Abstract
BACKGROUND
Venous thromboembolism (VTE) is a common complication in trauma patients. Pharmacologic prophylaxis is utilized in trauma patients to reduce their risk of a VTE event. The Eastern Association for the Surgery of Trauma guidelines recommend use of low-molecular-weight heparin (LMWH) as the preferred agent in these patients. However, there is literature suggesting that unfractionated heparin (UFH) is an acceptable, and less costly, alternative VTE prophylaxis agent with equivalent efficacy in trauma patients. We examined data from the Michigan Trauma Quality Improvement Program to perform a comparative effectiveness study of UFH versus LMWH on outcomes for trauma patients.
METHODS
We conducted an analysis of the Michigan Trauma Quality Improvement Program data from January 2012 to December 2014. The data set contains information on date, time, and drug type of the first dose of VTE prophylaxis. Thirty-seven thousand eight hundred sixty-eight patients from 23 hospitals were present with an Injury Severity Score of 5 or greater and hospitalization for more than 24 hours. Patients were excluded if they died within 24 hours or received no pharmacologic VTE prophylaxis or agents other than UFH or LMWH while admitted to the hospital. We compared patients receiving LMWH to those receiving UFH. Outcomes assessed were VTE event, pulmonary embolism, deep vein thrombosis, and mortality during hospitalization. We used a generalized estimating equation approach to fit population-averaged logistic regression models with the type of first dose of VTE prophylaxis as the independent variable. Unfractionated heparin was considered the reference value. Timing of the first dose of VTE prophylaxis was entered into the model in addition to standard covariates. Odds ratios were generated for each of the dependent variables of interest.
RESULTS
The analysis cohort consisted of 18,010 patients. Patients administered LMWH had a decreased risk of mortality (odds ratio, 0.64; confidence interval, 0.49–0.83), VTE (odds ratio, 0.67; confidence interval, 0.53–0.84), pulmonary embolism (odds ratio, 0.53; confidence interval, 0.35–0.79), and deep vein thrombosis (odds ratio, 0.73; confidence interval, 0.57–0.95) when compared with UFH following risk adjustment and accounting for hospital effect. The reduced risk of a VTE event for patients receiving LMWH was most pronounced for patients in the lower injury-severity categories.
CONCLUSIONS
In our examination of VTE prophylaxis drug effectiveness, LMWH was found to be superior to UFH in reducing the incidence of mortality and VTE events among trauma patients. Therefore, LMWH should be the preferred VTE prophylaxis agent for use in hospitalized trauma patients.
LEVEL OF EVIDENCE
Therapeutic, level III.
Keywords: Collaborative quality improvement, complications, quality improvement, trauma outcomes, venous thromboembolism, venous thromboembolism prophylaxis
Venous thromboembolism (VTE) is a common complication of hospitalization following traumatic injury.1,2 The American College of Surgeons Trauma Quality Improvement Program (ACS-TQIP) benchmark reporting system lists the rate of deep vein thrombosis (DVT) as 1.4% and pulmonary embolism (PE) at 0.6% in trauma patients admitted to the hospital with an Abbreviated Injury Scale (AIS) score of 3 or greater in at least one body region.3 Because DVT and PE are associated with an increased risk of morbidity and mortality in high-risk trauma patients, pharmacologic prophylaxis is strongly recommended in patients for whom there is no contraindication.4–6 The preferred pharmacologic agent for VTE prophylaxis in trauma patients is low-molecular-weight heparin (LMWH) in the form of enoxaparin at a dose of 30 mg twice daily.7,8 However, the primary prospective randomized clinical trial demonstrating the superiority of LMWH for VTE prophylaxis in trauma patients compared its efficacy to a regimen of 5,000 units of unfractionated heparin (UFH) every 12 hours, rather than the more accepted dosage schedule of 5,000 units every 8 hours.8
Because there is no large prospective randomized trial comparing LMWH to UFH at 5,000 units every 8 hours, guidelines and recommendations on appropriate VTE prophylaxis remain mixed. In fact, the most recent American College of Chest Physicians guidelines consider these two different regimens to be of equivalent efficacy and do not indicate a preference.9 Many hospitals are reluctant to support the use of LMWH in trauma patients because of cost concerns. A recent prospective randomized noninferiority trial of UFH (5,000 units every 8 hours) versus enoxaparin (30 mg every 12 hours) for prevention of VTE in trauma patients suggested that UFH may be noninferior to LMWH in the prevention of new VTE events in trauma patients.10 However, this clinical trial may have been underpowered, as the DVT rate used in the power calculation was 44% (UFH) versus 31% (LMWH), and a 10% noninferiority margin was selected for the power calculation. The actual total new difference in the VTE rate observed in the trial was 3.1% (UFH 8.2% vs. LMWH 5.1%).
Significant variability exists in the pharmacologic approach to VTE prophylaxis for trauma patients in the United States with regard to the agent used, the frequency of administration, and the dose given.11 Thus, controversy remains regarding whether LMWH is indeed superior to UFH. Consequently, we have undertaken a comparative effectiveness study of trauma patients within the Michigan Trauma Quality Improvement Program (MTQIP) evaluating UFH versus LMWH for the in-hospital outcomes of VTE, PE, DVT, and mortality.
METHODS
Data Collection
The MTQIP is a collaborative quality initiative (CQI) composed of 29 American College of Surgeons Committee on Trauma–verified Levels I and II trauma centers in Michigan. The MTQIP is sponsored by Blue Cross Blue Shield of Michigan and Blue Care Network (BCBSM/BCN) as part of its Value Partnerships Program aimed at improving health care quality and value.12 Data are collected using the existing trauma registry at each participating hospital. The MTQIP utilizes a standardized data definitions dictionary, which is published online and updated annually.13 Trauma registrars and data abstractors from participating trauma centers all completed training in MTQIP and National Trauma Data Standard data definitions. Data are transmitted to the coordinating center at 4-month intervals. A comprehensive data validation program is accomplished through site visits and targeted audits of 7 to 10 randomly selected patient charts at each participant trauma center once per year.
