Abstract
Cancer decision-making interventions commonly utilize narratives as a persuasive strategy to increase identification with the message source, promote involvement with the topic, and elicit greater willingness to adopt recommended behaviors. However, there is little empirical research examining the mechanisms underlying the effectiveness of this strategy in the context of cancer research participation. Data for the current manuscript were collected as part of a larger study conducted with cancer patients (N =413) from the USA, UK, and the Republic of Ireland. Participants viewed and evaluated video-recorded vignettes, illustrating different strategies for discussing clinical trials participation with family members. Results showed nationality was a significant predictor of identification with the main character (i.e., patient) in the vignette. Unexpectedly, these cross-national differences in identification disappeared when patients currently undergoing treatment had higher perceived susceptibility of their cancer. Identification with the main character in the vignettes was a significant predictor of intentions to participate in cancer research, but only when the mediating role of narrative transportation was considered. The findings demonstrate the importance of considering how individual and social identities influence identification with characters in cancer narratives and yield practical guidance for developing arts-based interventions to increase cancer research participation.
Keywords: Cancer clinical trials, Cancer research registry, Entertainment education, Identification, Narrative transportation, Perceived susceptibility
Introduction
Cancer clinical trials (CCTs) are vital to identifying new and more effective cancer treatments; however, approximately 40% of federally funded CCTs in the United States (US) are not completed due to failure to accrue an adequate number of participants [1]. As a result of the challenges associated with identifying, recruiting, and consenting patients in the context of cancer treatment studies, less than 5% of cancer patients in the US enroll in CCTs, with even lower rates for ethnic minorities [2]. These participation rates are particularly poor when put into a global context. In the United Kingdom (UK), for example, approximately 20% of cancer patients enroll in CCTs [3]. Given the importance of CCTs, combined with low rates of US participation, the National Cancer Institute’s Cancer Moonshot has identified increasing participation in CCTs as a national priority [4]. A key challenge to achieving this goal is the fact that many cancer patients have little awareness of and poor attitudes toward cancer research [3, 5].
Entertainment Education
In order to increase participation in CCTs, the information needs of patients must be carefully considered. While many behavioral interventions to increase CCT enrollment rely on didactic approaches for patient education, there is growing interest in the potential for the arts and storytelling to promote greater attention to, interest in, and comprehension of intervention content. Commonly known as entertainment education (E-E), narrative approaches to message construction harness the power of stories to gain the audience’s attention and temporarily transport them into a fictional scenario in which a main character (or characters) experiences a decisional conflict, and eventually, a resolution [6].
Two important factors associated with the success of narratives for facilitating decision-making are identification with the main character and narrative transportation. Identification with a character is the extent to which a message receiver perceives that they are similar to the character, causing them to like the character, or feel as if they know the character [7]. While demographic matching (e.g., race, ethnicity) of characters is strongly associated with identification, there is evidence that more subtle similarities can also increase identification [6]. Narrative transportation refers to a process by which individuals become immersed in the story and develop heightened cognitive and affective engagement with events in the story [8]. This process can change real-world beliefs and behaviors, as individuals elaborate on the decisional conflict and the resolution discussed (or implied) within the story. Existing literature has shown that narrative transportation may not require identification with a specific character, and as such is considered to influence decision-making by a mechanism independent of identification [9].
E-E approaches offer a novel and perhaps effective approach for designing communication tools in the context of CCTs. Patients offered the opportunity to participate in a CCT often experience some degree of decisional conflict, whether due to limited knowledge of what participating in a CCT entails, preexisting negative perceptions of the patient experience within a CCT, or the practical considerations of participating for them and their loved ones [10]. However, there is little empirical evidence on the efficacy of narratives in the context of CCT decision-making to date. Consequently, it is unknown what factors constitute an effective message in this context, such as what influences audience identification with characters and their transportation into a narrative.
According to social identity theory, perceived risk to a patient’s identity increases identification with salient social groups who possess a similar identity [11, 12]. Thus, in the case of cancer treatment decision-making, we expect that greater perceptions of cancer susceptibility should increase patients’ identification with a character in a narrative conveying a strong “patient” identity. Importantly, the influence of illness on identity is not stable across the cancer survivorship continuum. It is possible that patients actively undergoing treatment may experience a different level of identification with a character with cancer, as compared to patients in remission. Finally, while social group memberships other than illness (e.g., nationality) might be important for facilitating identification, it is unknown whether the ability to relate to the patient experience is strong enough to overcome differences in demographic group membership and encourage transportation into a narrative.
Another challenge associated with increasing CCT participation is the inability of researchers to easily identify and connect with potential participants about CCTs for which they may be eligible to enroll. The emergence of consent to contact registries has the potential to overcome the challenge of recruiting patients for a specific CCT. Consent to contact registries enable patients to give informed consent for researchers to contact them about research opportunities for which they might be eligible [13] and have been demonstrated to improve recruitment and enrollment in clinical trials as compared to other common recruitment strategies [14]. At present, consent to contact registries are especially useful for recruiting patients with familial cancer syndromes (e.g., hereditary pancreatic or colorectal cancers). As genetic testing and hypothesis driven clinical trials evolve that offer opportunities to better define the molecular abnormalities that result in cancer family syndromes and therapies (e.g., immunotherapy or targeted molecular treatments), consent to contact registries will become increasingly important for identifying and contacting potential participants [15]. We argue that patients who identify with a character who has been offered a CCT will be more likely to want to have similar treatment choices and will opt to participate in a cancer research registry. Thus, the purpose of the current cross-national study is to examine what factors influence audience identification with a protagonist in the context of narratives about CCT participation, and how identification influences patients’ intentions to participate in a consent to contact registry for cancer research.
