Abstract
The advancement of high-resolution metabolomics (HRM) and metabolome-wide-association study (MWAS) enables the readout of environmental effects in human specimens. We used HRM to understand DDT-induced alterations of in utero environment and potential health effects. Endogenous metabolites were measured in 397 maternal perinatal serum samples collected during 1959–1967 in the Child Health and Development Studies (CHDS) and in 16 maternal postnatal serum samples of mice treated with or without DDT. MWAS was performed to assess associations between metabolites and p,p’-DDT, o,p’-DDT and p,p’-DDE levels, followed by pathway analysis. Distinct metabolic profiles were found with p,p’-DDT and p,p’-DDE. Amino acids such arginine had a strong association with p,p’-DDT and o,p’-DDT in both women and mice, whereas lipids and acyl-carnitine intermediates were found exclusively associated with p,p’-DDE in CHDS women indicating mitochondrial impairment. It suggests that the role of serine and fatty acid metabolism on the causal disease pathway should be examined.
Keywords: DDT, DDE, metabolomics, in utero, CHDS
Introduction
Dichlorodiphenyltrichloroethane (DDT) chemically known as 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane was one of the most widely used organochlorine pesticides around the world for both agricultural and non-agricultural purposes [1, 2]. Despite the ban of use in many countries included the United States, DDT and its degradation products (including dichlorodiphenyldichloroethylene, DDE) are persistent and transplacental toxicants that remain in the environment and present a significant threat to human health. Known effects of DDT and DDE in animals include cancer and toxicity to the reproductive, developmental, neurological and metabolic systems [1–3]. In recent years, concerns have been raised that the increased incidence of breast cancer during the past 50 years is due to exposure to endocrine disruptors such as DDT [2, 4]. In particular, multiple studies have proposed in utero as a key timing during development; exposure to hormonally active agents at this time induces massive changes in composition of the extracellular matrix and anatomy of the mammary gland thus predisposing the offspring to neoplastic development at later life stage [4, 5]. Nevertheless, the actual effect of fetal exposure to environmental endocrine disruptors in humans remains difficult to assess.
More recently, several studies have reported link between early-life DDT exposure and breast cancer risk based on the Child Health and Development Studies (CHDS) pregnancy cohort [6, 7]. The CHDS is a multi-generational follow-up of pregnancies enrolled during 1959–1967 when DDT was most heavily used. It was found that women who were exposed to p,p’-DDT prior to age 14 had 5-fold or even higher increase in the risk of breast cancer before age 50 in their daughters, and the DDT association was only observed for women with exposure before puberty [7]. A follow-up study found that high maternal serum levels of o,p’-DDT (an isomer of DDT that was a contaminant found in technical DDT) predicted a nearly 4-fold increase in the daughter’s risk of developing breast cancer [6]. Several meta-analysis of multiple human epidemiology studies also showed implications of in utero exposure to DDT and/or DDE on obesity and type 2 diabetes [3, 8]. These findings clearly support the need to further investigate the role of early-life DDT exposure in human health.
Development of a number of systems biology methodologies including metabolomics has provided new technological and conceptual tools to understand the complex mechanisms underlying environmentally mediated diseases. High-resolution metabolomics (HRM) measures metabolites in most metabolic pathways and provides a comprehensive and quantitative phenotyping of global metabolic alterations at molecular level [9–13]. Metabolic profiling of maternal samples can reflect the alterations in fetal environment and be further linked to the health outcomes of the offspring [14–16]. In addition, our past research work with environmental heavy metals and volatile organic compounds have consistently demonstrated the value of using HRM to study cellular mechanisms in response to exposure in animal models [12, 17–19].
In this study, we used HRM of 397 maternal serum samples collected during pregnancy and early postpartum to determine the metabolic effects associated with DDT and DDE exposure and to provide implications for the influence on fetal health. Because the most prevalent exposures include the isomer of p,p’-DDT (~85% of technical DDT), its degradation product p,p’-DDE, and o,p’-DDT (~15% of technical DDT), we built separate regression models to examine metabolic effects associated with three individual compounds (p,p’-DDT, o,p’-DDT and p,p’-DDE). This is especially useful as they may exhibit different toxicities. For example, o,p’-DDT has shown a much stronger estrogenic activity than p,p’-DDT while the persistent metabolite p,p’-DDE has been shown as an androgen receptor antagonist in animals [1, 2]. Our results in the present study showed that maternal p,p’-DDT, o,p’-DDT and p,p’-DDE exposure were associated with differential metabolic characteristics suggesting crosstalk between non-essential amino acid and lipid metabolism. Using a mouse model, we utilized experimental doses within the range of the top tertile of CHDS mothers in order to validate our human findings prospectively while free from confounding that typifies human studies. The result shows that the associations of DDT with urea cycle and amino acids are consistent between the human data and the mouse data.
Methods
Study population and exposures
The 397 subjects that participated in this study were part of the Child health and Development Studies (CHDS) cohort. These are a subset of the samples that were the basis of a recent report of associating maternal perinatal DDT exposure and daughter’s breast cancer risk [6]. The CHDS was designed to examine the association between prenatal exposures and health and development over the life course for parents and children. The CHDS recruited women residing in the Oakland, California area who were members of the Kaiser Foundation Health Plan and received obstetric care for pregnancies between 1959 and 1967 [20]. This paper includes 50 randomly selected cases (breast cancer in daughters) and 347 controls, roughly approximating the disease prevalence of 1/8 of the US population. The cases and controls were matched on birth year and trimester of maternal blood draw. All blood specimens were collected throughout all trimesters and early postpartum (1–3 days after delivery). The present study was reviewed and approved by The Institutional Review Board of the Public Health Institute and it has complied with all federal guidelines governing use of human subjects.
