Abstract
Primary human bronchial epithelial cell (HBEC) cultures are a useful model for studies of lung health and major airway diseases. However, mechanistic studies have been limited by our ability to selectively disrupt specific genes in these cells. Here we optimize methods for gene targeting in HBECs by direct delivery of single guide RNA (sgRNA) and rCas9 (recombinant Cas9) complexes by electroporation, without a requirement for plasmids, viruses, or antibiotic selection. Variations in the method of delivery, sgRNA and rCas9 concentrations, and sgRNA sequences all had effects on targeting efficiency, allowing for predictable control of the extent of gene targeting and for near-complete disruption of gene expression. To demonstrate the value of this system, we targeted SPDEF, which encodes a transcription factor previously shown to be essential for the differentiation of MUC5AC-producing goblet cells in mouse models of asthma. Targeting SPDEF led to proportional decreases in MUC5AC expression in HBECs stimulated with IL-13, a central mediator of allergic asthma. Near-complete targeting of SPDEF abolished IL-13–induced MUC5AC expression and goblet cell differentiation. In addition, targeting of SPDEF prevented IL-13–induced impairment of mucociliary clearance, which is likely to be an important contributor to airway obstruction, morbidity, and mortality in asthma. We conclude that direct delivery of sgRNA and rCas9 complexes allows for predictable and efficient gene targeting and enables mechanistic studies of disease-relevant pathways in primary HBECs.
Keywords: CRISPR, human bronchial epithelial cells, SPDEF, goblet, MUC5AC
Clinical Relevance
We believe that the method of gene targeting described here will have a significant impact for a large group of AJRCMB readers who perform mechanistic studies in primary lung cells and that our findings regarding the role of SPDEF will be of substantial interest to those interested in airway epithelial cell biology and in asthma pathogenesis.
The bronchial epithelium is critical for normal lung function, and altered epithelial function is central to the pathogenesis of major airway diseases including asthma, chronic obstructive pulmonary disease, and cystic fibrosis (1, 2). The mechanisms underlying normal bronchial epithelial cell function and pathophysiological alterations leading to disease have been studied in various model systems. Mouse and other animal model systems have made indispensable contributions to our understanding of airway epithelial cells, but intraspecies differences (3) require that we complement these studies with others in human systems. In vivo investigations in humans are important but are largely confined to observational studies until preclinical work justifies clinical trials of therapeutic agents. Studies in immortalized cell lines are also valuable, but these systems are quite limited in their ability to model differentiation of ciliated cells, mucus-producing goblet cells, and other specialized airway epithelial subpopulations with critical roles in human health and disease (4). Primary air–liquid interface (ALI) cultures of human bronchial epithelial cells (HBECs) have proven to be valuable models because of their ability to recapitulate many in vivo aspects of airway epithelial cell differentiation and function of the human airway epithelium (5, 6). Although gene targeting has enabled elegant mechanistic studies in transgenic mice, analyses of the contributions of specific genes in highly differentiated HBEC models have been limited because of challenges in efficiently targeting genes in these cells.
The advent of CRISPR/Cas9 gene editing technology has transformed our ability to investigate gene function in many model systems and has great potential for therapeutic applications. CRISPR/Cas9 gene editing makes use of a programmable guide RNA (gRNA) as well as a Cas9 nuclease to form a ribonucleoprotein (RNP) complex, which in turn binds and cleaves the target site in genomic DNA (7, 8). gRNA and Cas9 have been delivered to human cells using viral and nonviral delivery systems (9). Viral delivery of gRNA and Cas9 transgenes together with an antibiotic resistance gene has been used for gene targeting in many cell types, including HBECs (10). Viral delivery allows for selection of transduced cells, which improves efficiency but requires the use of more starting cells or more rounds of cell division. Moreover, continued expression of Cas9 may increase the likelihood of undesired effects, including chromosome rearrangements (11). An alternative to viral delivery is direct delivery of RNP complexes of gRNA and rCas9 (recombinant Cas9) via electroporation. This approach has been shown to target genes at high efficiency in many cell types, including primary cells (12–14).
Here, we describe methods for direct delivery of RNP complexes to robustly target the genome of primary HBECs. As an illustration of the value of these methods, we targeted the SAM pointed domain containing ETS transcription factor SPDEF. Previous studies with Spdef−/− transgenic mice demonstrated that this transcription factor is required for airway epithelial cell mucus metaplasia in mouse models of asthma (15). Spdef is induced by IL-13, a key mediator in type 2 asthma, and regulates the expression of a substantial set of transcripts found in mucus-producing goblet cells. By targeting SPDEF in HBECs, we were able to examine its contributions to IL-13–induced changes in gene expression and epithelial function in primary human cells. Some of the results of these studies have been previously published in preprint form (https://www.biorxiv.org/content/10.1101/716670v1).
