Abstract
Responsible implementation of engineered nanomaterials (ENMs) into commercial applications is an important societal issue, driving demand for new approaches for rapid and comprehensive evaluation of their bioactivity and safety. An essential part of any research focused on identifying potential hazards of ENMs is the appropriate selection of biological endpoints to evaluate. Herein, we use a tiered strategy employing both targeted biological assays and untargeted quantitative proteomics to elucidate the biological responses of human THP-1 derived macrophages across a library of metal/metal oxide ENMs, raised as priority ENMs for investigation by NIEHS’s Nanomaterial Health Implications Research (NHIR) program. Our results show that quantitative cellular proteome profiles readily distinguish ENM types based on their cytotoxic potential according to induction of biological processes and pathways involved in the cellular antioxidant response, TCA cycle, oxidative stress, endoplasmic reticulum stress, and immune responses as major processes impacted. Interestingly, bioinformatics analysis of differentially expressed proteins also revealed new biological processes that were influenced by all ENMs independent of their cytotoxic potential. These included biological processes that were previously implicated as mechanisms cells employ as adaptive responses to low levels of oxidative stress, including cell adhesion, protein translation and protein targeting. Unsupervised clustering revealed the most striking proteome changes that differentiated ENM classes highlight a small subset of proteins involved in the oxidative stress response (HMOX1), protein chaperone functions (HS71B, DNJB1), and autophagy (SQSTM), providing a potential new panel of markers of ENM-induced cellular stress. To our knowledge, the results represent the most comprehensive profiling of the biological responses to a library of ENMs conducted using quantitative mass spectrometry-based proteomics. The results provide a basis to identify the patterns of a diverse set of cellular pathways and biological processes impacted by ENM exposure in an important immune cell type, laying the foundation for multivariate, pathway-level structure activity assessments of ENMs in the future.
Keywords: engineered nanomaterials, proteomics, cytotoxicity, oxidative stress, macrophage
Graphical Abstract

1. Introduction
As advances in nanoscience continue to drive transformations in consumer products, energy efficiency, medical imaging, drug delivery and other important applications, the responsible implementation of these technologies remains a critical societal issue. In particular, the enhanced potential for human exposure to engineered nanomaterials (ENMs) through ingestion, inhalation, or dermal penetration due to their increased prevalence in commercial applications is an important concern. The vast diversity of ENMs under development is also driving demand for new approaches to comprehensively assess bioactivity and potential hazard of ENMs, increasing the importance of in vitro (cellular) test systems that can be conducted with rapid throughput. Accordingly, the U.S. National Institutes of Environmental Health Sciences implemented the Nanomaterials Health Implications Research (NHIR) Consortium, to advance research linking the physical and chemical properties of ENMs to biological responses, with the goal of predicting potential health impacts associated with high priority and emerging classes of ENMs. ENMs composed of metal and metal oxides remain high priority materials for investigation due to their broad applications including food packaging (Pulizzi 2016; Sharma et al. 2017), as anti-microbial agents (Arias et al. 2018), as medical diagnostics and imaging agents (Parveen et al. 2012), and in a variety of semiconductor applications (Waiskopf et al. 2016).
An essential aspect of any research focused on identifying bioactivity and potential hazard of ENMs is the appropriate selection of biological endpoints to evaluate. Unfortunately, the primary focus of many nanotoxicity studies is limited to measures of the direct cytotoxic or pro-inflammatory effects of ENMs, with little attention to more subtle alterations in biological function. For instance, previous studies from us and others have shown that ENMs which are often categorized as biologically inert based on cytotoxicity analysis alone can modulate the expression of hundreds of gene products (Kodali et al. 2013; Perkins et al. 2012; Waters et al. 2009). In the case of macrophages, an important immune cell type critical for clearance of foreign particles, exposure to some classes of ENMs even at subcytotoxic levels can disrupt important innate immune functions and hinder cellular phagocytic capacity against foreign pathogens (Kim et al. 2011; Kodali et al. 2013; Thrall et al. 2019). Identifying the underlying pathways involved in these responses to engineered particulates is not only important to advance predictive nanotoxicology, but could provide new insight into the mechanisms by which ambient air particulate exposures increase risk of lung infections such as pneumonia, particularly in children and elderly (Loeb et al. 2009; Neupane et al. 2010). Similar mechanisms of bio-nano interactions may also underlie the increased risks of morbidity and lung infections observed in welders exposed to fumes that are rich in nanoscale metal oxide particles (Andujar et al. 2014; Coggon et al. 1994; Palmer et al. 2003).
Omics-based technologies offer attractive approaches for both an unbiased and multivariate system-level characterization of nano-bio interactions. Although transcriptomics studies have provided insights into the cellular responses to a variety of ENMs (Costa et al. 2018; Frohlich 2017; Kodali et al. 2013; Zhao et al. 2015a), it is also widely understood that changes in mRNA abundance are not necessarily predictive of changes at the protein level. However, assessing global proteome responses to broad types of ENMs at both a quantitative and comprehensive level remains challenging, despite providing a more direct read-out of signaling and cell phenotypes than provided by transcriptomics. While proteomics approaches have been commonly used to qualitatively characterize the corona of adsorbed proteins on ENMs (Shannahan et al. 2013; Zhang et al. 2011), quantitative mass spectrometry-based proteomics is only beginning to be fully explored as a strategy for hazard assessment and mechanistic studies of ENM toxicity (Zhang et al. 2018). Inclusion of multivariate omics endpoints as part of a hierarchical structure-activity assessment for ENMs has the potential to link intrinsic properties of ENMs with specific biological processes and/or pathway-level responses (Cai et al. 2018). Since regulation of key biological processes such as phagocytic function and endoplasmic reticulum (ER) stress involve multiple proteins and mediators, it is anticipated that compared to targeted biological endpoints, omics measurements of the impact of ENM exposure will reduce uncertainty in assessing the potential bioactivity of ENMs.
The ability of ENMs to induce cellular oxidative stress has emerged as one of the leading paradigms for predicting the toxicity of ENMs, particularly for metals and metal oxides (Kodali and Thrall 2015; Meng et al. 2009; Thrall et al. 2019). Previous work from our laboratory comparing response of macrophages to three types of metal oxide ENMs revealed a clear relationship between the level of cellular protein oxidative modification (S-glutathionylation) induced and the degree of altered macrophage function (Duan et al. 2016). These studies also demonstrated that oxidative modifications across the proteome following low levels of ENM-induced oxidative stress were not stochastic, but selective to proteins involved in cellular adaption pathways (e.g., protein translation and ER stress). In contrast, ENMs which displayed cytotoxic potential showed reduced specificity for the proteins targeted by oxidative stress across a broader set of pathways associated with classical stress responses, mitochondrial energetics (e.g., glycolysis), and apoptosis (Duan et al. 2016).
In this study, we extend our previous studies and use a tiered strategy employing both targeted biological endpoints and untargeted quantitative proteomics to elucidate the biological responses of human THP-1 derived macrophages across a broader library of metal and metal oxide ENMs. Twenty metal and metal oxide ENMs were selected as priorities for investigation by the NHIR consortium due to their increasing prevalence in commercial applications and human exposure potential. These ENMs varied widely in their ability to induce cytotoxicity, inflammasome activation, or ER stress, as determined by targeted endpoint assays. Quantitative proteomic profiling of a subset of 11 representative ENMs not only distinguished ENMs with different cytotoxic potential, confirming the role of important stress-response pathways, but also revealed a subset of common biological processes that were generically influenced by particle exposure independent of the cytotoxic potential of the ENM. Our results suggest new sets of adaptive response pathways that are broadly triggered by particle interactions that could contribute to altered macrophage functions and reveal interesting new protein markers which could serve as sensitive markers of cellular redox stress in response to ENMs.
2. Material and Methods
2.1. Preparation and characterization of ENMs
All ENMs used in this study (Table 1) were provided by the Engineered Nanomaterials Resource and Coordination Core (ERCC), which is the primary resource for ENM synthesis and characterization for the NIEHS Nanotechnology Health Implications Research (NHIR) Consortium. ENM suspensions were prepared as described previously (DeLoid et al. 2017). Briefly, ENMs were subjected to sonication in ultra-pure water to form a stable suspension and subsequently measured by dynamic light scattering (DLS). Dispersed ENMs were then diluted in RPMI-1640 culture media supplemented with 10% fetal bovine serum to the desired concentration for cell dosing. Characterization of ENMs provided by the ERCC and our in-house DLS measurements are summarized in Table 1. Details on physicochemical characterization of the ENMs has also been described previously (Ahn et al. 2018; Beltran-Huarac et al. 2018; Zimmerman et al. 2019).
Table 1.