Within the MTQIP, DVT is defined as the formation, development, or existence of a blood clot or thrombus within the vascular system, which may be coupled with inflammation. This diagnosis may be confirmed by venogram, ultrasound, or computed tomography (CT) scan. The patient should be treated with anticoagulation therapy and/or placement of a vena cava filter or clipping of the vena cava. Also included as positive result were patients with DVT in whom the attending physician documents therapeutic anticoagulation contraindication because of bleeding risk. Not included as a positive result was thrombosis of superficial veins of the upper or lower extremities, such as the cephalic or greater saphenous vein. Pulmonary embolism is defined as lodging of a blood clot in the pulmonary artery with subsequent obstruction of blood supply to the lung parenchyma. The blood clots usually originate from the deep leg veins or the pelvic venous system. Consider the condition present if the patient has a V-Q scan interpreted as high probability of PE or a positive pulmonary arteriogram or positive spiral CT or CT angiogram. Venous thromboembolism is a composite variable indicating presence of either a DVT or PE. A process measures module is added to each trauma registry to allow for data collection on aspects of VTE prophylaxis for all admitted trauma patients. Within the MTQIP process measures data, information is collected on the date, time, and drug type of the first dose of pharmacologic VTE prophylaxis. Outcome data are also collected for mortality, DVT, and PE.
Patient cohort inclusion criteria were the following: age 16 years or older, at least one valid trauma International Classification of Diseases, Ninth Revision, Clinical Modification code in the range of 800–959.9 excluding late effects (905–909.9), superficial injuries (910–924.9), and foreign bodies (930–930.9); primary mechanism of injury classified as either blunt or penetrating; calculated Injury Severity Score (ISS) of 5 or greater; and known patient disposition after emergency department (ED) discharge. Blunt injury is defined as one in which where the primary external-cause-of-injury code (E-code) is mapped to one the following categories: fall, machinery, motor vehicle traffic, pedestrian, pedal cyclist, and struck by, against. Penetrating is defined as an injury where the primary E-code is mapped to the following categories: cut/pierce and firearm.
Data Analysis
The analysis cohort consisted of acutely injured patients admitted to the trauma service between January 1, 2012, and December 31, 2014. A total of 23 hospitals were participating in MTQIP during this time period. Patients were excluded if they were admitted directly from another hospital (bypassing the ED), died within 24 hours of hospital arrival, or were transferred to another hospital or if time to VTE prophylaxis was more than 1,000 hours. The analysis was performed only on those patients who received UFH or LMWH for VTE prophylaxis. Patients receiving no pharmacologic VTE prophylaxis or other medications in lieu of UFH or LMWH for VTE prophylaxis were excluded. All ISS values were derived from registrar-abstracted and -recorded AIS 2005 codes with 2008 updates.
For the type of VTE prophylaxis drug used, we extracted from the database the medication administered at the time of first pharmacologic VTE prophylaxis. Specific dosing schedules utilized for UFH and LMWH at each hospital during the study period were determined by surveying trauma program medical directors and program managers at the MTQIP member trauma centers. Data were obtained regarding protocols, preferred agent, dosage amounts, and frequencies used for types of pharmacologic VTE prophylaxis in their trauma center. Patients were assumed to have received the drug dosing and frequency of VTE prophylaxis that matched the type of the first agent administered as VTE prophylaxis.
The primary outcome of interest was the risk-adjusted development of VTE, defined as the occurrence of a DVT or PE event during hospitalization. Risk adjustment covariates at the patient levelwere selected using a three-step process: first, those known to be clinically relevant were selected from a full set of baseline characteristics and injury severity variables; next, comorbidities exhibiting a difference between the UFH and LMWH groups were added; and lastly, we added comorbidities potentially associated with the outcome (p < 0.2, univariate) to construct the final model. In some instances, specific incidents had missing values for potentially important covariates (Glasgow Coma Scale motor score, systolic blood pressure, and pulse rate). To minimize bias, these values were accounted for using indicator variables for missing status. Patient covariates were entered into a generalized estimating equation logistic regression model, with patients clustered at the hospital level. For models run on a smaller sample, patient covariates were entered as a linear predictor. The independent variable in the model was the type of VTE prophylaxis, with UFH as the null value. Odds ratios were generated for each of the dependent variables being investigated.
This study was submitted to the University of Michigan Medical School Institutional Review Board and given a determination of “not regulated” status as a quality assurance and quality improvement clinical activity.
Statistical Analysis
Statistical analyses were performed using Stata 12.0 (StataCorp, College Station, TX). Results are presented as values ± 95% confidence intervals (95% CIs), mean, or mean ± SD. Statistical significance was defined as a p < 0.05. Sample sizes were analyzed for statistical power using an α of 5% and power of 80%.