Method
Study Design
An online message design experiment was approved by The University of Florida Institutional Review Board, with patients recruited through Qualtrics Panels, a proprietary opt-in online panel company. Recruitment was conducted over a 2-week period (January 23–February 6, 2017) and comprised of eligible patients who had to be full-time residents of the US or UK/Ireland (ROI), age 18 years or older, able to read and write in English, and been previously diagnosed with one of four cancer types (breast, n = 197; colorectal, n = 54; lung or bronchus, n = 32; prostate, n = 130). Patients who had self-reported as previously participating in a CCT as part of their treatment (n = 54) were removed from the data set prior to the analyses. Therefore, a total of 413 patients were randomized to either a no-video control condition or to watch one of five vignette message conditions. Patients randomized to the no-video control condition completed the survey in a median time of 15:13 min (interquartile range = 9:12 min), while patients randomized to the five vignette message conditions completed the survey in a median time of 21:13 min (interquartile range = 10:55 min) and answered an additional 65 post-test items.
Stimuli Development
Stimuli
The production of the vignettes was based on Applied Theater Theory. In Applied Theater Theory, dramatic situations pose problems, rather than offer solutions, and aim to provoke viewers to develop their own solutions based on their reactions to the content presented [16]. Presenting problematic situations in a non-judgmental format enables viewers to identify the underlying issues in scenarios and apply this new understanding to their own thinking [16]. The content of these scenarios was created using empirical data in order to provide realistic portrayals of family conversations about CCT participation [17]. The videos were produced in collaboration with the University of Florida’s Center for Arts in Medicine, and ranged in length from approximately 2 to 3 min (see Supplementary Material for example vignette).
Instrumentation
Predictors
Nationality
Nationality was dichotomized, with US patients coded as the referent (n = 219). Patients from the UK (n = 181) and ROI (n = 13) were coded together.
Covariates
Study covariates were measured using instruments adapted from the Health Information National Trends Survey and the UK’s Office for National Statistics Census [18, 19]. Demographic measures were dummy coded and controlled for in all analyses, including education (no college education as referent), sex (male as referent), income (less than $50 k/£40 k as referent), and cancer status (in remission as referent). Race was dummy coded with White US (n = 201) and White UK/ROI (n = 176) combined and coded as the referent. With other racial categories limited in membership (n = 36),and reporting no significant differences in the dependent measures across categories, they were collapsed and coded together. Patient age was controlled for, with cancer type also dichotomized and included with breast cancer as the referent condition (see Table 1 for study covariates by nationality).
Table 1.
Key study covariates of US and UK/ROI patients
| Patient characteristics | US (n = 219) | UK/ROI (n = 194) | ||
|---|---|---|---|---|
| N = 413 (%)a | n (%) | n (%) | χ2 | |
| Age, mean (SD)b | 60.22 (15.90) | 68.60 (9.80) | 50.71 (16.17) | 189.43*** c |
| Race | 0.15 | |||
| White | 377 (91.3) | 201 (91.8) | 176 (90.7) | |
| Other | 36 (8.7) | 18 (8.2) | 18 (9.3) | |
| Education | 0.29 | |||
| No college education | 222 (53.8) | 115 (52.5) | 107 (55.2) | |
| College education | 191 (46.2) | 104 (47.5) | 87 (44.8) | |
| Incomed | 17.34*** | |||
| Less than $50k/£40k | 213 (53.3) | 90 (43.3) | 123 (64.1) | |
| Greater than $50k/£40k | 187 (46.8) | 118 (56.7) | 69 (35.90) | |
| Sex | 1.22 | |||
| Male | 180 (43.6) | 101 (46.1) | 79 (40.7) | |
| Female | 233 (56.4) | 118 (53.9) | 115 (59.3) | |
| Cancer status | 53.32*** | |||
| Remission | 309 (74.8) | 196 (89.5) | 113 (58.2) | |
| Currently has cancer | 104 (25.2) | 23 (10.5) | 81 (41.8) | |
| Cancer type | 11.95** | |||
| Breast | 197 (47.7) | 93 (42.5) | 104 (53.6) | |
| Colorectal | 54 (13.1) | 27 (12.3) | 27 (13.9) | |
| Lung or bronchus | 32 (7.7) | 14 (6.4) | 918 (9.3) | |
| Prostate | 130 (31.5) | 85 (38.8) | 45 (23.2) | |
| Ask CCT | 1.61 | |||
| No | 397 (96.1) | 213 (97.3) | 184 (94.8) | |
| Yes | 16 (3.9) | 6 (2.7) | 10 (5.2) |
Ask CCT, patient previously asked to participate in a cancer clinical trial
Percentages totaled within column may exceed 100% due to rounding
Patients who failed to respond were removed (n = 1)
F statistic derived from a one-way analysis of variance
Patients who failed to respond were removed (n = 13)
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Moderators/Mediators
Perceived Susceptibility
For patients with cancer, four items measuring susceptibility to treatment failure (e.g., “It is highly likely that my cancer will not be cured”) were measured on a seven-point, Likert scale (M = 3.50, SD = 0.96, α = 0.75). For patients in remission, the same four items were modified to measure their perceived susceptibility to their cancer recurring (e.g., “It is highly likely my cancer will recur”) to create a seven-point, Likert scale (M = 2.58, SD = 1.03, α = 0.84).
