Summary
Background
Radiotherapy is standard of care for cervical cancer, but major global gaps in access exist, particularly in low-income and middle-income countries. We modelled the health and economic benefits of a 20-year radiotherapy scale-up to estimate the long-term demand for treatment in the context of human papillomavirus (HPV) vaccination.
Methods
We applied the Global Task Force on Radiotherapy for Cancer Control investment framework to model the health and economic benefits of scaling up external-beam radiotherapy and brachytherapy for cervical cancer in upper-middle-income, lower-middle-income, and low-income countries between 2015 and 2035. We estimated the unique costs of external-beam radiotherapy and brachytherapy and included a specific valuation of women’s caregiving contributions. Model outcomes life-years gained and the human capital and full income net present value of investment. We estimated the effects of stage at diagnosis, radiotherapy delivery system, and simultaneous HPV vaccination (75% coverage) up to a time horizon set at 2072.
Findings
For the period from 2015 to 2035, we estimated that 9·4 million women in low-income and middle-income countries required treatment with external-beam radiotherapy, of which 7·0 million also required treatment with brachytherapy. Incremental scale-up of radiotherapy in these countries from 2015 to meet optimal radiotherapy demand by 2035 yielded 11·4 million life-years gained, $59·3 billion in human capital net present value (–$1·5 billion in low-income, $19·9 billion in lower-middle-income, and $40·9 billion in upper-middle-income countries), and $151·5 billion in full income net present value ($1·5 billion in low-income countries, $53·6 billion in lower-middle-income countries, and $96·4 billion in upper-middle-income countries). Benefits increased with advanced stage of cervical cancer and more efficient scale up of radiotherapy. Bivalent HPV vaccination of 12-year-old girls resulted in a 3·9% reduction in incident cases from 2015·2035. By 2072, when the first vaccinated cohort of girls reaches 70 years of age, vaccination yielded a 22·9% reduction in cervical cancer incidence, with 38·4 million requiring external-beam radiotherapy and 28·8 million requiring brachytherapy.
Interpretation
Effective cervical cancer control requires a comprehensive strategy. Even with HPV vaccination, radiotherapy treatment scale-up remains essential and produces large health benefits and a strong return on investment to countries at different levels of development.
Introduction
Cervical cancer is a major cause of morbidity and mortality in women in low-income and middle-income countries (LMICs). In 2018, the International Agency for Research on Cancer estimated that 569 847 women were diagnosed with and 311 365 died from cervical cancer worldwide, with 85% of diagnoses and 88% of deaths occurring in LMICs.1 Globally, it is the most commonly diagnosed cancer in 28 countries and the predominant cause of cancer mortality in 42 countries, most of which are in sub-Saharan Africa and Southeast Asia.1,2 International efforts to address cervical cancer in LMICs and to reduce global disparities have focused on early detection of precancerous lesions and prophylactic vaccination against human papillomavirus (HPV) infections, the primary cause of nearly all cervical cancers. However, this emphasis on primary and secondary prevention has not been matched by provision of accessible treatment and palliative care.3,4
In countries without effective coverage of cervical cancer screening, many women, especially those living in poverty, do not seek medical attention until their disease is advanced and no longer amenable to surgical intervention.3,5 Access to treatment and financial protection is inadequate and too costly for most low-income families.6 When they do seek care, treatment is often unavailable, which contributes to the disproportionately high mortality from the disease in LMICs.
Radiotherapy is the standard of care for stages IB3-III cervical cancer7 and is a highly effective and curative treatment, even for patients with advanced disease (stage IB2-IVA).8 However, radiotherapy is largely unavailable in LMICs, where the need is often greatest. These disparities were well documented in the 2015 Lancet Oncology Commission on Radiotherapy.4 The Commission delineated the substantial health and economic benefits that might accrue from a global investment in expansion of radiotherapy access and laid out a call for action to include radiotherapy in cancer control planning and universal health coverage schemes, as well as to invest in human resources and technical capacity, especially in LMICs.4
The objective of this study was to inform policy by quantifying the health and economic effects of scaling up radiotherapy over a 20-year period to achieve optimal use for patients with cervical cancer by 2035, in the context of HPV vaccination. We extended previous modelling approaches4,9 by evaluating the unique costs of treating patients who require both external-beam radiotherapy (EBRT) and brachytherapy, an essential component of curative treatment for cervical cancer.10 We also included the economic value of women’s contributions as health-care providers and caregivers, applying the methods from the Lancet Global Health 2035 Commission11 and the Lancet Commission on Women and Health.12 Additionally, we aimed to estimate the direct effect of implementing universal HPV vaccination for 12-year-old girls on the future demand for radiotherapy from 2015 to 2072, at which time the first vaccinated cohort (girls vaccinated in 2014) will reach 70 years of age.
Methods
Radiotherapy investment model
Three independent models, which estimate demand, cost, and survival benefit of EBRT and brachytherapy, were integrated into an investment framework developed by the Global Task Force on Radiotherapy for Cancer Control for the 2015 Lancet Oncology Commission on Radiotherapy to estimate the health and economic effects of scaling up access to radiotherapy for cervical cancer in low-income, lower-middle-income, and upper-middle-income countries (figure 1; appendix pp 1–8).4 This framework relied on a parsimonious Markov model, in which patients diagnosed between 2015 and 2035 were simulated over their remaining lifetime from diagnosis in a scenario of radiotherapy scale-up compared with no scale-up. In line with the Commission,4 we assumed that radiotherapy capacity would be scaled up at a linear rate from initial capacity in 2015 to optimal capacity by 2035 (for assumptions specific to this analysis, see tables 1 and 2 and appendix pp 1–8).
