Supplemental Digital Content is available in the text.
Key Words: glaucoma, brimonidine, timolol, visual field, MD slope
Abstract
Précis:
Instillation of brimonidine or timolol slowed visual field deterioration in patients with open-angle glaucoma; both brimonidine and timolol might improve the mean deviation (MD) slopes.
Purpose:
The purpose of this study was to investigate and compare the effects of 0.1% brimonidine and 0.5% timolol on the progressing visual field defects in open-angle glaucoma.
Patients and Methods:
We evaluated 1 eye each of 68 glaucoma patients who were treated with at least 1 prostaglandin analog. Their baseline MD slopes were < −0.5 dB/y based on at least 5 Humphrey field analyzer measurements within 3 years. Eligible eyes were randomly assigned to brimonidine or timolol treatment groups and treatments were administered without the wash-out period. Clinical examinations were performed every 4 months for 2 years. We designated the MD slope as the primary endpoint.
Results:
Ultimately, 56 eyes (brimonidine:timolol=26:30) were included in the present study (mean age=65.2 y). Dropout rates of brimonidine and timolol treatment groups were 27.8% and 6.3%, respectively. There were no significant differences in baseline intraocular pressure or MD slopes between brimonidine and timolol groups (12.7 and 12.9 mm Hg, P=0.77, and −1.22 and −1.08 dB/y, P=0.43, respectively). Intraocular pressure decreased significantly in the brimonidine group at 4, 8, 12, and 16 months, and in the timolol group at 4 months, without significant differences between the drugs (P=0.20). MD slopes significantly improved in both groups (brimonidine: −0.38 dB/y, P<0.001; timolol: −0.52 dB/y, P=0.04). Furthermore, there was no significant difference between groups in the primary endpoint (P=0.59).
Conclusion:
Brimonidine and timolol treatments improved MD slopes in open-angle glaucoma.
Glaucoma is an important cause of blindness that is characterized by pathognomonic visual field defects corresponding to changes in the morphology of the optic nerve head. Although glaucoma is a multifactorial disease, elevated intraocular pressure (IOP) is 1 of the most important risk factors for visual field deterioration.1–5 Several studies have shown an association between glaucoma and elevated IOP; the Advanced Glaucoma Intervention Study evaluated the effect of IOP control on the progression of visual field defects in open-angle glaucoma and found that patients with lower average IOP had better preservation of visual field scores than did patients with higher average IOP.6 The United Kingdom Glaucoma Treatment Study (UKGTS) used a randomized, triple-masked, placebo-controlled design to show that patients with open-angle glaucoma exhibited longer preservation of the visual field when they received treatment with 0.005% latanoprost, compared with when no treatment was provided.7 Finally, the Collaborative Normal-Tension Glaucoma Study (CNTGS) showed that a 30% reduction in IOP prevented the progression of visual field defects in normal-tension glaucoma (NTG)—even when IOP remained in the normal range.8 As a result, lowering IOP has become the gold standard for glaucoma treatment and is accepted medical management for preventing the progression of visual field defects, regardless of baseline IOP.
Nevertheless, past studies have also shown the limitations of IOP-lowering treatment for glaucoma. Some glaucoma patients who receive IOP-lowering treatment continue to experience deterioration of the visual field.9 This continued progression might be attributable in part to insufficient IOP reduction; however, given the multifactorial nature of glaucoma and the poor current understanding of its pathogenesis, factors other than IOP may play an important role in these patients with continued deterioration.5 Candidate alternative factors include decreased blood flow to the optic nerve head,10,11 oxidative stress,12 and genetic background13–16; however, it remains difficult to target these factors for antiglaucoma therapy. Considering this clinical problem, there is an urgent need for alternative approaches in patients with glaucoma.
The Low-Pressure Glaucoma Treatment Study (LoGTS) found evidence that brimonidine, a topical antiglaucoma medication that decreases IOP, also exerts neuroprotective effects.17–21 The LoGTS was a randomized, double-masked clinical trial that included glaucoma patients aged 30 years or older with untreated IOP ≤21 mm Hg and a Humphrey field analyzer (HFA)-measured mean deviation (MD) ≥−16 dB. Patients were assigned to receive monotherapy with either brimonidine tartrate 0.2% or timolol maleate 0.5%, twice daily in both eyes. After treatment, both groups exhibited similar reductions in mean IOP; however, patients who received brimonidine experienced reduced progression of visual field defects compared with those who received timolol. Thus far, few clinical trials have specifically examined the ability of brimonidine to preserve the visual field. Therefore, the purpose of our study was to confirm that adjunctive topical brimonidine treatment could slow the progression of visual field loss in a group of Japanese patients with open-angle glaucoma who had received IOP-lowering treatment but continued to show the progression of visual field defects while their IOP remained in the normal range.
