Abstract
Pre‐market/prospective environmental risk assessments (ERAs) contribute to risk analyses performed to facilitate decisions about the market introduction of regulated stressors. Robust ERAs begin with an explicit problem formulation, which involves among other steps: (1) formally devising plausible pathways to harm that describe how the deployment of a regulated stressor could be harmful; (2) formulating risk hypotheses about the likelihood and severity of such events; (3) identifying the information that will be useful to test the risk hypotheses; and (4) developing a plan to acquire new data for hypothesis testing should tests with existing information be insufficient for decision‐making. Here, we apply problem formulation to the assessment of possible adverse effects of RNA interference‐based insecticidal genetically modified (GM) plants, GM growth hormone coho salmon, gene drive‐modified mosquitoes and classical biological weed control agents on non‐target organisms in a prospective manner, and of neonicotinoid insecticides on bees in a retrospective manner. In addition, specific considerations for the problem formulation for the ERA of nanomaterials and for landscape‐scale population‐level ERAs are given. We argue that applying problem formulation to ERA maximises the usefulness of ERA studies for decision‐making, through an iterative process, because: (1) harm is defined explicitly from the start; (2) the construction of risk hypotheses is guided by policy rather than an exhaustive attempt to address any possible differences; (3) existing information is used effectively; (4) new data are collected with a clear purpose; (5) risk is characterised against well‐defined criteria of hypothesis corroboration or falsification; and (6) risk assessment conclusions can be communicated clearly. However, problem formulation is still often hindered by the absence of clear policy goals and decision‐making criteria (e.g. definition of protection goals and what constitutes harm) that are needed to guide the interpretation of scientific information. We therefore advocate further dialogue between risk assessors and risk managers to clarify how ERAs can address policy goals and decision‐making criteria. Ideally, this dialogue should take place for all classes of regulated stressors, as this can promote alignment and consistency on the desired level of protection and maximum tolerable impacts across regulated stressors.
Keywords: biodiversity, ecosystem services, GMO, hypothesis testing, pathway to harm, pesticides, protection goals
1. Introduction
As in most jurisdictions, pre‐market/prospective environmental risk assessments (ERAs) are a key tool contributing to risk analyses performed to facilitate decisions about the market introduction of new products in the European Union (EU). These ERAs are conducted for a variety of products, such as plant protection products (PPPs), chemicals, pharmaceuticals, feed additives and genetically modified organisms (GMOs) (hereafter referred to as regulated stressors), some of which are in the remit of the European Food Safety Authority (EFSA). In this process, EFSA's role is to assess and provide scientific advice to risk managers on any possible risk that the deployment of regulated stressors may pose to the environment.
ERA addresses the question to what extent the use of a regulated stressor poses risks to the environment. This risk is characterised by testing specific hypotheses about the probability that harm (i.e. an adverse effect on something of value) will occur and the severity of that harm should it occur. Risk managers decide on the level of acceptable risk and weigh policy options to accept, minimise, reduce or reject characterised risks with other relevant information such as the economic, social or political implications of the proposed activity.
The methods used and data requirements for the ERA of each type of regulated stressor can be very different, as they typically fall under different regulatory frameworks. For some regulated stressors, a very specific set of studies and methods have been established based on experience, and triggers and criteria for the acceptability of risk have been set, building on familiarity and a wealth of scientific data available to support these approaches (e.g. Rortais et al., 2017).
However, scientific advances are quickly evolving, resulting in a range of new products moving to commercial application, ranging from RNA interference (RNAi)‐based genetically modified (GM) plants to systemic insecticides, GM fish, gene drive‐modified insects, biological control agents and nanomaterials. For some of these regulated stressors, there may be challenges in directly applying pre‐existing requisite risk assessment tools such that ways in which they can be made fit‐for‐purpose will have to be found. One option that has been identified is ‘problem formulation’. This is a methodology that has been used implicitly for many years in chemical risk assessments. More recently, EFSA proposed a more explicit use of problem formulation for the ERA of GMOs (EFSA, 2010, 2013a), mostly because GMOs can be very diverse, and their assessments are conducted on a case‐by‐case basis. Since an initial elaboration and adoption by the USA's Environmental Protection Agency in 1998 (US EPA, 1998), problem formulation has gained support around the world and is now widely used (e.g. Raybould, 2006; Nelson et al., 2007; Gray, 2012; Garcia‐Alonso, 2013; Tepfer et al., 2013; Devos et al., 2018). In the EU, Directive (EU) 2018/350, which amends Directive 2001/18/EC on the deliberate release of GMOs in the environment, formally introduces problem formulation as a key first step and requirement for the ERA of GMOs.
Problem formulation frames the ERA process and does so by clarifying policy goals and decision‐making criteria for assessing risks and devising tests of hypotheses that meet those criteria (Raybould, 2006). It is a method that enables identifying potential harms derived from the deployment of a regulated stressor and potential pathway(s) to such harm, and defining the actual information genuinely needed to assess the likelihood of the harm to occur and its seriousness. This helps to focus the risk assessment on those phenomena that are important for decision‐makers and steer it from those that are less important or irrelevant (Raybould, 2006, 2007, 2010; Tepfer et al., 2013; Garcia‐Alonso and Raybould, 2014; Gray, 2014; Devos et al., 2016a). In essence, problem formulation is concerned with maximising the practical utility of ERA in decision‐making (Raybould and Macdonald, 2018).
Here, we first describe the general concepts behind problem formulation as a tool to frame ERA. Then, to assess its merits and challenges, we apply problem formulation to various regulated stressors expected to emerge from development in the near future. Finally, we discuss suggestions on how to overcome some of these challenges, as well as areas of future work.
This publication builds upon presentations made and discussions held during the breakout session ‘Advancing risk assessment science – Environment’ at EFSA's third Scientific Conference ‘Science, Food and Society’ (Parma, Italy, 18–21 September 2018).1
2. Problem formulation in theory: concepts
Problem formulation involves amongst other steps: (1) formally devising plausible pathways to harm that describe how a proposed activity could be harmful (i.e. impact a protected value adversely); (2) formulating risk hypotheses (i.e. hypotheses of no more harm or risk than the existing activity) about the likelihood and severity of such events; (3) identifying the information that will be useful to test the risk hypotheses; and (4) developing a plan to acquire new data for hypothesis testing should tests with existing information be insufficient for decision‐making (Raybould, 2006; Craig et al., 2017). In this context, it is important to consider whether a proposed activity may lead to new harms, or only to different ways of causing harm that already result from current practice. This helps to put the impact of regulated stressors derived from new technology in the context of those caused by current and past practices.
A crucial step in problem formulation is to define what qualifies as harm under the relevant regulations (Sanvido et al., 2012). Protecting everything, everywhere, and forever is rarely, if ever, tenable. Unfortunately, policy protection goals are typically very broadly defined (e.g. protection of biodiversity). Consequently, operational protection goals must delineate the environmental components that are valued and need to be protected (e.g. species, ecosystem services, habitats), where and over what time period, and the maximum tolerable impact (Garcia‐Alonso and Raybould, 2014; Devos et al., 2015, 2016a; EFSA, 2016b; Maltby et al., 2017a,b).
To further frame ERA, plausible pathways (also called conceptual models) are constructed to describe how the proposed activity could lead to possible harm to operational protection goals (Raybould, 2006, 2007, 2010; Johnson et al., 2007; Nickson, 2008; Wolt et al., 2010; Gray, 2012; Tepfer et al., 2013; Layton et al., 2015; Sauve‐Ciencewicki et al., 2019). As with adverse outcome pathways (Lanzoni et al., 2019), a pathway to harm is a causal chain of events that need to occur for a harm to be realised (Tepfer et al., 2013; Craig et al., 2017).
The steps in the pathway enable the formulation of risk hypotheses that can then be tested to characterise risk (Figure 1). A risk assessment may include many pathways because the proposed activity could lead to different harms, or because a particular harm could arise from different pathways, or both. Each step in the pathway leads to a risk hypothesis that harm will not arise. Consider a pathway in which Event A must lead to Event B for harm to occur. A conservative risk hypothesis would be that Event A never leads to Event B. A less conservative risk hypothesis might be that Event B does not occur at a certain time or place or does not occur above a certain frequency or magnitude (Raybould, 2010). The precise form of risk hypotheses will depend on how harm is defined and how decisions on the acceptability of risk will be made.
Figure 1.
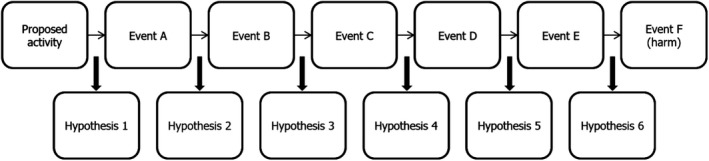
Pathway to harm and risk hypotheses
A careful first scrutiny of the pathway, based on existing expert knowledge, can usually help identify which of the step(s) may be the most decisive or easiest to test in attempting to disrupt the pathway with the highest degree of certainty (Craig et al., 2017). Corroboration of risk hypotheses will build confidence that risk is negligible via the pathway in question, and corroboration following a rigorous test gives greater confidence than does a weak test (Raybould, 2006, 2007). A particularly useful feature of this strategic analysis is that it suffices to decisively determine with sufficient confidence that a single step is highly unlikely, to conclude that the likelihood that harm will result via the pathway is negligible and that no other step will require analysis.
Risk hypotheses may be tested with existing information, which can come from many sources, including published scientific literature, expert opinions, research data and relevant data gathered during product development, and does not necessarily require experimentation. If the available information is inconclusive for decision‐making, new studies may be undertaken. It is important to note that information on the ecology of regulated stressors reported in the scientific literature is only relevant for ERA when it helps to address the formulated risk hypotheses. This is because basic research, which includes ecological research, and ERA differ in the sources of problems addressed, the nature of hypotheses under examination, the precision anticipated, and even the methods for testing hypotheses (Raybould, 2006, 2007, 2010; Tepfer et al., 2013; Gray, 2014; Layton et al., 2015). It is therefore important to demarcate ERA studies from ecological research (Raybould, 2006, 2007, 2010; Johnson et al., 2007).
Testing of relevant risk hypotheses in risk assessment studies should be as rigorous and objective as hypothesis testing in any other branch of science, but the construction of those risk hypotheses needs to be guided by policy rather than an exhaustive attempt to address any possible differences. This testing needs to comply with quality standards to increase confidence in the results and add certainty to the conclusions. Moreover, any study should be carried out in such a way that it minimises the probability of erroneous (i.e. false negatives and false positives), or inconclusive results. Adhering to quality standards will facilitate study reproducibility and peer review of tests. It will also benefit regulatory authorities by enhancing the quality of information generated for use in risk assessments. Furthermore, high confidence in the study results is a precondition for the acceptance of data across regulatory jurisdictions and should encourage risk assessors to share useful information and thus avoid redundant testing.
Because risk assessment is a decision‐making tool and not basic research, rigorous tests of risk hypotheses under unrealistically conservative conditions, i.e. ‘worst‐case scenarios’ are generally preferred (Hokanson et al., 2018, but see Vandersteen et al., 2019). If risk cannot be ruled out with sufficient certainty, further testing under more realistic conditions is required (Raybould, 2006). Following this tiered approach, information collected in lower tiers directs the extent and nature of any experimentation conducted in higher tiers: hazards are evaluated within different tiers that progress from worst‐case exposure scenario conditions, framed in highly controlled laboratory environments, to more realistic scenarios under semi‐field or field conditions (Garcia‐Alonso et al., 2006; Romeis et al., 2008). Progression to larger scale experiments in higher tiers aims to provide increasingly refined estimates of exposure. Within each tier, all relevant data are gathered to determine whether there is enough information to conclude the risk assessment at that tier. The conclusion can only be made if any residual uncertainty has been defined; otherwise additional investigations to generate further data at a higher tier(s) are conducted. Should potential hazards be detected in early tier tests or if unacceptable uncertainties concerning possible hazards remain, additional information is required to confirm whether the observed effect might still be detected at more realistic rates and routes of exposure. In the case that risk cannot be ruled out with enough certainty, risk management measures can be implemented.
3. Problem formulation in practice: case studies
In this section, problem formulation is applied to the assessment of possible adverse effects of RNAi‐based insecticidal GM plants, GM growth hormone (GH) coho salmon, gene drive‐modified mosquitoes and classical biological weed control agents on non‐target organisms (NTOs) in a prospective manner, and of neonicotinoid insecticides on bees in a retrospective manner. In addition, specific considerations for the problem formulation for the ERA of nanomaterials and for landscape‐scale population‐level ERAs of PPPs are given.
3.1. Problem formulation for the assessment of adverse effects of RNAi‐based insecticidal GM plants on non‐target arthropods – a prospective analysis
3.1.1. Case study
RNAi is an emerging and powerful technology that offers new opportunities for arthropod pest control through the silencing of target genes in arthropod pests (Burand and Hunter, 2013). RNAi in arthropods involves short‐interfering RNAs (siRNAs), derived from double‐stranded RNA (dsRNA), that bind to complementary messenger RNAs (mRNAs) leading to cleavage of the duplex by an enzyme complex, thus silencing expression of the mRNA‐encoding gene. Evidence suggests that contiguous sequence matches to the target gene of ≥ 21 nucleotides are typically necessary for dsRNA to have an RNAi effect in insects (Bachman et al., 2013, 2016). Because the RNAi effect is sequence‐specific, dsRNA can be designed to have a very narrow spectrum of activity, thus allowing highly targeted pest control. While functional RNAi has been reported for several arthropod species belonging to various orders, the impact of dietary RNAi (i.e. RNAi response when dsRNA is ingested) is more limited (Baum and Roberts, 2014). RNAi effects in pest control may, for example, be achieved by providing dsRNA in GM plants, GM microorganisms, or in baits or foliar sprays. While we focus on dsRNA‐producing GM plants in the following, what is described will be equally applicable to other dsRNA applications.
As for any other pest control method, one of the main concerns related to the use of dsRNA is that this could cause adverse effects to valued non‐target (NT) species, including arthropods. Arthropods form a major part of the biodiversity in agricultural landscapes and contribute to important ecosystem services (Devos et al., 2019). This includes regulating services such as biological control of herbivores and pollination, supporting services such as nutrient cycling and cultural services in the case of species of conservation concern (Sanvido et al., 2012). Consequently, assessing the potential impacts that GM plants or PPPs may have on valued NT arthropods is legally required before entering the market.
3.1.2. Problem formulation considerations
For harm to be realised, the plants must express a dsRNA and the NT arthropods must be exposed to (i.e. ingest) it. (In the case of PPPs, contact exposure might also be possible (see Zheng et al., 2019). The greatest exposure is expected to occur to NT arthropods feeding directly on living plant material or consumption of plant‐fed herbivores (in the case of natural enemies), although exposure from consumption of pollen, cuttings, leaf litter, or other plant materials or exudates into soil or aquatic environments is also a possibility provided the dsRNA persists in these environments in sufficient concentrations (Romeis et al., 2019). Following consumption, the dsRNA must resist degradation in the gut, and the NT arthropod must be competent to uptake the dsRNA in sufficient quantities to activate its endogenous RNAi machinery. This can occur either locally at the point of uptake (i.e. in cells lining the gut), or systemically if the NT arthropod is capable of triggering systemic RNAi (Ivashuta et al., 2015; Chan and Snow, 2017). Once the endogenous RNAi machinery is active, it must lead to the degradation or translational suppression of a corresponding mRNA in a sequence‐dependent fashion (Whyard et al., 2009; Baum and Roberts, 2014). And finally, the loss of that transcript must have an adverse impact on the NT arthropod (Bolognesi et al., 2012; Baum and Roberts, 2014).