Narrative Transportation
Narrative transportation was measured using seven items from Green and Brock’s [20] narrative transportation scale. These items assessed how engaged patients were in the events taking place in the video (e.g., “I was mentally involved in the video while watching it”). Items were rated on a seven-point Likert scale, with response categories ranging from “strongly disagree” to “strongly agree” (M = 4.54, SD = 1.32, α = 0.88).
Intent to Seek Information About a Cancer Research Registry
Patient intent to seek information about a cancer research registry was adapted from previous study measuring behavioral intent to participate in randomized clinical study (e.g., “I intend to search for more information about cancer research registries) [21]. Five items were rated on a seven-point Likert scale, with response categories ranging from “strongly disagree” to “strongly agree” (M = 3.79, SD = 1.72, α = 0.93).
Dependent Variables
Patient Identification
Patient identification was adapted from Cohen [22] and measured how strongly the patient felt connected to the patient (e.g., “I was able to understand the problem faced by the patient”). Four items were rated on a seven-point Likert scale, with response categories ranging from “strongly disagree” to “strongly agree” (M = 4.93, SD = 1.42, α = 0.87).
Cancer Research Registry Link Click
A patient clicking on a link to find out how to enroll in a cancer research registry was tracked and dichotomized as a binary behavioral outcome. After being given a description of a cancer research registry, patients were asked, “If you would like to find out more information about how to enroll in a cancer research registry, please click here.” Patients who did not click on the link were coded as the referent.
Results
Statistical Analyses
Main Effects of Message Design Experiment
Bivariate correlations were conducted to identify key study covariates and E-E predictor variables. An analysis of covariance, employing a Bonferroni correction, determined that there were no differences between patients across the vignette message conditions and the no-video control condition in the rates of clicking the link [F (4, 384) = 1.91, p = .09, ηp2 = 0.02]. There were also no significant differences across the vignette message conditions on key study variables: patient identification [F (4, 315) = .82, p = .51, ηp2 = 0.01], narrative transportation [F (4, 315) = 1.50, p = .20, ηp2 = 0.02], intent to seek information about a cancer research registry [F (4, 315) = .47, p = .76, ηp2 = 0.01], or on link clicking [F (4, 315) = .65, p = .62, ηp2 = 0.01]. As UK/ROI patients reported greater identification [F (1, 338) = 2.82, M = 5.07 vs. 4.81, p = 0.09], narrative transportation [F (1, 338) = 19.56, M = 4.79 vs. 4.31, p = 0.001], and intent to seek information about a cancer research registry [F (1, 338) = 29.98, M = 4.30 vs. 3.32, p < 0.001], but not link clicking [χ2 (1, N = 340) = 0.02, n = 29 vs. 33, p = 0.88], a series of supplementary analyses probed for the effect of patient nationality and E-E variables on differences in patient link clicking behavior.
Supplementary Analyses
Version 2.15 of the SPSS PROCESS macro was used to test for both direct and indirect effects through an ordinary least-squares path analytical framework for simple moderation (i.e., Model 1), and a logistic-based path analytical framework for serial mediation (i.e., Model 6). In the simple moderation model, nationality was set as the focal predictor, perceived susceptibility as the moderator, and patient identification as the dependent variable. Age, education, sex, race, income, cancer type, previously being asked to participate in a CCT, and vignette message conditions were included as covariates. The simple moderation analysis used indicator coding to ascertain differences in patient identification between US patients and UK/ROI patients for varying levels of perceived susceptibility. Analyses for patients in remission and those currently with cancer were conducted separately. In the serial mediation model, narrative transportation and intent to seek information on cancer research registries were tested as mediators, operating in sequence to explain the indirect effect of patient identification on the outcome of clicking on a link to find out more information about how to enroll in a cancer research registry. In PROCESS, moderated mediation analysis cannot be implemented with mediators in sequence, so bootstrapping was used to construct percentile-based, bias-corrected 95% confidence intervals (CIs) for specific and total indirect effects while controlling for age, education, sex, race, income, nationality, cancer status, cancer type, previously being asked to participate in a CCT, and message conditions [21].
