Abstract
Larsen syndrome is chronic debilitating disease that presents with multiple joint dislocations and severely affects the cervical spine in the form of cervical kyphosis and atlantoaxial dislocation. Children usually present in early with a myriad of deficits, compressive myelopathy being the most common. In addition to a bony compression, there is sometimes a soft tissue component, which is seldom addressed. We present here a case of atlantoaxial dislocation with cervical kyphosis due to Larsen syndrome, and along with our previous experience on syndromic atlantoaxial dislocations, we try to define an algorithm for the treatment approach of these onerous challenges. The importance of early intervention is also emphasized with a literature review of similar cases. In addition to the obvious physical damage, early intervention can also avoid the more sinister socioeconomic face of this debilitating disease.
Keywords: cervical kyphosis, atlantoaxial dislocation, Larsen syndrome
Introduction
In 1950, Larsen et al described the first series of cases with distinctive facial features, multiple joint dislocations, and spinal anomalies. 1 2 3 4 5 6 7 8 9 10 11 12 There is also a risk of dramatic cervical instability and sudden neurological deficit and death, as reported by Larsen himself. Since 1950, several authors have reported similar findings and yet a consensus regarding timing of correction, surveillance, pre- and postoperative bracing, and even the preferred surgical approach is missing. This is due to the wide spectrum of presentation and difference in severity at initial clinical evaluation.
Larsen syndrome occurs in 1 in 100,000 newborns, which is caused by mutations in the gene encoding filamin B (FLNB; 603381) on chromosome 3p14 that is important in regulating the structure and activity of the cytoskeleton. 13 14 15 We present a case of Larsen syndrome, which was managed, at our institute along with a possible protocol for the management of such cases in the future.
Case Report
History and Examination
“Dish face” they used to call him. A 15-year-old boy, studying in eighth grade, right-handed, presented with a prominent forehead and flattened nose ( Fig. 1A ). He never used to play with the other boys, lest he risk getting injured, a lesson he had learned early in his life. They used to ridicule him for his long thin “spider”-like limbs ( Figs. 1B C ). Social stigma apart, he started noticing that the school bag was getting heavier. It became nearly impossible to walk to school with it. Gradually, feeding oneself became a task. The food would often slip through the fingers like grains of sand. Assistance was needed to drink water or even go to the toilet. Eventually, he was bed ridden for the better part of last month. However, he had retained function of his bladder and bowel, with inability to walk to the toilet. On inquiring from his teachers, they would describe him as a student with an average scholastic performance.
Fig. 1.
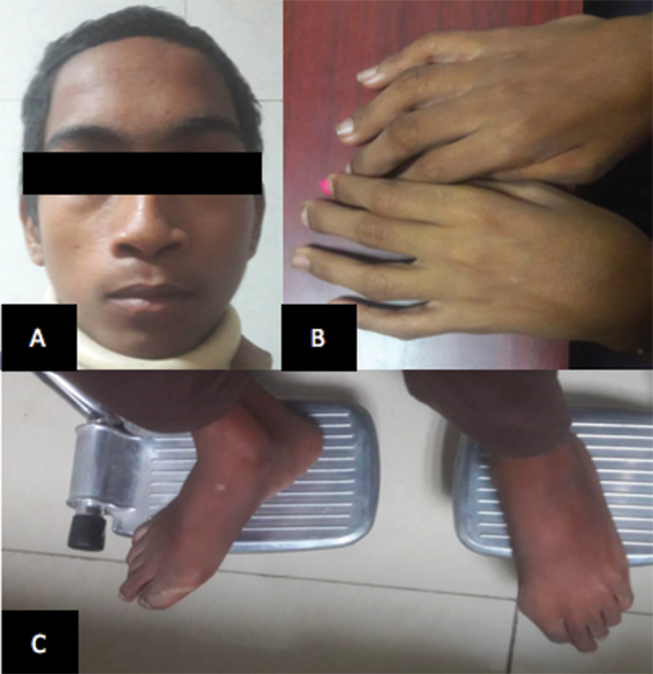
Clinical photograph of Larsen syndrome with prominent forehead and flattened nose ( A ) and spider-like limbs ( B and C ).
