenic growth pattern that preserves the stroma and lacks hypoxia. Br. J. Cancer 90, 1429–1436 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kienast Y et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med 16, 116–122 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Kusters B et al. Differential effects of vascular endothelial growth factor A isoforms in a mouse brain metastasis model of human melanoma. Cancer Res 63, 5408–5413 (2003). [PubMed] [Google Scholar]
- 57.Maniotis A et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol 155, 739–752 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sottnik JL et al. Integrin α2β1 (α2β1) promotes prostate cancer skeletal metastasis. Clin. Exp. Metastasis 30, 569–578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou B et al. Integrin α3 β1 can function to promote spontaneous metastasis and lung colonization of invasive breast carcinoma. Mol. Cancer Res 12, 143–154 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shibue T, Brooks MW & Weinberg RA An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24, 481–498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oku N et al. Liposomal ARG-GLY-ASP analogs effectively inhibit metastatic B16 melanoma colonization in murine lungs. Life Sci 58, 2263–2270 (1996). [DOI] [PubMed] [Google Scholar]
- 62.Hardan I et al. Inhibition of metastatic cell colonization in murine lungs and tumor-induced morbidity by nonpeptidic Arg-Gly-Asp mimetics. Int. J. Cancer 55, 1023–1028 (1993). [DOI] [PubMed] [Google Scholar]
- 63.Tentori L et al. The integrin antagonist cilengitide increases the antitumor activity of temozolomide against malignant melanoma. Oncol. Rep 19, 1039–1043 (2008). [PubMed] [Google Scholar]
- 64.Yamada S et al. Effect of the angiogenesis inhibitor cilengitide on glioblastoma growth in nude mice. Neurosurgery 59, 1304–1312 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Mason WP End of the road: confounding results of the CORE trial terminate the arduous journey of cilengitide for glioblastoma. Neuro-Oncol 17, 634–635 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manegold C et al. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Investigat. New Drugs 31, 175–182 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alva A et al. Phase II study of cilengitide (EMD 121974, NSC 707544) in patients with nonmetastatic castration resistant prostate cancer, NCI-6735. A study by the DOD/PCF prostate cancer clinical trials consortium. Investigat. New Drugs 30, 749–757 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim KB et al. A randomized phase II study of cilengitide in patients with metastatic melanoma. Melanoma Res 22, 294–301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim LC, Song LX & Haura EB Src kinases as therapeutic targets for cancer. Nat. Rev. Clin. Oncol 6, 587–595 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Yori JL et al. Combined SFK/mTOR inhibition prevents rapamycin-induced feedback activation of AKT and elicits efficient tumor regression. Cancer Res 74, 4762–4771 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gucalp A et al. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin. Breast Cancer 11, 306–311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Finn RS et al. Dasatinib as a single agent in triple-negative breast cancer: results of an open-label phase 2 study. Clin. Cancer Res 17, 6905–6913 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Mayer EL et al. A phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancer. Clin. Cancer Res 17, 6897–6904 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Schilder RJ et al. Phase II evaluation of dasatinib in the treatment of recurrent or persistent epithelial ovarian or primary peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol 127, 70–74 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharma MR et al. Dasatinib in previously treated metastatic colorectal cancer: a phase II trial of the University of Chicago Phase II Consortium. Investigat. New Drugs 30, 1211–1215 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kluger HM et al. A Phase 2 trial of dasatinib in advanced melanoma. Cancer 117, 2202–2208 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gangadhar TC, Clark JI, Karrison T & Gajewski TF Phase II study of the Src kinase inhibitor saracatinib (AZD0530) in metastatic melanoma. Investigat. New Drugs 31, 769–773 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molina JR et al. A phase II trial of the Src-kinase inhibitor saracatinib after four cycles of chemotherapy for patients with extensive stage small cell lung cancer: NCCTG trial N-0621. Lung Cancer 85, 245–250 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fury MG et al. Phase II study of saracatinib (AZD0530) for patients with recurrent or metastatic head and neck squamous cell carcinoma (HNSCC). Anticancer Res 31, 249–253 (2011). [PMC free article] [PubMed] [Google Scholar]
- 80.Mackay HJ et al. A phase II trial of the Src kinase inhibitor saracatinib (AZD0530) in patients with metastatic or locally advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: a trial of the PMH phase II consortium. Investigat. New Drugs 30, 1158–1163 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Pusztai L et al. Gene signature-guided dasatinib therapy in metastatic breast cancer. Clin. Cancer Res 20, 5265–5271 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Yang JC et al. Effect of the specific Src family kinase inhibitor saracatinib on osteolytic lesions using the PC-3 bone model. Mol. Cancer Ther 9, 1629–1637 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Koreckij T et al. Dasatinib inhibits the growth of prostate cancer in bone and provides additional protection from osteolysis. Br. J. Cancer 101, 263–268 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Twardowski PW et al. A phase II trial of dasatinib in patients with metastatic castration-resistant prostate cancer treated previously with chemotherapy. Anti-Cancer Drugs 24, 743–753 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu EY et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology 77, 1166–1171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu EY et al. Phase II study of dasatinib in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res 15, 7421–7428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Araujo JC et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol 14, 1307–1316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gerlinger M et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med 366, 883–892 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braun S et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med 353, 793–802 (2005). [DOI] [PubMed] [Google Scholar]
- 90.Schumacher T & Schreiber R Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015). [DOI] [PubMed] [Google Scholar]; This study undertook neoantigen quantification in multiple cancer types and demonstrated its potential relevance to immunotherapy.