If any of the aforementioned steps is unlikely or impossible, then the risk to a NT arthropod from a RNAi‐based GM plant is negligible. Some elements of this generic pathway will be case specific – for example, the sequence of a dsRNA will determine whether mRNA with a complementary sequence is available for silencing. However, there are steps in this pathway that will be common to most or all cases, and these represent potential targets for conducting basic research, to gather baseline data in support of the risk assessment of RNAi‐based GM plants (Roberts et al., 2015). This includes, for example, the ability of dsRNA to persist in the environment, the importance of sequence independent effects, and the use of bioinformatics to predict adverse effects.
Multiple factors can affect RNAi efficiency in arthropods, including: dsRNA concentrations; lengths of dsRNA fragments; the timing and duration of exposure; dsRNA uptake and degradation activities; activation of RNAi machinery; and the life stage of the target organisms (reviewed by Christiaens et al., 2018). Information on barriers to exposure such as the potential degradation of dsRNA prior to ingestion, instability of the dsRNA within the recipient organism following ingestion, barriers to cellular uptake, and the inherent sensitivity of the organism to ingested dsRNA could facilitate risk assessment predictions across NT taxa, refine exposure estimates, or allow assumptions of minimal exposure in certain organisms. At present, however, there is insufficient understanding of specific barriers to make any generalisations (Ramon et al., 2014; US EPA, 2014; Christiaens et al., 2018). An improved understanding of the ability of susceptible arthropods to take up dsRNA from the environment as well as a more complete picture of which insect orders possess the capacity for systemic RNAi will be informative for identifying species that may require consideration during risk assessment, and those species that can be eliminated from consideration owing to their inability to respond to dietary RNAi.
In cases where a NT arthropod is known to be susceptible and is predicted to be exposed to the dsRNA in the environment in sufficient quantity (Romeis et al., 2014), it is useful to understand how likely off‐target gene effects are to be realised. There are two ways to approach this question (Roberts et al., 2015), and examples of both approaches are available in the literature (Whyard et al., 2009; Bolognesi et al., 2012; Bachman et al., 2013, 2016; Pan et al., 2017; Haller et al., 2019). The first is a bioinformatics‐based approach where one analyses the sequence complementarity between the pool of siRNAs and the target gene in NT species as this would indicate potential bioactivity. This approach could guide the selection of NT species harbouring genes that share a certain level of homology with the target gene in the pest, and those species should therefore be the focus of further assessment. Moreover, if reliable bioinformatic data indicate that the minimum sequence requirements for RNAi activity are not met, further assessment may not be necessary as the likelihood of adverse effects is low. However, this approach is currently subject to substantial limitations (EFSA, 2014; Ramon et al., 2014; US EPA, 2014; Casacuberta et al., 2015; Christiaens et al., 2018). The bioinformatics‐based approach requires knowledge of sequence information that may not be available for all species of interest. It may also be subject to differences between organisms in terms of how the RNAi machinery functions in relation to base pair mismatches. Moreover, scientific uncertainty remains on the exact rules governing siRNA–mRNA matches/interactions. Hence, progress of basic research on RNAi mechanisms, production of suitable genome data for relevant species and design of efficient algorithms to make reliable predictions will increase the usability of bioinformatic data in support of ERAs of RNAi‐based GM plants.
The other way to approach the problem is to conduct NT feeding studies using dsRNA that is perfectly homologous to the target gene in the target organism. These should be done with a range of organisms, starting with close relatives, and then moving outward to see how phylogenetically distant organisms respond (Bachman et al., 2013, 2016; Pan et al., 2017; Haller et al., 2019). This approach enables characterisation of the activity spectrum of dsRNAs and can be done without sequence information from the tested species. Bachman et al. (2013, 2016) suggest that, in tested arthropods, close phylogenetic relationships are required for off‐target gene effects. The experience gained will indicate whether such bioassays are appropriate to assess the effects of RNAi on the fitness and performance of NT arthropods. The timing and duration of exposure necessary to achieve the RNAi response are uncertain, as are the most sensitive endpoints to be measured, and a more thorough investigation of dose–response relationships for siRNA targets would therefore be necessary for RNAi susceptible NT arthropods. Since the usefulness of bioassays with plant material to capture unknown complexities and variability in the RNAi‐based GM plant remains a contentious point of debate (Romeis et al., 2011, 2014; Lundgren and Duan, 2013; US EPA, 2014; Devos et al., 2016b; Arpaia et al., 2017), it would be helpful to investigate whether such bioassays will add weight of evidence to the NT risk assessment, and what determines their need.
3.1.3. Conclusions and recommendations
Although current knowledge may well be enough to conduct case‐specific ERAs, we presently have an incomplete understanding of the susceptibility of arthropods to environmental exposure to dsRNA, as well as the parameters that influence the likelihood of off‐target gene effects. Additional research addressing these areas is warranted to improve the certainty associated with ERAs of RNAi applications and contribute to reducing the burden of case‐specific data collection and testing (Roberts et al., 2015).
3.2. Problem formulation for the assessment of adverse effects of GMGH coho salmon to wild coho salmon – a prospective analysis
3.2.1. Case study
Growth hormone transgenesis can produce accelerated growth and improved feed conversion efficiency in many species of fish (Devlin et al., 2015). It is being explored as a method to improve aquaculture production of several species of food fish, and GMGH Atlantic salmon (Salmo salar) are now approved for land‐based commercial production and human consumption in Canada and the USA. GH transgenesis in fish is reported to produce a pleiotropy of unintended (off‐target) effects, includingaltered behaviour, life‐history timing, gene expression levels and disease resistance (Devlin et al., 2015). When considering potential environmental risks associated with use of GMGH fish, effects from both targeted and off‐target phenotypes are considered.
As a test case on potential environmental risks of GMGH fish, the problem formulation for the ERA of a line of GMGH coho salmon (Oncorhynchus kisutch; Devlin et al., 1994, 2004a) developed for non‐commercial research on effects of accelerated growth in salmonids is discussed. A theoretical use scenario of land‐based aquaculture production of sterile (triploid) GMGH coho salmon within the natural migratory range of wild coho salmon populations is considered.
3.2.2. Problem formulation considerations
The goal of the case study is to determine if commercial production of GMGH coho salmon could cause harm to wild populations of coho salmon. Wild coho salmon are key contributors to both marine and riparian ecosystems and have strong socioeconomic importance. Consequently, the maintenance of wild stocks is a key protection goal, with negative deviations from historic population sizes being considered harmful. The problem formulation focuses on potential impacts of GMGH coho salmon to wild salmon populations through hybridisation, although harm could arise through trophic interactions, as a vector of disease, and through impacts to habitat and biodiversity.
For GMGH, coho salmon to cause harm to wild populations of coho salmon through hybridisation, several steps must occur: release of GM fish from land‐based facilities; survival in nature; migration to wild spawning grounds; successful reproduction with wild fish; and negative impacts to wild populations as a result of hybridisation and introgression (Figure 2). If the biology of the GM fish or other factors prevent or partially influence any one of these steps from occurring, this would affect potential harm occurring to wild populations through hybridisation. Ideally, the potential success of GMGH fish at each step in the pathway would be examined under natural conditions. As this is not considered feasible for numerous reasons, comparisons of GMGH fish with non‐GM fish should be conducted in contained conditions that simulate relevant natural conditions as closely as possible. An advantage of using GMGH coho salmon as a test case is that the fitness and impacts of this model have been extensively examined in both standard laboratory and simulated natural conditions (Devlin et al., 2015). Consequently, this model can be used to demonstrate the information needed to determine the likelihood of pathways to harm.
Figure 2.
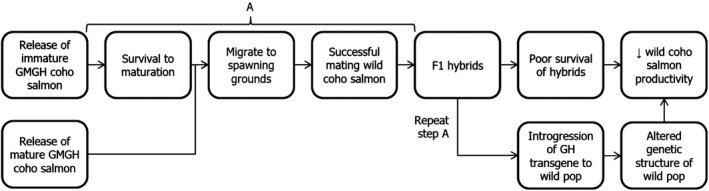
Pathway to harm from genetically modified growth hormone (GMGH) coho salmon to wild coho salmon populations through hybridisation (modified from Leggatt et al., 2010) (pop: populations)
Assuming release of GMGH coho salmon from land‐based facilities, survival of transgenic fish in semi‐natural freshwater or marine conditions relative to non‐transgenic fish has been shown to vary depending on environmental factors (e.g. disease load, food type and availability, predation, time of simulated escape, habitat complexity, etc.; Sundström et al., 2007; Leggatt et al., 2017; Vandersteen et al., 2019), but is possible in all but the most extreme conditions (e.g. Devlin et al., 2004b). Spawning migration has not been examined in any GMGH fish and, because of the large‐scale and complex migration that occurs at this stage, it is difficult to examine under contained conditions. In another fast‐growing salmonid, the domesticated Atlantic salmon, triploid female salmon do not migrate to spawning grounds (Glover et al., 2016), do not mature, and so would not attempt to mate with wild salmon. Consequently, only fertile transgenic broodstock or diploid fish from failed triploidy (e.g. Devlin et al., 2010) have potential to migrate and spawn with wild salmon. In domesticated Atlantic salmon, while migration to spawning grounds is diminished, large numbers of escaped domesticated aquaculture fish are reported at wild Atlantic salmon spawning grounds (Glover et al., 2017), which suggests a fast‐growing phenotype does not prevent spawning migration. In terms of reproductive success, GMGH fish had extremely poor reproductive success when reared in small‐scale culture, but had similar reproductive success as non‐GM coho salmon when both were reared under semi‐natural conditions and could successfully mate with wild‐reared hatchery fish (Bessey et al., 2004; Fitzpatrick et al., 2011; Leggatt et al., 2014). Should successful reproduction between GMGH and wild coho salmon occur, studies in simulated stream conditions indicate the presence of the transgene may decrease survival under some conditions (Vandersteen et al., 2019), with potential impacts on wild salmon productivity. However, impacts from the presence of the transgene have been found to be context‐specific, where GM fish may have lesser, equal or greater survival than non‐GM siblings dependent on numerous factors (see above, and Vandersteen et al., 2019). Consequently, the impacts of hybridisation on wild populations may vary depending on the environmental conditions present. Regarding the impacts of introgression of the transgene into wild salmon populations, computer modelling simulations found presence of the transgene could potentially shift genetic backgrounds and phenotypes of both GM and non‐GM individuals away from the naturally selected optima (Ahrens and Devlin, 2011). Quantitative trait loci mapping demonstrated the presence of the GH transgene altered the genetic basis of growth‐related traits in coho salmon, which may indicate the potential for the transgene to influence evolutionary changes in coho salmon, with potential ecological consequences (Kodama et al., 2018).
Overall, assuming GMGH coho salmon escape aquaculture facilities, there is no step in the examined pathway that would completely prevent harm to wild coho salmon populations through hybridisation. It is important to note that the likelihood and level of harm are expected to be context‐specific. Studies in laboratory and varying semi‐natural conditions demonstrate the steps in the pathway may be influenced by numerous factors including time or life stage of escape and biological conditions present in the natural environment. Consequently, there is significant uncertainty in final predictions of harm to wild populations from GMGH salmon. These studies have also demonstrated major difficulties associated with using data solely from culture conditions to predict environmental risk. For example, genotype‐by‐environment interactions have been noted for most phenotypes examined, where wild and GM fish respond to different environments in different ways (Devlin et al., 2015). GMGH and non‐GM salmon have been shown to respond to cultured and simulated‐natural conditions very differently (e.g. Sundström et al., 2007). These strong genotype‐by‐environment interactions make problematic any predictions of success or impacts of GMGH fish in nature using data from culture conditions and contribute significantly to uncertainty in ERA (Vandersteen et al., 2019).
3.2.3. Conclusions and recommendations
Under the theoretical use scenario, should GMGH coho salmon escape land‐based facilities, they could conceivably harm important populations of wild coho salmon through hybridisation. All post‐release steps in the examined pathway to harm (Figure 2) have been corroborated to some extent through laboratory studies or use of surrogate organisms, although likelihood and level of effects for many steps may be context specific, with significant uncertainty in measures of harm. Differing predictions of success and harm of GMGH fish when examined under culture versus semi‐natural conditions demonstrate the importance of simulating relevant natural conditions when examining environmental risk. The examined pathway to harm is only one of several potential interacting pathways where GH transgenic fish may harm wild fish populations and natural ecosystem, should they escape aquaculture facilities. This demonstrates the importance of full containment of GMGH fish to prevent harm to wild fish populations and the environment during commercial production of GM fish.
3.3. Problem formulation for the assessment of adverse effects of gene drive‐modified mosquitoes on NTOs – a prospective analysis
3.3.1. Case study
Any genetic element that is inherited at a greater frequency than predicted through Mendelian genetics can be referred to as a ‘gene drive’. Gene drives positively bias their own inheritance and thus spread rapidly through populations, even if they incur a fitness cost (North et al., 2019). First reported in the 1950s, natural gene drives have been well‐characterised (Hammond and Galizi, 2017). The idea of harnessing gene drives to address challenges related to disease vectors, agricultural pests, invasive species and conservation is not new, but received new impetus with the discovery of driving‐endonuclease genes and with recent molecular advances that allow their introduction into insects. Most recently, the use of CRISPR‐Cas9 technology to create novel driving‐endonuclease genes has further spurred interest, suggesting that a practical application of gene drive mechanisms could be more readily achievable than previously believed. Several different projects are ongoing to develop engineered gene drives, and these have captured a great deal of public attention.
Strategies to use gene drives in the context of GM insects can be differentiated based on (e.g. Eckhoff et al., 2017; James et al., 2018; Scott et al., 2018):
desired outcome: population suppression2 vs. population replacement,3
ability of the trait to establish or spread: self‐sustaining vs. self‐limiting drives.
Within each category, different technical approaches [e.g. homing‐based drives using homing endonuclease genes; sex‐linked meiotic drives; Medea, the maternal effect dominant embryonic arrest system; underdominance or heterozygote inferiority drives; heritable microorganisms as illustrated by Wolbachia; Frieß et al., 2019)] with diverse characteristics are possible [e.g. conventional vs. integral gene drives (Nash et al., 2019); toxin‐antidote recessive embryo gene drives (Champer et al., 2019); allelic gene drives (Guichard et al., 2019)].
Population suppression strategies aim to reduce the size of a target population by imposing a fitness cost via the inactivation of important genes involved in the survival or reproduction of the target population (e.g. reducing fertility of progeny, bias of the sex ratio toward males). This causes population decline or even collapse. Population replacement strategies for controlling vector populations are used to replace a current genotype with one less able to transmit disease (disease refractory). These strategies are based on inactivation of a gene or genes involved in pathogen survival in the vector (e.g. pathogen resistance) or that are required for the target organism to transmit the pathogen (e.g. a tendency to feed on humans in the case of mosquitoes). They can involve the introduction of a new gene or genes, such as those that produce molecules that will kill the pathogen in the vector. For strategies aiming for population suppression, modified target insects are expected to decrease to low numbers over the period of a few years as the overall target population is reduced. Strategies aiming for population replacement require the modification to persist: the drive mechanism must be capable of overcoming any fitness costs and increase in frequency in the target population from low initial levels to fixation, or near fixation, within a meaningful time frame (James et al., 2018).
