Abstract
Microbes alter their transcriptomic profiles in response to the environment. The physiological conditions experienced by a microbial community can thus be inferred using meta-transcriptomic sequencing by comparing transcription levels of specifically chosen genes. However, this analysis requires accurate reference genomes to identify the specific genes from which RNA reads originate. In addition, such an analysis should avoid biases in transcript counts related to differences in organism abundance. In this study we describe an approach to address these difficulties. Sample-specific meta-genomic assembled genomes (MAGs) were used as reference genomes to accurately identify the origin of RNA reads, and transcript ratios of genes with opposite transcription responses were compared to eliminate biases related to differences in organismal abundance, an approach hereafter named the “diametric ratio” method. We used this approach to probe the environmental conditions experienced by Escherichia spp. in the gut of 4 premature infants, 2 of whom developed necrotizing enterocolitis (NEC), a severe inflammatory intestinal disease. We analyzed twenty fecal samples taken from four premature infants (4–6 time points from each infant), and found significantly higher diametric ratios of genes associated with low oxygen levels in samples of infants later diagnosed with NEC than in samples without NEC. We also show this method can be used for examining other physiological conditions, such as exposure to nitric oxide and osmotic pressure. These study results should be treated with caution, due to the presence of confounding factors that might also distinguish between NEC and control infants. Nevertheless, together with benchmarking analyses, we show here that the diametric ratio approach can be applied for evaluating the physiological conditions experienced by microbes in situ. Results from similar studies can be further applied for designing diagnostic methods to detect NEC in its early developmental stages.
Introduction
Physiological conditions within the gut are important metrics to measure when studying gut inflammatory diseases [1,2], yet are notoriously difficult to measure in vivo. Transcriptional profiling provides information on the pool of genes that microbial cells express, and therefore can reveal the physiological conditions experienced by these cells. Microbial transcriptional patterns have been analyzed using many methods, including reverse transcription quantitative PCR [3], microarrays [4], and in recent years, RNA sequencing [5]. Analyzing transcription patterns within microbial communities, i.e. meta-transcriptomics, is challenging because it is necessary to specifically identify the microbial species from which transcripts originate. In addition, it is also important to account for changes in the abundance of different microbial species from which transcripts originate, as changes in transcript abundance can also be related to changes in organismal abundances [6].
A relevant case in which such an approach would be useful is the study of necrotizing enterocolitis (NEC) in premature infants. The hallmark of NEC is inflammation of the small and/or large bowel that can progress rapidly to intestinal necrosis, sepsis, and death [7,8]. Because the onset of the disease is often fulminant, treatment options for severe cases are limited and often futile. Thus, the need for disease biomarkers, to enable early and accurate diagnosis, motivates ongoing research on early stages of NEC development. A recent meta-analysis of 14 DNA-based studies reported that fecal samples from preterm infants, later diagnosed with NEC, contained modest but statistically significant increased abundance of facultative anaerobes from the Proteobacteria phylum and a modest decrease in abundance of strict anaerobes [9]. As others have previously noted, an understudied approach to effectively study the bacterial response to local conditions within the infant gut is to pair taxonomic profiling with functional information [9,10]. This understudied approach can be addressed by applying the transcriptomic analysis approaches described here.
Here we established a new method of measuring transcriptomic data, named the diametric ratio, to measure physiological conditions from microbial community transcriptomic data. We used this approach to study NEC in a pilot cohort of premature infants, to infer about the transcriptional patterns associated with physiological conditions occurring before NEC is diagnosed, specifically targeting genes related to oxygen exposure.
Methods
Study design and sampling
This study made use of a previously analyzed dataset of metagenomic DNA sequencing of premature infant stool samples [10–13]. In this study a subset of these stool samples from four infants, two of which were diagnosed with NEC, were additionally subjected to RNA sequencing. As previously described [12], stool samples for establishing these datasets were collected after perineal stimulation [14], so that fecal samples were collected under direct vision immediately upon evacuation, to minimize changes in the transcription pattern of gut microbes outside the intestine. After collection of the samples, they were stored at -80°C until DNA and RNA extraction. Medical information for these infants is presented in Table 1, and sampling schedule for each infant is available in Table 2. To analyze changes in transcription patterns over time and with a balanced distribution of samples, five samples from both NEC and Control infants from day of life (DOL) 10–19 were grouped over the 1st time block, and those from DOL 20–37 were grouped over the 2nd time block (Table 2).
Table 1. Infant medical information.
| Infant | Gestational age (Weeks) | Study | Gender | Delivery | Weight (g) | Feeding | Condition | NEC Diagnosis (DOL) |
|---|---|---|---|---|---|---|---|---|
| 64 | 28 | NIH2 | M | Vaginal | 1100 | Combination | Control | – |
| 66 | 28 | NIH2 | F | Vaginal | 1028 | Breast | Control | – |
| 69 | 26 | NIH2 | M | C-section | 637 | Combination | NEC | 32 |
| 71 | 25 | NIH2 | M | C-section | 754 | Combination | NEC | 31 |
Table 2. Infant stool sampling scheme.
| Diagnosis | infant | 1st time block | 2nd time block | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NEC | 71 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | * | |||||||||||
| 69 | ✓ | ✓ | ✓ | ✓ | * | ||||||||||||||
| Control | 66 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||||||
| 64 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||||||
| 10 | 11 | 13 | 14 | 15 | 16 | 17 | 19 | 20 | 21 | 25 | 27 | 28 | 31 | 32 | 33 | 36 | 37 | ||
| Days of life | |||||||||||||||||||
✓ indicate dates stool samples were taken
* indicate NEC diagnosis
DNA and RNA sequencing
Procedures for DNA extraction and sequencing were previously described [13]. RNA was extracted from selected stool samples using MOBIO PowerMicrobiome RNA isolation kit. The only modification to the manufacturer’s protocol was that phenol:chloroform:isoamyl alcohol was added to the glass bead tubes prior to the addition of the stool sample. RNAseq libraries were prepared with Illumina's 'TruSeq Stranded RNAseq Sample Prep kit'. Prior to library preparation, eukaryotic rRNA was removed using the 'Ribo-Zero rRNA Removal Kit (Human/Mouse/Rat)', and bacterial rRNA was removed using the 'Ribo-Zero rRNA Removal Kit (Bacteria)' from Illumina. DNA and RNA were sequenced on HiSeq2500, RNA sequencing was performed at the Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign. DNA sequencing yielded an average of 35,127,007 reads per sample, while RNA sequencing of corresponding samples yielded an average of 13,786,716 reads per sample.
