Abstract
In traditional medicine, Cannabis sativa has been prescribed for a variety of diseases. Today, the plant is largely known for its recreational purpose, but it may find a way back to what it was originally known for: a herbal remedy. Most of the plant's ingredients, such as Δ9-tetrahydrocannabinol, cannabidiol, cannabigerol, and others, have demonstrated beneficial effects in preclinical models of intestinal inflammation. Endogenous cannabinoids (endocannabinoids) have shown a regulatory role in inflammation and mucosal permeability of the gastrointestinal tract where they likely interact with the gut microbiome. Anecdotal reports suggest that in humans, Cannabis exerts antinociceptive, anti-inflammatory, and antidiarrheal properties. Despite these reports, strong evidence on beneficial effects of Cannabis in human gastrointestinal diseases is lacking. Clinical trials with Cannabis in patients suffering from inflammatory bowel disease (IBD) have shown improvement in quality of life but failed to provide evidence for a reduction of inflammation markers. Within the endogenous opioid system, mu opioid receptors may be involved in anti-inflammation of the gut. Opioids are frequently used to treat abdominal pain in IBD; however, heavy opioid use in IBD is associated with opioid dependency and higher mortality. This review highlights latest advances in the potential treatment of IBD using Cannabis/cannabinoids or opioids.
INTRODUCTION
The marijuana plant, Cannabis sativa, has long been used by mankind for medical, ritual, and recreational purposes. Because of its anti-inflammatory, antiemetic, antidiarrheal, and analgesic properties, Cannabis extracts were prescribed for a variety of diseases until the beginning of the past century (1). At the same time, recreational use of the plant became increasingly popular, eventually leading to its banning (2). However, with the detection of Δ9-tetrahydrocannabinol (THC) as the psychotropic ingredient of Cannabis (3) and the discovery of “endogenous cannabinoids” (and consequently of the endocannabinoid system) (4), the focus on Cannabis has shifted back to its role as a herbal remedy. More Cannabis ingredients except for THC are known nowadays, e.g., cannabidiol (CBD), tetrahydrocannabivarin, cannabichromene, and cannabigerol (5). In general, the term cannabinoid encompasses herbal cannabinoids, i.e., the ingredients of the Cannabis plant (C. sativa contains more than 100 cannabinoid ingredients (6)) and the synthetic cannabinoids, e.g., CP-55,940 and WIN55, 212 (7). In many preclinical models of intestinal inflammation, Cannabis/cannabinoids proved being highly effective in improving inflammation (8), supporting the notion of a potential treatment of people suffering from inflammatory bowel diseases (IBDs). Meanwhile, a small number of clinical trials and observational studies that investigated the effects of Cannabis/cannabinoids in patients with IBD have been completed. Beside the mechanistic understanding of cannabinoid actions, these studies will be discussed together with the adverse effects of Cannabis. In addition, caveats of opioid treatment will be highlighted.
INFLAMMATORY BOWEL DISEASES
Crohn's disease (CD) and ulcerative colitis (UC) represent 2 forms of IBD. Despite their unknown etiology, it is widely assumed that an exaggerated and misdirected immune response against bacterial antigens causes structural damage of the intestinal mucosa in genetically predisposed people (9). According to a recent systematic review, IBD has become a global burden, with incidences accelerating in newly industrialized countries (10). The prevalence of IBD now exceeds 0.3% in North America and many countries in Europe and Oceania. IBD poses a high symptomatic and psychological burden to the patient and a challenge to the society. It has been estimated that direct healthcare costs for IBD in Europe amount to 4.6–5.6 bn Euros/year (11). Between 15% and 40% of the patients with IBD display extraintestinal manifestations (e.g., peripheral arthropathies or cutaneous manifestations, such as erythema nodosum), and 30%–50% of patients with CD need surgical intervention (11). Depending on the severity of the flares and the response to medication, IBD therapy includes anti-inflammatory agents, such as aminosalicylic acid (5-ASA), corticosteroids, as well as immunosuppressants, azathioprine, and methotrexate. Antitumor necrosis factor (TNF)-α antibodies, vedolizumab (anti-α4β7 integrin antibody), and ustekinumab (interleukin [IL]-12/IL-23 antibody) are effective biological agents to treat severe forms of IBD. Conventional treatment with biologicals, however, can come with side effects such as opportunistic infections, malignancies, and infusion/injection reactions (12). Patients, therefore, often seek alternative forms of treatment. More than 50% of patients with IBD reportedly have sought treatment with complementary and alternative medicine, for instance, treatment with prebiotics, vitamins, probiotics, and Medical Cannabis, at some point of the disease (13).
