Abstract
OBJECTIVES:
We tested the hypothesis that a genetic deletion (Del) variant in the REPIN1 gene is associated with the severity of nonalcoholic fatty liver disease (NAFLD) in humans.
METHODS:
Sixty-three donors of liver biopsies from individuals with obesity and different degrees of NAFLD and fibrosis were screened for a Del REPIN1 gene variant and liver REPIN1 mRNA expression.
RESULTS:
In 8 homozygous Del carriers, we found significantly lower NAFLD activity and fibrosis scores compared with 55 wild-type allele carriers.
DISCUSSION:
A Del variant of REPIN1 may be associated with a lower risk of the development of NAFLD.
INTRODUCTION
Pathological accumulation of hepatic fat can lead to nonalcoholic fatty liver disease (NAFLD), including simple steatosis and nonalcoholic steatohepatitis (NASH), which may progress to cirrhosis and increase the risk to develop hepatocellular carcinoma (1). Although obesity is an important risk factor for NAFLD, not all patients with obesity develop steatosis hepatis (2). The mechanisms underlying hepatic steatosis and its progression to more severe stages are complex and involve nutritive, behavioral, genetic, epigenetic, as well as environmental factors. In this context, prospective twin studies estimated the heritable component of hepatic steatosis at ∼50% (3). Recently, we found that a genetic variant, a 12 base pair (bp) deletion (Figure 1a), within the REPIN1 gene causes a loss of function and is associated with alterations in glucose and lipid metabolism (4). Functional consequences of this variant were confirmed in HepG2 cells in vitro (4). In addition, studies in mice lacking hepatocellular Repin1 provided evidence that loss of Repin1 in the liver attenuates progression of NAFLD most likely by reducing fat accumulation and alleviating chronic tissue inflammation and injury (5). Moreover, Repin1-deficient mice exhibited lower NAFLD-related tumor incidence accompanied by a lower liver weight/body weight index (5). Beneficial effects of a liver-specific REPIN1 small interfering RNA (siRNA) treatment confirmed the potential of REPIN1 as a target gene for the prevention and therapy of NAFLD (5). These results prompted us to search for homozygous carriers of the 12 bp deletion in the REPIN1 gene in a cohort of human liver biopsy donors (N = 63). In a cross-sectional study, we compared homozygous deletion carrier (Del) with wild-type carrier.
Figure 1.
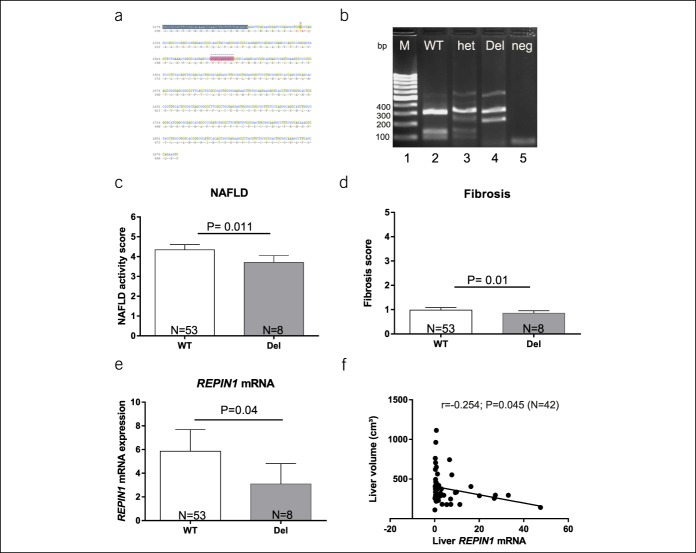
(a) Sequence of REPIN1 region with the12 bp deletion marked in gray and (b) REPIN1 genotyping by agarose gel after RFLP for the human genotyping. Lane 1 marker (M, 100bp marker), lane 2 WT, homozygote for wild-type (WT, 3 fragments, 99bp, 143bp, 322bp), lane 3 het, heterozygote (het, 4 fragments, 99 bp, 143 bp, 230 bp, 322 bp), lane 4 homozygote for 12 bp deletion (Del, 2 fragments, 230 bp, 322 bp), and lane 5 neg (negative control). (c and d) The NAFLD activity score and fibrosis score in subjects with REPIN1 wildtype allele (WT, N = 53) and homozygous deletion (Del, N = 8) variant. (c) The NAFLD activity score and (d) fibrosis score are significantly reduced in subjects with REPIN1 Del variant compared with wildtype allele carrier. (e) Significantly reduced REPIN1 mRNA expression in liver biopsies in subjects with REPIN1 Del variant compared with wildtype allele carrier. Results are expressed as means ± SE. (f) Liver volume (cm3) correlation of all subjects with hepatic mRNA level of REPIN1 (N = 42). bp, base pair; NAFLD, nonalcoholic fatty liver disease; RNA, ribonucleic acid; RFLP, restriction fragment length polymorphism.