On examination , his height was a 150 cm and weight 40 kg (BMI = 17.8 kg/m 2 ). There was a definite kyphotic deformity of the spine without any local tenderness. He was able to lift all four limbs against gravity and had definite signs of myelopathy in the four of exaggerated deep tendon reflexes with upgoing plantars. Handgrip was worse on the right (30–40%) side than left (50%). Superficial reflexes were absent with graded sensory loss to all four modalities below C4 dermatome. Single breath count was 7 with a breath holding time of 20 seconds.
Past history was suggestive of multiple fracture dislocations of shoulders and knee often while playing in school after which the child had started to refrain from contact sports. There was no history of consanguinity in the family with birth history being unremarkable apart from the fact the child was home delivered. No history of recent trauma or tuberculosis could be elicited. The history, characteristic facial features, and physical findings were suggestive of a compressive myelopathy due to congenital atlantoaxial dislocation.
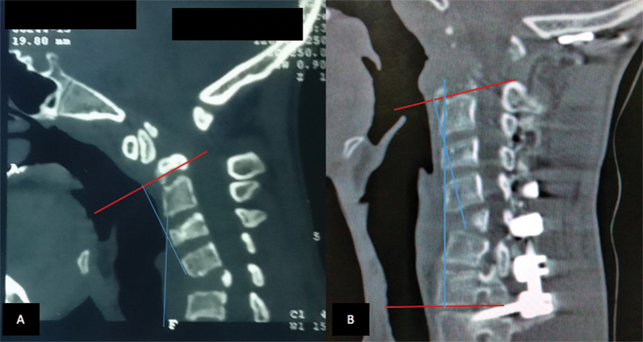
X-ray of the cervical spine revealed a gross kyphotic deformity at C6 to C7 and atlantoaxial dislocation. Careful evaluation of computed tomography (CT) of the cervical spine showed kyphotic deformity ( Fig. 2A B ) due to anterior wedging of C5 vertebrae. The atlantoaxial joints showed degenerative changes. Magnetic resonance imaging demonstrated significant compression of the cord with signal changes at the craniovertebral junction and minimal retroflexion of the odontoid, although the soft tissue compression anterior to the cervical cord was significant.
Fig. 2.
Plain computed tomographic scan of sagittal section of patient demonstrating improvement in preoperative kyphotic deformity (Cobb angle 27 degrees to postoperative 14 degrees).
Surgical Planning
There were two major considerations while doing the surgical planning: Soft tissue compressing the cervical cord at the cervicomedullary junction and the kyphosis at C5 to 7 levels. Both of the pathologies were significant and hence a decision was made for a transoral decompression of the soft tissue component followed by posterior fixation, including the two transition zones at craniovertebral junction and C7 to T1 levels. However, since more than three levels were involved a completely anterior approach with C5 corpectomy was abandoned as a single posterior fusion and decompression would deal with all the levels adequately. Since the compression was due to kyphosis and a soft tissue component, without any dislocation, preoperative traction was not considered.
Surgery
The patient was placed supine with neuromonitoring in the form of motor-evoked potentials and somatosensory potentials. He underwent a transoral–transpharyngeal approach that allowed lateral exposure of roughly 15 to 20 mm bilaterally off the midline from the inferior clivus to the C3 body. The anterior arch of C1 was drilled, laterally up to the lateral margins of the odontoid (~15 mm from midline). Safety margins lie within 11 mm from the midline at the foramen magnum, 24 mm at the atlas, and 14 mm at the lower border of the axis. Following this the odontoid drilling was done in a top down fashion. This avoided formation of a free-floating fragment of the dens as it was always attached at its base. A thick pannus was seen below the odontoid composed of elastic collagenous tissue and was gradually teased out using nibblers till pulsating dura was visible. After hemostasis, the closure proceeded sequentially using vicryl 2–0 and then monofilament 2–0 suture in an intermittent pattern. Immediate posterior stabilization was done by occipitocervical fixation including T1. Intraoperative manipulation was done to correct the kyphotic deformity ( Figs. 2 3 ) with the neuromonitoring parameters remaining unchanged throughout.