Genome reconstruction
DNA reads were trimmed using Sickle (v1.33) (https://github.com/najoshi/sickle), and assembled into scaffolds with IDBA_UD (v1.1.1) [15]. Scaffolds were binned into genomes using DAS tool (v1.0), which uses a combination of established binning algorithms [16]. Reconstructed genomes from each infant were de-replicated according to 99% average nucleotide identity using dRep (v2.3.2) [17]. One genome of each identified bacterial species was manually chosen from each infant gut microbiome for downstream RNA analysis. Scaffolds from MAG’s are accessible on ggkbase interface (https://ggkbase.berkeley.edu/), with a ‘scaffold to bin’ file available as supporting information files that can be used to reconstruct all selected genome bins used in this study (S3–S6 Files). In addition, Table A in S1 File shows accessions for the different Escherichia spp. genes used in this study.
Phylogenetic profiling
Taxonomic classification was done according to ‘shared affiliation of predicted proteins’ procedure, which compares each predicted protein on genome scaffolds, assembled from metagenomic sequences, to UniProt database, as described previously [11]. When more than 50% of predicted proteins on a scaffold shared the same taxonomic affiliation, which can be on any taxonomic level (from species to kingdom), this scaffold was classified according to the shared taxonomic affiliation [11].
Gene identification and annotation
Open reading frames (ORFs) were predicted using Prodigal (v2.6.3) [18] with the option to run in metagenome mode selected. Sequences of predicted ORFs were annotated using Hidden Markov Models (HMM) [19]. Annotations of the set of genes later analyzed in transcript ratio analyses were also confirmed by aligning against UniProtKB and UNIREF100 databases.
Calculating diametric ratios (DR)
Before analysis RNA reads were trimmed using Cutadapt [20], these RNA reads are available on the short-read archive (SRA) on NCBI, Bio-project ID PRJNA505710. To calculate Diametric Ratios (DR), RNA reads were mapped to the nucleotide sequence of all open reading frames, identified by Prodigal, in each scaffold of the de-replicated genomes sets, of each infant, using Bowtie2.
Further filtering of RNA reads, mapped to each gene sequences, was performed using the script ‘mapped.py’ (https://github.com/christophertbrown/bioscripts/blob/master/ctbBio/mapped.py). This script filters out any reads that map with more than one mismatch to the reference genome.
Transcript abundance was measured as coverage depth, by calculating the number of RNA reads per gene length. To calculate RNA reads coverage depth on each gene sequence we used the script ‘calculate_coverage.py’ (https://github.com/christophertbrown/bioscripts/blob/master/ctbBio/calculate_coverage.py). mapped.py and calculate_coverage.py are part of ctbbio version 0.45.
Gene expression levels were measured using diametric transcript ratios (DR), calculated according to Eq (1):
| q | (1) |
Where α is the transcript (RNA) abundance of a certain gene/genes (average abundances if abundances of several genes are considered, as denoted with the overbar) in a specific genome (G), and β is the transcript (RNA) abundance of a different gene/genes in the same genome (G) with an opposite transcriptional response to a surrounding physiological condition (E).
For example, if a certain surrounding physiological condition (E; e.g. oxygen level) increases transcription of gene α in genome G than for the same physiological condition (E) transcription of gene β in genome G decreases (Table 3). Because of the opposite transcriptional responses of examined genes these transcript ratios are referred to as diametric ratios (DR). Coupled genes for DR must have a transcriptional response that is opposite to a given physiological condition (such as norVW vs norR; Table 3) or respond to opposing physiological condition (such as ompC vs ompF Table 3). Calculating transcriptional responses this way allows measuring shifts in transcriptional responses while minimizing biases associated with changes in genome abundance. As noted, transcripts of the two sets of genes (α and β) originate from the same genome (G). Thus, given that the values of α and β are composed from transcript abundance per genome multiplied by genome abundance, the DR formula factors out genome abundance and the DR are comparable between samples.
Table 3. Genes examined in this study and the factors controlling their transcription.
| Coupled genes for DR | Genes | Factors controlling transcription§ | Variable in Eq (1) | Reference |
|---|---|---|---|---|
| 1) | cydAB | Micro-aerobic↑ | α | [21] |
| 1) | cyoABCD | Aerobic↑ | β | [21] |
| 2) | arcA | Anaerobic ↑ | α | [22] |
| 2) | fnr | Oxygen in-depended | β | [23] |
| 3) | nrdDG | Anaerobic and Micro-aerobic↑ | α | [24] |
| 3) | nrdAB | Aerobic↑ | β | [24] |
| 4) | norVW | Nitric oxide ↑ | α | [25] |
| 4) | norR | Nitric oxide ↓ | β | [25] |
| 5) | ompC | High osmolarity ↑ | α | [26] |
| 5) | ompF | Low osmolarity ↑ | β | [26] |
§ Arrow direction indicates whether the controlling factor up or down regulate transcription of the gene.
In each sample of our DR analysis we discarded genes that didn’t have any RNA reads mapped to them after the stringent filtering, to avoid α or β values of 0 leading to extreme DR values of 1 or 0.
Mapping to cytochrome oxidases from infant MAG’s compared to cytochrome oxidases from KEGG
In order to compare between mapping of RNA reads to genes from assembled genomes from premature infant’s gut microbiome to mapping of RNA reads to genes from available databases, E. coli (K-12 MG1655) cytochrome oxidases sequences were downloaded from KEGG (Kyoto Encyclopedia of Genes and Genomes) database. RNA reads were mapped to these genes and filtered as described previously for genes from assembled genomes. Diametric ratios were created for those mappings.
Statistical analyses
Differences in transcript ratios were visualized with boxplots constructed with R software for statistical computing (v3.5.1), using ggplot2 package (v3.1.1) [27].
Differences in DR of examined microbial genes were compared within time blocks between NEC and control infants. The first-time block was from 10th day of life till 19th day of life and a second time block was from 20th day of life till 36th day of life. In addition, differences in the DR of examined microbial genes were compared between all NEC and all control infants. Comparisons were done with Welch's t-test. To avoid type 1 error due to multiple comparisons, p values were adjusted with Bonferroni correction.
