Abstract
BACKGROUND AND PURPOSE: MR imaging has revealed putative evidence of subclinical cerebrovascular disease (CVD) as reflected by white matter signal changes and infarct-like lesions (ILLs). Nonetheless, the prevalence of this condition in the general population has been defined only to a limited extent. We herein report the prevalence and anatomic characteristics of ILLs seen on cranial MR images obtained as part of a population-based study of cardiovascular disease in middle-aged adults. These results are contrasted to those of previous similar studies, particularly those of an elderly population in the Cardiovascular Health Study (CHS).
METHODS: This Atherosclerosis Risk in Communities (ARIC) cohort consists of a probability sample of community-living persons who were 55 to 72 years old at the time of MR examination. MR imaging of 1890 participants was performed at two ARIC field centers, based on a common protocol. MR studies were evaluated by trained readers at the MR Reading Center using original digital data displayed on a high-resolution workstation. The measures of lesion size, anatomic location, and signal intensity were collected. The definition for an ILL was a non-mass, hyperintense region with an arterial vascular distribution on spin-density and T2-weighted images.
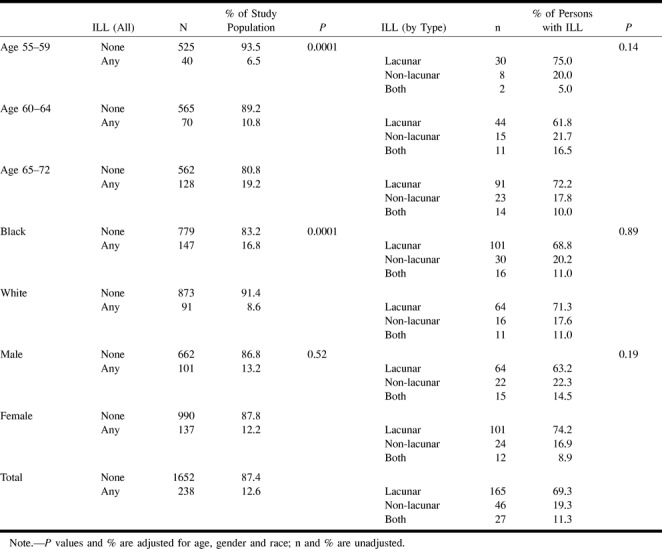
RESULTS: Two hundred ninety participants had ILLs, for an overall prevalence of 15.3%. Eighty-two percent of participants with ILLs had lesions that were 3 mm or larger in maximal dimension, although 87% of these lesions were 20 mm or smaller in maximal dimension. The prevalence of ILLs increased with age, from 7.9% in the 55- to 59-year-old age group to 22.9% in the 65- to 72-year-old age group (P < .001). Lesion prevalence was greater in black (20.7%) than in white persons (10.2% [P < .0001]), but did not differ significantly between male and female participants. The basal ganglia and thalamic region was the most commonly affected anatomic site, accounting for 78.9% of the lesions.
CONCLUSION: Considering that the prevalence of self-reported stroke or transient ischemic attack in ARIC participants is 1.5%, these results suggest that there is significantly more subclinical than clinical CVD in the general population. Furthermore, the prevalence of this subclinical disease increases with age, and is greater in black persons. ILLs are dominated by “lacunae” in the basal ganglia and thalamus. These results are, in general, similar to those of a comparable study of elderly participants in the CHS, except for a 60% lower prevalence of ILLs in this younger population.
Cerebrovascular disease (CVD) is the third-leading cause of morbidity and mortality in the United States, with its impact primarily on the older population (1). Despite these well-documented facts, there are relatively little data on the prevalence of CVD in the general population. A recent report from the Cardiovascular Health Study (CHS) suggests a prevalence of clinical CVD in the elderly (older than 65 years) of approximately 5% and 1.5% for stroke and transient ischemic attack, respectively (2). There is much less information on the prevalence of subclinical CVD that is disease-detected without respect to clinical signs or symptoms. A major limitation for ascertaining the prevalence of subclinical CVD has been a lack of methods for detecting the condition in large populations. Nonetheless, with the advent of X-ray CT and, more recently, MR imaging, it has become possible to evaluate putative subclinical markers of CVD, including “silent” infarction, ischemic white matter disease, and cerebral atrophy (3, 4). This method only recently has been applied in population-based studies, with reported cohorts remaining limited in either sample size or age range (5, 6). Prevalence of subclinical CVD, as reflected by MR imaging–defined infarct-like lesions (ILLs) in the elderly CHS population has been reported to be 36%, approximately six times the prevalence of clinical disease in that same population (6). The primary purpose of this study was to determine the prevalence of subclinical CVD, as reflected by ILLs seen on cerebral MR images in middle-aged adults, a population for which such data are meager. These data are contrasted to those of previous reports of subclinical CVD prevalence in the elderly, indicating a lower prevalence in this younger population.