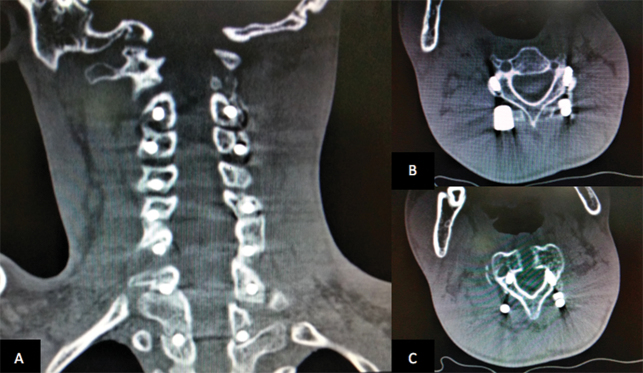
Fig. 3.
Postoperative coronal ( A ) and axial ( B , C ) computed tomographic scan with posterior stabilization.
Postoperative Course
The patient was transferred to the postoperative intensive care unit and was extubated the next day followed by Ryles tube feeding started on day 2. He was transferred to the rehabilitation unit, from which he was later discharged neurologically intact with reduced spasticity on day 7 after surgery. Postoperative CT demonstrated complete correction of the deformity ( Fig. 2 ). Cervical collar was maintained for 3 weeks. Follow-up at 18 months showed excellent recovery of power to 4+/5 with independent ambulation and ability to take care of daily needs with return to school.
Discussion
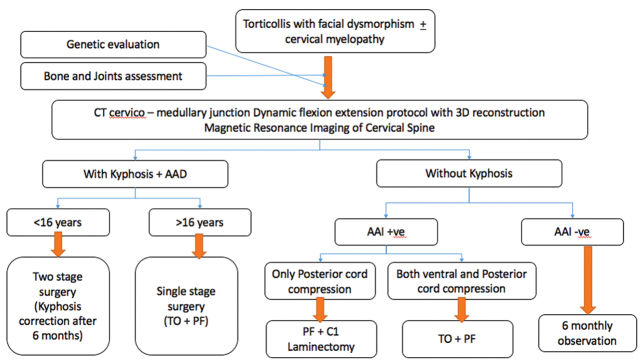
Atlantoaxial dislocation is different in this case when compared with other syndromic and nonsyndromic varieties. We have found, in our case, that not only does the bony kyphosis cause compression and myelopathy, but also there was a soft tissue component to the compression. 16 17 18 During anterior decompression after removal of the odontoid, there was no dura visible and neither any pulsations were seen. On removal of soft cartilaginous tissue using rongeurs and nibblers, the dura was finally seen and compression relieved. Consequently, to achieve complete decompression of the cord, a 360-degree approach is necessary. Madera et al 14 were the first to perform a synchronous anterior decompression and fixation, posterior fusion, and fixation for a case of Larsen syndrome. 14 On extensive review, there have been 22 cases of surgically treated cervical deformity in patients with Larsen syndrome ( Table 1 ). The authors have significant previous experience in managing syndromic atlantoaxial dislocation and proposing an algorithmic approach ( Fig. 4 ) for the management of such cases. 2 3 8 9 19
Table 1. Review of all cases of surgically corrected cervical kyphosis in patients of Larsen syndrome.
