Abstract
Summary: A patient with a glioblastoma multiforme and mild sensorimotor deficits had significantly less activation of the motor and sensory cortices on the side with the tumor than on the contralateral side on blood oxygen level–dependent (BOLD) functional MR images. This difference, which may be due to pressure effects or loss of vascular autoregulation, should be considered in preoperative planning in which BOLD functional MR imaging is used to identify eloquent cortices to be avoided during brain tumor surgery.
In malignant gliomas, the length and quality of survival are improved with maximum tumor resection (1–4). Therefore, the goal of neurosurgery in the treatment of malignant gliomas is to resect as much of the tumor as possible while preserving important brain functions (3–5). Anatomic identification of the eloquent cortices to be avoided during surgery is complicated by distortion of the anatomy of mass lesions (5–9) and by the fact that the functional cortex does not always correspond to its expected anatomic location (5, 9–14). Presurgical blood oxygen level–dependent (BOLD) functional MR imaging has been used to define functional cortices of the brain in preoperative planning for glioma surgery (3, 4, 15, 16).
BOLD functional MR imaging is based on the premise that brain activation is inherently linked to an increase in blood flow in the area of activation that is disproportionate to the increase in oxygen consumption. This accounts for the changes in signal intensity registered by the BOLD functional MR imaging technique (17). However, a number of factors are known to affect the blood flow in areas of the brain adjacent to a tumor. For example, it is know that vascular autoregulation can be lost in and around gliomas (18). It is also possible that the mass effect of a large tumor mass may affect the adjacent circulation. If such autoregulation is lost in brain tissue that is still functioning, then this area may not respond to increased neural activity by a corresponding increase in blood flow. Consequently, the area in question may not show a statistically significant change on the BOLD functional MR images. This may cause false-negative results, which may lead the neurosurgeon to resect an area of functioning cortex.
Case Report
A 40-year-old man with a right-sided glioblastoma multiforme, diagnosed 14 months earlier, presented with a generalized seizure. No focal deficit was evident on examination. The patient underwent stereotactic biopsy and fractionated radiotherapy (54 Gy in 30 fractions to the enhancing margins plus 3 cm), along with intravenous chemotherapy with BCNU. Stereotactic radiosurgery was performed 6 months after diagnosis as an adjuvant therapy.
On the current admission, the patient was disoriented, had a dense left hemiparesis, and left-sided neglect. A routine diagnostic MR study showed a large peripherally enhancing mass in the right temporoparietal area. The mass measured 5.5 × 4.5 × 4.0 cm and had a central area of hypointensity, presumably representing central necrosis. The area of enhancement was surrounded by edema, which extended to involve almost the entire right hemisphere, including the white matter of the right pre- and postcentral gyri. The areas of enhancement and central necrosis did not extend to involve the gyri in the expected locations of the homunculi of the arm and leg. Instead, the area of enhancement involved the brain just posterior to the expected location of the sensory cortex for the left hand. There was significant mass effect and midline shift. The right pre- and postcentral gyri were displaced anteriorly by the mass.
High-dose steroids significantly improved the patient's symptoms within 2 days, to the point where functional MR imaging could be performed. Just before the imaging examination, the patient exhibited mild weakness in the left arm, including a left pronator drift. The sensory changes in the left arm were limited to a mild degree of numbness and tingling.
Surgical resection was planned in order to reduce mass effect and implant BCNU-impregnated wafers (Gliadel, Rhone-Poulenc Rorer, Collegeville, PA). Functional MR imaging was done to define the location of the sensorimotor cortex in relation to the lesion, and to register it to a surgical navigation system for real-time intraoperative localization, as described by Maldjian et al and Schulder et al (3, 4). The patient underwent paradigms to identify both the motor and sensory cortices. The motor paradigm consisted of alternating periods of 30 seconds of rest and 30 seconds of self-paced finger tapping for a total of three periods of rest and two periods of activation. The patient was observed to make sure that he performed the paradigm accurately. No difference was detected in the performance of the motor paradigms between the right and left hands. The sensory paradigm consisted of having a physician rhythmically squeeze the patient's fingers at a frequency of 1 Hz. The timing of the periods of rest and activation was identical to the motor paradigm. Likewise, the sensory paradigms were performed by one of the authors in an identical manner for both hands.
The functional data were acquired using the BOLD technique (gradient-echo echo-planar imaging) with parameters of 2000/60 (TR/TE), 14 sections, 64 × 64 matrix, and a section thickness of 5 mm with no gap. A contrast-enhanced (0.1 mmol/kg gadopentetate dimeglumine) T1-weighted image was obtained immediately afterward. The raw functional MR imaging data were analyzed off-line using a SPARC20 workstation and software written in IDL, described previously (3, 4). Functional MR imaging maps were generated using a cross-correlation technique (19). The functional MR imaging data were coregistered to the high-resolution contrast-enhanced image. These fused images were transferred to a neurosurgical guidance system over an Ethernet connection, allowing the neurosurgeon to identify not only the anatomy but also the functional areas of the brain by viewing the display in the operating room (3, 4).