Results
Establishment of the diametric ratio as a quantitative metric
In this study, a new approach was examined to circumvent potential biases associated with meta-transcriptomic analysis. This approach involved accurate mapping of RNA reads to metagenomic assembled genomes (MAG’s) from the same pool of samples from which RNA reads were retrieved, to be more confident to the genomic origin of the transcripts. Next, transcript abundance ratios of genes that are known to have opposing transcriptional responses to a specific environmental exposure were calculated. To avoid biases related to changes in genome abundances, ratios were calculated only using transcripts belonging to the same genome within the same sample. This approach was designated as Diametric Ratios (DR) analysis, due to the expected opposite expression patterns of chosen genes. The approaches described here are hypothesis-driven, and are distinct from other recent approaches described for meta-transcriptomic observations, which aim to achieve a wide-spread comprehensive view of the changes in relative abundances of gene families and pathways [6,28].
To assess this approach, we analyzed 20 meta-transcriptomic datasets paired with MAG’s from gut microbiomes of 4 premature infants. Two of those premature infants were eventually diagnosed with NEC.
We focused our analysis to Escherichia spp. as this genus was ubiquitous in almost all of the analyzed samples (as representatives of this genus occurred in most of the samples, allowing statistical analysis between NEC and control samples), while other species or genera were not adequately ubiquitous for conducting comparisons between NEC and control samples (Fig A in S1 File). More than 85% of the predicted proteins on the same scaffolds of each of Escherichia spp. genomes had shared affiliation with Escherichia genus. On the species taxonomic level more than 84% of the predicted proteins on the same scaffolds had shared affiliation with either E. coli or E. vulneris (Table B in S1 File). Relative abundances of different bacterial genera were calculated by the ratio of genome coverage to sum of coverages of all genomes in a sample (Fig A in S1 File). Coverage depth and relative abundances data of other MAG’s in each sample is also available in S3–S6 Files. Each sample included about 15 MAG’s (max-24, min-10), with the genus Escherichia occurring in most samples. It is important to note, that RNA reads from each sample were mapped to the Escherichia sp. genome found in the same sample, as not all of the infants had E.coli species. Subsequent analyses were carried out on the genus level of Escherichia. Therefore, calculated DRs for all Escherichia spp. found in the different samples were gathered and compared between NEC infants and control infants. As Escherichia spp. are facultative anaerobes, they can adjust their life style according to available oxygen, which was well represented in its transcription pattern [3]. Thus, by examining Escherichia spp. we addressed a longstanding idea that inadequate oxygen tension in the intestine, due to reduced regional blood flow (ischemia), may be a key contributor to NEC development [29,30]. In vivo patterns of microbial gene expression in the infant gut may be an indicator of insufficient oxygen supply to the intestine and the progression of NEC. Furthermore, diametric ratios of different sets of genes related to other physiological conditions, such as exposure to different nitric oxide (NO) and osmolarity levels, were examined as well.
We examined transcripts abundances of cydAB and cyoABCD, genes encoding cytochrome oxidases with high and low affinity to oxygen (respectively; see below ‘cydAB cyoABCD diametric ratio’ section). Transcript abundances of each of these two sets of genes (either cydAB or cyoABCD, without any normalization accounting for genome abundance) was extremely variable within all-time blocks of either NEC or control infants. This high variation was exemplified through the high standard deviation compared to the average transcript abundance, yielding high coefficients of variance (between 93–174; Table 4). However, in NEC samples there was a trend showing differences between abundances of cydAB genes compared to cyoABCD genes while in Control infants no differences were found, inspiring the idea of diametric ratios. After DR calculation, much lower variation was found, yielding much lower coefficients of variance (20 and 24 for NEC and control infants, respectively; Table 4).
Table 4. Comparison between transcript abundances and diametric ratio (DR) of Escherichia spp. cytochrome oxidases across all time blocks in NEC and control infants.
| Infants | Genes/DR | Average | Standard Deviation | Coefficient of Variance | F-TEST§ | T-TEST§ |
|---|---|---|---|---|---|---|
| NEC | cydAB | 233 | 217 | 93.0 | 1.53E-12 | 0.008 |
| cyoABCD | 71.7 | 125 | 174 | 2.03E-09 | 0.117 | |
| cydAB-cyoABCD DR | 0.82 | 0.17 | 20.3 | 0.226 | 2.11E-05 | |
| Control | cydAB | 2.18 | 2.73 | 125 | ||
| cyoABCD | 3.09 | 4.40 | 142 | |||
| cydAB-cyoABCD DR | 0.43 | 0.10 | 23.9 |
§ p-values
cydAB cyoABCD diametric ratio
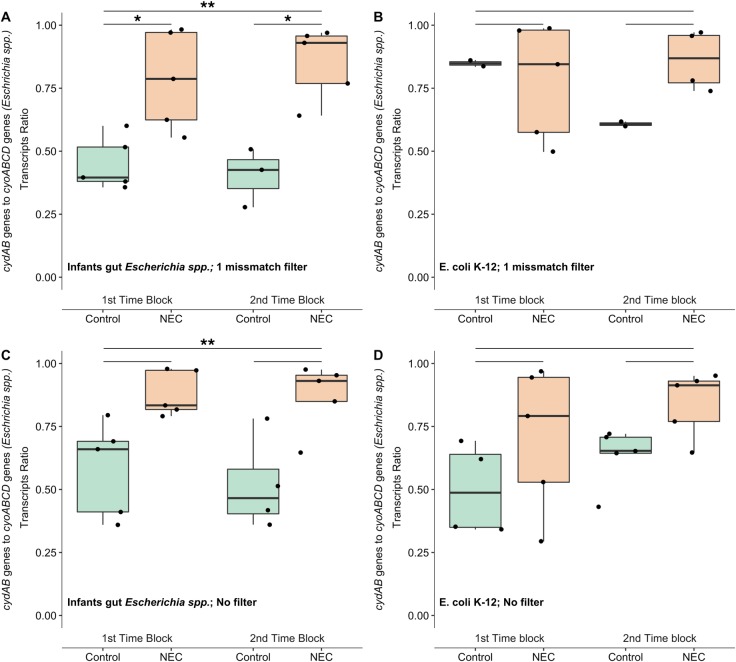
To examine the association between microbiome oxygen exposure and NEC development in the gut of premature infants, we first examined genes encoding for cytochrome oxidases. These protein complexes are a part of the electron transport chain that pass electrons to O2 during aerobic respiration. We constructed DR from transcript abundances of bacterial cytochrome oxidase genes that have different affinities for oxygen: cytochrome bd oxidase (cydAB) with high affinity for oxygen and cytochrome o oxidase (cyoABCD) with low affinity for oxygen [31,32]. Consistent with these biochemical predictions, a previous study showed that under microaerophilic conditions there was higher expression of cydAB whereas under aerobic conditions expression of cyoABCD genes increases [21]. Our results showed that Escherichia spp. had higher cydAB to cyoABCD transcript ratio in the gut of NEC infants compared to control infants (Fig 1A), and these differences were significant within both time blocks (p < 0.05) and also across all time blocks (p < 0.01).