RESULTS
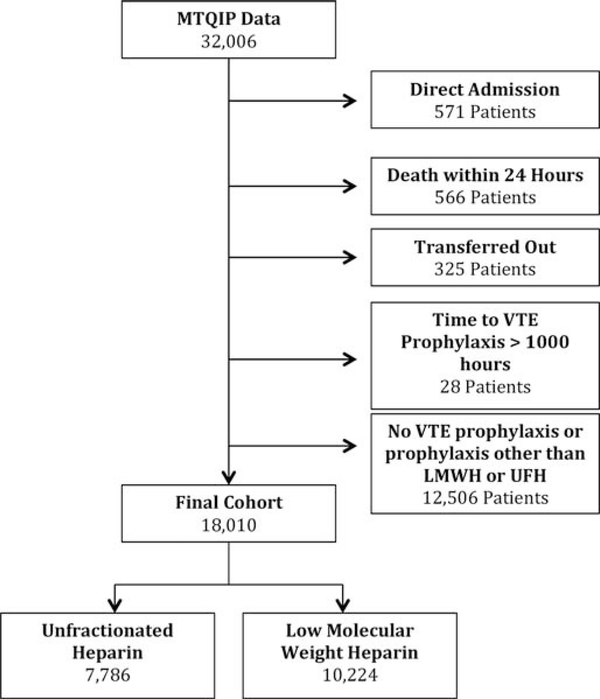
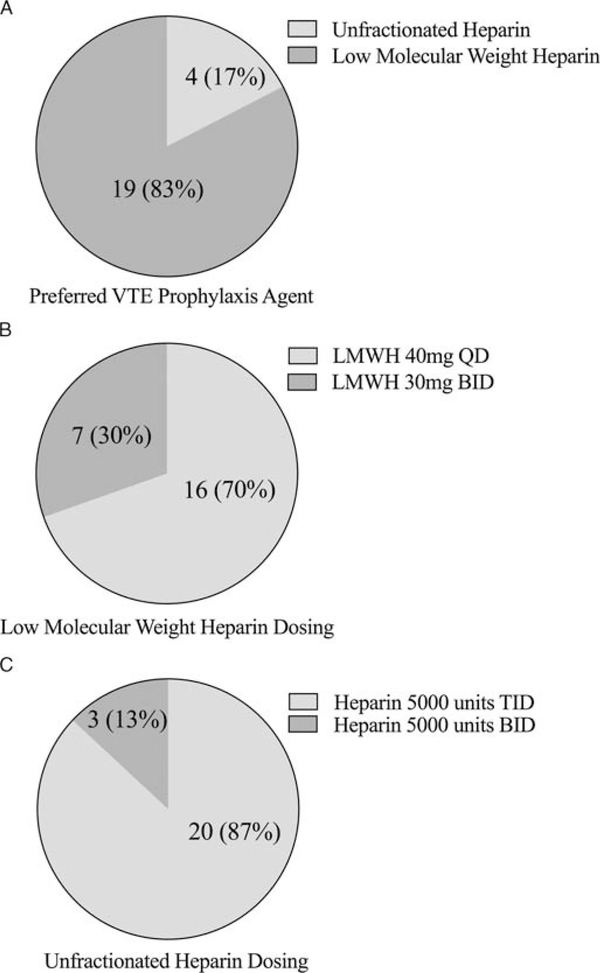
We identified 32,006 trauma patients who met inclusion criteria within the MTQIP database (Fig. 1). Seven thousand seven hundred eighty-six patients were identified as receiving UFH as their first dose of VTE prophylaxis, and 10,224 patients were identified as receiving LMWH as their first dose of VTE prophylaxis. Thirteen thousand nine hundred ninety-six patients were excluded in whom no VTE prophylaxis was administered, or who received VTE prophylaxis with a drug other than prophylaxis dosage UFH or LMWH, were admitted directly from another hospital (bypassing the ED), died within 24 hours of hospital arrival, were transferred out to another hospital, or had a time to VTE prophylaxis of more than 1,000 hours. Patient characteristics for the two different VTE prophylaxis agents are detailed in Table 1. Survey results for the preferred VTE prophylaxis agent, dosage, and timing by MTQIP hospital are illustrated in Figure 2. In addition, agent type and dosages were obtained for all VTE prophylaxis choices so that the drug dosage amounts and frequencies were known for both UFH and LMWH at each of the 23 trauma centers represented in the analysis cohort. Based on the first dosage data and survey results, 7,207 patients received UFH 5,000 units three times daily, 579 patients received UFH 5,000 units twice daily, 6,357 received enoxaparin (LMWH) 30 mg twice daily, and 3,867 received enoxaparin (LMWH) 40 mg daily.
Figure 1.
Cohort diagram for exclusions and VTE prophylaxis regimens.
TABLE 1.