Characterization of metal and metal oxide ENMs used in this study
| ENM type | Serial Number | Particle description | Primary particle diameter (nm)ab | Effective Diameter (nm)c | Zeta Potential (mV)a | Polydispersity Index | Agglomeration State | Density (g/mL)a | Cell-deposited dose fractione |
|---|---|---|---|---|---|---|---|---|---|
| Ag | AG-20NM- JB20170316–1:T10 | Silver (20 nm) | 25.6±6.5 | 211.1±16.5 | −10.5±0.5 | 0.371 | agglomerate | 2.16 | 0.138 |
| Ag_Cit | AG-20NM-CITRATECONV:PC20170324–1:T10 | Silver Citrate Capped (20 nm) | 21.6±2.8 | 115.5±0.9 | −17.2±0.4 | 0.259 | protein-coated primary particles | 1.583 | 0.166 |
| 1%Ag_SiO2 | 1%AG-SIO2-JB20161109–1:T10 | Silver (∼8 nm) Supported on Silica (∼7 nm) | 10.6±7.1 | 219.2±3.1 | −10.4±0.6 | 0.322 | agglomerate | 1.074 | 0.089 |
| 10%Ag_SiO2 | 10%AG-SIO2-JB20161109–1:T10 | Silver (∼7 nm) Supported on Silica (∼10 nm) | 7.8±4.3 | 213.1±19.5 | −12.9±0.2 | 0.309 | agglomerate | 1.087 | 0.09 |
| Al2O3 | AL2O3–30NM:JB20161003–1:T10 | Aluminum Oxide (30 nm) | 28.2±13.1 | 150.9±3.3 | −14.0±1.3 | 0.358 | agglomerate | 1.943 | 0.151 |
| Au | AU-15NM-CITRATECONV:PC20160930–1:T10 | Gold Citrate Capped (15 nm) | 18.4±1.8 | 38.6±0.6 | −17.7±2.4 | 0.341 | non-porous agglomerate | 19.3 | 0.384 |
| CdS | CdS-60NM-NS-B-1 | Cadmium Sulfide (60 nm) | 10.7±3.1 | 162.2±17.4 | −9.6±1.1 | 0.343 | agglomerate | 2.982 | 0.413 |
| CeO2_10nm | CEO2–10NM-JB201611205–1:T10 | Cerium (IV) Oxide (10 nm) | 10.5±5.0 | 219.3±13.3 | −14.7±0.9 | 0.295 | agglomerate | 1.375 | 0.092 |
| CeO2_30 nm | CEO2–30NM-JB20161126–1:T10 | Cerium (IV) Oxide (30 nm) | 34.8±22.9 | 336.3±27.5 | −11.2±1.9 | 0.266 | agglomerate | 1.774 | 0.138 |
| CuO | CUO-50NM-SA-B-1 | Copper Oxide (50 nm) | 50.2±11.0 | 341.9±58.1 | −11.9±1.5 | 0.368 | agglomerate | 1.227 | 0.075 |
| Fe2O3_10nm | FE2O3–10NM-JB20161128–1:T10 | Iron (III) Oxide (10 nm) | 9.9±3.8 | 181.5±0.5 | −13.3±0.7 | 0.338 | agglomerate | 1.226 | 0.099 |
| Fe2O3_100nm | FE2O3–100NM-JB20161128–1:T10 | Iron (III) Oxide (100 nm) | 108.9±47.6 | 2408.9±488.2 | −10.7±0.2 | 0.328 | agglomerate | 1.504 | 1 |
| MgO | MGO-20NM-SC-B-1 | Magnesium Oxide (20 nm) | 23.8±7.6 | 40.9±2.2 | −12.0±1.3 | 0.27 | protein-coated primary particles | 1.125 | 0.205 |
| SiO2 | SIO2–15NM:GP20160930–1:T10 | Silicon Oxide (15 nm) | NAd | 120.0±19.5 | −11.3±2.0 | 0.37 | agglomerate | 1.135 | 0.117 |
| TiO2_E171 | TIO2-E171–100NM-PF-B-1 | Titanium Oxide Food Grade E171 (100 nm) | 113.4±37.2 | 389.7±33.0 | −11.0±1.2 | 0.268 | agglomerate | 1.512 | 0.169 |
| TiO2_P25 | TIO2-P25–30NM-AO-B-1 | Titanium Oxide Degussa P25 (30 nm) | 28.8±11.1 | 342.3±6.5 | −12.8±0.5 | 0.271 | agglomerate | 1.512 | 0.13 |
| V2O5 | V2O5–100NM-B-NS | Vanadium Pentoxide (100 nm) | 310.8±214.7 | 656.9±118.5 | −11.4±1.6 | 0.366 | agglomerate | 1.453 | 1 |
| WO3 | WO3–15NM- JB20170929–1 | Tungsten Oxide (15 nm) | 20.9±9.4 | 65.6±1.1 | −12.4±1.3 | 0.364 | protein-coated primary particles | 1.588 | 0.182 |
| ZnO | ZNO-50NM-MT-B-1 | Zinc Oxide (50 nm) | 45.7±17.4 | 57.8±5.7 | −12.4±1.6 | 0.365 | protein-coated primary particles | 1.552 | 0.181 |
| ZnS | ZnS-100NM-B-NS | Zinc Sulfide (100 nm) | 211.4±77.6 | 243.5±5.0 | −11.9±0.5 | 0.373 | protein-coated primary particles | 1.557 | 0.35 |
Note: All measurements were done in triplicate. Data represented as mean ± standard deviation. Both effective diameter and density were measured in the presence of the RPMI-1640 culture media with 10% FBS.
Data provided by ERCC.
Primary size was determined by TEM.
Effective diameter was determined by DLS.
SiO2 lacks discrete particle borders and therefore is not possible to define TEM based diameter.
Determined by the ISD3 model (see Methods for details)
2.2. Cell dosimetry analysis
The agglomeration state and the cell-deposited dose fraction of each ENM (Table 1) after dilution in cell culture medium were determined by our in vitro dosimetry model, ISD3 (Thomas et al. 2018), which is an experimentally-validated particokinetic model for predicting the combined effects of particle sedimentation, diffusion and dissolution on cellular dosimetry for in vitro systems. The agglomeration state of the particles was assessed by determining if the measured density and size values are consistent with the formula used in the ISD3 model for the agglomeration density,
where, ρp is the agglomerate density, ε is the agglomerate porosity, ρp1 is the density of the primary particle, and ρf is the density of the liquid media (1g/mL). The porosity is calculated using the equation,
where, DF is the fractal dimension of the agglomerate (1 < DF ≤ 3), Dp is the agglomerate diameter, and Dp1 is the primary particle diameter. The DF value for each nanoparticle is estimated using the measured values (listed in Table 1) for Dp, Dp1, ρp1, and ρp, and by setting ρf to 1 g/mL. If the DF value fell outside its valid range (between 1 and 3), then the nanoparticle was not considered an agglomerate, but a protein-coated primary particle, because lower density values and larger sizes of the non-agglomerates compared to that of the primary particles can be attributed to the presence of protein corona on the surface of the primary particles (Thomas et al. 2018). Based on our analysis, the nanoparticles that behave as protein-coated primary particles were MgO, WO3, ZnO, ZnS, and Ag_Cit. The calculated thickness of the protein layers ranged from 6.05 for the ZnO nanoparticles to 47 nm for the citrate-stabilized silver (Ag_Cit) nanoparticles.
The cell-deposited dose fraction (proportion of administered particles that reach the cell monolayer) was also calculated using the ISD3 model for ENMs suspended in RPMI media over a 24-hour time period, based on a media height, volume and temperature equal to 6.3 mm, 0.2 ml, and 310 K, respectively (Table 1).
2.3. Cell culture and cell viability
THP-1 human monocytes were obtained from the American Type Culture Collection (ATCC TIB-202). Cells were maintained in RPMI-1640 culture media supplemented with 10% fetal bovine serum (Gibco, Gaithersburg, MD), 1% antibiotic/antimycotic mixture (Gibco, Gaithersburg, MD), and 2 mM of L-glutamine (Gibco, Gaithersburg, MD) at 37°C with 5% CO2. For all experiments, cells were differentiated into macrophages with 80 nM of phorbol 12-myristate 13-acetate (PMA) (Sigma) for 48 h followed by another 24 h incubation without PMA (Gatto et al. 2017; Zhao et al. 2015b). Cell viability was defined using the MTT assays as described previously (Lanone et al. 2009; Waters et al. 2009). Briefly, THP-1 cells were seeded in 96-well plates at 8.0 × 104 cells per well in 200 μl culture media and differentiated as described above. Cells were treated with ENMs at concentrations of 6.25, 12.5, 25, and 50 μg/mL in 200 μl total volume for 24 hr. After incubation with ENMs culture medium was removed and each well was rinsed with 200 μl of Hank’s Balanced Salt Solution (HBSS). Cells were then incubated with 200 μl of MTT solution (0.5 mg/ml in HBSS) for 1.5 h at 37 °C. MTT solution was then removed and 100 μl of dimethyl sulfoxide was added to each well. Plates were shaken prior to reading absorbance spectrophotometrically with a SpectraMax Plus 384 microplate reader (Molecular Devices) at a wavelength of 570 nm. Four biological replicates were performed for the MTT assay and the following gene expression and proteomics experiments.
2.4. Gene expression analysis
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) assays were designed to measure mRNA expression of reporter gene products for oxidative stress response (HMOX1), ER stress (CHOP), inflammasome activation (IL1B) based on results from our previous studies (Duan et al. 2016; Kodali et al. 2013). For gene expression analysis THP-1 cells are seeded in 6-well plates at 1.0 × 106 cells per well in 3 mL culture media and differentiated as described above. Cells were treated with ENMs at concentrations of 12.5 or 25 μg/mL in 3 mL total volume for 6 or 24 hr. After incubation with ENMs the culture medium was removed and each well was washed with 3 mL HBSS, and RNA extraction was performed using the Qiagen RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol for adherent mammalian cells. For reverse transcription, 1 μg total RNA was used as template with the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions. qRT-PCR was performed using SYBR Green PCR Master Mix per the manufacturer’s instructions (Applied Biosystems). Relative mRNA levels were calculated using the (2−ΔΔCt) method using CYPA as the internal reference control using the following forward and reverse primers respectively: CYPA 5’-atgctggacccaacacaaat-3’; CYPA 5’-tctttcactttgccaaacacc-3’; CHOP 5’-gacctgcaagaggtcctgtc-3’; CHOP 5’-gcagggtcaagagtggtgaa-3’; IL1B 5’-tacctgtcctgcgtgttgaa-3’; IL1B 5’-tctttgggtaatttttgggatct-3’; HMOX1 5’-ggcagagggtgatagaagagg-3’; HMOX1 5’-agctcctgcaactcctcaaa-3’.
2.5. Proteomics sample processing
For mass spectrometry analysis THP-1 cells were seeded in 60 mm plates at 1.5 × 106 cells per plate in 5 mL culture media and differentiated as described above. Cells were treated with ENMs at concentrations of 12.5 and 25 μg/mL in 5 mL total volume for 12 hr. After incubation with ENMs the culture medium was removed, and each well was washed with 3 mL HBSS. Cell lysis was performed in 100 μl of lysis buffer (8 M urea in 100 mM NH4CO3, pH 7.8) by sonication and vortexing. The lysate was centrifuged at 14,000 rpm for 10 min at 4°C, and the supernatant was collected. The bicinchoninic acid assay (BCA) (Thermo Fisher Scientific) was used to determine the protein concentration, and an aliquot of 75 μg protein was transferred to a 96-well plate. Reduction of protein thiols was performed with 10 mM dithiothreitol (DTT) for 30 min at 37°C, followed by alkylation with 40 mM iodoacetamide (IAA) for 1 h in the dark. The samples were diluted to a urea concentration of 1 M using 50 mM NH4CO3, pH 7.8, and subjected to trypsin digestion (1:50 mass ratio of enzyme to proteins) at 37°C for overnight. Desalting of the peptide samples was performed with 96-Well SPE Plates (Phenomenex).