ARIC is a population-based, multicenter study of risk factors for cardiovascular disease, including transient ischemic attack and stroke, in middle-aged adults (7). The study protocol includes prospective, protocol-defined cranial MR imaging of a subset of eligible ARIC participants. We previously presented an objective method for reporting various clinical and subclinical indicators of CVD on these MR studies, including the presence of ILLs, degree of cerebral atrophy, and white matter signal changes (6, 8, 9). We now report the prevalence of ILLs in this population, as well as characteristics of the lesions, including size and anatomic location.
Methods
The population for this report consisted of 1890 persons who participated in the cerebral MR investigation at two study sites of the ARIC study. The ARIC is a longitudinal study of cardiovascular disease sponsored by the National Heart, Lung and Blood Institute. The ARIC cohort was selected as a probability sample of 15,800 men and women between the ages of 45 and 64 years at four study centers in the United States. Three of these four study centers enumerated and enrolled an ethnically diverse population (Washington County, MD; Forsyth County, NC; and selected suburbs of Minneapolis, MN). The fourth quarter of the ARIC cohort was sampled from black persons who were residents of Jackson, MS. Details of sampling, study design, and cohort examination procedures have been published (7). Eligible participants were interviewed at home, and then invited to a baseline clinical examination. The baseline examination of the ARIC cohort was conducted from 1987 through 1989. Every 3 years after the baseline examination, all participants were invited to a follow-up clinical examination. The first follow-up examination was conducted from 1990 through 1992 and the second follow-up examination from 1993 through 1995.
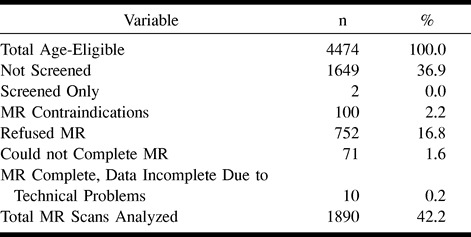
During the third triennial examination conducted from 1993 through 1995, a sample of cohort members who were 55 years old and older (n = 2825 [Table 1]) at the Forsyth County, NC, and Jackson, MS, study sites was screened for eligibility for cerebral MR examination. For participant safety, the following criteria were used to exclude ineligible persons from the MR study. Persons were excluded for: 1) having ever undergone surgery for an aneurysm in the brain; 2) having metal fragments in the eyes, brain, or spinal cord; 3) having a valvular prosthesis, a cardiac pacemaker, cochlear implant, spinal cord stimulator, or other internal electrical device; 4) being pregnant; and 5) having occupational exposure to metal fragments. Two percent of the participants seen from 1993 through 1994 were ineligible. Of those meeting inclusion criteria, 27.6% declined to undergo the cerebral MR examinations. The final sample size for this study was 1890 (1127 women; 763 men; 964 white persons; 926 black persons).
TABLE 1:
Study population
Although the MR examinees were drawn from only the first 2 years of the 1993 through 1995 cohort reexamination, this subgroup is a random sample of the full cohort because examination dates were allocated at baseline through randomly selected induction cycles. Reexamination visits were scheduled according to the anniversary date, to the degree possible.
MR imaging and analytic methods were the same as those for CHS (8). MR images included 500/20/1 (TR/TE/excitations) T1-weighted images and 5/0/24 sagittal localizing images with a 5-mm section thickness, 0-mm section gap, 24-cm field of view, and 128 × 256 matrix. Midline sagittal images were used to identify the anterior commissure-posterior commissure line, along which all oblique axial images were aligned. Spin-echo, spin-density/T2-weighted (3000/30–90/1) and T1-weighted (500/20/1) oblique axial images with a 5-mm section thickness, 0-mm section gap, 24-cm field of view, and 192 × 256 matrix were acquired from the vertex to the foramen magnum.
The resulting images were displayed on workstation monitors for evaluation by trained readers. Each study had a primary and a secondary interpretation rendered by different readers blinded to any information except that the studies were from the ARIC. All primary readers were board-certified radiologists with subspecialty neuroradiology training. Secondary readers included the same radiologists plus an experienced neuroimaging technologist.
ILLs were defined as focal, non-mass lesions having arterial vascular distribution and being hyperintense to gray matter on both spin-density and T2-weighted images. The intensity of the lesions on the T1-weighted images relative to normal gray matter was recorded. To be considered an ILL in cerebral white matter and the brain stem, lesions were required to be hypointense on T1-weighted images, similar to CSF (6) (Fig 1).
fig 1.