Methods
CRISPR-based Gene Targeting
gRNA sequences were selected from the CRISPR Targets 10K track on the University of California Santa Cruz Genome Browser (https://genome.ucsc.edu, hg38 human genome build) and are shown in Table E1 in the data supplement. RNAs were resuspended in 150 mM KCl and 10 mM TRIS-HCl, pH 7.4. For experiments involving CRISPR RNA (crRNA) and trans-activating CRISPR RNA (tracrRNA) (Thermo Fisher Scientific), crRNA (160 μM, 1 μl) and tracrRNA (160 μM, 1 μl) were mixed, hybridized at 37°C for 30 minutes, combined with rCas9 (40 μM, 2 μl; MacroLab) and electroporation enhancer DNA oligonucleotide (100 μM, 1 μl), and incubated at 37°C for 15 minutes before electroporation. Unless otherwise indicated, for experiments involving single guide RNAs (sgRNAs; Synthego), sgRNA (53.3 μM, 3 μl) was combined with rCas9 (40 μM, 2 μl) and incubated at room temperature for 10 minutes before electroporation.
After reaching 80% confluence, HBECs or BEAS-2B, an immortalized human airway epithelial cell line, cells were harvested for electroporation.
Unless otherwise indicated, 150,000 cells were resuspended in 20 μl of P3 Primary Cell Nucleofector Solution with Supplement 1 and mixed with the gRNA/rCas9 solutions before electroporation (4D-Nucleofector System; Lonza; program DC-100). For experiments involving the Neon Transfection System (Thermo Fisher Scientific), ∼150,000 cells were resuspended in Resuspension Buffer R, mixed with the gRNA/rCas9 solutions, and electroporated at 1,400 V, 20 milliseconds, and two pulses. For experiments involving two sequential electroporations, HBECs were seeded on human placental collagen-coated 6-well dishes and propagated in bronchial epithelial cell growth medium (BEGM; Lonza) supplemented with the rho-associated protein kinase inhibitor Y-27632 (10 μM; Enzo Life Sciences), which enhances cell proliferation (16). After 3 days, cells were harvested for the second electroporation. After completing the electroporation(s), HBECs were seeded on human placental collagen–coated 6.5-mm Transwell inserts (Corning) and cultured at ALI as described previously (17). In some experiments, targeting efficiency was assessed by harvesting HBECs 3 days after electroporation(s). In other experiments, cells were maintained in ALI culture for 23 days before harvesting to allow cells to differentiate. Where indicated, IL-13 (10 ng/ml; Peprotech) was added to the culture medium for the final 7 days of the 23-day ALI culture period. In experiments with BEAS-2B cells, cells were harvested 3 days after electroporation.
Measuring Targeting Efficiency
Genomic DNA was extracted using the Quick-DNA Miniprep Plus Kit (Zymo Research) or the RNA/DNA/Protein Purification Plus Kit (Norgen Biotek) according to manufacturers’ protocols. Approximately 1-kb regions containing the gRNA target were amplified using appropriate PCR primers (Table E2) and Q5 DNA Polymerase (New England Biolabs) and purified using a PCR Purification Kit (Qiagen). In cases where one gRNA was used per sample, PCR products were analyzed by Sanger sequencing (MCLAB; sequencing primers are shown in Table E2), and sequencing read files from cells treated with gene-targeting gRNAs (experimental) and with nontargeting gRNAs (control) were analyzed using the Inference of CRISPR Edits (ICE) tool (18) to determine targeting efficiency, defined as percentage of insertions or deletions (indels). In experiments involving deletion of a larger genomic DNA region using a pair of sgRNAs, targeting efficiency was measured via 2% agarose gel electrophoresis followed by quantitation of PCR product band intensities using Fiji (19). Band intensities were adjusted according to the estimated masses of each PCR product.
Additional information regarding materials and methods can be found in the data supplement.
Results
Optimizing Genome Targeting in Human Primary Bronchial Epithelial Cells
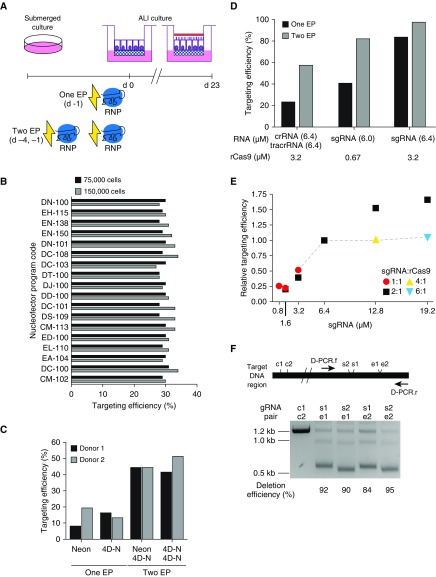
Our general approach to gene targeting in HBECs via electroporation of gRNA/rCas9 RNP complex is shown in Figure 1A. After 6 days in submerged culture, cells were placed in suspension and electroporated using rCas9 complexed with a crRNA designed to target SPDEF and a tracrRNA. Cells were then placed in culture on Transwell inserts. After reaching confluence (Day 0), the apical medium was removed, and cells were maintained in ALI culture. Gene targeting efficiency was analyzed by ICE-coupled Sanger sequencing (18). We began by using rCas9, crRNA, and tracrRNA concentrations previously used with primary human T cells (14). In initial experiments, we used the 4D-Nucleofector system and found similar targeting efficiencies (∼30%) using each of 18 nucleofection programs (Figure 1B). Targeting efficiency was similar when cell numbers were doubled (from 75,000 to 150,000 cells per electroporation). We compared the 4D-Nucleofector and Neon nucleofection systems and did not observe consistent differences in efficiency (Figure 1C). However, addition of a second round of electroporation, performed 3 days after the first electroporation, substantially increased efficiency. The 4D-Nucleofector system, which allows for up to 96 simultaneous electroporations, was used for subsequent experiments.