Fig 1. Transcriptional response to oxygen by Escherichia spp. in the gut of NEC and control premature infants.
(A) Diametric ratios were compared between NEC and control infants in each time block (short lines above) and across all time points (longer lines). Distributions of diametric ratios were compared using Welch’s t-test with Bonferroni correction. Asterisks and double asterisks (*, **) represent p < 0.05 and p <0.01, respectively. (B) Diametric ratios of cydAB and cyoABCD transcript abundances of RNA reads mapped to gene sequences of Escherichia spp. genomes found in infants’ gut. Filtering of reads with more than 1 miss matches was applied. (C) Diametric ratios of cydAB and cyoABCD transcript abundances of RNA reads mapped to gene sequences of E. coli (K-12 MG1655) downloaded from KEGG (Kyoto Encyclopedia of Genes and Genomes) database. Filtering of reads with more than 1 miss matches was applied. (D) Diametric ratios of cydAB and cyoABCD transcript abundances of RNA reads mapped to gene sequences of Escherichia spp. genomes found in infants’ gut. No filtering of reads with miss matches was applied.
To evaluate whether mapping RNA reads to genes from sample-specific MAG’s could improve results sensitivity compared to mapping to genes retrieved from KEGG databases, we examined results of cydAB and cyoABCD diametric ratios between the two mapping approaches. We found that mapping RNA reads to genes from Escherichia spp. from each infant microbiome MAG’s had more significantly distinguishable DR between NEC and control infants then DR found for RNA reads mapped to genes retrieved from KEGG database (Fig 1A and Fig 1B, respectively). Further evaluation of differences between MAG’s and E.coli K12 gene sequences through alignments of cydA genes showed variable number of mismatches (S8 File), ranging from 1 to 19 in E.coli species and 269 mismatches with E.vulneris. This signal was enhanced when filtering out reads that had more than 1 base mismatch (Fig 1A compared to Fig 1C). After filtering, mapping to KEGG database genes was even less assured as there were less data points due to filtering out of reads that were inaccurately mapped (Fig 1B compared to Fig 1D). These results highlighted the necessity for having MAG’s retrieved from the same sample set as RNA reads were retrieved, as well as accurate mapping of RNA reads.
fnr arcA and nrdGD nrdAB diametric ratios
To further strengthen our observations that Escherichia spp. in the gut of NEC infants were exposed to lower oxygen levels, we analyzed another set of genes that are differentially transcribed in response to oxygen levels, fnr and arcA (Fumarate and nitrate reductase, and Aerobic respiration control protein, respectively). fnr transcription is consistent in both aerobic and anaerobic conditions [22], and the regulatory mode is through changes in FNR protein conformation at different oxygen levels [33]. However, the transcription of arcA, which is regulated by fnr, increases when low oxygen conditions prevail [22].
A third set of genes that their transcription is associated to oxygen levels, nrd, encodes ribonucleotide reductase, which catalyzes the enzymatic reduction of ribonucleotides to deoxyribonucleotides. Escherichia spp. have two sets of nrd genes that are differently expressed depending on prevailing oxygen levels. Under aerobic conditions transcription of nrdAB is upregulated, while under anaerobic condition transcription of nrdDG is upregulated [24].
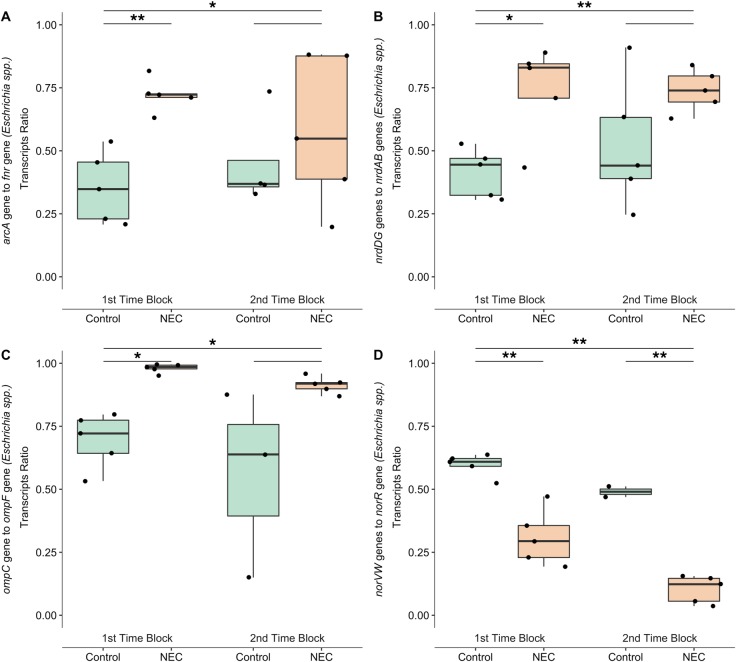
According to the cydAB and cyoABCD diametric ratios we hypothesized that diametric ratios of arcA and fnr genes and nrdDG and nrdAB genes transcribed by Escherichia spp. in the gut of NEC infants would be higher compared to control infants. Indeed, results of these diametric ratios confirmed the results of cydAB and cyoABCD diametric ratios, showing that higher ratios of both arcA and fnr genes nrdDG and nrdAB genes of Escherichia spp. in the gut of the NEC premature infants were significantly higher, across all time blocks (p < 0.05 and p < 0.01, respectively; Fig 2A and 2B) and specifically in the 1st time block (p < 0.01 and p < 0.05, respectively; Fig 2A and 2B). These results further suggested that Escherichia spp. in the gut of the NEC premature infants were exposed to lower oxygen levels.