DDT levels and serum lipids were measured in non-fasting maternal perinatal serum samples. All serum samples were stored at −20°C at the repository and shipped frozen to the laboratory. An aliquot of 1.5 mL was assayed for dichlorodiphenyltrichloroethane (DDTs), including p,p’-DDT, (85% of technical-grade DDT manufactured); o,p’-DDT (15% of technical-grade DDT) and the primary metabolite, p,p’-DDE, in the laboratory of the California Department of Toxic Substances Control using methods developed previously [21]. Briefly, serum samples spiked with surrogate standards (tetrachloro-m-xylene, PCB-65, PCB-166) were extracted using Oasis HLB SPE cartridges (Waters Corp.; Milford, MA), and subsequently cleaned up with 33% sulfuric acid silica. DDT compounds were analyzed on a DB-5ms column (30 m x 0.25 mm I.D., 0.25 μm film thickness; Agilent Technologies; Sunnyvale, CA) using gas chromatograph-tandem mass spectrometer (Agilent, Santa Clara, CA). A calibration curve, ranging from 0.1 to 30 pg/μL (DDTs) or 0.1 to 800 pg/μL (DDE) (R2 >0.99), was used for quantitation. The method detection limit of o,p’-DDT, p,p’-DDT and p,p’-DDE is 0.012, 0.010 and 0.027 ng/mL as previously reported [21]. Total lipids were measured by total cholesterol and triglycerides assayed enzymatically as previously described [6] at the Clinical & Epidemiologic Research Laboratory (CERLab) at Boston Children’s Hospital (certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization).
Animal exposure experiment
The 1.7 mg DDT mixture/kg BW/day was created to replicate the commercial pesticide formula of DDT used in the US before it was banned in the 1970s, which consisted of 77.2% p,p’-DDT (97.7% purity neat, AccuStandard, New Haven, CT) and 22.8% o,p’-DDT (98.6 % purity neat, AccuStandard). C57BL/6J female mice (Jackson Laboratory, Bar Harbor, ME) were mated. Gestational day (GD) 0.5 was identified by the existence of a vaginal plug. Dams were randomly assigned to two treatment groups (DDT and Control) at GD 11.5. The dams were orally administered 10 μl oil/g body weight of DDT mixture added certified organic olive oil in the DDT group (n = 7) or vehicle control in the control group (n =9) every morning during GD 11.5 to postnatal day (PND) 5. At PND 29, the dams were fasted for 6 hours before their blood was collected. Unlike overnight fasting, glucogenolysis remains the primary source for energy in the first 8 hours of fasting as liver glycogen was not depleted [22] and the catabolism of fatty acid and lipids was not yet activated [23, 24]. Thus we assume that the metabolic states in these mice remained physiologically comparable to non-fasting women for us to test the metabolome association of DDT exposure [23, 24]. The serum levels measured in the dams during the perinatal period fell into the upper quartile of CHDS mothers evaluated here, as confirmed in a previous study [25]. These levels are not expected to change much at the time of metabolomics measures because sub-chronic DDT dosing produces a steady state of plasma levels [26] and low dose DDT has considerably slower excretion of DDTs (a much higher dose resulted in a ~25% elimination in ~24 day time period [27]. The serum of mice was kept in −80°C.
High-resolution LC-MS metabolomics
Metabolite extracts of sera were analyzed blinded to exposures and/or treatment as described previously to acquire untargeted metabolic profiling [12, 13, 17]. Briefly, 65 μL aliquots were taken from each thawed serum sample and maintained on ice. Ice-cold acetonitrile was added to the aliquots at a 2:1 ratio to precipitate the proteins. A mixture of 14 stable isotope internal standards was included for quality control prior to protein precipitation. Following 30 minute incubation on ice, samples were centrifuged for 10 minutes at 16,100×g at 4°C. Supernatants were then randomized, and injected in triplicates to C18 chromatography (Higgins Analytical, 100×2.1mm, 5uL) coupled with Q-Exactive HF mass spectrometer (Thermo Fisher). Mass spectral data were collected with positive electrospray ionization from m/z (mass-to-charge ratio) 85 to 1275. This data is available at the NIH Common Fund’s Metabolomics Data Repository and Coordinating Center (supported by NIH grant, U01-DK097430) website, the Metabolomics Workbench, http://www.metabolomicsworkbench.org, where it has been assigned Project ID (Project ID to be inserted later). The data can be accessed directly via the Project DOI: (Project DOI to be inserted later).
Bioinformatics and Biostatistics
Raw mass spectrometry data was extracted using apLCMS [28] and xMSanalyzer [29] for m/z, retention time, and integrated peak intensity. CHDS metabolomics data were pre-filtered from 12,855 to 3,121 metabolic features to retain only features with non-zero values in > 80% in all samples and >50% in each group. Metabolome-wide-association study (MWAS) was conducted using linear regression models, which associate the serum p,p’-DDT, o,p’-DDT and p,p’-DDE concentration (log 2 transformed) with metabolite intensities, adjusted for covariates including total serum lipids (continuous variable), age (continuous variable), sampled time (pregnancy vs postpartum) and parity (primiparous vs multiparous).
All metabolic features were annotated with xMSannotator [30] using HMDB (Human Metabolome Database, [31]) at 5 ppm tolerance; confidence scores for annotation by xMSannotator are derived from a multistage clustering algorithm. Identities of selected metabolites were confirmed by accurate m/z match and co-elution with authentic standards (level 1 identification by criteria of Schymanski et al [32]).
Each DDT variable (o,p′-DDT, p,p′-DDT, and p,p′-DDE) was categorized in quartiles of the logged distribution based on the cohort population. Student’s t test (two-tailed with Welch’s correction for unequal variance) were used to test statistical significance between quartile 4 (highest exposure category) and quartile 1 (considered as baseline exposure). Pathway enrichment analysis was performed in mummichog (version 2.0, raw P < 0.05 as cutoff), which uses a probabilistic algorithm different from feature level statistics and produces pathway p-values based on permutation [33, 34]. The version 2 of mummichog enforces retention time in grouping isotopes or adducts, thus producing more stringent results compared to version 1. All other bioinformatics analysis were performed in RStudio version 1.1.447 (RStudio, Inc.). The significance level was P < 0.05 for all tests; Benjamini and Hochberg false discovery rate was used for multiple comparisons [35].
Results
Distributions of study variables
Demographic information for this study is summarized in Supplemental Table 1. The present study sample has an average age of 25 years old, mostly non-obese and non-African-American. Because DDT compounds cross the placenta and accumulate in fetus, we used samples throughout pregnancy and postpartum yet with a preference of early postpartum (within 3 days of delivery) samples for maximal in utero exposure duration. Prior work has established that organochlorine levels are consistent across all trimesters of pregnancy and soon after delivery within women [36]. Other than age, we also adjusted for total serum lipids and parity, which affects DDT body burden, and the time of sampling (during pregnancy vs postpartum) which may affect endogenous metabolite levels.