2.6. LC-MS/MS analysis
Peptide samples were re-dissolved in 0.1% formic acid and adjusted to a concentration of 0.1 μg/ μl, and 7 μl was analyzed by a nanoAcquity ultra performance liquid chromatography (UPLC) system (Waters) coupled to a Q Exactive Plus (Thermo Scientific). The peptides were trapped on an in-house prepared C18 trapping column (4 cm x 150 μm i.d., 5 um particle size of Jupiter C18, Phenomenex), and LC separations were performed with a custom packed analytical C18 column (70 cm x 75 μm i.d., 3 um particle size of Jupiter C18, Phenomenex). Binary mobile phases comprising of buffer A (0.1% formic acid in water) and buffer B (0.1% formic acid in acetonitrile) were used at a flow rate of 300 nl/min. For peptide elution, the percentage of buffer B was increased linearly as follows: from 0.1–8% over 0–4 min; from 8–12% over 4–36 min; from 12–30% over 36–135 min; from 30–45% over 135–175min; from 45–95% over 175–180 min. A 10 min wash with 95% buffer B and a final 1 min wash with 100% buffer B was also included.
Full MS scans were conducted at 400–2000 m/z range with a resolution of 35, 000 in the Orbitrap followed by top 12 data-dependent MS/MS acquisitions. The automatic gain control (AGC) was set to 3e6, and the maximum injection time (IT) was 20 ms. The MS/MS isolation window was set as 2 m/z, and higher-energy collisional dissociation (HCD) of NCE (normalized collision energy) was 35. MS/MS settings were as follows: resolution of 17, 500; AGC of 1e5; and maximum IT of 100 ms, and a 30 s dynamic exclusion window.
2.7. Proteomics data processing and statistical analysis
The MS data were processed by MaxQuant (version 1.6.2.0). Raw data files were searched against the non-redundant human Uniprot database containing 20,198 entries. Carbamidomethylation on cysteine was set as fixed modification, while oxidation on methionine and acetylation on protein N-terminus were set as variable modifications. A tryptic rule with a maximum of two missed cleavages was applied. Mass tolerance of peptide for the first and second search was set as 20 and 4.5 ppm, respectively. The MS/MS search tolerance was 0.5 Da. The minimum peptide length was 7 amino acid residues, and the minimum score for a modified peptide was 40. The search also set the false discovery rate (FDR) at 0.01 for both peptide and protein identifications. Label-free quantification (LFQ) was used for all experiments with the “match between run” option enabled. A minimum LFQ ratio count was set to 2 with default normalization method enabled.
ANOVA (analysis of variance) tests with permutation-based FDR calculation were performed on all experimental groups using Perseus. Student t test for pair-wised comparison was performed to determine the statistical significance between each experimental group and controls. Statistically significant proteins were defined using strict criteria of FDR < 0.01 and p-value < 0.01. Gene ontology analysis was performed with the Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/).
2.9. Other methods
The role of ENM dissolution potential on oxidative stress was investigated by plotting HMOX1 expression levels as a function of , a quantum-mechanical description defined as the enthalpy of formation of a gaseous cation having the same oxidation state as that of the metal in the metal oxide structure. The values of , obtained by quantum-mechanical calculations, were described previously (Puzyn et al. 2011). For ENMs where the values of are not available, a regression model was used to derive a value for from the electronegativity scale (χi) and oxidation number (χox) of the metal cation as described previously (Li and Xue 2006; Thrall et al. 2019).
3. Results
3.1. Characterization of ENM properties and cell dosimetry
The physiochemical properties for all ENMs used in this study are summarized in Table 1. Particle size distributions as measured by transmission electron microscopy (TEM) showed that most ENMs were in the range of 7 to 100 nm. Effective particle diameters in the culture media, as determined by DLS, displayed much larger values compared to TEM. In most cases, the larger effective size values are indicative of particle agglomeration in medium. However, comparative analysis using the ISD3 model suggested that the increased effective diameter of a subset of ENMs (Ag_Cit, MgO, WO3, ZnO, ZnS) measured by DLS is likely due to decreased effective density resulting from formation of a protein corona rather than particle agglomeration. In addition, all ENMs in this study exhibited a net negative zeta potential in culture media, in agreement with metal oxide chemistry and the fact that ENMs adsorb serum proteins, which on average bear net negative charge on average.
Due to the number of diverse metal/metal oxide ENMs used in this study, it was not practical to quantify cell dose and uptake through direct experimental measures. Therefore, we applied the ISD3 model, which was previously experimentally validated using metal oxides, to estimate the relative cell-deposited dose fractions for each ENM (Thomas et al. 2018). As shown in Table 1, for most ENMs, less than 20% of the applied concentration of ENMs deposit on the cell monolayer over 24 hrs (based on culture conditions used in cytotoxicity assays, described below). Furthermore, the deposited dose fraction varied by over 13-fold across the library of ENMs. In particular, Fe2O3 and V2O5 ENMs displayed very high dose deposition rates, resulting in 100% deposition at 24 hrs. In fact, analysis of the fraction of deposited ENM as a function of time revealed that, all of the applied V2O5 particles are predicted to settle on the cell layer within only 2 h incubation time (not shown), in large contrast to the dosimetry behavior of the majority of the other ENMs.
3.2. Impact on cell viability and oxidative stress
The impact of each ENM on cell viability was assessed by the MTT assay. PMA-differentiated THP-1 macrophages were exposed with a range of ENM concentrations (6.25–50 μg/mL) for 24 h. Initial studies showed that 12 hr exposure was insufficient to reveal ENM-mediated cytotoxicity, thus subsequent experiments focused on the 24 hr time point. Significant effects on the percentage of metabolically viable cells was found for only a subset of ENMs, including Ag_Cit, ZnO, CuO, and V2O5 (Fig 1A). In contrast, many of the ENMs tested including ZnS, CdS, Au, SiO2, Fe2O3 (10 nm), Fe2O3 (100 nm), CeO2 (10 nm), CeO2 (30 nm), MgO, TiO2_P25, and 1% Ag_SiO2, did not significantly affect cell viability even at the highest concentration administered (50 μg/mL, p > 0.01). Other ENMs such as 10% Ag_SiO2, Al2O3, Ag, and WO3 showed a minor decrease in cell viability (around 20%) with high dosage exposure.
Figure 1.
Cell viability and HMOX1 expression of THP-1 cells exposed to ENMs. (A) MTT assay for cell viability. The cytotoxicity of 20 ENMs was evaluated in THP-1 cells under different dosages for 24 h. ENMs were ranked in the figure legend based on the percentage of cell viability at the highest dosage tested (50 μg/mL). The median value of coefficient of variations for MTT assays was 5.1%. Data points with a black circle denote p < 0.01. (B) Relative mRNA expression of HMOX1. The mRNA level in control samples was normalized to 1 (red dotted line). ENMs were ranked based on the mRNA level at the high dose (25 μg/ml). Data was represented as mean ± standard deviation. In both experiments, four biological replicates were conducted. *: p < 0.01.
One proposed mechanism of ENM-induced cytotoxicity is through reactive oxygen species (ROS)-dependent cellular stress. For instance, we and others have previously found that mRNA induction of heme-oxygenase 1 (HMOX1), a Nrf2-regulated gene product, is a good surrogate for assessing redox stress induced by ENMs in cells (Meng et al. 2009; Thrall et al. 2019). While the impact of ENMs on cellular redox state can also be assessed by GSH and GSSG/GSH ratio, our previous studies demonstrated mRNA induction of HMOX1 occurs at much lower levels of ENM-induced redox stress than is necessary for altering cellular GSH level (Duan et al. 2016). Thus, we used HMOX1 mRNA induction as a sensitive indicator to quantify cellular oxidative stress levels following ENM exposure. As expected, some of the ENMs which displayed the greatest cytotoxic potential, such as CuO, ZnO and Ag_Cit, induced marked increases in HMOX1 gene expression (Fig. 1B, Table S1). Significant increases in HMOX1 were also observed by several non-cytotoxic ENMs such as Fe2O3, AgSiO2 and Ag, albeit at lower levels. Interestingly, a decrease in HMOX1 was observed following V2O5 exposure, possibly due to a high degree of cytotoxicity observed with this ENM. As discussed in more detail, the high level of cytotoxicity and decrease in HMOX1 mRNA associated with V2O5 treatment must be interpreted with caution, given the rapid settling of these ENMs in culture systems. Moreover, statistical association between cell viability and HMOX1 change was also evaluated, which showed a moderate negative correlation (r = −0.565 at the high dose, Fig. S1).
The potential role of dissolution (metal cation release) in mediating the cytotoxicity of metal oxide ENMS has also been reported for several of the ENM types studied here, including Ag, ZnO and CuO ENMs (Mihai et al. 2015; Smith et al. 2018; Zhang et al. 2012). Since it was not practical to conduct ICP-MS analyses across the diverse types of ENMs within this library, we also investigated the potential role of ion dissolution as a factor for inducing oxidative stress using first principle quantum descriptors of physicochemical properties previously used in a quantitative structure-activity relationship model of metal oxide toxicity in bacteria (Puzyn et al. 2011). Specifically, we compared the level of HMOX1 induction as a measure of cellular oxidative stress with the physical property , which is a quantum mechanical estimate of the enthalpy of formation of a gaseous cation from a metal oxide (Puzyn et al. 2011). A general decrease in HMOX1 induction as increases was found for most ENMs except for MgO (Fig. S2). In other words, the lower the energy barrier necessary for the metal oxide nanoparticle to release its metal cation, the higher the observed HMOX1 response. For strongly cytotoxic ENMs in particular, this result is consistent with a role for cation release accompanied by release of electrons as a mechanism for the induction of oxidative stress underlying their cytotoxic effects and is consistent with previous experimental results (Mihai et al. 2015; Zhang et al. 2012).
