Abstract
Summary: We describe a rare case of multiple dural arteriovenous shunts (DAVSs) in a 5-year-old boy. MR imaging performed at 1 year of age showed only a dilated anterior part of the superior sagittal sinus; however, angiography at 5 years of age revealed an infantile-type DAVS there and two other DAVSs of the adult type. The pathophysiological evolution of DAVSs in children and their treatment strategies are discussed.
Dural arteriovenous shunts (DAVSs) are unusual in children. Their origin and pathophysiology are not yet well known, and treatment strategies are still controversial. We present a rare case of multiple DAVSs, each with different anatomic features, and discuss the pathophysiological evolution and treatment strategies for these lesions.
Case Report
A 5-year-old boy had developmental delay, which had been evident since infancy. MR imaging at 1 year of age showed dilatation of the anterior part of the superior sagittal sinus (Fig 1A and B), and no further investigation was performed at that time. The child could sit with no support at 12 months, started walking at 2 years, and uttered a few recognizable words at 4 years.
fig 1.
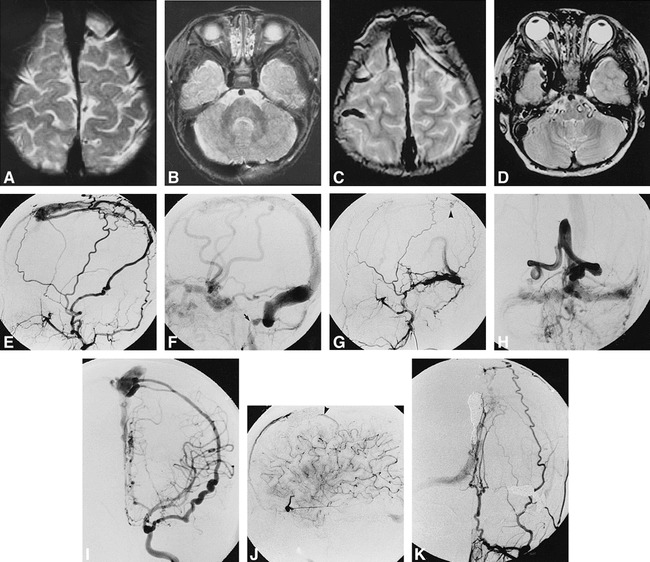
5-year-old boy with developmental delay and multiple DAVSs.
A and B, T2-weighted MR images (3200/80) at 1 year of age show dilatation of anterior part of the superior sagittal sinus.
C and D, T2-weighted MR images (3800/85) at 5 years of age show dilatation of cortical veins (C), posterior fossa veins, and right superior ophthalmic vein (D).
E and F, Right external carotid angiograms, lateral view, arterial (E ) and venous (F ) phase, show an infantile DAVS in the anterior part of the superior sagittal sinus. Venous drainage goes into the right jugular vein and via the cortical veins into the cavernous sinuses. Severe stenosis of the right jugular bulb is noted (arrow, F ).
G and H, Left external carotid angiograms, lateral view, arterial phase (G), and anteroposterior view venous phase (H), show adult-type DAVSs in the left transverse sinus and posterior part of the superior sagittal sinus (arrowhead, G). Venous drainage goes into the cavernous sinuses via the basal veins, right jugular vein, and spinal perimedullary veins through the posterior fossa veins. Occlusion of the left sigmoidal sinus is noted.
I, Left internal carotid angiogram, anteroposterior view, shows a direct AVS between the middle meningeal artery, originating from the ophthalmic artery, and the anterior part of the superior sagittal sinus.
J, After embolization, left internal carotid angiogram, lateral view, late arterial phase, shows a small DAVS in the anterior part of the superior sagittal sinus (arrowhead) supplied by the anterior falx branch of the ophthalmic artery.
K, After embolization, left external carotid angiogram, anteroposterior view, shows an adult-type DAVS in the posterior part of the superior sagittal sinus, which was not treated, and complete obliteration of the DAVS in the left transverse sinus.
At age 5 years, the patient's mother noticed discoloration around his eyes, nose, and mouth. A general physical examination revealed no evidence of cardiac failure, and chest radiographs showed no cardiac enlargement. Neurologic examination revealed moderate mental retardation. Bruit was heard over the cranial vault. An MR examination at this time revealed dilated cortical veins, deep basal veins, right superior ophthalmic vein, and posterior fossa veins (Fig 1C and D). There was no signal abnormality in the white matter. Angiography showed multiple intracranial DAVSs (Fig 1E–I). An infantile-type high-flow DAVS was present in the anterior part of the superior sagittal sinus. Arterial pedicles came from the right middle meningeal artery and left ophthalmic artery (Fig 1E and I). Pial arterial supply from the right anterior and posterior cerebral arteries was also noted. Venous drainage went both anterogradely, via the torcular into the right jugular vein, and retrogradely, via the cortical veins into the cavernous sinuses (Fig 1F). Two other adult-type DAVSs were present, supplied by the left external carotid artery, in the left transverse sinus and the posterior part of the superior sagittal sinus (Fig 1G). Venous drainage was into the cavernous sinuses via the basal veins, the right jugular vein, and the spinal perimedullary vein through the posterior fossa veins. Severe stenosis was present in the right jugular bulb and occlusion was seen in the left sigmoidal sinus. Venous drainage from both cerebral hemispheres was rerouted through the sylvian veins into the cavernous sinuses. Pial venous congestion was not definite, but the venous drainage transit time was longer than normal.
