Abstract
Summary: A 49-year-old woman had a small saccular aneurysm that was incompletely occluded with a Guglielmi detachable coil (GDC). She died from rupture of another aneurysm 42 days after the treatment. Autopsy for the embolized aneurysm revealed no neoendothelium at the aneurysmal neck, but an organized thrombus was observed limited to the periphery of the aneurysmal lumen. Although isolation of the aneurysm was not apparent, loose embolization with this method may help to reinforce the aneurysmal wall.
Embolization with Guglielmi detachable coils (GDCs) has become accepted as an effective and safe method for preventing rehemorrhage of ruptured cerebral aneurysms (1–3), but few clinical reports concerning tissue response in successfully treated aneurysms have been published (4, 5). We describe histologic findings in a small aneurysm that was incompletely occluded with a GDC.
Case Report
A 49-year-old hypertensive woman had been clipped for a ruptured right middle cerebral, unruptured right anterior choroidal, and left middle cerebral arterial aneurysm in 1992. On July 5, 1997 she presented with a sudden onset of severe headache, vomiting, and generalized convulsion. When admitted for treatment, she was hypertensive, drowsy and had preretinal hemorrhage. Laboratory data, including blood coagulability, were normal. CT findings showed subarachnoid hemorrhage located in the interhemispheric fissure and left ambient cistern. Angiography revealed no abnormality at the operated sites, but a small saccular aneurysm, considered to have developed de novo, was detected at the basilar-superior cerebellar artery bifurcation just distal to the connection with the primitive trigeminal artery (PTA) (Fig 1A).
fig 1.

A 49-year-old woman with a small saccular aneurysm.
A, Anteroposterior carotid angiogram shows the PTA (arrow) and a small saccular aneurysm at the basilar-superior cerebellar bifurcation (arrowhead).
B, Angiogram, after embolization with a GDC, shows partial opacification of the fundus of the aneurysm.
The aneurysm had a broad neck. Endovascular treatment for this lesion was performed with GDCs 2 days after the onset of symptoms. A GDC Tracker 18 catheter was placed in the aneurysm through the PTA via the right internal carotid artery, and a GDC (4 mm × 8 cm) was introduced into the fundus of the lesion. No other coil could be applied because of migration to the parent artery through the broad neck. At completion of the treatment, the fundus of the aneurysm was partially opacified (Fig 1B).
After endovascular treatment, the patient's condition was maintained in a normotensive state, and she regained consciousness gradually. She suffered intracerebral hemorrhage in the right frontal lobe twice, however, and died 42 days after GDC occlusion. Gross examination of the brain showed severe swelling with diffuse subarachnoid hemorrhage. At the circle of Willis, a small (2-mm diameter) ruptured saccular anterior communicating aneurysm was found that was probably the source of the fatal hemorrhage. The three aneurysms to which clips were applied and the basilar-superior cerebellar aneurysm showed no evidence of rupture. At the basilar-superior cerebellar small aneurysm (maximum and neck diameters, 3 mm) the coil could be observed through the thin wall. The inner lumen of the aneurysm was filled with the coil and clots, but no endothelial formation was found at the aneurysmal orifice. Microscopic observation of the aneurysm revealed the thrombus at the center of the aneurysmal lumen lacked tissue organization. On the other hand, an organized thrombus with capillaries, fibrous connective tissue, and infiltrating inflammatory cells existed at the margin of the aneurysmal lumen (Fig 2 A, B).
fig 2.
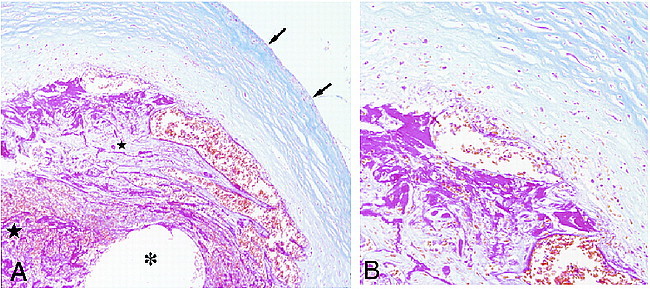
Microscopic examination of the small saccular aneurysm after GDC occlusion.