- 91.Fidler I Critical factors in the biology of human cancer metastasis. Am. Surg 61, 1065–1066 (1995). [PubMed] [Google Scholar]
- 92.Taichman RS et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS ONE 8, e61873 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawson MA et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun 6, 9983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aguirre-Ghiso JA, Estrada Y, Liu D & Ossowski L ERKMAPK activity as a determinant of tumor growth and dormancy: regulation by p38(SAPK). Cancer Res 63, 1684–1695 (2003). [PubMed] [Google Scholar]
- 95.Marshall JCA et al. Effect of inhibition of the lysophosphatidic acid receptor 1 on metastasis and metastatic dormancy in breast cancer. J. Natl Cancer Inst 104, 1306–1319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; An LPAR1 inhibitor prevented metastasis and induced aspects of metastatic dormancy.
- 96.Lawson DA et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 526, 131–135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu X et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 20, 701–714 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barkan D et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res 68, 6241–6250 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naumov G et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late developing metastases. Breast Cancer Res. Treat 82, 199–206 (2003). [DOI] [PubMed] [Google Scholar]; Metastases from an aggressive and a dormant cell line responded differently to chemotherapy.
- 100.Goss PE & Chambers AF Does tumour dormancy offer a therapeutic target? Nat. Rev. Cancer 10, 871–877 (2010). [DOI] [PubMed] [Google Scholar]
- 101.Albanese I et al. Heterogeneity within and between primary colorectal carcinomas and matched metastases as revealed by analysis of Ki-ras and p53 mutations. Biochem. Biophys. Res. Commun 325, 784–791 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Bartosch C et al. Endometrial endometrioid carcinoma metastases show decreased ER-α and PR-A expression compared to matched primary tumors. PLoS ONE 10, e0134969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jordan VC, Curpan R & Maximov PY Estrogen receptor mutations found in breast cancer metastases integrated with the molecular pharmacology of selective ER modulators. J. Natl Cancer Inst 107, djv075 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singhi AD et al. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Modern Pathol 25, 378–387 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Colombino M et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J. Clin. Oncol 30, 2522–2529 (2012). [DOI] [PubMed] [Google Scholar]
- 106.Eriksson H et al. BRAF(V600E) protein expression in primary cutaneous malignant melanomas and paired metastases. JAMA Dermatol 151, 410–416 (2015). [DOI] [PubMed] [Google Scholar]
- 107.Surriga O et al. Crizotinib, a c-Met inhibitor, prevents metastasis in a metastatic uveal melanoma model. Mol. Cancer Ther 12, 2817–2826 (2013). [DOI] [PubMed] [Google Scholar]
- 108.Moody SE et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2, 451–461 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Lenfert E et al. Mutant p53 promotes epithelialmesenchymal plasticity and enhances metastasis in mammary carcinomas of WAP-T mice. Int. J. Cancer 136, E521–E533 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Morton JP et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl Acad. Sci. USA 107, 246–251 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bandyopadhyay A, Wang L, Chin SH & Sun LZ Inhibition of skeletal metastasis by ectopic ERα expression in ERα-negative human breast cancer cell lines. Neoplasia 9, 113–118 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brastianos P et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov 5, 1164–1177 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; Fifty-three per cent of brain metastases harboured actionable mutations not detected in the matched primary tumour.