USE OF CANNABIS/CANNABINOIDS IN IBD
Surveys/questionnaires
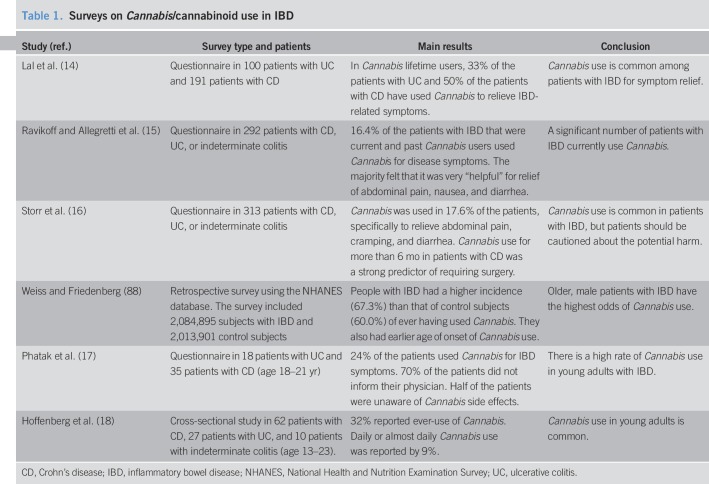
Several questionnaires and surveys have indicated that “self-treatment” with Cannabis for symptom relief is common in patients with IBD and that Cannabis is generally well tolerated (14–16). However, 1 study also noted that in patients with CD, Cannabis use was associated with a higher risk for surgery (16). Surveys in young adults showed that a high number of patients with IBD were using Cannabis (17,18), but that they were unaware of adverse effects and usually did not inform their physician about its use (17). Most surveys suggest that Cannabis provides some benefit for patients with IBD, although the caveats of adverse effects and a higher risk for surgery in patients with CD remain. Interestingly, legalization of Cannabis did not prompt more patients with IBD to seek medical Cannabis therapy, although this may have been expectable (19). An overview of surveys on the Cannabis use in IBD and the main findings is given in Table 1.
Table 1.
Surveys on Cannabis/cannabinoid use in IBD
Observational and prospective clinical studies
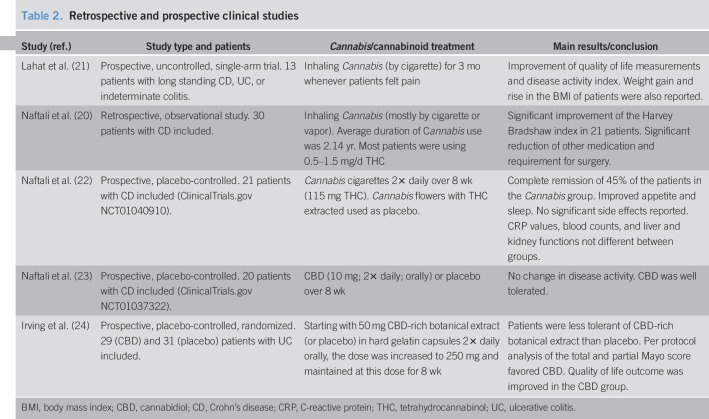
An early observational/retrospective study in patients with CD described Cannabis consumption as helpful (20). This observation was corroborated by Lahat et al. (21) who reported an improvement of disease activity index and quality of life in a small cohort of patients with IBD. Three prospective clinical studies followed; however, this time, placebo groups were included. Naftali et al. (22) investigated the effects of inhaled Cannabis in comparison to THC-free Cannabis (placebo group) in patients with CD and reported an improved CD activity index (<150) in 5 out of 11 subjects of the Cannabis group as compared to 1 of 10 subjects in the placebo group. However, there was no change in the levels of C-reactive protein, indicating symptomatic relief rather than reduction of inflammation. Two other trials investigated the effects of CBD in human IBD (23,24). Based on preclinical findings that CBD acts as an anti-inflammatory agent in models of intestinal inflammation (25–27), 2 clinical trials with CBD were carried out in patients with CD (23) and UC (24). CBD treatment did not have an influence on disease activity and C-reactive protein levels in patients with CD as compared to the placebo group (23). However, a trial that investigated the effects of CBD-rich botanical extract in patients with UC revealed that after per protocol analysis, quality of life outcomes were improved in patients taking the extract (24). So far, no clinical trial has investigated the effects of synthetic cannabinoids such as dronabinol or nabilone, but some case reports already suggest that nabilone, a THC analogue that is primarily used as an antiemetic, provides antidiarrheal effects which could be helpful in IBD treatment (28). An overview on clinical studies on Cannabis treatment in patients with IBD together with the main findings is given in Table 2.
Table 2.
