Abstract
Summary: We report functional neuroimaging studies of a 54-year-old man with Marchiafava-Bignami disease (MBD). Glucose metabolic images obtained by [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography showed diffusely reduced whole brain metabolism and strongly decreased metabolism in the frontal and parietal lobes, orbital gyrus, and thalamus. Cerebral perfusion images showed a similarly decreased radioactivity pattern as the metabolic images. Functional neuroimages would be useful for understanding the pathophysiologic processes of MBD.
Marchiafava-Bignami disease (MBD) is a rare disorder associated with chronic and massive alcoholism in which the corpus callosum characteristically is involved. X-ray CT and MR imaging can show demyelination and necrosis of the corpus callosum, which is a very useful sign for the clinical diagnosis of this disorder (1, 2). In addition to hemispheric disconnection syndrome, various cognitive impairments, including dementia, occur, but the mechanism underlying them remains undetermined. Although a few investigators have attempted to identify MBD-related functional abnormalities in the cerebral gray matter (3−5), findings are not inconsistent. One positron emission tomographic (PET) study failed to show clinically relevant abnormalities in CBF and oxygen metabolism (4). Another showed a marked decrease of glucose metabolism in the frontal and temporo-parieto-occipital association cortices (5), and a single-photon emission CT (SPECT) study showed a hypoperfusion in the frontoparietal region, including the primary sensorimotor strip (3). We report a patient with MBD, in whom cerebral metabolism and blood flow were studied with [18F]-2-fluoro-2-deoxy-D-glucose (FDG) PET and N-isopropyl-p-[123I]iodoamphetamine (IMP) SPECT.
Case Report
A 54-year-old man, with a 30-year history of heavy alcohol consumption, was referred to us because of speech and writing disturbance. Three years earlier, the patient sometimes felt clumsiness and stiffness of the hands and difficulty in gaze. Six months earlier, difficulties in calculation, handwriting, speech, and stiffness of the hands developed. At admission, disorientation, inattention, constructional disability, dyscalculia, and dysgraphia were apparent. Mild dysarthria, paratonia of the bilateral upper limb, and hyperreflexia of the lower limbs also were noted. Signs of callosal disconnection were not noted, and aphasia, apraxia, and agnosia were not evident. The patient's intellectual function was low; he scored 19 on the Mini Mental State Examination, 18.3 on the Alzheimer's Disease Assessment Scale, 64 on a full-scale IQ test, 68 on a verbal IQ test, and 62 on a performance IQ test using the Wechsler Adult Intelligence Scale-Revised. He also showed a mild degree of memory impairment and achieved scores of 68 for the general memory index, 67 for the verbal memory index, 89 for the visual memory index, and 68 for the delayed recall index using the Wechsler Memory Scale-Revised. The results of the laboratory tests were not remarkable except for the presence of mild anemia. On sagittal T1-weighted images, atrophy of the corpus callosum and linear hypointense lesions in the genu and trunk of the corpus callosum were noted (Fig 1A). On axial T2-weighted images, the deep frontal white matter, including the forceps minor and a part of the centrum semiovale, was hyperintense, as was the corpus callosum (Fig 1B).
fig 1.
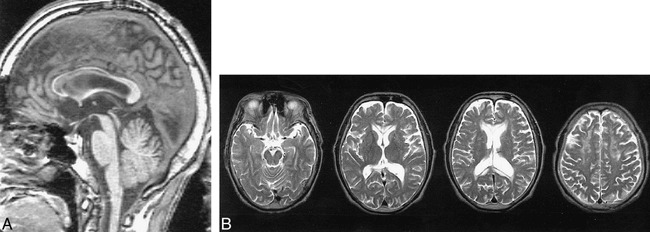
Images of a 54-year-old man, with a history of heavy alcohol drinking for 30 years, who was referred because of speech and writing disturbance.
A, Sagittal T1-weighted MR image shows atrophy of entire corpus callosum, except for the splenium. Hypointense bands are seen in the genu and truncus of the corpus callosum.
B, Axial T2-weighted MR images show a hyperintense area in the corpus callosum, periventricular hyperintensity, and hyperintense areas in the frontal white matter.