| Author | Age at first surgery | Trauma | Traction/Collar | Preoperative condition | Surgery | Collar/brace | Follow-up |
|---|---|---|---|---|---|---|---|
| Micheli et al 1976 15 | 10 mo | None | Semirigid cervical | UE and mild LE weakness | C3-T2 posterior fusion | Minerva jacket | Unknown |
| Muzumdar et al 1977 21 | 13.5 y | Fall | NA | Bilateral numbness, weakness in all four | Cervical decompression | NA | Minimal improvement, later deteriorated |
| Bowen et al 1985 5 | 19 y | None | NA | No deficit, progression of kyphosis | Occiput–C4 posterior fusion, later scoliosis correction | Minerva jacket | No deficit |
| Miz and Engler 1987 20 | 14 mo | Motor vehicle accident | NA | Hyperreflexia, decreased perineal sensation | Occiput–C2 posterior fusion | Minerva jacket | No recurrence |
| Francis and Noble 1988 7 | 5 y | None | NA | Weakness, inability to right self | Anterior cervical decompression, fibular strut placement | Halo vest | No deficit |
| Forsee et al 1995 6 | 5 y | None | NA | Arm weakness | Anterior, later posterior fusion | NA | No improvement |
| Johnston et al 1996 10 | 10 mo | Fall after first operation | NA | No deficit initially, fall after first operation induced quadriparesis | Posterior cervical fusion f/b 2 anterior decompression and fusion | Minerva jacket and Halo vest | Improvement in walking |
| 14 mo | None | NA | No deficit | Posterior fusion | Halo vest | No deficit | |
| 14 mo | None | NA | No deficit | Posterior fusion | Minerva jacket | No deficit | |
| 16 mo | None | NA | No deficit | Posterior fusion | Minerva jacket | No deficit | |
| 12 y | None | NA | Myelopathy, weakness | Anterior and posterior fusion | NA | No improvement | |
| Luk and Yip 2002 13 | 8 y | None | NA | No deficit at first surgery, sensory in UE later | Anterior T12-L3 fusion, anterior decompression and fusion, later posterior fusion | Halo vest | Transient weakness, later no deficit |
| 6 y | None | Halo | Myelopathy | Posterior C1-T1 fusion, anterior cervical fusion and repeated anterior fusion | Halo vest | Myelopathy resolved after first anterior cer vical fusion | |
| Banks et al 2003 1 | 13 y | Fall | Halo | Myelopathy, weakness in all extremities | Posterior C1-T1 fusion, anterior cervical decompression and fusion 4 d later | Halo vest and then hard cervical collar | Transient increased weakness postop w/ later improvement in better than preop status |
| Katz et al 2005 11 | 3 y | Falls | NA | Weakness before first operation, inability to walk before third operation | Two failed posterior cervical fusions, posterior decompression and fusion, later anterior fusion w/ post sublaminar wires | Halo vest and then collar after second operation, Halo vest through fourth operation, later collar | After fourth operation, transient weakness w/ improvement but persistent C5 and C6 weakness |
| Sakaura et al 2007 18 | 34 mo | None | Minerva Brace | Spastic quadriparesis with sleep apnea | Anterior decompression corpectomies C4–C5 arthrodesis C3–C6 using tibial strut bone grafts via a lateral approach and later revision of anterior fixation with C2–C7 fixation | NA | Quadriparesis and respiratory dysfunction improved |
| 58 mo | None | None | Hyperreflexia | Posterior fusion C3–C5 | Halo vest | No deficit | |
| 10 mo | None | Halo traction | No deficit, kyphosis worsening | Postcervical arthrodesis. At 29 mo: Anterior decompression C4–5 corpectomy, C3–C6 arthrodesis and later occiput-T4 arthrodesis | Halo vest | No deficit | |
| Madera et al 2008 14 | 2.5y | None | Hard collar | No deficit | Synchronous ant decompression and fusion/fixation, posterior fusion/fixation | Halo vest | Transient postoperative weakness and Horner syndrome resolved. |
| Kumar et al 2013 16 | 36 y | None | NA | Mild spasticity of all four limbs | Anterior C2–C5 decompression and fixation and later posterior C1–C6 fusion | Philadelphia collar | No deficit |
| Yonekura et al 2015 22 | 18 y | None | NA | Airway obstruction and repeated aspiration pneumonia | 3 y age: post cervical arthrodesis. 18 y: Anterior mediastinal tracheostomy | NA | No deficit |
| Sahoo et al 2016 17 | 56 y | None | NA | Neck pain with spastic quadriparesis | Posterior C1-C2 fusion | NA | Improvement in spasticity |
| Present case | 15 y | None | Hard cervical collar | Neck pain with spastic quadriparesis | Transoral decompression f/b occipito-T1 fusion | Hard cervical collar | Improvement in power and spasticity |
Fig. 4.