- 113.Amersi FF et al. Activation of CCR9/CCL25 in cutaneous melanoma mediates preferential metastasis to the small intestine. Clin. Cancer Res 14, 638–645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kitamura T et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med 212, 1043–1059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zheng J et al. Chemokine receptor CX3CR1 contributes to macrophage survival in tumor metastasis. Mol. Cancer 12, 141 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Biragyn A et al. Inhibition of lung metastasis by chemokine CCL17-mediated in vivo silencing of genes in CCR4+ Tregs. J. Immunother 36, 258–267 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kee JY et al. Chemokine CXCL16 suppresses liver metastasis of colorectal cancer via augmentation of tumor-infiltrating natural killer T cells in a murine model. Oncol. Rep 29, 975–982 (2013). [DOI] [PubMed] [Google Scholar]
- 118.Zhao L et al. Recruitment of a myeloid cell subset (CD11b/Gr1(mid)) via CCL2/CCR2 promotes the development of colorectal cancer liver metastasis. Hepatology 57, 829–839 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Phillips RJ et al. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am. J. Respir. Crit. Care Med 167, 1676–1686 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Kajiyama H et al. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int. J. Cancer 122, 91–99 (2008). [DOI] [PubMed] [Google Scholar]
- 121.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02179970.
- 122.Lok E, Chung AS, Swanson KD & Wong ET Melanoma brain metastasis globally reconfigures chemokine and cytokine profiles in patient cerebrospinal fluid. Melanoma Res 24, 120–130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Erler JT et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440, 1222–1226 (2006). [DOI] [PubMed] [Google Scholar]; This paper reports that LOX is a crucial component of metastatic colonization.
- 124.Canesin G et al. Lysyl oxidase-like 2 (LOXL2) and E47 EMT factor: novel partners in E-cadherin repression and early metastasis colonization. Oncogene 34, 951–964 (2015). [DOI] [PubMed] [Google Scholar]
- 125.Hecht JR et al. A phase II, randomized, double-blinded, placebo-controlled study of simtuzumab or placebo in combination with FOLFIRI for the second line treatment of metastatic KRAS mutant colorectal adenocarcinoma. J. Clin. Oncol 33 (Suppl.) abstract 3537 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Erler JT & Giaccia AJ Lysyl oxidase mediates hypoxic control of metastasis. Cancer Res 66, 10238–10241 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Ostenfeld MS et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res 74, 5758–5771 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Valencia K et al. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol. Oncol 8, 689–703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shimbo K et al. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun 445, 381–387 (2014). [DOI] [PubMed] [Google Scholar]
- 130.Costa-Silva B et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol 17, 816–826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peinado H et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med 18, 883–891 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le MTN et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J. Clin. Invest 124, 5109–5128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang L et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; Exosomes contribute to metastatic colonization of the brain by altering astrocyte–tumour interactions.
- 134.Pan QW et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut 61, 1330–1339 (2012). [DOI] [PubMed] [Google Scholar]
- 135.Marleau AM, Chen CS, Joyce JA & Tullis RH Exosome removal as a therapeutic adjuvant in cancer. J. Transl Med 10, 134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01779583 (2015).
- 137.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02393703 (2016).
- 138.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01550523 (2013).
- 139.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT01159288 (2010).
- 140.US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/ct2/show/NCT02439008 (2015).
- 141.Hu GH et al. MTDH activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell 15, 9–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang XHF et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 16, 67–78 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; SRC is a potential therapeutic target for dormant bone metastatic tumours.
- 143.Steeg PS, Camphausen KA & Smith QR Brain metastases as preventive and therapeutic targets. Nat. Rev. Cancer 11, 352–363 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fitzgerald D et al. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clin. Exp. Metast 25, 799–810 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lockman PR et al. Heterogeneous blood–tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res 16, 5664–5678 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kwon ED et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc. Natl Acad. Sci. USA 96, 15074–15079 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iwai Y et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Strome SE et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res 63, 6501–6505 (2003). [PubMed] [Google Scholar]
- 149.Peggs KS, Quezada SA, Chambers CA, Korman AJ & Allison JP Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med 206, 1717–1725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Parker C et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med 369, 213–223 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Milenic DE, Baidoo KE, Kim YS & Brechbiel MW Evaluation of cetuximab as a candidate for targeted alpha-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. Mabs 7, 255–264 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports a novel therapeutic strategy for established metastatic disease.