Figure 1.
Optimization of gene targeting via RNP (ribonucleoprotein) transfection of primary human bronchial epithelial cells (HBECs). (A) Overview of targeting protocol. After a period of submerged culture on tissue culture dishes, cells were mixed with guide RNA (gRNA):recombinant Cas9 (rCas9) RNP complexes that were delivered by electroporation (EP) before initiation of air–liquid interface (ALI) culture. In some experiments, cells underwent two EPs before initiation of ALI. For assessments of targeting efficiency, cells were harvested at 3 days unless otherwise specified. For studies that required analysis of differentiated cells, cells were cultured for 23 days to allow for formation of ciliated cells and a mucus layer (red) before harvesting. (B) Effects of 4D-Nucleofector (4D-N) program on targeting efficiency. RNP complexes comprising SPDEF-3 CRISPR RNA (crRNA; 6.4 μM), trans-activating CRISPR RNA (tracrRNA; 6.4 μM), and rCas9 (3.2 μM) were delivered to either 75,000 (black) or 150,000 (gray) primary HBECs using 18 different programs. All subsequent experiments were performed with program DC-100 and 150,000 cells. Each value is from a single well from one experiment with one HBEC donor. (C) Effects of nucleofection device and number of EPs on targeting efficiency. RNP complexes were prepared as in B and delivered to HBECs using either a Neon Nucleofection System or a 4D-N. After one EP, cells were either directly seeded on Transwells or propagated in submerged culture for 3 days before undergoing a second EP. Cells were cultured for 23 days prior to harvesting. One well from each of two HBEC donors was tested for each condition. All subsequent experiments were performed using the 4D-N device. (D) Comparison of crRNA:tracrRNA and single guide RNA (sgRNA)-based gene targeting. The crRNA and the sgRNA were designed based on the same SPDEF-3 gRNA sequence and delivered by a single electroporation or by two sequential electroporations. Each value is from a single well from one experiment with one HBEC donor. (E) Effects of varying sgRNA and rCas9 concentrations. Results are aggregated from three separate single electroporation experiments. In each experiment, a single well was used to test each condition. In one experiment, a KLF5 sgRNA was used to test sgRNA:rCas9 ratios of 4:1 and 6:1. In two other experiments, the SPDEF-3 sgRNA was used to test other ratios. In all experiments, one sample was electroporated with the reference concentrations (sgRNA, 6.4 μM; rCas9, 3.2 μM; 2:1 ratio). At the reference concentrations, absolute targeting efficiencies ranged from 55% to 70%. Relative targeting efficiencies were normalized to reference concentration targeting efficiencies from the same experiment to facilitate comparisons across experiments. Gray dashed lines connect data points of increasing ratios of sgRNA:rCas9 at the reference concentration (3.2 μM) of rCas9. The reference concentrations of sgRNA and rCas9 used in these experiments were used for all subsequent experiments. (F) Highly efficient deletion of a genomic DNA region. HBECs were transfected with 3.2 μM rCas9 and a pair of sgRNAs (s1 or s2 and e1 or e2; 3.2 μM of each of the pair of sgRNAs) by two electroporations, targeting two sites separated by ∼0.6 kb. Genomic DNA from targeted cells was amplified by PCR with primers D-PCR.f and D-PCR.r and analyzed by agarose gel electrophoresis. Control cells were transfected with c1 and c2 control sgRNAs, which targeted sequences located ∼15 kb outside of the amplified region. Band intensities were quantified by densitometry and adjusted according to the estimated masses of each PCR product, and deletion efficiencies were calculated as the ratio of the ∼0.6 kb band adjusted intensity to the total adjusted intensity for the ∼0.6 and 1.2 kb bands. Each lane is from a single well from one experiment with one HBEC donor. KLF5 = Kruppel-like factor 5; SPDEF = SAM pointed domain containing E26 transformation-specific transcription factor.