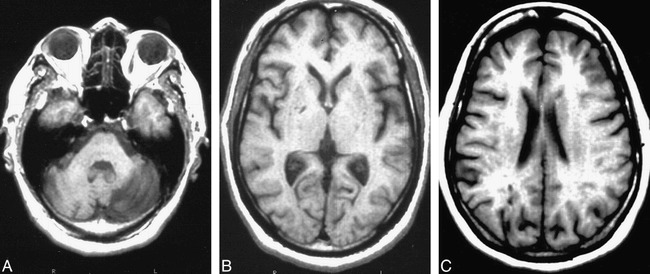
Examples of ILL.
A, Left cerebellar hemisphere ILL.
B, Right globus palidus ILL (arrow).
C, Right occipital lobe ILL.
Note.—Left to right: spin-echo, spin-density (3000/30), T2-weighted (3000/90), and T1-weighted (500/20) images.
The dimensions of the lesions were measured carefully using an electronic cursor; the maximal right-to-left and anterior-to-posterior dimension of each lesion was recorded. The superior-to-inferior dimension was reported by the number of axial sections on which the lesion appeared. Lesions, the greatest dimension of which was less than 3 mm, could not be measured accurately because of pixel resolution, and were recorded simply as less than 3 mm.
For anatomic localization, lesions were assigned to one or more of 23 anatomic regions defined by gross anatomic and vascular characteristics. For this report, the primary anatomic region occupied by the lesion was used for analysis, except for an additional analysis of “lacunar-like lesions,” which were defined as ILLs equal to or greater than 3 mm and less than 20 mm and located in the basal ganglia, internal capsules, thalamus, and deep cerebral white matter.
One hundred forty-four randomly selected cases were reread for the purpose of analyzing lesion detection reproducibility. These blinded double readings were used to determine intra- and inter-reader percent agreement and for calculation of the kappa statistic (10). Forty-two intrareader and 102 inter-reader interpretations were performed. Separate analysis was performed for those lesions that were less than 3 mm and for those that were greater than or equal to 3 mm.
MR interpretative results were sent to the ARIC coordinating center for statistical analysis; t-tests and χ2 tests were used to determine statistical relationships of age, gender, race, and ILLs and to compare participants who completed MR imaging with those who did not (10).
Results
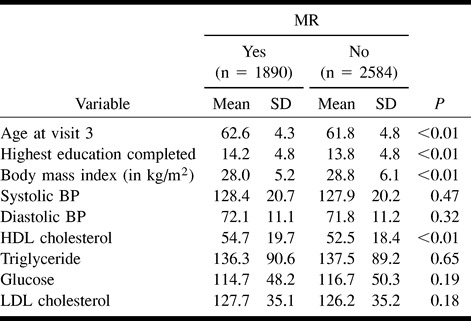
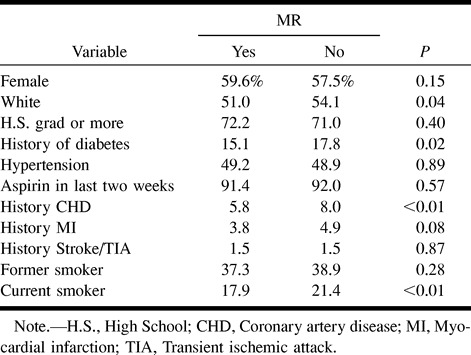
One thousand eight hundred ninety of the ARIC participants who were 55 to 72 years old successfully completed technically adequate MR examinations. Table 1 details the cohort groups that did or did not complete the MR examinations and the reasons for nonperformance of MR imaging. Sixty-seven percent of the eligible screened cohort participants satisfactorily completed the examinations. The most common reasons for screened participants not undergoing MR imaging included MR imaging contraindications, participant refusal attributed to lack of interest, and inability to complete the examination because of claustrophobia. Information regarding the presence of infarcts was unavailable for 10 participants because of incomplete or poor-quality images; these cases were omitted from the analysis. To determine whether completing the MR examinations resulted in a biased sampling of the age-eligible participants in these two centers of the ARIC cohort, comparisons of participant demographics and clinical characteristics were made between the MR and non-MR groups (Table 2). Compared with the remainder of the eligible cohort, participants completing the MR examinations were older, thinner, more educated, and had higher high-density lipoprotein cholesterol counts. They were also more likely to be black, but less likely to be diabetic, to have prevalent congestive heart disease, or to smoke cigarettes. Only 1.5% of both groups reported a physician-diagnosed history of stroke or transient ischemic attack.
TABLE 2a:
Characteristics of MR study population
Inter- and intrareader observer agreement was 64% and 75%, respectively, for ILLs less than 3 mm. When observed agreement was corrected for chance, the kappa statistics for those lesions less than 3 mm in maximal diameter were 0.25 and 0.54, respectively, for inter- and intra-reader agreement. Inter- and intrareader agreement rates for lesions less than 3 mm were 79% and 82%, respectively, resulting in kappa statistics of .52 and .78, respectively.