A, Low-power photomicrograph shows a cross-section of the aneurysm at the fundus, with a fibrotic component (small star) adjacent to the aneurysmal wall (arrows) and an unformed thrombus at the center of the aneurysm (large star). The location of the coil is indicated (asterisk). (Azan stain; magnification ×100)
B, Higher power magnification of the region adjacent to the wall shows fibrous connective tissue with rich capillaries. (Azan stain; magnification ×200)
Discussion
In experimental animal models, complete isolation of aneurysms from the parent circulation, with neointima at the orifice and organized thrombi filling the fundus, has been reported after GDC treatment (6–8). Such ideal findings were observed for experimental side-wall aneurysms 2 to 6 months after the procedure (6–8). Spetzger et al, however, have questioned the effectiveness of this method (9). Although these investigators employed GDCs for experimental bifurcation aneurysms which evince very similar hemodynamic patterns to human terminal portion aneurysms, they failed to obtain perfect isolation of these aneurysms even though complete occlusion was revealed by angiography (9). Clinical findings of giant bifurcation aneurysms treated with GDCs showed no formation of new endothelial layers at the orifices of aneurysms, and organized thrombi were mainly observed around the margins of the aneurysms 2 and 6 months after the treatment (5). In another case, an electron microscopic study showed thin layers of fibrin-covered coils and neoendothelium at the edge of the aneurysmal neck in a small aneurysm 4 weeks after coiling, which suggested subsequent isolation of the aneurysm (4). Thus, the reported histologic effects of GDC occlusion vary in experimental models and clinical cases of angiographically occluded aneurysms. In the Tenjin study, experimental observation of postoperative histologic changes showed accumulation of white blood cells and fibroblasts in clots around coils at 4 days, fibroblast invasion of clots at 2 weeks, and organizing aneurysms with media-like structures at 3 months (8). This study reported similar aneurysmal characteristics and degree of coiling to our case, except side-wall aneurysms were used in the experimental model. The experimental results may therefore predict the stages of histologic changes in the clinical setting, in aneurysms such as ours. Our case showed thrombic formation with rich capillaries and fibrous connective tissues reflecting endothelial organization adjacent to the aneurysmal wall. This organization was present very soon after the procedure in spite of loose coil application and unfavorable factors such as a thin aneurysmal wall with poor vascularity, direct blood flow associated with the PTA, a broad neck of the lesion, and hypertension. Although occlusion of bifurcation aneurysms was not confirmed histologically or angiographically (10), this case suggests that even flawed application of GDCs may reinforce the aneurysmal wall. This may be most evident shortly after GDC application in small aneurysms, and may be useful in those cases that preclude other treatment options. Additional histologic review of such cases is needed to indicate how degree of coiling, physical features of aneurysms (shape, size, anatomical location), the patient's general condition, and the period after the procedure are related.
Footnotes
Address reprint requests to Satoru Shimizu,1-15-1 Kitasato, Sagamihara, Kanagawa, 228-8555 Japan.
References
- 1.Graves VB, Strother CM, Duff TA, Perl J. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery 1995;37:640-648 [DOI] [PubMed] [Google Scholar]
- 2.Guglielmi G, Viñuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg 1991;75:8-14 [DOI] [PubMed] [Google Scholar]
- 3.Malisch TW, Guglielmi G, Viñuela F, et al. Intracranial aneurysms treated with the Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg 1997;87:176-183 [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MB, Purdy PD, Burns D, Bellotto D. Scanning electron microscopic findings in a basilar tip aneurysm embolized with Guglielmi detachable coils. . AJNR Am J Neuroradiol 1997;18:688-690 [PMC free article] [PubMed] [Google Scholar]
- 5.Molyneux AJ, Ellison DW, Morris J, Byrne JV. Histological findings in giant aneurysms treated with Guglielmi detachable coils. J Neurosurg 1995;83:129-132 [DOI] [PubMed] [Google Scholar]
- 6.Guglielmi G, Viñuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg 1991;75:1-7 [DOI] [PubMed] [Google Scholar]
- 7.Mawad ME, Mawad JK, Cartwright J Jr, Gokaslan Z. Long-term histopathologic changes in canine aneurysms embolized with Guglielmi detachable coils. AJNR Am J Neuroradiol 1995;16:7-13 [PMC free article] [PubMed] [Google Scholar]
- 8.Tenjin H, Fushiki S, Nakahara Y, et al. Effect of Guglielmi detachable coils on experimental carotid artery aneurysms in primates. Stroke 1995;26:2075-2080 [DOI] [PubMed] [Google Scholar]
- 9.Spetzger U, Reul J, Weis J, Bertalanffy H, Thron A, Gilsbach JM. Microsurgically produced bifurcation aneurysms in a rabbit model for endovascular coil embolization. J Neurosurg 1996;85:488-495 [DOI] [PubMed] [Google Scholar]
- 10.Reul J, Spetzger U, Weis J, Sure U, Gilsbach JM, Thron A. Endovascular occlusion of experimental aneurysms with detachable coils: influence of packing density and perioperative anticoagulation. Neurosurgery 1997;41:1160-1168 [DOI] [PubMed] [Google Scholar]