Perceived Susceptibility as a Moderator of Nationality and Patient Identification
Patients with Cancer
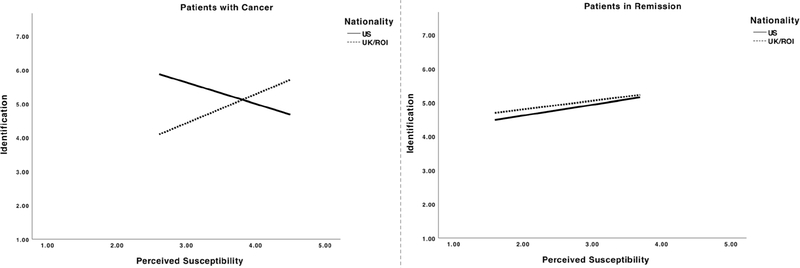
A moderation analysis was conducted to identify whether perceptions of susceptibility moderated the relationship between patient nationality and identification with the patient in the vignette. For patients with cancer, the overall model was significant F (16, 71) = 3.75, p < 0.001, R2 = 0.40 (Table 2). Within this model, greater perceived susceptibility of dying from cancer did not predict greater patient identification (b= − 0.63, t = − 1.60, p = 0.11), but nationality was a significant predictor with US patients more likely to identify with the patient in the vignette (b = − 5.62, t = − 3.61, p = 0.001). However, an inverse association emerged when perceived susceptibility was included as a moderator (b = 1.48, t = 3.39, p = 0.001), explaining a further 10.5% of the variance in the model, F (1, 71) = 11.47, p = 0.001 (Fig. 1). A Johnson-Neyman analysis probed the conditional effect of perceived susceptibility on nationality and patient identification, indicating that US patients who reported a perceived susceptibility value of 3.06 or below (26.14% of the overall reported values of the moderator) were significantly more likely to identify with the patient in the vignette than UK/ROI patients (b=− 1.11, t = − 1.99, p = 0.05). At values above this point, patient nationality was no longer a significant predictor of identification.
Table 2.
Linear regression of nationality on patient identification at differing levels of perceived susceptibility by cancer status (N = 329)
| Patients with cancer (n = 88) |
Patients in remission (n = 241) |
|||||
|---|---|---|---|---|---|---|
| Predictor variable | B (SE) | t | 95% CI | B (SE) | t | 95% CI |
| Age | ||||||
| Age | − 0.03 (0.02) | − 1.47 | − 0.06, 0.01 | 0.00 (0.01) | 0.37 | − 0.01, 0.02 |
| Race | − 0.05 (0.57) | − 0.08 | − 1.19, 1.10 | − 0.15 (0.34) | − 0.45 | − 0.83, 0.52 |
| Education | − 0.20 (0.34) | − 0.60 | − 0.89, 0.48 | − 0.17 (0.21) | − 0.81 | − 0.57, 0.24 |
| Income | − 0.25 (0.42) | − 0.59 | − 1.10, 0.59 | 0.14 (0.20) | 0.70 | − 0.25, 0.53 |
| Sex | − 0.25 (0.72) | − 0.35 | − 1.70, 1.19 | 0.14 (0.39) | 0.36 | − 0.62, 0.90 |
| Colorectal cancer | − 1.02 (0.85) | − 1.20 | − 2.71, 0.68 | 0.21 (0.33) | 0.62 | − 0.45, 0.87 |
| Lung cancer | − 1.56 (0.74)* | − 2.11 | − 3.03, −0.08 | 0.25 (0.36) | 0.70 | − 0.46, 0.97 |
| Prostate cancer | − 0.44 (0.76) | − 0.58 | − 1.95, 1.08 | 0.24 (0.44) | 0.53 | − 0.64, 1.11 |
| Ask CCT | − 0.35 (0.53) | − 0.67 | − 1.41, 0.71 | 1.05 (0.62) | 1.70 | − 0.17, 2.27 |
| Predictors | ||||||
| Perceived sus. (PS) | − 0.63 (.39) | − 1.60 | − 1.41, 0.16 | 0.32 (0.12)** | 2.79 | 0.09, 0.54 |
| Nationality | − 5.62 (1.56)*** | − 3.61 | − 8.73, − 2.52 | 0.32 (0.73) | 0.43 | − 1.13, 1.76 |
| Interaction | ||||||
| PS × nationality | 1.48 (0.44)*** | 3.39 | 0.61, 2.35 | − 0.07 (0.23) | − 0.28 | − 0.53, 0.39 |
| R2 | 0.40*** | 0.08 | ||||
| ΔR2 | 0.11*** | 0.00 | ||||
Cases with missing values across the variables were not included in the analysis (n = 14)
PS × nationality, perceived susceptibility x nationality; Ask CCT, patient previously asked to participate in a cancer clinical trial; R2, the variance in identification explained by the model; ΔR2, increase in variance due to interaction between perceived susceptibility and nationality
p ≤ 0.05;
p ≤ 0.01;
p ≤ .001
Fig. 1.
Interaction between nationality and patient identification at differing levels of perceived susceptibility by cancer status
Patients in Remission
Similarly, a moderation analysis was conducted to identify whether perceptions of susceptibility moderated the relationship between nationality and patient identification for patients in remission. The overall moderation model was not significant F (16, 224) = 1.13, p = .33, R2 = 0.08, but greater perceived susceptibility did predict greater identification (b= 0.32, t = 2.79, p < 0.01) (Table 2). However, there were no significant differences in the effects of nationality (b = 0.32, t = 0.43, p = 0.67) or their interaction (b = − 0.07 t = − 0.28, p = 0.78) on identification. Figure 2 illustrates the positive linear relationship between susceptibility and identification, regardless of nationality.