PATIENTS AND METHODS
Study Design
This study was designed as a randomized, parallel-group, open-label, clinical study. This prospective clinical study was approved by the Ethics Committee of the Tohoku University Graduate School of Medicine (study 2012-2-176-1) and was registered at the Japan Pharmaceutical Information Center (clinical trial registration number: JapicCTI-132111) on May 15, 2013. All experimental procedures were conducted in accordance with the tenets set forth in the Declaration of Helsinki. All data were collected at Tohoku University Hospital during the period from July 2013 to April 2016.
Patients
We recruited 72 patients aged 20 years or older who had open-angle glaucoma. The study purpose and protocols were explained to all participants, and all participants provided written informed consent. Given that this number of patients was higher than initially planned, the study was re-submitted to the Institutional Review Board for approval.
A diagnosis of glaucoma was based on a finding of glaucomatous visual field defects in reliable HFA data (Carl Zeiss Meditec Inc., Dublin, CA) with corresponding glaucoma optic neuropathy. Visual field defects were defined in accordance with the Anderson-Patella criteria, and glaucoma optic neuropathy was defined as an enlarged vertical cup-to-disc ratio, narrow neuroretinal rim width, notching, and nerve fiber layer defects. Cases of secondary glaucoma were excluded. Pretreatment IOP was determined retrospectively by examining clinical records or information from the referring hospital. All glaucoma patients in this study were already undergoing treatment with prostaglandin analogs, and some patients were also undergoing additional glaucoma treatments. The duration of this prospective study was 2 years and included 8 examinations as described below.
Inclusion and Exclusion Criteria
All eligible patients with open-angle glaucoma met the following inclusion criteria: (1) best-corrected visual acuity of 0.3 (logMAR) or better, (2) treated IOP<21 mm Hg in both eyes, (3) HFA-measured MD −20 dB or better, (4) history of glaucoma treatment with a prostaglandin analog for >1 year, (5) history of glaucoma treatment with a β-adrenergic antagonist or carbonic anhydrase inhibitor for >6 months if used, and (6) visual field deterioration faster than −0.5 dB/y based on at least 5 HFA measurements conducted within 3 years. The exclusion criteria were as follows: (1) presence of active eye disease other than glaucoma in either eye, (2) presence of retinal disease expected to progress during the study period in either eye, (3) hypersensitivity to β-adrenergic antagonists or α2-adrenergic agonists, (4) history of asthma or chronic obstructive pulmonary disease, (5) history of uncontrolled heart failure, sinus bradycardia, second-degree or third-degree atrioventricular block, or cardiogenic shock, (6) history of intraocular surgery, including laser therapy for glaucoma during the 3 months before baseline measurements, and (7) history of intraocular surgery for glaucoma. If both eyes met the inclusion criteria, the eye with more advanced glaucoma was selected for the study.
Study Treatment
Patients were assigned to treatment with brimonidine tartrate 0.1% (brimonidine; AIPHAGAN; Senju Pharmaceutical Co. Ltd, Osaka, Japan) or timolol maleate gel-forming solution 0.5% (timolol; TIMOPTOL XE; Santen Pharmaceutical Co. Ltd, Osaka, Japan) at random by severity of the visual field defect (mild: MD>−6 dB, moderate: −6 dB ≥MD ≥−12 dB, severe: MD<−12 dB). Brimonidine or timolol was applied to the registered eye without a wash-out period at regular times each day: brimonidine was applied twice per day and timolol was applied once per day in the morning.
Visual Field Testing
In this prospective study, visual field loss was measured every 4 months using the HFA 24-2 Swedish Interactive Threshold Algorithm. For the analysis, only reliable visual field data were used (ie, data from examinations with <33% false positives, <33% false negatives, and <33% fixation losses). We defined deterioration of the visual field as glaucoma progression. To estimate progression speed, we calculated the slope of MD values (dB/y) with the linear least-squares method. Data from at least 5 HFA examinations within the previous 3 years were used to calculate the baseline MD slope. MD slopes were also calculated after intervention in each treatment group.
As a post hoc analysis, visual field loss was determined with Guided Progression Analysis (GPA). Visual field progression was determined to have occurred when statistically significant (P<5%) worsening of visual sensitivity occurred at the same test point in at least 3 points and was confirmed in the following examination (ie, “possible progression”). Baseline data for the GPA included the results of 2 separate examinations: the initial HFA examination performed during this study and the most recent previous examination. If data from the previous examination were unavailable, data from the initial examination and the following examination were used.
Follow-Up Visits and Clinical Examinations
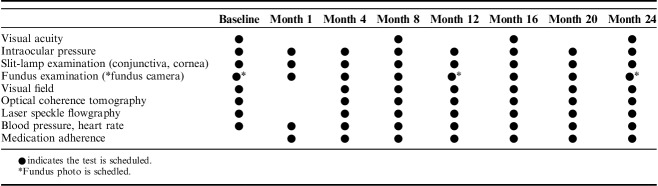
At baseline, all participants underwent full clinical ophthalmologic evaluations, including tests for visual acuity, refractive error, and IOP with Goldmann applanation tonometry, as well as slit-lamp, fluorescein staining, and fundus examinations. The second study visit took place 1 month±2 weeks after the first visit for checking adverse events, and the third to eighth visits took place every 4 months±2 weeks from the first visit. The study period examination schedule is summarized in Table 1.