All of the CHDS women serum samples contained detectable levels of p,p’-DDT, o,p’-DDT and p,p’-DDE. Although the levels of p,p’-DDE were higher than the other two DDTs, the relative dynamic range were similar (interquartile range in relative to median level, Supplemental Table S1). The concentration measurement of DDT compounds showed that p,p’-DDT and o,p’-DDT were highly correlated (ρ = 0.67), while p,p’-DDT was less correlated with its metabolite p,p’-DDE (ρ = 0.59). All of the three variables showed positive skewed distribution, yet the skewness was the lowest for p,p’-DDE (1.6) and highest for p,p’-DDT (6.4) with o,p’-DDT in between (3.3) (Figure 1). This suggested difference in sources of exposure to DDT and DDE, or differences in elimination rates of DDT/DDE among individuals. Median levels of p,p’-DDT and p,p’-DDE (12.97 and 42.81 μg/L, respectively) were comparable to levels found in occupational exposed workers, and fell into the high end of levels reported for the general population in recent decades [1]. This high exposure level was attributed to the heavy use of DDT compounds at the time of sampling between 1959 and 1967.
Figure 1.
Distribution of serum p,p’-DDT (A), o,p’-DDT (B) and p,p’-DDE (C) concentration in 397 subjects from the Child Health and Development Studies (CHDS) cohort, visualized by histogram and density curves.
Metabolome wide association of DDT compounds
In order to study the global metabolic alterations associated with increasing DDT concentrations, we performed a metabolome wide association study (MWAS) of the untargeted HRM data from the serum samples collected during pregnancy or early postpartum. A metabolic feature is defined by the accurate m/z, retention time and ion intensity [37]. Using our linear regression model, 278 out of 3121 metabolic features were found significantly associated with p,p’-DDT concentrations (P < 0.05, Figure 2A). Similarly, 212 features were associated with o,p’-DDT concentrations (Figure 2B), while the metabolite p,p’-DDE showed the strongest association with the metabolome among the three, with 466 features being significant (Figure 2C). With an FDR cutoff of Q = 0.05, 32 features remained significant in association with p,p’-DDE, while 4 remained significant in association with p,p’-DDT and none with o,p’-DDT. These Manhattan plots also showed both positively and negatively correlated metabolites over a broad range of m/z and retention time, indicating a wide spectrum of metabolic alteration (Figure 2). Reversed phase C18 chromatography separated metabolic features based on the lipophilicity of chemicals, allowing qualitative speculation on physicochemical properties of unknown metabolites [38, 39]. A more lipophilic chemical has greater affinity for the stationary phase and thereby has longer retention time [40]. MWAS of p,p’-DDT (Figure 2A) showed more positive association with less lipophilic metabolites (retention time: 20–180 s) while p,p’-DDE (Figure 2C) showed more association with more lipophilic metabolites (retention time: 420–600 s) than p,p’-DDT.
Figure 2.
Metabolome-wide association study (MWAS) of serum p,p’-DDT (A), o,p’-DDT (B) and p,p’-DDE (C) concentrations in 397 subjects from the Child Health and Development Studies (CHDS) cohort. The extracted data was pre-filtered to retain 3121 features with non-zero values in > 80% in all samples and >50% in each group. Results shows that p,p’-DDE exposure was associated with more metabolic alterations than the parent chemicals (indicated by the raw P value and FDR adjusted Q value cutoff). Type 1 Manhattan plot for –log P vs m/z features and type 2 Manhattan plot for –log P vs retention time with C18 chromatography separation are presented left and right, respectively. Red indicates positive correlation between significant features (P < 0.05) and DDT exposure and blue indicates negative correlation.
The results from the regression analysis were used to perform pathway enrichment analysis with mummichog, to understand the functional alterations of the metabolome. We used the raw P value cutoff instead of FDR the analysis to ensure the number of input features was sufficient and the coverage of metabolite was unbiased [33]. This approach has been shown to facilitate discovery of biologically important associations in exploratory studies which may be overlooked by using stringent FDR correction, especially when the effect was modest [11]. The mummichog pathway analysis uses statistical testing in permutation and enrichment to assess overall significance of pathway and provides an additional layer of protection against type I error [33]. Moreover, as demonstrated in transcriptomics research, pathway and network analysis based on biologic knowledge is relatively robust to errors at individual feature level [33, 38]. Therefore, we decided to use mummichog enrichment statistical test and a raw P value threshold as an effective compromise to protect against both type I and type II statistical error [38].
Results showed that p,p’-DDE had relatively distinguished metabolic pathway profiles compared to its parent compound p,p’-DDT. Only two metabolic pathways (glycine, threonine, alanine and serine metabolism, and phosphatidylinositol phosphate metabolism) were shared by both p,p’-DDT and p,p’-DDE, and these two pathways were also associated with o,p’-DDT (Figure 3, group 1). Three pathways (urea cycle/amino groups, arginine and proline metabolism, aspartate and asparagine metabolism) were found associated with p,p’-DDT and o,p’-DDT, but not with p,p’-DDE (Figure 3, group 2). On the other hand, a cluster of pathways including fatty acid metabolism and carnitine shuttle was uniquely associated with p,p’-DDE exposure. This cluster of pathways presented an indication of mitochondrial dysfunction that is commonly found in toxic responses to xenobiotic and disease mechanisms (Figure 3, group 3).
Figure 3.
Metabolic pathways enriched by significant features (raw P < 0.05) associated with p,p’-DDT, o,p’-DDT and p,p’-DDE concentrations. Circles are colored by the similarity between the chemicals: Dark red (group 1) indicates the most common pathways shared by all three chemical exposure and dark blue (group 3) indicates unique pathway found with p,p’-DDE only. Size of circles is proportional to the overlap size (i.e. number of significant metabolites in each pathway, ranging 3–13).