Retrospective and prospective clinical studies
CANNABIS/CANNABINOIDS AND IBD
The endocannabinoid system in the gut
Any IBD therapy with Cannabis/cannabinoids is based on cannabinoids such as THC acting through the body's endocannabinoid system (ECS) through activation of cannabinoid 1 (CB1) and 2 (CB2) receptors. In general, the ECS includes receptors (CB1 and CB2), their endogenous ligands (the so-called endocannabinoids, e.g., anandamide [AEA] and 2-arachidonoylglycerol [2-AG]), and the ligands' synthesizing (diacylglycerol lipase and N-acylphosphatidylethanolamine phospholipase D) and degrading enzymes (fatty acid amide hydrolase [FAAH] and monoacylglycerol lipase [MGL]). There are several other receptors—non-CB1/CB2 receptors—such as GPR55, GPR119, PPARα, PPARγ, and TRPV1 that are phylogenetically unrelated to the 2 CB receptors but have the ability to respond to (endo)cannabinoids (review. in Ref. (8)). They modulate CB receptor's actions and are considered part of a broader system, the “endocannabinoidome,” a term that has been coined to unite the ECS, non-CB1/CB2 receptors, and endocannabinoid-like ligands (e.g., oleoylethanolamide and palmitoylethanolamide) in 1 system (29). All components of the “endocannabinoidome” are found in the gastrointestinal (GI) tract (30–32). CB1 is primarily present in enteric cholinergic neurons where it inhibits neuronal hyperactivity (33), thus alleviating strong bowel contractions and secretion. CB1 receptors are also present in enterocytes (30), where they are most likely involved in the regulation of mucosal permeability and wound healing (34,35). Unlike CB1, CB2 receptors are only little found in enteric neurons (30) but rather in B and T cells (30) as well as macrophages (35). One of the main purposes of the ECS in the gut is the maintenance of immune tolerance, and CB2 plays an active role in it (36). We also found a high expression of MGL, the 2-AG degrading enzyme, in macrophages of the colon (30). Because pharmacological blockade of MGL was shown to reduce experimentally induced colitis, high MGL activity may promote intestinal inflammation (37). It should be noted that ECS components are also abundantly present in the brain and that centrally mediated cannabinoid effects may be involved in cannabinoid-induced improvement of intestinal inflammation. For instance, intracerebroventricular activation of CB receptors by WIN55, 212-2 was shown to inhibit whole gut transit in mice (38) and genetic deletion of CB1 in the vagus caused an increase in GI motility (39), suggesting that the gut-brain axis can be manipulated by cannabinoids. In agreement with this concept, central application of CB receptor agonists was able to improve inflammation in a mouse model of colitis (40).
ECS and microbiota
Studies of adipogenesis and obesity have revealed a link between the ECS and the microbiome (41,42). By prebiotic and antibody treatment, authors demonstrated that gut microbiota could selectively modulate colonic CB1, FAAH, and MGL expression in mice (41). CB1 antagonists improved diet-induced metabolic dysfunction correlating with an increase in Akkermansia municiphila (43)—a strain known to have beneficial effects in the dextrane sulfate sodium (DSS)-induced colitis model (44). Most interestingly, genetic deletion of N-acylphosphatidyl-ethanolamine phospholipase D (and therefore, the production of AEA and related N-acylethanolamines) in intestinal epithelial cells drastically altered the composition of the gut microbiota (45), suggesting a physiological ECS-microbiota relationship. Another important observation for the presence of ECS-microbiota interaction is that Lactobacillus acidophilus induced CB2 receptor expression in intestinal epithelial cells and in the rodent gut mucosa (46). Manipulation of the ECS by Cannabis/cannabinoids may, therefore, contribute to an improvement of IBD symptoms.
Preclinical evidence of beneficial effects of Cannabis/cannabinoids in IBD
Preclinical evidence for a beneficial effect of Cannabis/cannabinoids in colitis has been mostly received from mouse models and recently reviewed in a meta-analysis (47). There is a huge amount of literature that confirms the observation that Cannabis/cannabinoids diminish inflammation in rodent models of colitis. Cannabis extracts, phytocannabinoids (e.g., CBD), synthetic CB1/CB2 agonists (e.g., WIN55, 212-2), endocannabinoids (e.g., AEA), and inhibitors of FAAH and MGL (to raise endocannabinoid levels) all effectively diminished macroscopic disease index and myeloperoxidase activity (47).
Evidence that the ECS is involved in human IBD comes from studies in which blood and intestinal mucosal biopsies from patients with IBD were used to measure endocannabinoid levels (48) and expression of several other ECS components (31). From these studies, it can be assumed that the ECS is deranged in patients with IBD and could even play some role in the development of IBD. In intestinal mucosal biopsies of patients with CD, gene expression levels of diacylglycerol lipase α, the 2-AG synthesizing enzyme, were increased, whereas those of CB1 and GPR119 were decreased in comparison to controls (31), indicating a potential role for endocannabinoids and their receptors in IBD. How cannabinoids directly affect human tissue was shown in ex vivo assays with cultured human intestinal mucosal biopsies: CBD (49) and Δ9-tetrahydrocannabinolic acid (50) prevented production of inflammatory cytokines, Cox-2, and metalloproteinase-9 in the colonic mucosal samples from patients with IBD. Interestingly, CBD and palmitoylethanolamide also strengthened the mucosal barrier in humans in vivo as evidenced by the inhibition of aspirin-induced absorption of lactulose and mannitol (51). Preclinical evidence, therefore, strongly suggests that ingredients of Cannabis are of benefit for patients with IBD, warranting further studies. Potential mechanisms of how Cannabis/cannabinoids may exert anti-inflammatory effects in the human GI tract are shown in Figure 1.
Figure 1.
