Abstract
BACKGROUND AND PURPOSE: Proton MR spectroscopy has recently been applied to the evaluation of seizures, but few comparisons have been made between different clinical spectroscopic techniques. Our goal was to determine whether there is a significant difference between hippocampal NAA/(Cho+Cr) ratios obtained by single-voxel spectroscopy (SVS) and by chemical-shift imaging (CSI).
METHODS: Twelve healthy adults and eight patients with complex partial seizures were studied on a 1.5-T MR scanner using a proton SVS method. Another 12 healthy adults and 10 patients with complex partial seizures were recruited for a proton CSI study, which was performed on a different 1.5-T MR system. The NAA/(Cho+Cr) ratio was calculated from the integral peak areas by curve fitting. The two-tailed t-test was used for statistical analysis.
RESULTS: The mean value ± standard deviation of the hippocampal NAA/(Cho+Cr) ratio in healthy control subjects was 0.63 ± 0.07 by SVS, with 0.62 ± 0.15 for the anterior hippocampus and 0.65 ± 0.11 for the posterior hippocampus by CSI. There was no significant difference between the control group data obtained by SVS and those by CSI, nor was there a regional difference in the CSI NAA/(Cho+Cr) ratio in the hippocampus. Relative to the control group, the patients with seizures had a significant decrease in the NAA/(Cho+Cr) ratio in the abnormal hippocampus: −28% by SVS, and −24% in the anterior hippocampus and −18% in the posterior hippocampus by CSI. Proton SVS and CSI detected hippocampal abnormalities, unilateral or bilateral, in all patients of each group.
CONCLUSION: Under similar measurement conditions, proton SVS and CSI provide similar NAA/(Cho+Cr) ratios among healthy control subjects, and they possess comparable ability for detecting hippocampal abnormalities in patients with complex partial seizures.
MR spectroscopy can be used to detect the presence of cerebral metabolites and to measure their amounts noninvasively (1). In vivo proton MR spectroscopy is now being applied in many clinical fields owing to its easy integration into clinical MR systems and the high magnetization and natural abundance of protons (1, 2). In addition to the structural information provided by MR imaging, MR spectroscopy can give spatially encoded biochemical information. There are many metabolites readily identified by proton MR spectroscopy, including N-acetylaspartate (NAA), choline-containing compounds (Cho), creatine-phosphocreatine (Cr), lactate, glutamine-glutamate, and myo-inositol (2). The two different techniques for spatially defining MR spectroscopy are single-voxel spectroscopy (SVS) (3, 4) and chemical-shift imaging (CSI) (5, 6). Generally speaking, SVS has the advantages of more explicit spatial localization, more homogeneous shimming, better water suppression, and shorter acquisition time; but only one spectrum can be obtained from one data acquisition (3–7). On the other hand, CSI has the advantage of obtaining multiple spectra simultaneously during one measurement. This technique is useful for evaluating an area or a lesion with multiple regions of interest. However, the postprocessing of CSI data is more complex (3–7).
Both proton SVS (8–11) and CSI (12–17) have been used to evaluate metabolic abnormalities in patients with complex partial seizures, especially those with mesial temporal sclerosis. A decrease in hippocampal NAA intensity has been demonstrated in most patients and indicates the neuronal loss underlying mesial temporal sclerosis (8–18). Increased intensities of Cho or Cho+Cr have also been reported and probably reflect the associated astrocytosis in the affected hippocampus (8, 10, 15, 16). Although the ability of proton SVS and CSI to detect metabolic abnormalities in seizure patients is reportedly satisfactory, diverse quantitative or semiquantitative results due to different measurement techniques, acquisition parameters, and data presentation have been noted (8, 11–17). This methodological inconsistency may hinder the widespread application of MR spectroscopy in clinical practice and prevent comparison of data from different research centers (19).
Using similar parameters for spectral acquisition and consistent positioning of the volume of interest (VOI), we compared the semiquantitative data obtained by proton SVS with those obtained by proton CSI. Our purpose was to compare hippocampal NAA/(Cho+Cr) ratios measured by these two different MR spectroscopic techniques to evaluate their effectiveness in detecting hippocampal abnormalities in patients with complex partial seizures.
Methods
Eight patients (five men and three women, ages 15 to 36 years) with symptoms of complex partial seizures and 12 healthy volunteers (eight men and four women, ages 25 to 34 years) were recruited for the proton SVS study. Another 10 patients (six men and four women, ages 15 to 54 years) with a diagnosis of complex partial seizures and 12 healthy adults (six men and six women, ages 20 to 35 years) were included in the proton CSI study. All patients underwent neurologic examination, neuropsychological evaluation, and interictal scalp electroencephalography (EEG), including sphenoidal electrodes. Informed consent was obtained from all the subjects before the start of the study.
MR Imaging and Proton SVS Study
For this group, MR studies were performed on a 1.5-T whole-body MR scanner (Signa, General Electric, Milwaukee, WI) using the standard quadrature bird cage head coil. The MR imaging study consisted of sagittal and transaxial T1-weighted images (450–550/12–30/1–2 [TR/TE/excitations], 5-mm section thickness, 2.5-mm intersection gap, 256 × 192 image matrix), transaxial T2-weighted images (3000–3500/90/1–2, 5-mm section thickness, 2.5-mm intersection gap, 256 × 192 image matrix), and oblique coronal T2-weighted images perpendicular to the long axis of the hippocampus (4000/102/4, 3-mm interleaved scan, 256 × 256 image matrix). Atrophy of the hippocampus by visual inspection or hyperintensity inside the hippocampus on T2-weighted images was taken as criteria for the diagnosis of mesial temporal sclerosis. For the volunteers, only transaxial T2-weighted images were obtained, and any intracranial abnormality would exclude the subject from the study.