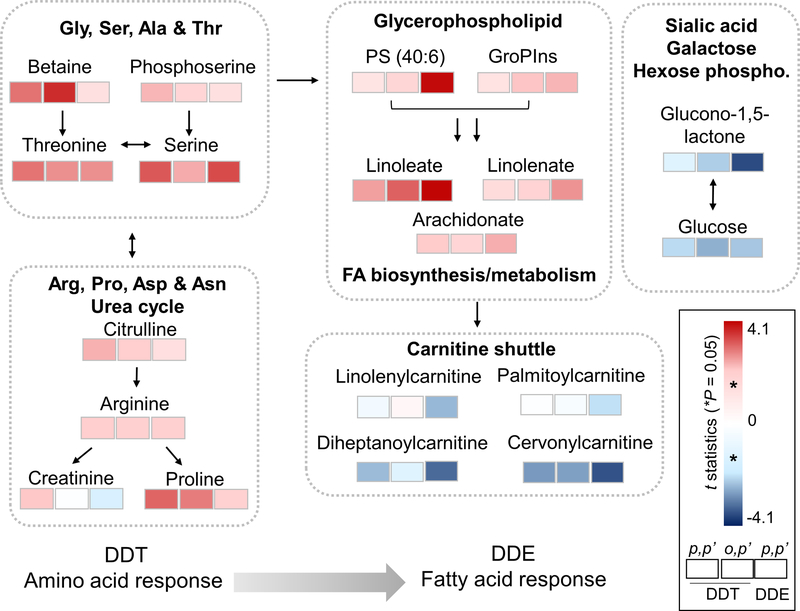
Metabolic network associated with DDT and DDE exposure
The pathways associated with p,p’-DDT, o,p’-DDT and p,p’-DDE exposure can be summarized by a simplified schematic (Figure 4). This network included five clusters of pathways: 1) glycine, serine, alanine and threonine metabolism; 2) Urea cycle and non-essential amino acid catabolism; 3) glycerophospholipid and fatty acid metabolism; 4) carnitine shuttle; 5) glucose related pathways including sialic acid, hexose and galactose. We found that p,p’-DDT exposure was strongly associated with increased levels of non-essential amino acids like serine, proline and arginine; their importance in cell proliferation and signaling in cancers have been previously recognized [41–44]. Serine metabolism is also important in glycerophospholipid formation and also participate in synthesis of other lipid species [45]. In our results, we found that p,p’-DDE exposure had a strong association with glycerophospholipid metabolites. Several long-chain fatty acid were increased and acyl-carnitines were decreased in association with p,p’-DDE, but not with p,p’-DDT. This metabolic signature was consistent with pathway enrichment analysis indicating an impairment in mitochondrial energy production from fatty acids [17, 46–48]. Common metabolites involved in metabolic pathways of sialic acid, galactose and hexose (Figure 3 and 4) were also found in association with p,p’-DDE and o,p’-DDT. These associations suggested alteration in glucose metabolism, which may also reflect mitochondrial bioenergetics defect [49, 50].
Figure 4.
Key metabolites (raw P < 0.05) in enriched metabolic pathways associated with DDT and DDE exposure. Left to right: p,p’-DDT, o,p’-DDT and p,p’-DDE. Colors are coded by significance of the association (t statistics) showing differences in metabolic responses associated with the exposure: In addition to amino acid metabolism, the metabolite p,p’-DDE was uniquely associated with metabolites involved in lipid and fatty acid metabolism. Red indicates positive association and blue indicates negative association quantitated by t statistics. The metabolites here are annotated by accurate mass (5 ppm, Supplemental Table S2), and selected metabolites were further confirmed by authentic chemical standards (Figure 5).
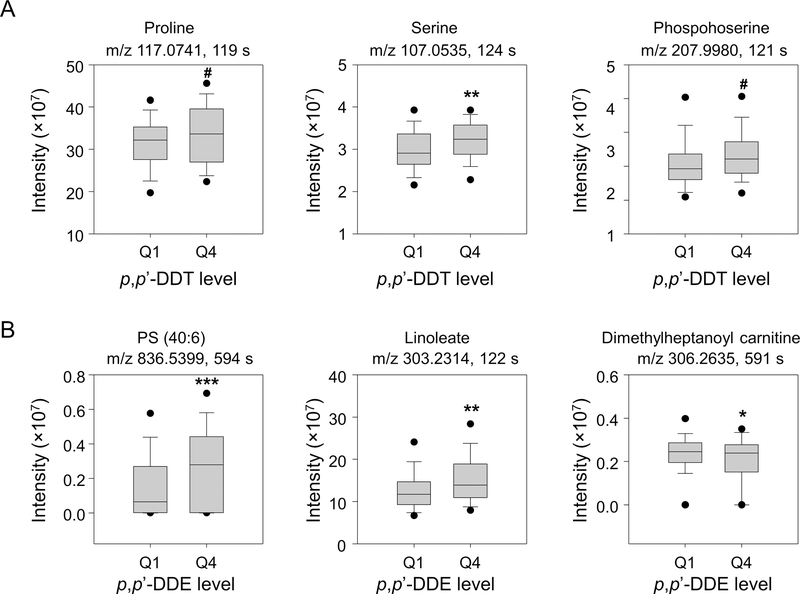
The corresponding levels of representative metabolites for the women in the fourth and first quartile of p,p’-DDT and p,p’-DDE exposure are presented in Figure 5. We showed women with higher p,p’-DDT levels (quartile 4) had higher intensities of proline, serine and phosophserine adducts compared to women with baseline exposure levels (quartile 1), and those with higher p,p’-DDE levels had increased intensities of phosphatidyl serine and linoleic acid, and decreased intensities of dimethylheptanoyl carnitine. These results were consistent with the pathway enrichment analysis showing differential metabolic pathway profiles between p,p’-DDT and p,p’-DDE (Figure 3).
Figure 5.
Selected metabolite levels in the Child Health and Development Studies (CHDS) subjects categorized by quartile 1 and 4 of p,p’-DDT (A) and p,p’-DDE (B) serum levels. The significant association of represented amino acid metabolites with p,p’-DDT exposure, and lipid metabolites with p,p’-DDE exposure are presented. A: proline and serine, adduct M(C13)+H, confirmed by accurate m/z match and co-elution with standard; phosphoserine, adduct M+Na, high confidence feature by isotope clustering; B: phosphotidyl serine and dimethylheptanoyl carnitine, adduct M+H, high confidence feature by isotope clustering; linoleate, adduct M+Na, confirmed by accurate m/z match and co-elution with standard. *P < 0.05, #P =0.07, by student’s t test comparing Q1 and Q4.