3.3. Cellular proteome responses following ENM exposure
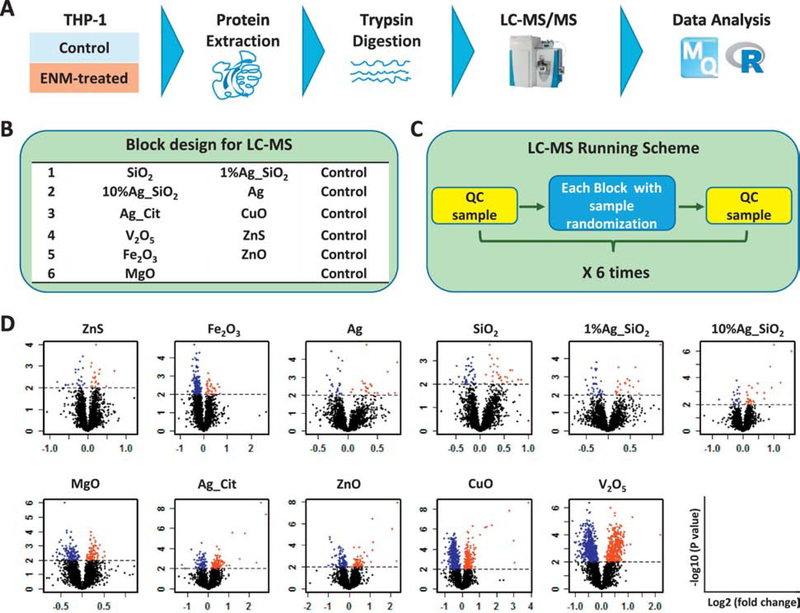
To systematically evaluate cellular responses to ENMs, we selected a subset of ENMs based on the targeted screening results and performed quantitative proteomics profiling analyses (Fig. 2A). Eleven ENMs were selected, including Ag, Ag_Cit, AgSiO2 (comprised of 1% and 10% Ag, respectively), CuO, Fe2O3, MgO, SiO2, V2O5, ZnO and ZnS, providing a representative subset of ENMs that display a broad range of physicochemical properties (Table 1), cytotoxic potential, and ability to induce oxidative stress (Fig. 1). For each ENM, both a low (12.5 μg/ml) and a moderate (25 μg/ml) concentration were used to investigate dose-dependent cellular responses. Cell exposures were conducted for 12 hr, based on pilot time course analyses (data not shown) which found this time period was sufficient to allow for gene and protein expression changes that precede any significant cytotoxicity (measured at 24 hr). Four biological replicates were included for each ENM exposure. The panel of 11 ENMs were assigned into 6 analytical blocks, with each block containing a nested set of untreated samples as the experimental control to both enable large-scale quantification and ensure quality control between blocks (Fig. 2B). The analysis yielded the identification and quantification of an average of 4800 proteins in each analytical block (Supplemental Data File 1) with quantitative data for 1505 proteins obtained in all samples (Supplemental Data File 2).
Figure 2.
ENMs exposures cause different levels of proteomic changes. (A) Schematic overview of the proteomics workflow. A) PMA-differentiated THP1 macrophages were treated with 11 ENMs at two different doses: 12.5 (low) or 25 (high) μg/mL for 12 h. Samples were subjected to protein extraction, digestion and LC-MS analysis. (B) Two ENMs, along with a set of controls, were assigned to each block for LC-MS analysis. (C) QC samples were run before and after each block, samples in which were completely randomized. (D) Volcano plots displaying the overall change of the proteome induced by ENMs. The extent of protein abundance change, expressed as log2 fold change (ENM treatment over the control), is shown in the x axis. Significance of the pair-wised comparisons, expressed as negative logarithm of the p values, is shown on the y axis. Blue and red dots represent proteins that are down- and up-regulated, respectively, with p value of 0.01 as a cutoff (horizontal black dashed lines). Note that data with the high dose (25 μg/ml) treatment were shown. For volcano plots with low dose (12.5 μg/ml) treatment, see Fig. S2.
To visualize the overall levels of proteome changes induced by ENMs, volcano plots were created that display the statistical significance (y axis) and the extent of change in protein abundances (x axis) under each treatment condition (Fig. 2C, Fig. S3). As anticipated, treatment with non-cytotoxic ENMs induced relatively small perturbations in the proteome with a narrower distribution of the log2 fold change (typically −0.5 to 0.5). In contrast, a larger portion of the overall proteome was found to be significantly altered following exposure to ENMs which had greater cytotoxic potential.
To investigate whether concentration-dependence can be observed from the overall proteome changes, we plotted the extent of protein abundance changes between high concentration and low concentration for each ENM (Fig. S4). The directionality of changes in the cellular proteome between the two exposure concentrations were consistent for all ENMs. While the cytotoxic ENMs displayed a general concentration-dependent alteration of the cellular proteome, there was no obvious trend observed for the non-cytotoxic ENMs, possibly due to an overall small change in the proteome under these treatments. In summary, the overall levels of proteome abundance changes correlate with both ENM cytotoxic potential and the administered ENM concentrations, which supports the robustness of the label-free global quantitative proteomics approach.
3.4. Unsupervised clustering of proteomics data differentiates cytotoxic potential of ENMs
To assess global cellular proteome responses to various ENMs, we first attempted a data-driven approach to group and visualize the complex dataset collected (Fig. 3). Unsupervised hierarchical clustering analysis using 1505 proteins that were quantified in all samples revealed six distinct sample (or dataset) clusters (A-F) corresponding to different ENM types and treatment concentrations. Of note, the high treatment concentration of V2O5 and ZnO formed two separate clusters (A and B, respectively), while the low concentration of these two ENMs grouped into one cluster (cluster D). Samples treated with CuO and Ag_cit at both the high and the low concentrations clustered as a single group (cluster C). Collectively, these four ENM types were grouped together and comprised the ENMs that display the greatest cytotoxic potential. On the other hand, the remaining seven ENMs, which were predominantly weakly or noncytotoxic, clustered separately within two related groups (cluster E and F, respectively). While all four biological replicates for each treatment condition (ENM by concentration) were within the same cluster in the cytotoxic group, biological replicates were not always within the same cluster E or F for the non-cytotoxic group, presumably due to the more subtle differences in the proteome changes observed between these treatment conditions. Thus, at the level of global response profiles, the data readily distinguished ENM classes based on bioactivity and support the hypothesis that some common mechanisms (protein pathways) are likely involved in the response across ENM types.
Figure 3.
Hierarchical clustering of THP-1 proteomes following exposure to ENMs. The relative protein abundance, expressed as log2 fold change between each experiment group and control, was used for clustering. Rows represent proteins quantified in all samples (1505 in total), which are hierarchically clustered in into five groups (Cluster I-V). Columns represent THP-1 samples treated with 11 ENMs at two dosages (12.5 and 25 μg/ml as the low and the high concentration, respectively), which are segregated to two major groups based on the cytotoxicity of ENMs: cytotoxic (Cluster A-D) and non-cytotoxic groups (Cluster E and F).Labeling of columns (experiment groups): name of ENM, followed by dose (L: low; H: high), followed by replicates number (1–4).
3.5. Biological processes impacted by ENM exposure
Unsupervised clustering of the protein abundance patterns also revealed five protein clusters that displayed distinct patterns of abundance change across the library of ENM treatment groups (Fig. 3, see y-axis ). We hypothesize that this pattern may indicate distinct functional processes that are differentially affected by ENM physicochemical properties. To further evaluate this concept, proteins from each cluster, except cluster Ⅱ, were subjected to gene ontology (GO) based enrichment analysis in order to identify biological processes represented in these clusters. The top 10 biological processes represented by each group were shown in Fig. 4. Proteins in cluster Ⅱ were analyzed separately since this cluster did not contain a sufficient number of proteins for a statistical-based enrichment analysis. For cluster I, the protein abundances were impacted across a broad set of ENMs and the top two enriched biological processes were translational initiation and cell-cell adhesion, both of which showed down-regulation following ENM exposure (Fig. 4A). For cluster III, proteins were statistically enriched in metabolic processes such as tricarboxylic acid (TCA) cycle and fatty acid beta-oxidation and were generally up-regulated following exposure to ENMs (Fig. 4B). For cluster IV, the top enriched biological processes were mRNA splicing and tRNA export from the nucleus, where proteins were both up- or down-regulated depending on treatment conditions (Fig. 4C). Cluster V featured mRNA processing and protein folding processes, which were predominantly up-regulated (Fig. 4D). It is noteworthy that the protein abundances changes for biological processes represented in clusters III-V were most strongly impacted by cytotoxic classes of ENMs. Nonetheless, significant downregulation of proteins involved in translational initiation and cell adhesion (Cluster I) was observed broadly across ENM types.
Figure 4.
Distinctive biological processes in ENM-perturbed THP-1 proteomes from four protein clusters. (A-D) The enriched biological processes of protein cluster I, III, IV and V, respectively. For the top two enriched biological process in each protein cluster, a heat map was created using the top five proteins ranked by average protein fold change.
Interestingly, cluster II was driven by robust changes in four proteins (HMOX1, HS71B, DNJB1, and SQSTM1). These proteins displayed an overall significant increase in abundance mainly in response to the cytotoxic ENMs, except V2O5. Fig. 5A shows the fold change values of these proteins as a function of ENM type. Similar to the mRNA expression screening data, we observed a 5–16 fold increase of HMOX1 protein abundance in THP-1 cells following exposure to ENMs which display stronger cytotoxic potential, with the exception of V2O5, but much smaller, yet significant changes with some of the non-cytotoxic ENMs. Similar trends were observed for the other three proteins (Fig. 5A), all of which showed strong positive correlations with HMOX abundance in terms of protein fold change in response to ENM treatments (Fig. S5), suggesting that all four of these proteins are responsive to oxidative stress. Both HS71B and DNJB1 are heat shock-related molecular chaperons that function in a wide range of processes in response to stress (37, 38). DNJB1 interact with HSP70, and the up-regulation of DNAJB1 mRNA expression in response to ENMs has been reported (Moos et al. 2011). Heat shock proteins have long been recognized as cytoprotective molecules involved in the facilitation of protein folding, assembly of transportation of protein complex, and proteolysis of misfolded proteins under oxidative stress (Ikwegbue et al. 2017; Tang et al. 2007). SQSTM1, also known as p62 or A170, is a multifunctional adaptor protein that plays an essential role in the delivery of the polyubiquitinated protein cargo to the autophagosome in the process of selective macroautophagy (Jeong et al. 2019). While general roles of these proteins in stress response pathways have been described, our discovery that they are highly responsive to ENM exposure is novel.
Figure 5.
Cluster II proteins and their associations with the overall proteome change and cell viability. (A) Fold change of cluster II proteins following exposure to ENMs. (B) The degree of overall proteome perturbation indicated by the number of significantly changed proteins. (C) Correlation between the protein fold change of HMOX1 and the number of significantly changed proteins. (D) Correlation between the protein fold change of HMOX1 and cell viability. In C and D, red lines indicate linear model fit, and r denote the Pearson’s correlation coefficient. Blue lines denote the normalized HMOX1 expression levels of control samples as 1. V2O5 are represented as triangles since it a cytotoxic particle that causes significant proteome changes and cell death, but a decrease in HMOX1 expression. All the other ENMs were represented as circles and used for the Pearson’s correlation analysis.