In an attempt to improve targeting efficiency, we focused on the type and amount of guide RNA. As an alternative to using the two-component crRNA:tracrRNA system, we tested an sgRNA approach. Unlike the crRNA:tracrRNA system, which requires hybridization of two smaller RNA molecules that then complex with rCas9, each sgRNA molecule contains both the crRNA sequence and the Cas9-binding sequence. Compared with a crRNA:tracrRNA targeting the same sequence, delivery of sgRNA resulted in a higher targeting efficiency (up to 97% with two electroporations; Figure 1D). When using sgRNA, increasing the amounts of RNA and rCas9 from levels recommended by the manufacturer (6.0 and 0.67 μM, respectively) to the amount used in our standard crRNA:tracrRNA protocol (6.4 and 3.2 μM) appeared to increase targeting efficiency. To evaluate this in more detail, we tested a range of concentrations and ratios of sgRNA:rCas9 (Figure 1E). On the basis of limited testing with our standard concentration of rCas9 (3.2 μM), targeting efficiency reached near-maximal levels when sgRNA was present in a twofold molar excess. Efficiency increased when the amount of sgRNA was increased while maintaining a 2:1 ratio.
Strand breaks generated by targeting a single DNA sequence generally result in short indels, but for some applications, such as analysis of noncoding regions of the genome, it is useful to delete larger regions. To test whether our approach could be used to delete larger regions, we introduced pairs of sgRNAs targeting regions that were separated by ∼600 nt (Figure 1F). We found that this approach efficiently introduced large deletions in HBECs.
Predictable Control of Gene-Targeting Efficiency in HBECs
Targeting efficiency also depends on the gRNA sequence. Despite the availability of numerous prediction algorithms, currently available computational tools cannot robustly predict gRNA targeting efficiencies (20). Because the supply of HBECs can be a limiting factor for some experiments, we investigated whether HBEC targeting efficiency could be estimated by testing gRNAs in BEAS-2B, an immortalized human airway epithelial cell line. Four different gRNAs targeting the same SPDEF exon were selected using the MIT Guide Design Tool, and all had high specificity scores. We delivered each to BEAS-2B cells (single electroporation) and primary HBECs (single or double electroporation) (Figure 2A). The relative targeting efficiency for the four gRNAs was the same in all cases, with gRNA 2 having the lowest efficiency and gRNA 4 the highest. For each sgRNA, targeting efficiency in BEAS-2B cells (one electroporation) was similar to efficiency found after two electroporations of HBECs.
Figure 2.
Predictable control of gene targeting efficiencies in primary HBECs. (A) Efficiencies of four sgRNA targeting sequences in the SPDEF gene delivered to BEAS-2B (an immortalized human airway epithelial cell line) cells or HBECs by EP. BEAS-2B cells were harvested 3 days after electroporation, and HBECs were cultured at ALI for 23 days with IL-13 stimulation for the last 7 days of culture. Each value is from a single well from one experiment with one HBEC donor. (B) Targeting efficiencies of the same gRNAs delivered to HBECs by lentiviral transduction. Transduced cells were cultured at ALI for 23 days with IL-13 stimulation for the last 7 days of culture. Each value is from a single well from one experiment with one HBEC donor. (C) Duplicate wells of HBECs electroporated as in A or transduced as in B were differentiated at ALI and stimulated with IL-13, and mucin 5AC (MUC5AC)-producing cells were quantified by flow cytometry. Reduction in MUC5AC-producing cells was computed by comparison with cells transfected with nontargeting control gRNAs in the same experiment. MUC5AC measurements are paired with targeting efficiency measurements from duplicate wells of cells from the same transfection or transduction. R = Pearson correlation coefficient.
Although virus-free gene targeting offers many advantages, virus-based methods are also useful for some applications. Direct determination of virus-based gene targeting efficiency in HBECs requires cloning, virus production, and testing in cell culture. To determine whether the efficiency of virus-based targeting in HBECs could be predicted from results with virus-free targeting with RNPs, we produced lentiviruses that drove expression of each of the four SPDEF-targeting gRNAs. Lentiviral delivery of each of these four gRNAs followed by antibiotic selection for transduced cells (Figure 2B) produced targeting efficiencies that were remarkably similar to efficiencies obtained with the same sequences delivered using the virus-free system without antibiotic selection (Figure 2A). This suggests that sequence-dependent effects on targeting efficiency are largely independent of method of delivery and that the virus-free methods can be useful for rapid screening of gRNA efficiency before subsequent experiments using either virus-free or virus-based methods.
In most cases, the goal of gene targeting is to eliminate functional protein production by the targeted gene. We attempted to use commercially available antibodies against SPDEF but were unable to detect this protein in untargeted cells. As an alternative, we measured the effect of SPDEF targeting on SPDEF function. Previous studies in mice demonstrated that Spdef is required for IL-13–induced production of the airway mucin 5AC (MUC5AC) (15). We used both virus-free and lentiviral-based methods to target SPDEF in HBECs, allowed cells to differentiate at ALI, stimulated cells with IL-13 for 7 days, and then measured MUC5AC-producing cells by flow cytometry. We found excellent correlation between gene targeting efficiency and reduction in MUC5AC-producing cells (Figure 2C). This correlation was seen across four distinct gRNA sequences, providing strong evidence that the effect results from elimination of functional SPDEF protein and not from off-target effects. The most efficient gRNA sequence, SPDEF-4, resulted in >90% SPDEF targeting and >95% reduction in MUC5AC-producing cells with both virus-free and lentiviral delivery.