The metabolic association of p,p’-DDT recapulated in mouse model
To validate the MWAS result from the CHDS cohort, we carried out a set of mouse experiments to model the DDT exposure, where pregnant mice were treated with DDT or vehicle control. After dosing commenced and pups were weaned, each maternal serum sample was analyzed for metabolomics similarly as human serum samples. After pre-filtering, more metabolic features remained compared to human samples (5950 vs 3121). A total of 132 features were different as assessed by student’s t test (raw P < 0.05) and 361 features met a cut off of P < 0.1. This relatively small effect compared to CHDS women may result from 1) small samples size of animals; 2) the relatively short gestation time and thus exposure term of mice; and 3) difference in sensitivity of statistical testing.
At the feature level, we are aware that it is still technically challenging to compare these metabolomics data with those from the human study, especially because the human samples underwent several decades of storage. Nonetheless, the pathway analysis of the mouse metabolomics data showed that DDT treatment caused perturbation of metabolome and the altered metabolites were most enriched in metabolic pathways of urea cycle/amino group, and metabolism of alanine, aspartate, glycine, serine, threonine, tyrosine, arginine and proline (Figure 6 and Supplemental Table S3). The overlapping metabolites compared to CHDS women included arginine, betaine, and acetamidobutanoate, while sacrosine, hydroxyglutamate semialdehyde, 2-amino-3-oxobutanoic acid were also directly related. Although the overlapping at individual feature level between mice and women metabolomics was small, these results confirmed the DDT-induced metabolic effects on amino acid metabolism and provided validation of our exploratory finding in human cohort while using a less stringent approach to select significant metabolites.
Figure 6.
Metabolic pathways associated with DDT treatment in mouse model. Serum samples from DDT-treated and control mice were analyzed by high resolution metabolomics. The extracted data was pre-filtered to retain only features with non-zero values in > 80% in all samples and >50% in each group. Out of the 5950 remaining metabolite features, 361 were different between the two groups by P < 0.1. The comparison result was used as input for pathway enrichment analysis using mummichog v2.0. Overlap size indicates the number of significant metabolites detected in that pathway.
Discussion
Metabolic phenotyping with high-resolution metabolomics provides a global and unbiased approach to investigate biological changes in response to environmental exposures. Our study presented a unique opportunity to determine the metabolic effects associated with serum DDT and DDE levels in women during pregnancy and early postpartum. All MWAS presented here (e.g. those of p,p’-DDT, o,p’-DDT, and p,p’-DDE exposure in women and in mice) showed alterations to metabolic features of the non-essential amino acid metabolism pathway glycine, serine, alanine and threonine. MWAS of p,p’-DDT and o,p’-DDT exposure in women and in mice, showed alterations to metabolic features of the urea cycle and another non-essential amino acid metabolism pathway, arginine and proline. MWAS of p,p’-DDE showed an metabolic signature that was distinct from the human DDT signatures and that of mice exposed to DDT, revealing a DDE association with numerous mitochondrial defects including disruption of carnitine shuttle, fatty acid biosynthesis and metabolism [17, 46–48].
Exposure to all of the three DDT target compounds in both women and mice was associated with non-essential amino acids via their shared common pathway: glycine, serine, alanine, and threonine metabolism. Although nutritionally non-essential, amino acids such as serine are metabolically indispensable and have been shown to play a critical role providing mechanisms for cell survival and proliferation in breast cancer [41, 42, 51] and environmental stress [52, 53]. Recent studies have reported essential roles for the serine synthesis pathway in breast cancer [43], and for glycine (synthesized from serine) in rapid cancer cell proliferation [54] in nucleotide biosynthesis and redox homeostasis [41, 42]. Metabolites within glycine, serine, and threonine metabolism pathway are now considered central to cancer metastasis and cellular transformation [43, 54–57]. Thus it is possible that metabolic alterations with these amino acids can be indicative of a cell’s transformation to a malignant state or responses to activation of specific oncogenes. This supports the previous findings that maternal DDT exposure is associated with increased risk of breast cancer in mothers and daughters of the CHDS [6, 7].
The association of p,p’-DDE with multiple lipid metabolic pathways and metabolites including lipids, fatty acids and acyl-carnitines was indicative of mitochondrial functional defect [17, 46–48]. Our findings were consistent with two previously reported alterations of glycerophospoholipids, sphingolipids, fatty acids and glycerolipids observed in association with p,p’-DDE levels in humans [58, 59] and suggested that lipid metabolism dysregulation can become a reproducible metabolic signature for p,p’-DDE exposure. Indeed a recent meta-analysis of prospective studies of prenatal p,p’-DDE exposure and later adiposity concluded that p,p’-DDE is a presumed human obesogen [3]. Consistent with these human studies, direct exposure of rat tissue to p,p’-DDE experimentally demonstrated its potential to reduce mitochondrial membrane potential and oxidative respiration including ATP synthesis [60]. After perinatal exposure to DDT, adult mice had disruptions in fatty acid oxidation and oxidative respiration which may have reflected effects of the persistence DDE metabolite [25].
These congruent results across DDT isomers and metabolites suggest crosstalk between amino acid and lipid metabolism in response to environmental exposure, and this connection may be attributed to glucogenesis and/or serine-derived lipid biosynthesis [61]. Serine is necessary for the production of sphingolipids and provides headgroup for phospholipids. It has also been found that serine biosynthesis pathway regulates mitochondrial TCA cycle [43]. Deprivation of serine compromises mitochondrial function and in turn affects lipid and glucose metabolism [61]. Changes in glucose metabolism may further impact other important carbohydrate structures such as sialic acid metabolism, one of the pathways associating with DDT and DDE exposure, which play essential roles in physiological processes as well as cancer metastasis. The congruence in our findings supports previous mouse studies and suggests more mechanistic research is warranted to understand on DDT and DDE induced energy metabolism dysregulation.
As p,p’-DDE is a metabolic result of p,p’-DDT exposure and is also acquired directly as a result of dietary and environmental contamination, the p,p’-DDT/ p,p’-DDE ratio can be used a marker of recent exposure, with higher ratios observed among occupationally exposed and recently exposed persons [1]. MWAS of p,p’-DDT/p,p’-DDE ratio in the CHDS women showed with the top metabolic pathways being arginine and proline metabolism, purine metabolism and urea cycle/amino group metabolism (data not shown). This metabolic pattern involving alterations in arginine, proline and urea cycle was similar to that of p,p’-DDT in the women (Figure 3) and in mice (Figure 6), suggesting this pattern can be a reproducible and relatively unique marker discriminating recent p,p’-DDT from p,p’-DDE exposure. This may also provide insights for further characterization of individual differences in metabolism associated with breast cancer risk and obesity.