Self‐sustaining drives are gene drives that are designed to cause desirable genes to increase in frequency in a population (or populations) and ideally become fixed in the population (or populations). These drives will ideally sustain the high frequency of the desirable gene indefinitely unless actions are taken to reverse the impact and/or frequency of the drive through release of another transgenic strain. Natural resistance to a self‐sustaining drive could evolve among wild individuals in a population and reverse its impact and/or frequency. A self‐sustaining drive can be designed to be spatially unrestricted and move to any population that has gene flow with the population where the drive was released, or a self‐sustaining drive can be designed to only spread within a single population or region. These are referred to as spatially restricted gene drives. Examples of spatially unrestricted drives are some CRISPR‐based, simple homing drives and Medea drives when developed to have very low thresholds for release. Examples of spatially restricted drives are under dominance‐based drives and Medea drives when developed to have high thresholds for release. Self‐limiting drives are gene drives that are designed to cause desirable genes to increase in frequency in a population for a limited period of time after which the genes decrease in frequency and are ideally lost from the population. The desirable genes could either be those that change the population's characteristics or suppress the population density. These type of gene drives are also referred to as temporally limited drives. Examples are daisy‐chain gene drives (Noble et al., 2019) and killer–rescue drives (Gould et al., 2008).
The nature of gene drive applications, which involve the deliberate spread of GM traits into interbreeding populations, is demonstrably different from other large scale applications of genetic technologies that are generally intended to be limited to specific uses in controlled environments (as is the case with GM crops for agriculture) or limited in exposure over space and time (as is the case with the release of sterile GM insects). The nature of gene drives to spread into populations, at least in part, has led some environmental groups to call for a global moratorium on the field application of this technology as they argue it may lead to undesired side effects and alter ecosystems in irreversible ways. However, in a recent open letter, a group of more than 100 scientists, including many studying gene drives, urged governments to reject this moratorium. Others have called for the establishment of different forms of governance so that any gene drive releases are done safely and responsibly, and with early input into the decision‐making process by all concerned parties (NASEM, 2016; Kofler et al., 2018; Bartumeus et al., 2019; Brossard et al., 2019; Hartley et al., 2019; Kuzma, 2019; Thizy et al., 2019).
As with any technology, however, true understanding of the potential risks to the environment must be informed by a case‐specific risk assessment, not a generalised view of the technology (James et al., 2018; Frieß et al., 2019). One specific case example where the use of gene drive mechanisms has been proposed is to target vector mosquitoes in order to reduce or eliminate the spread of malaria (Gantz et al., 2015; Burt et al., 2018; Kyrou et al., 2018). This is a long‐standing public health goal, and the eradication of human malaria would have tremendous economic and social benefits, particularly in sub‐Saharan Africa where the most efficient vectors transmit the deadliest malaria parasite.
To ensure that the development and potential deployment of a gene drive as part of a malaria eradication strategy is fully informed by an evaluation of risks to the environment and human health, researchers, donor organisations and stakeholders have embarked on a series of consultations, workshops and public engagements aimed at problem formulation for the use of gene drive‐modified mosquitoes to reduce malaria incidence (Murray et al., 2016; Roberts et al., 2017; Hayes et al., 2018; James et al., 2018).
3.3.2. Problem formulation considerations
In May 2016, the Foundation for the National Institutes of Health and the ILSI Research Foundation organised an expert workshop to consider the potential risks related to the use of gene drives in Anopheles gambiae for malaria control in Africa. The resulting discussions yielded a series of consensus points that are reported in Roberts et al. (2017). Subsequently, in 2016–2018, the New Partnership for African Development (NEPAD) organised a series of regional consultations in Africa addressing the same issue. It is impossible to fully capture the richness of these discussions in a few paragraphs, but this section will identify some important highlights common to most or all of these events.
Like all risk assessments, any ERA for a gene drive‐modified mosquito will need to be case‐specific – focusing on the species, the trait introduced and the environment where the species will be released. In the case of gene drive‐modified A. gambiae intended to reduce the incidence of malaria, quite a lot is known about the species based on a long history of control programmes intended to reduce its interactions with humans and subsequent disease transmission. Although this mosquito is a prey species for many generalist predators, experience with control programmes does not suggest removing it from the environment will have serious impacts on ecosystems (Collins et al., 2019). Nevertheless, it was generally agreed that future risk assessments should consider the potential for the introduction of a gene drive to affect biodiversity, for example, through the introduction of a toxic protein.
Perhaps unsurprisingly, most participants agreed that some attention was required to address the vectorial capacity of the organism and indicated that future risk assessment would need to incorporate consideration of whether the genetic modification could result in any increase in transmission or severity of malaria or other diseases that are vectored by the mosquito.
Because A. gambiae spends part of its life cycle in aquatic habitats, some participants identified the need to assess possible effects on water quality. However, it is worth noting that pathways to harm by which this might occur were difficult for most participants to generate, primarily because these mosquitoes live in small, frequently transient bodies of water closely associated with human habitation. Soil quality, air quality, agriculture and natural resources were by and large not considered pertinent.
3.3.3. Conclusions and recommendations
The introduction of any new technology is accompanied by questions about associated risks. The potential to use a gene drive incorporated into a disease vectoring mosquito to reduce the incidence of malaria represents a departure from earlier methods of vector control (such as chemical sprays, use of physical barriers, and environment modification to eliminate breeding habitat), as well as from earlier releases of GMOs into the environment, which have primarily involved the use of GMOs for spatially limited uses, including crops for agriculture or sterile insect releases (Frieß et al., 2019). Because this application of genetic technology is novel, a great deal of attention is being given to considerations around ERA. Efforts to engage with stakeholders around regulatory considerations will continue, and as they do so, it will be important to keep in mind what has been learned through earlier work.
First, although the particular application is novel, it can still be informed by experiences with classical vector control, and especially the use of exotic biological control organisms. While it may be true that engineered gene drives (excluding insects harbouring artificially acquired strains of Wolbachia) have not previously been released into the environment, there is ample experience with releasing organisms (and their genomes) into environments where they were not earlier present, and these experiences provide a good basis to identify potential risks. Second, many of the discussions around risk assessment for gene drive‐modified mosquitoes identified risks that are not associated with this specific technology but are common to any vector control programme. These include the potential negative consequences of removing the mosquito from the environment, and human health consequences if malaria is removed from the environment only to return after local immunity is reduced. These are important considerations as part of any control effort, but they should not be unnecessarily linked to any technology. Finally, as with any other ERA for a new technology, it will be critical for regulators and governments to clearly identify their environmental policy goals (and protection priorities). Agreement on protection goals will be a prerequisite for producing ERAs that can address them.
3.4. Problem formulation for the assessment of adverse effects of classical biological weed control agents on NTOs – a prospective analysis
3.4.1. Case study
When invasive alien weed species become established and spread to an extent that cannot be addressed by traditional control methods, classical biological control (CBC) can be considered. When invasive the biological control agent is sought from the suite of herbivores that have co‐evolved with the target weed in its native range (Thomas and Reid, 2007). The intent of this action, which has a long history, is for the biological control agent to become established, ideally after a single release, and persist in the environment, while bringing the target weed under permanent control with little or no further intervention (McFadyen, 1998). This is normally a public good activity funded by governments as there is no real opportunity for sales aside from redistribution of agents to areas from which they are absent. CBC against weeds has a very long history of application in much of the world, where over 500 biological control agents have been released against at least 220 species of weed in 130 countries (Winston et al., 2014). In contrast, there has been very little take up of CBC in Europe, and the only successful examples are the results of accidental introductions (Shaw et al., 2018).
3.4.2. Problem formulation considerations
CBC could be considered to have the potential for direct and indirect effects on the environment. Most of the focus in CBC is on the likelihood of NT impacts post‐release. The questions that may be raised and how they can be assessed – at least for an arthropod agent – are, in order of scale, direct effects (Figure 3) and indirect effects (Figure 4).
Figure 3.
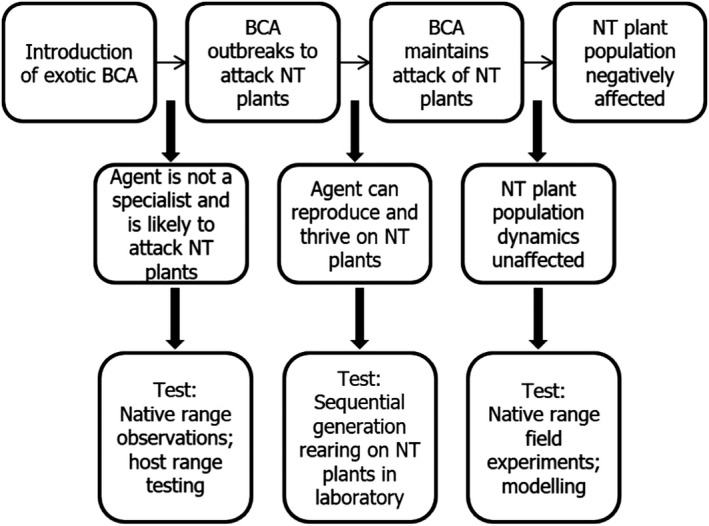
Pathway to harm for direct effects of a classical biological weed control agent (BCA: biological control agent; NT: non‐target)
Figure 4.
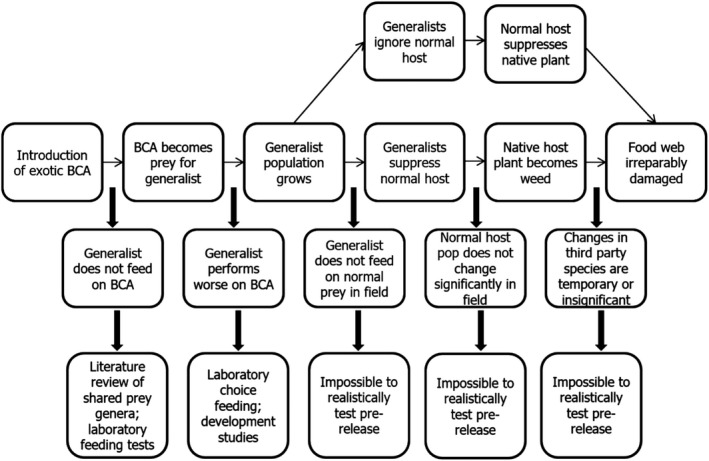
Pathway to harm for potential indirect effects of releasing a classical biological weed control agent (BCA: biological control agent; pop: populations)
For direct effect‐related questions, study methods for assessing specificity in the laboratory are well‐established. Moreover, they are recognised as being predictive of safety in the field, although rather conservative. The physiological host range of a herbivore, as revealed by feeding and survival studies carried out in the laboratory, tends to be larger than the one realised in the field post‐release and can be considered as rather conservative when assessing risk. It is common that false positives emerge that suggest a threat that is not realised once the agent is released.
While direct feeding effects are relatively easy to establish, the complexity of many food webs makes any indirect effects of a new organism much harder to predict and even study (Figure 4). Such effects are often described as apparent competition since the arrival of an organism that becomes a food source for another organism (predator/parasitoid) may have ripple effects on a tertiary organism(s) that the natural enemies normally feed upon and these can be negative or positive for that organism.
Many of the abovementioned questions have been considered during the research and licensing phase of the Japanese knotweed biocontrol programme which culminated in the release of the psyllid Aphalara itadori (Shinji) in England and Wales in 2010. The traditional host range testing carried out in CBC meant the rejection of many of the 186 species of arthropod herbivores that were recorded during the study in Japan (Shaw et al., 2009). Questions were raised about the potential secondary, tertiary and community‐level impacts of releasing the psyllid into the environment, i.e. indirect effects. The fact that it was a quarantine organism at the time made it impossible to carry out much more than preference studies using commercially available natural enemies in the laboratory, which showed no preference for such alternative prey over aphids, for example. Once the psyllid had been cleared for release, these results were reinforced by field cage studies with heavily augmented psyllid populations applied to natural knotweed stands. The psyllid has yet to establish reliably at most release sites and a new stock is likely to be needed.
CBC agents also come in the form of fungi (exotic and intended for single release) and for these the risks are arguably even lower as obligate biotrophs are the normal candidates. Rusts, smuts and powdery mildews are often remarkably specific, even to a subspecies level. Also, the likelihood of indirect effects is very low as they are a food source for very few organisms, and these are of little significance. A recent example is the release of the rust fungus Puccinia komarovii var. glanduliferae against Himalayan balsam (Impatiens glandulifera) in England and Wales (Tanner et al., 2015) which revealed variations in the target Himalayan balsam population and is effectively too specific for wide‐scale control. While establishment and spread are promising at some release sites, other varieties are being sought to target currently unsusceptible populations.
3.4.3. Conclusions and recommendations
Risk assessment in weed biocontrol can be straightforward as far as host range and direct NT safety is concerned, but once considerations of food web and community level predictions are required, our ability to assess these meaningfully all but vanishes. This is a consequence of the organisms being subject to quarantine requirements and the complexity of the field environment. One challenge facing researchers is how to detect and overcome false positives in safety testing due to laboratory artefacts, i.e. distinguishing the physiological vs the realised host range and therefore avoiding the unnecessary rejection of perfectly safe agents. There are numerous effective and safe weed biocontrol agents that were released decades ago that continue to do their job effectively and without significant negative impacts that would not have passed the current regulatory requirements. Unfortunately, the only successful weed biocontrol examples in the EU are the results of unintentional introductions of biological control agents (Shaw et al., 2018), and this may lead to more unauthorised introductions if the regulatory pathway is perceived as a hindrance.
Perhaps the most fundamental issue is to find a way for the benefits to be included in any risk assessment relating to invasive species since doing nothing is a high‐risk response. If we were to assess the risk of not releasing a biological control agent against an invasive weed that has not yet filled the whole of its potential ecological range, then it is virtually guaranteed that negative environmental impacts would be predicted to continue to increase along with the direct and indirect economic cost. Thankfully, this was recognised during the recent EFSA assessment of the proposal to release Trichilogaster acacealongifoliae against long‐leaved wattle Acacia longifolia in Portugal where it was recommended that experts should review the risk assessment, not recreate it, and that the review process needed to be rapid and include consideration of the potential benefits of the biological control agent introduction (EFSA, 2015b).
3.5. Problem formulation for the assessment of adverse effects of neonicotinoid insecticides on bees – a retrospective analysis
3.5.1. Case study
Pesticides, together with biocides, are the only chemicals deliberately introduced in the environment with the aim of exerting a toxic action. When used in agricultural settings in the form of PPPs, their targets are pests, i.e. any living organism that may cause a reduction of crop yield. However, once introduced in the environment, pesticides may pose a risk to NTOs.
Bees represent an important group of NTOs; they provide important ecological functions, sustaining ecosystem services (e.g. pollination, food production). Wild bee populations and managed bee colonies in Europe and in North America have been found to be under great pressure in the last decade, due to exposure to a wide range of stressors, some of which contributed to honeybee colony losses in many parts of the worlds. Since bees are often strongly linked to agricultural settings, assessing the risk posed by pesticide exposure to their health is extremely important.
EFSA is responsible for producing guidance documents for the ERA of PPPs in the EU. The role of these guidance documents is to provide clear and prescriptive indications on how to carry out ERA for NTOs. Therefore, problem formulation is not determined for every specific case, as the guidance documents define it a priori for all cases in most of its aspects. These include: identification of hazard and exposure pathways; definition of operational protection goals; formulation of risk hypotheses; and assessment methodology comprising different tiers.
The guidance document on the risk assessment of PPPs on bees (EFSA, 2013b; see also Rortais et al., 2017) has been used by EFSA for its recent conclusions on three neonicotinoid substances (EFSA, 2018a,b,c). This experience forms the basis for the analysis below, which, contrary to the previously presented case studies, has a retrospective focus.
3.5.2. Problem formulation considerations
The problem formulation for the ERA of PPPs on bees has been reviewed for three main areas: (1) identification of routes of exposure; (2) quantification of exposure; and (3) quantification of effects.