Figure 1: Analytical structure.
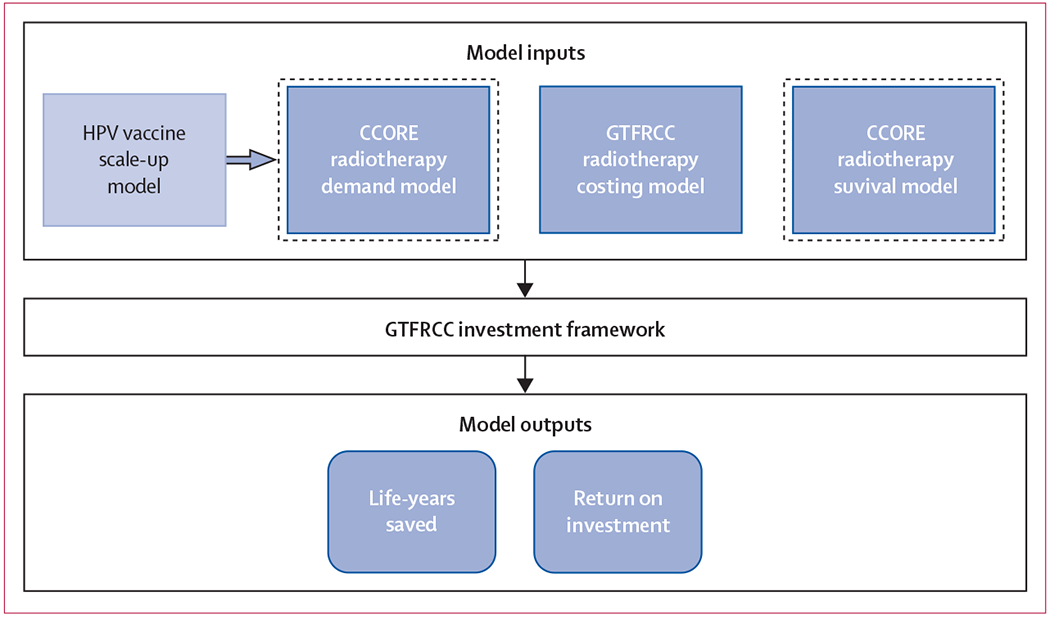
The model inputs box displays the three models in dark blue that served as the core inputs for the GTFRCC radiotherapy investment framework: demand, costing, and survival. The demand and survival models were based on the work of the CCORE, and the costing model was developed in collaboration with the International Atomic Energy Agency. The light blue HPV vaccine scale-up model box identifies how the Papillomavirus Rapid Interface for Modelling and Economics model best case parameters were integrated into the GTFRCC model input structure by modulating the demand model. The dotted lines around the demand and survival boxes represent the model inputs that were varied in our sensitivity analysis on the population distribution of cervical cancer stage at diagnosis. The model outputs included the life years saved and return on investment (human capital and full income net present value). CCORE=Collaboration for Cancer Outcomes Research and Evaluation. GTFRCC=Global Task Force on Radiotherapy for Cancer Control. HPV=human papillomavirus.
Table 1:
Model input parameters for cervical cancer and HPV
| Base case value (GTFRCC parameters) | Sensitivity analysis (advanced stage distribution) | |
|---|---|---|
|
Cervical cancer parameters | ||
| Demand for radiotherapy | ||
| External-beam radiotherapy | 71% | 86% |
| Brachytherapy | 53% | 68% |
| 5-year population survival benefit | 18% | 35% |
| Stage distribution | ||
| IA1–IA2 | 23% | 9% |
| IB1–IIA1 | 20% | 29% |
| IB2–IIA2 | 9% | 13% |
| IIB | 12% | 21% |
| III–IVA | 24% | 25% |
| IVB | 12% | 3% |
|
HPV parameters | ||
| Cases attributable to HPV 16 and 18 (%) | .. | 75% |
| Vaccine efficacy (HPV 16 and 18) | .. | 100% |
| Vaccine coverage of 3-dose schedule | .. | 75% |
| Vaccinated cohort | .. | 12-year-old girls |
HPV=human papillomavirus. GTFRCC=Global Task Force on Radiotherapy for Cancer Control.
Table 2:
Model input cost parameters
| Nominal model |
Efficient model |
|||
|---|---|---|---|---|
| Capital cost of scale-up | Operating cost | Capital cost of scale-up | Operating cost | |
|
External-beam radiotherapy | ||||
| Low-income countries | $277·81 | $61 | $133·86 | $29·43 |
| Lower-middle-income countries | $277·48 | $67 | $133·81 | $33·29 |
| Upper-middle-income countries | $292·14 | $90 | $142·86 | $49·90 |
|
Brachytherapy | ||||
| Low-income countries | $461·00 | $132 | $206·33 | $84·33 |
| Lower-middle-income countries | $459·67 | $179 | $233·00 | $121·67 |
| Upper-middle-income countries | $487·67 | $367 | $292·00 | $271·67 |
All costs are presented in 2015 US$ per radiation fraction.