- 152.Hurvitz SA et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J. Clin. Oncol 31, 1157–1163 (2013). [DOI] [PubMed] [Google Scholar]
- 153.Gould SE, Junttila MR & De Sauvage FJ Translational value of mouse models in oncology drug development. Nat. Med 21, 431–439 (2015). [DOI] [PubMed] [Google Scholar]
- 154.DeRose YS et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med 17, 1514–1520 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that PDXs provided new metastasis models that compared closely to patient outcomes.
- 155.Yi B, Williams PJ, Niewolna M, Wang Y & Yoneda T Tumor-derived platelet-derived growth factor-BB plays a critical role in osteosclerotic bone metastasis in an animal model of human breast cancer. Cancer Res 62, 917–923 (2002). [PubMed] [Google Scholar]
- 156.Gu B, Espana L, Mendez O, Torregrosa A & Sierra A Organ-selective chemoresistance in metastasis from human breast cancer cells: inhibition of apoptosis, genetic variability and microenvironment at the metastatic focus. Carcinogenesis 25, 2293–2301 (2004). [DOI] [PubMed] [Google Scholar]
- 157.Bhang HEC et al. Studying clonal dynamics in response to cancer therapy using high-complexity barcoding. Nat. Med 21, 440–448 (2015). [DOI] [PubMed] [Google Scholar]
- 158.Lange J & Ananworanich J The discovery and development of antiretroviral agents. Antiviral Ther 19 (Suppl. 3), 5–14 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Weber G Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett 328, 207–211 (2013). [DOI] [PubMed] [Google Scholar]
- 160.Steeg PS Perspective: the right trials. Nature 485, S58–S59 (2012). [DOI] [PubMed] [Google Scholar]
- 161.Steeg P & Theodorescu D Metastasis: a therapeutic target for cancer. Nat. Clin. Pract. Oncol 5, 206–219 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li S, Wang N & Brodt P Metastatic cells can escape the proapoptotic effects of TNF-α through increased autocrine IL-6/STAT3 signaling. Cancer Res 72, 865–875 (2012). [DOI] [PubMed] [Google Scholar]
- 163.Casar B et al. Blocking of CDCP1 cleavage in vivo prevents Akt-dependent survival and inhibits metastatic colonization through PARP1-mediated apoptosis of cancer cells. Oncogene 31, 3924–3938 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Woditschka S et al. DNA double-strand break repair genes and oxidative damage in brain metastasis of breast cancer. J. Natl Cancer Inst 106, dju145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peng YF et al. Promoting colonization in metastatic HCC cells by modulation of autophagy. PLoS ONE 8, e74407 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peng YF et al. Autophagy inhibition suppresses pulmonary metastasis of HCC in mice via impairing anoikis resistance and colonization of HCC cells. Autophagy 9, 2056–2068 (2013). [DOI] [PubMed] [Google Scholar]
- 167.Sahni S et al. The metastasis suppressor, N-myc downstream-regulated gene 1 (NDRG1), inhibits stress-induced autophagy in cancer cells. J. Biol. Chem 289, 9692–9709 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kelber JA et al. KRas induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res 72, 2554–2564 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang Y et al. Lifetime exposure to a soluble TGF-β antagonist protects mice against metastasis without adverse side effects. J. Clin. Invest 109, 1607–1615 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]; Preclinical validation of the prevention of metastasis and safety of potential TGFβ-directed therapeutics.
- 170.Kang Y et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell 3, 537–549 (2003). [DOI] [PubMed] [Google Scholar]
- 171.Sadok A et al. Rho kinase inhibitors block melanoma cell migration and inhibit metastasis. Cancer Res 75, 2272–2284 (2015). [DOI] [PubMed] [Google Scholar]
- 172.Tenbaum SP et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat. Med 18, 892–991 (2012). [DOI] [PubMed] [Google Scholar]
- 173.Yin T et al. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J. Surg. Res 141, 196–203 (2007). [DOI] [PubMed] [Google Scholar]
- 174.Gupta GP et al. ID genes mediate tumor reinitiation during breast cancer lung metastasis. Proc. Natl Acad. Sci. USA 104, 19506–19511 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper identifies the early steps in metastatic colonization.