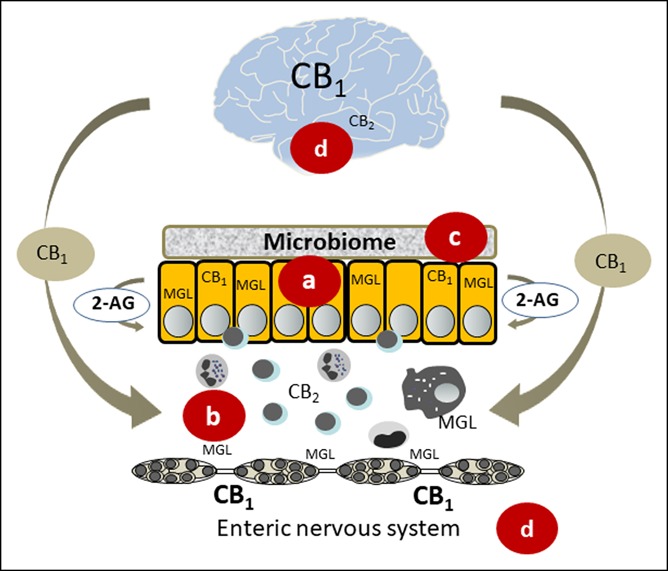
Potential mechanisms underlying beneficial effects of Cannabis/cannabinoids in inflammatory bowel disease. (a) Cannabinoids protect the mucosal barrier and promote wound healing (35). Blockade of MGL increases 2-AG levels, which promotes protection in colitis (37). (b) Cannabis/cannabinoids influence the activity of immunocytes and promote apoptosis in T cells (86). (c) The microbiome interacts with the endocannabinoid system in maintaining a healthy barrier (41,42). (d) CB1 receptors are found in the brain (87) along the gut brain axis (39) and the enteric nervous system. CB1 receptors in the brain may be essential in the protection against intestinal inflammation (40). 2-AG, 2-arachidonoylglycerol; CB1, cannabinoid 1 receptor; CB2, cannabinoid 2 receptor; and MGL, monoacylglycerol lipase.
EVIDENCE, SAFETY, AND ADVERSE EFFECTS OF CANNABIS/CANNABINOID TREATMENT
Despite overwhelming preclinical data showing beneficial effects of cannabinoids in mouse models of intestinal inflammation, clinical data are only beginning to emerge. So far, clinical evidence for an improvement of patients with IBD by Cannabis is only based on surveys and a few clinical trials (Tables 1 and 2); most of the trials are uncontrolled and underpowered. Based on these few studies, Cannabis could have some therapeutic potential. Cannabis is particularly helpful in alleviating symptoms such as cramping, abdominal pain, and diarrhea. Patients also report an improvement of overall well-being, which (because effects on inflammation markers were unchanged) may be caused by central effects (22). Although significant side effects were not reported in that study (22), short-term side effects have been reported for Cannabis/cannabinoid treatment in other diseases and reviewed in a meta-analysis (52). Dizziness, dry mouth, nausea, somnolence, and euphoria were the most common short-term side effects; however, anxiety and hallucinations also occurred (52). These effects are caused by the activation of central CB1 receptors by THC and are likely to be dose dependent, suggesting that application of low, but effective, doses might be a way to prevent/reduce them. In this context, nabilone, a THC analogue, decreased spasticity-related pain in a clinical trial at a dose as low as 1 mg/d causing only mild but well-tolerable side effects (53). Concerning the mode of application of THC, another caveat has recently emerged. After an outbreak of acute lipoid pneumonia associated with the use of electronic cigarettes (e-cigarettes) (54), the Center for Disease Control and Prevention has recommended that, because of the possibility of severe lung injury, persons should not use e-cigarette, or vaping, products that contain THC (55).
Little is known on medicinal long-term use of Cannabis. From recreational Cannabis use, we know that there is an 8.9% cumulative probability of a transition to dependence (22.7% for alcohol users) (56). Long-term Cannabis use increases the risk of psychotic outcomes (57) and of cardiovascular (58) and lung diseases (59). Male reproductive functions are also negatively affected by Cannabis use (60). Regular Cannabis use has been associated with a loss in grey matter in brain areas rich in CB1 expression in people between 18 and 30 years (61); therefore, adolescence is a strong caveat for long-term use of medical Cannabis, which may be necessary for the an effective treatment of IBD. There is also evidence that Cannabis use during pregnancy may cause problems in neurological development and cognitive functions of the child (62). The authors recommend that unless more evidence is available to counsel pregnant women on Cannabis use, they should not use Cannabis in pregnancy or while lactating (62).
USE OF OPIOIDS IN IBD
Similar to the ECS, the endo-opioid system, which is composed of mu- (MOR), kappa-, and delta-opioid receptors and their endogenous ligands (met-enkephalin, leu-enkephalin, β-endorphin, and dynorphin) regulates GI functions (63,64). Opioid receptors are expressed in smooth muscle cells, neurons, and blood vessels of the gut but are also encountered on lymphocytes and macrophages (64). Several preclinical studies suggest that the endo-opioid system could play an important role in intestinal inflammation. MOR, for instance, is increased in patients with active CD and UC (65), and MOR agonists have shown beneficial effects in preclinical models of intestinal inflammation (66–69). However, intestinal inflammation can also increase the antinociceptive tolerance to morphine (70).
