Abstract
BACKGROUND AND PURPOSE: Since the approval of intravenous tissue plasminogen activator for acute ischemic stroke, great interest has been generated in cerebral fibrinolysis. Our purpose was to assess long-term outcome and hemorrhagic risk in patients with anterior circulation ischemic stroke treated with intraarterial urokinase.
METHODS: Twenty-six patients were treated within 6 hours of ictus; of these, 21 were followed up for an average of 23 months. Angiographic reperfusion was classified according to thrombolysis in myocardial infarction (TIMI) grades. The Rankin Scale (RS) and the modified Barthel Index (mod BI) were used as outcome measures (good outcome: RS = 0–2, mod BI = 16–20; poor outcome: RS = 3–5, mod BI ≤ 15).
RESULTS: Ten of the 21 patients (average age, 48 years) had a good outcome; three (average age, 71 years) had a poor outcome; eight patients (average age, 78 years) died. Partial/complete (successful) recanalization was observed in 11 of 26 patients and minimal or no (unsuccessful) recanalization in 15. Recanalization favored a better outcome: nine of 21 had successful recanalization, with a good outcome in seven; 12 of 21 had unsuccessful reperfusion, with poor outcome/death in nine. Poor outcome was noted in five patients with internal carotid artery (ICA) bifurcation occlusions, four of whom had unsuccessful recanalization and poor outcome or death. Hemorrhage occurred in 10 of the 26 patients, with clinical deterioration in three. The average dose of urokinase was higher in the hemorrhage group, and mortality was higher in patients who hemorrhaged.
CONCLUSION: Intraarterial thrombolysis is feasible in the setting of acute stroke. Successful reperfusion is associated with a better outcome, and the prevalence of hemorrhage does not exceed that which occurs in the natural history of embolic stroke. Poor outcome or death is associated with nonrecanalization, older age, hemorrhage, and ICA bifurcation occlusions.
Stroke is the third most common cause of death in the United States following heart disease and cancer (1, 2). More than 500,000 new strokes occur each year, with over 150,000 deaths (3). Annually, in the United States, stroke is estimated to cost $30 billion in medical expenses, rehabilitation, and loss of employment (4, 5). The majority of ischemic strokes are due to thromboembolic arterial occlusions (6), with 75% in the distribution of the carotid artery (3). Angiographic studies performed in stroke patients within 8 hours of symptom onset have shown arterial occlusions corresponding to symptoms in more than 80% of cases (7). The role of thrombus in stroke, combined with the success of thrombolysis in acute myocardial infarction, has generated great interest in cerebral fibrinolysis. Intravenous recombinant tissue plasminogen activator (rTPA) is now approved by the Food and Drug Administration (FDA) for the treatment of acute ischemic stroke within 3 hours of symptom onset (8). Compared with intravenous thrombolysis, local intraarterial fibrinolysis can theoretically achieve faster, more complete recanalization with less fibrinolytic agent. However, concern about hemorrhagic risk, confusion about how to select the patients for treatment, and the technical and logistic difficulties of the procedure currently limit the widespread use of the local intraarterial approach.
We retrospectively studied 26 patients with ischemic stroke in the carotid territory treated at our institution with local intraarterial fibrinolysis. The long-term outcome in 21 of these patients is reported. We studied the sites of angiographic occlusion on entry into the study, the effects of treatment on recanalization, and hemorrhagic transformation. Factors affecting outcome, recanalization, and hemorrhagic conversion are discussed.
Methods
Patient Demographics
Between January 1993 and January 1998, 26 patients with ischemic stroke in the distribution of the carotid artery were treated with intraarterial thrombolysis. All patients presented within 6 hours of symptom onset. Fifteen patients were women and 11 were men; the average age was 64 years (range, 25–85 years). The patient's neurologic status was assessed at admission using the National Institutes of Health Stroke Scale (NIHSS).
CT Findings
A head CT scan was obtained in all patients prior to angiography to exclude hemorrhage and other mimics of ischemic stroke. The unenhanced preprocedural CT scans were reviewed retrospectively by a staff neuroradiologist who was unaware of the clinical symptoms, clinical outcome, or follow-up CT results. CT hypodensities were classified as occurring in the basal ganglia, the insular cortex, or the frontal, temporal, or parietal lobe. Hemispheric hypodensity seen in more than 33% of the middle cerebral artery (MCA) territory was recorded as a major early sign of ischemia (MESI) (9, 10).
All patients also had postprocedural head CT scans to exclude hemorrhage and to evaluate the extent of ischemia.
Thrombolytic Procedure
An initial angiogram was obtained to document an arterial occlusion corresponding to the patient's neurologic symptoms. Nonionic contrast material was used in all cases. Because time was of the essence, the circle of Willis and the presence of collateral channels were not always demonstrated. The thrombolytic drug used in all cases was urokinase, delivered by a microcatheter without side holes and introduced coaxially with a guide catheter. Along with the thrombolytic agent, each patient received a bolus of 5000 units of heparin intravenously followed by 1000 units every hour thereafter to prevent thromboembolic complications related to catheterization. The dose of urokinase used was 150,000 to 1.5 million units. The end points for treatment with urokinase were complete recanalization, maximum of 1 million units of urokinase, or 6 hours elapsed since symptom onset. The only exceptions to these were in three patients who received greater than 1 million units of urokinase (which was considered safe at the time of treatment, since excellent collateral circulation had been documented and symptom onset was very recent), and in one patient who was treated 7.5 hours after symptom onset (in this case, excellent collaterals were noted with nearly complete filling of the occluded territory by leptomeningeal collateral circulation).