The proton SVS study was performed in a separate MR examination session without knowledge of the results of EEG and MR imaging. A series of coronal T2-weighted images of the hippocampus (3000/85/1, 3-mm section thickness, 1.5-mm intersection gap, 256 × 192 image matrix) was obtained for localization. A 20 × 20 × 20 mm3 VOI was placed over the anterior portion of the hippocampus to encompass part of the hippocampal head and body (Fig 1A). The position was meticulously adjusted and repeatedly checked in each coronal image to include as much of the hippocampus as possible so as to decrease the degree of tissue contamination. Care was taken to ensure the standard placement in all subjects, and positioning was performed by a single neuroradiologist for technique consistency. In this study, water-suppressed SVS was applied by the method of point-resolved spectroscopy (20). After automated transmitter and receiver adjustment, the signal over the VOI was shimmed to within a linewidth of 3 to 5 Hz. Optimal water suppression, 98% to 99%, was achieved by preirradiation of the water resonance by applying three chemical-shift selected pulses (21) and spoiled gradients. A proton spectrum was then acquired using parameters of 1600/135/256, a spectral width of 2500 Hz, and data points of 2K. The whole procedure was repeated on the contralateral hippocampus. The acquisition time for each hippocampus was 7 minutes 52 seconds. The total time required in the magnet was 30 to 40 minutes.
fig 1.
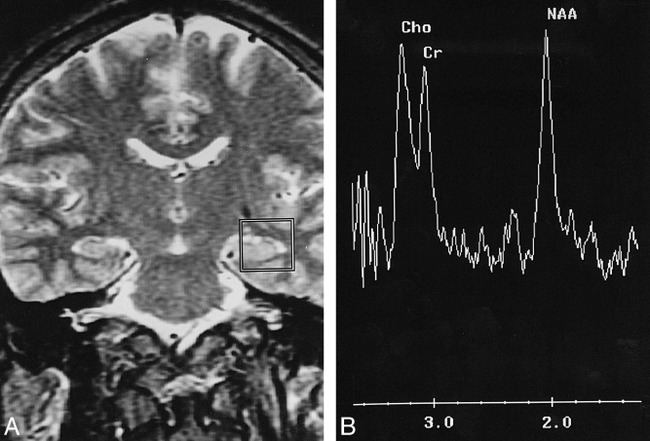
Proton SVS study of the left hippocampus of a healthy adult.
A, This coronal T2-weighted image (3000/85/1) is the central section for voxel positioning, about 1 to 2 mm anterior to the ipsilateral internal acoustic canal. The voxel is 20 × 20 × 20 mm3. Part of the hippocampal head and body are included for measurement.
B, There are three major peaks in this proton spectrum: NAA at 2.0 ppm, Cr at 3.0 ppm, and Cho at 3.2 ppm. The NAA peak is usually, but not necessarily, higher than the other two.
The raw data were transferred to an off-line workstation (Sparc 20, Sun Microsystems, Mountain View, CA), and automated postprocessing was performed using a spectroscopic analysis package (SA/GE, General Electric) according to the following steps: 1.0-Hz exponential apodization, zero-filling to 8K, baseline correction, Fourier transformation, auto-phasing, location of the creatine peak at 3.0 parts per million (ppm), and acquisition of the peak integrals by curve fitting. The NAA peak could be identified at 2.0 ppm, the Cr peak at 3.0 ppm, and the Cho peak at 3.2 ppm. The integral value of each peak was dimensionless and represented relative measurement of the amount of each metabolite. The semiquantitative result of each hippocampus was expressed as NAA/(Cho+Cr) ratio. The time required for data postprocessing was less than 10 minutes.
MR Imaging and Proton CSI Study
For this group, MR studies were performed on another 1.5-T clinical MR system (Magnetom Vision, Siemens, Erlangen, Germany), using a standard circularly polarized head coil. The MR imaging study was similar to that described for the SVS study group.
Proton CSI of the hippocampus was acquired in a separate examination session without knowledge of the results of EEG and MR imaging. A series of orthogonal T2-weighted images for localization were obtained by using the fast imaging with steady-state precession sequence (6.32/3.00/2, 70° flip angle, 5- or 7-mm section thickness, 5- or 7-mm intersection gap, and 200 × 256 image matrix). The transaxial CSI plane was approximately parallel to the long axis of the hippocampus. The VOI, about 20 × 40 × 15 mm3 in size, was positioned over one hippocampus for each measurement (Fig 2A). The position was carefully checked and meticulously adjusted in all three orthogonal sections to ensure standard placement in all subjects. After automated shim and water suppression, consecutive two-dimensional proton CSI was performed in each hippocampus using a spin-echo sequence. The acquisition parameters included 1500/135/2, a field of view of 16 × 16 cm2, a phase encoding of 16 × 16, and data points of 1024. The resultant voxel size was 10 × 10 × 15 mm3. The acquisition time for each hippocampus was 12 minutes 55 seconds. The total time required in the magnet was 40 to 50 minutes.
fig 2.