Optimal demand and 5-year overall population survival benefit from EBRT and brachytherapy were based on a model of optimum use developed by the Australian Collaboration for Cancer Outcomes Research and Evaluation (appendix pp 1–3),13 which assumed that patients were treated according to evidence-based guidelines. The distribution of cancer stage at diagnosis used in the Commission was applied, which was derived from Australian population-based models (table 1).13 Curative (radical and adjuvant) and palliative indications were included, but indications for brachytherapy alone were not considered. Information about cervical cancer stage in LMICs is poor or incomplete, but individual hospital-based reports suggest more advanced disease at diagnosis compared with high-income countries.14 Because radiotherapy indications increase with stage of disease, use of Australian estimates in our base case are expected to yield conservative estimates of health and economic benefits. The effects of more advanced stage distribution were modelled as a sensitivity analysis based on the stage at diagnosis in the 26th International Federation of Gynecology and Obstetrics annual report,14 in which a greater proportion of patients presented with locally advanced, non-metastatic disease (table 1; appendix p 3).
Global cervical cancer incidence data were derived from country-specific GLOBOCAN estimates15 categorised by the 2017 World Bank income groups.16 Baseline radiotherapy capacity was determined for each country on the basis of the number of treatment fractions that could be delivered with the existing EBRT machines recorded in the Directory of Radiotherapy Centres maintained by the International Atomic Energy Agency. Model simulations were developed for patients from a median age at diagnosis of 50 years in low-income and lower-middle-income countries and 47 years in upper-middle-income countries, based on GLOBOCAN 2012 age-specific incidence data.15 A background mortality rate to account for the probability of dying from other causes was modelled using representative life tables17 from each income group and proportionately weighted, accounting for increasing mortality as the population ages.
The funding needed for capital and operating investments was estimated from the Commission’s time-driven, activity-based, costing model (table 2; appendix p 4).4,18 The capital costs reflect the additional investment in equipment and human resources needed to achieve optimal use by 2035 (scale-up period). Capital costs related to maintenance and machine and source replacement were included in the operating budget (table 2), with the assumption that equipment (eg, CT simulators and linear accelerators) is replaced every 12 years, or 5 years for computer equipment.
Unlike many other malignancies, brachytherapy is an indispensable component of cervical cancer treatment.10 Therefore, we separately estimated the cost of EBRT and high dose-rate brachytherapy (table 2), which have unique technical, operational, and educational considerations. Although 2D brachytherapy is used in many centres around the world,8 we modelled the cost of scaling up to 3D brachytherapy with CT simulation based on international recommendations, assuming diffusion of these technologies over the time horizon of the analysis. We also assumed that EBRT was delivered using 3D-conformal radiotherapy planning and portal image guidance.
Two radiotherapy investment scenarios were considered: a nominal scenario with essential equipment and typical operating parameters and an efficiency scenario with lower costs. The efficiency scenario was based on a 50% reduction in time spent on EBRT and brachytherapy treatment planning and quality assurance, a reduction in EBRT treatment time from an average of 15 min to 12 min, an increase in machine operating hours from 12 h to 16 h, and a 30% reduction in capital costs from bulk purchasing. These technological efficiencies, which were estimated by the Global Task Force on Radiotherapy for Cancer Control and published in the Commission, are expected to be derived from increased use of automation in routine clinical practice (appendix pp 4–6).
Health and economic benefits
Economic benefits were calculated using both the human capital and full income methods.11 These complementary approaches estimate the economic value of treating cancer and allowing patients to productively return to the workforce. Model outcomes were the life-years gained and the economic benefits of added years of worker productivity, measured in term of gross domestic product (GDP) per person using 2015 US$. Projections for GDP growth were made using a weighted average of country-specific GDP for each income group, according to estimates provided by the International Monetary Fund’s World Economic Outlook for 2012–22;19 projections beyond 2022 were estimated based on average annual growth over the preceding 10-year period. Patients were assumed not to make further contributions to the economy beyond the age of 70 years. Radiotherapy return on investment was calculated as the net present value divided by cost. All benefits and costs were discounted by 3% per annum to adjust for their value at the time at which they occur.20
The human capital model estimates the monetary value of health as the contribution to national income accounts as a result of recovery from illness and avoidance of premature mortality.11 We calculated this value by multiplying the additional life-years gained with radiotherapy scale-up by the GDP per person of each World Bank income group. However, most economic analyses underestimate the full value of a year of life by zero-valuing unpaid caregiving and domestic labour, hence defining these hours of work in the home as outside the productive sphere of activities that contribute to economic growth.12 A robust analysis should consider both the value of time and the fact that improved health at home drives productivity of all household members. This is an especially important oversight for any analysis of the value of women’s time and, therefore, an important additional benefit to estimate for our study of cervical cancer. The Lancet Commission on Women and Health specifically analysed the value of women’s unpaid caregiving contributions as part of women’s contribution to health. They found that, globally, the hours of unpaid caregiving work are worth almost as much as paid health-care work.12
For our study, the human capital model included the economic value of women’s unpaid work in health in the home that would be realised through radiotherapy scale-up, which was estimated by adding the average wage equivalency per person to the human capital benefits (appendix pp 7–8).12 The average wage was calculated by the Lancet Commission on Women and Health for each income group by using the average hourly earnings of all life science and health professionals as a proxy wage. These wages do not incorporate the value of women’s domestic work that does not directly contribute to health.