Clinical implications
There are a majority of patients with IBD who experience pain (mostly abdominal and back pain) during the course of the disease (71). Opioids are, therefore, prescribed for the treatment of pain and diarrhea (72). However, IBD has also been identified as a risk factor for opioid misuse questioning the legitimacy of the wide-spread use of opioids in IBD (73). One study found that almost 45% of the patients with IBD received an opioid prescription during the study period (between 2009 and 2015) (72). Of the opioid-naïve patients in that study, more than one-third became persistent opioid users (72). In addition to the risk of opioid misuse in IBD, use of strong opioids in patients with IBD is also associated with an increased all-cause premature mortality (74). Significant side effects of opioid intake primarily consist of constipation and nausea (75), whereas serious adverse events can include fractures, cardiovascular events, and bowel obstruction (76). Persistent use of opioids may lead to narcotic bowel syndrome, which is characterized by abdominal pain that worsens with continued or escalating dosages of narcotics (77).
Not only activation but also blockade of opioid receptors may provide some benefit for patients with IBD. A low dose of naltrexone (LDN), a MOR receptor antagonist, has been suggested to improve IBD. In a study by Lie et al. (78), LDN (4.5 mg naltrexone once daily) induced clinical improvement in almost 75% and remission in ∼25% of conventional therapy-refractory patients with IBD. However, a systematic review in the Cochrane Database revealed that from the existing literature, there was not enough evidence to draw conclusions on efficacy and safety of LDN in CD (79). Persistent LDN usage may, however, favor a decline in the use of anti-inflammatory agents and immunosuppressants (80).
CONCLUSIONS AND RECOMMENDATION FOR CLINICAL USE OF CANNABIS/CANNABINOIDS AND OPIOIDS IN IBD
There is strong preclinical evidence that Cannabis and its ingredients improve experimentally induced intestinal inflammation. Although studies indicate a significant improvement of quality of life in patients with IBD after Cannabis use, a reduction of independent anti-inflammatory markers has not been shown so far. As a major hurdle of treatment, THC is a psychotropic substance and causes strong side effects. As recently pointed out by 2 reviews from the Cochrane Library, the effects and safe application of Cannabis, Cannabis oil, and CBD in CD and UC are still uncertain until larger trials assess efficacy and safety. In addition, optimal doses and routes of application still need to be investigated (81,82), leaving health professionals with no clear recommendation. For more information on the legal status of medical Cannabis and practical advice for clinicians, the reader is referred to a position paper by the Crohn's and Colitis Foundation (83). As for medical Cannabis, its use as a complementary medicine for conventional therapy-resistant patients with IBD is quite possible, but the hope that (endo)cannabinoids find a way into the treatment of IBD probably lies in the future use of compounds that manipulate the ECS, such as FAAH and MGL inhibitors, with then minimal central side effects. It remains unclear whether cannabinoids may be a valuable tool as an add-on treatment when functional symptoms such as abdominal pain (despite good control of the inflammation) are a problem because the present clinical trials clearly show that cannabinoids are helpful in relieving functional symptoms in patients with IBD.
Prolonged opioid treatment in IBD not only increases the risk of misuse, severe adverse effects, and even premature mortality but also is an important predictor of emergent encounters and is associated with higher total health care costs (84). Therefore, pain, anxiety, and depression should be carefully assessed in patients with IBD to reduce comorbidities and adverse events. A recommendation by the Canadian IBD Network for Research and Growth in Quality Improvement in collaboration with Crohn's and Colitis Canada and the Canadian Association of Gastroenterology points out that while opioids can be used in select acute settings to manage abdominal pain, their long-term use for managing IBD-related abdominal pain should be avoided (85).
CONFLICTS OF INTEREST
Guarantor of the article: Rudolf Schicho, PhD.
Specific author contributions: Melanie Kienzl, MSc and Rudolf Schicho, PhD, wrote and contributed equally to the manuscript. M.S. wrote and critically revised the manuscript. All authors read and approved the final version of the manuscript.
Financial support: This work was supported by the grants P30144 and KLI521 to R.S. from the Austrian Science Fund (FWF).
Potential competing interests: None to report.