Treatment with urokinase was initiated, on average, 4.3 hours after symptom onset (range, 2.8–7.5 hours). The thrombolytic procedure was carried out as follows: the microcatheter was initially placed at the proximal aspect of the clot, where 30,000 to 40,000 units of urokinase was delivered at a rate of 10,000 U/min. With the microcatheter/guidewire combination, the clot was then traversed until its distal aspect was reached, where an additional 30,000 to 40,000 units of urokinase was delivered. The microcatheter was then withdrawn into the clot and additional urokinase delivered within the clot at several points until the proximal aspect of the clot was reached. This routine was repeated until complete lysis of the clot was achieved. In cases in which the clot was located in distal branches not reached by the microcatheter (for example, distal MCA branch occlusions), superselective catheterization of the occluded vessel was performed with infusion of urokinase. After urokinase infusion had been completed, arteriograms were obtained in all cases to assess recanalization. All patients were transferred to an intensive care unit until neurologically stable.
Evaluation of Angiograms
Angiograms were evaluated by a neuroradiologist in a nonblinded manner to determine the site of occlusion before initiation of thrombolysis. The location of the clot was classified according to the vessel or vessels involved. Thus, seven types of occlusions were identified: 1) any occlusion of the cervical ICA without involvement of the ICA bifurcation; 2) occlusion of the ICA with embolus to the MCA; 3) occlusion of the ICA bifurcation (carotid “T” occlusion, CTO); 4) combined MCA and anterior cerebral artery (ACA) occlusions; 5) MCA trunk occlusion; 6) occlusion involving an MCA division just beyond the bifurcation or trifurcation of the vessel; and 7) occlusions of the distal branches of the MCA.
The extent of angiographic perfusion before and after treatment was classified according to thrombolysis in myocardial infarction (TIMI) grades (11). Recanalization after thrombolysis was then graded as follows (12): no recanalization, TIMI grade 0; minimal recanalization, TIMI grade 1; partial recanalization, improvement from TIMI grade 0 or 1 to grade 2; complete recanalization, TIMI grade 3.
No or minimal recanalization was considered unsuccessful reperfusion; partial or complete recanalization, successful reperfusion.
Clinical Outcome and Follow-up
Follow-up studies were obtained in 21 patients, with an average follow-up period of 23 months (range, 6–44 months). The Rankin Scale (RS) and the modified Barthel Index (mod BI) were used as outcome measures. A good outcome was defined as RS = 0 to 2 and mod BI = 16 to 20. A poor outcome was defined as RS = 3 to 5 and mod BI = 15 or less.
Five patients were lost to follow-up, in that up to the time of this writing we were unable to contact these patients nor were they seen in our clinic at any time after discharge.
Statistical Analysis
Statistical comparisons were made using the Fisher exact test (two-tailed P), the Jonckheere-Terpstra test, or a two-sample t-test, where appropriate. Values less than .05 were considered statistically significant.
In the analysis of CT interpretations, the effectiveness of CT hypodensity as a predictor of hemorrhage and poor outcome was assessed by using the Cohen κ statistic (13).
Results
Clinical Outcome
Twenty-six patients were treated with intraarterial thrombolysis (Table 1). The median NIHSS score on admission was 20 (range, 3–35). Follow-up data were available for 21 patients, seen for an average of 23 months. Ten patients (48%) had a good outcome (RS = 0–2, mod BI = 16–20), three patients (14%) had a poor outcome (RS = 3–5, mod BI = <15), and eight patients (38%) died. The average age of the patients who had a good outcome was 48 years (range, 25–68 years); in those who died, the average age was 78 years (range, 67–85 years); and in those who had a poor outcome, the average age was 71 years (range, 57–80 years).
TABLE 1:
Outcome of patients with acute stroke post intraarterial thrombolysis
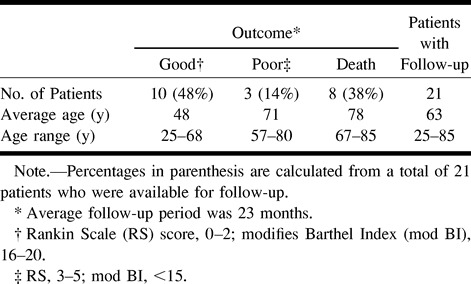
Overall, patients with right hemispheric strokes had a better outcome. In the 21 patients who were followed up, nine had right hemispheric strokes and 12 had left hemispheric strokes. Of the nine with right-sided strokes, six (67%) had a good outcome and three (33%) had a poor outcome or died (two died and one had a poor outcome). Of the 12 with left-sided strokes, four (33%) had a good outcome and eight (67%) had a poor outcome or died (six died and two had a poor outcome).
Four patients died of brain swelling and transtentorial herniation; all four during hospitalization, within 1 week after thrombolysis. Two patients died of medical complications of stroke shortly after discharge to a chronic nursing facility. And two patients died at a chronic nursing facility of unknown causes 5 months after discharge.
Recanalization
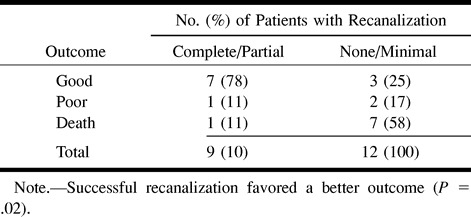
We observed partial or complete recanalization in 11 (42%) of the 26 patients (Table 2). In 15 (58%), there was minimal or no recanalization, with recanalization favoring a better outcome (P = .02). Of the 21 patients for whom clinical follow-up data were available, nine had complete or partial recanalization and 12 had no or minimal recanalization (Table 3). Of the nine who had successful recanalization, seven (78%) had a good outcome and two (22%) had a poor outcome or died. Of the 12 patients who had unsuccessful reperfusion, three (25%) had a good outcome and nine (75%) had a poor outcome or died.