TABLE 1.
Visits and Examination Schedule For the 24-Month Study Period
IOP was defined as the average of 2 measurements made using Goldmann applanation tonometry. Images of the optic nerve head were taken with a stereo fundus camera (nonmyd WX; Kowa Company Ltd, Nagoya, Japan). The visual field was evaluated using the HFA. Retinal nerve fiber layer thickness was measured using optical coherence tomography (OCT, Topcon 3D-OCT 2000; Topcon Corp., Tokyo, Japan). Laser speckle flowgraphy (LSFG-NAVI; Softcare Co. Ltd, Fukuoka, Japan) was performed after blood pressure and heart rate measurements.
Discontinuation of the Study
Attending physicians assessed all patient data. If there was uncontrolled IOP elevation, an intolerable adverse event, or deterioration of the visual field, physicians discussed whether the patient in question should be discontinued from the study until a consensus was reached. Deterioration of the visual field was defined as a significant worsening (P<5%) compared with baseline for the same ≥3 test locations confirmed at 2 subsequent examinations. Because patients in our cohort had progressive visual field defects at baseline, judgments about discontinuation were made following consideration of all available clinical data, including baseline MD slope and OCT data.
Sample Size
With a 2-sided significance level (α) of 0.05 and power (1−β) of 80%, assuming that the mean of the baseline MD slope is −0.75±0.25 dB, and that brimonidine and timolol reduce the MD slope by 50% and 20%, respectively, we calculated a requirement of 21 subjects per group. Allowing for a withdrawal/dropout rate of 30%, we planned to include 30 subjects in each group.
Randomization
Investigational drugs (brimonidine or timolol) were randomized in permuted blocks of size 2 by the study drug coordinator at a ratio of 1:1. The randomization code list was managed and retained independently until study completion.
Statistics Analysis
Statistical analyses were performed using Stata MP 15.0 (StataCorp LLC, College Station, TX). P-values <0.05 were considered to be statistically significant. Study participant characteristics were summarized and compared between the 2 treatment groups.
Primary Endpoint
Using linear regression models, a slope for the MD values (dB/y) between baseline and 24 months was calculated for each subject. The mean MD slope value was then calculated for both groups; a 2-sided t test was used to compare the mean MD slope values between the 2 treatment groups.
Secondary Endpoint
Changes in IOP and MD values were assessed using mixed linear regression models for repeated measurements. IOP and MD values were compared between baseline and 4, 8, 12, 16, 20, or 24 months. Then, we evaluated whether there were interactions among treatment groups over time by including an interaction term in the models. The same analyses were repeated for logMAR visual acuity, systolic blood pressure, diastolic blood pressure, and heart rate.
We compared the MD slope at baseline to that calculated during the trial with 2-sided t tests. A survival curve analysis was performed to examine GPA progression. A log-rank test was used to compare progression in the 2 groups.
Finally, we recorded adverse events in each treatment group during the study period. For this comparison, the severities of conjunctival hyperemia were classified into 5 categories: 0 (none), 0.5 (slight), 1 (mild), 2 (moderate), and 3 (severe). The severities of conjunctival follicle were classified into 4 categories: 0 (none), 1 (mild), 2 (moderate), and 3 (severe). The severity of superficial punctate keratopathy was assessed by area-density classification with fluorescence staining.22
RESULTS
This study initially recruited 72 patients. Four patients were immediately excluded from the study, before randomization, due to problems with data management and because they did not meet this study’s definition of open-angle glaucoma (Fig. 1). Therefore, 68 patients were randomized; 36 eyes were included in the brimonidine group and 32 eyes were included in the timolol group. During the follow-up period, 11 patients in the brimonidine group and 7 patients in the timolol group dropped out of the present study for various reasons: undergoing cataract surgery, undergoing capsulotomy using YAG laser, adverse events, and accidental death. Of the dropout patients, 10 in the brimonidine group and 2 in the timolol group were out of the examination schedule after the dropout. Thus, in the present study, we performed an intent to treat (ITT) analysis on all 56 patients (26 patients in the brimonidine group and 30 patients in the timolol group) who completed the 24-month examination schedule, regardless of their adherence to the allocated medication. The 50 patients without protocol deviations (ie, 25 patients in each treatment group) were further analyzed [per protocol set (PPS) analysis].
FIGURE 1.