3.5.2.1. Identification of relevant routes of exposure
EFSA's bee risk assessment guidance considers several routes of exposure, some of which were already addressed in other guidance documents (EC, 2002; EPPO, 2010). These include: (1) contact exposure due to contamination of plant surfaces after sprayed pesticide applications; and (2) oral exposure due to pollen and nectar contamination following either spray applications or upward translocation (from soil, roots or seeds) of pesticides with systemic properties. Apart from these, explicit consideration of other routes of exposure, such as due to dust drift and guttation, has been introduced in EFSA (2013b).
Dust drift generally refers to mechanical abrasion of granular formulations or pesticide‐treated seeds that, during the sowing process, results into the blowing out of dust containing the active substance. The dust can deposit onto the vegetation surrounding the treated field, contaminating plant surfaces as well as nectar and pollen. The relevance of such a route of exposure became apparent in the late 2000s, when some important episodes of hive poisoning took place (Pistorius et al., 2008; Forster, 2010). Nonetheless, the quantification of exposure via dust drift depends on multiple variables, and hence, current estimations are complex and present considerable uncertainties.
Guttation is the exudation of drops of xylem sap on leaves of some vascular plants. Pesticides with systemic properties can be found in very high concentration in guttation fluids (Tapparo et al., 2011) and thus honeybees using guttation fluids as water supply may be harmed. As such, this route of exposure is explicitly considered in EFSA (2013b). However, while exposure via guttation fluids can be important for single honeybees, its relevance at the colony level is debatable for most crops, as the extent to which honeybees use guttation fluids for water provisioning is unclear. In addition, the current scheme for refining the exposure values for both dust deposition and guttation has proven impractical as it requires information on several variables for which data are difficult to gather at the EU level.
Despite the novelties introduced in EFSA (2013b), routes of exposure currently considered are mainly based on experience with honeybees and other bees with similar bioecological traits. Bee diversity is significant, and little is known on whether the evaluated routes of exposure are protective of others. Joint efforts coordinated by the USA's EPA (Bireley et al., 2019) recently produced important advancements in this respect (Gradish et al., 2018; Sgolastra et al., 2018), which should be considered in future developments.
3.5.2.2. Quantification of exposure
For a rather long time, the ERA for bees relied on the concept of hazard quotient, where exposure and effects are compared using different units (e.g. application rates in terms of weight/area vs. doses in terms of intake weight/bee), and the identification of trigger values implied the use of uncertain and complex calibration. The need to solve this mismatch had been recognised (e.g. see Barmaz et al., 2012; Rortais et al., 2017) before being addressed in EFSA (2013b), at least for oral exposure. Indeed, EFSA (2013b) introduced a quantification of exposure in terms of intake per bee, which is the same unit used to express thresholds of effects (i.e. ecotoxicological endpoints). Such quantification of the exposure makes an explicit consideration of multiple factors (residue levels, sugar content in nectar, sugar/pollen consumption, etc.), most of which can be refined through direct measurement. In addition, EFSA (2013b) introduced the concept of the exposure assessment goal, i.e. a use‐specific exposure level to consider in ERA. Exposure varies between different colonies/hives surrounding a certain treated area. Within such distribution of exposure, the assessment goal always represents the 90th percentile. The selection of this level is consistent across tiers. However, the actual quantification makes use of default values at tier 1, while it could be refined via direct measurements for higher tiers.
3.5.2.3. Quantification of effects
The core endpoint for the risk assessment is the colony strength/population abundance, for which EFSA (2013b); specifies clear thresholds of effects in the definition of operational protection goals. This endpoint is generally measured in higher tier studies (i.e. semi‐field and field studies), where actual hives/nests/populations are monitored. At the lower tiers of the assessment, the main measured effect is mortality at the level of individual organisms. For lethal effects to honeybees, the link with operational protection goals has been addressed via a simple deterministic model (Khoury et al., 2011). Hence, the link with the main operational protection goal is explicit and rather straightforward for lower tiers, albeit being somewhat oversimplified. While the conceptual link between mortality and colony strength/population abundance remains unaltered for higher tier studies, mortality measurements are more uncertain and unreliable in such experiments, especially for forager bees, owing to practical challenges posed by the experimental setup.
In contrast, the influence of lethal effects measured at lower tiers on colony/population performances is not explicitly addressed for non‐Apis bees, i.e. bumble bees and solitary bees.
Finally, for all bees, EFSA (2013b) recommends considering a wide range of sublethal effects. However, the link between such effects and the operational protection goal is not clear, and their use in ERAs is not explicitly addressed. Furthermore, reference thresholds for these effects are not explicitly mentioned.
3.5.3. Conclusions and recommendations
For the most recent ERAs of three neonicotinoid substances, EFSA performed an extensive literature review considering more than 1,500 documents (EFSA, 2018d). This review confirmed that the assessment scheme proposed in EFSA (2013b) considers all relevant exposure routes for honeybees. However, the relevance of some exposure routes currently considered (e.g. consumption of contaminated guttation fluids) still needs further investigation. Furthermore, other exposure routes potentially relevant for other bees need to be considered further in future developments. In general, the exposure characterisation presented in EFSA (2013b) made a huge advancement compared to the previously available assessment schemes. The refinement strategy for the exposure is logical and consistent. However, while the suggested approach is rather straightforward for oral exposure to contaminated pollen and nectar, it is significantly more problematic for other routes of exposure (i.e. guttation and dust drift), because of issues pertaining to the collection of the necessary amount of data.
Regarding effects characterisation, problem formulation resulted in a consistent scheme across tiers for endpoints (e.g. mortality) that have a direct link to the effects mentioned in the operational protection goal (colony strength/population abundance and forager mortality). However, the use of all other endpoints (mainly sub‐lethal) in ERA as recommended by EFSA (2013b) is considered is more problematic, requiring revision.
3.6. Considerations for the problem formulation for the ERA of nanomaterials
3.6.1. Case study
The foundation of nanotechnology is that engineering the size and shape of materials at the nanometre scale produces distinct, novel properties with potentially functional and commercial value (EC, 2011). The specific properties of nanomaterials and their resulting unique environmental behaviour and potential effects have led to concerns that current chemical‐based ERA methods, endpoints and approaches may not be adequate, and consequently, regulations [e.g. Registration, Evaluation, Authorisation and Restriction of Chemicals (EU REACH)] are being updated to cover nanospecific elements (EC, 2018). Progress is needed in the prediction of environmental distribution, concentration and form (speciation) of nanomaterials, to allow early assessment of potential environmental and human exposure, thereby ensuring the relevance of hazard data and subsequent potential risk calculations to facilitate safe product design.
In recent years, it has become clear that ranking toxicities of nanomaterials through the testing of ‘pristine’ or ‘as made’ particles in standard test media may not always provide relevant answers in terms of the environmental risk their released forms might represent. However, dealing with detailed physical and chemical characterisation of the multiple forms in which nanomaterials may be released from all stages (particle production, incorporation, use and disposal phases) of a nanoenabled product's life cycle is impossible. Also, it is clear that adequate, realistic and efficient risk assessment cannot be done by simply comparing predicted no‐effect concentrations (PNECs) from ‘laboratory tests with pristine nanomaterials’ with the predicted exposure concentrations (PECs) from mass flow‐based models that do not take into account how transformations of nanomaterials both pre‐ and post‐release to the environment may affect their fate and thus exposure forms and patterns. Fate processes and behaviour of the released materials depend on the new physical and chemical properties developed in such transformations. So, tracking such transformations in detail and doing so in environmentally relevant media and concentrations is technically challenging and resource intensive beyond most available means.
To address the abovementioned challenges, the EU H2020 project NanoFASE (http://www.NanoFASE.EU) has worked towards moving focus away from just the physical and chemical properties of pristine engineered nanomaterials (ENMs) as the driving parameters for fate modelling and towards developing an understanding of the functional and behaviour patterns of ‘release relevant ENMs’ in exposure‐relevant environments and chemistries, and how to include them in exposure modelling (see Williams et al., 2019). Achieving good ERAs means aiming to provide more realistic exposure scenarios and at the same time ensuring that the hazard test results used for PNECs do represent worst‐case exposure to the relevant nanoforms.
3.6.2. Problem formulation considerations
The problem formulation for the ERA of nanomaterials must ensure that the most relevant exposure predictions (form and concentrations) are considered, and that relevant and matching hazard data are derived and selected. For EFSA, nanomaterials are relevant in several food related products and applications (Peters et al., 2016). The sections below highlight key issues related to the ERA of nanomaterials through a series of recent (often non‐standardised) experiments and studies aimed at getting nanomaterial exposure and hazard estimates that are as relevant as possible. Issues will be highlighted through two examples falling respectively under plant protection and chemical contaminants.
3.6.2.1. Identification of relevant routes of exposure – release and environmental fate issues
Engineered nanomaterials have by now reached many everyday products and can be released from many points in ‘product value chains’; e.g. nanomaterial manufacture, incorporation into the product, daily use, recycling or waste handling (Pomar and Vazquez, 2019). Many of these releases will enter managed waste streams rather than go directly to the environment, but numerous nanomaterials will eventually enter wastewater treatment plants (WWTPs), and the transformation processes there drive the exposure‐relevant release forms (Adam et al., 2018). Within the WWTP, nanomaterials will react and transform, and the majority will sediment and be captured in the sludge, which is then used in many countries across Europe as valuable fertiliser in agriculture. The processes that affect EMNs, both in waste facilities and later in the environment, are currently not widely included either in research or regulatory fate and exposure models. At present, such inclusion is largely limited to aggregation processes and, to a lesser extent, dissolution. Transformation processes (e.g. sulphidation), the manufactured coatings, particle size distribution, shape and state are still usually excluded (Williams et al., 2019). These transformation and behavioural processes (e.g. dissolution, agglomeration and sedimentation) will in most cases completely transform the core material during the transit time in WWTPs (Gogos et al., 2017, 2018), and the WWTP will retain most of the nanomaterial mass within the sludge phase. The sludge that is transferred to agricultural land will contain an exposure‐relevant form of the ENM that is very different from the pristine material that was originally manufactured. Similar transformation issues will occur in soils and waters, and they will be important for driving the environmental exposure, uptake and eventual effects as detailed below (Hendren et al., 2013; Williams et al., 2019).
3.6.2.2. Quantification of exposure, uptake kinetics and effects
Nanomaterials can behave very differently from ‘classical chemicals’. Since the fate, availability, uptake and transport of the dissolved forms of classical chemicals follow mainly equilibrium kinetics, exposure can be expressed in concentrations. Nanomaterials on the other hand behave as suspended forms; most often they do not follow equilibrium, meaning that the kinetic fate descriptors are fluxes, which are based and often involve co‐transport with other suspended particulates (Praetorius et al., 2014).
The complexity of the problem formulation depends on whether nanomaterials are intentionally used as PPPs or unintentionally added, e.g. contained in sludge used as fertilisers. For the specific issues that relate to the ERA of nanoenabled pesticides, an overview undertaken by researchers and the Australian authorities can be found in Walker et al. (2018). As an example, the work of Elmer and White (2016) on the application side for plant protection using different fate and uptake behaviours of nanoforms is illustrative. The authors address the problem of interplay between plant nutrition and the ability of plants to combat root diseases. Many micronutrients (e.g. Cu, Mn, Zn, Mg, B, Si) stimulate or are part of plant defence systems, but these nutrients have limited availability to roots when delivered in soil. The study undertaken by Elmer and White (2016), investigated whether the metals could be added via topical leaf application and translocated to roots. It was found that a single foliar application of CuO (100 mg/L) to seedlings in the greenhouse before transfer to infested outdoor soils provided higher root Cu concentrations when applied in nanoform than as ions (dissolved salts) or in bulk form (large particles), and, importantly, that the nanoform resulted in greater disease suppression. Further work by Borgatta et al. (2018) shows that not only is nanoscale copper more effective, but also that it is tuneable: changing the composition and morphology of the nanoscale copper can enhance the effect. This clearly demonstrates that the fate and uptake kinetics of nanoforms of metals can lead to higher uptake and tissue concentration that can be employed beneficially. It also highlights that such differences must be considered during ERA if trying to cross read from data on dissolved forms.
On the chemical contaminant side, WWTP sludge being applied to agricultural land provides a good example for the implications for ERA processes. As covered in the previous section, the nanoforms released to the environment are likely very different from the originally produced so‐called ‘pristine’ ENM forms. For example, labile cores of Cu or Ag will often have been sulfidised and the original coatings and shapes degraded or modified (Gogos et al., 2017, 2018). The flow to various environmental compartments needs to be modelled carefully taking the different fate parameter of the different forms into account (Adam and Nowack, 2017; Adam et al., 2018; Williams et al., 2019). Once within the soil, the nanomaterial forms relevant to environmental exposure (i.e. the nanoforms actually added with the sludge) taken up by soil biota may vary significantly from those seen for dissolved forms or the pristine nanoforms. This has been demonstrated for earthworms exposed to the Ag2S forms of ENM for which body concentrations of silver reached only a tenth of that seen in the treatments exposed to pristine versions of the Ag ENM and ionic Ag forms (Baccaro et al., 2018). However, such effects are not simple or clear‐cut and, while saying that the aged nanoforms are less available and hazardous than the ionic counter parts may be true in most laboratory hazard studies, the paradigm does not always hold in more realistic exposure experiments. In a set of studies where the uptake and effects of metals (Ag and Zn) were studied in earthworms (Lahive et al., 2017) and clover plants (Judy et al., 2015) exposed to sludge generated in a pilot‐scale WWTP with three parallel lines with respectively, no metals (control), ionic metals or metals in nanoform added to the inflow. In the earthworms, uptake of Ag was higher after exposure to soil with sludge from the nanotreated line than the ionic, while for zinc, body concentrations matched for ionic and nano treatments, and the observed reduction in earthworm reproduction was significantly greater for the nanotreated sludge. Similarly, in the clover plants, the nanotreatment inhibited root nodulation more than the ionic metals and caused increased metal uptake. It is thus clear that the form in which the nanomaterials are presented to the organisms is of great importance. It should also be considered that, while standard hazard tests are designed to provide worst‐case exposure and hazard results to serve ERA conservativism, this may not be the case for nanomaterials owing to the slow dissolution kinetics in soils. A study by Diez‐Ortiz et al. (2015) undertook the standard 4‐week OECD 222 earthworm reproduction test in soils aged for up to a year after spiking with Ag in nano or ionic forms before testing. This showed the nanoform increasing in toxicity over time, with the EC50 reducing from 1,450 mg Ag/kg in the freshly spiked soils to 34 mg Ag/kg in soils aged 12 months before testing. In contrast, the EC50 for the ionic Ag treatment increased slightly from 49 mg Ag/kg in the freshly spiked soils to 104 mg Ag/kg in soils aged 12 months, as would be expected from the normal aging effects of metals in soils. Thus, hazard testing of nanomaterials must be undertaken bearing in mind the transformation and behavioural fate processes and ensuring the most exposure‐relevant forms are included in order to provide meaningful hazard data for ERAs.
3.6.3. Conclusions and recommendations
In conclusion, we must be aware that the ‘nano‐specific elements’ employed and the forms and their release routes to the environment will differ between intended use and the materials involved, and that this must be clearly addressed in the problem formulation for the ERA of nanomaterials. Because of this and the widespread use of nanomaterials in other sectors, exposure in the food supply may become significant. An understanding of fate processes, mechanisms of action and (biological) interaction is needed to enable accurate and responsible ERAs. Nanotechnology has the potential to improve agriculture, and trade‐offs against safety and uncertainty should be discussed when considering ERAs for these products.