Fig. 2.
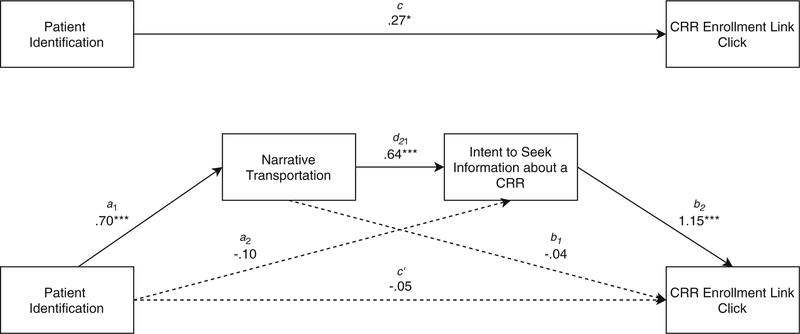
A serial mediation model showing the significant indirect effect of patient identification on cancer research registry enrollment link clicking through narrative transportation and intent to seek information about a cancer research registry
Serial Mediation Through Narrative Transportation and Intent to Seek Information About a Cancer Research Registry
A logistic-based path analytical framework for serial mediation was conducted to evaluate whether greater patient identification predicted a greater likelihood that patients would click on a link about enrolling in a cancer research registry. To test this model, identification was included as a predictor, with narrative transportation and intent to seek information on how to enroll in a cancer research registry included as the mediators in serial (see Table 3 for regression models). The model predicting narrative transportation was significant, and was able to explain a large amount of the variance in narrative transportation, F (16, 312) = 32.38, p < 0.001, R2 = 0.67. Greater patient identification was significantly associated with greater transportation (b = .70, t = 19.84, p < .001), as were younger patients (b = − 0.01, t = − 2.15, p < 0.05) and more educated patients (b = 0.32, t = 3.40, p = 0.001). The model predicting intent to seek information about a CRR was significant, F (17, 311) = 13.88, p < .001, R2 = 0.37, with narrative transportation significantly, positively associated with greater intent to seek information (b = 0.64, t = 5.71, p < 0.001). Younger patients were again more likely to intend to seek information about a CRR (b = − .01, t = − 2.16, p < 0.05), as were current cancer patients (b = 0.60, t = 2.99, p < 0.01). Patient identification did not significantly predict intent to seek information (b = − 0.01, t = − 1.06, p = 0.29).
Table 3.
Regression models predicting cancer research registry enrollment link clicking (N = 329)
| Narrative transportation |
Intent to seek information about a CRR |
Direct effects model |
Total effects model |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor variable | B (SE) | t | 95% CI | B (SE) | t | 95% CI | B (SE) | Z | 95% CI | B (SE) | Z | 95% CI |
| Age | − 0.01 (0.00)* | − 2.15 | − 0.02, − 0.00 | − 0.01 (0.01)* | − 2.16 | − .28, − 0.00 | 0.05 (0.02)** | 3.11 | .02, .08 | .02 (.01) | 1.74 | −.00, .05 |
| Race | − 0.10 (0.17) | − 0.57 | − 0.43, 0.24 | 0.18 (0.28) | 0.66 | − 0.37, 0.74 | − 0.41 (0.65) | − 0.64 | − 1.68, 0.85 | − 0.35 (0.60) | − 0.59 | − 1.52, 0.82 |
| Education | 0.32 (0.10)*** | 3.40 | 0.14, 0.51 | 0.02 (0.18) | 0.11 | − 0.34, 0.37 | 0.60 (0.41) | 1.47 | − .20, 1.40 | 0.43 (0.33) | 1.31 | − .22, 1.09 |
| Income | 0.07 (0.10) | 0.67 | − 0.13, 0.26 | 0.36 (0.18) | 1.93 | − 0.01, 0.72 | − 0.09 (0.41) | − 0.22 | − .89, 0.71 | 0.39 (0.35) | 1.10 | − 0.30, 1.07 |
| Sex | 0.05 (0.18) | 0.30 | − 0.30, 0.41 | − 0.60 (0.33) | − 1.85 | − 1.25, 0.04 | − 1.15 (0.83) | − 1.39 | − 2.78, 0.48 | − 1.52 (.82) | − 1.86 | − 3.12, 0.08 |
| Colorectal cancer | 0.15 (0.17) | 0.88 | − 0.19, 0.49 | − 0.33 (0.33) | − 1.01 | − 0.98, 0.31 | − 2.31 (0.91)* | − 2.54 | − 4.09, − 0.53 | − 2.19 (.91)* | − 2.41 | − 3.96, − 0.41 |
| Lung cancer | 0.18 (0.17) | 1.03 | − 0.16, 0.51 | 0.09 (0.36) | 0.24 | − 0.63, 0.80 | − 1.54 (0.89) | − 1.74 | − 3.28, 0.20 | − 1.03 (.83) | − 1.24 | − 2.66, 0.60 |
| Prostate cancer | − 0.04 (0.21) | − 0.21 | − 0.45, 0.36 | − 0.67 (0.36) | − 1.85 | − 1.37, 0.04 | − 2.00 (0.93)* | − 2.15 | − 3.83, − 0.17 | − 2.17 (.88)* | − 2.46 | − 3.91, 0.44 |
| Ask CTT | − 0.06 (0.20) | − 0.30 | − 0.44, 0.33 | − 0.44 (0.49) | − 0.89 | − 1.40, 0.53 | − 0.22 (0.99) | − 0.22 | − 2.17, 1.73 | − .46 (.87) | − 0.52 | − 2.17, 1.26 |
| Nationality | 0.04 (0.12) | 0.36 | − 0.19, 0.27 | 0.21 (0.21) | 1.00 | − 0.20, 0.62 | − 0.58 (0.50) | − 1.16 | − 1.56, .401 | − .22 (.42) | − 0.51 | − 1.05, 0.61 |
| Cancer status | 0.18 (0.11) | 1.61 | − 0.04, 0.40 | 0.60 (0.20)** | 2.99 | 0.21, 0.99 | 0.45 (0.43) | 1.05 | − 0.39, 1.29 | 1.05 (.36)** | 2.88 | 0.34, 1.76 |
| Patient identification | 0.70 (0.04)*** | 19.84 | 0.63, 0.77 | − 0.10 (0.09) | − 1.06 | − 0.28, 0.09 | − 0.05 (0.23) | − 0.20 | − 0.50, 0.40 | 0.27 (.12)* | 2.25 | 0.03, 0.50 |