Our full income model was based on the Lancet Commission on Investing in Health, which considered the benefits of improved health and longevity by also considering the value that individuals place on changes in their own life expectancy (the value of a statistical life-year) and, correspondingly, the amount of wealth that individuals in a country are willing to expend for a reduction in the probability of death. This approach captures the behavioural changes and broader economic effects that occur when people live in a society where they can expect to live a longer and healthier life.11 The Commission on Investing in Health’s 2013 report11 calculated full income benefits by multiplying the human capital US$ projections by the value of a statistical life-year, estimated as 2·3—the value adopted for this study.11
HPV vaccination and radiotherapy investment
To explore the interaction of intensification of HPV vaccination programmes and demand for treatment capacity for patients with established locally advanced disease, we modelled the effect of a universal rollout of bivalent HPV vaccination (vaccination against HPV-16 and HPV-18) in low-income, lower-middle-income, and upper-middle-income countries on the annual burden of cervical cancer and the demand for radiotherapy treatment (appendix pp 9–10). We modelled the best case validation scenario defined by Jit and colleagues9 in their Papillomavirus Rapid Interface for Modelling and Economics (PRIME) study, which assumed routine bivalent vaccination of all 12-year-old girls before sexual debut with 75% coverage, with the expectation of immediate, lifelong protection against HPV-16 and HPV-18 infections (table 1). The proportion of cervical cancer cases worldwide that are attributable to HPV-16 and HPV-18 infections ranges from 65% in South and Central America to 76% in North America.21,22
Under the assumption that the first cohort of girls was vaccinated in 2014, we modelled the annual age-specific reduction in cervical cancer incidence relative to GLOBOCAN projections15 from 2015 to 2035 in the base case analysis. The timeframe was extended to 2072 in the sensitivity analysis, which represents the year in which the first vaccinated cohort would reach the age of 70 years. Catch-up vaccination, vaccination of boys, cross-protection against other HPV strains not targeted by the vaccine, and HPV herd immunity were not considered. The stage distribution of cervical cancer in our base case analysis conservatively reflected the burden of disease in a population with ongoing screening and effective health system performance.
Role of the funding source
There was no funding source for this study. DR received fellowship support from the Canadian Association of Radiation Oncology and the The Commonwealth Fund during the preparation of this report, but neither organisation had a role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data and had final responsibility for the decision to submit for publication.
Results
For the period from 2015 to 2035, we estimated that 9·4 million women in lower-middle-income countries required treatment with EBRT, of which 7·0 million also required treatment with brachytherapy (table 3). Incrementally scaling up radiotherapy in these countries using the nominal costing scenario over the time horizon of the study resulted in 11·4 million life-years gained and a net present value of $59·3 billion using the human capital model (–$1·5 billion in low-income, $19·9 billion in lower-middle-income, and $40·9 billion in upper-middle-income countries), and $151·5 billion using the full income model ($1·5 billion in low-income, $53·6 billion in lower-middle-income, and $96·4 billion in upper-middle-income countries; figure 2).
Table 3:
Population health and economic effects of scaling up radiotherapy capacity for cervical cancer treatment, 2015–35
| Health parameters |
Human capital return on investment |
Full income return on investment |
|||||
|---|---|---|---|---|---|---|---|
| Patients requiring external-beam radiotherapy | Patients requiring brachytherapy | Life-years gained with radiotherapy scale-up | Nominal model | Efficient model | Nominal model | Efficient model | |
|
Base case values | |||||||
| Low-income countries | 1 340 413 | 1 005 310 | 2 348 608 | −0·4 | 0·3 | 0·3 | 1·8 |
| Lower-middle-income countries | 4 892 966 | 3 669 725 | 6 188 537 | 1·6 | 4·2 | 4·3 | 9·7 |
| Upper-middle-income countries | 3 120 872 | 2 340 654 | 2 861 813 | 6·1 | 12·3 | 14·2 | 27·7 |
| Total | 9 354 251 | 7 015 689 | 11 398 958 | 2·5 | 6·0 | 6·5 | 13·8 |
|
Sensitivity analysis: advanced stage distribution | |||||||
| Low-income countries | 1 623 599 | 1 282 643 | 3 359 445 | 0·0 | 1·1 | 1·1 | 3·3 |
| Lower-middle-income countries | 5 926 692 | 4 682 086 | 9 655 660 | 3·0 | 7·0 | 7·2 | 15·4 |
| Upper-middle-income countries | 3 780 211 | 2 986 367 | 4 480 428 | 8·7 | 17·3 | 20·0 | 38·6 |
| Total | 11 330 502 | 8 951 096 | 17 495 533 | 4·0 | 9·5 | 9·9 | 22·1 |
|
Sensitivity analysis: integrated radiotherapy and vaccination scale-up | |||||||
| Low-income countries | 1 284 243 | 963 183 | 2 041 140 | −0·4 | −0·1 | 0·3 | 0·9 |
| Lower-middle-income countries | 4 729 919 | 3 547 440 | 5 936 526 | 1·1 | 3·2 | 3·2 | 7·5 |
| Upper-middle-income countries | 3 014 362 | 2 260 772 | 2 694 691 | 5·6 | 11·4 | 13·4 | 25·87 |
| Total | 9 028 525 | 6 771 394 | 10 672 357 | 2·2 | 5·1 | 5·6 | 11·7 |
Return on investment is a ratio defined as the net present value (itself defined as the economic return on investment minus the cost of investment) divided by the cost of investment, all in US$.
Figure 2: Net present value of radiotherapy scale-up (nominal and efficient scenarios) to universal access for patients with cervical cancer in low-income and middle-income countries, 2015–35.