To reduce the shunt flow, embolization was performed in three stages, all with the patient under general anesthesia. In the first session, we performed transarterial embolization of the right middle meningeal artery using n-butyl 2-cyanoacrylate (NBCA), followed by transvenous embolization of the anterior part of the superior sagittal sinus with platinum coils from a transfemoral venous access. In the second session, transvenous embolization of the left transverse sinus was performed from the femoral vein. In the third session, a microcatheter was advanced just into the fistulous portion of the anterior superior sagittal sinus through the left ophthalmic artery, and the fistula was occluded with platinum coils. The DAVS in the posterior part of the superior sagittal sinus was not treated.
Postembolization angiography (Fig 1J and K) revealed complete obliteration of the DAVS in the left transverse sinus. There was a tiny residual DAVS in the anterior part of the superior sagittal sinus supplied by the anterior falx branches of the bilateral ophthalmic arteries, but the shunt flow was markedly decreased and the venous drainage transit time was shorter than that at angiography performed before treatment. The postoperative course was uneventful. The patient's clinical condition and discoloration around the eyes remained unchanged during the 6-month follow-up period.
Discussion
DAVSs in children are rare lesions, with few cases reported in the literature (1–11). However, it seems that some different entities with different anatomic or pathophysiological characteristics are included in them. Recently, considering the clinical history, anatomic features, and pathophysiological characteristics, Lasjaunias et al (12) divided DAVSs in children into three groups: 1) dural sinus malformation (DSM) with AVS, 2) infantile DAVS (IDAVS), and 3) adult-type DAVS. The DSM and IDAVS types are specific to children. The DSM is a developmental anomaly of the dural sinus and usually seen as a giant dural sinus lake. IDAVSs are the direct dural AV shunts with high flow and low venous sinus pressure. Adult-type dural AVSs are usually acquired lesions. The pathophysiological mechanism is the same as that in adults and the prognosis is usually excellent.
Typically, it is difficult to depict DAVSs directly with MR imaging, and only indirect manifestations, such as cortical venous dilatation or venous infarction caused by stenoocclusive change of venous outlets, can be seen (13, 14). In the present case, the initial MR study, performed at 1 year of age, showed only dilatation of the anterior part of the superior sagittal sinus, which indicated the existence of the IDAVS there, and there was neither cortical venous dilatation nor any evidence of the other DAVSs. However, angiography performed 4 years later revealed adult-type DAVSs in the left transverse sinus and posterior part of the superior sagittal sinus. Occlusion of the left sigmoidal sinus and severe stenosis of the right jugular bulb were also noted. Venous drainage was rerouted through the cavernous sinuses.
It is difficult to determine when and how the DAVSs in the left transverse sinus and posterior part of the superior sagittal sinus developed. There are some possible explanations for the pathogenesis. The venous sump effect produced by the persistence of the high flow in the sinus may have caused secondary AV shunts to develop (9, 11, 12). Another possibility is that some unknown remote factors triggered the development of multiple AV shunts in different locations individually (9, 12). Then, after the development of multiple DAVSs, thrombosis of the venous outlets progressed and the cortical venous dilatation became definite. On the other hand, it has been suggested that dural sinus thrombosis and venous hypertension play important roles in the pathogenesis of DAVS in adults (15–18). Therefore, it can be speculated that preexisting high-flow IDAVSs caused the restriction of venous outlets, and the elevated sinus pressure resulted in the development of secondary adult-type DAVSs. Evolution of both multifocal lesions and venous outlet obstruction may cause venous hypertension and lead to progressive neurologic deficits, venous infarction, hydrodynamic disorders, and a poor prognosis (11, 12).
The outcome for children with DAVS is poor. Morita et al (11) reported that the overall mortality in the literature, including their own cases, was 38%. They suggested that total surgical resection of the lesions provides a better result and should be considered in severe, symptomatic cases. It was also stated that preoperative transarterial and transvenous embolization is useful in reducing surgical risks.
On the other hand, Lasjaunias et al (12) suggested that, in patients with IDAVS, multiple sinuses are usually involved and transvenous sacrifice or surgical resection is not suitable. They prefer transarterial partial embolization using a permanent embolic material (NBCA), which offers remission of symptoms. However, the long-term outcomes were disappointing, as early recurrence of the shunts near the embolized regions was common.