- 175.Chou J et al. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat. Cell Biol 15, 201–213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Korpal M et al. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med 17, 1101–1108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu YN et al. Loss of androgen-regulated microRNA 1 activates SRC and promotes prostate cancer bone metastasis. Mol. Cell. Biol 35, 1940–1951 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Tavazoie SF et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature 451, 147–152 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yang F et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol. Cell 50, 303–304 (2013). [DOI] [PubMed] [Google Scholar]
- 180.Liao JQ et al. Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS ONE 9, e84941 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bartucci M et al. TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells. Oncogene 34, 681–690 (2015). [DOI] [PubMed] [Google Scholar]
- 182.Malanchi I et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2012). [DOI] [PubMed] [Google Scholar]; A POSTN-WNT mediated interaction between the microenvironment and tumour cells controls stemness and metastatic colonization.
- 183.Steeg P Metastasis suppressors alter the signal transduction of cancer cells. Nat. Rev. Cancer 3, 55–63 (2003). [DOI] [PubMed] [Google Scholar]
- 184.Meehan W et al. The BRMS1 metastasis suppressor forms complexes with RBP1 and the mSin3 histone deacetylase complex and represses transcription. J. Biol. Chem 279, 1562–1569 (2003). [DOI] [PubMed] [Google Scholar]
- 185.Bandyopadhyay S et al. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res 63, 1731–1736 (2003). [PubMed] [Google Scholar]
- 186.Horak CE et al. Nm23-H1 suppresses metastasis by inhibiting expression of the lysophosphatidic acid receptor EDG2. Cancer Res 67, 11751–11759 (2007). [DOI] [PubMed] [Google Scholar]
- 187.Shtivelman E A link between metastasis and resistance to apoptosis of variant small cell lung carcinoma. Oncogene 14, 2167–2173 (1997). [DOI] [PubMed] [Google Scholar]
- 188.Titus B et al. Endothelin axis is a target of the lung metastasis suppressor gene RhoGDI2. Cancer Res 65, 7320–7327 (2005). [DOI] [PubMed] [Google Scholar]
- 189.Szmulewitz RZ et al. MKK4 suppresses metastatic colonization by multiple highly metastatic prostate cancer cell lines through a transient impairment in cell cycle progression. Int. J. Cancer 130, 509–520 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fournier P et al. TGF-β signaling regulator PMEPA1 suppresses prostate cancer metastases to bone. Cancer Cell 27, 809–821 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dai JL et al. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer Res 65, 8274–8285 (2005). [DOI] [PubMed] [Google Scholar]
- 192.Mohammad KS et al. TGF-β-RI kinase inhibitor SD-208 reduces the development and progression of melanoma bone metastases. Cancer Res 71, 175–184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ostapoff KT et al. Neutralizing murine TGFβ R2 promotes a differentiated tumor cell phenotype and inhibits pancreatic cancer metastasis. Cancer Res 74, 4996–5007 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kang Y et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl Acad. Sci. USA 102, 13909–13914 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yin J et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest 103, 197–206 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Calon A et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22, 571–584 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stankic M et al. TGF-β-Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Rep 5, 1228–1242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pang YL et al. TGF-β signaling in myeloid cells is required for tumor metastasis. Cancer Discov 3, 936–951 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Northey JJ et al. Distinct phosphotyrosine-dependent functions of the ShcA adaptor protein are required for transforming growth factor beta (TGFβ)-induced breast cancer cell migration, invasion, and metastasis. J. Biol. Chem 288, 5210–5222 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kohn EA et al. Biological responses to TGF-β in the mammary epithelium show a complex dependency on Smad3 gene dosage with important implications for tumor progression. Mol. Cancer Res 10, 1389–1399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xu J et al. 14-3-3 ζ turns TGF-β’s function from tumor suppressor to metastasis promoter in breast cancer by contextual changes of Smad partners from p53 to Gli2. Cancer Cell 27, 177–192 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; 14-3-3-ζ interaction with p53 or GLI2 determines SMAD binding and TGFβ tumour suppressive versus pro-metastatic function.
- 202.Sato M et al. Differential proteome analysis identifies TGF-β-related pro-metastatic proteins in a 4T1 murine breast cancer model. PLoS ONE 10, e0126483 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yang L TGF beta, a potent regulator of tumor microenvironment and host immune response: implication for therapy. Curr. Mol. Med 10, 374–380 (2010). [DOI] [PubMed] [Google Scholar]
- 204.Le D et al. PD-1 blockade in tumors with mismatch-repair deficiency. N.Engl. J. Med 372, 2509–2520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; Pembrolizumab, an immune checkpoint inhibitor, produced responses and extended PFS in mismatch repair-deficient metastatic colorectal cancer.