Patient Characteristics
| Characteristic | Heparin | LMWH | p |
|---|---|---|---|
| Patients, n | 7,786 | 10,224 | — |
| Age, mean, y | 51.8 ± 22.0 | 51.3 ± 21.6 | 0.09 |
| Age, % | |||
| 16–25 y | 14.7 | 15.2 | 0.07 |
| 26–45 y | 26.3 | 26.2 | |
| 46–65 y | 30.2 | 30.9 | |
| 66–75 y | 9.9 | 10.3 | |
| >75 y | 18.9 | 17.4 | |
| Male sex, % | 65.6 | 65.1 | 0.5 |
| Race, % | |||
| White | 58.8 | 76.6 | <0.001 |
| Black | 37.4 | 18.1 | |
| Other | 3.8 | 5.3 | |
| Private insurance, % | 46.6 | 52.2 | <0.001 |
| Blunt mechanism, % | 85.7 | 90.9 | <0.001 |
| ED pulse, % | |||
| 51–120 beats/min | 90.8 | 91.5 | 0.002 |
| >120 beats/min | 7.3 | 6.5 | |
| 1–50 beats/min | 1.0 | 0.7 | |
| Missing | 0.9 | 1.3 | |
| ED systolic blood pressure, % | |||
| >90 mm Hg | 94.7 | 95.2 | 0.001 |
| 61–90 mm Hg | 3.7 | 3.1 | |
| ≤60 mm Hg | 0.6 | 0.3 | |
| Missing | 1.0 | 1.4 | |
| ED GCS motor score, % | |||
| 6 | 85.4 | 87.9 | <0.001 |
| 5–2 | 7.2 | 5.1 | |
| 1 | 4.6 | 3.6 | |
| Missing | 2.8 | 3.4 | |
| ISS, mean | 12.3 ± 8.0 | 12.8 ± 8.1 | <0.001 |
| ISS, % | |||
| 5–15 | 74.8 | 73.4 | <0.001 |
| 16–24 | 15.7 | 17.7 | |
| 25–35 | 7.8 | 6.8 | |
| >35 | 1.7 | 2.1 | |
| AIS head/neck >2, % | 20.8 | 16.3 | <0.001 |
| AIS chest >2, % | 25.8 | 29.0 | <0.001 |
| AIS abdomen >2,% | 7.8 | 8.1 | 0.4 |
| AIS extremity >2, % | 19.0 | 23.7 | <0.001 |
| Intubated, % | 46.5 | 47.5 | 0.2 |
| Transfer in, % | 13.4 | 20.9 | <0.001 |
| Congestive heart failure, % | 2.3 | 2.8 | 0.02 |
| Dialysis | 1.2 | 0.4 | <0.001 |
| Drug use | 13.1 | 11.4 | <0.001 |
| Hypertension, % | 33.0 | 29.7 | <0.001 |
| Obesity, % | 13.7 | 12.7 | 0.05 |
| Psychiatric history, % | 10.4 | 11.9 | 0.002 |
| Hours to VTE prophylaxis, mean | 35.4 ± 54.9 | 43.7 ± 57.6 | <0.001 |
| Hours to VTE prophylaxis, median | 13.9 | 26.4 | <0.001 |
| Timely VTE prophylaxis, % | 79.6 | 73.8 | <0.001 |
GCS indicates Glasgow Coma Scale.
Figure 2.
The MTQIP hospital survey results for VTE prophylaxis regimens. A, Preferred type of VTE prophylaxis agent, (B) UFH dosing, (C) LMWH dosing. Total of 23 MTQIP participant hospitals. N indicates number of MTQIP hospitals (% of MTQIP hospitals out of 23 total).
Overall unadjusted rates of mortality, DVT, PE, and VTE were greater in the UFH group compared with the LMWH group (Table 2). The sample sizes required, based on power analysis for each outcome, were 2,287 (mortality), 3,177 (DVT), 4,711 (PE), and 2,275 (VTE). The rate of VTE in trauma patients admitted to the hospital, with an ISS of 5 or greater, who did not die in the first 24 hours, and received pharmacologic VTE prophylaxis was 2.2% among the MTQIP participant hospitals. Timely initiation of VTE prophylaxis was considered to be the administration of the first dose less than 48 hours after ED admission; 79.6% of UFH patients versus 73.8% of LMWH patients received their first dose of VTE prophylaxis in a timely fashion (Table 1).
TABLE 2.
Unadjusted Outcomes
| Outcome | Heparin | LMWH | p |
|---|---|---|---|
| Patients, n | 7,786 | 10,224 | — |
| Mortality, % (n) | 2.1 (166) | 1.4 (139) | <0.001 |
| DVT, % (n) | 2.1 (161) | 1.5 (153) | <0.001 |
| PE, % (n) | 0.8 (66) | 0.5 (52) | 0.01 |
| VTE, % (n) | 2.7 (207) | 1.9 (190) | <0.001 |
The covariates and odds ratios for the VTE event generalized estimating equation model are shown in Table 3. Similar models were constructed for the outcomes PE, DVT, and mortality. After adjusting for patient factors and variances in characteristics between the UFH and LMWH groups, significant differences were found for the following outcomes: VTE event, PE, DVT, and mortality (Table 4). Results for all of these outcomes favoured VTE prophylaxiswith LMWH following risk adjustment. The advantage of LMWH over UFH in the prevention of VTE was most pronounced in the less severely injured patient groups (ISS 5–15 and 16–24). No difference was evident between UFH and LMWH for VTE, DVT, or PE in highly injured patients (ISS ≥25). In contrast, the positive effect on mortality was reversed. Low-molecular-weight heparin was favored in the high-ISS group and demonstrated no difference compared with UFH in the trauma patients with less severe injury.
TABLE 3.