Highly Efficient Gene Targeting Dissects the Role of SPDEF in IL-13–induced Effects on the HBEC Transcriptome
Having established the capacity for efficient targeting of SPDEF, we used the HBEC ALI culture system to study downstream effects of sgRNA/rCas9-mediated nonviral deletion of this critical transcription factor. Analyses of effects of SPDEF targeting on mRNA transcripts showed that SPDEF is required for IL-13–induced increases of MUC5AC (Figure 3A). SPDEF was also required for the IL-13–induced increases in the goblet cell–related mRNAs FOXA3 (forkhead box A3) and CLCA1 (chloride channel accessory 1) (Figures 3B and 3C) and the IL-13–induced decrease in expression of club cell secretory protein SCGB1A1 (Figure 3D). Analyses of transcripts expressed primarily outside of the secretory cell lineage revealed no detectable effects of SPDEF targeting on the induction of POSTN (periostin) by IL-13 or on the expression of the ciliated cell marker TUBB4B (tubulin beta 4B class IVb) and the basal cell marker KRT5 (keratin 5) (Figures 3E–3G).
Figure 3.
SPDEF-dependent and SPDEF-independent effects of IL-13 on gene expression in HBECs. (A–H) Primary HBECs from three donors were electroporated twice with RNPs containing nontargeting control sgRNAs (NT) or SPDEF-4 sgRNA and cultured at ALI for 23 days with IL-13 stimulation for the last 7 days of culture. (A–H) Gene expression changes of MUC5AC (A), FOXA3 (forkhead box A3) (B), CLCA1 (chloride channel accessory 1) (C), SCGB1A1 (secretoglobin family 1A member 1) (D), POSTN (periostin) (E), TUBB4B (tubulin β 4B class IVb) (F), KRT5 (keratin 5) (G), and MUC5B (mucin 5B) (H) were measured using quantitative real-time PCR. Values are log2-fold differences relative to cells electroporated with NT control sgRNAs and cultured without IL-13 stimulation (dashed line). *P < 0.05 and not significant (ns) for comparison between IL-13–stimulated NT and SPDEF-4 sgRNA by Student’s t test. †P < 0.05 for comparison between unstimulated controls and IL-13–stimulated HBECs electroporated with NT control sgRNAs by Student’s t test. (I) To further analyze how MUC5B expression is regulated by SPDEF, we analyzed effects of SPDEF targeting on MUC5B expression in unstimulated as well as IL-13–stimulated cells from three donors. Gray dashed lines connect values from the same HBEC donor. One of the three donors was the same as in A–H. *P < 0.05 by Student’s t test for comparisons indicated by horizontal lines.
In addition to MUC5AC, the other major airway mucin is mucin 5B (MUC5B) (21). Previous reports indicate that MUC5B, unlike MUC5AC, decreased by IL-13 stimulation. We found that SPDEF targeting led to a further decrease in MUC5B expression below the level seen with IL-13 treatment alone (Figure 3H). Further analysis showed that IL-13 stimulation reduced MUC5B mRNA level in both nontargeted cells (70% reduction) and SPDEF-targeted cells (83% reduction; Figure 3I). Analogously, SPDEF targeting reduced MUC5B expression in both unstimulated cells (60% reduction) and IL-13–stimulated cells (77% reduction). These results indicate that SPDEF expression increases expression of MUC5B and that the IL-13–induced decrease in MUC5B expression does not require SPDEF.
Targeting SPDEF Prevents IL-13–induced Goblet Cell Metaplasia and Mucostasis
We used histologic staining and immunofluorescence to examine effects of RNP-based gene targeting on the epithelium (Figure 4). As expected, IL-13 induced a marked change in the appearance of the epithelium, with the appearance of a large number of Alcian blue–periodic acid-Schiff and MUC5AC-stained goblet cells. Electroporation with rCas9 and a nontargeting control sgRNA had no obvious effects on the appearance of either untreated or IL-13–treated cultures and did not have a significant effect on number of MUC5AC-producing cells after IL-13 treatment (mean fold difference of 1.11 ± 0.22; P = 0.62; N = 5). In contrast, use of an SPDEF-targeting sgRNA resulted in dramatic protection from the effects of IL-13 on mucous metaplasia and MUC5AC staining, whereas the population of acetylated α-tubulin–containing ciliated cells was maintained.
Figure 4.
SPDEF targeting blocks IL-13–induced mucous cell metaplasia. HBECs were cultured at ALI for 23 days without prior electroporation sgRNA− or after electroporation with NT or SPDEF-4 sgRNA. Some cultures were stimulated with IL-13 for the final 7 days as indicated. (A) Hematoxylin and eosin (H&E) staining. (B) Alcian blue–periodic acid–Schiff (AB-PAS), pH 2.5, staining for mucopolysaccharides. (C) Immunofluorescence staining for MUC5AC (cyan) and MUC5B (red) mucins and the ciliated cell marker acetylated α-tubulin (ac-tubulin, yellow). Nuclei were stained with DAPI (purple). Scale bars: 10 μm. All images are from the same experiment with cells from the same donor. These images are representative of 3 wells per condition (1 to 3 HBEC donors per condition).
