Abstract
Summary: We report the unusual phenomenon of abrupt intraventricular contrast medium leakage from the choroid plexus occurring during ethanol embolization of a periventricular arteriovenous malformation. There was no evidence of any associated intraventricular hemorrhage to suggest that leakage arose from a vessel perforation, as was first suspected. Intraventricular contrast medium leakage has been reported previously in the setting of ependymitis, and it is likely that similar pathogenetic mechanisms apply in this case. To our knowledge, this is the first reported case of intraventricular contrast medium leakage occurring during an embolization procedure.
Intraventricular leakage of contrast material has been reported previously in association with ependymitis (1). Using opacified pure ethanol, we encountered leakage of contrast material from the choroid plexus into the ventricles during embolization of a small, periventricular arteriovenous malformation (AVM). The unusual radiographic appearances were interpreted intraprocedurally as an iatrogenic, small-vessel perforation with intraventricular extravasation. A postprocedural, nonenhanced brain CT scan, however, revealed intraventicular contrast medium, but with no evidence of new hemorrhage. We discuss the pathogenetic mechanisms involved in this case, thus alerting the endovascular surgeon to interventional scenarios in which this uncommon but potentially alarming phenomenon may be encountered.
Case Report
A 5-year-old, previously healthy boy presented with headache, disorientation, and somnolence. A CT scan showed a small (< 1.5 cm), deep left periventricular hematoma that extended into the atrium of the adjacent left lateral ventricle. An MR scan and x-ray cerebral angiogram revealed an underlying 2.5-cm AVM adjacent to the atrium of the left lateral ventricle (Fig 1A and B). Its arterial supply arose from the enlarged left anterior choroidal artery, lateral lenticulostriate branches of the left middle cerebral artery, and the lateral posterior choroidal branch of the left posterior cerebral artery. The nidus was predominantly plexiform with unrestricted, deep venous drainage into an ipsilateral atrial vein connecting to the vein of Galen (Fig 2A and B). Intravenous dexamethasone (4 mg, every 6 hours), fosphenytoin (45 mg, every12 hours), and ranitidine (25 mg, every 6 hours) were administered, and the patient emergently transferred to our institution for preoperative embolization. At presentation, the child was neurologically intact. Specifically, there was no evidence of language impairment, motor weakness, or visual-field defect.

Fig 1. CT of previously healthy 5-year-old boy presenting with headache, disorientation, and somnolence is shown.
A and B, T1-weighted MR images of brain performed on the day of ictus show left periventricular hematoma with extension into atrium of adjacent left lateral ventricle. Note absence of blood within left occipital horn (arrow). Flow voids representing AVM feeding pedicles are present anteriorly within left basal ganglia.

Fig 2. Embolization procedure conducted under digital roadmap and fluoroscopic guidance is shown.
A and B, Anteroposterior (AP) Towne's and lateral views of left vertebral injection show an enlarged left lateral posterior choroidal artery supplying AVM nidus (arrow).
C, AP view of superselective injection into left lateral posterior choroidal artery supplying AVM is shown. Position of microcatheter tip from which ethanol embolization was performed (long arrow). Note unopacified inflow (flow reversal) from small medial branch supplying normal choroid plexus of glomus (small arrow).
D and E, AP and lateral unsubtracted views (immediately after test injection) show persistent contrast filling of small choroidal branch indicating flow arrest and contrast stain in the region of atrial choroid plexus.
F, Unsubtracted lateral view showing contrast material within dependent portion of left lateral ventricle (arrows). Contrast medium appears to outline wall of ventricle. This appearance is most likely caused by preexistent intraventricular hematoma acting as radiolucent “filling defect.” Radioopaque fiber-coated coils within occluded left lateral posterior choroidal artery are visible anteriorly.
Procedure
The procedure was performed under general anesthesia. An intravenous loading dose of 1400 units of heparin was administered and anticoagulation confirmed with an activated clotting time ([ACT] > 2× baseline). A 4-French UCSF-III pediatric cerebral catheter (Cordis, Miami Lakes, FL) was placed into the left vertebral artery as a guide catheter and connected to a continuous, metered, heparinized saline flush. A Prowler 0.014-in. microcatheter (Cordis, Miami Lakes, FL) primed with a Transend 0.010-in guidewire (Scimed, Maple Grove, MN) was passed through the guide catheter. Under digital roadmap and fluoroscopic guidance, the microcatheter was carefully navigated over the guidewire into the left lateral posterior choroidal artery. A small branch to the normal choroid plexus arose distally from this artery; however, the flow within this small branch was reversed (ie, retrograde flow into the main pedicle) as a result of the “sump” effect of the AVM. The final microcatheter tip position, from which embolization of the AVM was begun, was at this branch point (Fig 2C), and a partial flow arrest of this small choroidal branch resulted. Free flow from the microcatheter tip into the larger branch that supplied the AVM was confirmed on a blank roadmap image. Although no contrast extravasation was observed during this injection, a persistent contrast stain in the expected location of the atrial choroid plexus was observed (Fig 2D and E). An angiographic run (hand injection via the microcatheter) confirmed no evidence of contrast extravasation, but the choroidal contrast stain increased in density with anterior extension.