Fig 2. Transcriptional response to oxygen, Nitric oxide and osmotic conditions by Escherichia spp. in the gut of NEC and control premature infants.
(A) Diametric ratios were compared between NEC and control infants in each time block (short lines above) and across all time points (longer lines). Distributions of diametric ratios were compared using Welch’s t-test with Bonferroni correction. Asterisks and double asterisks (*, **) represent p < 0.05 and p <0.01, respectively.(A) Diametric ratios of arcA and fnr transcript abundances of RNA reads mapped to sequences of Escherichia spp. genomes found in infants’ gut. (B) Diametric ratios of nrdDG and nrdAB transcript abundances of RNA reads mapped to sequences of Escherichia spp. genomes found in infants’ gut. (C) Diametric ratios of ompC and ompF transcript abundances of RNA reads mapped to gene sequences of Escherichia spp. genomes found in infants’ gut. (D) Diametric ratios of norVW and norV transcript abundances of RNA reads mapped to gene sequences of Escherichia spp. genomes found in infants’ gut.
ompC ompF and norVW norR diametric ratios
The next set of genes we examined encode the Outer Membrane Proteins, ompC and ompF. These genes were previously shown to be transcribed under different osmotic conditions, with high abundance of ompC being associated with inflammatory bowel diseases [34]. High expression of ompC, which has small porin size, occurs during high osmotic conditions, while ompF, which has large porin size, occurs during low osmotic conditions [26]. These genes are reciprocally regulated by ompR, depending on its phosphorylation state [26]. Interestingly, significantly higher diametric ratios of ompC to opmF were found to be transcribed by Escherichia spp. in the gut of NEC premature infants compared to control premature infants across all time blocks (p > 0.05), specifically in the 1st time block (p < 0.05; Fig 2C).
The last set of genes we examined were the norVW genes, coding for NO detoxifying enzyme Nitric Oxide Reductase, and their oppositely transcribed regulating gene norR [25]. Nitric oxide binds to constitutively expressed NorR, which up-regulates the transcription of norVW and down-regulates norR expression [25]. Thus, to assess microbial transcriptional response to NO prior to NEC diagnosis, we measured the ratio of transcript abundances for norVW and norR. Higher diametric ratios of NO detoxifying enzymes compared to norR transcribed by Escherichia spp. were found in the guts of the control infants compared to NEC infants, in both time blocks and across time blocks (p < 0.01, for all cases; Fig 2D). Interestingly, the ratio of norVW to norR transcript abundances was higher at earlier compared to later time points, for both NEC and control infants (Welch’s t test, p = 0.009). This may reflect the response of the preterm infant gut to initial microbial colonization.
Discussion
Using diametric ratios and RNAseq mapping to MAG’s to infer physiological conditions in the gut of premature infants
Here we demonstrate that calculating diametric ratios of genes with opposite transcriptional responses to ambient physiological conditions is an approach that can be effectively used for analyzing meta-transcriptomic data (Table 4; Fig 1). In addition, we show that mapping to sample specific MAG’s provides the most clear and significant signal. Results based on three sets of genes (cytochrome oxidase genes, aerobic/anaerobic regulation genes and ribonucleotide reductase genes) suggest that Escherichia spp. in the gut of two premature infants that developed NEC were exposed to lower oxygen levels than Escherichia spp. in the gut of two premature infants without NEC. Together, these data suggest that hypoxic conditions may exist in the gut prior to NEC development.
Previous animal model studies and analyses of early microbial colonization of premature infant guts showed that at early gestational age the gut milieu was more aerobic [35–38]. During NEC, however, hypoxic conditions were observed in the gut tissue of many patients [39]. Furthermore, histologic examination of removed dead intestine tissue of NEC patients demonstrated coagulation necrosis, evidence for ischemic injury [40], in either small or large intestine. Yet, hypoxia is highly debated as a primary controlling factor of NEC [29,41–43]. Recent theories on NEC development point to the role of gut tissue immaturity, impairing intestinal microcirculation and oxygen delivery [44], which might explain the observed transcriptional response to lower O2 levels in the gut microbiomes of NEC infants (Fig 1A; Fig 2A and 2B). It should be noted also that these circumstances are distinct from those that occur in mature gut systems, where anaerobic conditions are linked to a healthy condition and aerobicity is linked to inflammation [45].
Another potential indicator for progression of inflammatory response in the infant gut is exposure of the gut microbiome to nitric oxide (NO). Increased expression of inducible nitric oxide synthase (iNOS) by host’s gut epithelial cells often occurs as a part of the inflammatory response, and recent studies have suggested that TLR4-mediated iNOS expression is a key element of NEC progression [46]. Thus, diametric ratios for nitric oxide reductase can help infer about exposure of Escherichia spp. to different nitric oxide levels in the gut of premature infants. Counter-intuitively to progression of an inflammatory response in the gut of NEC infants, results of this study indicate down regulation of bacterial genes for NO detoxification in the gut of NEC infants (Fig 2D), indicating lower NO levels in the gut lumen. This might be explained by low oxygen supply to the gut lumen, as suggested by results found by previous examined genes (Fig 1A; Fig 2A and 2B), reducing host epithelial cells iNOS activity to produce the antimicrobial agent NO, as these enzymes need oxygen to produce NO [47,48]. Alternatively, these results might also be explained by the report that norVW transcription decreases with combined oxidative and nitrosative stresses in contrast to nitrosative stress alone [49], as occurs during inflammation [50]. Inflammatory response inducing combined oxidative and nitrosative stresses also stimulates higher cydAB transcription (Fig 1A; [51,52]). An additional physiological condition that can be associated with inflammatory response is altered osmotic conditions [53].
Results of diametric ratios between transcripts of outer membrane protein genes indicate that Escherichia spp. in the gut of NEC premature infants might be exposed to high osmotic conditions (Fig 2C). Consistent with previous observation of high expression of ompC by E. coli during high osmolarity levels and increased adherence to host gut epithelial cell through the development of Crohn’s disease [34]. To the best of our knowledge, little is known about gut lumen osmolarity and the development of NEC. Osmolality of feeding formula and its association with NEC development has been studied, but no clear connection was found [54].