3.7. Considerations for the problem formulation for landscape‐scale population‐level ERAs of PPPs
3.7.1. Problem formulation considerations
Current guidance on the ERA of PPPs has its focus on toxicology and environmental fate, but it is becoming increasingly clear that ecological aspects pertaining to landscape and populations cannot be ignored (Dalkvist et al., 2009). While ecological aspects are included in a general way in higher tiers, these lack the descriptive ability to capture feedback consequences between environmental context and behaviour. Landscape, its structure and function, has a very important role to play in determining the outcome of risk assessment on populations of NTOs, as has been acknowledged in recent EFSA outputs (EFSA, 2015a, 2016a, 2018e). The incorporation of landscape‐scale ERA is also part of EFSA's 2020 strategy. In addition, there is a shift in focus with time in the regulations and outputs of the European Commission. From Directive 91/414/EEC via the current Regulation (EC) No 1107/2009, to the latest outputs of the European Commission's Scientific Advice Mechanism (Group of Chief Scientific Advisors, 2018), there is an increasingly broad view, i.e. from focus on individual toxicity, to populations and towards a ‘One Health/One Environment’ concept linking human and environmental health with socio‐economic considerations at large scales.
This amounts to a paradigm shift in ERAs of PPPs (e.g. Streissl et al., 2018), and naturally, there are both opportunities to grasp and challenges to overcome when addressing this. A key issue is to define problem formulation in a way that is commensurate with larger scale concepts but still workable as a regulatory process. A confounding factor is that current ERA guidance for PPPs (EC, 2002) is a mixture of approaches, often derived from Directive 91/414/EEC, with different ERA approaches for different NTO groups, and those approaches are variously inconsistent with landscape or systems approaches. Therefore, the starting point for landscape‐based ERAs is not easily defined. Hence, we assume here that future intermediate‐state ERAs will be aimed at population impacts at landscape scales, taking account of realistic patterns of PPP use. While this will not cover all future ERA needs, it is a useful and widely applicable approach that covers individual effects to population impacts. Some key issues to consider in this process are addressed below.
3.7.1.1. Realistic use of pesticides
Realistic use combines both multiple applications of multiple PPPs and a spatiotemporal context. Several studies show that there is the potential for multiple impacts (additive or interactive) caused by mixtures, multiple applications, and seasonal application of PPPs (e.g. Fryday et al., 2011; Luttik et al., 2017). This is particularly important at a landscape scale when considering long‐term impacts on organisms because interactions between the spatial dynamics of organisms, population dynamics and the patterns of PPP use will determine overall population exposure and thus population‐level impacts. The immediate consequences of this, even when considering regulation of a single substance, is that the combined impact of the other stressors cannot be ignored when determining the vulnerability of NT populations (EFSA, 2016a).
3.7.1.2. Integration of multiple ERAs
Currently, exposure is calculated differently across a range of ERAs, and anomalies are often encountered, e.g. when species have different life stages. When needing to develop a combined population approach, it is not possible to assess compartments of the receiving environment independently: the combined effect, including interactions, needs to be considered. To achieve this, ERAs could integrate common factors using a standard framework. A key step would be integration of terrestrial and aquatic fate models.
3.7.1.3. Definition of population and operational protection goals
At landscape scale, the definition of a population must be considered carefully. This is part of the definition of operational protection goals (EFSA, 2016b), which attempts to relate ecosystem services to service‐providing units and defines attributes to protect (Devos et al., 2019). At landscape scale, this differs from the current non‐spatial or local‐spatial conceptual approach. In current guidance, terrestrial impacts of PPPs have been considered separately as either ‘in‐crop’ or ‘off‐crop’, and ERA schemes are designed accordingly. However, many populations are distributed across landscapes both within and outside crop areas. Both locations can be impacted by PPPs, even indirectly via ‘action at a distance’, which results from population spatial dynamics. Consequently, populations must be defined carefully (Brock et al., 2010; Topping et al., 2015). One definition might consider a population as being those individuals contained within a defined spatial area (e.g. a landscape). In this case, there is also a need to consider how to interpret protection goals in this context. For example, population effects of a certain magnitude could be considered acceptable. However, if measured at the landscape scale, local effects (e.g. in‐crop) may easily exceed acceptable effects due to buffering of large areas of ‘off‐crop’. In the case of operational protection goals based on ecosystem services (Nienstedt et al., 2012; Devos et al., 2015, 2019), this may invalidate the approach, if, for example, generalist predators are eradicated from fields. Conversely, an overall population impact at acceptable levels may produce unacceptable effects off‐crop due to re‐immigration. These off‐crop effects may be small and sustainable, but if allowable off‐crop effects are low, then the ERA may be overprotective. Therefore, in all cases, a careful consideration of the spatiotemporal nature of operational protection goals is needed, as well as of the metrics used to properly assess impacts in space and time (Nienstedt et al., 2012; EFSA, 2016b).
The choice of species used in ERA also needs to be carefully considered. For example, the rabbit may be used rather than the hare in toxicological studies, but for population effects, the use of the rabbit over the hare would be highly questionable when considering vulnerability (Topping and Weyman, 2018). The selection of the focal species should therefore focus more on population dynamics and less on (predicted) toxicological aspects. This also brings some new challenges since many studies have been conducted with established surrogate species, and data for new species may be lacking. This may be particularly true for new groups of conservation concern such as amphibians and reptiles (EFSA, 2018e) or bats.
3.7.1.4. Landscape context
In all cases, the context in which ERA should be carried out is critical. At landscape‐scale, this context is considerably more complex, and factors such as topographical structure, spatial distribution of field and non‐field, distribution and type of farmer and the crops they grow need to be added to the more traditional regulatory scenario issues that focus mainly on PPP application and assumptions of fate and toxicity. In fact, the number of variables is so great that defining a single realistic worst‐case scenario is not feasible. Therefore, there is no real alternative but to take a range of situations covering the range expected over which the PPP is used.
A second reason for this is that the interactions between landscape context and the ecology and behaviour of species result in difficulties in predicting in which landscapes the PPP will have the highest impacts. For example, when the same landscapes were used for evaluating PPP scenarios for newts and skylarks, landscape was a major factor in impact, but the pattern of effects was different between the two species (Topping and Luttik, 2017; EFSA PPR Panel, 2018e). Therefore, a range of contexts is needed to determine realistic effects.
3.7.2. Conclusions and recommendations
The landscape‐based approach may be a radical departure from traditional ERA, although the concept of a landscape ecotoxicology is not new (Cairns and Niederlehner, 1996); it is also in line with recent outputs of the European Commission (Group of Chief Scientific Advisors, 2018) and EFSA (e.g. EFSA, 2015a). However, to make more realistic long‐term assessments of overall PPP impact on the environment, it will be necessary to move from individual‐based single substance ERAs to large spatiotemporal scales, populations and communities, and multiple stressors (e.g. Rortais et al., 2017; Streissl et al., 2018).
The opportunities presented by this change are primarily related to being better able to predict risk related to the context in which animals find themselves, and to the interactions between stressors and animal populations in space and time. This has several key potential benefits:
This approach uses real landscapes simulated under realistic conditions. There is therefore the potential to link ERAs with post‐market monitoring data. Landscapes for ERA could also be chosen for monitoring, and this would allow evaluation of long‐term impacts resulting from regulatory decisions, similar to the suggestions by Streissl et al. (2018). This provides both a test of the ERA as well as allowing validation of the underlying models.
Since real landscapes are considered, ERA tools can be used to directly evaluate mitigation options. These options could be tailored to landscape contexts, which would require additional labelling. For example, PPPs may be authorised for use only in landscapes with a certain minimum percentage of ‘off‐crop’ area, or when using buffer strips of a specified minimum width. These specifications could be based on the landscape's resilience.
Cross‐compliance could be dealt with through this system. Linkage between the regulatory control of PPPs and the Common Agricultural Policy subsidy schemes and Water Framework Directive could be made by considering landscape management as a whole. Hence, subsidy schemes may provide the basis for implementing mitigation options, as well as the methods for policing them.
The efficiency of the ERA process may be increased. When simulating the system, many of the disparate ERA procedures currently used could be neatly harmonised. For example, aquatic concentrations of PPPs would be a natural outcome of terrestrial applications and occur in realistic patterns over time. The need to make statistical assumptions regarding distribution and exposure would be dramatically reduced because they would be mechanistically modelled, and the driving factors behind ERAs and human risk assessment from environmental exposure would be common to all.
Challenges are both technical and conceptual. The technical challenges relate to the need for accurate simulation of landscape structure and function, the development and testing of models, and obtaining the volume of data required to support these activities. The conceptual issues relate primarily to definition of operational protection goals at this scale, and decisions regarding choice of representative species and regulatory scenarios. The latter require careful attention, but once an ERA framework is defined for landscape assessment, these challenges should be relatively easily addressed.
Technical challenges may be larger and need a longer term view since considerable resources will be required. To start this process, several steps towards development of models suitable for supporting landscape‐scale ERA have already been taken by EFSA. EFSA is developing ApisRAM, an individual‐based honeybee model that will utilise landscape structure and dynamics to predict impacts of PPPs and other stressors on bees. Other models for mammals, birds, aquatic organisms and terrestrial arthropods already exist and are being adapted for use.
Currently, EFSA is working towards implementation of landscape‐scale ERAs for PPPs using a systems approach (EFSA, 2016a). This endeavour will use those building blocks currently available, and adapt them to provide a systems model that includes relevant local (Member State and regional) context. These building blocks include current models of NT animals and environmental fate models. It is planned that landscape simulation will use existing EU datasets (e.g. those held by the Joint Research Centre) to generate regulatory scenarios, and existing landscape simulation and landscape capture methods provided by the ALMaSS framework (Topping et al., 2016). This process has been initiated for restricted set of terrestrial mammal, bird and insect species including honeybees, but the aim in later developmental steps is to expand to aquatic and other systems.
Steps towards fully integrated landscape simulations combining aquatic and terrestrial ERAs with detailed interactions between components are still some way off, however, and would require significant research and development input to make the approach operational.
4. Conclusions
We have argued that applying problem formulation helps to frame ERAs and maximise the usefulness of ERA studies for decision‐making, through an iterative process. This is because: (1) harm is explicitly defined from the start; (2) the construction of risk hypotheses is guided by policy rather than an exhaustive attempt to address any possible differences; (3) existing information is used effectively; (4) new data are collected with a clear purpose; (5) risk is characterised against well‐defined criteria of hypothesis corroboration or falsification; and (6) risk assessment conclusions can be communicated clearly. By clearly identifying the extent to which harm becomes unacceptable (not all harms are equal; some may be more acceptable than others), considering scenarios that might lead to them, and developing testable hypotheses when necessary, risk assessments can be conducted in an open and transparent manner. In each case, problem formulation can help break down complex scenarios into more manageable elements, and thus provide a strategic approach to ERA. Moreover, it provides an easy‐to‐understand approach and a common language. These elements are especially useful for deliberations held in the context of multidisciplinary expert panels, novice risk assessors, or community/stakeholder/public engagement activities, as they help to work collegiately to organise existing knowledge into effective risk assessments and easily sort tangible concerns from those that are highly unlikely. They also help to communicate what should be assessed, why it should be assessed, and how it should be assessed, thereby greatly assisting the dialogue between risk assessors and risk managers. Finally, problem formulation offers the necessary flexibility to adapt to new circumstances, allowing for further evolution and improvement of ERAs and their harmonisation.
Problem formulation is still often hindered by the absence of clear policy goals and decision‐making criteria (e.g. definition of protection goals and what constitutes harm, limits or thresholds of concern, trigger values for action or acceptability of risk, judging the sufficiency of scientific knowledge and the extent to which uncertainty should be reduced for decision‐making) that are needed to guide the interpretation of scientific information (Evans et al., 2006; Hokanson et al., 2018). Even in jurisdictions with well‐developed regulatory systems, policy goals and decision‐making criteria are often defined in general terms, requiring refinement to make them operational for use in ERAs (Garcia‐Alonso and Raybould, 2014; Devos et al., 2015; Maltby et al., 2017a,b; Faber et al., 2019). If what constitutes harm is not defined, risk assessors face an extremely difficult or impossible task because there are no criteria to determine whether certain potential effects of a proposed activity are relevant to the risk assessment. Natural sciences can help risk assessors to predict whether there will be consequences of an activity, but cannot determine whether those consequences are acceptable (Devos et al., 2019). The absence of such definitions of harm is a symptom of what Evans et al. (2006) called the risk assessment–policy gap: the lack of clear policy objectives from which risk managers can set decision‐making criteria that can focus risk assessment on certain effects and exclude others as less important or irrelevant.
Not only is the definition of harm subjective and rooted in societal values (Sanvido et al., 2012), but also it cannot be deduced scientifically (Sarewitz, 2004). Consequently, risk managers must interpret the objectives of policy and regulations to define harm and the degree at which harm becomes unacceptable. This will provide a useful framework in which risk assessors can operate, recognising that there will always be some areas of uncertainty. Alternatively, risk assessors can elaborate different management options from which risk managers can select the most suitable one(s) (EFSA, 2016b).
Once definitions of ‘harm’, ‘benefit’, and ‘acceptability’ are in place, science can estimate the probability and severity of any harmful effects (i.e. assess the risk), and the probability and value of any beneficial effects (i.e. assess the opportunity) (Raybould and Macdonald, 2018).
A similar challenge for risk assessors is to know whether or when a risk hypothesis has been tested with sufficient rigour for decision‐making, and thus whether scientific uncertainty is unacceptably high and needs to be reduced (Hokanson et al., 2018; Luján and Todt, 2018). In the absence of such policy guidance, risk assessors can always suggest further studies because no hypothesis can ever be proved; some uncertainty will always remain. Besides improving the science used for ERAs, however, it is equally important to ensure that the risk assessment is consistent with the objectives of the guiding policy. We therefore advocate further dialogue between risk assessors and risk managers to clarify how ERAs can address policy goals and decision‐making criteria. Ideally, this dialogue should take place for all classes of regulated stressors, as this can promote alignment and consistency on the desired level of protection and maximum tolerable impacts across regulated stressors.
Abbreviations
- BCA
biological control agent
- CBC
classical biological control
- dsRNA
double‐stranded RNA
- ENM
engineered nanomaterial
- ERA
environmental risk assessment
- GH
growth hormone
- GM
genetically modified
- GMO
genetically modified organism
- mRNA
messenger RNA
- NEPAD
New Partnership for African Development
- NT
non‐target
- NTO
non‐target organism
- PEC
predicted exposure concentration
- PNEC
predicted no‐effect concentration
- PPP
plant protection product
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- RNAi
RNA interference
- siRNA
short‐interfering RNA
- US EPA
US Environmental Protection Agency
- WWTP
wastewater treatment plant
Suggested citation: Devos Y, Craig W, Devlin RH, Ippolito A, Leggatt RA, Romeis J, Shaw R, Svendsen C and Topping CJ, 2019. Using problem formulation for fit‐for‐purpose pre‐market environmental risk assessments of regulated stressors. EFSA Journal 2019;17(S1):e170708, 31 pp. 10.2903/j.efsa.2019.e170708
Acknowledgements: The European Food Safety Authority (EFSA) and authors wish to thank the participants of the breakout session ‘Advancing risk assessment science – Environment’ at EFSA's third Scientific Conference ‘Science, Food and Society’ (Parma, Italy, 18–21 September 2018) for their active and valuable contributions to the discussion. We also thank Mónica García‐Alonso and Andrew Roberts for their contributions to this publication, and Melanie Kah, Hans Verhagen and Jason White for carefully proofreading (parts of) it.
Disclaimer: The views or positions expressed in this article do not necessarily represent in legal terms the official position of the European Food Safety Authority (EFSA). EFSA assumes no responsibility or liability for any errors or inaccuracies that may appear. This article does not disclose any confidential information or data. Mention of proprietary products is solely for the purpose of providing specific information and does not constitute an endorsement or a recommendation by EFSA for their use.