We previously showed that IL-13–induced changes in mucin composition and organization result in epithelial mucus tethering and impaired mucociliary clearance as measured by transport of fluorescent microspheres in HBEC ALI cultures (22). We investigated whether rCas9 and sgRNA-mediated gene targeting could be used to identify molecular mechanisms required for this critical function of HBECs (Figure 5). IL-13 stimulation of cells treated with rCas9 and a nontargeting control sgRNA led to a marked reduction in microsphere transport speed, as previously reported for untransfected cells (22). After targeting SPDEF, IL-13–stimulated cultures had transport speeds that were higher than those found in cells electroporated with control sgRNA and treated with IL-13 and were not significantly different from transport speeds found in untreated control cultures. We conclude that IL-13–induced mucostasis is SPDEF dependent and that RNP-directed gene targeting enables evaluation of the roles of specific genes in HBEC function.
Figure 5.
SPDEF targeting prevents IL-13–induced mucostasis. Mucociliary transport rates were determined from trajectories of fluorescent microspheres placed on gels atop unstimulated and IL-13–stimulated ALI–cultured HBECs electroporated with either NT or SPDEF-4 sgRNAs and cultured for 23 days at ALI. (A) Superimposition of 10 images acquired at 1-second intervals. Scale bars: 50 μm. (B) Microsphere speeds determined from 3 donors, 3 wells per donor, 3 fields per well (total of 27 fields per condition). Values represent median microsphere speed for each field. Boxes extend from the 25th to the 75th percentile, the horizontal line within the box indicates the mean, and the whiskers represent minimum and maximum values. **P < 0.01 and ***P < 0.001. ns = not significant by the Games-Howell test.
Discussion
We optimized nonplasmid, nonviral direct delivery of RNPs to HBECs and used this approach to show that the goblet cell transcription factor SPDEF is required for IL-13–induced mucostasis. The methods we developed should be broadly applicable for mechanistic experiments in the widely used HBEC ALI culture system, which has unique strengths for studies of airway epithelial function in human health and in airway diseases. Using these methods, we demonstrated that SPDEF’s central role in goblet cell differentiation is conserved between mouse and human and that disruption of SPDEF prevents IL-13–driven changes in secretory cell transcripts. Some effects of IL-13, including its effects on expression of Muc5b/MUC5B, are not conserved between mouse and human, and the use of the HBEC gene-targeting system allowed us to show that SPDEF disruption does not restore MUC5B expression in IL-13–treated human cells to unstimulated levels. Because the HBEC ALI culture system is well suited for functional studies, we were also able to show that SPDEF is required for IL-13–induced mucostasis, an important contributor to airway obstruction, morbidity, and mortality in asthma.
We identified several factors that affected gene-targeting efficiency in HBECs. By varying the amounts of sgRNA and rCas9, the sgRNA sequence, and the number of electroporations, we were able to obtain SPDEF-targeting efficiencies that approached 100% without the need for antibiotic selection. For the set of four SPDEF-targeting sgRNAs we tested, targeting efficiency in an immortalized cell line (BEAS-2B) was predictive of efficiency in HBECs. Furthermore, targeting efficiency resulting from direct delivery of RNPs by electroporation also predicted efficiency of targeting by lentiviruses expressing the same gRNA sequence. Although these experiments were limited to a small number of gRNAs targeting the same gene, the results suggest that testing gRNA sequences by direct delivery to cell lines provides an efficient path for selecting optimal gRNA sequences for subsequent use.
Compared with lentiviral delivery, direct delivery of RNPs by electroporation offers several advantages. With direct delivery of RNPs, there is no requirement for plasmid construction, virus production, or antibiotic selection of transduced cells, and there are no concerns about potential effects of viral infection or prolonged expression of Cas9 and gRNAs. By varying delivery conditions, we were able to precisely control targeting efficiency to examine the relationship between the expression of the targeted gene (e.g., SPDEF) and the downstream effects of targeting the gene (e.g., SPDEF) and the effects of gene targeting (e.g., reduction in MUC5AC expression). Although the limited duration of exposure to Cas9 and gRNA might be beneficial in reducing off-target effects, we did not directly assess off-target effects in our experiments. However, the finding that SPDEF targeting efficiency was highly correlated with downstream changes in MUC5AC expression across the set of four sgRNAs provides confidence that these changes did not result from off-target effects.
The direct delivery method also simplifies targeting of multiple sequences in the genome. We showed that targeting of two sites in the same region led to efficient deletion of larger segments of genomic DNA (>0.5 kb). This approach could be useful for analyzing the functions of regulatory regions, where it may be challenging or impossible to disrupt function with short insertions or deletions produced by individual gRNAs. Although not tested in these experiments, we expect that use of multiple sgRNAs targeting different genes in a single electroporation or in sequential electroporations will allow for efficient analysis of epistatic interactions in HBECs. Despite the advantages of direct delivery of RNPs, lentiviral delivery may be preferred for experiments involving very large numbers of cells, where cost of sgRNA and rCas9 may be an issue, or for testing large libraries of gRNA sequences in pooled screens (23).