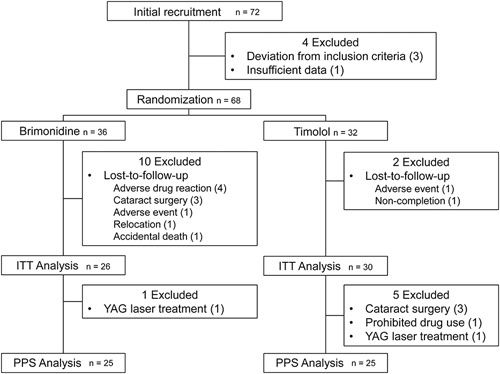
Flowchart of participant recruitment and treatment assignment. A total of 68 eyes were randomized to 2 treatment groups: 36 eyes to the brimonidine group and 32 eyes to the timolol group. During the follow-up period, 11 patients in the brimonidine group and 7 patients in the timolol group dropped out of the study for various reasons. We performed an ITT analysis on all 56 patients (26 patients in the brimonidine group and 30 patients in the timolol group) who completed the 24-month examination schedule, regardless of their adherence to the allocated medication. ITT indicates intent to treat; PPS, per protocol set.
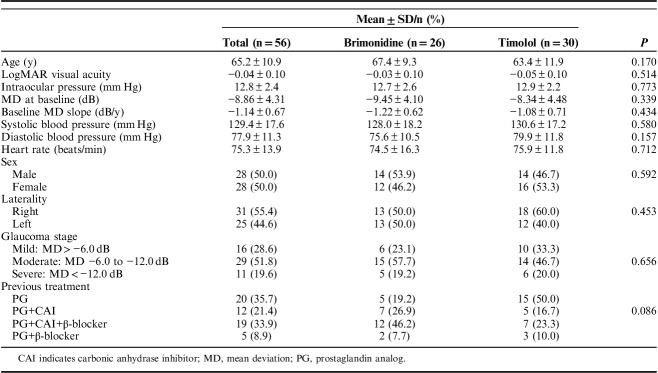
In the present study, the ITT analysis, the mean age was 65.2±10.9 years, and there were equal proportions of men and women (Table 2). They had no history of glaucoma surgery, including laser therapy. There were no significant differences in demographic or clinical characteristics between the brimonidine and timolol groups.
TABLE 2.
Baseline Patient Characteristics
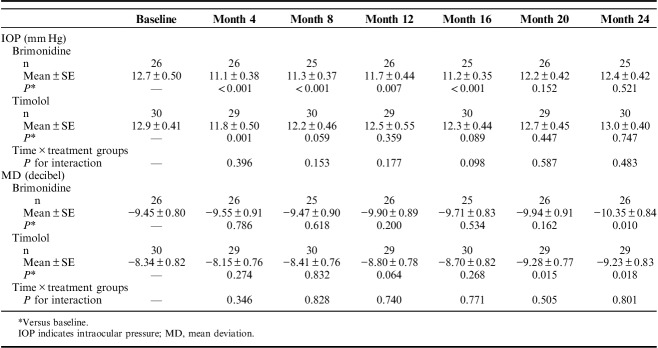
Figure 2 and Table 3 show changes in IOP in each group during the study period. There was a significant difference in IOP over time (P<0.001); however, there was no significant difference between treatment groups (P=0.195), and no significant interaction between treatment groups over time for IOP (P=0.663 for overall, and P>0.05 for all follow-up visits). IOP was decreased at 4 months compared with baseline in the timolol group, whereas IOP was continuously reduced in the brimonidine group from 4 to 16 months.
FIGURE 2.
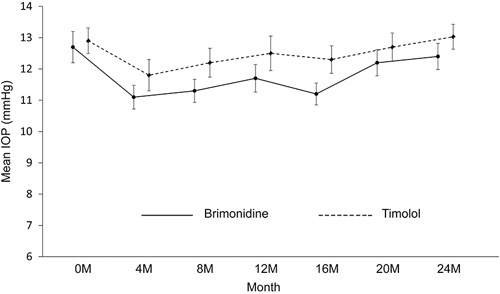
Changes in intraocular pressure (IOP) during the study period. Error bars represent SE. There were no significant interactions between treatment groups and visits.
TABLE 3.
Analyses of IOP and MD Using Mixed Linear Regression Models
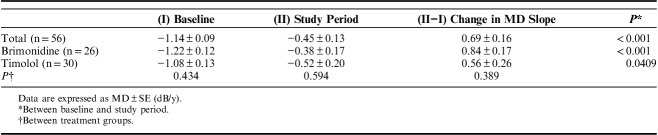
In the visual field-related analyses, the difference in MD slope during the study period was compared between each treatment group and served as the primary endpoint of the study. MD slope increased significantly compared with baseline in both treatment groups (Table 4). There was no difference in MD slope during the study period between the 2 treatment groups. Next, a mixed linear regression model was used to assess changes in IOP and MD; this showed a significant difference in MD over time (P<0.001). Furthermore, in the brimonidine group, MD decreased significantly for the first time at 24 months (P=0.010), whereas in the timolol group, MD decreased significantly at both 20 and 24 months (P=0.015 and 0.018, respectively) (Table 3). There was no significant difference in MD between the treatment groups (P=0.323), and no significant interaction between time and treatment group over time (P=0.774). Furthermore, in the survival curve analysis of visual field progression, better visual field preservation was noted in the brimonidine group than in the timolol group (P=0.0198, Fig. 3). In addition, the characteristics of the patients and the result of the PPS are shown in Supplementary Tables S1 to S3 available online (Supplemental Digital Content 1, http://links.lww.com/IJG/A279). In the PPS analysis, there was a significant difference in IOP over time (P<0.001); however, there was no significant difference between treatment groups (P=0.229), and no significant interaction between treatment groups over time for IOP (P=0.689 for overall, and P>0.05 for all follow-up visits). MD slopes during the study period were increased compared with baseline in both treatment groups; however, improvement in MD slopes was only significant in the brimonidine group. The survival curve analysis of visual field progression in PPS is shown in Supplementary Figure S1 available online (Supplemental Digital Content 2, http://links.lww.com/IJG/A280). Survival curve analysis of PPS, as well as the result of ITT analysis, revealed that brimonidine preserved the visual field for longer than did timolol.