Regional cerebral glucose metabolism was measured by FDG PET, and the results were compared with those of 10 age-matched healthy volunteers (mean age, 56.8 ± 1.7 years; four men and six women). The PET procedure was followed strictly according to our institution's protocol and was approved by the internal ethics committee. Informed consent was obtained from the patient and his family as well as from all volunteers. We placed more than one circular region of interest of 10-mm diameter on each of the 84 brain regions with the aid of registration on reconstructed MR images of identical 3D scales and coordinates of the PET images. The values of cerebral metabolic rates of glucose in each structure of each side were then averaged. The values were converted to a Z score against the normative data [(patient's value − mean value of controls)/standard deviation of controls]. The patient's regional cerebral blood flow imaging was obtained using N-isopropyl-p-123IMP SPECT. Regional cerebral blood flow also was evaluated by region of interest (16-mm diameter) analysis and was expressed as ratios relative to cerebellar blood flow. The ratios were also converted to a Z score against the normative data derived from another 10 age-matched healthy volunteers (mean age, 55.3 ± 2.8 years; five men and five women).
Regional cerebral glucose metabolism was reduced diffusely over the cerebral hemispheres. The reduction was accentuated in the frontal and parietal association cortices, primary sensorimotor cortices, occipital cortices, and thalamus (Table 1 and Fig 1C). Regional cerebral blood flow also was decreased in a similar manner (Fig 1D). Regional relative CBF ratios to CBF were 79% (Z score = −2.21) in the temporal lobes, 88% (−2.58) in the occipital lobes, 84% (−3.05) in the frontal lobes, 77% (−2.63) in the parietal lobes, and 82% (−1.46) in the thalami.
Discussion
In this patient, glucose metabolism and CBF were reduced markedly despite MR imaging evidence of lesions essentially limited to the corpus callosum and the white matter in the frontal lobes. These findings suggest that, in cases of MBD, perfusion and metabolism are affected in the cerebrum beyond the corpus callosum and that the widespread cerebral functional involvement accounts for the complicated neurologic and cognitive deficits. The cognitive deficits would be the most relevant to the functional depression of the frontal and parietal lobes. A transneural mechanism through the white matter, including the corpus callosum, is a plausible cause of functional depression of the association cortices. According to an experiment in baboons (6), a section of the corpus callosum induces a depression of cortical metabolism. Nevertheless, the involvement of the primary sensorimotor cortices cannot be attributed to a disconnection of commissural fibers, because commissural connection is lacking in the primary sensorimotor cortices. MBD lesions often appear in the corona radiata in addition to the corpus callosum on T2-weighted MR images (7, 8). An involvement of the extracallosal white matter was apparent also on the T2-weighted MR images of our patient. In cases of MBD, not only commissural fibers, but also the extracallosal projection and association fibers, are involved.
Disruption of cortico-cortical networks and cortico-subcortical projections crossing the corpus callosum presumably contributed to the clinical-metabolic findings in this patient. An additional role could be played by neocortical neuronal loss, which is occasionally observed in postmortem studies of MBD patients accompanied by Wernicke and pellagroid encephalopathies (9, 10), although the signs of them were not clinically evident in our patient.
Conclusion
Functional neuroimaging showed widespread cortical involvement in a patient with MBD, which would account for the patient's cognitive deficits. The findings suggested that a disconnection of not only commissural fibers but also the extracallosal projection and association fibers involving the cortico-cortical and cortico-subcortical connectivity cause transneural depression of the cortical metabolism and function.
fig 2.