BOLD functional MR images showed robust activation in the expected locations on the motor and sensory homunculi for the corresponding motor and sensory paradigms for the side of the brain without the tumor. Using a correlation coefficient of r = .60 and .48 (correlating the changes in signal intensity on the BOLD functional MR image to the paradigm), we identified volumes of activation of 1.40 cm3 and 2.31 cm3, respectively, in the motor homunculus (P < .01) (Fig 1). For the sensory homunculus, the respective volumes of activation were 0.49 cm3 for r = .60 and 2.03 cm3 for r = .40 (P < .01) (Fig 2).
fig 1.
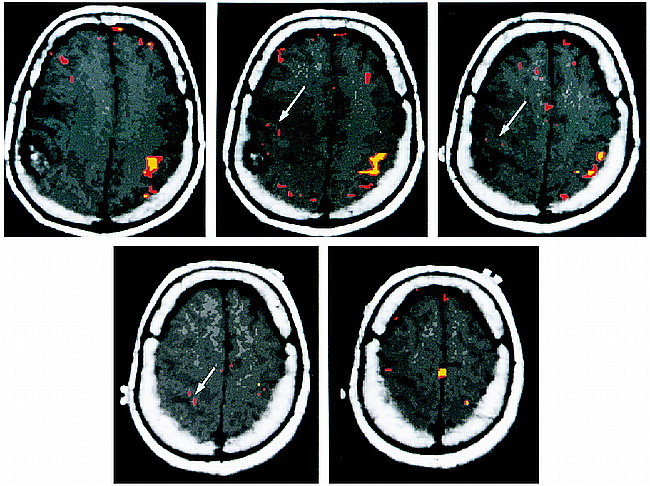
Axial T1-weighted images with coregistered functional MR images obtained during a bilateral motor paradigm show a larger volume of activation on the normal side than on the side with the tumor (arrows). This effect is seen for different correlation coefficients (r). The red areas indicate significant activation for r = .48, P < .01. The yellow areas indicate significant activation for r = .60, P < .01. Notwithstanding the difference in the volume of activation, one is still able to identify the motor cortex on the side with the tumor. The motor cortex on the right is displaced anteriorly and superiorly by the tumor mass. The accessory motor area is seen at the midline in the superiormost image (bottom right)
fig 2.
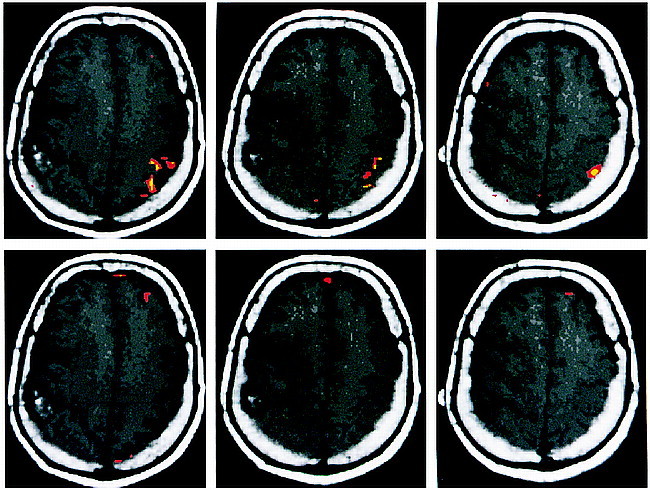
Axial T1-weighted images with coregistered functional MR images obtained during right-hand (top row, left sensory cortex) and left-hand (bottom row, right sensory cortex) sensory paradigms. The right-hand sensory paradigm shows robust activation in the left postcentral gyrus at different correlation coefficients (r). The red areas indicate activation for r = .48, P < .01. The yellow areas indicate activation for r = .60, P < .01. The left-hand sensory paradigm fails to show activation in the right postcentral gyrus (located just anterior to the tumor mass), even with P < .10
For the side of the brain with the tumor, the location of the motor cortex, as defined by functional MR imaging, corresponded to its expected anatomic location. However, the volume of activation was reduced, especially when the higher correlation coefficient was used. For r = .60, there was no activation; for r = .48, the volume of activation was 0.63 cm3 (Fig 1). For the sensory cortex, there was no activation for either correlation coefficient for up to P < .1 (Fig 2). At surgery, the motor cortex was identified by using direct bipolar stimulation as well as phase reversal of somatosensory evoked potentials. This location corresponded to that predicted by functional MR imaging registered to the surgical navigation device. Frozen section was consistent with radiation necrosis and tumor. A gross total resection of the tumor was performed, followed by Gliadel implantation. The patient was discharged several days later with no change in the sensorimotor deficits as compared with the prefunctional MR imaging examination.
