Abstract
BACKGROUND AND PURPOSE: T1-weighted MR images show high signal intensity in the pallidum of many patients with liver cirrhosis. The purpose of this study was to evaluate quantitative changes in MR signals in patients with liver cirrhosis by using the magnetization transfer technique.
METHODS: Magnetization transfer ratios were measured in seven different regions of the brain in 37 patients with liver cirrhosis and in 37 healthy volunteers.
RESULTS: The magnetization transfer ratios in patients with liver cirrhosis were significantly lower than those in control subjects in the globus pallidus, putamen, thalamus, corona radiata, and subcortical white matter.
CONCLUSION: Abnormal magnetization transfer ratios may be found in otherwise normal-appearing cerebral regions.
T1-weighted MR images show high signal intensity in the pallidum of many patients with liver cirrhosis (1–4). It was recently suggested that this abnormality may be related to an accumulation of manganese (2–4). We previously reported that the magnetization transfer ratio (MTR) is decreased in the pallidum of patients with liver cirrhosis (4). Norenberg (5, 6) has shown that histologic changes occur in the cortex, thalamus, and pallidum of patients with cirrhosis. In the present study, we investigated the MTR values in various regions of the brain in patients with liver cirrhosis.
Methods
The study included 37 Japanese patients with liver cirrhosis (24 men and 13 women with a mean age ± SD of 63 ± 11 years). None of the patients exhibited overt hepatic encephalopathy at the time of examination. The cause of liver cirrhosis was viral infection in 30 patients (type B in two patients and type C in 28 patients) and alcoholism in seven patients. There were no cases of cirrhosis due to metabolic or autoimmune disease. Child's classification of the patients was as follows: group A, 24 patients; group B, 10 patients; and group C, three patients (7). Thirty-three patients had no clinical history of hepatic encephalopathy, two had a single time-limited episode, and two had recurrent episodes. At the time of the study, 11 patients were receiving oral disaccharides and branched-chain amino acids. Details of their profile were reported previously (4).
The control group consisted of 37 age-matched healthy volunteers. Informed consent was obtained from all patients, and the study was performed in accordance with the Helsinki Declaration.
A Gyroscan ACS-NT MR scanner (Philips, Best, Netherlands) with a magnetic field strength of 1.5 T was used. The 3D fast-field echo (FFE) technique and the magnetization transfer contrast (MTC) technique were used to obtain MTC-FFE images. Fifteen coronal sections were made, each 3 mm thick. The pallidum was placed in the center section. Other imaging conditions were 50/3.5/1 (TR/TE/excitations); flip angle, 20°; matrix, 256 × 256; and field of view, 230 mm, as described previously (4). The off-resonance pulse was centered 1100 Hz below the water frequency with a duration of 15 milliseconds. This pulse was in the shape of the sinc function, with an average field intensity equal to 620°. The interval between the end of the saturation pulses and the beginning of each excitation was approximately 1 millisecond. To measure the signal intensity, a radiologist who was unaware of the subjects' backgrounds set a region of interest of 3 mm in diameter in the globus pallidus, putamen, thalamus, hippocampus, corona radiata, parietal gray matter, and subcortical parietal white matter. The MTRs in seven different cerebral regions were calculated by using the following formula:
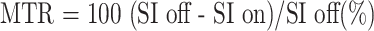 |
where SI off is the signal intensity of FFE images in various regions of the brain, and SI on is the signal intensity of MTC-FFE images. The MTR was defined as the mean of the right and left sides.
Data are expressed as the mean ± SD. The significance of differences between the two groups was evaluated by the Wilcoxon rank test. A level of P < .05 was accepted as statistically significant.
Results
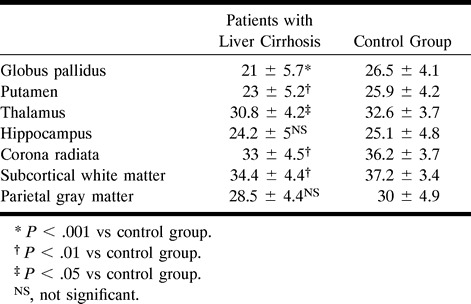
The MTR values in patients with liver cirrhosis were significantly lower than those in control subjects in the globus pallidus (P < .001), putamen (P < .01), thalamus (P < .05), corona radiata (P < .001), and subcortical parietal white matter (P < .01). However, no measurable differences were observed in the hippocampus or parietal gray matter (see Table).
Magnetization transfer ratio values in various regions of the brain
Discussion
MTC, a new MR imaging technique based on the interactions between macromolecules and water, provides quantitative molecular-based information about tissues (8–13). Measurement of MTR is increasingly being used in the evaluation of disorders of the CNS (8–12). In a previous study, we found that manganese reduces MTR in manganese chloride phantoms and that MTR of the pallidum decreases with severity of the liver disease (as reflected by the serum total bilirubin, indocyanine green 15-minute retention rate, and plasma ammonia) (4, 13). Homeostasis of manganese is maintained by rapid transport into tissue and enterohepatic circulation (2, 3). In patients with liver cirrhosis, manganese is increased in the systemic circulation and deposited in the pallidum, owing to portosystemic shunts and decreased hepatic clearance.