Confounding factors of this study dataset
It should be noted, however, that this data set contains confounding factors limiting the interpretation of Escherichia spp. transcriptional differences between NEC and control infants solely as a result of NEC development. First of all, the limited number of infants (two NEC and two control) examined in this study impaired our ability to draw conclusions. Low sample number also restricted our ability to define cutoffs to optimize DR analysis, as discarding more data points would have limited statistical analysis on the time block level. Secondly, other factors, such as gestational age, mode of delivery and birth weight, also differed between NEC cases and controls (Table 1). These factors were previously shown to affect the development of microbial communities [36,55], suggesting that occurrence of different physiological conditions bring about such alterations in infant gut microbiomes.
Nevertheless, transcription results shown here were opposite than expected according to some of those factors, such as gestational age. According to previous studies, younger gestational age infants might have more aerobic conditions as their microbial communities are associated with a more facultative anaerobic life style compared to older gestational age infants associated with an obligate anaerobic life style [36,38,56]. Whereas our results show that lower oxygen exposure occurred at earlier gestational age, in infants that develop NEC (Table 1; Fig 1A; Fig 2A and 2B). Although, larger sized and higher time point resolution gut microbiome meta-transcriptomic studies would be fundamental to confirm shifts in aerobicity state in the intestine of premature infants. In addition, many of the studies examining how these factors affect microbial communities were done on full term infants and data on gut microbiome of preterm infants is still very scarce. A recent paper examining gut microbiome in preterm infants showed that cesarean or vaginal birth mode did not significantly affect microbial communities [57], unlike full-term infant where delivery mode is a major factor shaping infant gut microbiome [55,58].
Although it is hard to conclude whether observed differences were associated with NEC development or other factors, the methodological approach described here still shows a clear and significant signal distinguishing between NEC and control premature infants. A larger study is needed to verify these results and confirm that the gut microbiome in early stages of NEC development senses and responds to different physiological conditions compared to microbiomes in guts of premature infant where NEC is not developing. Further experiments can verify this approach, either by experiments where stool samples are inoculated into artificial media with varying physiological condition (e.g. oxygen levels or NO) or experiments with animal models inducing wanted physiological conditions in-vivo.
Concluding remarks on examined approaches
The approach described here can add new insights into gut microbiome analysis. It is important to note that this approach relies upon prior knowledge on the transcriptional responses of different genes to physiological conditions. It is, thus, essential to do a preliminary literature survey on gene expression pathways of dominant species in examined samples to decide on genomes and genes from which to calculate diametric ratios. In addition, a significant factor affecting the accuracy of DR measurements is proper read mapping. Mapping RNA reads to sample-specific MAG’s is shown here to give more significantly distinguishable signal compared to mapping to genes retrieved from an outside database (Fig 1). The mapping exercise shown here might potentially describe the extreme end of strain heterogeneity, as K-12 E. coli is a laboratory strain that can be different enough from gut E. coli to result in inquorate RNA read mapping. Choosing genomes from databases originating from gut microbiome databases might be more adequate for RNA mapping than KEGG database, as long as chosen genomes are most similar to those genomes for which RNA was sequenced. Genome databases are valuable for taxonomic identification, as done here for confident identification of MAG’s in our samples. However, based on propositions put forth in this study, further research might further enforce the idea that MAG’s enable more accurate mapping and promote better quantification of RNA reads than genomes from databases. It is also important to note that potentially fewer metagenomes are required per individual than meta-transcriptomes, as it was previously shown that specific strains can remain stable within an individual human host [12,59].
In conclusion, we show here how meta-transcriptomic data combined with sample-specific MAG’s can be applied effectively to probe the physiological conditions that gut microbial communities experience by comparing diametric ratios. Further application of this approach can bring new insights on microbe-host interactions within the GI tract systems, and potentially help identify biomarkers for early detection gut diseases, such as NEC, onset and progression.
Supporting information
Fig A. Relative abundances of different Bacterial genera; Table A. Accession phrases for Escherichia spp. genes in on ggkbase interface; Table B. Percent of shared affiliation of the predicted proteins.
(DOCX)
(XLSX)
(TSV)
(TSV)
(TSV)
(TSV)
(XLSX)
(TXT)
Acknowledgments
We wish to thank two anonymous reviewers for their critical and constructive reviews.
Data Availability
RNA reads are available on the short-read archive (SRA) on NCBI, Bio-project ID PRJNA505710. Scaffolds from assemblies are accessible on ggkbase interface (https://ggkbase.berkeley.edu/), with a ‘scaffold to bin’ file available as a supplementary file that can be used to reconstruct all genome bins used in this study.
Funding Statement
This research was supported by the National Institutes of Health (NIH) under award no. RAI092531A to JFB. This work was supported in part by the March of Dimes Foundation research Grant no. 5-FY10-103 (to MJM). YS work was supported by postdoctoral fellowship grant no. 2016-67012-24717 from the USDA National Institute of Food and Agriculture. The funder Enview, inc., provided support in the form of salaries for the author RS, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Rivera-Chávez F, Lopez CA, Bäumler AJ. Oxygen as a driver of gut dysbiosis. Free Radical Biology and Medicine. 2017;105: 93–101. 10.1016/j.freeradbiomed.2016.09.022 [DOI] [PubMed] [Google Scholar]