Embolization was commenced using 2 cc of pure ethanol opacified with metrizamide contrast medium (Nycomed, Princeton, NJ). During embolization, which lasted a few minutes, staining of the choroid plexus extended anteriorly to involve the posterior part of the left lateral ventricle body. To confirm that this staining was an accumulation of contrast medium by the choroid plexus and not an intraventricular or intraparenchymal extravasation (contrast was not layering within the dependent part of the ventricle) a saline injection through the microcatheter was performed on a blank roadmap image. This showed a slow washout of choroid plexus density rather than the gradual diffusion into the surrounding space that would be expected with extravasation. An additional 0.8 cc of metrizamide-opacified ethanol then was injected slowly, causing an abrupt change in the distribution and appearances of the intraventricular contrast. Although the contrast staining had conformed previously to the expected position of the choroid plexus within the atrium and posterior body of the left lateral ventricle, there was now contrast filling the dependent portion of the left lateral ventricle, implying a free flow of intraventricular contrast medium (Fig 2F). Perforation of the small choroidal branch with intraventricular extravasation was considered the cause for this new appearance; therefore, heparinization was immediately reversed with 20-mg intravenous protamine sulphate administration. An ACT was obtained to ensure complete reversal of anticoagulation. The left lateral posterior choroidal artery then was occluded with five straight, fiber-coated coils.
During the embolization procedure, the patient's vital signs remained stable. An immediate postprocedural CT scan of the brain was obtained that showed high attenuation material compatible with the contrast medium within the left lateral ventricle but no evidence of ventricular expansion or fresh hematoma (Fig 3A and B). The child recovered from anesthesia without any neurologic impairment but did complain of a mild headache. The postprocedural course was uncomplicated. A nonenhanced brain CT performed 20 hours later depicted significant resorption of intraventricular high-attenuation fluid, which was compatible with contrast medium and not blood (Fig 3C).
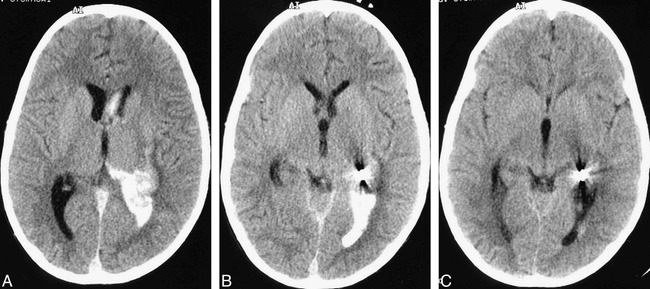
Fig 3. Postprocedural CT of brain is shown.
A and B, Immediate postembolization nonenhanced brain CT scan showing high-attenuation material consistent with iodinated contrast medium within dependent portion of left lateral ventricle. Size of the intraventricular hematoma has not increased.
C, Postembolization (at 20 hr), nonenhanced brain CT scan demonstrating almost complete resorption of high-attenuation iodinated contrast medium.
Discussion
The unusual phenomenon reported in this case is the unexpected passage of iodinated contrast medium between the intravascular space and ventricular CSF. Intraprocedurally, this was thought to arise from an iatrogenic vascular perforation with intraventricular hemorrhage. Hence, anticoagulation was reversed, and the suspected perforated vessel was sealed with coils. The immediate postprocedural, nonenhanced CT scan of the brain, however, depicted high-attenuation material, which was compatible with iodinated contrast within the dependent part of the left lateral ventricle, and it showed no evidence of fresh hemorrhage compared with an MR image obtained a few days earlier (Fig 3). Furthermore, the intraventricular high-attenuation material had almost completely cleared the following day, which was consistent with it being iodinated contrast medium. The fact that the patient's vital signs remained unchanged after the suspected perforation likewise argues against a vascular perforation. Enlargement of the left lateral ventricle secondary to hematoma may have been expected with a vascular perforation, but subtle ventricular dilatation was difficult to gauge accurately intraprocedurally.
Embolization of the left lateral posterior choroidal artery, using pure ethanol opacified with metrizamide, resulted in contrast staining of the choroid plexus of the left lateral ventricle that progressively increased in density and extent and eventually involved the atrium and posterior body. The AVM did not directly involve the choroid plexus; however, a small branch to the choroid plexus arose from the feeding pedicle, and there was partial occlusion or wedging of this small choroidal branch by the microcatheter. This segment of choroid plexus therefore received a significant dose of the ethanol/metrizamide mixture. The choroid plexus constitutes the principal interface between the blood and CSF (2). The capillaries of the choroid plexus are fenestrated, unlike those of the brain parenchyma, thus allowing the ultrafiltration of plasma and the passage of contrast medium into the choroidal interstitium (3). This passage accounts for the enhancement of the choroid plexus normally observed with CT (4). Nevertheless, passage of contrast media and other solutes between the choroidal interstitium and CSF is restricted by the choroid plexus epithelium. This imperfect barrier consists of a single layer of cuboidal cells connected by apical tight junctions that rest on a thin basement membrane. The histologic appearance is similar to that of the epithelial cells of the proximal tubule of the kidney (5).
