Abstract
Background The role of oxidative stress in neuronal injury due to ischemic stroke has been an interesting topic in stroke research. Malondialdehyde (MDA) has emerged as a sensitive oxidative stress biomarker owing to its ability to react with the lipid membranes. Total antioxidant power (TAP) is another biomarker to estimate the total oxidative stress in stroke patients. We aimed to determine the oxidative stress in acute stroke patients by measuring MDA and TAP.
Materials and Methods MDA and TAP were determined in 100 patients with ischemic stroke and compared with that in 100 age- and sex-matched healthy adults. Demographic data, stroke severity measured by the National Institutes of Health Stroke Scale (NIHSS), and disability measured by the Barthel index (BI) were recorded. The association of MDA and TAP with other variables was analyzed by paired t -test.
Results Of the whole sample, 74% represented males. The mean NIHSS score was 13.11 and BI was 38.87. MDA was significantly higher in stroke patients (7.11 ± 1.67) than in controls (1.64 ± 0.82; p = 0.00). TAP was significantly lower in stroke patients (5.72 ± 1.41) than in controls (8.53 ± 2.4; p = 0.00). The lipid profile and blood sugar levels were also significantly higher in stroke patients. There was no association of MDA and TAP with other variables.
Conclusion We found that oxidative stress was associated with acute ischemic stroke. However, we could not establish an association between oxidative stress and the severity of acute stroke.
Keywords: acute stroke treatment, ischemic stroke, stroke, oxidative stress, total antioxidant power, malondialdehyde
Introduction
Stroke is one of the leading causes of mortality and morbidity, with a huge economic burden on the society. Understanding the pathophysiological mechanisms associated with neuronal injury due to stroke is of considerable importance. Oxidative stress with reactive oxygen species (ROS) has been considered as one of the underlying mechanisms of injury for many types of disease processes. Furthermore, it is one of the major mechanisms for inducing neuronal damage by ischemia and reperfusion. 1 Brain has an abundance of lipid membranes, and oxidative stress has a specific predilection for these. Importantly, oxidative stress occurs when the balance shifts in favor of oxidants than antioxidants.
Malondialdehyde (MDA) has been increasingly used as an oxidative stress biomarker due to its characteristic ability to react with the lipid membranes. Total antioxidant power (TAP) gives us a global estimate about the oxidative stress in a given patient. 2 Well-designed studies on these biomarkers in stroke patients are, however, lacking, and there has been a great interest in this area. We aimed to determine oxidative stress by measuring MDA and TAP in patients with acute stroke within 24 hours of occurrence using a case–control design. Furthermore, we investigated if oxidative stress was related to stroke severity.
Materials and Methods
This case–control study included 100 consecutive acute ischemic stroke patients admitted to a stroke unit, and 99 age- and sex-matched healthy volunteers served as controls. All patients underwent detailed evaluation, which included the collection of demographic data, assessment of stroke risk factors, assessment of stroke severity measured by the National Institutes of Health Stroke Scale (NIHSS) at admission, and assessment of disability measured by the (BI) at discharge. Stroke severity by NIHSS was scored as 0 with no symptoms, and 21- 42 as having severe stroke.
Stroke was classified according to the Oxfordshire Community Stroke Project (OCSP) classification system. The OCSP is a simple classification method that predicts the site and size of the infarct on imaging in ischemic stroke patients. Total anterior circulation infarct, partial anterior circulation infarct, posterior circulation infarct, and lacunar infarct were classified based on their neurologic deficits and imaging. BI is scored from 0 to 100, with lower scores representing greater nursing dependency.
Patients with intracerebral bleed, ischemic stroke of more than 24 hours of duration, renal or hepatic impairment, underlying neoplasm, and congestive heart failure were excluded. Patients with fever during admission were also excluded.
Blood Sample Collection
We collected blood samples in the morning after overnight fasting. Venous blood (10 mL) was collected from each patient, and part of it was transferred to the EDTA (ethylenediaminetetraacetic acid) coated vacutainers for the estimation of hematological parameters. The remaining blood was allowed to clot and centrifuged at 300 rpm for 10 minutes to obtain serum and stored at–20°C to measure the rest of the analytes.
Hematological analysis included the estimation of hemoglobin, total white blood cells, red blood cells, platelets, packed cell volume, mean corpuscular volume, mean corpuscular hemoglobin, and erythrocyte sedimentation rate. These were analyzed using the Ac T 5diff Hematology Analyzer (Beckman Coulter). Biochemical analysis included plasma glucose, serum creatinine blood urea, liver enzymes, and lipid profile (total cholesterol, triglycerides, high-density lipoprotein cholesterol, and very low density lipoprotein cholesterol).