TABLE 2:
Rate of recanalization

TABLE 3:
Recanalization vs outcome
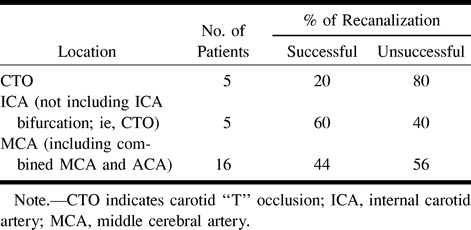
The distribution of clot location is presented in Table 4 along with the median NIHSS score and mean recanalization score for each category. The majority of patients presented with occlusions of the MCA trunk followed by CTO and MCA division occlusion. There was a trend toward lower recanalization rates in patients with CTO (P = .6) (Table 5). Five patients had CTO, and, of these, four (80%) had no recanalization and only one (20%) had successful reperfusion. Patients with occlusions at other sites had rates of successful recanalization similar to those of the overall population. When there was occlusion of the intracranial vessels, MCA or combined MCA and ACA reperfusion was favorable in 44% and unsuccessful in 56%. When the clot was in the ICA (not involving the ICA bifurcation), successful recanalization was achieved in 60% of cases.
TABLE 4:
Clot location
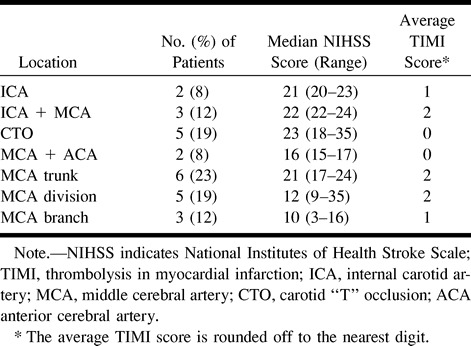
TABLE 5:
Clot location vs recanalization
There was also a trend toward poor clinical outcome in patients with CTO (P = .7). Of five patients with CTO, four (80%) died or had a poor outcome and one (20%) had a good outcome. Of four patients with ICA occlusions (not involving the ICA bifurcation) for whom follow-up data were available, three (75%) had a good outcome and one (25%) had a poor outcome; there were no mortalities in this group. Of the 13 patients with MCA or combined MCA and ACA occlusions for whom follow-up data were available, six (46%) had a good outcome and seven (54%) had a poor outcome or died.
We found no correlation between time to treatment and recanalization.
Hemorrhage
Overall, hemorrhages occurred in 10 (38%) of the 26 patients, all in the distribution of the occluded vessels. Hemorrhagic transformation causing clinical deterioration occurred in three (12%) of the patients, all within 24 hours of treatment. The average dose of urokinase in the patients with hemorrhage was higher (P = .08) than in those without hemorrhage. For the 10 patients who hemorrhaged, the average dose was 840,000 units; in the 16 patients without hemorrhage, the average dose was 593,000 units.
There were four basal ganglia and six hemispheric hemorrhages. Seven patients had occlusion of the lenticulostriate arteries on the prethrombolytic angiograms, with three resulting in basal ganglia hemorrhage. Although the population size was too small to show statistical significance, the time to treatment appeared to be an important factor in the lenticulostriate occlusions and basal ganglia hemorrhages. In the three patients with lenticulostriate occlusion and basal ganglia hemorrhage, the average time to treatment was 5.2 hours; in the four patients with lenticulostriate occlusion without hemorrhage, the average time to treatment was 3.9 hours (P = .5). In the overall population of 26 patients, however, no significant difference in time to treatment was noted in the hemorrhage (average time to treatment, 4.6 hours) vs nonhemorrhage (average time to treatment, 4.2 hours) groups.
Hemorrhagic transformation was noted both among patients who had successful and unsuccessful reperfusion. In the group with successful recanalization, five (45%) of 11 patients had hemorrhagic transformations. Of the 15 patients who had unsuccessful reperfusion, five (33%) had hemorrhages. This difference was not statistically significant (P = .26).
Mortality was noted to be higher among those who had hemorrhage. Of the 10 patients who hemorrhaged, follow-up was available in eight; and, of these, five (62%) died. Of the 16 patients who did not hemorrhage, follow-up was available in 13; and, of these, three (23%) died. There were no systemic hemorrhagic complications.
CT scans were reviewed retrospectively by a neuroradiologist without knowledge of the patients' clinical history. In cases in which hypodensity was noted in more than one third of the MCA territory, correlation with respect to outcome and hemorrhage was assessed. No significant association was found between MESI and hemorrhage or poor outcome. With respect to outcome, κ = .16 (P = .64); similarly, in predicting hemorrhage; κ = .16 (P = .66).
Discussion
Clinical Outcome
Conservative medical management of nonhemorrhagic stroke results in severe neurologic deficit or death in many patients. The 30-day and 5-year mortality rates for stroke in the carotid distribution are 17% and 40%, respectively (2). Saito et al (14), in a study of clinical outcome in 33 patients with M1 occlusions, reported that 26 (78%) died or were severely disabled and only three (9%) had a good outcome. Clinical outcome of patients with vertebrobasilar occlusion is even less favorable, with death in the majority of patients and severe deficit in most survivors (15–17). Because of the very poor outcome of patients with vertebrobasilar occlusion, Zeumer et al (18), in 1983, treated five patients with local intraarterial fibrinolysis, achieving successful recanalization in three, all of whom experienced subsequent neurologic improvement. One year later, Zeumer et al (19) reported treating two patients who had occlusion of the distal ICA with urokinase, both of whom improved clinically. Since then, a number of small series have been published (20–23). Neurologic improvement was variable in these studies, with minimal or no neurologic deficit reported in 15% to 75% of patients. This wide variation in outcome is most likely due to several factors, including 1) the grading system used for assessing outcome, 2) dose of thrombolytic agent used, 3) differences in baseline patient demographics (ie, age, baseline neurologic status), and 4) site of arterial occlusion.