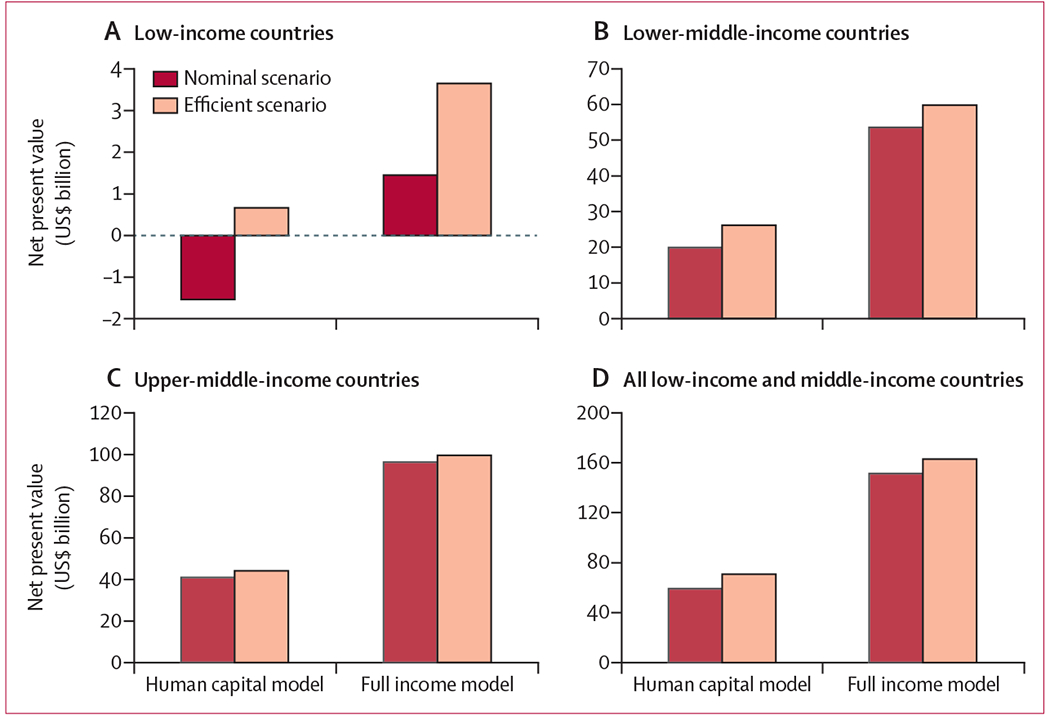
Axes reflect the individual data ranges of each graph and are not uniform. All costs are presented in 2015 US$.
Under the efficient radiotherapy scenario, the human capital model suggests a net present value of $68·8 billion ($0·67 billion in low-income, $26·1 billion in lower-middle-income, and $43·6 billion in upper-middle-income countries) and, using the full income scenario, a net present value of$163·0 billion ($3·7 billion in low-income, $59·8 billion in lower-middle-income, and $99·5 billion in upper-middle-income countries). In LMICs overall, worldwide investments in radiotherapy yielded positive returns on investment in all scenarios, ranging from 2·5 to 13·8 (table 3).
In a sensitivity analysis, when we assumed a more advanced cancer stage distribution, the number of patients requiring radiotherapy increased by 21% (11·3 million for EBRT and 9·0 million for brachytherapy). Scaling up radiotherapy over this period yielded 17·5 million life-years gained across LMICs, with a strong return on investment in all scenarios, ranging from 4·0 to 22·1 (table 3). Applying the nominal costing model, this scale-up resulted in $103·2 billion human capital net present value gains and $257·6 billion in net present value full income gains. Under the efficiency scenario, the net present value increased to $117·4 billion from the human capital approach and $271·8 billion from the full income approach.
Over the 20-year analytic period (2015–35), HPV vaccination of 12-year-old girls resulted in a 3·9% reduction in cervical cancer incidence compared with 2012 GLOBOCAN projections (4·2% in low-income, 3·3% lower-middle-income, and 4·8% reduction in upper-middle-income countries; figure 3). After 20 years with an HPV vaccination programme in place, we projected that 9·0 million women would require EBRT and 6·8 million women would require brachytherapy to treat their cancer (table 3). Increasing the time horizon to 2072, HPV vaccination is estimated to cumulatively reduce cervical cancer by 22·9% (24·6% in low-income, 22·8% in lower-middle-income, and 21·9% in upper-middle-income countries; figure 3); however, 38·4 million women would require EBRT and 28·8 million would require brachytherapy (figure 4).
Figure 3: Effect of universal human papillomavirus vaccination strategy on cervical cancer incidence.
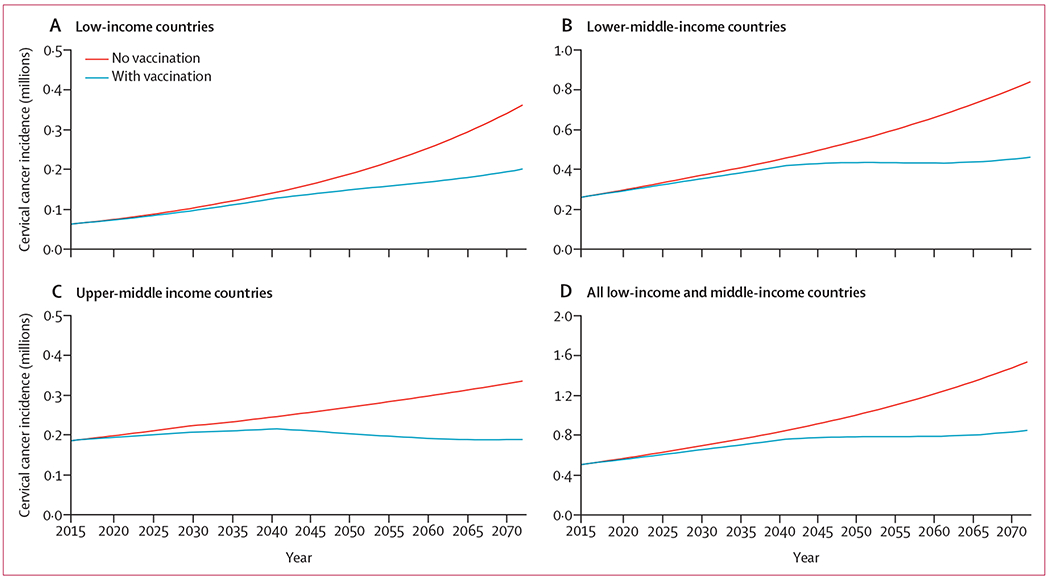
Vaccination strategy reflects the implementation of bivalent vaccination of 12-year-old girls beginning in 2014 with 75% coverage. Full assumptions are detailed in the Methods section and in the appendix (pp 9–10). Axes reflect the individual data ranges of each graph and are not uniform.