- 2.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, et al. Correlation Between Intraluminal Oxygen Gradient and Radial Partitioning of Intestinal Microbiota. Gastroenterology. 2014;147: 1055–1063.e8. 10.1053/j.gastro.2014.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge JD, Sanguinetti G, Dibden DP, Roberts RE, Poole RK, Green J. Transition of Escherichia coli from Aerobic to Micro-aerobic Conditions Involves Fast and Slow Reacting Regulatory Components. J Biol Chem. 2007;282: 11230–11237. 10.1074/jbc.M700728200 [DOI] [PubMed] [Google Scholar]
- 4.Tjaden B, Saxena RM, Stolyar S, Haynor DR, Kolker E, Rosenow C. Transcriptome analysis of Escherichia coli using high‐density oligonucleotide probe arrays. Nucleic Acids Res. 2002;30: 3732–3738. 10.1093/nar/gkf505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazen TH, Michalski J, Luo Q, Shetty AC, Daugherty SC, Fleckenstein JM, et al. Comparative genomics and transcriptomics of Escherichia coli isolates carrying virulence factors of both enteropathogenic and enterotoxigenic E. coli. Scientific Reports. 2017;7: 3513 10.1038/s41598-017-03489-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schirmer M, Franzosa EA, Lloyd-Price J, McIver LJ, Schwager R, Poon TW, et al. Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nature Microbiology. 2018;3: 337 10.1038/s41564-017-0089-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackam D, Caplan M. Necrotizing enterocolitis: Pathophysiology from a historical context. Seminars in Pediatric Surgery. 2018;27: 11–18. 10.1053/j.sempedsurg.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neu J, Walker AW. Necrotizing Enterocolitis. The New England Journal of Medicine. 2011;38: 552–559. 10.1542/pir.2017-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome. 2017;5 10.1186/s40168-016-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown CT, Xiong W, Olm MR, Thomas BC, Baker R, Firek B, et al. Hospitalized premature infants are colonized by related bacterial strains with distinct proteomic profiles. mBio. 2018;9 10.1128/mBio.00441-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raveh-Sadka T, Thomas BC, Singh A, Firek B, Brooks B, Castelle CJ, et al. Gut bacteria are rarely shared by co-hospitalized premature infants, regardless of necrotizing enterocolitis development. eLife. 2015;2015: 1–25. 10.7554/eLife.05477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raveh-Sadka T, Firek B, Sharon I, Baker R, Brown CT, Thomas BC, et al. Evidence for persistent and shared bacterial strains against a background of largely unique gut colonization in hospitalized premature infants. ISME J. 2016;10: 2817–2830. 10.1038/ismej.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks B, Olm MR, Firek BA, Baker R, Thomas BC, Morowitz MJ, et al. Strain-resolved analysis of hospital rooms and infants reveals overlap between the human and room microbiome. Nature Communications. 2017;8: 1–7. 10.1038/s41467-016-0009-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morowitz MJ, Denef VJ, Costello EK, Thomas BC, Poroyko V, Relman DA, et al. Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. PNAS. 2011;108: 1128–1133. 10.1073/pnas.1010992108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y, Leung HCM, Yiu SM, Chin FYL. IDBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics. 2012;28: 1420–1428. 10.1093/bioinformatics/bts174 [DOI] [PubMed] [Google Scholar]
- 16.Sieber CMK, Probst AJ, Sharrar A, Thomas BC, Hess M, Tringe SG, et al. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nature Microbiology. 2018;3: 836–843. 10.1038/s41564-018-0171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olm MR, Brown CT, Brooks B, Banfield JF. DRep: A tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME Journal. 2017;11: 2864–2868. 10.1038/ismej.2017.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyatt D, Chen GL, LoCascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond S, Andeer PF, Li Z, Crits-Christoph A, Burstein D, Anantharaman K, et al. Mediterranean grassland soil C–N compound turnover is dependent on rainfall and depth, and is mediated by genomically divergent microorganisms. Nat Microbiol. 2019;4: 1356–1367. 10.1038/s41564-019-0449-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17: 10–12. 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 21.Tseng CP, Albrecht J, Gunsalus RP. Effect of microaerophilic cell growth conditions on expression of the aerobic (cyoABCDE and cydAB) and anaerobic (narGHJI, frdABCD, and dmsABC) respiratory pathway genes in Escherichia coli. J Bacteriol. 1996;178: 1094–1098. 10.1128/jb.178.4.1094-1098.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Compan I, Touati D. Anaerobic activation of arcA transcription in Escherichia coli: roles of Fnr and ArcA. Molecular Microbiology. 1994;11: 955–964. 10.1111/j.1365-2958.1994.tb00374.x [DOI] [PubMed] [Google Scholar]
- 23.Spiro S, Guest JR. Regulation and Over-expression of the fnr Gene of Escherichia coli. Microbiology. 1987;133: 3279–3288. 10.1099/00221287-133-12-3279 [DOI] [PubMed] [Google Scholar]
- 24.Torrents E, Grinberg I, Gorovitz-Harris B, Lundström H, Borovok I, Aharonowitz Y, et al. NrdR Controls Differential Expression of the Escherichia coli Ribonucleotide Reductase Genes. Journal of Bacteriology. 2007;189: 5012–5021. 10.1128/JB.00440-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarboe LR, Hyduke DR, Liao JC. Chapter 4—Systems Approaches to Unraveling Nitric Oxide Response Networks in Prokaryotes Second Edi In: Ignarro LJ, editor. Nitric Oxide: Biology and Pathobiology. Second Edi. San Diego: Academic Press; 2010. pp. 103–136. 10.1016/B978-0-12-373866-0.00004-6 [DOI] [Google Scholar]
- 26.Yoshida T, Qin L, Egger LA, Inouye M. Transcription Regulation of ompF and ompC by a Single Transcription Factor, OmpR. J Biol Chem. 2006;281: 17114–17123. 10.1074/jbc.M602112200 [DOI] [PubMed] [Google Scholar]
- 27.Wickham H. ggplot2: elegant graphics for data analysis. Second edition. Cham: Springer; 2016. [Google Scholar]
- 28.Abu-Ali GS, Mehta RS, Lloyd-Price J, Mallick H, Branck T, Ivey KL, et al. Metatranscriptome of human faecal microbial communities in a cohort of adult men. Nature Microbiology. 2018;3: 356 10.1038/s41564-017-0084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young CM, Kingma SDK, Neu J. Ischemia-reperfusion and neonatal intestinal injury. Journal of Pediatrics. 2011;158: e25–e28. 10.1016/j.jpeds.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 30.Morowitz MJ, Poroyko V, Caplan M, Alverdy J, Liu DC. Redefining the Role of Intestinal Microbes in the Pathogenesis of Necrotizing Enterocolitis. Pediatrics. 2010;125: 777–785. 10.1542/peds.2009-3149 [DOI] [PubMed] [Google Scholar]