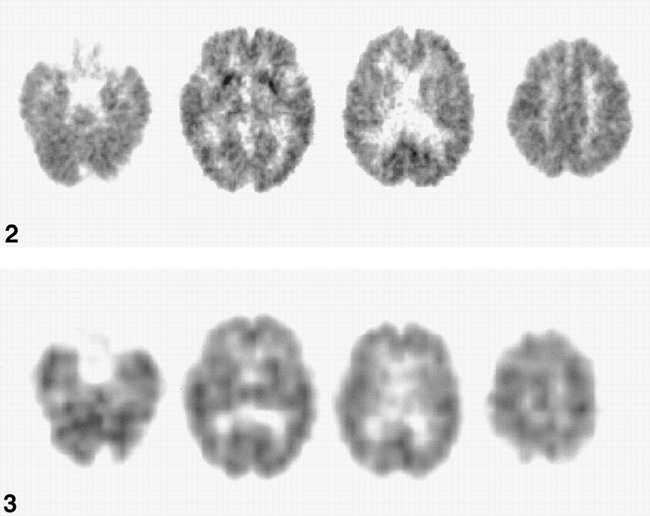
Whole-brain cerebral metabolic rate of glucose (CMRglc) is reduced diffusely and strongly in the frontal and parietal lobe and the thalamus. CMRglcs in the basal ganglia are preserved. The white matter lesions in the frontal lobes do not seem to influence the frontal CMRglc, because the frontal CMRglc is affected as severely as the parietal CMRglc.fig 3. IMP CBF images show the same distributions of the affected area of CMRglc
Acknowledgments
We thank Toru Kida and Hiroto Sakai (Radiology Service, Hyogo Institute for Aging Brain and Cognitive Disorders) for technical assistance. The SPECT examination was performed at Nuclear Medicine Service, Himeji Brain and Heart Center.
Footnotes
Address reprint requests to Kazunari Ishii, MD, Division of Neuroimaging Research, Hyogo Institute for Aging Brain and Cognitive Disorders, 520 Saisho-Ko, Himeji 670–0981, Japan.
References
- 1.Chang KH, Cha SH, Han MH, et al. Marchiafava-Bignami disease. Serial changes in corpus callosum in MRI. . Neuroradiology 1992;34:480-482 [DOI] [PubMed] [Google Scholar]
- 2.Caparros-Lefebvre D, Pruvo JP, Josien E, Pertuzon B, Clarisse J, Petit H. Marchiafava-Bignami disease. Use of contrast media in CT and MRI. . Neuroradiology 1994;36:509-511 [DOI] [PubMed] [Google Scholar]
- 3.Humbert T, De Guilhermier P, Maktouf C, Grasset G, Lopez FM, Chabrand P. Marchiafava-Bignami disease. A case studied by structural and functional brain imaging. . Eur Arch Psychiatry Clin Neurosci 1992;242:69-71 [DOI] [PubMed] [Google Scholar]
- 4.Kamaki M, Kawamura M, Moriya H, Hirayama K. “Crossed homonymous hemianopia” and ‘crossed left hemispatial neglect’ in a case of Marchiafava-Bignami disease. . J Neurol Neurosurg Psychiatry 1993;56:1027-1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pappata S, Chabriat H, Levasseur M, Legault-Demare F, Baron JC. Marchiafava-Bignami disease with dementia. Severe cerebral metabolic depression revealed by PET. . J Neural Transm 1994;8:131-137 [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Kunimoto M, Pappata S, et al. Effects of anterior corpus callosum section on cortical glucose utilization in baboons. . Brain 1990;113:937-951 [DOI] [PubMed] [Google Scholar]
- 7.Izquierdo G, Quesada MA, Chacon J, Martel J. Neuroradiologic abnormalities in Marchiafava-Bignami disease of benign evolution. . Eur J Radiol 1992;15:71-74 [DOI] [PubMed] [Google Scholar]
- 8.Berek K, Wagner M, Chemelli AP, Aichner F, Benke T. Hemispheric disconnection in Marchiafava-Bignami disease. Clinical, neuropsychologicaland MRI findings. . J Neurol Sci 1994;123:2-5 [DOI] [PubMed] [Google Scholar]
- 9.Serdaru M, Hausser-Hauw C, Laplane D, et al. The clinical spectrum of alcoholic pellagra encephalopathy. A retrospective analysis of 22 cases studied pathologically. . Brain 1988;111:829-842 [DOI] [PubMed] [Google Scholar]
- 10.Leong AS. Marchiafava-Bignami disease in a non-alcoholic Indian male. . Pathology 1979;11:241-249 [DOI] [PubMed] [Google Scholar]