We chose to target SPDEF on the basis of prior work in transgenic mice that demonstrated a critical role of this transcription factor in goblet cell differentiation (15). In mouse and human systems, IL-13 produced by type 2 lymphocytes and innate lymphoid cells drives an increase in Spdef/SPDEF expression and a large change in the secretory cell population, with a decrease in club cells and an increase in mucus-producing goblet cells (24, 25). IL-13 increases expression of the major mucin gene Muc5ac/MUC5AC in both mice and humans (26, 27). Experiments in human cell lines found a role for SPDEF in regulation of MUC5AC expression (15, 28), but these systems do not provide effective models for studying important aspects of bronchial epithelial cell differentiation or mucociliary function. Targeting SPDEF in HBECs allowed us to investigate the role of this transcription factor in a differentiated model of the bronchial epithelium.
We found that targeting SPDEF in HBECs led to a proportional reduction in IL-13–induced expression of MUC5AC. Using conditions that led to highly efficient targeting showed that SPDEF was also required for expression of other genes that are characteristic of goblet cells, including FOXA3 and CLCA1. These findings are consistent with prior work indicating that Foxa3 is downstream of Spdef in mouse airway epithelial cells (15) and that CLCA1 triggers a MAPK13-dependent pathway responsible for mucus production (29). In addition to increases in these goblet cell transcripts, SPDEF targeting restored IL-13–suppressed expression of the club cell gene SCGB1A1 (also known as CCSP), indicating that SPDEF is required for the IL-13–induced shift of secretory cells from a predominantly club cell phenotype to a predominantly goblet cell phenotype. Induction of POSTN, which is highly induced by IL-13 and is expressed predominantly in basal cells (30), was unaffected by targeting SPDEF, which indicates that the targeting protocol did not have global effects on IL-13 signaling outside of the secretory cell lineage.
The availability of a system for efficient gene targeting in HBECs allowed us to investigate an important species difference in IL-13 effects on mucin expression. In mice, IL-13 increases expression of both major airway mucins, Muc5ac and Muc5b (27). However, in human cells, IL-13 increases MUC5AC expression but decreases MUC5B expression (22). This is likely relevant to asthma, because individuals with type 2–high asthma have reduced MUC5B expression as well as increased MUC5AC expression (31). Although targeting SPDEF prevented other IL-13–induced changes in secretory cells, including the increase in MUC5AC and the decrease in the club cell marker SCGB1A1 (CCSP), targeting SPDEF did not prevent the IL-13–induced decrease in MUC5B. On the contrary, targeting SPDEF reduced MUC5B expression both in unstimulated cells and in IL-13–stimulated cells. Previous mouse studies found that Spdef deficiency reduced both Muc5ac and Muc5b expression (32, 33). SPDEF/Spdef therefore appears to have a similar ability to increase both MUC5AC/Muc5ac and MUC5B/Muc5b in human and mouse airway epithelial cells. Our work indicates that the difference in MUC5B/Muc5b responses to IL-13 in humans and mice is not due to species differences in SPDEF/Spdef function but instead is attributable to an as-yet-unidentified IL-13–induced SPDEF-independent pathway that decreases MUC5B expression in humans but not in mice. The gene targeting method that we developed should be useful for investigating this and other pathways that contribute to asthma and other human airway diseases.
We found that SPDEF is required for IL-13–induced mucostasis. We previously showed that IL-13–induced changes in mucus production and organization result in tethering of MUC5AC-rich mucus domains to the airway epithelium, which dramatically impairs mucociliary clearance (22). The ability to efficiently target genes in the HBEC ALI culture system without apparent nonspecific effects on cell differentiation or mucociliary function allowed us to test the requirement for SPDEF in IL-13–induced mucostasis. Our results suggest that SPDEF could be a valuable target for therapies designed to decrease mucus production and improve mucociliary clearance in asthma or other airway diseases. However, given the important role of MUC5B in airway homeostasis and protection from infection (34), the finding that SPDEF targeting exacerbates rather than reverses IL-13–induced decreases in MUC5B expression suggests a potential limitation of this approach.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Michael Matthay for supplying human bronchial specimens and Jane Gordon (UCSF Laboratory for Cell Analysis), Kari Herrington (UCSF Nikon Imaging Center), Theodore Roth, Alexander Marson, and Olivier Le Tonqueze for technical advice and assistance.
Footnotes
Supported by National Institutes of Health grants U19 AI 077439, R35 HL145235, and R01 HL138424 (D.J.E.); National Institutes of Health grant DK072517 and Cystic Fibrosis Foundation grant DR613-CR11 (W.E.F.); and China Scholarship Council Fund 201806275100 and National Natural Science Foundation of China grant 81600023 (D.C.).