Measurement of Total Antioxidant Power
TAP is a measure of the total antioxidant capacity of the system. Higher oxidants levels in the system will lead to reduced TAP. TAP was determined by ferric reducing antioxidant power (FRAP) assay. At low pH, reduction of a ferric tripyridyltriazine (Fe3 + TPTZ) complex (Sigma Aldrich, St. Louis, Missouri, United States) to a ferrous form has an intense blue color that can be monitored by measuring the absorbance at 593 nm using a spectrophotometer. It is directly related to the combined or total reducing power of the electron-donating antioxidants present in the reaction mixture. The results were expressed as micromole per liter (μmol/L). The sensitivity of TAP assay is high and can measure low levels (0.2 μmol/L) of TAP. 3
Measurement of Malondialdehyde
Lipid peroxidation was estimated by measuring the MDA levels using an ultraviolet–visible spectrophotometer. 4 We used the TBARS (thiobarbituric acid reactive substances) assay, which determines MDA, the end products of lipid peroxidation at 535 nm using a spectrophotometer. The values are expressed in μmol/L. The levels of MDA indirectly indicate the level of oxidative stress. Sensitivity is generally good and can measure 0.5 to 100 μmol/L of high linearity in patients and controls.
Statistical Analyses
The data were analyzed using the SPSS statistical package for Mac, version 24 (SPSS Inc., Chicago, Illinois, United States). Descriptive analyses are expressed as means ± standard deviation. Paired Student’s t -test was performed for comparison between patients and controls for continuous variables. A p- value of <0.05 was considered statistically significant. As our controls were matched for age and gender, we did not perform a logistic regression analysis.
Results
Our stroke patients and controls were matched for age and gender. The participants’ demographic measures and clinical characteristics are presented in Table 1 . Of the whole sample, 74% were males. For the stroke severity index measured by the NIHSS, the mean score was 13.11, and for the Barthel index, the mean score was 38.87.
Table 1. Age, sex, and health characteristics of the sample.
| Stroke patients ( n = 100) | Controls ( n = 99) |
|
|---|---|---|
| Abbreviations: HT, hypertension; DM, diabetes mellitus; LACI, lacunar infarct; NIHSS, National Institutes of Health Stroke Scale; OCSP, Oxfordshire Community Stroke Project; PACI, partial anterior circulation infarct; POCI, posterior circulation infarct; SD, standard deviation; TACI, total anterior circulation infarct. | ||
| Age (years), mean ± SD | 55.54 ± 11.57 | 57.39 ± 10.53 |
| Sex (male %) | 74 | 74 |
| HT (%) | 71 | – |
| DM (%) | 31 | – |
| Smoking (%) | 47 | – |
| Alcohol (%) | 30 | – |
| OCSP | ||
| TACI | 28 | |
| PACI | 30 | |
| LACI | 33 | |
| POCI | 9 | |
| NIHSS (mean ± SD) | 13.11 ± 6.49 | – |
| Barthel index (mean ± SD) | 38.87 ± 35.05 | – |
The results of independent samples t -tests are shown in Table 2 . The oxidative stress biomarker, MDA, was significantly higher in stroke patients (7.11 ± 1.67 μmol/L) than in controls (1.64 ± 0.82) ( p = 0.00) ( Fig. 1 ). The TAP was significantly lower in stroke patients (5.72 ± 1.41 μmol/L) than in controls (8.53 ± 2.41; p = 0.00) ( Fig. 2 ). The lipid profile and blood sugar levels were also significantly higher in stroke patients. None of the other variables were significant.