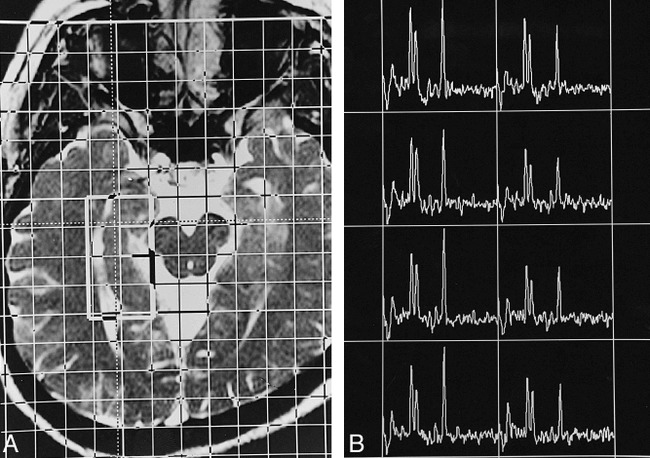
Proton CSI study of the right hippocampus of a healthy adult.
A, This transverse T2-weighted MR image (6.32/3.00/2), approximately parallel to the long axis of the hippocampus, illustrates the position of the VOI (about 40 × 20 mm2) inside the field of view (160 × 160 mm2), which has 16 × 16 phase encoding. The section thickness is 15 mm, the in-plane resolution is 10 × 10 mm2, and the voxel size is 10 × 10 × 15 mm3. The lateral margin of the VOI is about 0 to 2 mm lateral to the lateral border of the ipsilateral temporal horn, and the anterior margin is 5 to 10 mm anterior to the peduncles.
B, The resultant spectral map has eight voxels arranged in a two by four matrix. The spectral profile is similar to that of a single-voxel study. Owing to field inhomogeneity caused by anatomic complexity, in many cases the spectral resolution is worse in the more anterior voxels than in the posterior ones.
Automated postprocessing of the spectral raw data was performed on the on-line workstation (Magnetom Vision, Siemens, Erlangen, Germany). The postprocessing procedures included apodization with a Hamming filter, Fourier transformation, phase and baseline correction, and acquisition of peak areas by fitting to a sum of gaussian curves. The spectra and integral values of each peak were displayed on the base image with an overlaid grid indicating the anatomic location from which the results were derived. There were eight spectra in each hippocampus, arranged in four rows and two columns. The average of the NAA/(Cho+Cr) ratios from the anterior two rows represented the semiquantitative result of the anterior hippocampus; that of the posterior two rows, the posterior hippocampus. Spectra were excluded from the analysis if curve fitting was in vain. The time required for data postprocessing was 20 to 30 minutes.
Student's t-test (two-tailed) was used for comparison of data between groups. A P value of < .01 was considered statistically significant.
Results
Control Group
Twenty-four proton spectra were obtained from the 12 healthy volunteers included in the SVS study. The NAA peak at 2.0 ppm, Cr at 3.0 ppm, and Cho at 3.2 ppm could be readily recognized (Fig 1B). The distribution of hippocampal NAA/(Cho+Cr) ratios for the healthy control subjects is shown in Figure 3A. There was no statistically significant difference between the NAA/(Cho+Cr) ratios of the left and right sides of the hippocampus (P = .280), so each ratio was considered as a separate control value. The mean value ± standard deviation (SD) of the control group's hippocampal NAA/(Cho+Cr) ratios derived from proton SVS was 0.63 ± 0.07.
fig 3.
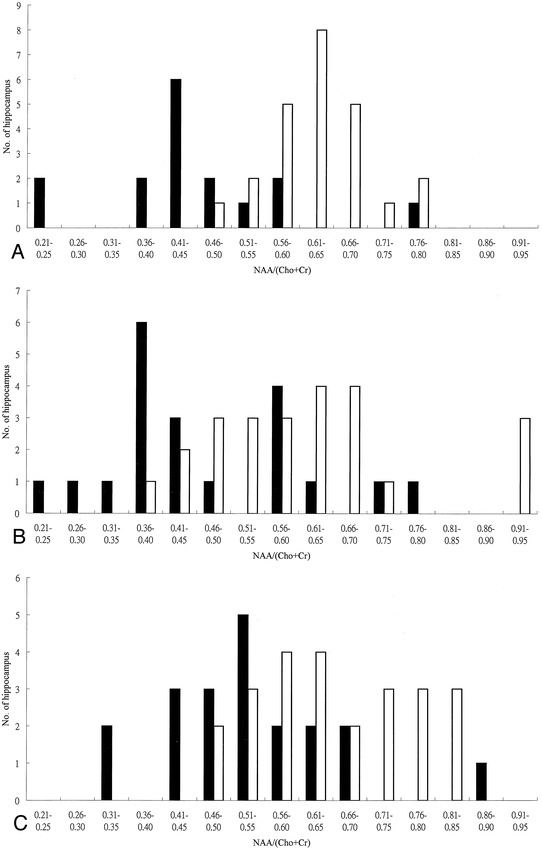
Distribution of hippocampal NAA/(Cho+Cr) ratios in control subjects (white bars) and in patients with complex partial seizures (black bars).