Algorithmic approach to a case of Larsen syndrome. AAD, atlantoaxial dislocation; CT, computed tomography; PF, posterior fixation
The treatment is dictated by the natural history of the disease. Although advocates of nonsurgical management have proposed continuous cervical traction and total spinal column bracing in patients with severe deficits since birth and have noted improvement in ventilator and motor functions, they too hypothesized that surgical intervention might be needed later. 20 21 Regarding the approach to subaxial cervical spine fusion, there is a simple approach that can be followed. In cases of minimal kyphosis with no myelopathy, a short segment fusion posteriorly may suffice. However, if severe kyphosis or myelopathy is present, anterior decompression followed by a 360-degree fixation should be aimed for. 22 23
One thing that is noncontroversial in this syndrome is the need for intervention. With the review of literature and our own personal experience, it is clear that the stage of intervention matters. 5 Patients who were operated earlier or at a stage where they had minimal to no deficits fared much better than those allowed to deteriorate. Further the impact of multiple falls or chronic cord compression cannot be overstated. In severe cases with apnea and respiratory distress, an even earlier decompression with Halo stabilization followed by the earliest allowable opportunity for fixation may be a safe alternative.
Conclusion
Larsen syndrome cases have several defects, the most severe of them being cervical kyphosis and atlantoaxial dislocation. A 360-degree decompression and fixation of the atlantoaxial dislocation followed by deformity correction can prevent further deterioration due to chronic cord compression or trivial falls. We attempt to delineate here that anterior decompression at every level may not be necessary and a single posterior fusion is sufficient. Decompression anteriorly is only needed in case of significant soft tissue compression that otherwise cannot be addressed posteriorly. Neurological condition at presentation dictates outcome.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Banks J T, Wellons J C, III, Tubbs R S, Blount J P, Oakes W J, Grabb P A. Cervical spine involvement in Larsen’s syndrome: a case illustration. Pediatrics. 2003;111(01):199–201. doi: 10.1542/peds.111.1.199. [DOI] [PubMed] [Google Scholar]
- 2.Behari S, Bhargava V, Nayak S et al. Congenital reducible atlantoaxial dislocation: classification and surgical considerations. Acta Neurochir (Wien) 2002;144(11):1165–1177. doi: 10.1007/s00701-002-1009-3. [DOI] [PubMed] [Google Scholar]
- 3.Behari S, Kiran K umar, MV, Banerji D, Chhabra D K, Jain V K. Atlantoaxial dislocation associated with the maldevelopment of the posterior neural arch of axis causing compressive myelopathy. Neurol India. 2004;52(04):489–491. [PubMed] [Google Scholar]
- 4.Bicknell L S, Farrington-Rock C, Shafeghati Y et al. A molecular and clinical study of Larsen syndrome caused by mutations in FLNB. J Med Genet. 2007;44(02):89–98. doi: 10.1136/jmg.2006.043687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowen J R, Ortega K, Ray S, MacEwen G D. Spinal deformities in Larsen’s syndrome. Clin Orthop Relat Res. 1985;(197):159–163. [PubMed] [Google Scholar]
- 6.Forese L L, Berdon W E, Harcke H T et al. Severe mid-cervical kyphosis with cord compression in Larsen’s syndrome and diastrophic dysplasia: unrelated syndromes with similar radiologic findings and neurosurgical implications. Pediatr Radiol. 1995;25(02):136–139. doi: 10.1007/BF02010328. [DOI] [PubMed] [Google Scholar]