REFERENCES
- 1.Mechoulam R. The pharmacohistory of Cannabis sativa. In: Cannabinoids as Therapeutic Agents. CRC Press: Boca Raton, FL, 1986, pp 1–19. [Google Scholar]
- 2.Belenko SR. Drugs and Drug Policy in America: A Documentary History. Greenwood Press: Westport, CT, 2000. [Google Scholar]
- 3.Mechoulam R, Gaoni Y. A total synthesis of DL-delta-1-tetrahydrocannabinol, the active constituent of hashish. J Am Chem Soc 1965;87:3273–5. [DOI] [PubMed] [Google Scholar]
- 4.Di Marzo V, Fontana A, Cadas H, et al. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994;372:686–91. [DOI] [PubMed] [Google Scholar]
- 5.Izzo AA, Borrelli F, Capasso R, et al. Non-psychotropic plant cannabinoids: New therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 2009;30:515–27. [DOI] [PubMed] [Google Scholar]
- 6.ElSohly MA, Radwan MM, Gul W, et al. Phytochemistry of Cannabis sativa L. Prog Chem Org Nat Prod 2017;103:1–36. [DOI] [PubMed] [Google Scholar]
- 7.Console-Bram L, Marcu J, Abood ME. Cannabinoid receptors: Nomenclature and pharmacological principles. Prog Neuropsychopharmacol Biol Psychiatry 2012;38:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasenoehrl C, Taschler U, Storr M, et al. The gastrointestinal tract: A central organ of cannabinoid signaling in health and disease. Neurogastroenterol Motil 2016;28:1765–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest 2007;117:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 11.Burisch J, Jess T, Martinato M, et al. The burden of inflammatory bowel disease in Europe. J Crohn's Colitis 2013;7:322–37. [DOI] [PubMed] [Google Scholar]
- 12.Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol 2010;24:167–82. [DOI] [PubMed] [Google Scholar]
- 13.Basson AR, Lam M, Cominelli F. Complementary and alternative medicine strategies for therapeutic gut microbiota modulation in inflammatory bowel disease and their next-generation approaches. Gastroenterol Clin North Am 2017;46:689–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lal S, Prasad N, Ryan M, et al. Cannabis use amongst patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2011;23:891–6. [DOI] [PubMed] [Google Scholar]
- 15.Ravikoff Allegretti J, Courtwright A, Lucci M, et al. Marijuana use patterns among patients with inflammatory bowel disease. Inflamm Bowel Dis 2013;19:2809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Storr M, Devlin S, Kaplan GG, et al. Cannabis use provides symptom relief in patients with inflammatory bowel disease but is associated with worse disease prognosis in patients with Crohn's disease. Inflamm Bowel Dis 2014;20:472–80. [DOI] [PubMed] [Google Scholar]
- 17.Phatak UP, Rojas-Velasquez D, Porto A, et al. Prevalence and patterns of marijuana use in young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2017;64:261–4. [DOI] [PubMed] [Google Scholar]
- 18.Hoffenberg EJ, McWilliams S, Mikulich-Gilbertson S, et al. Cannabis oil use by adolescents and young adults with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2019;68:348–52. [DOI] [PubMed] [Google Scholar]
- 19.Merker AM, Riaz M, Friedman S, et al. Legalization of medicinal marijuana has minimal impact on use patterns in patients with inflammatory bowel disease. Inflamm Bowel Dis 2018;24:2309–14. [DOI] [PubMed] [Google Scholar]
- 20.Naftali T, Lev LB, Yablecovitch D, et al. Treatment of Crohn's disease with Cannabis: An observational study. Isr Med Assoc J 2011;13:455–8. [PubMed] [Google Scholar]
- 21.Lahat A, Lang A, Ben-Horin S. Impact of Cannabis treatment on the quality of life, weight and clinical disease activity in inflammatory bowel disease patients: A pilot prospective study. Digestion 2012;85:1–8. [DOI] [PubMed] [Google Scholar]
- 22.Naftali T, Bar-Lev Schleider L, Dotan I, et al. Cannabis induces a clinical response in patients with Crohn's disease: A prospective placebo-controlled study. Clin Gastroenterol Hepatol 2013;11:1276–80.e1. [DOI] [PubMed] [Google Scholar]
- 23.Naftali T, Mechulam R, Marii A, et al. Low-dose cannabidiol is safe but not effective in the treatment for Crohn's disease, a randomized controlled trial. Dig Dis Sci 2017;62:1615–20. [DOI] [PubMed] [Google Scholar]
- 24.Irving PM, Iqbal T, Nwokolo C, et al. A randomized, double-blind, placebo-controlled, parallel-group, pilot study of cannabidiol-rich botanical extract in the symptomatic treatment of ulcerative colitis. Inflamm Bowel Dis 2018;24:714–24. [DOI] [PubMed] [Google Scholar]
- 25.Borrelli F, Aviello G, Romano B, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med (Berl) 2009;87:1111–21. [DOI] [PubMed] [Google Scholar]
- 26.De Filippis D, Esposito G, Cirillo C, et al. Cannabidiol reduces intestinal inflammation through the control of neuroimmune axis. PLoS One 2011;6:e28159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schicho R, Storr M. Topical and systemic cannabidiol improves trinitrobenzene sulfonic acid colitis in mice. Pharmacology 2012;89:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pellesi L, Verga MC, De Maria N, et al. Nabilone administration in refractory chronic diarrhea: A case series. BMC Gastroenterol 2019;19:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Marzo V, Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics 2015;12:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grill M, Hasenoehrl C, Kienzl M, et al. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem Cell Biol 2019;151:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grill M, Högenauer C, Blesl A, et al. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci Rep 2019;9:2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: From pathophysiology to therapeutic opportunity. Trends Mol Med 2012;18:615–25. [DOI] [PubMed] [Google Scholar]