Figure 4: Effect of universal human papillomavirus vaccination strategy on demand for external-beam radiotherapy and brachytherapy in low-income and middle-income countries.
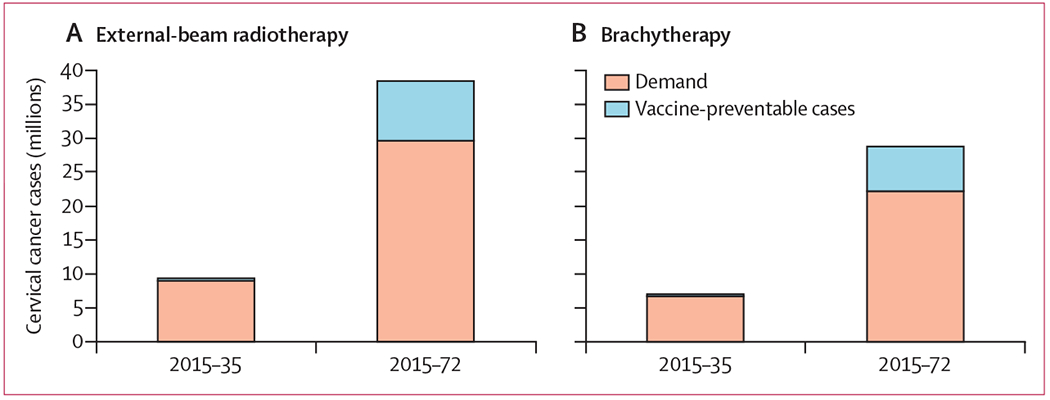
The columns on each graph represent the total number of cervical cancer cases projected by GLOBOCAN for the periods indicated (2015–35 and 2015–72). The blue portion of each column represents the number of cases estimated to be prevented through implementation of a bivalent vaccination strategy with 75% coverage of 12-year-old girls, beginning in 2014.
Discussion
This modelling study provides quantitative evidence on the health and economic benefits of investing in life-saving radiotherapy treatment for women with cervical cancer across a wide range of country income settings and alongside investment in HPV vaccination. Although ongoing operational costs remain low, the existing scarcity of radiotherapy access in low-income countries required large upfront capital expenditure, leading to negative returns for the nominal scale-up model. This finding highlights the limitations of cost–benefit analyses in appropriately accounting for other endpoints that have legitimate normative value to economists, such as equity and household protection. Nevertheless, our findings compare favourably with investment frameworks developed for other diseases.23,24 The Global Investment Framework for Women’s and Children’s Health found that, in 74 countries with 95% of the global maternal and child mortality burden, the return on investment of scaling up an evidence-based package of reproductive, maternal, newborn, and child health interventions was 8·7 times at a 3% discount rate.23 An analysis24 of childhood immunisation in 94 LMICs from 2011 to 2020 estimated a return on investment of 16 times using the human capital approach and 44 times using the full income approach.
Although great momentum exists within the global health community to scale up HPV vaccination and move toward cervical cancer elimination, this study demonstrates the need for a comprehensive approach to cervical cancer prevention and treatment. Incomplete vaccine coverage, the long time lag for carcinogenesis, and a growing population led to a cumulative 22·9% reduction in cervical cancer incidence by 2072. Based on GLOBOCAN projections for 2015–35,15 nearly 9·4 million women globally will be diagnosed with cervical cancer and will require radiotherapy to prevent death. Women of all ages and at all stages of life are affected and, as outlined here, the individual, social, and economic consequences are devastating. The direct economic growth generated from treating and hopefully curing cervical cancer with radiotherapy is compounded by the unique contributions that healthy and empowered women make to the prosperity of their families, communities, and society. Appropriately valuing women’s time reconceptualises them as both users and providers of health care and is key to making the case for investing in their health, as well as to showing why a gender-neutral distribution of paid and unpaid work should be a societal goal.12
The inclusion of gender equality and cancer in the Sustainable Development Goals is a clear mandate to tackle the challenges facing women beyond childbirth, including access to appropriate cervical cancer treatment. The resurgence of a false dichotomy between prevention and treatment risks hindering progress and obscuring the mutually reinforcing effect of a comprehensive approach to cervical cancer control.25 An important step forward was the formation of the UN Joint Global Programme on Cervical Cancer Prevention and Control,26 a collaboration established in 2016 among seven UN agencies to eliminate cervical cancer as a global public health concern. This initiative, and the 2018 WHO Call to Action,27 recognised the opportunity for multidisciplinary collaboration to improve cancer care across the continuum of disease and across the life course of women.