VTE Event Model
| Variable | Odds Ratio (95% CI) | p |
|---|---|---|
| Age | ||
| 16–25 y | 1.0 | — |
| 26–45 y | 1.5 (1.04–2.11) | 0.03 |
| 46–65 y | 2.1 (1.48–3.02) | <0.001 |
| 66–75 y | 3.2 (2.02–4.99) | <0.001 |
| >75 y | 2.4 (1.49–3.94) | <0.001 |
| Male sex | 1.4 (1.08–1.75) | 0.009 |
| Race | ||
| White | 1.0 | — |
| Black | 0.9 (0.66–1.20) | 0.4 |
| Other | 0.8 (0.51–1.38) | 0.5 |
| Private insurance | 1.1 (0.86–1.37) | 0.5 |
| Blunt mechanism | 0.7 (0.47–0.93) | 0.02 |
| Fall | 1.0 (0.73–1.27) | 0.8 |
| ED pulse | ||
| 51–120 beats/min | 1.0 | — |
| >120 beats/min | 1.8 (1.34–2.37) | <0.001 |
| 1–50 beats/min | 1.0 (0.43–2.42) | 1.0 |
| Missing | 2.7 (0.86–8.21) | 0.09 |
| ED systolic blood pressure | ||
| >90 mm Hg | 1.0 | — |
| 61–90 mm Hg | 1.5 (1.02–2.09) | 0.04 |
| ≤60 mm Hg | 2.9 (1.38–6.00) | 0.005 |
| Missing | 0.7 (0.23–2.13) | 0.5 |
| ED GCS motor score | ||
| 6 | 1.0 | — |
| 5–2 | 1.4 (1.04–1.96) | 0.03 |
| 1 | 1.4 (1.02–1.99) | 0.04 |
| Missing | 0.9 (0.50–1.81) | 0.9 |
| ISS | ||
| 5–15 | 1.0 | — |
| 16–24 | 2.0 (1.46–2.66) | <0.001 |
| 25–35 | 2.6 (1.79–3.84) | <0.001 |
| >35 | 5.0 (3.01–8.39) | <0.001 |
| AIS head/neck >2 | 1.1 (0.78–1.42) | 0.7 |
| AIS chest >2 | 0.9 (0.73–1.21) | 0.6 |
| AIS abdomen >2 | 1.2 (0.84–1.60) | 0.4 |
| AIS extremity >2 | 1.5 (1.22–1.95) | <0.001 |
| Intubated | 2.8 (2.03–3.78) | <0.001 |
| Transfer in | 1.1 (0.83–1.41) | 0.6 |
| Acquired coagulopathy | 1.3 (0.90–1.91) | 0.2 |
| Congestive heart failure | 1.0 (0.50–1.82) | 0.9 |
| Dialysis | 0.3 (0.05–2.40) | 0.3 |
| Documented history of cirrhosis | 1.7 (0.65–4.31) | 0.3 |
| Drug use | 1.1 (0.77–1.48) | 0.7 |
| Hypertension | 0.9 (0.69–1.15) | 0.4 |
| Obesity | 1.3 (0.98–1.73) | 0.06 |
| Psychiatric history | 1.2 (0.90–1.68) | 0.2 |
| Smoking | 0.7 (0.57–0.94) | 0.02 |
| Timely VTE prophylaxis | 0.5 (0.37–0.59) | <0.001 |
GCS, Glasgow Coma Scale; VTE, Venous Thromboembolism.
TABLE 4.
Adjusted Patient Outcomes
| Outcome | n | Odds Ratio | 95% CI | p |
|---|---|---|---|---|
| VTE | 18,010 | 0.67 | 0.53–0.84 | 0.001 |
| VTE by ISS categories | ||||
| 5–15 | 13,328 | 0.70 | 0.49–0.99 | 0.047 |
| 16–24 | 3,035 | 0.46 | 0.31–0.70 | <0.001 |
| ≥25 | 1,647 | 1.05 | 0.72–1.53 | 0.8 |
| PE | 18,010 | 0.53 | 0.35–0.79 | 0.002 |
| PE by ISS categories | ||||
| 5–15 | 13,328 | 0.41 | 0.23–0.73 | 0.002 |
| 16–24 | 3,035 | 0.41 | 0.19–0.87 | 0.02 |
| ≥25 | 1,647 | 1.2 | 0.60–2.38 | 0.6 |
| DVT | 18,010 | 0.73 | 0.57–0.95 | 0.02 |
| DVT by ISS categories | ||||
| 5–15 | 13,328 | 0.82 | 0.54–1.25 | 0.4 |
| 16–24 | 3,035 | 0.50 | 0.32–0.80 | 0.004 |
| ≥25 | 1,647 | 1.18 | 0.79–1.77 | 0.4 |
| Mortality | 18,010 | 0.64 | 0.49–0.83 | 0.001 |
| Mortality by ISS categories | ||||
| 5–15 | 13,328 | 0.81 | 0.56–1.18 | 0.3 |
| 16–24 | 3,035 | 0.75 | 0.43–1.30 | 0.3 |
| ≥25 | 1,647 | 0.55 | 0.36–0.84 | 0.006 |
Lastly, we compared each dosing regimen of enoxaparin (30 mg twice daily and 40 mg once daily) to UFH 5,000 units three times daily as the reference (Table 5). We found that either dosing regimen of LMWH conferred benefit for reduction of VTE, PE, and mortality when compared with UFH. For prevention of DVT, enoxaparin 40 mg once daily was better than UFH, but enoxaparin 30 mg twice daily was equivalent to UFH. In a direct comparison, the once-daily dosing regimen of 40 mg enoxaparin reduced the risk of VTE (odds ratio, 0.6; p = 0.03) and DVT (odds ratio, 0.6; p = 0.04) when compared with the twice-daily regimen of 30 mg. No difference was established between once-daily and twice-daily dosing of LMWH for PE and mortality.
TABLE 5.
Drug Type and Dosage
| n | Odds Ratio | 95% CI | p | |
|---|---|---|---|---|
| VTE | ||||
| Heparin 5,000 units TID | 7,207 | 1.0 | — | Reference |
| Enoxaparin 30 mg BID | 6,357 | 0.77 | 0.60–0.99 | 0.04 |
| Enoxaparin 40 mg daily | 3,867 | 0.47 | 0.32–0.71 | <0.001 |
| PE | ||||
| Heparin 5,000 units TID | 7,207 | 1.0 | — | Reference |
| Enoxaparin 30 mg BID | 6,357 | 0.57 | 0.37–0.89 | 0.01 |
| Enoxaparin 40 mg daily | 3,867 | 0.37 | 0.19–0.72 | 0.004 |
| DVT | ||||
| Heparin 5,000 units TID | 7,207 | 1.0 | — | Reference |
| Enoxaparin 30 mg BID | 6,357 | 0.86 | 0.65–1.15 | 0.3 |
| Enoxaparin 40 mg daily | 3,867 | 0.53 | 0.34–0.82 | 0.005 |
| Mortality | ||||
| Heparin 5,000 units TID | 7,207 | 1.0 | — | Reference |
| Enoxaparin 30 mg BID | 6,357 | 0.64 | 0.47–0.88 | 0.005 |
| Enoxaparin 40 mg daily | 3,867 | 0.67 | 0.47–0.98 | 0.04 |
BID indicates twice a day; TID, three times a day.