TABLE 4.
Mean Deviation (MD) Slopes at Baseline and During the Study Period in the 2 Treatment Groups
FIGURE 3.
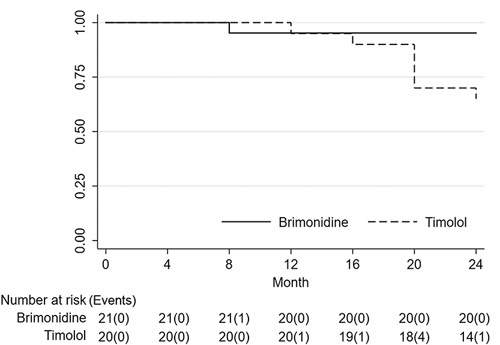
Kaplan-Meier survival estimates of the cumulative probability of visual field progression. The numbers of subjects with Guided Progression Analysis progression were 1 and 7 in the brimonidine and timolol groups, respectively. This is a statistically significant result according to the log-rank test (P=0.0198).
Details of other clinical conditions during the study period are presented in Supplementary Tables S4 to S6 available online (Supplemental Digital Content 3, http://links.lww.com/IJG/A281), including visual acuity, the occurrence of disc hemorrhage, number of subjects who experienced conjunctival hyperemia and follicular or corneal changes, blood pressure, and heart rate. Visual acuity showed no significant change over time. Cumulative numbers of disc hemorrhages were 1 in the brimonidine treatment group and 4 in the timolol treatment group (P=0.359).
Adverse drug reactions during the study period are summarized in Table 5. Conjunctival hyperemia, blepharitis, lacrimation, and discharge were more frequently observed in the brimonidine group than in the timolol group.
TABLE 5.
Adverse Drug Reactions During the Study Period

DISCUSSION
In this study, we compared the progression of visual field defects in patients with glaucoma who were treated with brimonidine or timolol. Although the 2 treatment groups were comparable at baseline and both treatments produced reductions in IOP, brimonidine maintained these reductions for a longer period of time than did timolol; however, this difference between treatment groups was not significant. There was no significant difference in MD slopes between the treatment groups, whereas a survival curve analysis showed that brimonidine was better than timolol for preventing the progression of visual field defects.
As mentioned earlier, increased IOP is a major risk factor for glaucoma. The beneficial effects of lowering IOP on glaucoma progression have been established in several studies.6–8,23–25 Timolol was the gold standard for lowering IOP before the development of prostaglandin analogs26,27; timolol reduced IOP values by 19% to 29% in clinical studies.28–30 Brimonidine 0.2% and timolol 0.5% have similar efficacies for lowering IOP.19,31,32 Moreover, brimonidine 0.1% has previously demonstrated efficacy for lowering IOP.33–35 In the present study, IOP was reduced by ∼10% in both treatment groups. One reason for this result may have been that all patients recruited to this study exhibited progressive visual field defects at baseline and had already undergone previous aggressive treatment for glaucoma. Therefore, the mean baseline IOP of patients in our cohort was 12.8 mm Hg, which is lower than the average IOP in Japanese subjects.36 As a result, it was difficult to achieve additional reductions in IOP in our cohort.
In the present study, we recruited patients with progressive open-angle glaucoma. The baseline MD slopes were −1.22 dB/y in the brimonidine group and −1.08 dB/y in the timolol group. These speeds of progression in patients with treated glaucoma were more rapid than the natural course of open-angle glaucoma reported in clinical studies. The Early Manifest Glaucoma trial reported that the speed of visual field decline in 46 patients with high-tension glaucoma (mean IOP, 24.7 mm Hg) was −1.31 dB/y, whereas that in 57 patients with NTG (mean IOP, 17.7 mm Hg) was −0.36 dB/y.37 This is consistent with the CNTGS, in which the speed of visual field decline in patients with untreated NTG (mean IOP, 16.1 mm Hg) was −0.40 dB/y.38 The present study included eyes with severe glaucoma that had more rapid disease progression than that in eyes examined in previous studies. Previously, we investigated the characteristics of glaucoma patients at our hospital, which is a large, central hospital that mainly receives advanced cases in Japan, and reported that the mean MD slope of patients with open-angle glaucoma and treated IOP was 14.3 mm Hg, with a progression of −0.77 dB/y.9 This progression was much more rapid than those reported in previous studies.