Discussion
The development of a system by which functional MR imaging data coregistered to high-resolution anatomic images can be viewed intraoperatively to guide resection has recently been described (3, 4). Such a system, in which the neurosurgeon can identify the eloquent cortices adjacent to a tumor by the use of a probe, has obvious advantages. Since the eloquent cortices are identified preoperatively, the exposure during surgery is limited. In addition, the length of the operation is curtailed, since the time-consuming and cumbersome intraoperative physiological tests to identify the appropriate functional areas of the brain are eliminated. Recent publications have indicated excellent correlation between BOLD functional MR imaging and intraoperative physiological tests to identify the motor cortex (3, 4).
In this case, BOLD functional MR images showed diminished activation in the motor cortex and no activation in the sensory cortex on the side with the tumor in a patient with mild sensorimotor deficits. This finding raises an important issue regarding the ability of functional MR imaging to define eloquent cortices adjacent to glioblastoma multiforme. Specifically, what are the limits of BOLD functional MR imaging data used to guide resection of a tumor near an eloquent cortex?
Notwithstanding the difference in the volume of activation, the motor gyrus was still identified in the present case using preoperative BOLD functional MR imaging. By extrapolation, the sensory gyrus was identified as the gyrus directly posterior to the motor gyrus, even though the sensory gyrus on the side with the tumor was not directly identified on MR images. Surgery based on intraoperative identification of the motor gyrus by physiological methods (which corresponded to the motor gyrus on BOLD functional MR images) resulted in no deficits. Nevertheless, a word of caution must be raised. If the present technology is applied indiscriminately to guide resection, especially in areas directly adjacent to areas of activation or within the motor gyrus itself, it may lead to resection of a cortex that has lost the ability to be detected by BOLD functional MR imaging but that is still functioning.
The phenomenon described in this report may be accounted for by the following mechanism. It has been demonstrated by angiography as well as by MR imaging that there is a loss of autoregulation of the tumor vasculature in malignant gliomas. For example, the vasculature of gliomas has shown an abnormal response to various physiological and pharmacologic challenges, including hypocapnia induced by hyperventilation (18, 20), hypercapnia (20), induced hypertension (21), and papaverine injection (21). These differences in tumor vasculature were more notable in malignant gliomas than in benign gliomas (18, 21). What may have occurred in the present situation is that the tumor vasculature in the motor and sensory cortices had diminished or absent capability for autoregulation. This may have precluded an increase in blood flow in the expected area of activation that normally occurs subsequent to motor or sensory activity. A lack of increased blood flow to the expected area of activation would significantly limit the ability of BOLD functional MR imaging to detect activation.
It is also known that there is increased pressure in the area adjacent to gliomas. Radiologically, this is manifest as mass effect and midline shift. A significant percentage, if not the majority, of the signal change from BOLD functional MR imaging is due to a change in the oxygenation state of the hemoglobin in the venules and larger veins, as opposed to the capillaries (22, 23). Venous structures are normally under low pressure and are imminently compressible. What might have occurred to explain the present situation is that the increased mass effect compressed the venules and larger veins, thereby speeding the egress of oxyhemoglobin-laden blood from the area of activation. This led to a decrease in the relative concentration of oxyhemoglobin in the area of activation, which in turn resulted in an effective decrease in the difference in the concentration of oxyhemoglobin between the resting and active states. This would lead to a decreased ability of functional MR imaging to detect changes between the resting and active states. It is possible that the two mechanisms described above are both responsible for the phenomenon described.
Conclusion
This case raises a number of fundamental questions regarding the validity of BOLD functional MR imaging as a reliable indicator of functioning cortex adjacent to a malignant glioma. Until now, BOLD functional MR imaging has had excellent correlation with intraoperative physiological tests and postoperative results. However, it is axiomatic that no test is 100% reliable, especially when it pertains to something as complex as brain function. It is, therefore, important to determine whether the present case is an anomaly or whether it is indicative of a fundamental limitation of this method. If such a limitation is identified, future studies should determine its nature, under what circumstances it occurs, and what can be done to avoid it.
Footnotes
Presented at the annual meeting of the American Society of Neuroradiology, Philadelphia, May 1998.
Address reprint requests to Andrei I. Holodny, MD, Department of Radiology, UMDNJ-New Jersey Medical School, University Hospital C-320, 150 Bergen St, Newark, NJ 07103.