| Narrative transportation | 0.64 (0.11)*** | 5.71 | 0.42, 0.86 | − 0.04 (0.28) | − 0.15 | − 0.59, 01.50 | ||||||
| Intent to seek information about a CRR | 1.15 (0.19)*** | 6.08 | 0.78, 1.52 | |||||||||
Cases with missing values across the variables were not included in the analysis (n = 11); both patients with cancer and patients in remission were included, with cancer status controlled for in the model; the total effects model depicts the predictive power of patient identification with covariates included, and the direct effects model depicts the predictive power of patient identification with covariates and mediators included. Tests of indirect mediation effects are included in text
Ask CCT, patient previously asked to participate in a cancer clinical trial; CRR, cancer research registry
p ≤ 0.05;
p ≤ 0.01;
p ≤ .001
The direct effects model was significantly better than the null model at predicting link clicking, − 2 log likelihood = 212.21, p < 0.001, and successfully explained between 26.9% (Cox & Snell R2) and 43.7% (Nagelkerke R2) of the variance in link clicking. Patient identification (b = − 0.05, Z = − 0.20, p = 0.84) and narrative transportation (b = − 0.04, Z = − 0.15, p = 0.81) did not have a significant direct effect on link clicking, but intent to seek information about a CRR did (b = 1.15, Z = 6.08, p < 0.001). Patients diagnosed with colorectal cancer or prostate cancer were significantly less likely than breast cancer patients to click on the link (colorectal: b = − 2.31, Z = − 2.54, p < 0.05; prostate: b = − 2.00, Z = − 2.15, p < 0.05). Older patients were significantly more likely to click on the link (b = 0.50, Z = 3.11, p < 0.01). The Total Effects Model was also significant, indicating that the model was able to successfully predict link clicking better than the model based on a simple constant, Cox & Snell R2 = 0.10, − 2 log likelihood = 279.34, p < 0.01. When controlling for study covariates, patient identification had a significant total effect at predicting link clicking (b = .27, Z = 2.25, p < 0.05). Patients diagnosed with colorectal cancer or prostate cancer were significantly less likely to click on the link than breast cancer patients (colorectal: b = − 2.19, Z = − 2.41, p < 0.05; prostate: b = − 2.17, Z = − 2.46, p < 0.05), with current cancer patients significantly more likely to click on the link (b = 1.05, Z = 2.88, p < 0.01).
Figure 2 provides a visual representation of the entire model, showing the significant and non-significant pathways. Specifically, there is a significant indirect effect of patient identification through narrative transportation and intent to seek information on a cancer research registry on link clicking (a1d21b2=0.51, SE=0.15, 95% bootstrap CI=0.22, 0.73), with statistically significant positive associations between all variables in serial. This suggests that, as patient identification increases, so does narrative transportation, which promotes greater intent to seek information about a cancer research registry, resulting in greater likelihood to click on a link to find out how to enroll in a cancer research registry. Other indirect pathways, a1b1 (i.e., patient self-identification → narrative transportation → link clicking; b = − 0.03, SE = 0.28, 95% bootstrap CI = − 0.64, 0.46) and a2b2 (i.e., patient identification →intent to seek information about a cancer research registry→link clicking; b=− 0.11, SE=0.13, 95% bootstrap CI=−0.35, 0.15) did not significantly predict link clicking, confirming this process as one of serial mediation (total indirect effects model, b=0.37, SE=0.27, 95% bootstrap CI=−0.22, 0.84).
Discussion
Increasing patient involvement in cancer research is a global priority; however, many patients are unaware of opportunities to participate in CCTs and lack understanding about the degree of personal agency they may have in the decision process [23]. Although previous literature suggests that narratives could offer a promising strategy for increasing the efficacy of patient education interventions designed to increase awareness and facilitate effective decision-making about CCTs, the current cross-national study is the first to our knowledge to provide empirical evidence on the efficacy of this approach in the CCT context.