Of the 1890 participants with readable images, 290 had at least one ILL, for an overall prevalence of ILLs of 15.3 % in this population (Table 3). Fifty-two had only lesions that were 3 mm in maximal dimension. One hundred ninety-nine had only lesions that were 3 mm or larger. Thirty-nine participants had both sized lesions. Expressed differently, of 290 participants with ILLs, 238 (82%) had lesions that were equal to or greater than 3 mm in greatest dimension. Because reader reproducibility for ILLs less than 3 mm was poor, and because they accounted for a relatively small percentage of all ILLs, most further analyses will concentrate on those ILLs that are equal to or greater than 3 mm in maximal dimension.
TABLE 3:
Age-, race-, and sex-adjusted prevalence of infarct-like lesions
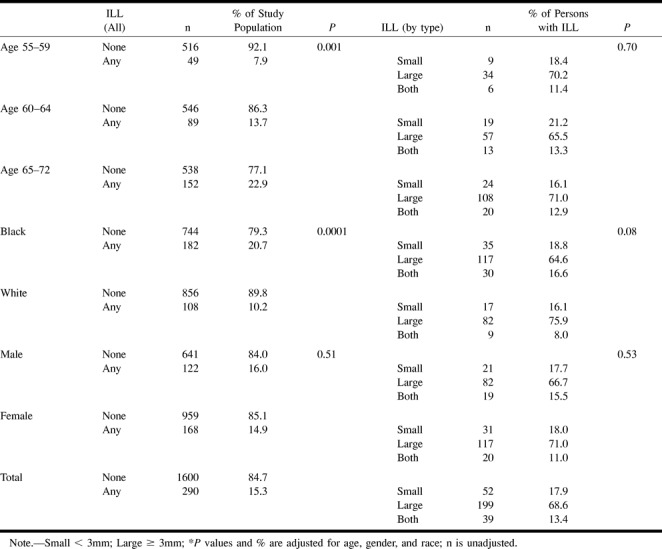
Table 3 presents age-, gender-, and race-adjusted prevalence of ILLs. There was a higher prevalence of lesions in older participants (Table 3 and Fig 2). The prevalence of lesions increased from 7.9% in the 55- to 59-year-old age group to 22.9% in the 65- to 72-year-old age group. This age association with ILL prevalence was significant (P < .001). There was also a higher prevalence of ILL in black than in white participants (20.7% versus 10.2% [P < .0001]). There was no significant difference in ILL prevalence between male and female participants.
fig 2.
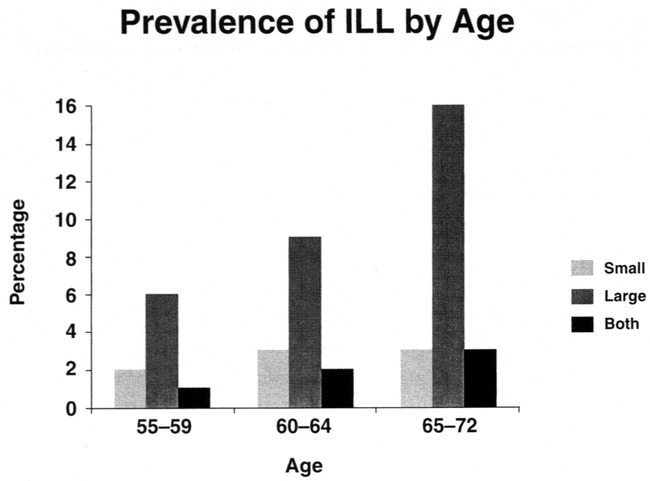
Prevalence (unadjusted) of ILL by type and age
One hundred fifty-seven (66%) of the 238 persons with at least one ILL equal to or greater than 3 mm had only one lesion, whereas 81 (34%) had more than one such lesion (Table 4). A breakdown of ILLs equal to or greater than 3 mm by maximal dimension is presented in Table 5. Eighty-seven percent of ILLs equal to or greater than 3 mm were 20 mm or less in maximal dimension.
TABLE 4:
Number of large infarct-like lesions per participant
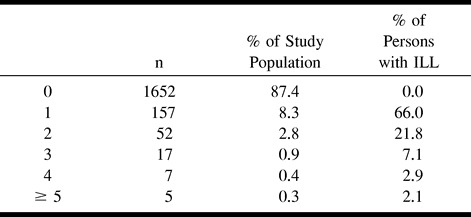
TABLE 5:
Large infarct-like lesions by size
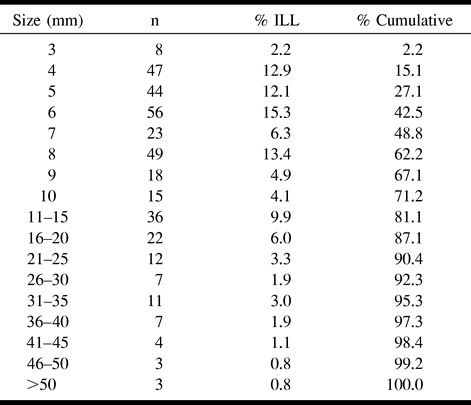
Table 6 details the locations of ILLs equal to or greater than 3 mm in the study population. The vast majority (78.9%) of these lesions were in the deep nuclear region (caudate and lentiform nuclei, internal capsule, and thalamus) and deep cerebral white matter, with the lentiform nuclei and thalamus accounting for 39% of all ILLs equal to or greater than 3 mm in size. ILLs less than 3 mm in size were also predominantly in the deep nuclear region. Figure 3 shows the relationship between size and gross anatomic location. In general, the deep nuclear region lesions were smaller than the cerebral lesions. Posterior fossa lesions had a more uniform size distribution.