Table 2. Antioxidants and biochemical characteristics of the sample.
| Characteristics | Patients ( n = 100) | Control ( n = 99) | p -Value |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein; MDA, malondialdehyde; SD, standard deviation; TAP, total antioxidant power; VLDL, very low density lipoprotein. | |||
| MDA (μmol/L) | 7.11 ± 1.67 | 1.64 ± 0.82 | 0.00 |
| TAP (μmol/L) | 5.72 ± 1.41 | 8.53 ± 2.41 | 0.00 |
| Hemoglobin (gm/dL) | 12.91 ± 2.10 | 13.15 ± 1.70 | 0.36 |
| Fasting blood sugar (mg/dL) | 105 ± 43.71 | 83.31 ± 7.44 | 0.00 |
| Total cholesterol (mg/dL) | 168.25 ± 46.65 | 167.70 ± 37.74 | 0.92 |
| HDL (mg/dL) | 41.43 ± 11.48 | 45.40 ± 10.05 | 0.01 |
| LDL (mg/dL) | 98.09 ± 32.25 | 102.60 ± 29.04 | 0.30 |
| Triglyceride (mg/dL) | 146.31 ± 64.93 | 106.25 ± 45.53 | 0.00 |
| VLDL (mg/L) | 27.94 ± 11.76 | 20.98 ± 9.05 | 0.00 |
Fig. 1.

Boxplot showing malondialdehyde for stroke cases and controls.
Fig. 2.
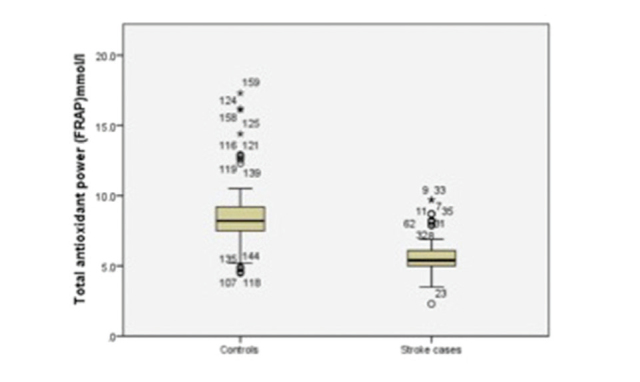
Boxplot showing total antioxidant power for stroke cases and controls. FRAP, ferric reducing antioxidant power.
Discussion
Stroke is a medical emergency where millions of neurons die within seconds and is one of the leading causes of disability. Thrombolytic therapy is the only evidence-based treatment available till date to prevent disability. However, some patients do not respond to this treatment and are left with residual neurologic deficits. In this subgroup of patients, oxidative stress or reperfusion injury after recanalization might play a significant role in further exacerbating the injury. This study evaluated the role of oxidative stress in patients with ischemic stroke and found that there were high levels of MDA and low levels of TAP as compared with controls, suggesting oxidative injury in stroke patients.
The brain is particularly sensitive to the oxidative injury because of the high content of polyunsaturated fatty acids. Ischemic stroke induces the generation of ROS and nitrogen molecules. 5 Furthermore, ROS has been implicated to have an important role in the genesis of reperfusion injury. 6
MDA is a frequently used biomarker of oxidative stress in various diseases including stroke. It is an end product formed during lipid peroxidation due to the degradation of cellular membrane phospholipids. After the release of MDA into the extracellular space, it reaches the blood; hence it has been used as an effective biomarker of lipid oxidation. MDA reacts to proteins and nucleic acids, which leads to further damage of the cell components. It has been shown that after 24 hours of permanent middle cerebral occlusion, the ischemic core had high levels of lipid peroxidation, which was highly reactive to phospholipase A2, highly suggestive of oxidative injury in acute stroke. 7 Our study did not show any significant association of MDA level with gender, body mass index, and other cardiovascular risk factors. Although our findings are relevant, we believe there are several intraindividual factors that are relevant in terms of the evolution and recovery of stroke-related neuronal injury.
We found low levels of TAP in stroke patients compared with controls. Previous studies have shown conflicting results of the TAP in ischemic stroke patients. 8 9 10 This difference could be because of the different etiological risk factors in each individual. Measurement of a single antioxidant variable may fail to reflect the antioxidant protective capacity due to the different interactions in vivo. Hence, we measured the TAP by measuring the FRAP, which reflects the altered redox status of the injured tissues in ischemic stroke. This provided us with information on the cumulative antioxidant capacity in the blood in our stroke patients. Furthermore, as with MDA levels, we did not find any association of TAP with demographic, NIHSS scores, BI scores, and various risk factors. Nonetheless, a previous study found a relationship between severe malignant middle cerebral artery infarction, serum TAP levels, and 30-day mortality. 11 Similarly, another study concluded that TAP levels were more in diabetic stroke than in nondiabetic stroke patients, although we did not find any such association in our sample. 12 In a previous study, TAP has been associated with the volume of ischemic cerebral infarction and the degree of neurologic impairment. 13 Our study, however, failed to demonstrate such an association. The oxidative stress is known to change as time progresses, which has been clearly demonstrated in a previous study. 14 One of the reasons for the lack of a correlation may be due to the different times of MDA and TAP measurements in our patients. A previous study evaluating antioxidant levels within 24 hours of stroke showed that a majority of antioxidants showed low levels immediately after an acute ischemic stroke and tended to increase subsequently. 15 In fact, the Rotterdam study showed that high dietary antioxidant intake lowered the risk of dementia and stroke; however, there could be variation in individual diet structure. Also, inflammation does seem to play an important role during cerebral ischemia. Therefore, we believe, though there is emerging evidence of oxidative stress in stroke patients, as we demonstrated in our study, the relation of oxidative stress with stroke severity is a complex process and needs to be further explored in detail in future studies.