Author Contributions: K.D.K., S.S., L.R.B., P.G.W., and D.J.E. contributed to study conception and design. K.D.K. and S.S. developed novel experimental methods. K.D.K., D.C., L.R.B., D.I.S., and L.T.Z. performed experiments. K.D.K. and D.C. analyzed data. K.D.K., S.S., D.C., L.R.B., and D.J.E. interpreted data. W.E.F., P.G.W., and D.J.E. supervised study execution. K.D.K. drafted the manuscript. K.D.K., S.S., D.C., L.R.B., D.I.S., L.T.Z., W.E.F., P.G.W., and D.J.E. revised the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0266OC on October 9, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Erle DJ, Sheppard D. The cell biology of asthma. J Cell Biol. 2014;205:621–631. doi: 10.1083/jcb.201401050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Rose V, Molloy K, Gohy S, Pilette C, Greene CM. Airway epithelium dysfunction in cystic fibrosis and COPD. Mediators Inflamm. 2018;2018:1309746. doi: 10.1155/2018/1309746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983;128:S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- 4.Pezzulo AA, Starner TD, Scheetz TE, Traver GL, Tilley AE, Harvey B-G, et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2011;300:L25–L31. doi: 10.1152/ajplung.00256.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaya M, Finkbeiner WE, Chun SY, Widdicombe JH. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 6.Awatade NT, Wong SL, Hewson CK, Fawcett LK, Kicic A, Jaffe A, et al. Human primary epithelial cell models: promising tools in the era of cystic fibrosis personalized medicine. Front Pharmacol. 2018;9:1429. doi: 10.3389/fphar.2018.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lino CA, Harper JC, Carney JP, Timlin JA. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 2018;25:1234–1257. doi: 10.1080/10717544.2018.1474964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu HW, Rios C, Huang C, Wesolowska-Andersen A, Burchard EG, O’Connor BP, et al. CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther. 2015;22:822–829. doi: 10.1038/gt.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765–771. doi: 10.1038/nbt.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S, Kim D, Cho SW, Kim J, Kim J-S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 2014;24:1012–1019. doi: 10.1101/gr.171322.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gundry MC, Brunetti L, Lin A, Mayle AE, Kitano A, Wagner D, et al. Highly efficient genome editing of murine and human hematopoietic progenitor cells by CRISPR/Cas9. Cell Rep. 2016;17:1453–1461. doi: 10.1016/j.celrep.2016.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth TL, Puig-Saus C, Yu R, Shifrut E, Carnevale J, Li PJ, et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature. 2018;559:405–409. doi: 10.1038/s41586-018-0326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, et al. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol. 2013;49:341–347. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin MH, Sullivan S, Nielson D, Yang B, Finkbeiner WE, Verkman AS. Hypertonic saline therapy in cystic fibrosis: evidence against the proposed mechanism involving aquaporins. J Biol Chem. 2006;281:25803–25812. doi: 10.1074/jbc.M604332200. [DOI] [PubMed] [Google Scholar]
- 18.Hsiau T, Maures T, Waite K, Yang J, Kelso R, Holden K, et al. Inference of CRISPR edits from Sanger trace data [preprint] bioRxiv. 2019 doi: 10.1089/crispr.2021.0113. [accessed 2019 Jul 26]. Available from: https://www.biorxiv.org/content/10.1101/251082v1. [DOI] [PubMed] [Google Scholar]
- 19.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson LOW, O’Brien AR, Bauer DC. The current state and future of CRISPR-Cas9 gRNA design tools. Front Pharmacol. 2018;9:749. doi: 10.3389/fphar.2018.00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonser LR, Erle DJ. Airway mucus and asthma: the role of MUC5AC and MUC5B. J Clin Med. 2017;6:E112. doi: 10.3390/jcm6120112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonser LR, Zlock L, Finkbeiner W, Erle DJ. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J Clin Invest. 2016;126:2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laoukili J, Perret E, Willems T, Minty A, Parthoens E, Houcine O, et al. IL-13 alters mucociliary differentiation and ciliary beating of human respiratory epithelial cells. J Clin Invest. 2001;108:1817–1824. doi: 10.1172/JCI13557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 27.Kuperman DA, Huang X, Nguyenvu L, Hölscher C, Brombacher F, Erle DJ. IL-4 receptor signaling in Clara cells is required for allergen-induced mucus production. J Immunol. 2005;175:3746–3752. doi: 10.4049/jimmunol.175.6.3746. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Li Q, Kolosov VP, Perelman JM, Zhou X. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes. 2010;17:83–92. doi: 10.3109/15419061.2010.551682. [DOI] [PubMed] [Google Scholar]
- 29.Alevy YG, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122:4555–4568. doi: 10.1172/JCI64896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-β activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen G, Volmer AS, Wilkinson KJ, Deng Y, Jones LC, Yu D, et al. Role of Spdef in the regulation of Muc5b expression in the airways of naive and mucoobstructed mice. Am J Respir Cell Mol Biol. 2018;59:383–396. doi: 10.1165/rcmb.2017-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajavelu P, Chen G, Xu Y, Kitzmiller JA, Korfhagen TR, Whitsett JA. Airway epithelial SPDEF integrates goblet cell differentiation and pulmonary Th2 inflammation. J Clin Invest. 2015;125:2021–2031. doi: 10.1172/JCI79422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.