References
- 1.Ammirati M, Vick N, Liao Y, et al. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery 1987;21:201-206 [DOI] [PubMed] [Google Scholar]
- 2.Devaux BC, O'Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms: a retrospective study of clinical parameters, therapy, and outcome. J Neurosurg 1993;78:767-75 [DOI] [PubMed] [Google Scholar]
- 3.Maldjian JA, Schulder M, Liu WC, et al. Intraoperative functional MRI using a real-time neurosurgical navigation system. J Comput Assist Tomogr 1997;21:910-912 [DOI] [PubMed] [Google Scholar]
- 4.Schulder M, Maldjian JA, Liu WC, et al. Functional image guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg 1998;89:412-418 [DOI] [PubMed] [Google Scholar]
- 5.Rezai AR, Hund M, Kronberg E, et al. The interactive use of magnetoencephalography in stereotactic image-guided neurosurgery. Neurosurgery 1996;39:92-102 [DOI] [PubMed] [Google Scholar]
- 6.Orrison WW, Rose DF, Hart BL, et al. Noninvasive preoperative cortical localization by magnetic source imaging. AJNR Am J Neuroradiol 1992;13:1124-1128 [PMC free article] [PubMed] [Google Scholar]
- 7.Bucholz RD. The central sulcus and surgical planning. AJNR Am J Neuroradiol 1993;14:926-927 [PMC free article] [PubMed] [Google Scholar]
- 8.Sobel DF, Gallen CC, Schwartz BJ, et al. Central sulcus localization in humans: comparison of MR anatomic and magnetoencephalographic functional methods. AJNR Am J Neuroradiol 1993;14:915-925 [PMC free article] [PubMed] [Google Scholar]
- 9.Seitz RJ, Yanxiong H, Knorr U, et al. Large-scale plasticity in the human motor cortex. Clin Neurosci Neuropathol 1995;6:742-744 [DOI] [PubMed] [Google Scholar]
- 10.Berger MS, Kincaid J, Ojemann GA, Leitich E. Brain mapping technique to maximize resection, safety and seizure control in children with brain tumors. Neurosurgery 1989;25:786-792 [DOI] [PubMed] [Google Scholar]
- 11.Cohen L, Pascual-Leone A, Hallet M. Plasticity of cortical motor output organization following deafferentation, cerebral lesion and skill acquisition. In: Devinsky O, Beric A, Dogali M, eds. Advances in Neurology: Electrical and Magnetic Stimulation of the Brain and Spinal Cord. New York: Raven Press; 1993;63:187–201 [PubMed]
- 12.Mogilner A, Grossman JA, Ribary U, et al. Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proc Natl Acad Sci U S A 1993;90:3593-3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ojemann GA. Individual variability in cortical localization of language. J Neurosurg 1979;50:164-169 [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran VS, Rogers-Ramachandran D, Stewart M. Perceptual correlates of massive cortical reorganization. Science 1992;258:1159-1160 [DOI] [PubMed] [Google Scholar]
- 15.Jack CR, Thompson RM, Butts Rk, et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology 1994;190:85-92 [DOI] [PubMed] [Google Scholar]
- 16.Atlas SW, Howard RS, Maldjian JA, et al. Functional magnetic resonance imaging of regional brain activity in patients with intracerebral gliomas: findings and implications for clinical management. Neurosurgery 1996;38:329-338 [DOI] [PubMed] [Google Scholar]
- 17.Owaga S, Lee T, Kay A, Tank D. Brain magnetic resonance imaging with contrast dependant on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868-9872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pronin IN, Holodny AI, Kornienko VN, Petraikin AV, Golovanov AV, Lee HJ. The use of hyperventilation in contrast-enhanced MR of brain tumors. AJNR Am J Neuroradiol 1997;18:1705-1708 [PMC free article] [PubMed] [Google Scholar]
- 19.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Processing strategies for time-course data sets for functional MRI of the human brain. Magn Reson Med 1993;30:161-173 [DOI] [PubMed] [Google Scholar]
- 20.Bradac GB, Simon RS, Heidieck CH. Angiographically verified transient alteration of the intracranial arteries and veins in dependence on different CO2 tensions. Neuroradiology 1976;10:257-262 [DOI] [PubMed] [Google Scholar]
- 21.Huber P. Functional tests in angiography of brain tumors. Neuroradiology 1970;1:132-141 [Google Scholar]
- 22.Boxerman JL, Bandettini PA, Kwong KK, et al. The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Reson Med 1995;34:4-10 [DOI] [PubMed] [Google Scholar]
- 23.Gao JH, Miller I, Lai S, Xiong J, Fox PT. Quantitative assessment of blood inflow effects in functional MRI signals. Magn Reson Med 1996;36:314-319 [DOI] [PubMed] [Google Scholar]