- 205.Larkin J et al. Combined novolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med 373, 23–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; Phase III trial demonstrating superiority of two, versus one, immune checkpoint inhibitors as first-line treatment for stage III or IV melanoma.
- 206.Kaplan RN et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that bone marrow-derived cells arrive in potential metastatic sites before tumour cells and begin to modify the microenvironment.
- 207.Yan HH et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res 70, 6139–6149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hiratsuka S, Watanabe A, Aburatani H & Maru Y Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol 8, 1369–1375 (2006). [DOI] [PubMed] [Google Scholar]
- 209.Hiratsuka S et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a premetastatic phase. Nat. Cell Biol 10, 1349–1355 (2008). [DOI] [PubMed] [Google Scholar]
- 210.Cox TR et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 211.Chafe SC et al. Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res 75, 996–1008 (2015). [DOI] [PubMed] [Google Scholar]
- 212.Sceneay J et al. Primary tumor hypoxia recruits CD11b+/Ly6C(med)/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res 72, 3906–3911 (2012). [DOI] [PubMed] [Google Scholar]
- 213.Fong MY et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol 17, 183–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gonzalez-Zubeldia I et al. Co-migration of colon cancer cells and CAFs induced by TGF beta(1) enhances liver metastasis. Cell Tissue Res 359, 829–839 (2015). [DOI] [PubMed] [Google Scholar]
- 215.Sawada S, Murakami K, Murata J, Tsukada K & Saiki I Accumulation of extracellular matrix in the liver induces high metastatic potential of hepatocellular carcinoma to the lung. Int. J. Oncol 19, 65–70 (2001). [PubMed] [Google Scholar]
- 216.Kitamura T et al. Inactivation of chemokine (C-C motif) receptor 1 (CCR1) suppresses colon cancer liver metastasis by blocking accumulation of immature myeloid cells in a mouse model. Proc. Natl Acad. Sci. USA 107, 13063–13068 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Kim S et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–108 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Liu L et al. Reductions in myeloid-derived suppressor cells and lung metastases using AZD4547 treatment of a metastatic murine breast tumor model. Cell. Physiol. Biochem 33, 633–645 (2014). [DOI] [PubMed] [Google Scholar]
- 219.Li H et al. Activation of PPARγ in myeloid cells promotes lung cancer progression and metastasis. PLoS ONE 6, e28133 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sawant A & Ponnazhagan S Myeloid-derived suppressor cells as osteoclast progenitors: a novel target for controlling osteolytic bone metastasis. Cancer Res 73, 4606–4610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen Q, Zhang XHF & Massague J Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell 20, 538–549 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that tumour VCAM1 interaction with macrophages provides a pro-survival signal in metastatic colonization.
- 222.Chapon M et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J. Invest. Dermatol 131, 1300–1307 (2011). [DOI] [PubMed] [Google Scholar]; This study shows that immune checkpoint expression varies in metastases.
- 223.Spranger S et al. Up-regulation of PD-L1, IDO, and T-regs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl Med 5, 200ra116 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Abiko K et al. IFN-gamma from lymphocytes induces PD-L1 expression and promotes progression of ovarian cancer. Br. J. Cancer 112, 1501–1509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports that IFNγ regulates PDL1 immune checkpoint expression and function in peritoneal colonization of ovarian cancer.
- 225.Coffelt SB et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 522, 345–348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Olkhanud PB et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res 69, 5996–6004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chen LM et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun 5, 5241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Donnem T et al. Vessel co-option in primary human tumors and metastases: an obstacle to effective anti-angiogenic treatment? Cancer Med 2, 427–436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bos P et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Carbonell WS, Ansorge O, Sibson N & Muschel R The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 4, 5241 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of the perivascular niche as a crucial microenvironment for metastatic colonization of the brain.