DISCUSSION
In this comparative effectiveness study, we found that trauma patients who received LMWH as their first dose of VTE prophylaxis had a significantly reduced risk of VTE, PE, DVT, and mortality when compared with UFH. We also found that the reduced risk of a VTE event for patients receiving LMWH was most pronounced for patients in the lower injury-severity categories. Enoxaparin at 30 mg twice daily or 40 mg once daily were both better than UFH 5,000 units three times daily in the prevention of VTE events and mortality. We found that administration of LMWH 40 mg once daily was superior to UFH 5,000 units three times daily for the avoidance of a DVT. However, similar to the findings of Olson et al.,10 we found no difference between LMWH 30 mg twice daily and UFH 5,000 units three times daily for the prevention of a DVT.
Because there has been no prospective randomized clinical trial of UFH at 5,000 units three times daily compared with enoxaparin 30 mg twice a day or 40 mg once per day, recommendations continue to equivocate regarding the preferred pharmacologic agent for VTE prophylaxis in trauma patients between UFH and LMWH. Pragmatic clinical trials using observational databases offer an alternative means to answer this clinical question. Examination of ACS-TQIP data for patients with severe traumatic brain injury revealed that LMWH was associated with a significantly lower odds ratio of VTE and mortality compared with UFH.14 Our study exhibited similar findings, favoring VTE prophylaxis with LMWH when applied to the entire cohort of trauma patients.
An unexpected finding in our study was that the patients in the UFH group had a decreased mean and median time to the first dose of VTE prophylaxis compared with the LMWH patients. There was a statistically significant difference in timely VTE prophylaxis (UFH 79.6 vs. LMWH 73.8%); however, the clinical relevance of this difference may not be consequential. It would be interesting to know why initiation of VTE prophylaxis with LMWH appears delayed compared with UFH. Perhaps the difference is due to a perceived increased risk of bleeding and concomitant difficulty in reversing the effect of LMWH when compared with UFH. Another possibility is that the initiation point of three-times-daily drug dosing (e.g., 6 am, 2 pm, and 10 pm) may occur earlier in the patient course following admission than twice-daily (9 am and 9 pm) or once-daily (9 am) dosing because of institutional scheduling standardization.
We did identify a greater potential reduction in risk of VTE, PE, and DVT for the once-daily dosing regimen of enoxaparin compared with UFH versus the risk reduction observed when twice-daily dosing was compared with UFH. A similar finding was discovered by Riha et al.,15 who found that 25% of patients on enoxaparin 30 mg twice daily developed a DVT, whereas 2.9% of patients on 40 mg once daily developed a DVT. In these patients, those who were administered enoxaparin 30 mg twice daily also had significantly lower anti-Xa levels. In another study, changing from a regimen of 30 mg of enoxaparin twice per day to weight-based dosing at 0.6 mg/kg twice per day resulted in an increase in the number of patients achieving a goal anti-Xa level from 8% to 61%.16 A higher percentage of missed VTE prophylaxis doses is associated with increased rates of VTE.17 This finding in our study seems enigmatic as the 30-mg twice-daily regimen should theoretically lead to fewer missed doses of VTE prophylaxis. However, this may be an incorrect assumption, and the impact of missed doses merits further investigation in the future.
The determination that LMWH is associated with a lower incidence of mortality is interesting. Low-molecular-weight heparin was found to be associated with reduced mortality in patients with severe traumatic brain injury when compared with UFH, using ACS-TQIP data.14 Our study and the ACS-TQIP study both examined large groups of patients available in quality improvement databases. Bryne et al. postulated that the finding of decreased mortality points to evidence that LMWH modulates postinjury inflammation, which could lead to improved outcomes.18,19 We did find that the significant effect of LMWH on mortality was confined to the group with higher ISS (≥25) of patients. These are patients who should presumably be exposed to considerable postinjury inflammatory changes.20
Recent work from our group indicated that trauma patients receiving enoxaparin demonstrated a VTE rate that was one-half the rate of those receiving UFH (odds ratio, 0.46; 95% CI, 0.25–0.85) within our hospital system.21 Based on analysis of MTQIP data, a local performance improvement action plan was created for the University of Michigan Health System adult trauma service emphasizing the preferential use of LMWH for VTE prophylaxis. After implementation of our focused performance improvement plan, the use of LMWH increased from 24% to 68%, and VTE rate decreased from 6.1% (n = 36 per year) to 1.7% (n = 8 per year).
An important economic fact is that the patent on enoxaparin (Lovenox) has expired, and generic versions of this medication have become available. Thus, the cost differential of LMWH compared with UFH should become less of a concern over time for hospitals involved in trauma care. Examination of current costs revealed that for the University of Michigan Health System UFH 5,000 units three times daily has a pharmacy cost of $2.70 per day and enoxaparin 30 mg twice daily costs $7.92 per day.
Life-threatening VTE events have a tendency to occur early during the hospital stay of trauma patients.22–24 Therefore, initiation of the medication most likely to be efficacious in a timely fashion is critically important for VTE prophylaxis to have maximal impact on improving outcomes.25 Because of the findings produced in conducting this study, and the existing evidence in the literature, MTQIP has prioritized VTE prophylaxis as a performance improvement initiative within the CQI. Each trauma center in MTQIP is scored on participation and quality improvement efforts annually.12 Timely initiation of VTE prophylaxis is included as a performance metric within the MTQIP CQI hospital performance index. To monitor progress, feedback is provided to participant trauma centers at every MTQIP meeting on rates of timely VTE prophylaxis and VTE outcomes. Information is also provided on rates of patients receiving no VTE prophylaxis, UFH, and LWMH for each hospital in comparison to the collaborative mean in a graphical form that allows trending over time.