We found results similar to those reported for patients who underwent carotid territory intraarterial fibrinolysis, with 48% of our patients experiencing a good outcome (RS = 0–2). The older age group was associated with a worse outcome and increased risk of dying. The average age in the 10 patients who had a good outcome was 48 years; in the eight who died, the average age was 78 years.
Published studies of patients with carotid distribution stroke report a mortality rate of 5% to 45% (14, 24, 25). Our results are within this range (38%) and do not exceed the mortality rates in other published reports of intraarterial thrombolytic treatment for stroke (20–23).
Early phase II results of prolyse in acute cerebral thromboembolism (PROACT), the first randomized, double-blind, multicenter trial comparing an intraarterial plasminogen activator with a placebo in the treatment of patients with acute MCA territory stroke, were published in 1998 (26). Patients with occlusion of the M1 or M2 segment of the MCA were randomized to receive intraarterial recombinant prourokinase (rpro-UK) versus a placebo. Outcome was assessed at 90 days, with good outcome defined as RS = 0 to 1. The study showed 31% of patients treated with rpro-UK had a good outcome compared with 21% in the placebo group. In our analysis, 29% of the patients had a good outcome if defined as RS = 0 to 1. This is comparable to the results of the PROACT trial. Mortality in the PROACT study was reported at 27% in patients treated with rpro-UK, as compared with 43% in the placebo group. We found a mortality rate of 38%, which is higher than that in the treated group in the PROACT study but does not exceed that in their placebo group. The reason for the higher mortality in our series may be due to differences in baseline demographics between the two groups of patients. For example, with respect to occlusion site, in the PROACT trial, all patients had M1 or M2 occlusions. In our series, five patients had CTO, and, of these, four died. Other studies have shown a higher mortality in patients with CTO as compared with occlusions in other locations (9, 21).
The intravenous rTPA trial conducted by the National Institute of Neurological Disorders and Stroke (NINDS) has provided evidence of the only proved therapy for stroke to date (8). Although the number of patients in our study is small, it is interesting to compare them with the treated group in the NINDS trial. The average age in the NINDS trial (combined results of parts 1 and 2) was 68 years, with a median NIHSS score of 14 on entry into the study. With good outcome defined as an RS score of 0 to 2, 41% of the treated group in the NINDS trial had a good outcome at the 1-year follow-up (27). In our study, the average age of the 21 patients who were followed up was 63 years, with a median NIHSS score of 20 on entry into the study. Ten (48%) of the 21 patients had a good outcome. Thus, the patients in our series had more severe neurologic deficits at entry and a later time to treatment than did the NINDS patients and yet experienced good long-term outcomes, at a rate equal to or better than those treated in the NINDS trial. The prevalence of symptomatic hemorrhage was reported at 6% in the treated group in the NINDS trial, whereas three (12%) of the 26 patients in our study had symptomatic hemorrhage. The concomitant use of heparin during intraarterial thrombolysis may account for the higher rate of bleeding in our series. In addition, the patients in our series had more severe neurologic deficits at baseline, with a median NIHSS score of 20, as compared with a score of 14 in the NINDS trial. The severity of neurologic deficit has been shown to be associated with an increased risk of symptomatic hemorrhage (28).
We found no adverse events resulting from the pretreatment angiographic procedure, indicating that cerebral angiography in the acute phase of stroke can be safely carried out. Other authors have reported similar findings (9, 12, 29).
Recanalization
Thrombolytic therapy will theoretically promote reopening of obstructed arteries, thus establishing blood flow to the ischemic brain tissue and limiting the size of infarction. A metaanalysis of several clinical trials has shown that early recanalization is effective in improving the outcome of acute stroke patients (30). Intravenous rTPA has been approved by the FDA for treatment of acute stroke within 3 hours of symptom onset (8). Compared with intravenous therapy, localized intraarterial thrombolysis has the theoretical advantage of achieving faster, more complete recanalization with less fibrinolytic agent. The disadvantage is the small risk associated with catheterization of intracranial vessels (7, 31–34). Another disadvantage of intraarterial thrombolysis is the delay to treatment. The neurointerventional team has to be gathered and the angiography suite prepared to perform the thrombolytic procedure, which can lead to a delay in treatment. In this series, the angiographic procedure was initiated, on average, 2.3 hours after the arrival of the patient in the emergency department. The average time to initiation of thrombolytic therapy after ictus was 4.3 hours (range, 2.8–7.5 hours).
Spontaneous recanalization is part of the natural history of acute ischemic stroke. Arterial occlusions are present in approximately 76% of patients when examined in the first 6 hours of ictus (35) and in 59% when examined at 24 hours (36). Spontaneous reperfusion is partly responsible for this reduction in occlusions seen in angiographic studies of patients with acute ischemic stroke. Exactly how or when spontaneous recanalization occurs is unclear. However, it appears that in most patients spontaneous recanalization occurs too late in the course to have a favorable impact on outcome.
We know of no prospective studies comparing the recanalization rate between intraarterial thrombolysis and conventional stroke therapy. The best information is perhaps provided by the PROACT trial. In their low-dose heparin group, successful recanalization at 120 minutes was obtained in 40% of treated patients versus 22% in the placebo group (26). Note, however, that the placebo group in the PROACT trial did not undergo conventional stroke therapy but rather selective microcatheterization of the occluded vessel with infusion of saline. In our study, successful recanalization was achieved in 42% of patients within 6 hours of symptom onset (Table 2). Therefore, at 6 hours, 58% were left with a large clot burden with complete or nearly complete occlusion of the vessel. This compares with 76% at 6 hours in the natural history of the disease (35). Our results are comparable to those of the PROACT trial, in which successful recanalization was reported in 40% of the low-dose heparin group (26).