TABLE 6:
Location of infarct-like lesions ≥ 3 mm by anatomic location
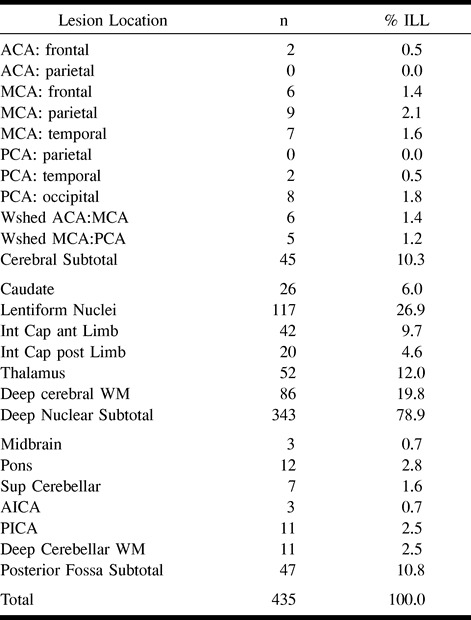
fig 3.
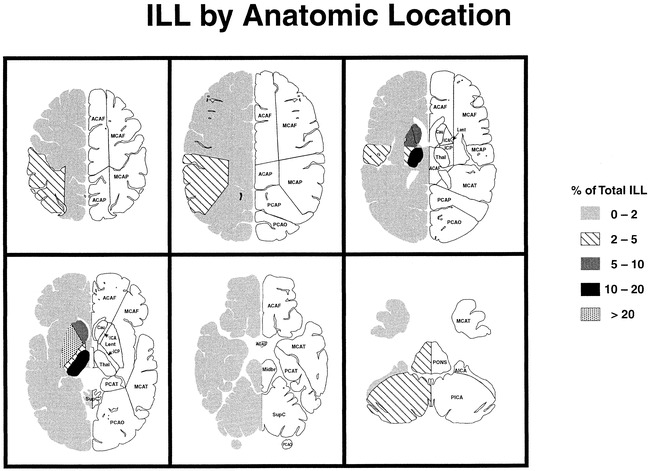
Percentage of ILL by anatomic location
Table 7 summarizes the age-, gender-, and race-adjusted prevalence of lacunar-like lesions in our study population based on a definition of those ILLs that were equal to or greater than 3 mm and less than 20 mm in maximal diameter and located in the basal ganglia, internal capsule, thalamus, brain stem, and deep cerebral white matter. By this definition, lacunar-like lesions accounted for approximately 80.6% of the lesions, and had an overall prevalence of 10%.
TABLE 7:
Prevalence of lacunar-like lesions by age, gender, race
By definition, all ILLs in the cerebral white matter and brain stem were hypointense to gray matter on the T1-weighted images; others could be isointense. Approximately 59% of deep nuclear lesions were hypointense on the T1-weighted images in this study, whereas 87% of cerebral and 98% of posterior fossa lesions were hypointense.
Discussion
MR imaging previously has revealed unsuspected lesions of the brain in a large percentage of our adult, particularly elderly, population. Although the nature and significance of these lesions (some of which resemble well-documented infarcts, whereas others are only reflected by white matter T2-weighted hyperintensities) were initially unknown, it has become increasingly clear that they reflect small-vessel ischemic disease of the brain (4, 6, 11–15). Although obviously common, the actual prevalence of this condition has been documented by only a few studies of small or restricted populations. In particular, the CHS reported a prevalence of MR imaging–defined ILLs of 36%, a six-times greater prevalence than clinical stroke/transient ischemic attack in that elderly population (6). The prevalence of MR imaging–defined ILLs in younger populations has been poorly documented, leading to the main focus of this report.
We have shown that approximately 15% of a population-based cohort of relatively healthy, predominantly middle-aged Americans have at least one ILL revealed by MR imaging. Considering that the clinical prevalence of stroke or transient ischemic attack was 1.5% in this cohort, and assuming that ILLs are secondary to CVD, these MR results suggest a significantly greater prevalence of subclinical CVD than that found in the ARIC. The prevalence of this subclinical disease is greater in older persons and in black rather than in white persons, but does not differ among men and women. These results confirm a long-standing suspicion that there is a large population of adults with CVD that is not obvious clinically.