We also compared the number of statistically significant proteins that show altered abundance levels as a function of the ENM type. In line with the clustering analysis, more significantly altered proteins were identified in samples treated with cytotoxic ENMs (Fig. 5B, Supplemental Data File 3). Given that cellular oxidative stress represents a major initiator of the adverse biological outcomes of ENMs (Kodali and Thrall 2015; Meng et al. 2009; Zhang et al. 2012) and that HMOX1 expression has been commonly used as a reporter of cellular oxidative stress levels (Di Cristo et al. 2016; Lenz et al. 2013; Tabei et al. 2016; Thrall et al. 2019), we aimed to correlate HMOX1 protein abundance with proteome changes. As expected, a strong positive correlation (r = 0.90) was observed between the fold change of HMOX1 and the number of significant proteins observed across different ENM treatment conditions (Fig. 5C). Moreover, a good correlation between the mRNA expression (by qRT-PCR) and protein abundance (by LC-MS) of HMOX1 was observed for all ENM treatments (Fig. S6), which provides orthogonal validation of our proteomics analyses. We also observed a negative correlation (r = − 0.51) between the fold change of HMOX1 protein abundance (at 12 h) and cell viability following exposure to ENMs measured later at 24 h (Fig. 5D), which supports the role of oxidative stress in initiating cellular responses to ENMs. V2O5 appears to be an exception to these observed correlations, as discussed later. However, our overall data support the conclusion that the observed cellular proteome responses are ROS-dependent or ROS-driven, which in turn leads to different levels of cytotoxicity.
3.6. Common and specific cellular pathways impacted by ENM exposure
To further assess whether there are unique pathways initiated by cytotoxic ENMs versus non-cytotoxic ENMs, we performed pathway enrichment analysis for the significant proteins identified from each ENM condition. The top significantly enriched biological processes from each ENM treatment were identified, and their significance (p-value) across all ENM groups were plotted in Fig. 6. This analysis revealed two general categories of biological processes, one broadly altered by most ENMs, and the other more uniquely impacted by ENMs which display cytotoxicity after 24 hr exposure. The commonly altered biological processes include cell-cell adhesion, translation, and mRNA catabolic processes, which involved proteins that were generally downregulated in abundance (Fig. S7). The impact on these biological processes is further amplified in the case of cytotoxic ENMs, suggesting that these processes may reflect a general adaptive cellular response to oxidative stress induced by ENM exposure, even at low levels of ENM-induced stress. The biological processes that were uniquely altered in response to cytotoxic ENMs included the TCA cycle, fatty acid beta-oxidation, response to unfolded protein, and NIK/NF-κB signaling (Fig. 6 and Fig. S8). These uniquely induced biological processes likely reflect pathological responses triggered by the higher level of oxidative stress induced by this subset of ENMs that ultimately lead to the loss of cell regulation and the observed cytotoxicity. The biological processes identified here are also consistent with our previous study (Duan et al. 2016), in which we found that mitochondrial proteins involved in key cellular metabolism and energetics pathways (including TCA cycle and beta-oxidation), are substrates for oxidative modification at high levels of ENM-induced oxidative stress.
Figure 6.
Biological processes responsive to ENMs exposure. The significance of the gene ontology enrichment is represented by the negative logarithm of the p value (0.01 as the significance cutoff). White blocks denote the absent of significant biological processes in enrichment.
Some of the specific protein signatures that distinguish the cytotoxic class of ENMs discovered in this study are shown in Fig. 7. Proteins showing induced expression by the cytotoxic ENMs include enzymes involved in cellular redox processes of ROS such as SODM, TRXR2, PRDX3, and PRDX5 (Fig. 7A), as well as proteins involved in the unfolded protein response such as ERO1A, CH60, HS106, and SERPH, (Fig. 7B). Interestingly, a down regulation of protein expression was found for proteins involved in NIK/NF-κB signaling (HMGB1, PSB6, PSME1, and ACTN4, Fig. 7C).
Figure 7.
Protein signatures in different cellular responses to ENMs. The relative abundance of proteins involved in responses to ROS (A), unfolded protein responses in the ER (B), and NF-kB signaling (C), were plotted. The boxplots show the range of protein fold change under the treatment of non-cytotoxic and cytotoxic ENMs, respectively. Each dot represents the average from four biological replicates. Signature proteins were selected based on the statistical significance between the two treatment groups (Student t test, *: p < 0.1; **: p < 0.05; ***: p < 0.01). For each boxplot, Q1 and Q3 define the boundaries of the box, while the minimum and maximum are the whiskers. The median is shown as the central black line. All data points including outliers are plotted.
To further confirm the observed ER stress response and inflammatory response, we used qRT-PCR to measure induction of CHOP and interleukin-1beta (IL1B) mRNA, as markers for ER stress and inflammation, respectively (Duan et al. 2016; Khan et al. 2013; Kodali et al. 2013; Li et al. 2014). Increased cellular ROS has been shown to induce inflammasome activation and IL-1β secretion (Duan et al. 2016; Yazdi et al. 2010). Indeed, CHOP and IL1B mRNA expression were induced following exposure to cytotoxic ENMs as compared to non-cytotoxic ENMs (Fig. 8, Table S1). The mRNA levels for these reporter genes were tightly distributed following exposure to non-cytotoxic ENMs, but showed a much wider distribution of expression following exposure to cytotoxic ENMs. It should be noted that the THP-1 cells used here were differentiated using PMA treatment, but were not further ‘primed’ with LPS prior to ENM exposure, and hence are referred to as a “M0” phenotype. The induction of IL-1β mRNA expression following ENM exposure is thereby consistent with a shift toward a proinflammatory M1-like polarization state. However, in pilot proteomic studies (data not shown) comparing the profile of THP-1 cells that were polarized with either classical M1 stimuli (LPS/IFN-γ) or M2 stimuli (IL-4/IL-13), no clear pattern of polarization was observed following ENM exposure, suggesting ENM-mediated effects on the proteome are more complex than a simple shift in classical polarization states. Further studies are warranted to investigate the potential role that macrophage polarization has in modulating the response to ENM exposure.
Figure 8.
Gene expression of CHOP (A) and IL1B (B) in response to non-cytotoxic and cytotoxic ENMs. The relative mRNA expression was shown fold change normalized to the untreated control, and the boxplots show the range of mRNA fold change under the treatment of non-cytotoxic and cytotoxic ENMs, respectively. Each dot represents the average from four biological replicates. Statistical signature was found between the two treatment groups for IL1B (Student t test, **: p < 0.05).
4. Discussion
While transcriptomics and proteomics have been previously applied to analyze the cellular responses to ENMs (Kodali et al. 2013; Tarasova et al. 2017; Zhang et al. 2018), the current work, to our best knowledge, represents the most comprehensive profiling of the broad biological responses to a library of ENMs using global quantitative proteomics. The comprehensive data (Fig. 3) provide a basis to identify the patterns of a diverse set of cellular pathways and biological processes impacted by ENM exposure in an important immune cell type, laying the foundation for more comprehensive pathway-level structure activity-based assessments of ENMs in the future.
Interestingly, a clear correlation was observed between the extent of proteome changes, the levels of induction of ROS-responsive proteins (e.g., HMOX1, HS71B, DNJB1, SQSTM), and the eventual cytotoxic outcome from ENM exposure (Fig. 5). These data collectively suggest that the cellular responses of THP-1 macrophages to the metal and metal-oxide ENMs are likely modulated through an ROS-dependent mechanism where common adaptive pathways are initially activated by particle interactions, and may progress to pathological responses such as cell death depending on the levels of ROS or oxidative stress induced by the ENMs. This observation is consistent with the notion that oxidative stress represents an initiating mechanism that is predictive of ENM cytotoxicity, as we and others have proposed (Duan et al. 2016; Meng et al. 2009; Nel et al. 2006; Thrall et al. 2019). Indeed, pathway analyses revealed clearly two distinctive categories of biological processes (Fig. 6) in response to the gradient of cellular oxidative stress elicited by various ENMs, as estimated by HMOX1 expression levels: 1) biological processes commonly impacted by many ENMs independent of cytotoxic potential, and 2) biological processes that ultimately invoke cytotoxicity, which are uniquely observed in response to cytotoxic ENMs.
The set of biological processes (e.g., translation, cell adhesion) commonly impacted by the majority of ENMs most likely represent cellular processes that facilitate adaption to low levels of oxidative stress triggered by particle interactions. These adaptive stress responses are critical for cellular homeostasis regulation and cell survival. The observation of adaptive stress responses with low levels of oxidative stress from non-cytotoxic ENMs is also consistent with our previous findings that ENMs that lack cytotoxic activity caused transcriptional reprogramming and dysregulation of various biological pathways, such as those involved in the oxidative stress response (Kodali et al. 2013). Significantly, alterations in these biological processes could resulted in an impaired innate immune response such as reduced phagocytic function. This has already been demonstrated in our previous work, in which bone marrow-derived macrophages pretreated with non-cytotoxic amorphous silica or superparamagnetic iron oxide showed a diminished phagocytic activity toward the lung pathogen Streptococcus pneumoniae (Kodali et al. 2013). One of the most commonly reported adaptive responses to cellular stress is translational inhibition. The inhibition of global protein synthesis was previously observed as a common adaptive response under stress conditions, including exposure to particles (Lin et al. 2019; Shenton et al. 2006; Simpson and Ashe 2012; Topf et al. 2018). The observation that translation-related proteins are primarily downregulated suggests that ENM-induced stress similarly contributes to the attenuation of translation, possibly to prevent accumulation of misfolded proteins. Indeed, oxidative stress has been shown to elicit multiple levels of translational regulation including inhibition of both translational initiation and elongation, as well as selective increase of translation of stress protective mRNAs (Grant 2011; Shenton et al. 2006). For example, our data revealed that ENM exposure caused downregulation of GCN1, which has been previously shown to regulate protein translation in response to H2O2 by acting as a positive activator of the eukaryotic initiation factor-2a protein kinase (Shenton et al. 2006). In addition, many ribosomal proteins are down-regulated after ENM exposure, but to a greater extent following exposure to cytotoxic ENMs, suggesting that the inhibition of translation is dependent on the level of oxidative stress. Related to translation, our analyses also identify SRP-dependent co-translational protein targeting to membrane as a commonly regulated process. In this process, nascent proteins with membrane-targeting sequences are recognized by SRP on the ribosome during translation and are then translocated into the ER (Nyathi et al. 2013). The down-regulation of key regulators of protein synthesis and protein loading into the ER under ENM-induced stress is consistent with our previous findings that showed proteins within the ER are highly sensitive to oxidative modification following exposure to metal oxide ENMs (Duan et al. 2016). Whether redox modification of these proteins is directly linked to a change in their abundance, and the underlying mechanisms involved, warrants additional investigation.