- 31.D’Mello R, Hill S, Poole RK. The Cytochrome bd Quinol Oxidase in Escherichia coli Has an Extremely High Oxygen Affinity and Two-Oxygen-binding Haems: Implicaitons for Regulation of Activity in vivo by Oxygen Inihibition. Microbiology. 1996;142: 755–763. 10.1099/00221287-142-4-755 [DOI] [PubMed] [Google Scholar]
- 32.D’Mello R, Hill S, Poole RK. The oxygen affinity of cytochrome bo’ in Escherichia coli determined by the deoxygenation of oxyleghemoglobin and oxymyoglobin: K(m) values for oxygen are in the submicromolar range. Journal of Bacteriology. 1995;177: 867–870. 10.1128/jb.177.3.867-870.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unden G, Achebach S, Holighaus G, Wackwitz B, Zeuner Y. Control of FNR Function of Escherichia coli by O2 and Reducing Conditions. J Mol Microbiol Biotechnol. 2002;4: 6. [PubMed] [Google Scholar]
- 34.Rolhion N, Carvalho FA, Darfeuille‐Michaud A. OmpC and the σE regulatory pathway are involved in adhesion and invasion of the Crohn’s disease-associated Escherichia coli strain LF82. Molecular Microbiology. 2007;63: 1684–1700. 10.1111/j.1365-2958.2007.05638.x [DOI] [PubMed] [Google Scholar]
- 35.Nankervis CA, Reber KM, Nowicki PT. Age-Dependent Changes in the Postnatal Intestinal Microcirculation. Microcirculation. 2001;8: 377–387. 10.1038/sj/mn/7800110 [DOI] [PubMed] [Google Scholar]
- 36.La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-moore CM, et al. Patterned progression of bacterial populations in the premature infant gut. Proceedings of the National Academy of Sciences. 2014;111: 17336–17336. 10.1073/pnas.1420817111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brooks B, Mueller RS, Young JC, Morowitz MJ, Hettich RL, Banfield JF. Strain-resolved microbial community proteomics reveals simultaneous aerobic and anaerobic function during gastrointestinal tract colonization of a preterm infant. Frontiers in Microbiology. 2015;6: 1–10. 10.3389/fmicb.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharon I, Morowitz MJ, Thomas BC, Costello EK, Relman DA, Banfield JF. Time series community genomics analysis reveals rapid shifts in bacterial species, strains, and phage during infant gut colonization. Genome Research. 2013;23: 111–120. 10.1101/gr.142315.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Chang KTE, Lian DWQ, Lu H, Roy S, Laksmi NK, et al. The role of ischemia in necrotizing enterocolitis. Journal of Pediatric Surgery. 2016;51: 1255–1261. 10.1016/j.jpedsurg.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 40.Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. The Journal of Pediatrics. 1990;117: 6–13. 10.1016/S0022-3476(05)81124-2 [DOI] [PubMed] [Google Scholar]
- 41.Neu J. The “myth” of asphyxia and hypoxia-ischemia as primary causes of necrotizing enterocolitis. Biology of the Neonate. 2005;87: 97–98. 10.1159/000081898 [DOI] [PubMed] [Google Scholar]
- 42.Crissinger K. Regulation of hemodynamics and oxygenation in developing intestine: insight into the pathogenesis of necrotizing enterocolitis. Acta P\a ediatrica. 1994;83: 8–10. 10.1111/j.1651-2227.1994.tb13233.x [DOI] [PubMed] [Google Scholar]
- 43.Nowicki PT, Nankervis CA. The Role of the Circulation in the Pathogenesis of Necrotizing Enterocolitis. Clinics in Perinatology. 1994;21: 219–234. 10.1016/S0095-5108(18)30342-7 [DOI] [PubMed] [Google Scholar]
- 44.Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS–NO–nitrite signaling. Proceedings of the National Academy of Sciences. 2013;110: 9451–9456. 10.1073/pnas.1219997110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Litvak Y, Byndloss MX, Tsolis RM, Bäumler AJ. Dysbiotic Proteobacteria expansion: a microbial signature of epithelial dysfunction. Current Opinion in Microbiology. 2017;39: 1–6. 10.1016/j.mib.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 46.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The Roles of Bacteria and TLR4 in Rat and Murine Models of Necrotizing Enterocolitis. The Journal of Immunology. 2006;177: 3273–3282. 10.4049/jimmunol.177.5.3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson MA, Baumgardner JE, Otto CM. Oxygen-dependent regulation of nitric oxide production by inducible nitric oxide synthase. Free Radical Biology and Medicine. 2011;51: 1952–1965. 10.1016/j.freeradbiomed.2011.08.034 [DOI] [PubMed] [Google Scholar]
- 48.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. 2015;36: 34–45. [DOI] [PubMed] [Google Scholar]
- 49.Baptista JM, Justino MC, Melo AMP, Teixeira M, Saraiva LM. Oxidative Stress Modulates the Nitric Oxide Defense Promoted by Escherichia coli Flavorubredoxin. Journal of Bacteriology. 2012;194: 3611–3617. 10.1128/JB.00140-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17: 497–511. 10.1038/s41579-019-0213-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindqvist A, Membrillo-Hernández J, Poole RK, Cook GM. Roles of respiratory oxidases in protecting Escherichia coli K12 from oxidative stress. Antonie Van Leeuwenhoek. 2000;78: 23–31. 10.1023/a:1002779201379 [DOI] [PubMed] [Google Scholar]
- 52.Hyduke DR, Jarboe LR, Tran LM, Chou KJY, Liao JC. Integrated network analysis identifies nitric oxide response networks and dihydroxyacid dehydratase as a crucial target in Escherichia coli. PNAS. 2007;104: 8484–8489. 10.1073/pnas.0610888104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brocker C, Thompson DC, Vasiliou V. The role of hyperosmotic stress in inflammation and disease. BioMolecular Concepts. 2012;3: 345–364. 10.1515/bmc-2012-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramani M, Ambalavanan N. Feeding Practices and Necrotizing Enterocolitis. Clinics in Perinatology. 2013;40: 1–10. 10.1016/j.clp.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562: 583 10.1038/s41586-018-0617-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown CT, Sharon I, Thomas BC, Castelle CJ, Morowitz MJ, Banfield JF. Genome resolved analysis of a premature infant gut microbial community reveals a Varibaculum cambriense genome and a shift towards fermentation-based metabolism during the third week of life. Microbiome. 2013;1: 30 10.1186/2049-2618-1-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart CJ, Embleton ND, Clements E, Luna PN, Smith DP, Fofanova TY, et al. Cesarean or Vaginal Birth Does Not Impact the Longitudinal Development of the Gut Microbiome in a Cohort of Exclusively Preterm Infants. Front Microbiol. 2017;8 10.3389/fmicb.2017.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. 2016;8: 343ra82–343ra82. 10.1126/scitranslmed.aad7121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, et al. The Long-Term Stability of the Human Gut Microbiota. Science. 2013;341: 1237439 10.1126/science.1237439 [DOI] [PMC free article] [PubMed] [Google Scholar]