METHODS
Study population
The middle-aged cohort was recruited at the University Hospital of Leipzig, Germany, and includes 63 (men, n = 21; women, n = 42) subjects. We selected small liver biopsies in patients, who underwent abdominal surgery for Roux-en-Y gastric bypass, sleeve gastrectomy, or elective cholecystectomy (6). All participants gave their written informed consent before taking part in the study. All investigations were approved by the Ethics Committee of the University of Leipzig, Germany (363-10-13122010 and 017-12-230112), and performed in accordance with the Declaration of Helsinki.
Anthropometric measures and HbA1c levels
All patients underwent anthropomorphic measurements (weight and height) using standardized methods, and body mass index (BMI) was calculated as weight (kg)/height (m2). HbA1c levels were measured in an automated clinical chemistry analyzer (Hitachi/Roche Diagnostics, Grenzach-Wyhlen, Germany) at the Institute of Laboratory Medicine, University Hospital Leipzig.
Genotyping and REPIN1 mRNA expression analysis
Screening for the 12 bp deletion was performed by restriction fragment length polymorphism as described recently (4). Briefly, genomic DNA was amplified by polymerase chain reaction (PCR), and the corresponding product (564 bp) was subsequently digested with the enzyme ApaI. The products were visualized using gel electrophoresis (Figure 1b).
A small liver biopsy was taken during the surgery, immediately snap frozen in liquid nitrogen, and stored at −80°C until further preparations. The hepatic expression of REPIN1 mRNA has been measured by quantitative PCR using specific REPIN1 probe (Hs00274221_s1) and calculated relative to 18S rRNA (Hs99999901_s1; both Applied Biosystems, Warrington, GB). Specific mRNA expression was calculated relative to 18S rRNA which was used as reference because of its resistance to glucose-dependent regulation (7). mRNA expression levels were quantified by using the second derivative maximum method.
NASH, NAFLD activity, and fibrosis scoring
The NASH score and fibrosis score were assessed on liver sections by a certified pathologist as described elsewhere (8,9). The NASH Clinical Research Network system for scoring activity and fibrosis in NAFLD was used to calculate the NAFLD Activity Score ranging 0–8 (10).The activity score is graded according to the intensity of necroinflammatory lesions (A0 = no activity, A1 = mild activity, A2 = moderate activity, and A3 = severe activity), and the fibrosis score is assessed on a five-point scale (F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = few septa, F3 = numerous septa without cirrhosis, and F4 = cirrhosis) (11). Liver volume was quantified by magnetic resonance imaging (Achieva XR, Philips Healthcare, Best, the Netherlands; N = 42) and calculated by an adapted software package (Matlab; MathWorks, Natick, MA).
Statistical analysis
Statistical significance between the groups was evaluated using the unpaired 2-tailed Student t test. Differences were considered statistically significant at P < 0.05. Correlation between REPIN1 mRNA expression in human liver and liver volume (cm3) was assessed by the Spearman rank correlation analysis after the Kolmogorov-Smirnov test was performed to assess normality of the data. Statistical analysis of diabetes frequency was assessed with the χ2 test. Logistic linear regression analysis was performed to estimate relationship between REPIN1 genotype, BMI, age, diabetes frequency, and gender. All statistical analyses were performed using SPSS Statistics (v24; IBM Corp., Armonk, NY).
RESULTS
The study included 63 liver biopsy donors with mean age of 49 years. Among the 63 donors, we identified 8 homozygous carriers of the 12 bp deletion in the REPIN1 gene (Figure 1a,b) and no heterozygous carriers.