This study has many strengths that enhance the applicability of the results obtained. The data collection methodology of MTQIP is robust, complete, and credible.12,26,27 Recording and benchmark reporting of process measures data have been performed since 2011. Annual training of data abstractors is conducted with regard to data definition modifications and new data definitions. We included time to first dose of VTE prophylaxis in the risk adjustment model to minimizing confounding from differences exhibited in the initiation of UFH versus LMWH. A difference in timing for the first dose of UFH versus LMWH was also evident in the ACS-TQIP severe traumatic brain injury study.14 The size of our study, with 18,010 patients examined, reduced the likelihood of a Type II statistical error. Lastly, we were able to include data from multiple trauma centers and a wide range of injured patients in the analyses, which enhances the generalizability of our findings to a broad-based trauma population.
There are some limitations to our study. Within MTQIP, data are recorded for each patient on the type of medication and time of administration for only the first dose of VTE prophylaxis. No ongoing data are collected on missed or held doses of medication. Data are not collected on later changes in medication type, dosage, or frequency, if these occur. We made an assumption that the medication dose and frequency matched each institution’s survey results for UFH and LMWH dosing protocols. Our results can be applied only to hospitalized trauma patients, as we did not collect outcome information on patients after discharge. Reported rates of DVTare influenced by screening practices, and we did not attempt to collect data on or correct for this surveillance bias.28,29 We assumed that DVT screening practices applied similarly to patients receiving VTE prophylaxis with UFH or LMWH within an individual hospital. Based on information shared at MTQIP meetings, no trauma centers participating in the collaborative have active, focused screening programs for asymptomatic DVT or PE. Hence, most of the VTE events identified in this study are likely symptomatic. Lastly, no attempt was made to collect data on hemorrhage and transfusion events specifically attributed to VTE prophylaxis. However, we did include an analysis of mortality to offset these concerns.
CONCLUSIONS
In our examination of VTE prophylaxis drug effectiveness, LMWH was found to be superior to UFH in reducing the incidence of mortality and VTE events among trauma patients. Therefore, LMWH should be the preferred VTE prophylaxis agent for use in hospitalized trauma patients. Further investigation should be conducted regarding the efficacy of the once-daily versus a twice-daily dosing regimen of enoxaparin.
Acknowledgments
DISCLOSURE
B.N.J. is supported by Training Grant NIH/NHLBI “Multidisciplinary Cardiovascular Research Training” T32HL007853. M.R.H., J.L.J., A.H.C.-N., and J.N.M. are supported by a Collaborative Quality Initiatives grant from Blue Cross Blue Shield of Michigan and Blue Care Network (a nonprofit mutual company) for the MTQIP. The remaining authors declare no conflicts of interest.
Contributor Information
Benjamin N. Jacobs, Department of Surgery, University of Michigan, Ann Arbor, Michigan.
Anne H. Cain-Nielsen, Department of Surgery, University of Michigan, Ann Arbor, Michigan.
Jill L. Jakubus, Department of Surgery, University of Michigan, Ann Arbor, Michigan.
Judy N. Mikhail, Department of Surgery, University of Michigan, Ann Arbor, Michigan.
John J. Fath, Department of Surgery Beaumont Hospital–Dearborn, Dearborn, Michigan..
Scott E. Regenbogen, Department of Surgery, University of Michigan, Ann Arbor, Michigan.
Mark R. Hemmila, Department of Surgery, University of Michigan, Ann Arbor, Michigan.
REFERENCES
- 1.Velmahos GC, Kern J, Chan LS, Oder D, Murray JA, Shekelle P. Prevention of venous thromboembolism after injury: an evidence-based report—part I: analysis of risk factors and evaluation of the role of vena caval filters. J Trauma. 2000;49:132–138. [DOI] [PubMed] [Google Scholar]
- 2.Velmahos GC, Kern J, Chan LS, Oder D, Murray JA, Shekelle P. Prevention of venous thromboembolism after injury: an evidence-based report—part II: analysis of risk factors and evaluation of the role of vena caval filters. J Trauma. 2000;49:140–144. [DOI] [PubMed] [Google Scholar]
- 3.ACS-TQIP Benchmark Report, Fall 2016. Chicago, IL: American College of Surgeons Committee on Trauma, Released October 2016. [Google Scholar]
- 4.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240:490–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schunemann HJ,American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(Suppl):7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrera LM, Perel P, Ker K, Cirocchi R, Farinella E, Morales Uribe CH.Thromboprophylaxis for trauma patients. Cochrane Database Syst Rev. 2013:CD008303. [DOI] [PubMed] [Google Scholar]
- 7.Rogers FB, Cipolle MD, Velmahos G, Rozyki G, Luchette FA. Venous thromboembolism: role of low-molecular weight heparin in VTE prophylaxis. J Trauma. 2002;53:142–164. [DOI] [PubMed] [Google Scholar]
- 8.Geerts WH, Jay RM, Code KI, Chen E, Szalai JP, Saibil EA. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med. 1996; 335:701–707. [DOI] [PubMed] [Google Scholar]
- 9.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA,Samama CM, for the American College of Chest Physicians. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence based clinical practice guidelines. Chest. 2012;141(Suppl):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson EJ, Bandle J, Calvo RY, Shackford SR, Dunne CE, Van Gent JM,Zander AL, Sikand H, Bongiovanni MS, Sise MJ, Sise CB. Heparin versus enoxaparin for prevention of venous thromboembolism after trauma: a randomized noninferiority trial. J Trauma Acute Care Surg. 2015;79:961–968. [DOI] [PubMed] [Google Scholar]
- 11.Bandle J, Shackford SR, Sise CB, Knudson MM. Variability is the standard: the management of venous thromboembolic disease following trauma. J Trauma Acute Care Surg. 2014;76:213–216. [DOI] [PubMed] [Google Scholar]
- 12.Hemmila MR, Jakubus JL. Trauma quality improvement. Crit Care Clin. 2017;33:193–212. [DOI] [PubMed] [Google Scholar]
- 13.Mtqip.org: Michigan Trauma Quality Improvement Program [Internet]. Available at: http://mtqip.org/resources/data-resources/#data-dictionary. Accessed November 21, 2016.