Approved: 1 April 2019
Notes
All conference materials are available at https://www.efsa.europa.eu/en/events/event/180918
Also termed: Population reduction.
Also termed: Population modification, population alteration, population transformation or population conversion.
References
- Adam V and Nowack B, 2017. European country‐specific probabilistic assessment of nanomaterial flows towards landfilling, incineration and recycling. Environmental Science: Nano, 4, 1961–1973. 10.1039/C7EN00487G [DOI] [Google Scholar]
- Adam V, Caballero‐Guzman A and Nowack B, 2018. Considering the forms of released engineered nanomaterials in probabilistic material flow analysis. Environmental Pollution, 243(Part A), 17–27. 10.1016/j.envpol.2018.07.108 [DOI] [PubMed] [Google Scholar]
- Ahrens RN and Devlin RH, 2011. Standing genetic variation and compensatory evolution in transgenic organisms: a growth‐enhanced salmon simulation. Transgenic Research, 20, 583–597. 10.1007/s11248-010-9443-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia S, Birch ANE, Kiss J, van Loon JJA, Messéan A, Nuti M, Perry JN, Sweet JB and Tebbe CC, 2017. Assessing environmental impacts of genetically modified plants on non‐target organisms: the relevance of in planta studies. Science of Total Environment, 583, 123–132. 10.1016/j.scitotenv.2017.01.039 [DOI] [PubMed] [Google Scholar]
- Baccaro M, Undas AK, de Vriendt J, van den Berg JHJ, Peters RJB and van den Brink NW, 2018. Ageing, dissolution and biogenic formation of nanoparticles: how do these factors affect the uptake kinetics of silver nanoparticles in earthworms? Environmental Science: Nano, 5, 1107–1116. 10.1039/C7EN01212H [DOI] [Google Scholar]
- Bachman P, Bolognesi R, Moar WJ, Mueller GM, Paradise MS, Ramaseshadri P, Tan J, Uffman JP, Warren JA, Wiggins BE and Levine SL, 2013. Characterization of the spectrum of insecticidal activity of a double‐stranded RNA with targeted activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). Transgenic Research, 22, 1207–1222. 10.1007/s11248-013-9716-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman PM, Huizinga KM, Jensen PD, Mueller G, Tan J, Uffman JP and Levine SL, 2016. Ecological risk assessment for DvSnf7 RNA: a plant‐incorporated protectant with targeted activity against western corn rootworm. Regulatory Toxicology and Pharmacology, 81, 77–88. 10.1016/j.yrtph.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Barmaz S, Vaj C, Ippolito A and Vighi M, 2012. Exposure of pollinators to plant protection products. Ecotoxicology, 21, 2177–2185. 10.1007/s10646-012-0971-7 [DOI] [PubMed] [Google Scholar]
- Bartumeus F, Costa GB, Eritja R, Kelly AH, Finda M, Lezaun J, Okumu F, Megan Quinlan M, Thizy DC, Paré Toé L and Vaughan M, 2019. Sustainable innovation in vector control requires strong partnerships with communities. PLoS Neglected Tropical Diseases, 13, e0007204 10.1371/journal.pntd.0007204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum JA and Roberts JK, 2014. Progress towards RNAi‐mediated insect pest management. Advances in Insect Physiology, 47, 249–295. 10.1016/B978-0-12-800197-4.00005-1 [DOI] [Google Scholar]
- Bessey C, Devlin RH, Liley R and Biagi CA, 2004. Reproductive performance of growth‐enhanced transgenic coho salmon. Transactions of the American Fisheries Society, 133, 1205–1220. 10.1577/T04-010.1 [DOI] [Google Scholar]
- Bireley R, Borges S, Cham K, Epstein D, Garber K, Hart C, Hou W, Ippolito A, Pistorius J, Poulsen V, Sappington K and Steeger T, 2019. Preface: workshop on pesticide exposure assessment paradigm for non‐Apis bees. Environmental Entomology, 48, 1–3. 10.1093/ee/nvy134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi R, Ramaseshadri P, Anderson J, Bachman P, Clinton W, Flannagan R, Illagan O, Lawrence C, Levine S, Moar W, Mueller F, Tan J, Uffman J, Wiggins E, Heck G and Segers G, 2012. Characterizing the mechanism of action of double‐stranded RNA activity against Western Corn Rootworm (Diabrotica virgifera LeConte). PLoS ONE, 7, e47534 10.1371/journal.pone.0047534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatta J, Ma C, Hudson‐Smith N, Elmer W, Plaza Pérez CD, De La Torre‐Roche R, Zuverza‐Mena N, Haynes CL, White JC and Hamers RJ, 2018. Copper based nanomaterials suppress root fungal disease in watermelon (Citrullus lanatus): role of particle morphology, composition and dissolution behavior. ACS Sustainable Chemistry & Engineering, 6, 14847–14856. 10.1021/acssuschemeng.8b03379 [DOI] [Google Scholar]
- Brock TCM, Belgers JDM, Roessink I, Cuppen JGM and Maund SJ, 2010. Macroinvertebrate responses to insecticide application between sprayed and adjacent nonsprayed ditch sections of different sizes. Environmental Toxicology and Chemistry, 29, 1994–2008. 10.1002/etc.238 [DOI] [PubMed] [Google Scholar]
- Brossard D, Belluck P, Gould F and Wirz CD, 2019. Promises and perils of gene drives: navigating the communication of complex, post‐normal science. Proceedings of the National Academy of Sciences, 116, 7692–7697. 10.1073/pnas.1805874115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burand JP and Hunter WB, 2013. RNAi: future in insect management. Journal of Invertebrate Pathology, 112, S68–S74. 10.1016/j.jip.2012.07.012 [DOI] [PubMed] [Google Scholar]
- Burt A, Coulibaly M, Crisanti A, Diabate A and Kayondo JK, 2018. Gene drive to reduce malaria transmission in sub‐Saharan Africa. Journal of Responsible Innovation, 5, S66–S80. 10.1080/23299460.2017.1419410 [DOI] [Google Scholar]
- Cairns J and Niederlehner BR, 1996. Developing a field of landscape ecotoxicology. Ecological Applications, 6, 790–796. 10.2307/2269484 [DOI] [Google Scholar]
- Casacuberta JM, Devos Y, du Jardin P, Ramon M, Vaucheret H and Nogué F, 2015. Biotechnological uses of RNA interference in plants: risk assessment considerations. Trends in Biotechnology, 33, 145–147. 10.1016/j.tibtech.2014.12.003 [DOI] [PubMed] [Google Scholar]
- Champer J, Lee YL, Yang E, Liu C, Clark AG and Messer PW, 2019. A toxin‐antidote CRISPR gene drive system for regional population modification. BioRxiv, 628354, 10.1101/628354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY and Snow JW, 2017. Uptake and impact of natural diet‐derived small RNA in invertebrates: implications for ecology and agriculture. RNA Biology, 14, 402–414. 10.1080/15476286.2016.1248329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens O, Dzhambazova T, Kostov K, Arpaia S, Joga MR, Urru I, Sweet J and Smagghe G, 2018. Literature review of baseline information on RNAi to support the environmental risk assessment of RNAi‐based GM plants. EFSA Supporting Publications 2018;15(5):EN‐1424, 173 pp. 10.2903/sp.efsa.2018.EN-1424 [DOI] [Google Scholar]
- Collins CM, Bonds JA, Quinlan MM and Mumford JD, 2019. Effects of the removal or reduction in density of the malaria mosquito, Anopheles gambiae s.l., on interacting predators and competitors in local ecosystems. Medical and Veterinary Entomology, 33, 1–15. 10.1111/mve.12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W, Ndolo DO and Tepfer M, 2017. A Strategy for integrating science into regulatory decision‐making for GMOs In: Adenle AA, Morris EJ. and Murphy DJ. (eds.). Genetically Modified Organisms in Developing Countries: Risk Analysis and Governance. 1st edition Cambridge University Press, Cambridge, UK: pp. 26–38. [Google Scholar]
- Dalkvist T, Topping CJ and Forbes VE, 2009. Population‐level impacts of pesticide‐induced chronic effects on individuals depend more on ecology than toxicology. Ecotoxicology and Environmental Safety, 72, 1663–1672. 10.1016/j.ecoenv.2008.10.002 [DOI] [PubMed] [Google Scholar]
- Devlin RH, Yesaki TY, Biagi CA and Donaldson EM, 1994. Extraordinary salmon growth. Nature, 371, 209–210. 10.1038/371209a0 [DOI] [Google Scholar]
- Devlin RH, Biagi CA and Yesaki TY, 2004a. Growth, viability and genetic characteristics of GH transgenic coho salmon strains. Aquaculture, 236, 607–632. 10.1016/j.aquaculture.2004.02.026 [DOI] [Google Scholar]
- Devlin RH, D'Andrade M, Uh M and Biagi CA, 2004b. Population effects of growth hormone transgenic coho salmon depend on food availability and genotype by environment interactions. Proceedings of the National Academy of Sciences, 101, 9303–9308. 10.1073/pnas.0400023101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RH, Sakhrani D, Biagi CA and Eom K‐W, 2010. Occurrence of incomplete paternal‐chromosome retention in GH‐transgenic coho salmon being assessed for reproductive containment by pressure‐shock‐induced triploidy. Aquaculture, 304, 66–78. 10.1016/j.aquaculture.2010.03.023 [DOI] [Google Scholar]
- Devlin RH, Sundström LF and Leggatt RA, 2015. Assessing ecological and evolutionary consequences of growth‐accelerated genetically engineered fishes. BioScience, 65, 685–700. 10.1093/biosci/biv068 [DOI] [Google Scholar]
- Devos Y, Romeis J, Luttik R, Maggiore A, Perry JN, Schoonjans R, Streissl F, Tarazona JV and Brock TCM, 2015. Optimising environmental risk assessments: accounting for biodiversity and ecosystem services helps to translate broad policy protection goals into specific operational ones for environmental risk assessments. EMBO Reports, 16, 1060–1063. 10.15252/embr.201540874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos Y, Gaugitsch H, Gray AJ, Maltby L, Martin J, Pettis JS, Romeis J, Rortais A, Schoonjans R, Smith J, Streissl F and Suter GW II, 2016a. Advancing environmental risk assessment of regulated products under EFSA's remit. EFSA Journal, 14(S1), s0508 10.2903/j.efsa.2016.s0508 [DOI] [Google Scholar]
- Devos Y, Álvarez‐Alfageme F, Gennaro A and Mestdagh S, 2016b. Assessment of unanticipated unintended effects of genetically modified plants on non‐target organisms: a controversy worthy of pursuit? Journal of Applied Entomology, 140, 1–10. 10.1111/jen.12248 [DOI] [Google Scholar]
- Devos Y, Ortiz‐García S, Hokanson KE and Raybould A, 2018. Teosinte and maize × teosinte hybrid plants in Europe – environmental risk assessment and management implications for genetically modified maize. Agriculture, Ecosystems & Environment, 259, 19–27. 10.1016/j.agee.2018.02.032 [DOI] [Google Scholar]
- Devos Y, Munns WR Jr, Forbes VE, Maltby L, Stenseke M, Brussaard L, Streissl F and Hardy A, 2019. Applying ecosystem services for pre‐market environmental risk assessments of regulated stressors. EFSA Journal, Special Issue July 2019, Third EFSA Conference on Science, Food and Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez‐Ortiz M, Lahive E, George S, Ter Schure A, Van Gestel CAM, Jurkschat K, Svendsen C and Spurgeon DJ, 2015. Short‐term soil bioassays may not reveal the full toxicity potential for nanomaterials: bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long‐term aged soils. Environmental Pollution, 203, 191–198. 10.1016/j.envpol.2015.03.033 [DOI] [PubMed] [Google Scholar]
- EC (European Commission, Health and Consumer Protection Directorate‐General), 2002. Guidance document on terrestrial ecotoxicology under Council Directive 91/414/EEC. Available online: https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_ppp_app-proc_guide_ecotox_terrestrial.pdf
- EC (European Commission), 2011. Commission Recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU). Official Journal of the European Union, L275, 38–40. Available online: https://ec.europa.eu/research/industrial_technologies/pdf/policy/commission-recommendation-on-the-definition-of-nanomater-18102011_en.pdf
- EC (European Commission), 2018. Commission Regulation (EU) 2018/1881 of 3 December 2018 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Annexes I, III, VI, VII, VIII, IX, X, XI, and XII to address nanoforms of substances. Official Journal of the European Union, L308, 1–20. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1881&from=EN [Google Scholar]
- Eckhoff PA, Wenger EA, Godfray HCJ and Burt A, 2017. Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics. Proceedings of the National Academy of Sciences, 114, E255–E264. 10.1073/pnas.1611064114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Guidance on the environmental risk assessment of genetically modified plants. EFSA Journal 2010;8(11):1879, 111 pp. 10.2903/j.efsa.2010.1879 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013a. Guidance on the environmental risk assessment of genetically modified animals. EFSA Journal 2013;11(5):3200, 190 pp. 10.2903/j.efsa.2013.3200 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2013b. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. International scientific workshop ‘Risk assessment considerations for RNAi‐based GM plants’ (4–5 June 2014, Brussels, Belgium). EFSA Supporting Publication 2014;11(12):EN‐705, 38 pp. 10.2903/sp.efsa.2014.en-705 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2015a. Scientific opinion addressing the state of the science on risk assessment of plant protection products for non‐target arthropods. EFSA Journal 2015;13(2):3996, 212 pp. 10.2903/j.efsa.2015.3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015b. Statement on the assessment of the risk posed to plant health in the EU territory by the intentional release of biological control agents of invasive alien plant species. EFSA Journal 2015;13(6):4134, 12 pp. 10.2903/j.efsa.2015.4134 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016a. Recovery in environmental risk assessments at EFSA. EFSA Journal 2015;14(2):4313, 85 pp. 10.2903/j.efsa.2016.4313 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016b. Guidance to develop specific protection goals options for environmental risk assessment at EFSA, in relation to biodiversity and ecosystem services. EFSA Journal 2016;14(6):4499, 50 pp. 10.2903/j.efsa.2016.4499 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018a. Evaluation of the data on clothianidin, imidacloprid and thiamethoxam for the updated risk assessment to bees for seed treatments and granules in the EU. EFSA Supporting Publication 2018;15(2):EN‐1378, 31 pp. 10.2903/sp.efsa.2018.en-1378 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2018b. Peer review of the pesticide risk assessment for bees for the active substance clothianidin considering the uses as seed treatments and granules. EFSA Journal 2018;16(2):5177, 86 pp. 10.2903/j.efsa.2018.5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018c. Peer review of the pesticide risk assessment for bees for the active substance imidacloprid considering the uses as seed treatments and granules. EFSA Journal 2018;16(2):5178, 113 pp. 10.2903/j.efsa.2018.5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018d. Peer review of the pesticide risk assessment for bees for the active substance thiamethoxam considering the uses as seed treatments and granules. EFSA Journal 2018;16(2):5179, 59 pp. 10.2903/j.efsa.2018.5179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues), 2018e. Scientific Opinion on the state of the science on pesticide risk assessment for amphibians and reptiles. EFSA Journal 2018;16(2):5125, 301 pp. 10.2903/j.efsa.2018.5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer WH and White JC, 2016. The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environmental Science: Nano, 3, 1072–1079. 10.1039/C6EN00146G [DOI] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), 2010. Chapter 10: honeybees. EPPO Bulletin, 40, 323–331. 10.1111/j.1365-2338.2010.02419.x [DOI] [Google Scholar]