A, Proton SVS study.
B, Proton CSI study: anterior hippocampus.
C, Proton CSI study: posterior hippocampus.
A significant decrease in the NAA/(Cho+Cr) ratio of the patient group is seen in all three data sets (P = .00003, P = .001, and P = .002, respectively).
A normal proton spectral map of the hippocampus obtained by CSI study is shown in Figure 2B. There were eight voxels in one hippocampus, and each voxel had one spectrum. The spectral profiles were similar to those described for the SVS group. The NAA/(Cho+Cr) ratio was calculated from the integral values of fitted curves for each voxel. There was a total of 192 voxels in the control group, of which metabolic ratios were not calculated from 17 voxels owing to poor spectral quality. Most of these voxels with poor spectral resolution were located in the anterior hippocampus, especially the voxels of the first row. There was no significant difference between the NAA/(Cho+Cr) ratios of the medial and lateral columns (P = .714). The NAA/(Cho+Cr) ratio derived from each voxel was grouped and averaged for the anterior and posterior hippocampus. The distribution of hippocampal NAA/(Cho+Cr) ratios for the control group by CSI study is shown in Figure 3. There was no significant side-to-side difference, either in the anterior (P = .687) or posterior (P = .198) hippocampus. The mean value ± SD of the anterior hippocampal NAA/(Cho+Cr) ratio measured by CSI was 0.62 ± 0.15, and that of the posterior hippocampus, 0.65 ± 0.11. Because the number of hippocampi studied was small and the SD was relatively large, the lowest value in each control group, instead of the more rigorous 2 SDs below the mean value, was taken as the lower limit of the normal range.
There was no significant difference between the NAA/(Cho+Cr) ratios obtained by SVS and those by CSI, in either the anterior or posterior hippocampus (P = .629 and .473, respectively). Although the lowest NAA/(Cho+Cr) ratio (0.48) and the mean value (0.65) of the posterior hippocampus were greater than those of the anterior hippocampus (0.40 and 0.62, respectively), there was no statistical significance (P = .346).
Patients in the SVS Study
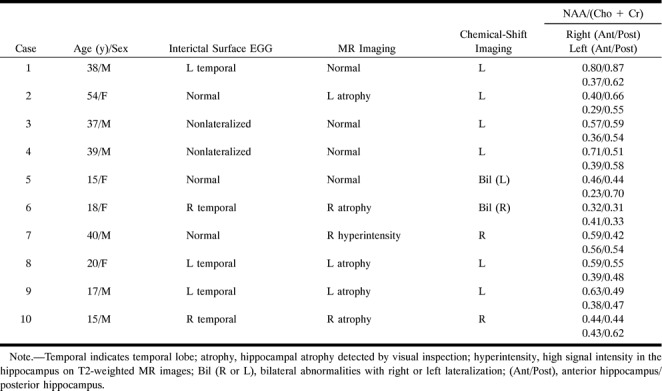
Repeated interictal surface EEG, including sphenoidal electrode recording, revealed lateralized epileptogenic foci in three patients, nonlateralization in one patient, and normal patterns in the other four patients (Table 1).
TABLE 1:
Findings in eight patients with complex partial seizures included for proton single-voxel spectroscopic study
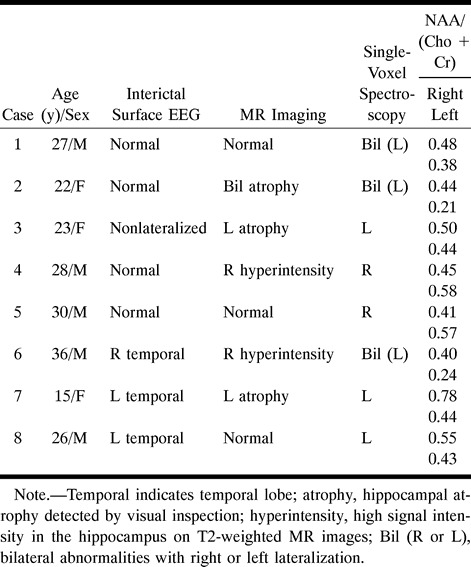
According to MR imaging studies, there were four patients with unilateral hippocampal abnormality, one patient with bilateral abnormalities, and three patients with normal results (Table 1).
The distribution of patients' NAA/(Cho+Cr) ratios is presented in Figure 3A. Compared with the healthy subjects, the metabolic ratios of this patient group were in a lower range. There was a significant difference in the hippocampal NAA/(Cho+Cr) ratio between control subjects and patients with complex partial seizures (P = .00003). The reduction in NAA/(Cho+Cr) ratio was −28% for the patient group, calculated as (patients' mean value − controls' mean value)/controls' mean value.
If the lowest NAA/(Cho+Cr) ratio of the healthy control subjects (0.50) was taken as the threshold for defining metabolic abnormality, there were unilateral hippocampal abnormalities in five of the eight patients and bilateral abnormalities in the other three (Table 1). An SVS study of a patient with unilateral hippocampal abnormality is shown in Figure 4. All three patients with normal MR imaging findings had abnormal proton SVS findings. In this study, lateralization by proton SVS was defined by a unilateral abnormal NAA/(Cho+Cr) ratio or bilateral abnormalities with a side-to-side difference greater than 0.07 (the SD). Using these criteria, lateralization could be achieved in all eight patients, including the three with bilateral hippocampal abnormalities.
fig 4.