- 33.Boesmans W, Ameloot K, van den Abbeel V, et al. Cannabinoid receptor 1 signalling dampens activity and mitochondrial transport in networks of enteric neurones. Neurogastroenterol Motil 2009;21:958-e77. [DOI] [PubMed] [Google Scholar]
- 34.Karwad MA, Couch DG, Theophilidou E, et al. The role of CB1 in intestinal permeability and inflammation. FASEB J 2017;31:3267–77. [DOI] [PubMed] [Google Scholar]
- 35.Wright K, Rooney N, Feeney M, et al. Differential expression of cannabinoid receptors in the human colon: Cannabinoids promote epithelial wound healing. Gastroenterology 2005;129:437–53. [DOI] [PubMed] [Google Scholar]
- 36.Acharya N, Penukonda S, Shcheglova T, et al. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci USA 2017;114:5005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhouayek M, Lambert DM, Delzenne NM, et al. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J 2011;25:2711–21. [DOI] [PubMed] [Google Scholar]
- 38.Li K, Fichna J, Schicho R, et al. A role for O-1602 and G protein-coupled receptor GPR55 in the control of colonic motility in mice. Neuropharmacology 2013;71:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vianna CR, Donato J, Jr, Rossi J, et al. Cannabinoid receptor 1 in the vagus nerve is dispensable for body weight homeostasis but required for normal gastrointestinal motility. J Neurosci 2012;32:10331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fichna J, Bawa M, Thakur GA, et al. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS One 2014;9:e109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muccioli GG, Naslain D, Bäckhed F, et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 2010;6:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cani PD, Plovier H, Van Hul M, et al. Endocannabinoids—at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol 2016;12:133–43. [DOI] [PubMed] [Google Scholar]
- 43.Mehrpouya-Bahrami P, Chitrala KN, Ganewatta MS, et al. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci Rep 2017;7:15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang CS, Ban M, Choi EJ, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One 2013;8:e76520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Everard A, Plovier H, Rastelli M, et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun 2019;10:457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rousseaux C, Thuru X, Gelot A, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 2007;13:35–7. [DOI] [PubMed] [Google Scholar]
- 47.Couch DG, Maudslay H, Doleman B, et al. The use of cannabinoids in colitis: A systematic review and meta-analysis. Inflamm Bowel Dis 2018;24:680–97. [DOI] [PubMed] [Google Scholar]
- 48.Di Sabatino A, Battista N, Biancheri P, et al. The endogenous cannabinoid system in the gut of patients with inflammatory bowel disease. Mucosal Immunol 2011;4:574–83. [DOI] [PubMed] [Google Scholar]
- 49.Couch DG, Tasker C, Theophilidou E, et al. Cannabidiol and palmitoylethanolamide are anti-inflammatory in the acutely inflamed human colon. Clin Sci 2017;131:2611–26. [DOI] [PubMed] [Google Scholar]
- 50.Nallathambi R, Mazuz M, Ion A, et al. Anti-inflammatory activity in colon models is derived from delta9-tetrahydrocannabinolic acid that interacts with additional compounds in Cannabis extracts. Cannabis Cannabinoid Res 2017;2:167–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Couch DG, Cook H, Ortori C, et al. Palmitoylethanolamide and cannabidiol prevent inflammation-induced hyperpermeability of the human gut in vitro and in vivo: A randomized, placebo-controlled, double-blind controlled trial. Inflamm Bowel Dis 2019;25:1006–18. [DOI] [PubMed] [Google Scholar]
- 52.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 2015;313:2456–73. [DOI] [PubMed] [Google Scholar]
- 53.Wissel J, Haydn T, Muller J, et al. Low dose treatment with the synthetic cannabinoid nabilone significantly reduces spasticity-related pain: A double-blind placebo-controlled cross-over trial. J Neurol 2006;253:1337–41. [DOI] [PubMed] [Google Scholar]
- 54.Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette-associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep 2019;68:784–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siegel DA, Jatlaoui TC, Koumans EH, et al. Update: Interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury—United States, October 2019. MMWR Morb Mortal Wkly Rep 2019;68:919–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez-Quintero C, Perez de los Cobos J, Hasin DS, et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: Results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend 2011;115:120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moore TH, Zammit S, Lingford-Hughes A, et al. Cannabis use and risk of psychotic or affective mental health outcomes: A systematic review. Lancet 2007;370:319–28. [DOI] [PubMed] [Google Scholar]
- 58.Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: What cardiologists need to know. Am J Cardiol 2014;113:187–90. [DOI] [PubMed] [Google Scholar]
- 59.Joshi M, Joshi A, Bartter T. Marijuana and lung diseases. Curr Opin Pulm Med 2014;20:173–9. [DOI] [PubMed] [Google Scholar]
- 60.Rossato M, Pagano C, Vettor R. The cannabinoid system and male reproductive functions. J Neuroendocrinol 2008;20(Suppl 1):90–3. [DOI] [PubMed] [Google Scholar]