T1 shortening in the pallidum of patients with liver cirrhosis is thought to be caused by the paramagnetic effect of manganese. It is unclear how manganese deposits can cause reduction of MTR in other areas of the brain without altering T1 relaxation. Histopathologic studies have previously demonstrated that, in regions in which MR changes occur, there is an increased proliferation of Alzheimer type II astrocytic cells (5, 6). Results reported by Norenberg (5, 6) have shown that astrocytic changes are predominantly observed in the basal ganglia, cortex, and thalamus, and that they are present to a lesser extent in the brain stem and spinal cord, and virtually absent in the hippocampus. He suggested that Alzheimer type II astrocytes represent the final stage of astrocytic changes in hepatic encephalopathy caused by advanced liver disease or hyperammonemia. The early phases of astrocytic changes are characterized by cytoplasmic hypertrophy, increased mitochondria, dense endoplasmic reticulum, and increased water content (5, 6). This increase in water content possibly decreases the amount of MT by diluting the number of structural protons in tissues, which may explain the reduced MTR values. However, the results reported by Norenberg regarding the histologic findings of astrocytosis in patients with liver cirrhosis are not completely consistent with our present MTR findings.
Myelinated fibers have demonstrated histologic degeneration with fragmentation of axis cylinders in chronic hepatocellular dysfunction. Dousset et al (11) reported that the MTRs are significantly decreased in white matter lesions in patients with multiple sclerosis. These authors hypothesized that lesions varied in MTR in proportion to the extent of myelin loss. Tomiak et al (12) also reported that the range of MTR values reflect the structural changes in the tissue resulting from demyelination and axonal loss. Demyelination may be another causative factor of low MTR of corona radiata and white matter.
Conclusion
Measurement of MTR throughout the brain of patients with liver cirrhosis has not been reported previously. The mechanism of regional decrease in the MTR values is unclear. The results of the present study suggest that microscopic abnormalities, which are not detectable by conventional MR imaging, occur in patients with subclinical hepatic encephalopathy. Reduced MTR may indicate manganese deposits, demyelination, Alzheimer type II astrocytosis, and/or increased water content in astrocytes.
Footnotes
Address reprint requests to Motoh Iwasa, MD, Third Department of Internal Medicine, Mie University School of Medicine, 514–8507 Edobashi 2–174, Tsu City, Mie, Japan.
References
- 1.Inoue E, Hori S, Narumi Y, et al. Porto-systemic encephalopathy: presence of basal ganglia lesions with high signal intensity on MR images. Radiology 1991;179:551-555 [DOI] [PubMed] [Google Scholar]
- 2.Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet 1995;346:270-274 [DOI] [PubMed] [Google Scholar]
- 3.Spahr L, Butterworth RF, Fontaine S, et al. Increased blood manganese in cirrhotic patients: relationship to pallidal magnetic resonance signal hyperintensity and neurological symptoms. Hepatology 1996;24:1116-1120 [DOI] [PubMed] [Google Scholar]
- 4.Iwasa M, Kinosada Y, Watanabe S, et al. Hepatic cirrhosis: magnetization transfer contrast in the globus pallidus. Neuroradiology 1998;40:145-149 [DOI] [PubMed] [Google Scholar]
- 5.Norenberg MD. The astrocyte in liver disease. Adv Cell Neurobiol 1981;2:303-352 [Google Scholar]
- 6.Norenberg MD. The role of astrocytes in hepatic encephalopathy. J Neurochem Pathol 1987;6:13-29 [DOI] [PubMed] [Google Scholar]
- 7.Pugh RNH, Murray-Lyon IM, Petroni MC, et al. Transection of the oesophagus by bleeding oesophageal varices. Br J Surg 1973;60:646-649 [DOI] [PubMed] [Google Scholar]
- 8.Lundbom N. Determination of magnetization transfer contrast in tissue: an MR imaging study of brain tumor. AJR Am J Roentgenol 1992;159:1279-1285 [DOI] [PubMed] [Google Scholar]
- 9.Matsushima S, Muroka M, Uchiyama Y, et al. Evaluation of basal ganglia in patients with hepatocellular carcinoma using magnetization transfer. Jpn J Magn Reson Med 1996;16:121-128 [Google Scholar]
- 10.Mehta RC, Pike GB, Enzmann DR. Measure of magnetization transfer in multiple sclerosis demyelinating plaques, white matter ischemic lesions, and edema. AJNR Am J Neuroradiol 1996;17:1051-1055 [PMC free article] [PubMed] [Google Scholar]
- 11.Dousset V, Grossman RI, Ramer KN, et al. Experimental allergic encephalomyelitis and multiple sclerosis: lesion characterization with magnetization transfer imaging. Radiology 1992;182:483-491 [DOI] [PubMed] [Google Scholar]
- 12.Tomiak MM, Rosenblum JD, Prager JM, Metz CE. Magnetization transfer: a potential method to determine the age of multiple sclerosis lesions. AJNR Am J Neuroradiol 1994;15:1569-1574 [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushima S, Kinosada Y. Quantitative evaluation and clinical application of magnetization transfer contrast using fast spin echo. Jpn J Med Electronics 1995;33:283-287 [Google Scholar]