Although there are many descriptive papers reporting putative CT and MR findings of CVD, including infarct, white matter signal changes, and cerebral atrophy (11–13, 16), it should be noted that much of this previous literature has focused on T2-weighted hyperintense and T1-weighted isointense white matter lesions, which would not have been classified as ILLs in this report. Although often based on examinations of elderly patients, few of these studies have been population-based. Lindgren et al (5) recently reported the prevalence of asymptomatic abnormalities on MR images in a Swedish population-based study of 77 participants who were 36 to 95 years old. Six of these participants had lesions resembling our ILLs, but their ages were not reported.
One of the most extensive population studies of the prevalence of CVD, clinical and subclinical, is that of the CHS (2, 6). The CHS MR study was similar in design to this ARIC study, except for its older population of 3658 participants between the ages of 65 and 97 years. The overall prevalence of ILLs on the MR images of the CHS participants was 36%, versus 15% for the ARIC participants (6). In both studies, ILLs increased with age, from 7.9% in participants who were 55 to 59 years old to 22.9% in participants who were 65 to 72 years old in the ARIC. The latter figure corresponds well with the 24.5% prevalence of ILLs in CHS participants who were 65 to 69 years old. In the latter study, the oldest group, ranging from 85 to 97 years old, had an ILL prevalence of 43%. These combined results highlight the strong association of ILLs and age.
The small (less than 3 mm) ILLs accounted for only 18% of ILLs in the ARIC, comparable with 15% small ILLs in the CHS. As in the CHS, approximately 80% of ILLs in the ARIC were less than 1.5 cm in diameter and were located in the deep nuclear region, characteristics consistent with lacunar infarcts. In both the CHS and ARIC, male participants had a slightly, but not statistically significant, higher prevalence of ILLs than did female participants. In the ARIC, black participants had a higher prevalence of ILLs than did white participants, in contrast to that found in the CHS, in which there was no significant racial difference. The number of black participants included in the CHS report, however, was probably too small to detect any difference. These comparisons show that the anatomic characteristics of ILLs in the ARIC and the CHS are very similar, suggesting a common pathophysiologic substrate. ILL prevalence, however, is strikingly different between the two studies, primarily on the basis of age, not sex or race.
Although the cause of ILLs is unproved, based on MR characteristics and previously demonstrated associations between ILLs and stroke/transient ischemic attack symptoms as well as cognitive and other neurologic abnormalities, a vascular cause seems most likely (8, 17). The appearance of the larger lesions in the cortex is consistent with the generally accepted descriptions of cerebral infarcts (14, 18, 19). Although the nature of the smaller lesions, particularly those in the deep nuclear regions, may be more controversial, we think that they are ischemic in origin and, in general, that they correspond to small, lacunar infarcts (20, 21). Like previous descriptions of lacunar infarctions, these lesions are bright on both spin-density and T2-weighted images (15, 22). The proton-density imaging parameters were selected such that gray matter and CSF had similar signal intensities. The hyperintensity of ILLs on spin-density images helps one distinguish these lesions from normal or dilated perivascular spaces (22–24). Furthermore, the lesions are most common in the lentiform nucleus and thalamus, as well as in the deep cerebral white matter zones, where small penetrating arteries terminate in vascular territories consistent with ILLs (eg, lenticulostriate arteries in the lentiform nucleus or thalamoperforating arteries in the thalamus) (25). These lesions are relatively small, with the majority being less than 20 mm in maximal diameter. Multiple lesions are frequent in affected persons, and they are associated with increased age.
By our definition, lacunar ILLs accounted for approximately 80% of the lesions, and had an overall prevalence of 10%. Thus, the percentage of ILLs consistent with lacunae (in contrast to non-lacunar ILLs) in this study is certainly greater than reports on the incidence of clinical lacunar infarction. Most reports suggest that lacunar infarcts account for approximately 20% to 30% of incident strokes (26, 27). This discrepancy between the MR prevalence and clinical incidence of lacunar infarction is consistent with C. Miller Fisher's observation that most lacunar infarcts are clinically unrecognized (20). The vast majority (more than 90%) of our lacunar-like lesions also occurred in participants without previous histories of stroke or transient ischemic attack.
Reports vary regarding the MR appearance of lacunar infarcts on T1-weighted images. Although some authors have indicated that lacunar infarcts are always hypointense on T1-weighted images, other authors have disagreed (15, 22). Sixty percent of ILLs equal to or greater than 3 mm in the deep nuclear region was hypointense on the T1-weighted images in this study. The T1-weighted isointense lesions could represent areas of ischemic demyelination, in contrast to the lesions hypointense on T1-weighted images, which may be actual lacunar infarctions with necrosis and cavitation. This explanation, along with thinner sections and a higher resolution examination, could explain the apparent increased sensitivity of MR imaging over gross autopsy in detecting these lesions.