Another impact of maternal metabolic alterations that might be more direct and better characterized is on fetal development via disruption of maternal-fetal nutrient transfer. Both essential and nonessential amino acids are transported and metabolized via placenta, providing health support for growth, development, regulation of immunological and neurological functions [62]. In intrauterine growth restriction, the transplacental flux of some essential amino acid (leucine, threonine and phenylalanine) is impaired [62, 63], and the degree of impairment is associated with the clinical severity of intrauterine growth restriction [64, 65]. On the contrary, it has shown suggested that unlike the bi-directionally transported leucine and phenylalanine, threonine is trapped within the fetal circulation with very little efflux and increase of maternal plasma level may lead to a large increase in the fetal environment [66, 67]. Similarly, it has been shown that dysregulation in multiple fatty acid and lipid species were also associated with poor fetal development [16, 68, 69]. Our findings shows that maternal exposure to pollutants may directly affect the nutrient supply, and consequently fetal development and infant health.
In conclusion, this study of a historic pregnancy population exposed to unbanned use of DDT at its peak shows that HRM is able to inform perturbation of maternal endogenous metabolism and in utero environment. The results showed differential metabolic effects associated with p,p’-DDT, o,p’-DDT and p,p’-DDE exposure, with p,p’-DDE linking to mitochondrial dysfunction and lipid dysregulation. Whereas our study was limited to lack in assessment of offspring health outcome in this first proof-of-concept study, the metabolic associations represent an important first step in identifying how DDT alters in utero environment and potentially leads to disease risk. As a future direction, we plan to fully utilize the additional samples and 59 years of multi-generational follow-up in CHDS, to better understand the pathways that link DDT exposure during pregnancy to breast cancer risk in mothers and their daughters. The results also establish the feasibility of using metabolomics to discriminate different effects of mixed exposure and underlying toxic mechanisms. Our study has shown that DDT exposure affects maternal metabolism and changes the in utero environment. These changes, in addition to transplacental DDT exposure, can affect the metabolome of both the mother and the fetus and predispose both to future disease.
Supplementary Material
Acknowledgement
This project has been funded in part by the California Breast Cancer Research Program 21UB-8002 (Cohn), through the HERCULES exposome research center (P30ES019776), the National Institutes of Health S10OD18006 (Jones), UH2AI132345 (Li) and U01OD026489 (Li).
Reference
- [1].Faroon O, Harris MO, Toxicological profile for DDT, DDE, and DDD, (2002). [Google Scholar]
- [2].Snedeker SM, Pesticides and breast cancer risk: a review of DDT, DDE, and dieldrin, Environ Health Perspect 109 Suppl 1 (2001) 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cano-Sancho G, Salmon AG, La Merrill MA, Association between Exposure to p,p’-DDT and Its Metabolite p,p’-DDE with Obesity: Integrated Systematic Review and Meta-Analysis, Environ Health Perspect 125(9) (2017) 096002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Soto AM, Sonnenschein C, DDT, endocrine disruption and breast cancer, Nature Reviews Endocrinology 11 (2015) 507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Paulose T, Speroni L, Sonnenschein C, Soto AM, Estrogens in the wrong place at the wrong time: Fetal BPA exposure and mammary cancer, Reproductive toxicology (Elmsford, N.Y.) 54 (2015) 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park J-S, Zimmermann L, Cirillo PM, DDT Exposure in Utero and Breast Cancer, The Journal of Clinical Endocrinology & Metabolism 100(8) (2015) 2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cohn BA, Wolff MS, Cirillo PM, Sholtz RI, DDT and breast cancer in young women: new data on the significance of age at exposure, Environ Health Perspect 115(10) (2007) 1406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Evangelou E, Ntritsos G, Chondrogiorgi M, Kavvoura FK, Hernandez AF, Ntzani EE, Tzoulaki I, Exposure to pesticides and diabetes: A systematic review and meta-analysis, Environ Int 91 (2016) 60–8. [DOI] [PubMed] [Google Scholar]
- [9].Jones DP, Park Y, Ziegler TR, Nutritional metabolomics: progress in addressing complexity in diet and health, Annual review of nutrition 32 (2012) 183–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roede JR, Uppal K, Park Y, Tran V, Jones DP, Transcriptome–metabolome wide association study (TMWAS) of maneb and paraquat neurotoxicity reveals network level interactions in toxicologic mechanism, Toxicology Reports 1 (2014) 435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Go Y-M, Walker DI, Soltow QA, Uppal K, Wachtman LM, Strobel FH, Pennell K, Promislow DEL, Jones DP, Metabolome-wide association study of phenylalanine in plasma of common marmosets, Amino acids 47(3) (2015) 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hu X, Chandler JD, Fernandes J, Orr ML, Hao L, Uppal K, Neujahr DC, Jones DP, Go Y-M, Selenium supplementation prevents metabolic and transcriptomic responses to cadmium in mouse lung, Biochimica et Biophysica Acta (BBA) - General Subjects (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chandler JD, Hu X, Ko E-J, Park S, Lee Y-T, Orr M, Fernandes J, Uppal K, Kang S-M, Jones DP, Go Y-M, Metabolic pathways of lung inflammation revealed by high-resolution metabolomics (HRM) of H1N1 influenza virus infection in mice, American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 311(5) (2016) R906–R916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Laine JE, Bailey KA, Olshan AF, Smeester L, Drobna Z, Styblo M, Douillet C, Garcia-Vargas G, Rubio-Andrade M, Pathmasiri W, McRitchie S, Sumner SJ, Fry RC, Neonatal Metabolomic Profiles Related to Prenatal Arsenic Exposure, Environ Sci Technol 51(1) (2017) 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu J, Liu G, Li Z, Importance of metabolomics analyses of maternal parameters and their influence on fetal growth, Experimental and Therapeutic Medicine 14(1) (2017) 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ciborowski M, Zbucka-Kretowska M, Bomba-Opon D, Wielgos M, Brawura-Biskupski-Samaha R, Pierzynski P, Szmitkowski M, Wolczynski S, Lipinska D, Citko A, Bauer W, Gorska M, Kretowski A, Potential first trimester metabolomic biomarkers of abnormal birth weight in healthy pregnancies, Prenatal diagnosis 34(9) (2014) 870–7. [DOI] [PubMed] [Google Scholar]