Our study has some limitations. We measured only two markers of oxidative stress. Although this may not be considered as representative of the complete oxidative stress state, the two important markers we selected are reasonably representative of the oxidative stress status.
Conclusion
Our study demonstrated the role of oxidative stress in patients with acute ischemic stroke. We did not find any association between oxidative stress and stroke severity, therefore, suggesting that oxidative stress is present in stroke patients, but its role in stroke severity needs to be further clarified. Of note, this study opens up avenues for exploring potentially innovative treatment targets of stroke patients in the form of antioxidants acting as neuroprotective agents. This is particularly relevant in the vast majority of patients who are not eligible for or responding to thrombolytic therapy and hence require new therapeutic alternatives.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Chen S D, Yang D I, Lin T K, Shaw F Z, Liou C W, Chuang Y C. Roles of oxidative stress, apoptosis, PGC-1 α and mitochondrial biogenesis in cerebral ischemia. Int J Mol Sci. 2011;12(10):7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000;29(11):1106–1114. doi: 10.1016/s0891-5849(00)00394-4. [DOI] [PubMed] [Google Scholar]
- 3.Benzie I FF, Strain J J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(01):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 4.Yagi K.Lipid peroxides and human diseases Chem Phys Lipids 198745(2-4)337–351. [DOI] [PubMed] [Google Scholar]
- 5.Traystman R J, Kirsch J R, Koehler R C. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol (1985) 1991;71(04):1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 6.Granger D N, Kvietys P R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AR . Muralikrishna Adibhatla R, Hatcher J F. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40(03):376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 8.Ciancarelli I, De Amicis D, Di Massimo C, Carolei A, Ciancarelli M G. Oxidative stress in post-acute ischemic stroke patients after intensive neurorehabilitation. Curr Neurovasc Res. 2012;9(04):266–273. doi: 10.2174/156720212803530717. [DOI] [PubMed] [Google Scholar]
- 9.Yang T H, Chang C Y, Hu M L. Various forms of homocysteine and oxidative status in the plasma of ischemic-stroke patients as compared to healthy controls. Clin Biochem. 2004;37(06):494–499. doi: 10.1016/j.clinbiochem.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Altamura C, Squitti R, Pasqualetti P et al. Ceruloplasmin/transferrin system is related to clinical status in acute stroke. Stroke. 2009;40(04):1282–1288. doi: 10.1161/STROKEAHA.108.536714. [DOI] [PubMed] [Google Scholar]
- 11.Lorente L, Martín M M, Pérez-Cejas A et al. Association between total antioxidant capacity and mortality in ischemic stroke patients. Ann Intensive Care. 2016;6(01):39. doi: 10.1186/s13613-016-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guldiken B, Demir M, Guldiken S, Turgut N, Turgut B, Tugrul A. Oxidative stress and total antioxidant capacity in diabetic and nondiabetic acute ischemic stroke patients. Clin Appl Thromb Hemost. 2009;15(06):695–700. doi: 10.1177/1076029608323087. [DOI] [PubMed] [Google Scholar]
- 13.Leinonen J S, Ahonen J P, Lönnrot K et al. Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke. 2000;31(01):33–39. doi: 10.1161/01.str.31.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Žitňanová I, Šiarnik P, Kollár B et al. Oxidative stress markers and their dynamic changes in patients after acute ischemic stroke. Oxid Med Cell Longev. 2016:9.761697E6. doi: 10.1155/2016/9761697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherubini A, Polidori M C, Bregnocchi M et al. Antioxidant profile and early outcome in stroke patients. Stroke. 2000;31(10):2295–2300. doi: 10.1161/01.str.31.10.2295. [DOI] [PubMed] [Google Scholar]