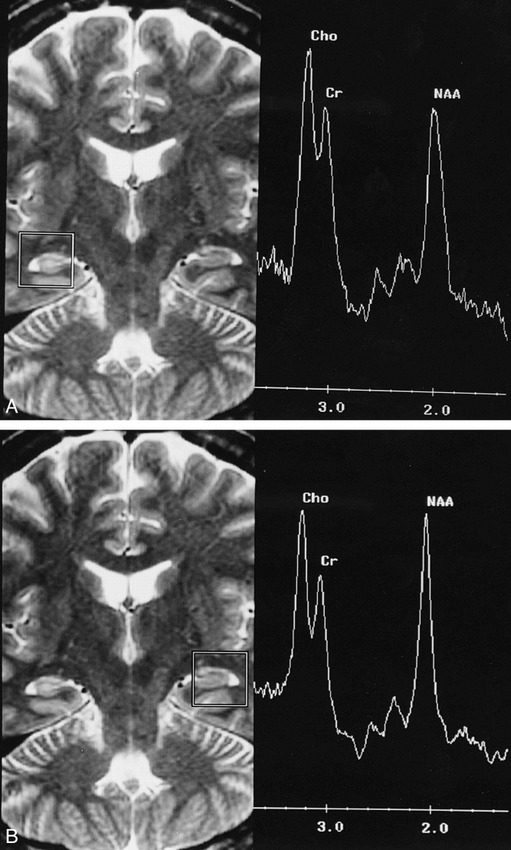
28-year-old man with complex partial seizures for 11 years.
A, The proton SVS study of the right hippocampus shows a low NAA peak, a high Cho peak, and a small NAA/(Cho+Cr) ratio (0.45).
B, The study of the left hippocampus has a normal spectral profile and a normal NAA/(Cho+Cr) ratio (0.58).
The T2-weighted MR images, 3000/85/1, also reveal abnormal hyperintensity in the right hippocampus.
Patients in the CSI Study
Repeated interictal surface EEG, including sphenoidal electrode recording, revealed lateralized epileptogenic foci in five patients, nonlateralization in two patients, and normal patterns in the other three patients (Table 2).
TABLE 2:
Findings in 10 patients with complex partial seizures included for proton chemical-shift imaging study
According to MR imaging studies, six patients had unilateral hippocampal abnormalities and four patients had normal results (Table 2).
In total, 160 voxels were obtained from 10 patients and 17 voxels were excluded from data analysis owing to poor spectral quality. As a result, at least two metabolic ratios were averaged for the anterior hippocampus, and at least three ratios were averaged for the posterior hippocampus. The distribution of anterior and posterior hippocampal NAA/(Cho+Cr) ratios is shown in Figure 3B and C. As compared with the control group, the patients had a significant reduction in NAA/(Cho+Cr) ratio in the anterior and posterior hippocampus (P = .001 and .002, respectively). Defined as (patients' mean − controls' mean)/controls' mean, the difference was −24% in the anterior hippocampus and −18% in the posterior hippocampus.
In the CSI study, a spectrum was abnormal if the NAA/(Cho+Cr) ratio was smaller than the lowest normal value: 0.40 for the anterior hippocampus and 0.48 for the posterior hippocampus. Proton CSI detected hippocampal abnormalities in all 10 of these patients, including four with normal MR imaging findings (Table 2). Unilateral abnormalities were noted in eight patients, one of which is shown in Figure 5, and bilateral abnormalities in the other two patients (cases 5 and 6). Lateralization could be achieved in these two patients according to the following criteria: abnormal NAA/(Cho+Cr) ratios involving both the anterior and posterior portion of the ipsilateral hippocampus and only one portion of the contralateral hippocampus (case 6) or abnormal NAA/(Cho+Cr) ratios involving only one portion of both hippocampi with their difference being more than 0.15 (the SD of the control group's anterior hippocampus) (case 5). All six patients with abnormal MR imaging findings had concordant proton CSI lateralization.
fig 5.
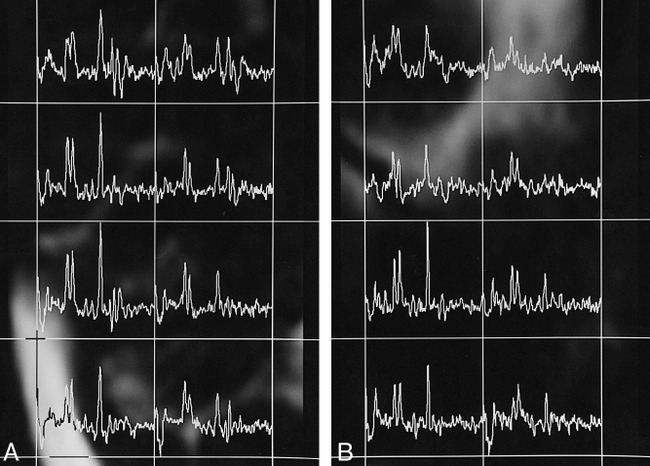
20-year-old woman with complex partial seizures for 14 years.