Successful recanalization after administration of thrombolytics is associated with a favorable outcome. Mori and coauthors (37) reported lower mortality rates in patients in whom recanalization was successful than in those in whom it was not (mortality, 0% versus 25%). Del Zoppo (38) reported that 10 (67%) of 15 patients in whom complete recanalization was achieved had nearly complete resolution of symptoms as compared with none of five patients in whom recanalization was not successful. In addition, of the 15 patients with successful recanalization, only one (7%) died, as compared with two (40%) of five in whom recanalization was unsuccessful. Our data support these findings, in that a statistically significant association was found between recanalization and outcome (P = .02). A good outcome was observed in 78% of patients with successful reperfusion versus in 25% of patients with unsuccessful reperfusion (Table 3). Conversely, poor outcome or mortality was seen in 22% of patients with successful reperfusion versus in 75% with unsuccessful reperfusion.
Clinical outcome also depends on the site of occlusion and the effectiveness of collateral circulation. Occlusion of a vessel supplying the brain activates compensatory mechanisms that attempt to reestablish blood flow and increase oxygen delivery to the neuronal tissue distal to the obstruction. These compensatory mechanisms include collateral circulation and increased oxygen extraction in the ischemic cerebral territory. Of the different types of occlusions seen in this study, CTO is most detrimental to effective collateral circulation. With occlusion of the ICA bifurcation (the M1 segment of the MCA and the A1 segment of the ACA), most collateral blood flow pathways are obstructed. This includes the circle of Willis channels and retrograde ophthalmic circulation. Effective leptomeningeal collaterals may be compromised as well. In CTO, the MCA can only be supplied by leptomeningeal collaterals from the ACA and PCA. However, the ipsilateral ACA territory can only be supplied by the anterior communicating artery, and the contralateral A1 segment may not have the capacity to provide adequate leptomeningeal blood supply to the most distal MCA territory. We found that patients with CTO had lower recanalization rates and worse clinical outcome than did those with occlusion of other vessels (Table 5). Of five patients with CTO, only one had successful reperfusion and four had no recanalization; all four patients with unsuccessful recanalization died or had a poor outcome, with fatalities in this group all due to hemispheric swelling and transtentorial herniation. Other authors have reported similar findings, with poor outcome in patients with ICA bifurcation occlusions. Theron et al (22) reported one death in their series, and this occurred in the only patient with ICA bifurcation occlusion. Zeumer et al (20), in a study of outcome versus type of occlusion, reported that all eight patients who had ICA bifurcation occlusions died. Jansen et al (21) reported a mortality of 53% and a poor outcome of 31% in their patients with CTO. More recently, Kucinski et al (9), in an evaluation of the predictive value of the site of angiographic occlusion as a prognostic indicator, found that CTO as determined by angiography is a strong predictor of fatal outcome. Of 19 patients with CTO, nine (47%) died early of fatal swelling, as compared with eight (15%) of 55 patients with occlusion in other areas.
The low recanalization rate of CTOs may be associated with the size and composition of these clots, which are thought to be large and rigid and composed of aged thrombotic material (20). Aging thrombi lose their intrinsically bound plasminogen, and retraction of the clot leads to chemical changes of the fibrin molecule, making it less reactive to proteolysis (20, 39). Furthermore, a large rigid clot will most likely occlude the carotid bifurcation, as this is the first significant normal arterial narrowing in the anterior circulation. Small emboli or large soft emboli will most likely squeeze through the narrow vascular segment of the ICA bifurcation or will fragment, resulting primarily in MCA occlusion.
Hemorrhage
Hemorrhagic transformation occurred in 38% of our patients after thrombolysis. The frequency of hemorrhage in other studies of carotid territory stroke treated with intraarterial thrombolysis has ranged from 17% to 56% (21, 22, 26, 37, 38, 40). In our study, no distinction was made between hemorrhagic infarction and parenchymatous hematoma. With respect to patient outcome, the important parameter was the frequency of symptomatic hemorrhage. Three (12%) of 26 patients in our study had symptomatic deterioration due to hemorrhage after thrombolysis, and in all three cases, this occurred within 24 hours of treatment; no symptomatic deterioration due to hemorrhage was seen after the initial 24 hours. Del Zoppo et al (26) reported symptomatic bleeding within 24 hours of treatment in 7% of their low-dose heparin group and in 15% of their overall population. In the patients receiving a placebo, no symptomatic deterioration occurred at 24 hours in the low-dose heparin group, and, in the overall placebo population, only one (7%) of 14 patients experienced symptomatic hemorrhage. However, by the end of the study, there was no significant difference between the rpro-UK and placebo groups in the frequency of hemorrhage with clinical deterioration.
All patients in our series received heparin during the thrombolytic procedure. This consisted of a 5000-unit bolus followed by 1000 units every hour thereafter to prevent thromboembolic complications related to the catheterization, and none occurred during our procedures. However, this may have contributed to the hemorrhagic complications that we observed. The potential contribution of concomitant heparin therapy to hemorrhagic transformation was examined in the PROACT study (26), and these researchers found that the high-dose heparin regimen resulted in a substantial increase in hemorrhagic transformation as compared with the placebo group (73% in the rpro-UK group versus 20% in the placebo group). Upon reduction of the heparin dose, the frequency of hemorrhagic complications within 24 hours of treatment dropped from 73% to 20% in the rpro-UK group and from 20% to 0% in the placebo group.
Hemorrhagic transformation is a common occurrence in the natural history of embolic stroke. Autopsy studies have shown that hemorrhagic transformation occurred in 51% to 71% of patients who died of embolic stroke (41–44). Fisher and Adams (44) described hemorrhagic transformation in 63 (51%) of 123 cases of cerebral embolism. In another autopsy study of 57 cases of brain embolism, two thirds were found to be hemorrhagic (45). In general, autopsy studies have established a high prevalence of hemorrhage after stroke. CT studies have shown a frequency of hemorrhagic transformation ranging from 5% to 43% (46–51). The very nature of postmortem data biases the conclusions toward the more serious infarcts, which most likely includes a larger percentage of patients with hemorrhage. Toni et al (50) reported that brain herniation accounted for 63% of deaths in their patients with hemorrhage as opposed to none in the patients who did not bleed. These authors contend that this might in part account for the higher rate of hemorrhage reported in autopsy series than in CT studies. In addition, petechial bleeding below the resolution of CT may account for the many more hemorrhagic transformations reported in postmortem studies.