In addition to protein translation, cell-cell adhesion was identified as another biological process commonly impacted by ENMs, where most proteins in this category were also down-regulated following exposure to ENMs (Fig. S7). Proteins involved in cell adhesion complexes are known to be required for many additional cellular functions such as phagocytosis, production of cytokines, and antigen presentation in macrophages (Prieto et al. 1994). The degree of down-regulation on these proteins also correlates with the level of alteration of HMOX1, again suggesting ROS plays a regulatory role in this process. ROS have been reported as essential mediators of cell adhesion through integrin signaling primarily via oxidative inhibition of protein tyrosine phosphatase (Chiarugi et al. 2003). We identified several significantly changed proteins including KTN1, RACK1, and SWP70 that interact or regulate integrins (Liliental and Chang 1998; Sivalenka and Jessberger 2004; Tran et al. 2002), suggesting that ENMs exposure could disrupt cell-cell adhesion via ROS-mediated down-regulation of integrin-associated processes. Additionally, a ROS-dependent loss of cell-cell adhesin was also found in endothelial cells from primary human umbilical veins, where phosphorylation on alpha cadherin was increased (van Wetering et al. 2002). Our data showed that IQGAP1, a Ras GTPase-activating-like protein and a key regulator of cadherin, was generally down-regulated (Fig. S7). The concept that ENMs exposure may induce an ROS-dependent disruption of cell adhesion is also consistent with our prior observation of reduced phagocytic activity of macrophages following exposure to a variety of metal oxide ENMs (Kodali et al. 2013; Duan et al. 2016; Thrall et al. 2019).
The second broad category of biological processes found to be uniquely impacted by exposure to cytotoxic ENMs included responses to ROS, unfolded protein responses, immune-related responses (e.g., antigen processing and NIK/NF-κB signaling), as well as energy metabolism processes such as the TCA cycle and fatty acid beta-oxidation. Collectively these data clearly illustrate activation of the classical antioxidant defense system, but with greater detail from a global proteome perspective. For instance, many antioxidant enzymes such as SODM, PRDX3, and PRDX5 and other redox-regulating proteins such as TRXR2 are up-regulated (Fig. S8). The activation of ROS response following exposure to cytotoxic ENMs is also closely connected to ER stress response or the unfolded protein response (UPR), which has been previously reported for ENM exposure by us and others (Chen et al. 2014; Chen et al. 2015; Duan et al. 2016; Tsai et al. 2011). In line with this, our study provided solid evidence for an up-regulation of UPR, suggesting an overload of the ER system under oxidative stress and an attempt of macrophages to improve or maintain ER homeostasis following exposure to ENMs.
Closely linked with the UPR is the observation that impaired immune-related responses such as NF-κB signaling were specifically impacted by cytotoxic ENMs. For instance, several proteins involved in NF-κB are down-regulated by cytotoxic ENMs. High mobility group box 1 (HMGB1), a DNA-binding protein, has been demonstrated to activate NF-κB signaling in bone marrow macrophages via the induction of gene expression and increase in protein abundance of RelB (Toia et al. 2015). Actin-binding protein alpha-actinin 4 (ACTN4) is an actin binding protein that can be localized to the nuclear to interact with and thus activate RelA/p65 subunit of NF-kB (Aksenova et al. 2013). Proteasome subunit beta type-6 (PSB6) is a component of the 20S proteasome complex which degrades intracellular misfolded or damaged proteins, and is predicted to be involved in the activation of NF-kB (predicted by Reactome, https://reactome.org/). Proteasome activator complex subunit 1 (PSME1) is implicated in the immunoproteasome assembly and its involvement in NF-kB signaling has also been predicted by Reactome. Significant down-regulation of these proteins (Fig. 7C) suggest that cytotoxic ENMs led to an impaired NF-κB signaling. Consistent with this prediction, previous studies had demonstrated that ENMs exposure can block the activation of NF-κB. For example, Ma et al showed that polyethylene glycol coated gold nanoparticles inhibit lipopolysaccharide (LPS)-induced activation of NF-κB in RAW264.7 cells (Ma et al. 2010). Similarly, the blocking effect on LPS-induced activation of NF-κB in RAW 264.7 macrophages following exposure to ZnO2 nanoparticles was also reported (Kim and Jeong 2015). Consistent with our results, the latter study also demonstrated that zinc oxide nanoparticles induce the expression of A20, a negative regulator of NF-κB. Collectively, the data suggest that ENMs could inhibit NF-κB signaling by down-regulating positive regulators in NF-κB signaling, leading to altered immune regulation. Regardless of the specific mechanism, the inhibition of NF-κB may also lead to the observed increase in IL-1β production, as it has been demonstrated that NF-κB is a negative regulator of IL-1β secretion (Greten et al. 2007).
Finally, our data revealed several highly sensitive protein signatures to ENM-induced oxidative stress. In addition to HMOX1 mRNA induction which has been used as a sensitive general readout for the relatively magnitude cellular oxidative stress, we identified molecular chaperons (HS71B and DNJB1) and autophagy receptor (SQSTM1) that were also highly sensitive. The induction of molecular chaperons by exposure to ENMs has also been reported (Safar et al. 2019; Sundarraj et al. 2017a; Sundarraj et al. 2017b). The degree of up-regulation of these proteins is consistent with the degree of cellular oxidative stress as indicated by the strong correlations with HMOX1 expression, suggesting potentially common regulatory mechanisms are involved. In addition to a general cytoprotective role, these proteins have also been shown to regulate the states of macrophage activation (Henderson and Henderson 2009) and phagocytosis (Wang et al. 2006). Under oxidative stress, misfolded proteins can aggregate, forming irreversibly oxidized molecules that can be recycled by autophagy (Filomeni et al. 2015; Vasconcellos et al. 2016). It has been demonstrated that ENMs could induce the formation of autophagosome (Li and Ju 2018; Ma et al. 2011). However, the role of SQSTM1 in the response to ENMs has not been reported previously. Our novel finding on the up-regulation of SQSTM1 following exposure to cytotoxic ENMs may suggest that SQSTM1-mediated autophagy is coordinated with the antioxidant defense system to control oxidative stress-induced cellular damage. Future studies are needed to investigate these potential mechanisms.
Besides the observations of distinctive cellular pathways in response to ENMs, potential limitations of this analysis should be acknowledged. First, we used HMOX1 expression as a reporter of cellular oxidative stress based on previous evidence (Meng et al. 2009; Thrall et al. 2019). This strategy is warranted over alternative measures of redox stress since fluorescent assays (e.g., DCF) have limited dynamic range and sensitivity, and our previous studies have directly demonstrated that protein redox modifications following metal oxide ENM exposure are detectable well below oxidative stress levels needed to alter glutathione status in cells (Duan et al. 2016). Second, although the same concentrations for 11 ENMs were used for cytotoxicity and proteomics profiling, the final dose of ENMs reaching the cells through diffusion and gravitation varied significantly across ENM types. In particular, the high cytotoxic effect of V2O5, which was an outlier for most correlation analysis, was likely due to the fact that it readily precipitates in culture systems and rapidly deposits on the cells (Table 1). Thus, while V2O5 and CuO appear to have similar cytotoxic potency (at higher concentrations), it is important to realize that the cells received up to 13-fold greater delivered dose of V2O5 as compared to CuO. In fact, dosimetry calculations using the experimentally validated ISD3 model show that essentially all of the applied V2O5 concentration is deposited on the cells within 2 hrs. In addition, the general pattern of higher increases of the cluster Ⅱ proteins (including HMOX1) upon exposure to cytotoxic ENMs does not apply to V2O5. This is unlikely due to a lack of cellular ROS production, as a previous study has shown that V2O5 generates long-lived O2•– radicals (Wang et al. 2017). Rather, it is likely that the rapid and high cell dose of V2O5 causes cell death through different mechanisms, which are unlikely to be relevant in vivo. Such observations point toward the critical importance of evaluating delivered cell dose as a part of any hazard analysis or mechanistic study of nanotoxicity.
5. Conclusions
The combined use of targeted endpoint assays and untargeted quantitative proteomics profiling used here reveals a breadth of biological pathways and processes that can be impacted by metal and metal oxide ENMs even when the ENMs have no apparent cytotoxicity. While our results support the oxidative stress paradigm as a predictive strategy, the use of global proteomics also sheds insights into new biological processes that have been previously implicated in adaptive cellular mechanisms at lower levels of redox stress, including translational inhibition and disruption of cell adhesion. To our knowledge this is the first study to apply quantitative LC-MS based proteomics across a library of ENMs this size. The results highlight the potential for extending this strategy to develop multivariate-based structure-function relationships at the cellular process or pathway level, rather than focus on single endpoints. From the perspective of hazard ranking approaches for emerging ENMs, the use of a simple endpoints such as cytotoxicity are clearly useful for rapidly assessing the potential acute potential of ENMs to induce toxicity. However, this approach must be balanced with the reality that typical human exposures to ENMs will be at low doses and occur in the context of mixed exposures to additional environmental challenges, such as bacteria. The important question of whether adverse effects of ENMs may be manifested by altering susceptibility to concurrent environmental exposures is often overlooked, despite mounting evidence that important health impact from environmental and occupational nanoparticle exposures in humans include indirect effects such as suppressed innate immune function (Andujar et al. 2014; Coggon et al. 1994; Neupane et al. 2010; Palmer et al. 2003; Thrall et al. 2019). Identification of the biological pathways and proteins that are broadly affected by ENMs, such as cell adhesion proteins that are also critical for normal macrophage function, can provide insight into the molecular mechanisms by ENM exposures may alter susceptibility to other environmental challenges. Consistent with this idea, our previous work found that oxidative modification of actin-binding proteins necessary for both cell adhesion and macrophage phagosomal function is associated with suppression of normal macrophage phagocytic clearance to bacterial pathogens (Duan et al. 2016). Additional research is needed to develop strategies for the integration of these ‘omics’-level pathway data into quantitative structure-activity relationship modeling frameworks. However, the pathway markers identified in this study lay an important foundation towards this future direction.
Supplementary Material
Highlights.
The biological responses of THP-1 derived macrophage to a library of metal/metal oxide engineered nanomaterials (ENMs) were evaluated by both conventional biological endpoint assays and proteomics.
The overall levels of ENMs-induced proteome changes and responses of specific pathways correlated positively with the level of oxidative stress they caused.
Adaptive responses for biological processes such as translation and cell adhesion were observed across all ENMs even for those ENMs which induced a low level of oxidative stress.
Specific biological processes such as unfolded protein response were only triggered by cytotoxic ENMs which induced a much higher level of oxidative stress.
In addition to HMOX1, several sensitive protein markers to ENM-induced oxidative stress were identified, including HS71B, DNJB1, and SQSTM.