A, Proton CSI study of the right hippocampus shows a normal NAA/(Cho+Cr) ratio in both anterior and posterior portions (0.59 and 0.55, respectively).
B, Conversely, the study of the left hippocampus shows a reduced NAA/(Cho+Cr) ratio in both anterior and posterior portions (0.39 and 0.48, respectively). The MR imaging study (not shown) revealed atrophy of the left hippocampus.
Discussion
In recent years, proton MR spectroscopy has been applied with more frequency to the area of seizure evaluation (8–18). Detectable changes in cerebral metabolites, such as NAA, Cho, and Cr, have been demonstrated in many research centers (8–18); however, several factors make comparison of spectral data from various centers difficult, such as different acquisition parameters, measurement techniques, voxel sizes and positioning, MR systems, and analytic methods (1, 19). The purpose of our study was to evaluate the consistency of spectral results under different conditions.
The variation between results obtained with different MR spectroscopic techniques is important, since it is the clinical applications that are being considered. It would have been better to perform SVS and CSI in the same hippocampus and during the same session so that direct comparison between the results could have been made. However, obtaining both SVS and CSI data from bilateral hippocampi during one session would have required the patient to remain in the magnet for more than 1 hour, and such repeated MR spectroscopic studies are not easily performed in patients with seizures. So, in this study, SVS and CSI were conducted in different groups of patients and control subjects, and comparison was made between their mean values rather than between the ratios.
In SVS studies, the voxel is large and encompasses not only the hippocampus but also the adjacent temporal lobe and cerebrospinal fluid. All these structures contribute to the signal intensity and influence the spectral profile (2, 12, 22). Different positioning of the voxel will thus result in different metabolic ratios. Extraneous tissue contamination seems to be of less severity in the CSI method owing to the small voxel size, but voxel bleeding resulting from point-spread function (23) is of concern: the signal of a neighboring voxel will contribute about 10% to the signal of adjacent voxels. The quality of shimming across the VOI is essential for good MR spectroscopy. In a CSI study, the large VOI and the anatomic complexity near the medial temporal lobe make it difficult to obtain field homogeneity over the measured region. Despite these problems, using our technique, the metabolic ratios of healthy control subjects obtained by SVS were consistent with those obtained by CSI, showing that consistent delineation of the region to be measured, similar acquisition parameters, and automated postprocessing can give comparable spectroscopic measurements on a clinical basis. Under such circumstances, different MR systems can give consistent semiquantitative data, whether SVS or CSI is used.
For the control groups, similar mean values could be obtained by different MR spectroscopic techniques, but the SDs of the CSI study (0.15 in the anterior and 0.11 in the posterior hippocampus) were much larger than those of the SVS study (0.07). This was most likely due to the poorer shimming across a larger VOI during CSI measurement compared with a smaller volume in the SVS study. Furthermore, the anatomic complexity around the anterior hippocampus makes the situation even worse, resulting in the poorest spectral resolution, the largest SD, and the greatest ratio distribution among the three groups. It is important, therefore, to further evaluate whether the measurements obtained with either the SVS or CSI technique for a given hippocampus during different sessions with the same magnet would give similar NAA/(Cho+Cr) ratios. The precision of MR spectroscopic measurements would thus be evaluated and the standard error of the observed NAA/(Cho+Cr) ratio would be defined.
There is no standard threshold between normal and abnormal hippocampal spectra that can be applied universally for seizure evaluation. The most common metabolic ratios used in the investigation of temporal lobe epilepsy are NAA/(Cho+Cr) (8, 11, 16, 24), NAA/Cho (13, 15), and NAA/Cr (14, 15, 17). In the present study, the NAA/(Cho+Cr) ratio was chosen for spectral analysis because of the association of low NAA, high Cho, and, probably, high Cr intensity, and the poor resolution between Cr and Cho peaks in hippocampal spectra (8, 11, 12, 16). It is possible to use metabolic ratios to detect unilateral or bilateral hippocampal abnormalities, to lateralize the epileptogenic focus, to predict the surgical outcome, and to differentiate neocortical from mesial temporal lobe epilepsy (24, 25). Although promising results have been reported, contradictory results were obtained in some cases (5, 26). The situation should be clarified by using similar acquisition protocols in a larger group of subjects. There are reports of absolute quantification of cerebral metabolic concentrations using proton MR spectroscopy (9, 16, 22, 24), but the process of in vivo quantification of metabolic concentration is rather complicated (27) and, in most clinical centers, its application for daily practice is not available.
In our CSI study, the voxels were divided into anterior and posterior groups for the evaluation of regional differences in the hippocampus. Both the lowest NAA/(Cho+Cr) ratio and the mean value of the posterior hippocampus were larger than those of the anterior hippocampus, but there was no statistical significance (P = .346). This finding is different from that in the study of Vermathen et al (28), who reported a strong anterior-posterior difference within the hippocampus using the CSI technique. Different measurement strategy, postprocessing procedure, and analytic method may, at least in part, lead to contrary observations. In our CSI study, approximately 10% of the voxels had to be discarded owing to poor quality, and many of these were located within the anterior hippocampus, where lower NAA/(Cho+Cr) ratios are more typical. Investigation of hippocampal spectra of different regions should take these factors into consideration.