- [17].Hu X, Chandler JD, Orr ML, Hao L, Liu K, Uppal K, Go Y-M, Jones DP, Selenium Supplementation Alters Hepatic Energy and Fatty Acid Metabolism in Mice, The Journal of Nutrition 148(5) (2018) 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walker DI, Uppal K, Zhang L, Vermeulen R, Smith M, Hu W, Purdue MP, Tang X, Reiss B, Kim S, Li L, Huang H, Pennell KD, Jones DP, Rothman N, Lan Q, High-resolution metabolomics of occupational exposure to trichloroethylene, International journal of epidemiology 45(5) (2016) 1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Walker DI, Pennell KD, Uppal K, Xia X, Hopke PK, Utell MJ, Phipps RP, Sime PJ, Rohrbeck P, Mallon CT, Jones DP, Pilot Metabolome-Wide Association Study of Benzo(a)pyrene in Serum From Military Personnel, Journal of occupational and environmental medicine 58(8 Suppl 1) (2016) S44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].van den Berg BJ, Christianson RE, Oechsli FW, The California Child Health and Development Studies of the School of Public Health, University of California at Berkeley, Paediatr Perinat Epidemiol 2(3) (1988) 265–82. [DOI] [PubMed] [Google Scholar]
- [21].Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C, Concentrations of persistent organic pollutants in California women’s serum and residential dust, Environ Res 136 (2015) 57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Geisler CE, Hepler C, Higgins MR, Renquist BJ, Hepatic adaptations to maintain metabolic homeostasis in response to fasting and refeeding in mice, Nutrition & Metabolism 13(1) (2016) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP, N.I.H.M.M.P.C. Consortium, Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice, Disease models & mechanisms 3(9–10) (2010) 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anton SD, Moehl K, Donahoo WT, Marosi K, Lee SA, Mainous AG 3rd, Leeuwenburgh C, Mattson MP, Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting, Obesity (Silver Spring, Md.) 26(2) (2018) 254–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].La Merrill M, Karey E, Moshier E, Lindtner C, La Frano MR, Newman JW, Buettner C, Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring, PLOS ONE 9(7) (2014) e103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tomiyama N, Watanabe M, Takeda M, Harada T, Kobayashi H, A comparative study on the reliablility of toxicokinetic parameters for predicting hepatotoxicity of DDT in rats receiving a single or repeated administration, The Journal of toxicological sciences 28(5) (2003) 403–13. [DOI] [PubMed] [Google Scholar]
- [27].Ando M, Dose-dependent excretion of DDE (1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene) in rats, Arch Toxicol 49(2) (1982) 139–47. [DOI] [PubMed] [Google Scholar]
- [28].Yu T, Park Y, Johnson JM, Jones DP, apLCMS—adaptive processing of high-resolution LC/MS data, Bioinformatics 25(15) (2009) 1930–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP, xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data, BMC Bioinformatics 14(1) (2013) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Uppal K, Walker DI, Jones DP, xMSannotator: An R Package for Network-Based Annotation of High-Resolution Metabolomics Data, Analytical Chemistry 89(2) (2017) 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, Fung C, Nikolai L, Lewis M, Coutouly MA, Forsythe I, Tang P, Shrivastava S, Jeroncic K, Stothard P, Amegbey G, Block D, Hau DD, Wagner J, Miniaci J, Clements M, Gebremedhin M, Guo N, Zhang Y, Duggan GE, Macinnis GD, Weljie AM, Dowlatabadi R, Bamforth F, Clive D, Greiner R, Li L, Marrie T, Sykes BD, Vogel HJ, Querengesser L, HMDB: the Human Metabolome Database, Nucleic Acids Res 35(Database issue) (2007) D521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J, Identifying small molecules via high resolution mass spectrometry: communicating confidence, Environ Sci Technol 48(4) (2014) 2097–8. [DOI] [PubMed] [Google Scholar]
- [33].Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, Pulendran B, Predicting Network Activity from High Throughput Metabolomics, PLOS Computational Biology 9(7) (2013) e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gardinassi LG, Xia J, Safo SE, Li S, Bioinformatics Tools for the Interpretation of Metabolomics Data, Current Pharmacology Reports 3(6) (2017) 374–383. [Google Scholar]
- [35].Benjamini Y, Hochberg Y, Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing, Journal of the Royal Statistical Society. Series B (Methodological) 57(1) (1995) 289–300. [Google Scholar]
- [36].Longnecker MP, Klebanoff MA, Gladen BC, Berendes HW, Serial levels of serum organochlorines during pregnancy and postpartum, Archives of environmental health 54(2) (1999) 110–4. [DOI] [PubMed] [Google Scholar]
- [37].Park YH, Shi YP, Liang B, Medriano CAD, Jeon YH, Torres E, Uppal K, Slutsker L, Jones DP, High-resolution metabolomics to discover potential parasite-specific biomarkers in a Plasmodium falciparum erythrocytic stage culture system, Malaria Journal 14(1) (2015) 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Uppal K, Walker DI, Liu K, Li S, Go Y-M, Jones DP, Computational Metabolomics: A Framework for the Million Metabolome, Chemical research in toxicology 29(12) (2016) 1956–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Karger BL, Snyder LR, Horvath C, Introduction to separation science, (1973). [Google Scholar]
- [40].Boswell PG, Schellenberg JR, Carr PW, Cohen JD, Hegeman AD, Easy and accurate high-performance liquid chromatography retention prediction with different gradients, flow rates, and instruments by back-calculation of gradient and flow rate profiles, Journal of chromatography. A 1218(38) (2011) 6742–9. [DOI] [PubMed] [Google Scholar]