Hemorrhage accompanying an infarct has been attributed to leakage from damaged vessels following recanalization or distal migration of the clot (44, 45, 52). We were concerned about the possible higher hemorrhagic rate among our patients in whom recanalization was successful. Overall, the number of patients with recanalization and subsequent hemorrhage was 45%, as compared with 33% of those without recanalization who hemorrhaged; however, the P value was .26, indicating that the difference was not significant. Other studies of intraarterial thrombolysis have also reported this lack of association between recanalization and hemorrhage (12, 21).
As found in other series, the time to treatment did not differ significantly in the hemorrhage (4.6 hours) versus nonhemorrhage groups (4.2 hours) (21). In patients with lenticulostriate occlusions, however, the time factor was more critical, although statistical significance could not be shown, owing to the small size of the populations. The time to treatment in patients with lenticulostriate occlusions and hemorrhage was 5.2 hours, versus 3.9 hours in those with lenticulostriate occlusions and no hemorrhage. The lenticulostriate arteries are terminal branches supplying the lenticular nuclei, with little possibility of collateral blood supply. After prolonged occlusion, the endothelium is thus likely to be damaged, with subsequent leakage and rupture of these vessels after reperfusion (22, 53).
We found a higher rate of mortality in the patients with hemorrhagic complications: 62% of patients with hemorrhage died, versus 23% of those without hemorrhage. Similarly, hemorrhage occurred more frequently in patients who died (63%) than in those who survived (28%). Hemorrhage in general has been found to be more frequently associated with a poor outcome or death after stroke. Kucinski et al (9) reported a 65% hemorrhagic transformation rate in their patients who died as compared with a 26% rate in the patients who survived. In another study, patients with hemorrhage more frequently incurred early neurologic deterioration than did those without hemorrhage (50). These patients also had a worse outcome in terms of mortality and residual deficit. By day 30, 77% of patients with hemorrhage had died or had major disability compared with 44% in the group without hemorrhage. In addition, in the hemorrhage group, 23% had normal neurologic findings or a nondisabling deficit at 30 days as compared with 56% of the nonhemorrhage patients.
In view of the worse outcome observed in patients with hemorrhage, identification of early predictors of bleeding might be of great value in planning interventional studies. Owing to the fact that CT is still the most widely used tool in daily clinical practice and in therapeutic trials, much has been published on the CT predictors of hemorrhagic transformation of stroke. Early focal hypodensity on CT scans has been reported to be predictive of an approaching hemorrhagic infarction. However, opinions on the predictability of early hypodensity as an indicator of hemorrhagic infarction remains controversial. Because of violation of the inclusion criteria in the “intent to treat” group in the European Cooperative Acute Stroke Study, hemorrhage-related complications were significantly associated with hypodensity of more than one third of the MCA territory (10). Toni et al (50) found signs of early hypodensity in 94% of their patients with hemorrhagic transformation as compared with 21% of those without hemorrhage. In fact, among baseline findings, logistic regression selected early focal hypodensity as the only independent predictor of hemorrhage. Bozzao et al (53) reported early CT hypodensity as a strong predictor of hemorrhagic transformation in ischemic stroke. Of 25 patients with early CT hypodensity, subsequent hemorrhage occurred in 18 (72%). Furthermore, hemorrhage did not occur in the 11 patients in whom hypodensity was not seen on early CT scans. The PROACT investigators reported similar findings, with hemorrhage developing in all their patients in whom hypodensity was exhibited in more than one third of the MCA territory (26). On the other hand, Kucinski et al (9) did not find a significant predictive value of early CT hypodensity, and Wolpert et al (12) found no relationship between early hypodensity and hemorrhagic transformation.
The CT scans in our series were reviewed by a neuroradiologist who was unaware of the clinical symptoms or follow-up CT results. The ability of CT hypodensity to predict hemorrhage and poor outcome was assessed. MESI was noted in eight (31%) of 26 patients. Of these eight patients, four (50%) suffered subsequent hemorrhage. This compares with six hemorrhages in the remaining 18 patients (33%) without MESI. Interestingly, no association was found between MESI and hemorrhage (κ = .16). Similarly, no association was found between MESI and outcome (κ = .16). We therefore did not find a significant correlation between CT hypodensity and poor outcome or hemorrhage.
Conclusion
Cerebral angiography and intraarterial thrombolysis in the setting of acute stroke are feasible and safe. We achieved successful reperfusion in 42% of patients, with a 48% overall good outcome. The patients with successful recanalization had a better outcome than those with unsuccessful recanalization (P = .02). Symptomatic hemorrhage occured in 12%. Hemorrhage following thrombolysis seems to occur in the area of infarction with the same frequency as it does in untreated patients. No hemorrhages occurred away from the site of infarction or systemically. No definite relationship was found between hemorrhage and recanalization or between hemorrhage and time to treatment, except in cases of lenticulostriate occlusion, in which the time factor appears to be more significant. Hemorrhage is related to higher doses of urokinase. Poor outcome or death is associated with nonrecanalization, older age, hemorrhage, left hemispheric stroke, and CTO. In our analysis of the CT scans, we found no relationship between hypodensity in more than one third of the MCA territory and poor outcome or hemorrhage.
Footnotes
Address reprint requests to Reza Jahan, MD, Interventional Neuroradiology, Department of Radiological Sciences, Center for the Health Sciences, Room B-220, UCLA School of Medicine, 10833 LeConte Ave, Los Angeles, CA 90095.