- 231.Xing F et al. Reactive astrocytes promote the metastatic growth of breast cancer stem-like cells by activating Notch signalling in brain. EMBO Mol. Med 5, 384–396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gril B et al. Pazopanib inhibits the activation of PDGFR β-expressing astrocytes in the brain metastatic microenvironment of breast cancer cells. Am. J. Pathol 182, 2368–2379 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Noda M et al. IL-6 receptor is a possible target against growth of metastasized lung tumor cells in the brain. Int. J. Mol. Sci 14, 515–526 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sartorius C et al. Estrogen promotes the brain metastatic colonization of triple negative breast cells via an astrocyte-mediated paracrine mechanism. Oncogene, 10.1038/onc.2015.353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Binder C et al. Relaxins enhance growth of spontaneous murine breast cancers as well as metastatic colonization of the brain. Clin. Exp. Metastasis 31, 57–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Silver DJ et al. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J. Neurosci 33, 15603–15617 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Louie E et al. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene 32, 4064–4077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pukrop T et al. Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58, 1477–1489 (2010). [DOI] [PubMed] [Google Scholar]
- 239.Liu Y et al. Premetastatic soil and prevention of breast cancer brain metastasis. Neuro-Oncol 15, 891–903 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Liu Y et al. Expression of antigen processing and presenting molecules in brain metastasis of breast cancer. Cancer Immunol. Immunother 61, 789–801 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen EI et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res 67, 1472–1486 (2007). [DOI] [PubMed] [Google Scholar]
- 242.Valiente M et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fitzgerald DP et al. Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Res 72, 144–153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Bock F et al. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Invest. Ophthalmol. Vis. Sci 48, 2545–2552 (2007). [DOI] [PubMed] [Google Scholar]
- 245.Ferrara N, Hillan KJ, Gerber HP & Novotny W Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov 3, 391–400 (2004). [DOI] [PubMed] [Google Scholar]
- 246.Bauerle T et al. Bevacizumab inhibits breast cancer-induced osteolysis, surrounding soft tissue metastasis, and angiogenesis in rats as visualized by VCT and MRI. Neoplasia 10, 511–520 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ninomiya S et al. Effect of bevacizumab, a humanized monoclonal antibody to vascular endothelial growth factor, on peritoneal metastasis of MNK-45P human gastric cancer in mice. J. Surg. Res 154, 196–202 (2009). [DOI] [PubMed] [Google Scholar]
- 248.Burke PA et al. Cilengitide targeting of αvβ3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res 62, 4263–4272 (2002). [PubMed] [Google Scholar]
- 249.Kurozumi K, Ichikawa T, Onishi M, Fujii K & Date I Cilengitide treatment for malignant glioma: current status and future direction. Neurol. Med.-Chirurg 52, 539–547 (2012). [DOI] [PubMed] [Google Scholar]
- 250.Shah NP et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science 305, 399–401 (2004). [DOI] [PubMed] [Google Scholar]
- 251.Dunn EF et al. Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab. Oncogene 30, 561–574 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Arcaroli JJ et al. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin. Cancer Res 16, 4165–4177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Levitt JM, Yamashita H, Jian W, Lerner SP & Sonpavde G Dasatinib is preclinically active against Src-overexpressing human transitional cell carcinoma of the urothelium with activated Src signaling. Mol. Cancer Ther 9, 1128–1135 (2010). [DOI] [PubMed] [Google Scholar]
- 254.Morton JP et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 139, 292–303 (2010). [DOI] [PubMed] [Google Scholar]
- 255.Chan CM et al. Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis. Clin. Cancer Res 18, 3580–3591 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yamaguchi H et al. Saracatinib impairs the peritoneal dissemination of diffuse-type gastric carcinoma cells resistant to Met and fibroblast growth factor receptor inhibitors. Cancer Sci 105, 528–536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Zhang SY et al. Src family kinases as novel therapeutic targets to treat breast cancer brain metastases. Cancer Res 73, 5764–5774 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Trevino JG et al. Inhibition of Src expression and activity inhibits tumor progression and metastasis of human pancreatic adenocarcinoma cells in an orthotopic nude mouse model. Am. J. Pathol 168, 962–972 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hingorani P, Zhang WD, Gorlick R & Kolb EA Inhibition of Src phosphorylation alters metastatic potential of osteosarcoma in vitro but not in vivo. Clin. Cancer Res 15, 3416–3422 (2009). [DOI] [PubMed] [Google Scholar]
- 260.Saltz LB et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol 26, 2013–2019 (2008). [DOI] [PubMed] [Google Scholar]
- 261.Hurwitz H et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med 350, 2335–2342 (2004). [DOI] [PubMed] [Google Scholar]
- 262.Yang JC et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med 349, 427–434 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Gilbert MR. et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med 370, 699–708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]