Acknowledgements
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number UO1ES027292 as part of the Nanotechnology Health Implications Research (NHIR) Consortium, and grant P41GM103494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The engineered nanomaterials used in the research presented in this publication have been procured/developed, characterized, and provided by the Engineered Nanomaterials Resource and Coordination Core established at Harvard T. H. Chan School of Public Health (NIH grant # U24ES026946) as part of the Nanotechnology Health Implications Research Consortium.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn S, Ardona HAM, Lind JU, Eweje F, Kim SL, Gonzalez GM, Liu Q, Zimmerman JF, Pyrgiotakis G, Zhang Z, Beltran-Huarac J, Carpinone P, Moudgil BM, Demokritou P, Parker KK, 2018. Mussel-inspired 3D fiber scaffolds for heart-on-a-chip toxicity studies of engineered nanomaterials. Anal Bioanal Chem 410(24), 6141–6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova V, Turoverova L, Khotin M, Magnusson K-E, Tulchinsky E, Melino G, Pinaev GP, Barlev N, Tentler D, 2013. Actin-binding protein alpha-actinin 4 (ACTN4) is a transcriptional co-activator of RelA/p65 sub-unit of NF-kB. Oncotarget 4(2), 362–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andujar P, Simon-Deckers A, Galateau-Salle F, Fayard B, Beaune G, Clin B, Billon-Galland MA, Durupthy O, Pairon JC, Doucet J, Boczkowski J, Lanone S, 2014. Role of metal oxide nanoparticles in histopathological changes observed in the lung of welders. Part Fibre Toxicol 11, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias LS, Pessan JP, Vieira APM, Lima T.M.T.d., Delbem ACB, Monteiro DR, 2018. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics (Basel, Switzerland) 7(2), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltran-Huarac J, Zhang Z, Pyrgiotakis G, DeLoid G, Vaze N, Hussain SM, Demokritou P, 2018. Development of reference metal and metal oxide engineered nanomaterials for nanotoxicology research using high throughput and precision flame spray synthesis approaches. NanoImpact 10, 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Dong J, Liu J, Zheng H, Kaweeteerawat C, Wang F, Ji Z, Li R, 2018. Multi-hierarchical profiling the structure-activity relationships of engineered nanomaterials at nano-bio interfaces. Nat Commun 9(1), 4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Huo L, Shi X, Bai R, Zhang Z, Zhao Y, Chang Y, Chen C, 2014. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano 8(3), 2562–2574. [DOI] [PubMed] [Google Scholar]
- Chen R, Ling D, Zhao L, Wang S, Liu Y, Bai R, Baik S, Zhao Y, Chen C, Hyeon T, 2015. Parallel Comparative Studies on Mouse Toxicity of Oxide Nanoparticle- and Gadolinium-Based T1 MRI Contrast Agents. ACS Nano 9(12), 12425–12435. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Pani G, Giannoni E, Taddei L, Colavitti R, Raugei G, Symons M, Borrello S, Galeotti T, Ramponi G, 2003. Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. The Journal of cell biology 161(5), 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggon D, Inskip H, Winter P, Pannett B, 1994. Lobar pneumonia: an occupational disease in welders. Lancet 344(8914), 41–43. [DOI] [PubMed] [Google Scholar]
- Costa PM, Gosens I, Williams A, Farcal L, Pantano D, Brown DM, Stone V, Cassee FR, Halappanavar S, Fadeel B, 2018. Transcriptional profiling reveals gene expression changes associated with inflammation and cell proliferation following short-term inhalation exposure to copper oxide nanoparticles. J Appl Toxicol 38(3), 385–397. [DOI] [PubMed] [Google Scholar]
- DeLoid GM, Cohen JM, Pyrgiotakis G, Demokritou P, 2017. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat Protoc 12(2), 355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo L, Movia D, Bianchi MG, Allegri M, Mohamed BM, Bell AP, Moore C, Pinelli S, Rasmussen K, Riego-Sintes J, Prina-Mello A, Bussolati O, Bergamaschi E, 2016. Proinflammatory Effects of Pyrogenic and Precipitated Amorphous Silica Nanoparticles in Innate Immunity Cells. Toxicol Sci 150(1), 40–53. [DOI] [PubMed] [Google Scholar]
- Duan J, Kodali VK, Gaffrey MJ, Guo J, Chu RK, Camp DG, Smith RD, Thrall BD, Qian WJ, 2016. Quantitative Profiling of Protein S-Glutathionylation Reveals Redox-Dependent Regulation of Macrophage Function during Nanoparticle-Induced Oxidative Stress. ACS Nano 10(1), 524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomeni G, De Zio D, Cecconi F, 2015. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ 22(3), 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich E, 2017. Role of omics techniques in the toxicity testing of nanoparticles. J Nanobiotechnology 15(1), 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto F, Cagliani R, Catelani T, Guarnieri D, Moglianetti M, Pompa PP, Bardi G, 2017. PMA-Induced THP-1 Macrophage Differentiation is Not Impaired by Citrate-Coated Platinum Nanoparticles. Nanomaterials (Basel, Switzerland) 7(10), 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant CM, 2011. Regulation of translation by hydrogen peroxide. Antioxid Redox Signal 15(1), 191–203. [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, Van Rooijen N, Xu Y, O’Cain T, Jaffee BB, Busch DH, Duyster J, Schmid RM, Eckmann L, Karin M, 2007. NF-κB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell 130(5), 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B, Henderson S, 2009. Unfolding the relationship between secreted molecular chaperones and macrophage activation states. Cell Stress Chaperones 14(4), 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikwegbue PC, Masamba P, Oyinloye BE, Kappo AP, 2017. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals (Basel) 11(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SJ, Zhang X, Rodriguez-Velez A, Evans TD, Razani B, 2019. p62/SQSTM1 and Selective Autophagy in Cardiometabolic Diseases. Antioxid Redox Signal 31(6), 458–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan HA, Abdelhalim MA, Alhomida AS, Al Ayed MS, 2013. Transient increase in IL-1beta, IL-6 and TNF-alpha gene expression in rat liver exposed to gold nanoparticles. Genet Mol Res 12(4), 5851–5857. [DOI] [PubMed] [Google Scholar]
- Kim JS, Adamcakova-Dodd A, O’Shaughnessy PT, Grassian VH, Thorne PS, 2011. Effects of copper nanoparticle exposure on host defense in a murine pulmonary infection model. Part Fibre Toxicol 8, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Jeong HJ, 2015. Zinc Oxide Nanoparticles Suppress LPS-Induced NF-κB Activation by Inducing A20, a Negative Regulator of NF-κB, in RAW 264.7 Macrophages. J Nanosci Nanotechnol 15(9), 6509–6515. [DOI] [PubMed] [Google Scholar]
- Kodali V, Littke MH, Tilton SC, Teeguarden JG, Shi L, Frevert CW, Wang W, Pounds JG, Thrall BD, 2013. Dysregulation of macrophage activation profiles by engineered nanoparticles. ACS Nano 7(8), 6997–7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali V, Thrall BD, 2015. Oxidative Stress and Nanomaterial-Cellular Interactions. Humana Press. [Google Scholar]
- Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J, Lacroix G, Hoet P, 2009. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part. Fibre Toxicol. 6, 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz A-G, Karg E, Brendel E, Hinze-Heyn H, Maier KL, Eickelberg O, Stoeger T, Schmid O, 2013. Inflammatory and oxidative stress responses of an alveolar epithelial cell line to airborne zinc oxide nanoparticles at the air-liquid interface: a comparison with conventional, submerged cell-culture conditions. BioMed research international 2013, 652632–652632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Xue D, 2006. Estimation of electronegativity values of elements in different valence states. J Phys Chem A 110(39), 11332–11337. [DOI] [PubMed] [Google Scholar]
- Li Y, Guo Y, Tang J, Jiang J, Chen Z, 2014. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin (Shanghai) 46(8), 629–640. [DOI] [PubMed] [Google Scholar]
- Li Y, Ju D, 2018. The Role of Autophagy in Nanoparticles-Induced Toxicity and Its Related Cellular and Molecular Mechanisms. Adv Exp Med Biol 1048, 71–84. [DOI] [PubMed] [Google Scholar]
- Liliental J, Chang DD, 1998. Rack1, a receptor for activated protein kinase C, interacts with integrin beta subunit. J Biol Chem 273(4), 2379–2383. [DOI] [PubMed] [Google Scholar]
- Lin L, Xu M, Mu H, Wang W, Sun J, He J, Qiu JW, Luan T, 2019. Quantitative Proteomic Analysis to Understand the Mechanisms of Zinc Oxide Nanoparticle Toxicity to Daphnia pulex (Crustacea: Daphniidae): Comparing with Bulk Zinc Oxide and Zinc Salt. Environ Sci Technol 53(9), 5436–5444. [DOI] [PubMed] [Google Scholar]
- Loeb M, Neupane B, Walter SD, Hanning R, Carusone SC, Lewis D, Krueger P, Simor AE, Nicolle L, Marrie TJ, 2009. Environmental risk factors for community-acquired pneumonia hospitalization in older adults. J Am Geriatr Soc 57(6), 1036–1040. [DOI] [PubMed] [Google Scholar]
- Ma JS, Kim WJ, Kim JJ, Kim TJ, Ye SK, Song MD, Kang H, Kim DW, Moon WK, Lee KH, 2010. Gold nanoparticles attenuate LPS-induced NO production through the inhibition of NF-κB and IFN-beta/STAT1 pathways in RAW264.7 cells. Nitric Oxide 23(3), 214–219. [DOI] [PubMed] [Google Scholar]
- Ma X, Wu Y, Jin S, Tian Y, Zhang X, Zhao Y, Yu L, Liang XJ, 2011. Gold nanoparticles induce autophagosome accumulation through size-dependent nanoparticle uptake and lysosome impairment. ACS Nano 5(11), 8629–8639. [DOI] [PubMed] [Google Scholar]
- Meng H, Xia T, George S, Nel AE, 2009. A predictive toxicological paradigm for the safety assessment of nanomaterials. ACS Nano 3(7), 1620–1627. [DOI] [PubMed] [Google Scholar]