The current message design experiment advances the literature in three important ways. First, and most importantly, it demonstrates the conditions under which narratives will be most effective for influencing intentions and behavior. Specifically, among those who viewed the vignettes, this study suggests a sequential relationship with the participant identifying with the patient in the story and then being transported into events in the story, and how this process then positively influenced their intentions to participate in future cancer research, reflected in a measureable behavioral outcome associated with participation. Extant literature has explicated the conceptual differences between identification and transportation, but has suggested that they are independent predictors [9]. In this experiment, a serial mediation model illuminated the potential for greater patient identification to influence narrative transportation, which predicted behavioral intent and action in sequence. In other words, patients’ assessment of their similarities to the patient in the vignette influenced their ability to be transported, or immersed, in the decisional conflict that the patient faced. Furthermore, transportation predicted intention to get more information about participation in a cancer consent to recontact registry, which predicted the behavioral outcome.
As increasing identification with the main character is essential to the success of the narrative, the current experiment also elucidates the circumstances under which cancer patients are likely to identify with a protagonist in a narrative. Previously, narrative research has focused on the influence of demographic similarity. In the current experiment, demographic similarity was manipulated using nationality. The E-E videos were filmed with US actors, so it was expected that US patients would identify more strongly than UK/ROI patients with the characters in the story, as an accent can function as a heuristic cue to strengthen perceptions of national identity. However, we found that this was only the case for patients who were currently in treatment. The differences in identification between patients currently in ‘treatment and those who were in remission are notable. The term “cancer survivor” is often used as a reminder of the life-long implications of cancer and cancer treatment on an individual’s physical, emotional, and mental well-being. However, the current data suggest that a shift in identity occurs at some point, as patients experienced less identification with the cancer patient if they were post-treatment. This suggests that messages about research participation should be designed differently for patients currently in treatment compared to those in remission.
Another interesting finding is the unique interaction between identification and perceived susceptibility associated with cancer. Although US patients currently in treatment identified more strongly with the characters than UK/ROI patients, this difference disappeared when perceived susceptibility to treatment failure was taken into account. In other words, patients who perceived the susceptibility of their cancer treatment failing to be high reported similar levels of identification with the protagonist. This suggests that high levels of perceived susceptibility alter which social identities are most salient in a message. Instead of demographic similarity, it appears that patients focused on a shared illness identity. Thus, not only can narratives be an effective method to promote identfication in the CCTcontext, but demographic differences can be superseded by other salient similarities, such as being worried about one’s health. This provides impetus for future health interventions that employ sophisiticated segmentation strategies to target audiences subgroups (i.e., through social media campaigns).
As with all research, the current experiment has several notable strengths and limitations. One strength is that it is the first study to explore the efficacy of narratives within the context of cancer research registry enrollment, an area highlighted as a priority by the National Institutes of Health [24, 25]. From a methodological standpoint, the study includes a cross-national sample of patients from the US and UK/ROI, providing unique understanding of larger social group membership, such as nationality, on patient identification. Similarly, the sample includes both patients currently with cancer and those in remission, which proved an important sampling strategy as these two patient subgroups presented different relationships between susceptibility and identity. The study stimuli included several different narratives, which enhances our confidence that the findings can be generalized beyond one particular type of message. This study also goes beyond behavioral intention as the study end point. The current study tracked whether patients clicked on a link to find out more about enrolling in a registry, which provided a more robust study end point. This is particularly interesting given that the stimuli focused on CCT decision-making, not consent to contact registries specifically.
There are also important limitations that should be noted. While the current study tracked patient link clicking, it did not track whether patients enrolled in a registry after participating in the study. There are several barriers that can inhibit completion of the enrollment process, with comprehending and agreeing to the informed consent document a frequent barrier to research participation. Enhanced informatics and linkage capabilities should enable future studies to explore whether narrative interventions improve the liklihood of a patient enrolling in a cancer CCT, as well as whether they remain in the study. Also, based on our finding that the vignette message conditions did not provide a significantly increased rate of link clicking when compared to the no-video control condition, an E-E attention-control condition that required a similar degree of patient survey burden should be included in future studies. Finally, the study limited participation to patients who had been previously been diagnosed with one of the four most prevalent cancer types in the US (colorectal, breast, lung, and prostate). Due to the greater clinical severity of certain other cancer types (e.g., pancreatic cancer), expanding the variety of cancer types may have presented a broader reflection of the differing relationships between perceived susceptibility and patient identity across a diverse spectrum of cancers.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s13187-018-1364-2) contains supplementary material, which is available to authorized users.