Many of these lesions were relatively subtle on MR images. Their detection challenged the resolution of the equipment and the observational powers of the interpreter. This was particularly true for the very small lesions (less than 3 mm in maximal dimension) that had relatively poor observational reproducibility. Nonetheless, the very small lesions accounted for a minority of the lesions, and observational reproducibility was good to very good for the lesions that were less than 3 mm in maximal diameter. These results are comparable with those of other reports on similar lesion detection reproducibility (6, 28, 29).
Conclusion
ILLs in this middle-aged population share most of the features of ILLs in older populations, including a predominance of lacunar ILLs, but are less prevalent. The ILLs in the ARIC cohort, however, are 10 times more prevalent than are clinical stroke and transient ischemic attack in the same population. The precise cause and significance of these lesions are currently unknown, although these lesions have been reported to occur more frequently with hypertension, smoking, depression, and, possibly, dementia, and are associated with cognitive and other neurologic abnormalities (17, 30, 31). Careful correlation with risk factors and subtle extrapyramidal function will be required to clarify better the cause and true significance of these lesions. If the lesions do represent infarctions or areas of ischemia, their prevalence in this study indicates that subclinical CVD is common in older middle-aged men and women, and is more common in black than in white persons.
TABLE 2b:
Characteristics of MR study population
Acknowledgments
The MR image readers included R. Nick Bryan, Azar Dagher, Carolyn Cidis Meltzer, Timothy J. Miller, Scott Wells, Sarah Whitehead, Linda Wilkins, Cindy Quinn, and Nancy Chang Yue. The participating institutions and principal staff members included the Bowman Gray School of Medicine of Wake Forest University, Forsyth County, NC; the University of Mississippi, Jackson, MS; the MR Reading Center of The Johns Hopkins University, Baltimore, MD, R. Nick Bryan, Carolyn Cidis Meltzer, Douglas Fellows, Melanie Hawkins, Patrice Holtz, Michael Kraut, Grace Lee, Larry Schertz, Earl P. Steinberg, Scott Wells, Linda Wilkins, and Nancy C. Yue; the Coordinating Center of the University of North Carolina, Chapel Hill, NC; and the NHLBI Project Office, A. Richey Sharrett and Lawton Cooper.
Footnotes
ARIC is supported by Contracts NO1-HC-35126, NO1-HC-55015, NO1-HC-55016, NO1-HC-55018, NO1-HC-55019, NO1-HC-55020, NO1-HC-55021, and NO1-HC-55022 from The National Heart, Lung, and Blood Institute.
Address reprint requests to Nick Bryan, MD, PhD, Diagnostic Radiology Department, Building 10, Room 1C660, 10 Center Drive MSC 1182, Bethesda, MD 20892-1182.
References
- 1. National Center for Health Statistics. Current Estimates from the National Health Interview Survey, 1991.. Hyattsville, MD: National Center for Health Statistics, 1992 [PubMed]
- 2.Mittelmark MB, Psaty BM, Rautaharju PM, et al. Prevalence of cardiovascular diseases among older adults. The Cardiovascular Health Study. Am J Epidemiol 1993;137(3):311-317 [DOI] [PubMed] [Google Scholar]
- 3.Laffey PA, Peyster RG, Nathan R, Haskin ME, McGinley JA. Computed tomography and aging. Results in a normal elderly population. Neuroradiology 1984;26:273-278 [DOI] [PubMed] [Google Scholar]
- 4.Brant-Zawadzki M, Fein G, Van Dyke C, Keirman R, Davenport L, De Groot J. MR imaging of the aging brain. AJNR Am J Neuroradiol 1985;6:675-782 [PMC free article] [PubMed] [Google Scholar]
- 5.Lindgren A, Roijer A, Rudling O, et al. Cerebral lesions on magnetic resonance imaging, heart disease, and vascular risk factors in subjects without stroke. A population based study. Stroke 1994;25:929-934 [DOI] [PubMed] [Google Scholar]
- 6.Bryan RN, Wells SW, Elster A, et al. Infarctlike lesions in the brain. Prevalence and anatomic characteristics at MR imaging of the elderly. Data from the Cardiovascular Health Study (CHS). Radiology 1997;202:47-54 [DOI] [PubMed] [Google Scholar]
- 7. The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study. Design and objectives. Am J Epidemiol 1989;129:687-702 [PubMed] [Google Scholar]
- 8.Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain. The Cardiovascular Health Study. AJNR Am J Neuroradiol 1994;15:1625-1633 [PMC free article] [PubMed] [Google Scholar]
- 9.Yue NC, Longstreth WT Jr, Elster AD, Jungreis CA, O'Leary DH, Poirier VC. Clinically serious abnormalities found incidentally at MR imaging of the brain. Data from the Cardiovascular Health Study. Radiology 1997;202:41-46 [DOI] [PubMed] [Google Scholar]
- 10. SAS, vol 6.11, SAS Institute Inc. 1996.