- Evans J, Wood G and Miller A, 2006. The risk assessment–policy gap: an example from the UK contaminated land regime. Environment International, 32, 1066–1071. [DOI] [PubMed] [Google Scholar]
- Faber JH, Marshall S, Van den Brink PJ and Maltby L, 2019. Priorities and opportunities in the application of the ecosystem services concept in risk assessment for chemicals in the environment. Science of the Total Environment, 651, 1067–1077. 10.1016/j.envint.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JL, Akbarashandiz H, Sakhrani D, Biagi CA, Pitcher TE and Devlin RH, 2011. Cultured growth hormone transgenic salmon are reproductively out‐competed by wild‐reared salmon in semi‐natural mating arenas. Aquaculture, 312, 185–191. 10.1016/j.aquaculture.2010.11.044 [DOI] [Google Scholar]
- Forster R, 2010. Bee poisoning caused by insecticidal seed treatment of maize in Germany in 2008. Proceedings of the Hazards of pesticides to bees – 10th International Symposium of the ICP‐Bee Protection Group, Bucharest (Romania), 126–131. Available online: https://www.researchgate.net/#
- Frieß JL, von Gleich A and Giese B, 2019. Gene drives as a new quality in GMO releases – a comparative technology characterization. PeerJ, 7, e6793 10.7717/peerj.6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryday S, Thompson H and Garthwaite D, 2011. Background information for considering risk of exposure to multiple pesticides. DEFRA Project PS2354, DEFRA, York, UK: 33 pp. Available online: http://randd.defra.gov.uk/Document.aspx?Document=sid5PS2354.doc [Google Scholar]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E and James AA, 2015. Highly efficient Cas9‐mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi . Proceedings of the National Academy of Sciences, 112, E6736–E6743. 10.1073/pnas.1521077112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Alonso M, 2013. Safety assessment of food and feed derived from GM crops: using problem formulation to ensure “fit for purpose” risk assessments. Collection of Biosafety Reviews, 8, 72–101. Available online: https://www.icgeb.org/wp-content/uploads/2019/02/4-Safety-Assessment-of-Food-and-Feed-Derived-from-GM-Crops_-Using-Problem-Formulation-to-Ensure-Fit-for-Purpose-Risk-Assessment-Monica-Garcia-Alonso.pdf [Google Scholar]
- Garcia‐Alonso M and Raybould A, 2014. Protection goals in environmental risk assessment: a practical approach. Transgenic Research, 23, 945–956. 10.1007/s11248-013-9760-1 [DOI] [PubMed] [Google Scholar]
- Garcia‐Alonso M, Jacobs E, Raybould A, Nickson TE, Sowig P, Willekens H, Van der Kouwe P, Layton R, Amijee F, Fuentes AM and Tencalla F, 2006. A tiered system for assessing the risk of genetically modified plants to non‐target organisms. Environmental Biosafety Research, 5, 57–65. 10.1051/ebr:2006018 [DOI] [PubMed] [Google Scholar]
- Glover KA, Bos JB, Urdal K, Madhun AS, Sørvik AGE, Unneland L, Seliussen BB, Skaala Ø, Skilbrei OT, Tang Y and Wennevik V, 2016. Genetic screening of farmed Atlantic salmon escapees demonstrates that triploid fish display reduced migration to freshwater. Biological Invasions, 18, 1287–1294. 10.1007/s10530-016-1066-9 [DOI] [Google Scholar]
- Glover KA, Solberg MF, McGinnity P, Hindar K, Verspoor E, Coulson MW, Hansen MM, Araki H, Skaala Ø and Svåsand T, 2017. Half a century of genetic interaction between farmed and wild Atlantic salmon: status of knowledge and unanswered questions. Fish and Fisheries, 18, 890–927. 10.1111/faf.12214 [DOI] [Google Scholar]
- Gogos A, Thalmann B, Voegelin A and Kaegi R, 2017. Sulfidation kinetics of copper oxide nanoparticles. Environmental Science: Nano, 4, 1733–1741. 10.1039/C7EN00309A [DOI] [Google Scholar]
- Gogos A, Voegelin A and Kaegi R, 2018. Influence of organic compounds on the sulfidation of copper oxide nanoparticles. Environmental Science: Nano, 5, 2560–2569. 10.1039/C8EN00523K [DOI] [Google Scholar]
- Gould F, Huang Y, Legros M and Lloyd AL, 2008. A killer‐rescue system for self‐limiting gene drive of anti‐pathogen constructs. Proceedings of the Royal Society: Biological Sciences, 275, 2823–2829. 10.1098/rspb.2008.0846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradish AE, van der Steen J, Scott‐Dupree CD, Cabrera AR, Cutler GC, Goulson D, Klein O, Lehmann DM, Lückmann J, O'Neill B, Raine NE, Sharma B and Thompson H, 2018. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): implications for risk assessments. Environmental Entomology, 48, 12–21. 10.1093/ee/nvy168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray AJ, 2012. Problem formulation in environmental risk assessment for genetically modified crops: a practitioner's approach. Collection of Biosafety Reviews, 6, 10–65. Available online: https://pdfs.semanticscholar.org/6007/dd3651aac058e3e40d796f3f9d2c32bd5aad.pdf [Google Scholar]
- Gray AJ, 2014. The policy chicken and the science egg. Has applied ecology failed the transgenic crop debate? Transgenic Research, 23, 923–932. 10.1007/s11248-013-9747-y [DOI] [PubMed] [Google Scholar]
- Group of Chief Scientific Advisors , 2018. EU authorisation processes of plant protection products – from a scientific point of view. Scientific Opinion, 05/2018. European Commission, Brussels, Belgium: Available online: https://ec.europa.eu/research/sam/pdf/sam_ppp_report.pdf [Google Scholar]
- Guichard A, Haque T, Bobik M, Xu X‐RS, Klanseck C, Kushwah RBS, Berni M, Kaduskar B, Gantz VM and Bier E, 2019. Efficient allelic‐drive in Drosophila . Nature Communications, 10, 1640 10.1038/s41467-019-09694-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller S, Widmer F, Siegfried BD, Zhou X and Romeis J, 2019. Responses of two ladybird beetle species (Coleoptera: Coccinellidae) to dietary RNAi. Pest Management Science. 10.1002/ps.5370 [DOI] [PubMed] [Google Scholar]
- Hammond AM and Galizi R, 2017. Gene drives to fight malaria: current state and future directions. Pathogens and Global Health, 111, 412–423. 10.1080/20477724.2018.1438880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley S, Thizy DC, Ledingham K, Coulibaly M, Diabate A, Dicko B, Diop S, Kayondo J, Namukwaya A, Nourou B and Paré Toé L, 2019. Knowledge engagement in gene drive research for malaria control. PLoS Neglected Tropical Diseases, 13, e0007233 10.1371/journal.pntd.0007233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes KR, Hosack GR, Dana GV, Foster SD, Ford JH, Thresher R, Ickowicz A, Peel D, Tizard M, De Barro P, Strive T and Dambacher JM, 2018. Identifying and detecting potentially adverse ecological outcomes associated with the release of gene‐drive modified organisms. Journal of Responsible Innovation, 5(Supl. 1), S139–S158. 10.1080/23299460.2017.1415585 [DOI] [Google Scholar]
- Hendren CO, Lowry M, Grieger KD, Money ES, Johnston JM, Wiesner MR and Beaulieu SM, 2013. Modelling approaches for characterizing and evaluating environmental exposure to engineered nanomaterials in support of risk‐based decision making. Environmental Science & Technology, 47, 1190–1205. 10.1021/es302749u [DOI] [PubMed] [Google Scholar]
- Hokanson KE, Ellstrand N and Raybould A, 2018. The integration of science and policy in regulatory decision‐making: observations on scientific expert panels deliberating GM crops in centers of diversity. Frontiers in Plant Science, 9, 1157 10.3389/fpls.2018.01157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashuta S, Zhang Y, Wiggins BE, Ramaseshadri P, Segers GC, Johnson S, Meyer SE, Kerstetter RA, McNulty BC, Bolognesi R and Heck GR, 2015. Environmental RNAi in herbivorous insects. RNA, 21, 840–850. 10.1261/rna.048116.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, Collins FH, Welkhoff PA, Emerson C, Godfray HCJ, Gottlieb M, Greenwood B, Lindsay SW, Mbogo CM, Okumu FO, Quemada H, Savadogo M, Singh JA, Tountas KH and Touré YT, 2018. Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in Sub‐Saharan Africa: recommendations of a scientific working group. The American Journal of Tropical Medicine and Hygiene, 98(6 Suppl.), 1–49. 10.4269/ajtmh.18-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Raybould A, Hudson MD and Poppy GM, 2007. How does scientific risk assessment of GM crops fit within the wider risk analysis? Trends in Plant Science, 12, 1–5. 10.1016/j.tplants.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Judy JD, McNear DH Jr, Chen C, Lewis RW, Tsyusko OV, Bertsch PM, Rao W, Stegemeier J, Lowry GV, McGrath SP, Durenkamp M and Unrine JM, 2015. Nanomaterials in biosolids inhibit nodulation, shift microbial community composition, and result in increased metal uptake relative to bulk/dissolved metals. Environmental Science & Technology, 49, 8751–8758. 10.1021/acs.est.5b01208 [DOI] [PubMed] [Google Scholar]
- Khoury DS, Myerscough MR and Barron AB, 2011. A quantitative model of honey bee colony population dynamics. PLoS ONE, 6, e18491 10.1371/journal.pone.0018491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Naish KA and Devlin RH, 2018. Influence of a growth hormone transgene on the genetic architecture of growth‐related traits: a comparative analysis between transgenic and wild‐type coho salmon. Evolutionary Applications, 11, 1886–1900. 10.1111/eva.12692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler N, Collins JP, Kuzma J, Marris E, Esvelt K, Nelson MP, Newhouse A, Rothschild LJ, Vigliotti VS, Semenov M, Jacobsen R, Dahlman JE, Prince S, Caccone A, Brown T and Schmitz OJ, 2018. Editing nature: local roots of global governance. Science, 362, 527–529. 10.1126/science.aat4612 [DOI] [PubMed] [Google Scholar]
- Kuzma J, 2019. Procedurally robust risk assessment framework for novel genetically engineered organisms and gene drives. Regulation & Governance. [Epub ahead of print: https://onlinelibrary.wiley.com/doi/epdf/10.1111/rego.12245]. 10.1111/rego.12245 [DOI] [Google Scholar]
- Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T and Crisanti A, 2018. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nature Biotechnology, 36, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahive E, Matzke M, Durenkamp M, Lawlor AJ, Thacker SA, Pereira MG, Spurgeon DJ, Unrine JM, Svendsen C and Lofts S, 2017. Sewage sludge from treated with metal nanomaterials inhibits earthworm reproduction more strongly than sludge treated with metal metals in bulk/salt forms. Environmental Science: Nano, 4, 78–88. 10.1039/C6EN00280C [DOI] [Google Scholar]
- Lanzoni A, Castoldi AF, Kass GEN, Terron A, De Seze G, Bal‐Price A, Bois FY, Delclos KB, Doerge DR, Fritsche E, Halldorsson T, Kolossa‐Gehring M, Hougaard Bennekou S, Koning F, Lampen A, Leist M, Mantus E, Rousselle C, Siegrist M, Steinberg P, Tritscher A, Van de Water B, Vineis P, Walker N, Wallace H, Whelan M and Younes M, 2019. Advancing human health risk assessment. EFSA Journal, Special Issue July 2019, Third EFSA Conference on Science, Food and Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton R, Smith J, Macdonald P, Letchumanan R, Keese P and Lema M, 2015. Building better environmental risk assessments. Frontiers in Bioengineering and Biotechnology, 3, 110 10.3389/fbioe.2015.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggatt RA, O'Reilly PT, Blanchfield PJ, Mckindsey CW and Devlin RH, 2010. Pathway of effects of escaped aquaculture organisms or their reproductive material on natural ecosystems in Canada. Fisheries and Oceans Canada, Canadian Science Advisory Secretariat. Research Document 2010/019, pp. vi + 70. Available online: http://waves-vagues.dfo-mpo.gc.ca/Library/342193.pdf
- Leggatt RA, Hollo T, Vandersteen WE, McFarlane K, Goh B, Prevost J and Devlin RH, 2014. Rearing in seawater mesocosms improves the spawning performance of growth hormone transgenic and wild‐type coho salmon. PLoS ONE, 9, e105377 10.1371/journal.pone.0105377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggatt RA, Biagi CA, Sakhrani D, Dominelli R, Eliason EJ, Farrell AP and Devlin RH, 2017. Fitness component assessments of wild‐type and growth hormone transgenic coho salmon reared in seawater mesocosms. Aquaculture, 473, 31–42. 10.1016/j.aquaculture.2017.01.022 [DOI] [Google Scholar]
- Luján JL and Todt O, 2018. The dilemmas of science for policy: scientific evidence and the consequences of regulatory options in risk and benefit assessment. EMBO Reports, 19, 194–196. 10.15252/embr.201744795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren JG and Duan JJ, 2013. RNAi‐based insecticidal crops: potential effects on nontarget species. BioScience, 63, 657–665. 10.1525/bio.2013.63.8.8 [DOI] [Google Scholar]
- Luttik R, Zorn MI, Brock TCM, Roex EWM and van der Linden AMA, 2017. Multiple stress by repeated use of plant protection products in agricultural areas. RIVM report, 2016‐0152. National Institute for Public Health and the Environment, Bilthoven, The Netherlands: 70 pp. Available online: https://www.rivm.nl/bibliotheek/rapporten/2016-0152.pdf [Google Scholar]
- Maltby LL, Duke C and van Wensem J, 2017a. Ecosystem services, environmental stressors and decision making: how far have we got? Integrated Environmental Assessment and Management, 13, 38–40. 10.1002/ieam.1796 [DOI] [PubMed] [Google Scholar]
- Maltby L, Jackson M, Whale G, Brown AR, Hamer M, Solga A, Kabouw P, Woods R and Marshall S, 2017b. Is an ecosystem services‐based approach developed for setting specific protection goals for plant protection products applicable to other chemicals? Science of the Total Environment, 580, 1222–1236. 10.1016/j.scitotenv.2016.12.083 [DOI] [PubMed] [Google Scholar]
- McFadyen REC, 1998. Biological control of weeds. Annual Review of Entomology, 43, 369–393. 10.1146/annurev.ento.43.1.369 [DOI] [PubMed] [Google Scholar]
- Murray JV, Jansen CC and De Barro P, 2016. Risk associated with the release of Wolbachia‐infected Aedes aegypti mosquitoes into the environment in an effort to control dengue. Frontiers in Public Health, 4, 43 10.3389/fpubh.2016.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASEM (National Academies of Sciences, Engineering, and Medicine), 2016. Gene drives on the horizon: advancing science, navigating uncertainty, and aligning research with public values. National Academies Press, Washington, DC: 230 pp. [PubMed] [Google Scholar]