Both proton SVS and CSI techniques showed a significantly lower NAA/(Cho+Cr) ratio in our patients. In comparison with the control subjects, the overall rate reduction was around 20% to 30%. Using the criteria defined previously, we could identify unilateral or bilateral abnormalities in all patients, including those with a normal MR imaging study, indicating that metabolic abnormalities might be detected earlier than the morphologic changes in the disease process accounting for complex partial seizures. The metabolic ratio NAA/(Cho+Cr) can be measured readily on a clinical MR scanner with the proton SVS or CSI technique, and can be used to differentiate a normal from an abnormal hippocampus in patients with complex partial seizures. Incorporated into the routine MR examination, proton MR spectroscopy can add invaluable metabolic information to the results of an MR imaging study. However, since only a few of our patients had undergone surgery when this article was submitted, the accuracy of lateralization by proton SVS and CSI cannot be determined from these data. A large-scale study with pathologic confirmation and surgical outcome is mandatory for defining the role of proton MR spectroscopy in the management of patients with seizures.
For patients with a diagnosis of complex partial seizures, the question is, which MR spectroscopic technique should be applied to evaluate the hippocampus, SVS or CSI? Both can be performed on most clinical MR systems and can efficiently detect metabolic changes in the hippocampus, as shown in this study and elsewhere in the literature. SVS can be accomplished in less time, which allows the subjects to feel more comfortable and makes body motion less of a problem. Shimming across the small VOI during SVS measurement always gives a more homogeneous field and better spectral profile. The CSI technique, on the other hand, has greater field inhomogeneity over a larger VOI and a longer acquisition time, leading to poor spectral resolution, difficult curve fitting, and a wider range of data distribution, as shown in this study. Data processing in CSI study is more complicated and time-consuming than in the SVS study. However, only one voxel can be studied during one SVS measurement, while multiple spectra can be obtained simultaneously during one CSI measurement, making comparison of a voxel of interest with others in and around the hippocampus possible. The CSI technique has the potential to better delineate the region of metabolic abnormality, which might be helpful in the planning of restricted surgery. At present, whether one uses SVS or CSI for seizure evaluation depends largely on the resources available and the expertise of the personnel involved. With rapid improvement of MR techniques and data processing, use of the CSI technique for seizure evaluation will undoubtedly increase, because it has the major advantages of multivoxel acquisition and metabolic image display.
Conclusion
Recently, more and more patients with complex partial seizures have been treated surgically, with good outcomes maintained during long-term follow-up periods (29). Tailored neuroimaging evaluation of the brain and accurate localization of the epileptogenic focus are proving to be helpful and important for a successful operation (30). Proton SVS and CSI techniques can be used to measure the cerebral metabolites on clinical MR systems, and, in patients with complex seizures, to detect the biochemical abnormalities before the appearance of morphologic changes. Our study has established that consistent hippocampal NAA/(Cho+Cr) ratios in healthy adults can be obtained by using similar measurement parameters, with either SVS or CSI. A standardized protocol for acquiring hippocampal proton spectra is desirable, as it will lead to more widespread application of MR spectroscopy in the field of complex partial seizures.
Footnotes
Presented in part at the annual meeting of the International Society for Magnetic Resonance in Medicine, Sydney, Australia, April 1998; and at the annual meeting of the American Society of Neuroradiology, Philadelphia, May 1998.
Address reprint requests to Dr. Yuan-Yu Hsu, Department II of Radiology, Chang Gung Memorial Hospital, No. 5 Fu-Shing St, Kwei-Shan, Taoyuan, Taiwan, R.O.C.
References
- 1.Cousins JP. Clinical MR spectroscopy: fundamentals, current applications, and future potential. AJR Am J Roentgenol 1995;164:1337-1347 [DOI] [PubMed] [Google Scholar]
- 2.Castillo M, Kwock L, Mukherji SK. Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol 1996;17:1-15 [PMC free article] [PubMed] [Google Scholar]
- 3.Luyten PR, Marien AJH, den Hollander JA. In vivo 1H-NMR spectroscopy of the human brain by spatial localization and imaging techniques. Presented at the annual meeting of the Society for Magnetic Resonance in Medicine, April 1988, Berkeley, CA
- 4.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized high-resolution H-1 NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med 1989;9:79-93 [DOI] [PubMed] [Google Scholar]
- 5.Maudsley AA, Hilal SK, Perman WH, Simon HE. Spatially resolved high resolution spectroscopy by “four-dimensional” NMR. J Magn Reson 1983;51:147-152 [Google Scholar]