Our study included many glaucoma patients who underwent deterioration of the visual field despite the successful lowering of IOP. However, in the present study, the MD slopes showed improvement in both treatment groups after the intervention, despite limited IOP reduction. Brimonidine improved the MD slope to −0.38 dB/y during the study period, whereas timolol only improved the MD slope to −0.52 dB/y. MD slope calculated using a limited number of tests may be inaccurate; therefore, we assessed the efficacy of brimonidine and timolol using survival curve analysis. This analysis showed that the survival rate of the brimonidine group was higher than that of the timolol group (P=0.0198). This result suggests that brimonidine was more effective than timolol at preserving the visual field. These outcomes were nearly identical to those of PPS analysis; however, the MD slope in the timolol treatment group improved after intervention, although not significantly.
The difference in dropout ratios between groups might have affected the result of ITT analysis. Because the dropout ratio of brimonidine was higher than that of timolol in LoGTS, our dropout ratio results were predictable. Considering that no patient dropped out due to events related to the insufficient reduction of IOP, the results of the endpoints appear to be at least somewhat reliable. Moreover, the results of PPS analysis suggest that brimonidine was beneficial, with respect to preventing visual field deterioration, in glaucoma patients who could continue brimonidine treatment.
The present results are consistent with those of the LoGTS, which compared the effects of brimonidine 0.2% and timolol 0.5% in glaucoma; yet, this study differed from the LoGTS in several respects.17,19 Most notably, glaucoma patients in the present study were of Japanese descent and had progressive visual field defect <−0.5 dB/y, despite treated IOP or IOP within the normal range. In addition, our study used brimonidine at a concentration of 0.1% in a commercially available preparation with sodium hypochlorite, whereas the LoGTS used 0.2% brimonidine in a benzalkonium chloride preparation. The concentration of brimonidine used in Japan (0.1%) is based on the results of a dose-response trial that confirmed its efficacy for lowering IOP. The LoGTS differed from our study with respect to assessing the progression of visual field defects. In the LoGTS, the authors used 2 criteria to define visual field progression: 1 was defined using a point-wise linear regression analysis with Progressor software (Medisoft Ltd, Leeds, UK) and the other was defined using Humphrey glaucoma change probability maps. Visual field progression required confirmation at the next 2 examinations, including a significant negative slope at the same 3 or more test locations. The LoGTS used a post hoc analysis with the 3-omitting method to verify the results of the primary Progressor outcome. Thus, the first detection of visual field deterioration could only occur at the 16-month follow-up visit; however, in analyses using each individual criterion, visual field progression was less frequent in the brimonidine 0.2% group than in the timolol 0.5% group, despite similar reductions in IOP. Conversely, in the present study, we used survival curve analysis as a secondary endpoint to confirm the results of MD slope analysis (ie, the primary endpoint). The different criterion for progression in survival curve analysis was adopted to increase the sensitivity of detection. Nevertheless, eyes treated twice daily with brimonidine 0.1% tended to progress more slowly than eyes treated with timolol 0.5%. Moreover, we found that the frequency of disc hemorrhage during the study period was higher in the timolol group (4 cases) than in the brimonidine group (1 case). In contrast, timolol or brimonidine treatment did not influence the occurrence or recurrence of disc hemorrhage in the LoGTS.21 Disc hemorrhage is an important sign of glaucoma progression. If we had defined glaucoma progression as the occurrence of disc hemorrhage in addition to visual field deterioration, glaucoma progression would have been clearly delayed in the brimonidine group compared with that in the timolol group.
In the present study, IOP values were significantly lower than baseline in the timolol group at 4 months, whereas IOP values were significantly lower in the brimonidine group at 4, 8, 12, and 16 months. However, considering that baseline IOP was relatively low in both groups as a result of IOP-lowering treatment and that there were no significant differences in IOP over time between the 2 treatment groups, we consider that mechanisms unrelated to IOP were responsible for at least a portion of the difference in effectiveness between the 2 treatments. This view is supported by the results of the LoGTS and the results of basic research showing neuroprotective effects in retinal ganglion cells (RGCs).39–41
Some basic research has demonstrated the protective effects of brimonidine on RGCs. Our study group also reported that intravitreal injection of brimonidine enhanced the survival and electrophysiological activity of RGCs in axotomized rat eyes.39 Semba et al40 found that brimonidine prevented retinal degeneration in excitatory amino-acid carrier 1-deficient mice, which exhibit progressive RGC loss due to glutamate neurotoxicity and oxidative stress, by stimulating multiple pathways, including glia-neuron interactions. Another previous study showed that brimonidine treatment rescued RGCs from ischemic damage induced by transient ligature of the ophthalmic vessels.41 Unfortunately, the potential neuroprotective effects of brimonidine are difficult to demonstrate clinically and thus remain controversial. Future clinical studies of brimonidine similar to the present study can better inform the therapeutic actions of brimonidine in glaucoma.