- Nash A, Urdaneta GM, Beaghton AK, Hoermann A, Papathanos PA, Christophides GK and Windbichler N, 2019. Integral gene drives for population replacement. Biology Open, 8, pii: bio037762 10.1242/bio.037762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KC, Basiao Z, Cooper A, Dey M, Fonticiella D, Hernandez ML, Kunawasen S, Leelapatra W, Li S, Ratner BD and Toledo MI, 2007. Problem formulation and options assessment (PFOA): science‐guided deliberation in ecological risk assessment of transgenic fish In: Kapuscinski AR, Hayes KR, Li S. and Dana G. (eds.). Environmental Risk Assessment of Genetically Modified Organisms: Methodologies for Transgenic Fish. 1st Edition CABI Publications, Wallingford, UK: pp. 29–60. Available online: https://gmoera.umn.edu/sites/gmoera.umn.edu/files/environmental_risk_assessment_volume_3.pdf [Google Scholar]
- Nickson TE, 2008. Planning environmental risk assessment for genetically modified crops: problem formulation for stress‐tolerant crops. Plant Physiology, 147, 494–502. 10.1104/pp.108.118422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienstedt KM, Brock TCM, van Wensem J, Montforts W, Hart A, Aagaard A, Alix A, Boesten J, Bopp SK, Brown C, Capri E, Forbes V, Köpp H, Liess M, Luttik R, Maltby L, Sousa JP, Streissl F and Hardy AR, 2012. Development of a framework based on an ecosystem services approach for deriving specific protection goals for environmental risk assessment of pesticides. Science of the Total Environment, 415, 31–38. 10.1016/j.scitotenv.2011.05.057 [DOI] [PubMed] [Google Scholar]
- Noble C, Min J, Olejarz J, Buchthal J, Chavez A, Smidler AL, DeBenedictis EA, Church GM, Nowak MA and Esvelt KM, 2019. Daisy‐chain gene drives for the alteration of local populations. Proceedings of the National Academy of Sciences of the United States of America, 116, 8275–8282. 10.1073/pnas.1716358116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North AR, Burt A and Godfray HCJ, 2019. Modelling the potential of genetic control of malaria mosquitoes at national scale. BMC Biology, 17, 26 10.1186/s12915-019-0645-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Yang X, Bidne K, Hellmich RL, Siegfried BD and Zhou X, 2017. Dietary risk assessment of v‐ATPase A dsRNAs on monarch butterfly larvae. Frontiers in Plant Science, 8, 242 10.3389/fpls.2017.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters RJB, Bouwmeester H, Gottardo S, Amenta V, Arena M, Brandhoff P, Marvin HJP, Mech A, Botelho Moniz F, Quiros Pesudo L, Rauscher H, Schoonjans R, Undas AK, Vittoria Vettori M, Weigel S and Aschberger K, 2016. Nanomaterials for products and application in agriculture, feed and food. Trends in Food Science & Technology, 54, 155–164. 10.1016/j.tifs.2016.06.008 [DOI] [Google Scholar]
- Pistorius J, Bischoff G, Heimbach U and Stähler M, 2008. Bee poisoning incidents in Germany in spring 2008 caused by abrasion of active substance from treated seeds during sowing of maize. Proceedings of the Hazards of pesticides to bees – 10th International Symposium of the ICP‐Bee Protection Group, Bucharest (Romania), 118–126. Available online: https://ojs.openagrar.de/index.php/JKA/article/view/142/127
- Pomar V and Vazquez S, 2019. NanoFASE deliverable D4.2: release estimations during ENMs and nano‐enabled products value chain. EU NanoPHASE Project. Available online: http://nanofase.eu/getatt.php?filename=NanoFASE_D4.2_Short_Deliverable_Release%20estimations%20during%20ENMs%20and%20nano-enabled%20products%20value%20chain_Web_Summary_1799.pdf
- Praetorius A, Tufenkji N, Goss K‐U, Scheringer M, von der Kammer F and Elimelech M, 2014. The road to nowhere: equilibrium partition coefficients for nanoparticles. Environmental Science: Nano, 1, 317–323. 10.1039/C4EN00043A [DOI] [Google Scholar]
- Ramon M, Devos Y, Lanzoni A, Liu Y, Gomes A, Gennaro A and Waigmann E, 2014. RNAi‐based GM plants: food for thought for risk assessors. Plant Biotechnology Journal, 12, 1271–1273. 10.1111/pbi.12305 [DOI] [PubMed] [Google Scholar]
- Raybould A, 2006. Problem formulation and hypothesis testing for environmental risk assessments of genetically modified crops. Environmental Biosafety Research, 5, 119–125. 10.1051/ebr:2007004 [DOI] [PubMed] [Google Scholar]
- Raybould A, 2007. Ecological versus ecotoxicological methods for assessing the environmental risks of transgenic crops. Plant Science, 173, 589–602. 10.1016/j.plantsci.2007.09.003 [DOI] [Google Scholar]
- Raybould A, 2010. The bucket and the searchlight: formulating and testing risk hypotheses about the weediness and invasiveness potential of transgenic crops. Environmental Biosafety Research, 9, 123–133. 10.1051/ebr/2011101 [DOI] [PubMed] [Google Scholar]
- Raybould A and Macdonald P, 2018. Policy‐led comparative environmental risk assessment of genetically modified crops: testing for increased risk rather than profiling phenotypes leads to predictable and transparent decision‐making. Frontiers in Bioengineering and Biotechnology, 6, 43 10.3389/fbioe.2018.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AF, Devos Y, Lemgo GNY and Zhou X, 2015. Biosafety research for non‐target organism risk assessment of RNAi‐based GE plants. Frontiers in Plant Sciences, 6, 958 10.3389/fpls.2015.00958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Paes de Andrade P, Okumu F, Quemada H, Savadogo M, Amir Singh J and James S, 2017. Results from the workshop “Problem formulation for the use of gene drive in mosquitoes”. The American Journal of Tropical Medicine and Hygiene, 96, 530–533. 10.4269/ajtmh.16-0726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J, Bartsch D, Bigler F, Candolfi MP, Gielkens M, Hartley SE, Hellmich RL, Huesing JE, Jepson PC, Layton R, Quemada H, Raybould A, Rose RI, Schiemann J, Sears MK, Shelton AM, Sweet J, Vaituzis Z and Wolt JD, 2008. Assessment of risk of insect‐resistant transgenic crops to nontarget arthropods. Nature Biotechnology, 26, 203–208. 10.1038/nbt1381 [DOI] [PubMed] [Google Scholar]
- Romeis J, Hellmich RL, Candolfi MP, Carstens K, De Schrijver A, Gatehouse AMR, Herman RA, Huesing JE, McLean MA, Raybould A, Shelton AM and Waggoner A, 2011. Recommendations for the design of laboratory studies on non‐target arthropods for risk assessment of genetically engineered plants. Transgenic Research, 20, 1–22. 10.1007/s11248-010-9446-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeis J, Meissle M, Álvarez‐Alfageme F, Bigler F, Bohan DA, Devos Y, Malone LA, Pons X and Rauschen S, 2014. Potential use of an arthropod database to support the non‐target risk assessment and monitoring of transgenic plants. Transgenic Research, 23, 995–1013. 10.1007/s11248-014-9791-2 [DOI] [PubMed] [Google Scholar]
- Romeis J, Naranjo SE, Meissle M and Shelton AM, 2019. Genetically engineered crops help support conservation biological control. Biological Control, 130, 136–154. 10.1016/j.biocontrol.2018.10.001 [DOI] [Google Scholar]
- Rortais A, Arnold G, Dorne J‐L, More SJ, Sperandio G, Streissl F, Szentes C and Verdonck F, 2017. Risk assessment of pesticides and other stressors in bees: principles, data gaps and perspectives from the European Food Safety Authority. Science of the Total Environment, 587–588, 524–537. 10.1016/j.scitotenv.2016.09.127 [DOI] [PubMed] [Google Scholar]
- Sanvido O, Romeis J, Gathmann A, Gielkens M, Raybould A and Bigler F, 2012. Evaluating environmental risks of genetically modified crops: ecological harm criteria for regulatory decision‐making. Environmental Science & Policy, 15, 82–91. 10.1016/j.envsci.2011.08.006 [DOI] [Google Scholar]
- Sarewitz D, 2004. How science makes environmental controversies worse. Environmental Science & Policy, 7, 385–403. 10.1016/j.envsci.2004.06.001 [DOI] [Google Scholar]
- Sauve‐Ciencewicki A, Davis KP, McDonald J, Ramanarayanan T, Raybould A, Wolf DC and Valenti T, 2019. A simple problem formulation framework to create the right solution to the right problem. Regulatory Toxicology and Pharmacology, 101, 187–193. 10.1016/j.yrtph.2018.11.015 [DOI] [PubMed] [Google Scholar]
- Scott MJ, Gould F, Lorenzen M, Grubbs N, Edwards O and O'Brochta D, 2018. Agricultural production: assessment of the potential use of Cas9‐mediated gene drive systems for agricultural pest control. Journal of Responsible Innovation, 5, S98–S120. 10.1080/23299460.2017.1410343 [DOI] [Google Scholar]
- Sgolastra F, Hinarejos S, Pitts‐Singer TL, Boyle NK, Joseph T, Lūckmann J, Raine NE, Singh R, Williams NM and Bosch J, 2018. Pesticide exposure assessment paradigm for solitary bees. Environmental Entomology, 48, 22–35. 10.1093/ee/nvy105 [DOI] [PubMed] [Google Scholar]
- Shaw RH, Bryner S and Tanner R, 2009. The life history and host range of the Japanese knotweed psyllid, Aphalara itadori Shinji: potentially the first classical biological weed control agent for the European Union. Biological Control, 49, 105–113. 10.1016/j.biocontrol.2009.01.016 [DOI] [Google Scholar]
- Shaw RH, Ellison CA, Marchante H, Pratt CF, Schaffner U, Sforza RFH and Deltoro V, 2018. Weed biological control in the European Union: from serendipity to strategy. BioControl, 63, 333–347. 10.1007/s10526-017-9844-6 [DOI] [Google Scholar]
- Streissl F, Egsmose M and Tarazona JV, 2018. Linking pesticide marketing authorisations with environmental impact assessments through realistic landscape risk assessment paradigms. Ecotoxicology, 27, 980–991. 10.1007/s10646-018-1962- [DOI] [PubMed] [Google Scholar]
- Sundström LF, Lõhmus M, Tymchuk WE and Devlin RH, 2007. Gene‐environment interactions influence ecological consequences of transgenic animals. Proceedings of the National Academy of Sciences, 104, 3889–3894. 10.1073/pnas.0608767104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner RA, Pollard KM, Varia S, Evans HC and Ellison CA, 2015. First release of a fungal classical biocontrol agent against an invasive alien weed in Europe: biology of the rust, Puccinia komarovii var. glanduliferae . Plant Pathology, 64, 1130–1139. 10.1111/ppa.12352 [DOI] [Google Scholar]
- Tapparo A, Giorio C, Marzaro M, Marton D, Solda L and Girolami V, 2011. Rapid analysis of neonicotinoid insecticides in guttation drops of corn seedlings obtained from coated seeds. Journal of Environmental Monitoring, 13, 1564–1568. 10.1039/c1em10085h [DOI] [PubMed] [Google Scholar]
- Tepfer M, Racovita M and Craig W, 2013. Putting problem formulation at the forefront of GMO risk analysis. GM Crops and Food, 4, 10–15. 10.4161/gmcr.22906 [DOI] [PubMed] [Google Scholar]
- Thizy DC, Emerson C, Gibbs J, Hartley S, Kapiriri L, Lavery J, Lunshof J, Ramsey J, Shapiro J, Amir Singh J, Paré Toé L, Coche I and Robinson B, 2019. Guidance on stakeholder engagement practices to inform the development of area‐wide vector control methods. PLoS Neglected Tropical Diseases, 13, e0007286 10.1371/journal.pntd.0007286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MB and Reid AM, 2007. Are exotic natural enemies an effective way of controlling invasive plants? Trends in Ecology & Evolution, 22, 447–453. 10.1016/j.tree.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Topping CJ and Luttik R, 2017. Simulation to aid in interpreting biological relevance and setting of population‐level protection goals for risk assessment of pesticides. Regulatory Toxicology and Pharmacology, 89, 40–49. 10.1016/j.yrtph.2017.07.011 [DOI] [PubMed] [Google Scholar]
- Topping CJ and Weyman GS, 2018. Rabbit population landscape‐scale simulation to investigate the relevance of using rabbits in regulatory environmental risk assessment. Environmental Modelling & Assessment, 23, 415–457. 10.1007/s10666-017-9581-3 [DOI] [Google Scholar]
- Topping CJ, Craig PS, de Jong F, Klein M, Laskowski R, Manachini B, Pieper S, Smith R, Sousa JP, Streissl F, Swarowsky K, Tiktak A and van der Linden T, 2015. Towards a landscape scale management of pesticides: ERA using changes in modelled occupancy and abundance to assess long‐term population impacts of pesticides. Science of the Total Environment, 537, 159–169. 10.1016/j.scitotenv.2015.07.152 [DOI] [PubMed] [Google Scholar]
- Topping C, Dalby L and Skov F, 2016. Landscape structure and management alter the outcome of a pesticide ERA: evaluating impacts of endocrine disruption using the ALMaSS European Brown Hare model. Science of the Total Environment, 541, 1477–1488. [DOI] [PubMed] [Google Scholar]
- US EPA (US Environmental Protection Agency), 1998. Guidelines for ecological risk assessment: EPA/630/R‐95/002F. Federal Register, 63, 26846–26924. EPA, Washington, DC, USA: 188 pp. Available online: http://rais.ornl.gov/documents/ECOTXTBX.PDF [Google Scholar]
- US EPA (US Environmental Protection Agency), 2014. Transmittal of the meeting minutes of the FIFRA SAP meeting held January 28, 2014 on the scientific issues associated with the use of “RNAi technology as a pesticide: problem formulation for human health and ecological risk assessment.” SAPanel minutes no. 2014‐02. Available online: https://www.epa.gov/sap/meeting-materials-january-28-2014-scientific-advisory-panel
- Vandersteen WE, Leggatt RA, Sundström LF and Devlin RH, 2019. Importance of experimental environmental conditions in estimating risks and associated uncertainty of transgenic fish prior to entry into nature. Scientific Reports, 9, 406 10.1038/s41598-018-35826-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker GW, Kookana RS, Smith NE, Kah M, Doolette CL, Reeves PT, Lovell W, Anderson DJ, Turney TW and Navarro DA, 2018. Ecological risk assessment of nano‐enabled pesticides: a perspective on problem formulation. Journal of Agricultural and Food Chemistry, 66, 6480–6486. 10.1021/acs.jafc.7b02373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyard S, Singh AD and Wong S, 2009. Ingested double‐stranded RNAs can act as species‐specific insecticides. Insect Biochemistry and Molecular Biology, 39, 824–832. 10.1016/j.ibmb.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Williams RJ, Harrison S, Keller V, Kuenen J, Lofts S, Praetorius A, Svendsen C, Vermeulen LC and van Wijnen J, 2019. Models for assessing engineered nanomaterial fate and behaviour in the aquatic environment. Current Opinion in Environmental Sustainability, 36, 105–115. 10.1016/j.cosust.2018.11.002 [DOI] [Google Scholar]
- Winston RL, Schwarzländer M, Hinz HL, Day MD, Cock MJW, Julien MH. (eds.), 2014. Biological control of weeds: a world catalogue of agents and their target weeds. 5th edition USDA Forest Service, Morgantown, WV: FHTET‐2014‐04. 848 pp. Available online: https://www.ibiocontrol.org/catalog/JulienCatalogueFHTET_2014_04.pdf [Google Scholar]
- Wolt JD, Keese P, Raybould A, Fitzpatrick JW, Burachik M, Gray A, Olin SS, Schiemann J, Sears M and Wu F, 2010. Problem formulation in the environmental risk assessment for genetically modified plants. Transgenic Research, 19, 425–436. 10.1007/s11248-009-9321-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Hu Y, Yan S, Zhou H, Song D, Yin M and Shen J, 2019. A polymer/detergent formulation improves dsRNA penetration through the body wall and RNAi‐induced mortality in the soybean aphid Aphis glycines . Pest Management Science. 10.1002/ps.5313 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