- [41].Amelio I, Cutruzzola F, Antonov A, Agostini M, Melino G, Serine and glycine metabolism in cancer, Trends in biochemical sciences 39(4) (2014) 191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mattaini KR, Sullivan MR, Vander Heiden MG, The importance of serine metabolism in cancer, The Journal of Cell Biology 214(3) (2016) 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG, Stransky N, Tsun ZY, Cowley GS, Barretina J, Kalaany NY, Hsu PP, Ottina K, Chan AM, Yuan B, Garraway LA, Root DE, Mino-Kenudson M, Brachtel EF, Driggers EM, Sabatini DM, Functional genomics reveal that the serine synthesis pathway is essential in breast cancer, Nature 476(7360) (2011) 346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Phang JM, Liu W, Hancock C, Christian KJ, The proline regulatory axis and cancer, Frontiers in oncology 2 (2012) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Engelking LR, Chapter 12 - Nonprotein Derivatives of Amino Acids, in: Engelking LR (Ed.), Textbook of Veterinary Physiological Chemistry (Third Edition), Academic Press, Boston, 2015, pp. 70–75. [Google Scholar]
- [46].Go YM, Fernandes J, Hu X, Uppal K, Jones DP, Mitochondrial network responses in oxidative physiology and disease, Free radical biology & medicine 116 (2018) 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance, Cell metabolism 7(1) (2008) 45–56. [DOI] [PubMed] [Google Scholar]
- [48].Go Y-M, Sutliff RL, Chandler JD, Khalidur R, Kang B-Y, Anania FA, Orr M, Hao L, Fowler BA, Jones DP, Low-dose cadmium causes metabolic and genetic dysregulation associated with fatty liver disease in mice, Toxicological Sciences 147(2) (2015) 524–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Eisenberg I, Novershtern N, Itzhaki Z, Becker-Cohen M, Sadeh M, Willems PHGM, Friedman N, Koopman WJH, Mitrani-Rosenbaum S, Mitochondrial processes are impaired in hereditary inclusion body myopathy, Human Molecular Genetics 17(23) (2008) 3663–3674. [DOI] [PubMed] [Google Scholar]
- [50].Lei S, Zavala-Flores L, Garcia-Garcia A, Nandakumar R, Huang Y, Madayiputhiya N, Stanton RC, Dodds ED, Powers R, Franco R, Alterations in energy/redox metabolism induced by mitochondrial and environmental toxins: a specific role for glucose-6-phosphate-dehydrogenase and the pentose phosphate pathway in paraquat toxicity, ACS chemical biology 9(9) (2014) 2032–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Phang J, The Proline Regulatory Axis and Cancer, Frontiers in oncology 2(60) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Douris N, Melman T, Pecherer JM, Pissios P, Flier JS, Cantley LC, Locasale JW, Maratos-Flier E, Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1852(10, Part A) (2015) 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Matthews DE, Battezzati A, Regulation of protein metabolism during stress, Current opinion in general surgery (1993) 72–7. [PubMed] [Google Scholar]
- [54].Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK, Metabolite Profiling Identifies a Key Role for Glycine in Rapid Cancer Cell Proliferation, Science 336(6084) (2012) 1040–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM, Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression, Nature 457(7231) (2009) 910–4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [56].Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B, Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis, Cell 148(1–2) (2012) 259–72. [DOI] [PubMed] [Google Scholar]
- [57].Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, Anastasiou D, Mullarky E, Vokes NI, Sasaki M, Beroukhim R, Stephanopoulos G, Ligon AH, Meyerson M, Richardson AL, Chin L, Wagner G, Asara JM, Brugge JS, Cantley LC, Vander Heiden MG, Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis, Nature Genetics 43 (2011) 869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Carrizo D, Chevallier OP, Woodside JV, Brennan SF, Cantwell MM, Cuskelly G, Elliott CT, Untargeted metabolomic analysis of human serum samples associated with exposure levels of Persistent organic pollutants indicate important perturbations in Sphingolipids and Glycerophospholipids levels, Chemosphere 168 (2017) 731–738. [DOI] [PubMed] [Google Scholar]
- [59].Salihovic S, Ganna A, Fall T, Broeckling CD, Prenni JE, van Bavel B, Lind PM, Ingelsson E, Lind L, The metabolic fingerprint of p,p′-DDE and HCB exposure in humans, Environment International 88 (2016) 60–66. [DOI] [PubMed] [Google Scholar]
- [60].Ferreira FM, Madeira VM, Moreno AJ, Interactions of 2,2-bis(p-chlorophenyl)-1,1-dichloroethylene with mitochondrial oxidative phosphorylation, Biochemical pharmacology 53(3) (1997) 299–308. [DOI] [PubMed] [Google Scholar]
- [61].Gao X, Lee K, Reid MA, Sanderson SM, Qiu C, Li S, Liu J, Locasale JW, Serine Availability Influences Mitochondrial Dynamics and Function through Lipid Metabolism, Cell reports 22(13) (2018) 3507–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Manta-Vogli PD, Schulpis KH, Dotsikas Y, Loukas YL, The significant role of amino acids during pregnancy: nutritional support, The Journal of Maternal-Fetal & Neonatal Medicine (2018) 1–7. [DOI] [PubMed] [Google Scholar]
- [63].Regnault TRH, de Vrijer B, Battaglia FC, Transport and metabolism of amino acids in placenta, Endocrine 19(1) (2002) 23–41. [DOI] [PubMed] [Google Scholar]
- [64].Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC, Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies, The Journal of Clinical Endocrinology & Metabolism 86(11) (2001) 5427–5432. [DOI] [PubMed] [Google Scholar]
- [65].Metcoff J, Cole TJ, Luff R, Fetal growth retardation induced by dietary imbalance of threonine and dispensable amino acids, with adequate energy and protein-equivalent intakes, in pregnant rats, J Nutr 111(8) (1981) 1411–24. [DOI] [PubMed] [Google Scholar]
- [66].Avagliano L, Gar, #xf2 C, Marconi AM, Placental Amino Acids Transport in Intrauterine Growth Restriction, Journal of Pregnancy 2012 (2012) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Marconi AM, Mariotti V, Teng C, Ronzoni S, D’Amato B, Morabito A, Battaglia FC, Effect of antenatal betamethasone on maternal and fetal amino acid concentration, American Journal of Obstetrics and Gynecology 202(2) (2010) 166.e1–166.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Horgan RP, Broadhurst DI, Walsh SK, Dunn WB, Brown M, Roberts CT, North RA, McCowan LM, Kell DB, Baker PN, Kenny LC, Metabolic profiling uncovers a phenotypic signature of small for gestational age in early pregnancy, Journal of proteome research 10(8) (2011) 3660–73. [DOI] [PubMed] [Google Scholar]
- [69].Heazell AE, Bernatavicius G, Warrander L, Brown MC, Dunn WB, A metabolomic approach identifies differences in maternal serum in third trimester pregnancies that end in poor perinatal outcome, Reproductive sciences (Thousand Oaks, Calif.) 19(8) (2012) 863–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.