- Mihai C, Chrisler WB, Xie Y, Hu D, Szymanski CJ, Tolic A, Klein JA, Smith JN, Tarasevich BJ, Orr G, 2015. Intracellular accumulation dynamics and fate of zinc ions in alveolar epithelial cells exposed to airborne ZnO nanoparticles at the air-liquid interface. Nanotoxicology 9(1), 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos PJ, Olszewski K, Honeggar M, Cassidy P, Leachman S, Woessner D, Cutler NS, Veranth JM, 2011. Responses of human cells to ZnO nanoparticles: a gene transcription study. Metallomics 3(11), 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N, 2006. Toxic potential of materials at the nanolevel. Science 311(5761), 622–627. [DOI] [PubMed] [Google Scholar]
- Neupane B, Jerrett M, Burnett RT, Marrie T, Arain A, Loeb M, 2010. Long-term exposure to ambient air pollution and risk of hospitalization with community-acquired pneumonia in older adults. Am J Respir Crit Care Med 181(1), 47–53. [DOI] [PubMed] [Google Scholar]
- Nyathi Y, Wilkinson BM, Pool MR, 2013. Co-translational targeting and translocation of proteins to the endoplasmic reticulum. Biochim Biophys Acta 1833(11), 2392–2402. [DOI] [PubMed] [Google Scholar]
- Palmer KT, Poole J, Ayres JG, Mann J, Burge PS, Coggon D, 2003. Exposure to metal fume and infectious pneumonia. Am J Epidemiol 157(3), 227–233. [DOI] [PubMed] [Google Scholar]
- Parveen S, Misra R, Sahoo SK, 2012. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed.-Nanotechnol. Biol. Med. 8(2), 147–166. [DOI] [PubMed] [Google Scholar]
- Perkins TN, Shukla A, Peeters PM, Steinbacher JL, Landry CC, Lathrop SA, Steele C, Reynaert NL, Wouters EF, Mossman BT, 2012. Differences in gene expression and cytokine production by crystalline vs. amorphous silica in human lung epithelial cells. Part Fibre Toxicol 9(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto J, Eklund A, Patarroyo M, 1994. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol 156(1), 191–211. [DOI] [PubMed] [Google Scholar]
- Pulizzi F, 2016. Nanotechnology in food: Silver-lined packaging. Nature Nanotechnology. [Google Scholar]
- Puzyn T, Rasulev B, Gajewicz A, Hu X, Dasari TP, Michalkova A, Hwang H-M, Toropov A, Leszczynska D, Leszczynski J, 2011. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nature Nanotechnology 6(3), 175–178. [DOI] [PubMed] [Google Scholar]
- Safar R, Doumandji Z, Saidou T, Ferrari L, Nahle S, Rihn BH, Joubert O, 2019. Cytotoxicity and global transcriptional responses induced by zinc oxide nanoparticles NM 110 in PMA-differentiated THP-1 cells. Toxicology Letters 308, 65–73. [DOI] [PubMed] [Google Scholar]
- Shannahan JH, Lai X, Ke PC, Podila R, Brown JM, Witzmann FA, 2013. Silver nanoparticle protein corona composition in cell culture media. PLoS One 8(9), e74001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma C, Dhiman R, Rokana N, Panwar H, 2017. Nanotechnology: An Untapped Resource for Food Packaging. Front. Microbiol. 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, Pavitt GD, Ashe MP, Grant CM, 2006. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem 281(39), 29011–29021. [DOI] [PubMed] [Google Scholar]
- Simpson CE, Ashe MP, 2012. Adaptation to stress in yeast: to translate or not? Biochem Soc Trans 40(4), 794–799. [DOI] [PubMed] [Google Scholar]
- Sivalenka RR, Jessberger R, 2004. SWAP-70 regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration. Mol Cell Biol 24(23), 10277–10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JN, Thomas DG, Jolley H, Kodali VK, Littke MH, Munusamy P, Baer DR, Gaffrey MJ, Thrall BD, Teeguarden JG, 2018. All that is silver is not toxic: silver ion and particle kinetics reveals the role of silver ion aging and dosimetry on the toxicity of silver nanoparticles. Part Fibre Toxicol 15(1), 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundarraj K, Manickam V, Raghunath A, Periyasamy M, Viswanathan MP, Perumal E, 2017a. Repeated exposure to iron oxide nanoparticles causes testicular toxicity in mice. Environ Toxicol 32(2), 594–608. [DOI] [PubMed] [Google Scholar]
- Sundarraj K, Raghunath A, Panneerselvam L, Perumal E, 2017b. Iron oxide nanoparticles modulate heat shock proteins and organ specific markers expression in mice male accessory organs. Toxicol Appl Pharmacol 317, 12–24. [DOI] [PubMed] [Google Scholar]
- Tabei Y, Sonoda A, Nakajima Y, Biju V, Makita Y, Yoshida Y, Horie M, 2016. Intracellular accumulation of indium ions released from nanoparticles induces oxidative stress, proinflammatory response and DNA damage. J Biochem 159(2), 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Xiao W, Jiang L, Liu M, Shi Y, Wang K, Wang H, Xiao X, 2007. Nuclear heat shock protein 72 as a negative regulator of oxidative stress (hydrogen peroxide)-induced HMGB1 cytoplasmic translocation and release. J Immunol 178(11), 7376–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasova NK, Gallud A, Ytterberg AJ, Chernobrovkin A, Aranzaes JR, Astruc D, Antipov A, Fedutik Y, Fadeel B, Zubarev RA, 2017. Cytotoxic and Proinflammatory Effects of Metal-Based Nanoparticles on THP-1 Monocytes Characterized by Combined Proteomics Approaches. J. Proteome Res. 16(2), 689–697. [DOI] [PubMed] [Google Scholar]
- Thomas DG, Smith JN, Thrall BD, Baer DR, Jolley H, Munusamy P, Kodali V, Demokritou P, Cohen J, Teeguarden JG, 2018. ISD3: a particokinetic model for predicting the combined effects of particle sedimentation, diffusion and dissolution on cellular dosimetry for in vitro systems. Part Fibre Toxicol 15(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall BD, Kodali V, Skerrett S, Thomas DG, Frevert CW, Pounds JG, Teeguarden JG, 2019. Modulation of susceptibility to lung bacterial infection by engineered nanomaterials: In vitro and in vivo correspondence based on macrophage phagocytic function. NanoImpact 14, 100155. [Google Scholar]
- Toia LM, Mariano J, Winter MW, Shand JC, 2015. Alarmin High Mobility Group Box 1 (HMGB1) Activates Alternative NFkB Signaling in Bone Marrow Macrophages in the Leukemia Microenvironment. Blood 126(23), 2204. [Google Scholar]
- Topf U, Suppanz I, Samluk L, Wrobel L, Boser A, Sakowska P, Knapp B, Pietrzyk MK, Chacinska A, Warscheid B, 2018. Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species. Nat Commun 9(1), 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, Pankov R, Tran SD, Hampton B, Burgess WH, Yamada KM, 2002. Integrin clustering induces kinectin accumulation. J Cell Sci 115(Pt 10), 2031–2040. [DOI] [PubMed] [Google Scholar]
- Tsai YY, Huang YH, Chao YL, Hu KY, Chin LT, Chou SH, Hour AL, Yao YD, Tu CS, Liang YJ, Tsai CY, Wu HY, Tan SW, Chen HM, 2011. Identification of the nanogold particle-induced endoplasmic reticulum stress by omic techniques and systems biology analysis. ACS Nano 5(12), 9354–9369. [DOI] [PubMed] [Google Scholar]
- van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL, 2002. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci 115(Pt 9), 1837–1846. [DOI] [PubMed] [Google Scholar]
- Vasconcellos LR, Dutra FF, Siqueira MS, Paula-Neto HA, Dahan J, Kiarely E, Carneiro LA, Bozza MT, Travassos LH, 2016. Protein aggregation as a cellular response to oxidative stress induced by heme and iron. Proc Natl Acad Sci U S A 113(47), E7474–E7482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiskopf N, Ben-Shahar Y, Galchenko M, Carmel I, Moshitzky G, Soreq H, Banin U, 2016. Photocatalytic Reactive Oxygen Species Formation by Semiconductor-Metal Hybrid Nanoparticles. Toward Light-Induced Modulation of Biological Processes. Nano Lett 16(7), 4266–4273. [DOI] [PubMed] [Google Scholar]
- Wang D, Zhao L, Ma H, Zhang H, Guo LH, 2017. Quantitative Analysis of Reactive Oxygen Species Photogenerated on Metal Oxide Nanoparticles and Their Bacteria Toxicity: The Role of Superoxide Radicals. Environ Sci Technol 51(17), 10137–10145. [DOI] [PubMed] [Google Scholar]
- Wang R, Kovalchin JT, Muhlenkamp P, Chandawarkar RY, 2006. Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens. Blood 107(4), 1636–1642. [DOI] [PubMed] [Google Scholar]
- Waters KM, Masiello LM, Zangar RC, Tarasevich BJ, Karin NJ, Quesenberry RD, Bandyopadhyay S, Teeguarden JG, Pounds JG, Thrall BD, 2009. Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicol Sci 107(2), 553–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, Couillin I, Tschopp J, 2010. Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1alpha and IL-1beta. Proc Natl Acad Sci U S A 107(45), 19449–19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ji Z, Xia T, Meng H, Low-Kam C, Liu R, Pokhrel S, Lin S, Wang X, Liao YP, Wang M, Li L, Rallo R, Damoiseaux R, Telesca D, Madler L, Cohen Y, Zink JI, Nel AE, 2012. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 6(5), 4349–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HZ, Burnum KE, Luna ML, Petritis BO, Kim JS, Qian WJ, Moore RJ, Heredia-Langner A, Webb-Robertson BJM, Thrall BD, Camp DG, Smith RD, Pounds JG, Liu T, 2011. Quantitative proteomics analysis of adsorbed plasma proteins classifies nanoparticles with different surface properties and size. Proteomics 11(23), 4569–4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Gaffrey MJ, Thrall BD, Qian WJ, 2018. Mass spectrometry-based proteomics for system-level characterization of biological responses to engineered nanomaterials. Anal Bioanal Chem 410(24), 6067–6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MZ, Li HY, Bu XL, Lei CN, Fang QJ, Hu ZY, 2015a. Quantitative Proteomic Analysis of Cellular Resistance to the Nanoparticle Abraxane. Acs Nano 9(10), 10099–10112. [DOI] [PubMed] [Google Scholar]
- Zhao P, Gao D, Wang Q, Song B, Shao Q, Sun J, Ji C, Li X, Li P, Qu X, 2015b. Response gene to complement 32 (RGC-32) expression on M2-polarized and tumor-associated macrophages is M-CSF-dependent and enhanced by tumor-derived IL-4. Cell Mol Immunol 12(6), 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JF, Ardona HAM, Pyrgiotakis G, Dong J, Moudgil B, Demokritou P, Parker KK, 2019. Scatter Enhanced Phase Contrast Microscopy for Discriminating Mechanisms of Active Nanoparticle Transport in Living Cells. Nano Lett 19(2), 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.