References
- 1.Wolf PA, Kannel WB, McGee DL. Epidemiology of strokes in North America. In: Barnett HJM, Stein BM, Mohr JP, Yatsu FM, eds. Stroke: Pathophysiology, Diagnosis and Management. New York: Churchill Livingstone; 1986:19–29
- 2.Chambers BR, Norris JW, Shurvell BL, Hachinski VC. Prognosis of acute stroke. Neurology 1987;37:221-225 [DOI] [PubMed] [Google Scholar]
- 3.Feussner JR, Matchar DB. When and how to study the carotids. Ann Intern Med 1988;109:805-818 [DOI] [PubMed] [Google Scholar]
- 4.Dobbin B. The economic impact of stroke. Neurology 1995;45(Suppl 1):S10-S14 [PubMed] [Google Scholar]
- 5.Barnaby W. Stroke intervention. Emerg Med Clin North Am 1990;8:267-281 [PubMed] [Google Scholar]
- 6.Zeumer H, Freitag HJ, Knospe V. Intravascular thrombolysis in central nervous system cerebrovascular disease. Neurol Clin North Am 1992;2:359-369 [Google Scholar]
- 7.del Zoppo GJ, Poeck K, Pessin M, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol 1992;32:78-86 [DOI] [PubMed] [Google Scholar]
- 8. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-1587 [DOI] [PubMed] [Google Scholar]
- 9.Kucinski T, Koch C, Gryzka U, Freitag HJ, Kromer H, Zeumer H. The predictive value of early CT and angiography for fatal hemispheric swelling in acute stroke. AJNR Am J Neuroradiol 1998;19:839-846 [PMC free article] [PubMed] [Google Scholar]
- 10.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke: the European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017-1025 [PubMed] [Google Scholar]
- 11. TIMI Study Group. Special report: the Thrombolysis in Myocardial Infarction (TIMI) Trial.. N Engl J Med 1985;312:932-936 [DOI] [PubMed] [Google Scholar]
- 12.Wolpert SM, Bruckmann H, Greenlee R, et al. Neuroradiologic evaluation of patients with acute stroke treated with recombinant tissue plasminogen activator. AJNR Am J Neuroradiol 1993;14:3-13 [PMC free article] [PubMed] [Google Scholar]
- 13.Fleiss J. Statistical Methods for Rates and Proportions.. 2nd ed. New York: Wiley; 1981:211–236
- 14.Saito I, Segawa H, Shiokawa Y, Taniguchi M, Tsutsumi K. Middle cerebral artery occlusion: correlation of computed tomography and angiography with clinical outcome. Stroke 1987;18:863-868 [DOI] [PubMed] [Google Scholar]
- 15.Hacke W, Zeumer H, Ferbert A, Bruckmann H, del Zoppo GJ. Intraarterial therapy improves outcome in patients with acute vertebrobasilar disease. Stroke 1988;19:1216-1222 [DOI] [PubMed] [Google Scholar]
- 16.Bruckmann H, Ferbert A, del Zoppo GJ, Hacke W, Zeumer H. Acute basilar thrombosis: angiologic-clinical comparison and therapeutic implications. Acta Radiol 1987;369(Suppl):38-42 [PubMed] [Google Scholar]
- 17.Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiographic correlation. Stroke 1977;8:383-391 [DOI] [PubMed] [Google Scholar]
- 18.Zeumer H, Hacke W, Ringelstein EF. Local intraarterial thrombolysis in vertebrobasilar thromboembolic disease. AJNR Am J Neuroradiol 1983;4:401-404 [PMC free article] [PubMed] [Google Scholar]
- 19.Zeumer H, Hundgen R, Ferbert A, Ringelstein EB. Local intraarterial fibrinolytic therapy in inaccessible internal carotid occlusion. Neuroradiology 1984;26:315-317 [DOI] [PubMed] [Google Scholar]
- 20.Zeumer H, Freitag HJ, Zanella F, Thie A, Arning C. Local intra-arterial fibrinolytic therapy in patients with stroke: urokinase versus recombinant tissue plasminogen activator (r-TPA). Neuroradiology 1993;35:159-162 [DOI] [PubMed] [Google Scholar]
- 21.Jansen O, von Kummer R, Forsting M, Hacke W, Sartor K. Thrombolytic therapy in acute occlusion of the intracranial internal carotid artery bifurcation. AJNR Am J Neuroradiol 1995;16:1977-1986 [PMC free article] [PubMed] [Google Scholar]
- 22.Theron J, Courtheoux P, Casasco A, et al. Local intraarterial fibrinolysis in the carotid territory. AJNR Am J Neuroradiol 1989;10:753-765 [PMC free article] [PubMed] [Google Scholar]
- 23.Barr JD, Mathis JM, Wildenhain SL, Wechsler L, Jungreis CA, Horton JA. Acute stroke intervention with intraarterial urokinase infusion. J Vasc Interv Radiol 1994;5:705-713 [DOI] [PubMed] [Google Scholar]
- 24.Moulin DE, Lo R, Chiang J, Barnett HJM. Prognosis in middle cerebral artery occlusion. Stroke 1985;7:282-284 [DOI] [PubMed] [Google Scholar]
- 25.Kaste M, Waltimo O. Prognosis of patients with middle cerebral artery occlusion. Stroke 1976;7:482-485 [DOI] [PubMed] [Google Scholar]
- 26.del Zoppo GJ, Higashida RT, Furlan AJ, Pessin MS, Rowley HA, Gent M. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT investigators: prolyse in acute cerebral thromboembolism. Stroke 1998;29:4-11 [DOI] [PubMed] [Google Scholar]
- 27.Kwiatkowski TG, Libman R, Frankel M, et al. The NINDS rt-PA stroke study: sustained benefit at one year. Stroke 1998;29:288 [Google Scholar]