The present study had several limitations. First, the study design might have contributed to potential bias. The study was a randomized, but open-label trial with respect to medications and IOP readings. It was performed at a single center without independent reading centers. In addition, the sample size was smaller than that in previous studies, such as the LoGTS. However, although intervention studies with large sample sizes can more easily show significant differences between treatments, it is unethical to include more than the minimum statistically necessary number of patients. Furthermore, we focused on the specific case of open-angle glaucoma (ie, patients with progressive disease despite treatment with IOP), to obtain results relevant to our interest. Thus, we believe that these results can provide meaningful clinical information if they are accurately interpreted. Second, the use of drugs before entry into the study could have influenced the progression of glaucoma during the study period. The duration of use of β-adrenergic antagonists or carbonic anhydrase inhibitor (ie, at least 6 mo) might have been insufficient for inclusion criteria, as these drugs might take effect after the entry. We reasoned that because we recruited patients with progressive glaucoma, the same combination of antiglaucomatous drugs was not used for an extended period of time. To minimize the effect of the drugs, we randomly assigned the patients into either of the 2 treatment groups. Although we could not precisely equalize the use of pretreatment drugs in the 2 groups, there was no significant difference between groups in the proportion of drugs that were used before entry into the study (P=0.086). Third, a few patients who had undergone cataract surgery were included in the ITT analysis. Therefore, we additionally performed PPS analysis, which excluded factors influencing the visual field. In this analysis, the MD slope in the brimonidine treatment group improved significantly as in ITT analysis, whereas improvement in the timolol treatment group was not significant. In addition, we confirmed that the results of the survival curve analysis were similar to that in the ITT analysis. Considering these results, PPS analysis might reveal more clearly that brimonidine preserved the visual field for a longer period than did timolol.
In the present study, we compared the efficacy of brimonidine 0.1% and timolol 0.5% in previously treated patients with open-angle glaucoma. Effects on IOP were similar between the treatment groups; furthermore, brimonidine and timolol preserved the visual field more effectively. Our research adds to the limited number of clinical studies informing the utility of low-concentration brimonidine treatment in glaucoma.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.glaucomajournal.com.
ACKNOWLEDGMENTS
The authors thank Seri Takahashi and Minami Yoshida for providing technical support.
Footnotes
Supported by Senju Pharmaceutical Co. Ltd which was involved in the research planning through provision of drug information.
Y.Y., R.K., H.T., S.M., S.T., K.O., and T.N. has received lecture fees from Senju Pharmaceutical Co. Ltd.
REFERENCES
- 1.Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–467. [DOI] [PubMed] [Google Scholar]
- 2.Coleman AL, Miglior S. Risk factors for glaucoma onset and progression. Surv Ophthalmol. 2008;53:3–10. [DOI] [PubMed] [Google Scholar]
- 3.McMonnies CW. Glaucoma history and risk factors. J Optom. 2017;10:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman DS, Wilson MR, Liebmann JM, et al. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am J Ophthalmol. 2004;138:S19–S31. [DOI] [PubMed] [Google Scholar]
- 5.Nakazawa T. Ocular blood flow and influencing factors for glaucoma. Asia Pac J Ophthalmol (Phila). 2016;5:38–44. [DOI] [PubMed] [Google Scholar]
- 6.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2010;130:429–440. [DOI] [PubMed] [Google Scholar]
- 7.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. [DOI] [PubMed] [Google Scholar]
- 8.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. [DOI] [PubMed] [Google Scholar]
- 9.Yokoyama Y, Maruyama K, Konno H, et al. Characteristics of patients with primary open angle glaucoma and normal tension glaucoma at a university hospital: a cross-sectional retrospective study. BMC Res Notes. 2015;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yokoyama Y, Aizawa N, Chiba N, et al. Significant correlations between optic nerve head microcirculation and visual field defects and nerve fiber layer loss in glaucoma patients with myopic glaucomatous disk. Clin Ophthalmol. 2011;5:1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiga Y, Kunikata H, Aizawa N, et al. Optic nerve head blood flow, as measured by laser speckle flowgraphy, is significantly reduced in preperimetric glaucoma. Curr Eye Res. 2016;41:1447–1453. [DOI] [PubMed] [Google Scholar]
- 12.Asano Y, Himori N, Kunikata H, et al. Age- and sex-dependency of the association between systemic antioxidant potential and glaucomatous damage. Sci Rep. 2017;7:8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souzeau E, Burdon KP, Dubowsky A, et al. Higher prevalence of myocilin mutations in advanced glaucoma in comparison with less advanced disease in an Australasian disease registry. Ophthalmology. 2013;120:1135–1143. [DOI] [PubMed] [Google Scholar]