References
- 1.Stensland KD, McBride RB, Latif A, et al. (2014) Adult cancer clinical trials that fail to complete: an epidemic? J Natl Cancer Inst 106. doi: 10.1093/jnci/dju229 [DOI] [PubMed] [Google Scholar]
- 2.Jimenez R, Zhang B, Joffe S, Nilsson M, Rivera L, Mutchler J, Lathan C, Paulk ME, Prigerson HG (2013) Clinical trial participation among ethnic/racial minority and majority patients with advanced cancer: what factors most influence enrollment? J Palliat Med 16:256–262. 10.1089/jpm.2012.0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moorcraft SY, Marriott C, Peckitt C, Cunningham D, Chau I, Starling N, Watkins D, Rao S (2016) Patients’ willingness to participate in clinical trials and their views on aspects of cancer research: results of a prospective patient survey. Trials 17:17 10.1186/s13063-015-1105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer DS, Jacks T, Jaffee E (2016) A U.S. “Cancer moonshot” to accelerate cancer research. Science 353:1105–1106. 10.1126/science.aai7862 [DOI] [PubMed] [Google Scholar]
- 5.Byrne MM, Tannenbaum SL, Glück S, Hurley J, Antoni M (2014) Participation in cancer clinical trials: why are patients not participating? Med Decis Mak 34:116–126. 10.1177/0272989X13497264 [DOI] [PubMed] [Google Scholar]
- 6.Murphy S, Frank L, Chatterjee J, Baezconde-Garbanati L (2013) Narrative versus nonnarrative: the role of identification, Transportation and Emotion in Reducing Health Disparities. J Commun 63:116–137. 10.1111/jcom.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyer-Gusé E (2008) Toward a theory of entertainment persuasion: explaining the persuasive effects of entertainment-education messages. Commun Theory 18:407–425. 10.1111/j.1468-2885.2008.00328.x [DOI] [Google Scholar]
- 8.Green MC, Clark JL (2013) Transportation into narrative worlds: implications for entertainment media influences on tobacco use. Addiction 108:477–484. 10.1111/j.1360-0443.2012.04088.x [DOI] [PubMed] [Google Scholar]
- 9.Moyer-Gusé E, Nabi RL (2010) Explaining the effects of narrative in an entertainment television program: overcoming resistance to persuasion. Hum Commun Res 36:26–52. 10.1111/j.1468-2958.2009.01367.x [DOI] [Google Scholar]
- 10.Cox K, Mcgarry J (2003) Why patients don’t take part in cancer clinical trials: an overview of the literature. Eur J Cancer Care (Engl) 12:114–122. 10.1046/j.1365-2354.2003.00396.x [DOI] [PubMed] [Google Scholar]
- 11.Jetten J, Haslam C, Haslam SA, Dingle G, Jones JM (2014) How groups affect our health and well-being: the path from theory to policy. Soc Issues Policy Rev 8:103–130. 10.1111/sipr.12003 [DOI] [Google Scholar]
- 12.Tajfel H (1974) Social identity and intergroup behaviour. Inf Int Soc Sci Counc 13:65–93 [Google Scholar]
- 13.Scherr CL, Dean M, Clayton MF, Hesse BW, Silk K, Street RL Jr, Krieger J (2017) A research agenda for communication scholars in the precision medicine era. J Health Commun 0:1–10. 10.1080/10810730.2017.1363324 [DOI] [PubMed] [Google Scholar]
- 14.Flood-Grady E, Clark VC, Bauer A, Morelli L, Horne P, Krieger JL, Nelson DR (2017) Evaluating the efficacy of a registry linked to a consent to re-contact program and communication strategies for recruiting and enrolling participants into clinical trials. Contemp Clin Trials Commun 8:62–66. 10.1016/j.conctc.2017.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richesson RL, Lee HS, Cuthbertson D, Lloyd J, Young K, Krischer JP (2009) An automated communication system in a contact registry for persons with rare diseases: scalable tools for identifying and recruiting clinical research participants. Contemp Clin Trials 30:55–62. 10.1016/j.cct.2008.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor P (2003) Applied theatre: creating transformative encounters in the community. Heinemann Drama [Google Scholar]
- 17.Krieger JL (2014) Family communication about cancer treatment decision making a description of the DECIDE typology. Ann Int Commun Assoc 38:279–305. 10.1080/23808985.2014.11679165 [DOI] [Google Scholar]
- 18.HINTS: Survey Instruments. https://hints.cancer.gov/instrument.aspx. Accessed 30 Sep 2017
- 19.2011. Census - Office for National Statistics. https://www.ons.gov.uk/census/2011census. Accessed 30 Sep 2017
- 20.Green MC, Brock TC (2000) The role of transportation in the persuasiveness of public narratives. J Pers Soc Psychol 79:701–721 [DOI] [PubMed] [Google Scholar]
- 21.Krieger JL, Neil JM, Strekalova YA, Sarge MA (2016) Linguistic strategies for improving informed consent in clinical trials among low health literacy patients. J Natl Cancer Inst 109:djw233 10.1093/jnci/djw233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen J (2001) Defining identification: a theoretical look at the identification of audiences with media characters. Mass Commun Soc 4:245–264. 10.1207/S15327825MCS0403_01 [DOI] [Google Scholar]
- 23.Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, Ellis P, Wright JR(2006) Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol 7:141–148. 10.1016/S1470-2045(06)70576-9 [DOI] [PubMed] [Google Scholar]
- 24.Blankshain KD, Moss HE (2016) Research registries: a tool to advance understanding of rare neuro-ophthalmic diseases. J Neuroophthalmol 36:317–323. 10.1097/WNO.0000000000000391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(2015) List of Registries. In: Natl. Inst. Health NIH https://www.nih.gov/health-information/nih-clinical-research-trials-you/list-registries. Accessed 30 Sep 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.