- 61.Battistella G, Fornari E, Annoni JM, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology 2014;39:2041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Metz TD, Stickrath EH. Marijuana use in pregnancy and lactation: A review of the evidence. Am J Obstet Gynecol 2015;213:761–78. [DOI] [PubMed] [Google Scholar]
- 63.Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept 2009;155:11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sobczak M, Sałaga M, Storr MA, et al. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: Current concepts and future perspectives. J Gastroenterol 2014;49:24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Philippe D, Chakass D, Thuru X, et al. Mu opioid receptor expression is increased in inflammatory bowel diseases: Implications for homeostatic intestinal inflammation. Gut 2006;55:815–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest 2003;111:1329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anselmi L, Huynh J, Duraffourd C, et al. Activation of mu opioid receptors modulates inflammation in acute experimental colitis. Neurogastroenterol Motil 2015;27:509–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fakhraei N, Javadian N, Rahimian R, et al. Involvement of central opioid receptors in protective effects of methadone on experimental colitis in rats. Inflammopharmacology 2018;26:1399–413. [DOI] [PubMed] [Google Scholar]
- 69.Salaga M, Mokrowiecka A, Jacenik D, et al. Systemic administration of sialorphin attenuates experimental colitis in mice via interaction with mu and kappa opioid receptors. J Crohn's Colitis 2017;11:988–98. [DOI] [PubMed] [Google Scholar]
- 70.Komla E, Stevens DL, Zheng Y, et al. Experimental colitis enhances the rate of antinociceptive tolerance to morphine via peripheral opioid receptors. J Pharmacol Exp Ther 2019, 370:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeitz J, Ak M, Müller-Mottet S, et al. Pain in IBD patients: Very frequent and frequently insufficiently taken into account. PLoS One 2016;11:e0156666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noureldin M, Higgins PDR, Govani SM, et al. Incidence and predictors of new persistent opioid use following inflammatory bowel disease flares treated with oral corticosteroids. Aliment Pharmacol Ther 2019;49:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Targownik LE, Nugent Z, Singh H, et al. The prevalence and predictors of opioid use in inflammatory bowel disease: A population-based analysis. Am J Gastroenterol 2014;109:1613–20. [DOI] [PubMed] [Google Scholar]
- 74.Burr NE, Smith C, West R, et al. Increasing prescription of opiates and mortality in patients with inflammatory bowel diseases in England. Clin Gastroenterol Hepatol 2018;16:534–41.e6. [DOI] [PubMed] [Google Scholar]
- 75.Furlan AD, Sandoval JA, Mailis-Gagnon A, et al. Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects. CMAJ 2006;174:1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Von Korff M, Kolodny A, Deyo RA, et al. Long-term opioid therapy reconsidered. Ann Intern Med 2011;155:325–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grunkemeier DM, Cassara JE, Dalton CB, et al. The narcotic bowel syndrome: Clinical features, pathophysiology, and management. Clin Gastroenterol Hepatol 2007;5:1126–39; quiz 1121–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lie MRKL, van der Giessen J, Fuhler GM, et al. Low dose Naltrexone for induction of remission in inflammatory bowel disease patients. J Transl Med 2018;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parker CE, Nguyen TM, Segal D, et al. Low dose naltrexone for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2018;4:CD010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raknes G, Simonsen P, Smabrekke L. The effect of low-dose naltrexone on medication in inflammatory bowel disease: A quasi experimental before-and-after prescription database study. J Crohn's Colitis 2018;12:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of Crohn's disease. Cochrane Database Syst Rev 2018;11:CD012853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kafil TS, Nguyen TM, MacDonald JK, et al. Cannabis for the treatment of ulcerative colitis. Cochrane Database Syst Rev 2018;11:CD012954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swaminath A, Berlin EP, Cheifetz A, et al. The role of cannabis in the management of inflammatory bowel disease: A review of clinical, scientific, and regulatory information. Inflamm Bowel Dis 2019;25:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alley K, Singla A, Afzali A. Opioid use is associated with higher health care costs and emergency encounters in inflammatory bowel disease. Inflamm Bowel Dis 2019;25:1990–5. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen GC, Boland K, Afif W, et al. Modified Delphi process for the development of choosing wisely for inflammatory bowel disease. Inflamm Bowel Dis 2017;23:858–65. [DOI] [PubMed] [Google Scholar]
- 86.Singh UP, Singh NP, Singh B, et al. Cannabinoid receptor-2 (CB2) agonist ameliorates colitis in IL-10(-/-) mice by attenuating the activation of T cells and promoting their apoptosis. Toxicol Appl Pharmacol 2012;258:256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience 1999;92:1177–91. [DOI] [PubMed] [Google Scholar]
- 88.Weiss A, Friedenberg F. Patterns of Cannabis use in patients with inflammatory bowel disease: A population based analysis. Drug Alcohol Depend 2015;156:84–9. [DOI] [PubMed] [Google Scholar]