- 14.Rezaie T, Child A, Hitchings R, et al. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–1079. [DOI] [PubMed] [Google Scholar]
- 15.Gharahkhani P, Burdon KP, Fogarty R, et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat Genet. 2014;10:1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramdas WD, van Koolwijk LM, Lemij HG, et al. Common genetic variants associated with open-angle glaucoma. Hum Mol Genet. 2011;20:2464–2471. [DOI] [PubMed] [Google Scholar]
- 17.Krupin T, Liebmann JM, Greenfield DS, et al. The low-pressure glaucoma treatment study (LoGTS) study design and baseline characteristics of enrolled patients. Ophthalmology. 2005;112:376–385. [DOI] [PubMed] [Google Scholar]
- 18.Greenfield DS, Liebmann JM, Ritch R, et al. Visual field and intraocular pressure asymmetry in the low-pressure glaucoma treatment study. Ophthalmology. 2007;114:460–465. [DOI] [PubMed] [Google Scholar]
- 19.Krupin T, Liebmann JM, Greenfield DS, et al. A randomized trial of brimonidine versus timolol in preserving visual function: Results from the low-pressure glaucoma treatment study. Am J Ophthalmol. 2011;151:671–681. [DOI] [PubMed] [Google Scholar]
- 20.De Moraes CG, Liebmann JM, Greenfield DS, et al. Risk factors for visual field progression in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2012;154:702–711. [DOI] [PubMed] [Google Scholar]
- 21.Furlanetto RL, De Moraes CG, Teng CC, et al. Risk factors for optic disc hemorrhage in the low-pressure glaucoma treatment study. Am J Ophthalmol. 2014;157:945–952. [DOI] [PubMed] [Google Scholar]
- 22.Miyata K, Amano S, Sawa M, et al. A novel grading method for superficial punctate keratopathy magnitude and its correlation with corneal epithelial permeability. Arch Ophthalmol. 2003;121:1537–1539. [DOI] [PubMed] [Google Scholar]
- 23.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. [DOI] [PubMed] [Google Scholar]
- 24.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 25.Anderson DR, Drance SM, Schulzer M. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. [DOI] [PubMed] [Google Scholar]
- 26.Li T, Lindsley K, Rouse B, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WY, Po AL, Dua HS, et al. Meta-analysis of randomised controlled trials comparing latanoprost with timolol in the treatment of patients with open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2001;85:983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strahlman E, Tipping R, Vogel R. A double-masked, randomized 1-year study comparing dorzolamide (Trusopt), timolol, and betaxolol. International Dorzolamide Study Group. Arch Ophthalmol. 1995;113:1009–1016. [DOI] [PubMed] [Google Scholar]
- 29.Mills KB. Blind randomised non-crossover long-term trial comparing topical timolol 0.25% with timolol 0.5% in the treatment of simple chronic glaucoma. Br J Ophthalmol. 1983;67:216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart RH, Kimbrough RL, Ward RL. Betaxolol vs timolol. A six-month double-blind comparison. Arch Ophthalmol. 1986;104:46–48. [DOI] [PubMed] [Google Scholar]
- 31.Katz LJ. Brimonidine tartrate 0.2% twice daily vs timolol 0.5% twice daily: 1-year results in glaucoma patients. Am J Ophthalmol. 1999;127:20–26. [DOI] [PubMed] [Google Scholar]
- 32.Cantor LB. The evolving pharmacotherapeutic profile of brimonidine, a 2-adrenergic agonist, after four years of continuous use. Expert Opin Pharmacother. 2000;1:815–834. [DOI] [PubMed] [Google Scholar]
- 33.Katz LJ. Twelve-month evaluation of brimonidine-purite versus brimonidine in patients with glaucoma or ocular hypertension. J Glaucoma. 2002;11:119–126. [DOI] [PubMed] [Google Scholar]
- 34.Cantor LB, Safyan E, Liu CC, et al. Brimonidine-purite 0.1% versus brimonidine-purite 0.15% twice daily in glaucoma or ocular hypertension: a 12-month randomized trial. Curr Med Res Opin. 2008;24:2035–2043. [DOI] [PubMed] [Google Scholar]
- 35.Cantor LB. Brimonidine in the treatment of glaucoma and ocular hypertension. Ther Clin Risk Manag. 2006;2:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: The Tajimi Study. Ophthalmology. 2004;111:1641–1648. [DOI] [PubMed] [Google Scholar]
- 37.Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–2276. [DOI] [PubMed] [Google Scholar]
- 38.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–497. [DOI] [PubMed] [Google Scholar]
- 39.Yukita M, Omodaka K, Machida S, et al. Brimonidine enhances the electrophysiological response of retinal ganglion cells through the Trk-MAPK/ERK and PI3K pathways in axotomized eyes. Curr Eye Res. 2017;42:125–133. [DOI] [PubMed] [Google Scholar]
- 40.Semba K, Namekata K, Kimura A, et al. Brimonidine prevents neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014;5:e1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lafuente López-Herrera MP, Mayor-Torroglosa S, Miralles de Imperial J, et al. Transient ischemia of the retina results in altered retrograde axoplasmic transport: neuroprotection with brimonidine. Exp Neurol. 2002;178:243–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.glaucomajournal.com.