- 11.Drayer BP. Imaging of the aging brain. Part 2. Pathologic conditions. Radiology 1988;166:797-806 [DOI] [PubMed] [Google Scholar]
- 12.Hendrie HC, Farlow MR, Austrom MG, Edwards MK, Williams MA:, Foci of increased T2 signal intensity on brain MR scans of healthy elderly subjects. AJNR Am J Neuroradiol 1989;10:703-707 [PMC free article] [PubMed] [Google Scholar]
- 13.Awad IA, Spetzler RF, Hodak JA, Awad CA, Carey R. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. I. Correlation with age and cerebrovascular risk factors. Stroke 1986;17:1084-1089 [DOI] [PubMed] [Google Scholar]
- 14.DeWitt LD, Kistler JP, Miller DC, Richardson EP, Buonanno FS:, NMR neuropathologic correlation in stroke. Stroke 1987;18:342-351 [DOI] [PubMed] [Google Scholar]
- 15.Brown JJ, Hesselink JR, Rothrock JF. MR and CT of lacunar infarcts. AJR Am J Roentgenol 1988;151:367-372 [DOI] [PubMed] [Google Scholar]
- 16.Drayer BP. Imaging of the aging brain. Normal findings. Radiology 1988;166:785-796 [DOI] [PubMed] [Google Scholar]
- 17.Price TR, Manolio TA, Kronmal RA, et al. Silent brain infarction on magnetic resonance imaging and neurological abnormalities in community-dwelling older adults. Stroke 1997;28:1158-1164 [DOI] [PubMed] [Google Scholar]
- 18.Yuh WTC, Crain MR, Loos DJ, Greene GM, Ryals TJ, Sato Y. MR imaging of cerebral ischemia. Findings in the first 24 hours. AJNR Am J Neuroradiol 1991;12:621-629 [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan RN, Levy LM, Whitlow WW, Killian JM, Preziosi TJ, Rosario JA. Acute stroke. Magnetic resonance imaging. AJNR Am J Neuroradiol 1991;12:611-620 [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher CM. Lacunes. Small deep cerebral infarcts. Neurology 1965;15:774-784 [DOI] [PubMed] [Google Scholar]
- 21.Fisher CM. Lacunar strokes and infarcts. A review. Neurology 1982;32:871-876 [DOI] [PubMed] [Google Scholar]
- 22.Rothrock JF, Lyden PD, Hesselink JR, Brown JJ, Healy ME. Brain magnetic resonance imaging in the evaluation of lacunar stroke. Stroke 1987;18:781-786 [DOI] [PubMed] [Google Scholar]
- 23.Jungreis CA, Kanal E, Hirsch WL, Martinez AJ, Moossy J. Normal perivascular spaces mimicking lacunar infarction. MR imaging. Radiology 1988;169:101-104 [DOI] [PubMed] [Google Scholar]
- 24.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MDF. Large Virchow-Robin spaces. MR-clinical correlation. AJNR Am J Neuroradiol 1989;10:929-936 [PMC free article] [PubMed] [Google Scholar]
- 25.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency. An anatomic study. AJNR Am J Neuroradiol 1990;11:431-439 [PMC free article] [PubMed] [Google Scholar]
- 26.Bamford J, Sandercock P, Jones L, Warlow C. The natural history of lacunar infarction. The Oxfordshire Community Stroke Project. Stroke 1987;18:545-551 [DOI] [PubMed] [Google Scholar]
- 27.Sacco SE, Whisnant JP, Broderick JP, Phillips SJ, O'Fallon WM. Epidemiological characteristics of lacunar infarcts in a population. Stroke 1991;22:1236-1241 [DOI] [PubMed] [Google Scholar]
- 28.Yetkin FZ, Haughton VM, Fischer ME, et al. High-signal foci on MR images of the brain. Observer variability in their quantification. AJR Am J Roentgenol 1992;159(1):185-188 [DOI] [PubMed] [Google Scholar]
- 29.Davis PC, Gray L, Albert M, Wilkinson W. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part III. Reliability of a standardized MR evaluation of Alzheimer's disease. Neurology 1992;42(9):1676-1680 [DOI] [PubMed] [Google Scholar]
- 30.Rabins PV, Pearlson GD, Aylward E, Kumar AJ, Dowell K. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry 1991;148:617-620 [DOI] [PubMed] [Google Scholar]
- 31.Davis PC, Mirra SS, Alazraki N. The brain in older persons with and without dementia. Findings on MR, PET and SPECT images. AJR Am J Roentgenol 1994;162:1267-1278 [DOI] [PubMed] [Google Scholar]