- 7.Francis W R, Jr, Noble D P. Treatment of cervical kyphosis in children. Spine. 1988;13(08):883–887. doi: 10.1097/00007632-198808000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Jain V K, Behari S. Management of congenital atlanto-axial dislocation: some lessons learnt. Neurol India. 2002;50(04):386–397. [PubMed] [Google Scholar]
- 9.Jain V K, Behari S, Banerji D, Bhargava V, Chhabra D K. Transoral decompression for craniovertebral osseous anomalies: perioperative management dilemmas. Neurol India. 1999;47(03):188–195. [PubMed] [Google Scholar]
- 10.Johnston C E, II, Birch J G, Daniels J L. Cervical kyphosis in patients who have Larsen syndrome. J Bone Joint Surg Am. 1996;78(04):538–545. doi: 10.2106/00004623-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Katz D A, Hall J E, Emans J B. Cervical kyphosis associated with anteroposterior dissociation and quadriparesis in Larsen’s syndrome. J Pediatr Orthop. 2005;25(04):429–433. doi: 10.1097/01.bpo.0000161091.85350.54. [DOI] [PubMed] [Google Scholar]
- 12.Larsen L J, Schottstaedt E R, Bost F C. Multiple congenital dislocations associated with characteristic facial abnormality. J Pediatr. 1950;37(04):574–581. doi: 10.1016/s0022-3476(50)80268-8. [DOI] [PubMed] [Google Scholar]
- 13.Luk K D, Yip D K. Congenital anteroposterior spinal dissociation in Larsen’s syndrome: report on two operated cases with long-term follow-up. Spine. 2002;27(12):E296–E300. doi: 10.1097/00007632-200206150-00023. [DOI] [PubMed] [Google Scholar]
- 14.Madera M, Crawford A, Mangano F T. Management of severe cervical kyphosis in a patient with Larsen syndrome. Case report. J Neurosurg Pediatr. 2008;1(04):320–324. doi: 10.3171/PED/2008/1/4/320. [DOI] [PubMed] [Google Scholar]
- 15.Micheli L J, Hall J E, Watts H G. Spinal instability in Larsen’s syndrome: report of three cases. J Bone Joint Surg Am. 1976;58(04):562–565. [PubMed] [Google Scholar]
- 16.Roopesh Kumar V R, Madhguiri V S, Sasidharan G M, Gundamaneni S K, Yadav A K. Larsen syndrome with C3-C4 spondyloptosis and atlantoaxial dislocation in an adult. Spine. 2013;38(01):E43–E47. doi: 10.1097/BRS.0b013e318278e59d. [DOI] [PubMed] [Google Scholar]
- 17.Sahoo S K, Deepak A N, Salunke P. Atlantoaxial dislocation adjacent to kyphotic deformity in a case of adult Larsen syndrome. J Craniovertebr Junction Spine. 2016;7(02):109–110. doi: 10.4103/0974-8237.181869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaura H, Matsuoka T, Iwasaki M, Yonenobu K, Yoshikawa H. Surgical treatment of cervical kyphosis in Larsen syndrome: report of 3 cases and review of the literature. Spine. 2007;32(01):E39–E44. doi: 10.1097/01.brs.0000250103.88392.8e. [DOI] [PubMed] [Google Scholar]
- 19.Sardhara J, Behari S, Jaiswal A K et al. Syndromic versus nonsyndromic atlantoaxial dislocation: do clinico-radiological differences have a bearing on management? Acta Neurochir (Wien) 2013;155(07):1157–1167. doi: 10.1007/s00701-013-1717-x. [DOI] [PubMed] [Google Scholar]
- 20.Miz G S, Engler G L. Atlanto-axial subluxation in Larsen’s syndrome. A case report. Spine. 1987;12(04):411–412. doi: 10.1097/00007632-198705000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Muzumdar A S, Lowry R B, Robinson C E.Quadriplegia in Larsen syndrome Birth Defects Orig Artic Ser 197713(3C)202–211. [PubMed] [Google Scholar]
- 22.Yonekura T, Kamiyama M, Kimura K et al. Anterior mediastinal tracheostomy with a median mandibular splitting approach in a Larsen syndrome patient with posterior cervical arthrodesis. Pediatr Surg Int. 2015;31(10):1001–1004. doi: 10.1007/s00383-015-3783-z. [DOI] [PubMed] [Google Scholar]
- 23.Martus J E, Griffith T E, Dear J C, Rathjen K E. Pediatric cervical kyphosis: a comparison of arthrodesis techniques. Spine. 2011;36(17):E1145–E1153. doi: 10.1097/BRS.0b013e3182039844. [DOI] [PubMed] [Google Scholar]