The dense choroid-plexus staining in our case may be explained by the superselective delivery of metrizamide via the lateral posterior choroidal artery and its accumulation in the choroidal interstitium. It is also likely there was concurrent choroidal interstitial accumulation of ethanol. The subsequent passage of contrast medium into the CSF probably occurred because of damage to the choroidal epthelium by the ethanol or hyperosmolar contrast medium or both. Ethanol embolization of normal tissues results in thorough devitalization by denuding and segmentally fracturing the epithelium. In the presence of blood, an acute thrombosis may be initiated (6). Hyperosmolar solutions have been shown to disrupt the usual blood-brain barrier (7). The preexistent AVM hematoma, which had an intraventricular component, also may have contributed by disrupting or disturbing the blood–CSF barrier.
To our knowledge, leakage of intravascular contrast material into the CSF during an embolization procedure has not been reported previously. In fact, reports of iodinated contrast leakage into the ventricles—excluding contrast opacified hemorrhage or extravasation—are rare. In 1983, Sullivan and Dorwart reported a case of intraventricular contrast leakage after CT of the brain in a 64-year-old man with meningitis. The postulated mechanism causing an inflammatory alteration of the blood-brain barrier of the supependymal vasculature was presumed to be ependymitis (1). Lee and Zimmer reported ventricular opacification in two infants, one asymptomatic and one with fatal anoxic brain damage, occurring after intraarterial and intravenous injections of contrast medium (Conray 60), respectively (8). Scotti and Harwood-Nash mention having observed ependymal enhancement and intraventricular contrast leakage with ventriculitis, but do not provide supporting case evidence (9). Kobayashi et al reported two cases of contrast enhancement of preexistent traumatic intraventricular hemorrhage on CT images, and proposed that it arose from active extravasation of injured ependymal or subependymal arteries (10).
To our knowledge we report for the first time leakage of iodinated contrast material into the ventricle of a patient undergoing ethanol embolization of a recently hemorrhaged periventricular AVM. The lateral posterior choroidal branch was embolized, and the presumed mechanism was accumulation of the opacified embolic agent within the choroid plexus interstitium, with ensuing toxic injury of the choroidal epithelium and consequent contrast spillage into the intraventricular CSF. Damage to the choroid plexus and ependyma of the left lateral ventricle by the preexistent parenchymal and intraventricular hematoma also may have been a predisposing factor. This case therefore shares some etiologic similarities to the case reported by Sullivan and Dorwart, in which an inflammatory ependymitis resulted in intraventricular leakage of contrast medium (1). The endovascular surgeon should be cognizant of the potential for this unusual and alarming phenomenon to occur when embolizing choroidal branches with ethanol or hyperosmolar embolic agents.
Footnotes
Address reprint requests to Constantine C. Phatouros MBBS, FRACR, Neurovascular Medical Group, University of California, San Francisco Medical Center, 505 Parnassus Ave, Room L-352, San Francisco, CA 94143-0628.
References
- 1.Sullivan WT, Dorwart RH. Leakage of iodinated contrast material into the cerebral ventricles in an adult with ependymitis. AJNR Am J Neuroradiol 1982;4:1251-1253 [PMC free article] [PubMed] [Google Scholar]
- 2.Sage MR, Turski PA, Levin A. CNS Imaging and the Brain Barriers. In: Neuwelt EA, ed. Implications of the Blood-Brain Barrier and its Manipulation, Vol. 2 Clinical Aspects. New York, NY: Plenum Press; 1989:1–51
- 3.Davson H. Physiology of the Cerebrospinal Fluid. Boston, Ma: Little Brown; 1967
- 4.Naidich TP, Pudlowski RM, Leeds NE, Naidich JB, Chiisholm AJ, Riekin MD. The normal contrast-enhanced computed axial tomography of the brain. J Comput Assist Tomog 1977;1:16-29 [DOI] [PubMed] [Google Scholar]
- 5.Bradbury M. The concept of a blood-brain barrier. Chichester, England: John Wiley & Sons, 1979
- 6.Yakes WF, Rossi P, Odink H. How I do it: Ateriovenous malformation management. Cardiovasc Intervent Radiol 1996;19:65-71 [DOI] [PubMed] [Google Scholar]
- 7.Pollary M. Effect of hypertonic solutions on the blood-brain barrier. Neurology (NY) 1975;25:852-856 [DOI] [PubMed] [Google Scholar]
- 8.Lee BCP, Zimmer J. Ventricular opacification after intravascular injections of contrast material. Radiology 1978;128:647-649 [DOI] [PubMed] [Google Scholar]
- 9.Scotti G, Harwood-Nash DC. Leakage of contrast into a post-meningitic subdural effusion: a CT finding. Neuroradiology 1980;20:95-98 [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S, Nakazawa S, Yano M, Otsuka T. Extravasation of contrast medium into blood clot in acute traumatic intraventricular hemorrhage. Neurolog Med Chirur 1985;25:32-35 [DOI] [PubMed] [Google Scholar]