Fortunately, in our patient, the IDAVS was located at the anterior part of the superior sagittal sinus; moreover, the left sigmoidal sinus was already occluded. Almost all the venous outflow, from both the AVS and normal brain, went into the cavernous sinuses and right jugular vein, where severe stenosis was recognized. Therefore, transvenous embolization could be performed successfully without deteriorating the venous drainage of the normal brain.
Conclusion
Our patient still has small residual AV shunts, and the long-term outcome is unknown; however, we believe that the reduction of AV shunt flow can lead to a better clinical prognosis by decreasing the hemodynamic stress on the venous outlets or by preventing further development of a new AVS or the progression of venous outlet restriction.
Footnotes
Address reprint requests to Satoshi Ushikoshi, MD.
References
- 1.Epstein BS, Platt N. Visualization of an intracranial arteriovenous fistula during angiocardiography in an infant with congestive heart failure. Radiology 1962;79:625-627 [Google Scholar]
- 2.Seljeskog EL, Rogers HM, French LA. Arteriovenous malformation involving the inferior sagittal sinus in an infant. J Neurosurg 1968;29:623-628 [DOI] [PubMed] [Google Scholar]
- 3.Robinson JL, Sedzimir CB. External carotid-transverse sinus fistula: case report. J Neurosurg 1970;33:718-720 [DOI] [PubMed] [Google Scholar]
- 4.Gordon IJ, Shah BL, Hardman DR, Chameides L. Giant dural supratentorial arteriovenous malformation. AJR Am J Roentgenol 1977;129:734-736 [DOI] [PubMed] [Google Scholar]
- 5.Tsugane R, Sato O, Watabe T. Non-communicating hydrocephalus caused by dural arteriovenous malformation. Surg Neurol 1979;12:393-396 [PubMed] [Google Scholar]
- 6.Albright AL, Latchaw RE, Price RA. Posterior dural arteriovenous malformations in infancy. Neurosurgery 1983;13:129-135 [DOI] [PubMed] [Google Scholar]
- 7.Ross DA, Walker J, Edwards MSB. Unusual posterior fossa dural arteriovenous malformation in a neonate: case report. Neurosurgery 1986;19:1021-1024 [DOI] [PubMed] [Google Scholar]
- 8.Chan S-T, Weeks RD. Dural arteriovenous malformation presenting as cardiac failure in a neonate. Acta Neurochir (Wien) 1988;91:134-138 [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Monaco R, Rodesch G, Terbrugge K, Burrows P, Lasjaunias P. Multifocal dural arteriovenous shunts in children. Childs Nerv Syst 1991;7:425-431 [DOI] [PubMed] [Google Scholar]
- 10.Cataltepe O, Berker M, Gurcay O, Erbengi A. An unusual dural arteriovenous fistula in an infant. Neuroradiology 1993;35:394-397 [DOI] [PubMed] [Google Scholar]
- 11.Morita A, Meyer FB, Douglas A, Nichols A, Patterson MC. Childhood dural arteriovenous fistulae of the posterior dural sinuses: three case reports and literature review. Neurosurgery 1995;37:1193-1200 [DOI] [PubMed] [Google Scholar]
- 12.Lasjaunias P, Magufis G, Goulao A, et al. Anatomoclinical aspects of dural arteriovenous shunts in children: review of 29 cases. Intervent Neuroradiol 1996;2:179-191 [DOI] [PubMed] [Google Scholar]
- 13.De Marco JK, Dillon WP, Halbach VV, Tsuruda JS. Dural arteriovenous fistulas: evaluation with MR imaging. Radiology 1990;175:193-199 [DOI] [PubMed] [Google Scholar]
- 14.Chen JC, Tsuruda JS, Halbach VV. Suspected dural arteriovenous fistula: results with screening MR angiography in seven patients. Radiology 1992;183:265-271 [DOI] [PubMed] [Google Scholar]
- 15.Chaudhary MY, Sachdev VP, Cho SH, Weitzner I, Puljic S, Huang YP. Dural arteriovenous malformation of the major venous sinuses: an acquired lesion. AJNR Am J Neuroradiol 1982;3:13-19 [PMC free article] [PubMed] [Google Scholar]
- 16.Nishijima M, Takaku A, Endo S, et al. Etiological evaluation of dural arteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg 1992;76:600-606 [DOI] [PubMed] [Google Scholar]
- 17.Herman JM, Spetzler RF, Bederson JB, Kurbat JM, Zabramski JM. Genesis of a dural arteriovenous malformation in a rat model. J Neurosurg 1995;83:539-545 [DOI] [PubMed] [Google Scholar]
- 18.Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg 1994;80:884-889 [DOI] [PubMed] [Google Scholar]