This study should be considered in the context of its limitations. Optimal management of cervical cancer requires an interdisciplinary team of surgeons, oncologists, pathologists, medical imagers, nurses, and others, as well as a general supportive health system infrastructure. As in the 2015 Lancet Oncology Commission on Radiotherapy report, we were unable to consider all of these components in this analysis. A previous study13 estimated that the addition of concurrent chemotherapy provides an additional overall survival benefit of 4–6% at 5 years, depending on the stage of disease, which is additive to the radiotherapy benefit estimates used in this analysis. Furthermore, because cervical cancer comprises up to 14% of all cancers, with variation by country,1 investment in radiotherapy for cervical cancer must occur alongside investment for other cancer types.
The scale-up scenario of full radiotherapy coverage by 2035 is ambitious and might not be realised in some countries owing to context-specific factors, such as limitations of political will or fiscal space for health. Although some countries are beginning to make progress, with the first radiotherapy centres now opening in Cambodia and Mozambique, such progress has not been uniform across LMICs.4,28 Budget impact analyses are essential for country-specific budgetary planning and represent important work that can follow this analysis. However, the advanced stage distribution of cervical cancer at presentation and the poor vital statistics in many countries, where many cases of cervical cancer are not recorded, suggest that our results might be conservative estimates. We also did not estimate radiotherapy’s substantial quality-of-life benefits in controlling pain and bleeding, especially in patients with incurable disease,5 whose lack of access mirrors the inadequate palliative care provided to most women with cancer in LMICs who die in pain.3,5
Our analysis is further limited by the unavailability of long-term data and we were unable to account for new treatment options or advances in technology over time or possible health system changes in our background mortality rate, which might change life expectancy for the population overall. However, previous validation efforts by the Global Task Force on Radiotherapy for Cancer Control to gain consensus about model inputs through an international Delphi process add to the generalisability of our findings.4 As in other published studies on vaccine cost-effectiveness, we were unable to reliably model the effects of herd immunity,9 which has been strongly linked to high population coverage of at least 50%. Therefore, we might have overestimated the radiotherapy needs following high vaccination coverage.29 Similarly, we did not include potential effects of the vaccine against HPV genotypes not targeted by the vaccine.30 Even so, our analysis is the first, to our knowledge, to quantify an estimate of remaining radiotherapy needs in the presence of high-coverage (75%) HPV vaccination. A systematic review of data published in 2006–14 on HPV vaccination coverage found that only 6·1% of females worldwide aged 10–20 years were estimated to have been vaccinated.31 In countries with organised vaccination programmes, coverage was 40% in targeted women, increasing to 54·9% among primary targets and organised catch-ups. However, most of the vaccinated women were from high-income (68%) or upper-middle-income (28%) countries.31
Cancer control plans have been developed by 158 countries, representing 82% of WHO Member States, either in conjunction with broader non-communicable disease strategies or as dedicated National Cancer Control Plans. A review32 of publicly available plans found that 85% (133/157) identified scale-up of cervical cancer screening and 57% (90/157) identified scale-up of HPV vaccination as priority areas. This study can be used to inform the National Cancer Control Plan implementation process to ensure effective prioritisation and collective action for tertiary care nationally and internationally. Comprehensive strategies that combine prevention, early detection, and treatment within universal health coverage schemes are needed to address the cervical cancer burden, prevent the premature loss of life, and stimulate economic growth by improving the health and productivity of women.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies published in English from Jan 1, 2008, to Dec 31, 2018, with the following key words: “cervical cancer AND radiotherapy” and “vaccination OR elimination”. No reported studies quantified the health and economic benefit of radiotherapy for cervical cancer across low-income and middle-income countries or estimated the effects of human papillomavirus (HPV) vaccination programmes on demand for treatment over short-term and medium-term time horizons in these regions. In May, 2018, the Director-General of WHO identified cervical cancer as one of the greatest public health challenges, but the resources that are needed for cervical cancer control and the ongoing benefit of investment in treatment capacity in the context of cervical cancer elimination targets have not been understood.
Added value of this study
We modelled the demand and benefit of a 20-year scale-up strategy for external-beam radiotherapy and brachytherapy fo the treatment of cervical cancer in low-income and middle-income countries from 2015 to 2035, alongside the implementation of a programme of bivalent HPV vaccination of 12-year-old girls. We found that by 2035, radiotherapy scale-up saved 11·4 million life-years with a return on investment of 2·5, using a human capital approach, to 6·5, using a full income approach, assuming a nominal radiotherapy delivery model, and of 6·0 to 13·8, using an efficient delivery model. By contrast, vaccination resulted in only a 3·9% reduction in incident cases of cervical cancer during this period. By 2072, at which point the first cohort of vaccinated girls would reach 70 years of age, HPV vaccination was estimated to decrease incident cases of cervical cancer by 22·9%, leaving 41·7 million women still in need of external-beam radiotherapy and 28·8 million in need of brachytherapy in the study period. To our knowledge, this is the first report of the results of a coordinated cervical cancer strategy that combines both prevention and treatment in low-income and middle-income countries with a practical and actionable time horizon.
Implications of all the available evidence
The findings of this study show that investment in radiotherapy for cervical cancer is both needed and feasible, generating millions of productive life-years for women who contribute in large and often underrecognised and undervalued ways to the economy and social wellbeing of their community. As the international community begins to work collectively toward cervical cancer elimination, these data highlight the very long time lag for vaccination strategies alone to contribute substantially to the elimination of cervical cancer. To achieve effective cervical cancer control, a multidisciplinary approach to policy planning is required, which addresses the need for life-saving radiotherapy treatment in the coming decades.
Acknowledgments
The authors gratefully acknowledge the fellowship support provided to DR by the Canadian Association of Radiation Oncology and The Commonwealth Fund during the preparation of this report.