- 28. The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997;28:2109-2118 [DOI] [PubMed] [Google Scholar]
- 29.Faught E, Trader SD, Hanna GR. Cerebral complications of angiography for transient ischemia and stroke: prediction of risk. Neurology 1979;29:4. [DOI] [PubMed] [Google Scholar]
- 30.Wardlaw JM, Warlow CP. Thrombolysis in acute ischemic stroke: does it work? Stroke 1992;23:1826-1839 [DOI] [PubMed] [Google Scholar]
- 31.Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Hieshima GB. Management of vascular perforations that occur during neurointerventional procedures. AJNR Am J Neuroradiol 1991;12:319-328 [PMC free article] [PubMed] [Google Scholar]
- 32.Mani RL, Eisenberg RL, McDonald EJ, Pollock JA, Mani JR. Complications of catheter cerebral arteriography: analysis of 5000 procedures, I: criteria and incidence. AJR Am J Roentgenol 1978;131:861-865 [DOI] [PubMed] [Google Scholar]
- 33.Mani RL, Eisenberg RL. Complications of catheter cerebral arteriography: analysis of 5000 procedures, II: relation of complication rates to clinical and arteriographic diagnosis. AJR Am J Roentgenol 1978;131:867-869 [DOI] [PubMed] [Google Scholar]
- 34.Mani RL, Eisenberg RL, McDonald EJ, Pollock JA, Mani JR. Complications of catheter cerebral arteriography: analysis of 5000 procedures, III: assessment of arteries injected, contrast medium used, duration of procedure, and age of patient. AJR Am J Roentgenol 1978;131:871-874 [DOI] [PubMed] [Google Scholar]
- 35.Fieschi C, Argentino C, Lenzi GL, Sacchetti ML, Toni D, Bozzao L. Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours.. Neurol Sci 1989;93:311-321 [DOI] [PubMed] [Google Scholar]
- 36.Solis OJ, Roberson GR, Taveras JM, Mohr J, Pessin M. Cerebral angiography in acute cerebral infarction. Rev Interam Radiol 1977;2:19-25 [PubMed] [Google Scholar]
- 37.Mori E, Tabuchi M, Yoshida T, Yamadori A. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke 1988;19:802-812 [DOI] [PubMed] [Google Scholar]
- 38.del Zoppo GJ, Ferbert A, Otis S, et al. Local intra-arterial fibrinolytic therapy in acute carotid territory stroke: a pilot study. Stroke 1988;19:307-313 [DOI] [PubMed] [Google Scholar]
- 39.Gottlob R. Die Grundlage für die Lyse alter Thromben. In: Pezold FA, ed. Fibrinolyse-Therapie Heute. Stuttgart: Schattauer; 1970:75–86
- 40.Ezura M, Kagawa S. Selective and superselective infusion of urokinase for embolic stroke. Surg Neurol 1992;38:353-358 [DOI] [PubMed] [Google Scholar]
- 41.Lodder J, Krijne-Kubat B, Broekman J. Cerebral hemorrhagic infarction at autopsy: cardiac embolic cause and the relationship to the cause of death. Stroke 1986;17:626-629 [DOI] [PubMed] [Google Scholar]
- 42.Jorgensen L, Torvik A. Ischemic cerebrovascular disease in an autopsy series, part 2. J Neurol Sci 1969;9:285-320 [DOI] [PubMed] [Google Scholar]
- 43.Adams RD, Vander Eecken HM. Vascular diseases of the brain. Ann Rev Med 1953;4:213-252 [DOI] [PubMed] [Google Scholar]
- 44.Fisher CM, Adams RD. Observations of brain embolism with special reference to mechanism of hemorrhagic infarction. J Neuropathol Exp Neurol 1951;10:92-94 [PubMed] [Google Scholar]
- 45.Fisher CM, Adams RD. Observations on brain embolism with special reference to hemorrhagic infarction. In: Furlan AJ, ed. The Heart and Stroke. New York: Springer; 1987:17–36
- 46.Fisher M, Zito T, Silva A, DeGirolami U. Hemorrhagic infarction: a clinical and CT study. Stroke 1984;15:192 [Google Scholar]
- 47.Hornig CR, Dorndorf W, Agnoli AL. Hemorrhagic cerebral infarction: a prospective study. Stroke 1986;17:179-185 [DOI] [PubMed] [Google Scholar]
- 48.Lodder J. CT detected hemorrhagic infarctions: relation with the size of the infarct, and the presence of midline shift. Acta Neurol Scand 1984;70:329-335 [DOI] [PubMed] [Google Scholar]
- 49.Okada Y, Yamaguchi T, Minematsu K, et al. Hemorrhagic transformation in cerebral embolism. Stroke 1989;20:598-603 [DOI] [PubMed] [Google Scholar]
- 50.Toni D, Fiorelli M, Bastianello S, et al. Hemorrhagic transformation of brain infarct: predictability in the first 5 hours from stroke onset and influence on clinical outcome. Neurology 1996;46:341-345 [DOI] [PubMed] [Google Scholar]
- 51.Weisberg LA. Nonseptic cardiogenic cerebral embolic stroke, clinical CT correlations. Neurology 1985;35:896-899 [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi T, Minematsu K, Choki J, et al. Clinical and neuroradiological analysis of thrombotic and embolic cerebral infarction. Jpn Circ J 1984;48:50-58 [DOI] [PubMed] [Google Scholar]
- 53.Bozzao L, Angeloni U, Bastianello S, Fantozzi LM, Pierallini A, Fieschi C. Early angiographic and CT findings in patients with hemorrhagic infarction in the distribution of the middle cerebral artery. AJNR Am J Neuroradiol 1991;12:1115-1121 [PMC free article] [PubMed] [Google Scholar]