- 14.Byrne JP, Mason SA, Gomez D, Hoeft C, Subacius H, Xiong W, Neal M,Pirouzmand F, Nathens AB. Timing of pharmacologic venous thromboembolism prophylaxis in severe traumatic brain injury: a propensity-matched cohort study. J Am Coll Surg. 2016;223:621–631. [DOI] [PubMed] [Google Scholar]
- 15.Riha GM, Van PY, Differding JA, Schreiber MA, Oregon Health & Science University Trauma Research Group. Incidence of deep vein thrombosis is increased with 30 mg twice daily dosing of enoxaparin compared with 40 mg daily. Am J Surg. 2012;203:598–602. [DOI] [PubMed] [Google Scholar]
- 16.Nunez JM, Becher RD, Rebo GJ, Farrah JP, Borgerding EM, Stirparo JJ,Lauer C, Kilgo P, Miller PR. Prospective evaluation of weight-based prophylactic enoxaparin dosing in critically ill trauma patients: adequacy of antiXa levels is improved. Am Surg. 2015;81:605–609. [PubMed] [Google Scholar]
- 17.Connelly CR, Van PY, Hart KD, Louis SG, Fair KA, Erickson AS, Rick EA,Simeon EC, Bulger EM, Arbabi S, Holcomb JB, Moore LJ, Schreiber MA. Thrombelastography-based dosing of enoxaparin for thromboprophylaxis in trauma and surgical patients: a randomized clinical trial. JAMA Surg. 2016; 151:e162069. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Eisenstadt R, Kumasaka K, Johnson VE, Marks J, Nagata K, Browne KD, Smith DH, Pascual JL. Does enoxaparin interfere with HMGB1 signaling after TBI? A potential mechanism for reduced cerebral edema and neurologic recovery. J Trauma Acute Care Surg. 2016;80:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luan ZG, Naranpurev M, Ma XC. Treatment of low molecular weight heparin inhibits systemic inflammation and prevents endotoxin-induced acute lung injury in rats. Inflammation. 2014;37:924–932. [DOI] [PubMed] [Google Scholar]
- 20.Almahmoud K, Namas RA, Abdul-Malak O, Zaaqoq AM, Zamora R,Zuckerbraun BS, Sperry J, Peitzman AB, Billiar TR, Vodovotz Y. Impact of injury severity on dynamic inflammation networks following blunt trauma. Shock. 2015;44:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado-Aranda DA, Jakubus JL, Wahl WL, et al. Reduction in venous thromboembolism events: trauma performance improvement and loop closure through participation in a state-wide quality collaborative. J Am Coll Surg. 2015;221:661–668. [DOI] [PubMed] [Google Scholar]
- 22.Owings JT, Kraut E, Battistella F, Cornelius JT, O’Malley R. Timing of the occurrence of pulmonary embolism in trauma patients. Arch Surg. 1997; 132:862–866. [DOI] [PubMed] [Google Scholar]
- 23.Menaker J, Stein DM, Scalea TM. Incidence of early pulmonary embolism after injury. J Trauma. 2007;63:620–624. [DOI] [PubMed] [Google Scholar]
- 24.Brakenridge SC, Toomay SM, Sheng JL, Gentilello LM, Shafi S. Predictors of early versus late timing of pulmonary embolus after traumatic injury. Am J Surg. 2011;201:209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nathens AB, McMurray MK, Cuschieri J, Durr EA, Moore EE, Bankey PE, Freeman B, Harbrecht BG, Johnson JL, Minei JP, McKinley BA, Moore FA, Shapiro MB, West MA, Tompkins RG, Maier RV. The practice of venous thromboembolism prophylaxis in the major trauma patient. J Trauma. 2007;62:557–562. [DOI] [PubMed] [Google Scholar]
- 26.Hemmila MR, Cain-Nielsen AH, Wahl WL, Vander Kolk WE, Jakubus JL, Mikhail JN, Birkmeyer NJ. Regional collaborative quality improvement for trauma reduces complications and costs. J Trauma Acute Care Surg. 2015; 78:78–87. [DOI] [PubMed] [Google Scholar]
- 27.Hemmila MR, Osborne NH, Henke PK, Kepros JP, Patel SG, Cain-Nielsen AH, Birkmeyer NJ. Prophylactic inferior vena cava filter placement does not result in a survival benefit for trauma patients. Ann Surg. 2015;262: 577–585. [DOI] [PubMed] [Google Scholar]
- 28.Haut ER, Chang DC, Pierce CA, Colantuoni E, Efron DT, Haider AH, Cornwell EE 3rd, Pronovost PJ. Predictors of posttraumatic deep vein thrombosis (DVT): hospital practice versus patient factors—an analysis of the National Trauma Data Bank (NTDB). J Trauma. 2009;66:994–999. [DOI] [PubMed] [Google Scholar]
- 29.Haut ER, Schneider EB, Patel A, Streiff MB, Haider AH, Stevens KA, Chang DC, Neal ML, Hoeft C, Nathens AB, Cornwell EE 3rd, Pronovost PJ, Efron DT. Duplex ultrasound screening for deep vein thrombosis in asymptomatic trauma patients: a survey of individual trauma surgeon opinions and current trauma center practices. J Trauma. 2011;70:27–33. [DOI] [PubMed] [Google Scholar]