Footnotes
For DIRAC (Directory of RAdiotherapy Centres) see https://dirac.iaea.org/
See Online for appendix
Declaration of interests
We declare no competing interests.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2018; 144: 1941–53. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3.Knaul FM, Farmer PE, Krakauer EL, et al. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: the Lancet Commission report. Lancet 2018; 391: 1391–454. [DOI] [PubMed] [Google Scholar]
- 4.Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015; 16: 1153–86. [DOI] [PubMed] [Google Scholar]
- 5.Rodin D, Grover S, Elmore SN, et al. The power of integration: radiotherapy and global palliative care. Ann Palliat Med 2016; 5: 209–17 [DOI] [PubMed] [Google Scholar]
- 6.Knaul F, Horton S, Yerramilli P, Gelband H, Atun R. Financing cancer care in low-resource settings, vol 3 In: Gelband H, Jha P, Sankaranarayanan R, Horton S eds. Cancer: disease control priorities, 3rd edn Washington, DC: The International Bank for Reconstruction and Development, The World Bank, 2015. [PubMed] [Google Scholar]
- 7.Bhatla N, Berek JS, Cuello Fredes et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019; 145: 129–35. [DOI] [PubMed] [Google Scholar]
- 8.Cibula D, Potter R, Planchamp F, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol 2018; 127: 404–16. [DOI] [PubMed] [Google Scholar]
- 9.Jit M, Brisson M, Portnoy A, Hutubessy R. Cost-effectiveness of female human papillomavirus vaccination in 179 countries: a PRIME modelling study. Lancet Glob Health 2014; 2: e406–14. [DOI] [PubMed] [Google Scholar]
- 10.Han K, Milosevic M, Fyles A, Pintilie M, Viswanathan AN. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013; 87: 111–19. [DOI] [PubMed] [Google Scholar]
- 11.Jamison DT, Summers LH, Alleyne G, et al. Global health 2035: a world converging within a generation. Lancet 2013; 382: 1898–955. [DOI] [PubMed] [Google Scholar]
- 12.Langer A, Meleis A, Knaul FM, et al. Women and Health: the key for sustainable development. Lancet 2015; 386: 1165–210. [DOI] [PubMed] [Google Scholar]
- 13.Hanna TP, Shafiq J, Delaney GP, Barton MB. The population benefit of radiotherapy for cervical cancer: local control and survival estimates for optimally utilized radiotherapy and chemoradiation. Radiother Oncol 2015; 114: 389–94. [DOI] [PubMed] [Google Scholar]
- 14.Quinn MA, Benedet JL, Odicino F, et al. Carcinoma of the cervix uteri. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 2006; 95 (suppl 1): S43–103. [DOI] [PubMed] [Google Scholar]
- 15.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86. [DOI] [PubMed] [Google Scholar]
- 16.World Bank. The world by income. World Bank Open Data, 2017. http://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed May 1, 2017).
- 17.WHO. Global Health Observatory data repository. Life tables by country. Geneva: World Health Organization, 2016. http://apps.who.int/gho/data/node.main.LIFECOUNTRY?lang=en (accessed July 1, 2017). [Google Scholar]
- 18.Van Dyk J, Zubizarreta E, Lievens Y. Cost evaluation to optimise radiation therapy implementation in different income settings: a time-driven activity-based analysis. Radiother Oncol 2017; 125: 178–85. [DOI] [PubMed] [Google Scholar]
- 19.International Monetary Fund. World Economic Outlook Database, October 2017 International Monetary Fund, 2017. https://www.imf.org/external/pubs/ft/weo/2017/02/weodata/index.aspx (accessed May 1, 2017).
- 20.McGuire A, Raikou M. Estimating health care treatment costs, vol 1 In: Scheffler RM, ed. World scientific handbook of global health economics and public policy. Singapore: World Scientific, 2016: 403–20. [Google Scholar]
- 21.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11: 1048–56. [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121: 621–32. [DOI] [PubMed] [Google Scholar]
- 23.Stenberg K, Axelson H, Sheehan P, et al. Advancing social and economic development by investing in women’s and children’s health: a new Global Investment Framework. Lancet 2014; 383: 1333–54. [DOI] [PubMed] [Google Scholar]
- 24.Ozawa S, Clark S, Portnoy A, Grewal S, Brenzel L, Walker DG. Return on investment from childhood immunization in low- and middle-income countries, 2011–20. Health Affairs 2016; 35: 199–207 [DOI] [PubMed] [Google Scholar]
- 25.Frenk J, Gomez-Dantes O. False dichotomies in global health: the need for integrative thinking. Lancet 2017; 389: 667–70. [DOI] [PubMed] [Google Scholar]
- 26.UN Inter-Agency Task Force on the Prevention and Control of Noncommunicable Diseases. UN Joint Global Programme on cervical cancer prevention and control. Geneva: World Health Organization, 2016. https://www.who.int/ncds/un-task-force/un-joint-action-cervical-cancer-leaflet.pdf (accessed May 1, 2017). [Google Scholar]
- 27.Ghebreyesus TA. Cervical cancer: an NCD we can overcome. Geneva: World Health Organization, 2018. [Google Scholar]
- 28.Grover S, Xu MJ, Yeager A, et al. A systematic review of radiotherapy capacity in low- and middle-income countries. Front Oncol 2015; 4: 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15: 565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17: 1293–302. [DOI] [PubMed] [Google Scholar]
- 31.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016; 4: e453–63. [DOI] [PubMed] [Google Scholar]
- 32.Romero Y, Trapani D, Johnson S, et al. National cancer control plans: a global analysis. Lancet Oncol 2018; 19: e546–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