- 6.Duijn JH, Matson GB, Maudsley AA, Weiner MW. 3D phase encoding 1H spectroscopic imaging of human brain. Magn Reson Imaging 1992;10:315-319 [DOI] [PubMed] [Google Scholar]
- 7.Sauter R, Schneider M, Wicklow K, Kolem H. Localized 1H MRS of the human brain: single-voxel versus CSI techniques (abstract). J Magn Reson Imaging 1991;1:241 [Google Scholar]
- 8.Gadian DG, Connelly A, Duncan JS, et al. 1H magnetic resonance spectroscopy in the investigation of intractable epilepsy. Acta Neurol Scand 1994;152:116-121 [DOI] [PubMed] [Google Scholar]
- 9.Breiter SN, Arroyo S, Mathews VP, Lesser RP, Bryan RN, Barker PB. Proton MR spectroscopy in patients with seizure disorders. AJNR Am J Neuroradiol 1994;15:373-384 [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly A, Jackson GD, Duncan JS, King MD, Gadian DG. Magnetic resonance spectroscopy in temporal lobe epilepsy. Neurology 1994;44:1411-1417 [DOI] [PubMed] [Google Scholar]
- 11.Achten E, Santens P, Boon P, et al. Single-voxel proton MR spectroscopy and positron emission tomography for lateralization of refractory temporal lobe epilepsy. AJNR Am J Neuroradiol 1998;19:1-8 [PMC free article] [PubMed] [Google Scholar]
- 12.Hugg JW, Laxer KD, Matson GB, Maudsley AA, Meiner MW. Neuron loss localizes human temporal lobe epilepsy by in vivo proton magnetic resonance spectroscopy imaging. Ann Neurol 1993;34:788-794 [DOI] [PubMed] [Google Scholar]
- 13.Thian C, Youssef GC, Min X, et al. Temporal lobe epilepsy: presurgical localization with proton chemical shift imaging. Radiology 1994;193:465-472 [DOI] [PubMed] [Google Scholar]
- 14.Cendes F, Andermann F, Preul MC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol 1994;35:211-216 [DOI] [PubMed] [Google Scholar]
- 15.Hetherington H, Kuzniecky R, Pan J, et al. Proton nuclear magnetic resonance spectroscopic imaging of human temporal lobe epilepsy at 4.1 T. Ann Neurol 1995;38:396-404 [DOI] [PubMed] [Google Scholar]
- 16.Ende GR, Laxer KD, Knowlton RC, et al. Temporal lobe epilepsy: bilateral hippocampal metabolite changes revealed at proton MR spectroscopic imaging. Radiology 1997;202:809-817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cendes F, Caramanos Z, Andermann F, Dubeau F, Arnold DL. Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol 1997;42:737-746 [DOI] [PubMed] [Google Scholar]
- 18.Peeling J, Sutherland G. 1H magnetic resonance spectroscopy of extracts of human epileptic neocortex and hippocampus. Neurology 1993;43:589-594 [DOI] [PubMed] [Google Scholar]
- 19.Bottomley PA. The trouble with spectroscopy papers. Radiology 1991;181:344-350 [DOI] [PubMed] [Google Scholar]
- 20.Bottomley PA. Spatial localization in NMR-spectroscopy in vivo. Ann N Y Acad Sci 1987;508:333-348 [DOI] [PubMed] [Google Scholar]
- 21.Haase A, Frahm J, Hanike W, Matthae D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol 1985;30:341-344 [DOI] [PubMed] [Google Scholar]
- 22.Michaelis T, Merboldt KD, Bruhn H, Hanicke W, Frahm J. Absolute concentrations of metabolites in the adult human brain in vivo: quantification of localized proton MR spectra. Radiology 1993;187:219-227 [DOI] [PubMed] [Google Scholar]
- 23.Bottomley PA, Hardy CJ, Roemer PB. Phosphate metabolite imaging and concentration measurements in human heart by nuclear magnetic resonance. Magn Reson Med 1990;14:425-434 [DOI] [PubMed] [Google Scholar]
- 24.Vermathen P, Ende G, Laxer KD, Knowlton RC, Matson GB, Weiner MW. Hippocampal N-acetylaspartate in neocortical epilepsy and mesial temporal lobe epilepsy. Ann Neurol 1997;42:194-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermathen P, Laxer KD, El Din M, Matson GB, Weiner MW. Hippocampal NAA loss in mesial temporal lobe epilepsy is not accompanied by changes in other brain regions: a combined multislice and PRESS MRSI study. Presented at the annual meeting of the International Society for Magnetic Resonance in Medicine, April 1997, Vancouver, BC, Canada
- 26.Choi CG, Lee HK, Suh DC, Whang YM, Yang SH. Lateralization of seizure focus by single-voxel proton MR spectroscopy in patients with definite unilateral hippocampal atrophy on MR imaging. Presented at the annual meeting of the International Society for Magnetic Resonance in Medicine, April 1997, Vancouver, BC, Canada
- 27.Henriksen Ole. In vivo quantitation of metabolite concentrations in the brain by means of proton MRS. NMR Biomed 1995;8:139-148 [DOI] [PubMed] [Google Scholar]
- 28.Vermathen P, Laxer KD, El Din M, Matson GB, Weiner MW. 1H MRSI of the hippocampus shows strong anterior-posterior differences in epilepsy patients and in controls (abstract). Presented at the annual meeting of the International Society for Magnetic Resonance in Medicine, April 1997, Vancouver, BC, Canada
- 29.Marsh WR. Epilepsy surgery. Neuroimaging Clin North Am 1995;5:729-738 [PubMed] [Google Scholar]
- 30.Vives KP, Al-Rodhan N, Spencer DD. Use of magnetic resonance imaging in surgical strategies for epilepsy. In: Cascino GD, Jack Jr CR, eds. Neuroimaging in Epilepsy: Principle and Practice. Newton, MA: Butterworth-Heinemann; 1996;16:235-259 [Google Scholar]