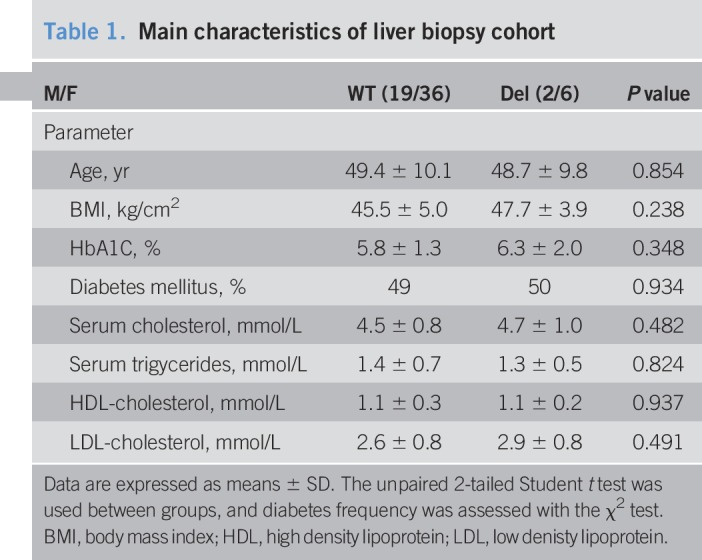
Interestingly, we observed a significant lower NAFLD activity score as well as fibrosis score in liver biopsies of subjects with Del compared with wildtype subjects (Figure 1c,d) (Table 1). There were no significant differences between carriers of the 12 bp deletion and wildtype allele carriers in HbA1c levels, diabetes frequency, and BMI as shown in Table 1. Moreover, logistic linear regression analysis confirmed these findings (data not shown). Hence, we suggest that carriers of the Del variant are more protected from hepatic fat accumulation and progression to fibrosis than wildtype subjects. Furthermore, we found significant lower REPIN1 mRNA level in human livers of Del carrier compared with wildtype allele carriers (Figure 1e). Interestingly, a negative correlation between hepatic REPIN1 mRNA expression level and liver volume was found as well (Figure 1f).
Table 1.
Main characteristics of liver biopsy cohort
DISCUSSION
Obesity is an important risk factor for NAFLD, but not all patients with obesity develop steatosis hepatis (2). The factors and mechanisms that cause progression from steatosis to hepatocellular carcinoma are not fully understood. Prospective twin studies estimated the heritable component of hepatic steatosis at ∼50% (3). Previous findings suggested that REPIN1 plays a significant role in lipid metabolism and glucose homeostasis (5,6,12,13). In the present cross-sectional study of middle-aged participants, we demonstrate in humans that the genetic 12 bp Del variant of REPIN1 is associated with a lower severity of NAFLD despite obesity and independently from diabetes mellitus, gender, and age. Our findings are supported by the in vivo data in mice with progressive NAFLD that hepatic REPIN1 deficiency attenuated NAFLD progression by alleviating systemic and hepatic lipid accumulation, chronic inflammation, and subsequently reducing liver injury (5). Consequently, and most strikingly, these mice exhibited lower NAFLD-related tumor incidence accompanied by a lower liver weight/body weight index (5). Moreover, REPIN1 siRNA treatment confirmed the potential of REPIN1 as a target gene for the prevention and therapy of NAFLD (5). Liver volume is known to be related to NAFLD and human obesity (14,15). However, not consistent is the negative correlation between hepatic REPIN1 mRNA expression level and liver volume. As REPIN1 deficiency was observed to be accompanied with less fat accumulation in recent studies (6,13), and a correlation between the degree of steatosis and liver volume in NAFLD exists (16), it should be expected that changes in hepatic fat content in human livers of Del carrier are also accompanied by changes in liver volume. Thus, this fact needs further investigations in larger prospective studies with more homozygous Del carriers to interpret and validate our study results. In summary, we conclude that REPIN1 might be an important genetic risk factor for the development of NAFLD and is an attractive therapeutic target for the treatment of NAFLD.
Study Highlights.
WHAT IS KNOWN
✓ Studies in mice lacking hepatocellular Repin1 provided evidence that loss of Repin1 in the liver attenuates progression of NAFLD.
WHAT IS NEW HERE
✓ Genetic variant, a 12 bp deletion of Repin1 is relevant for NAFLD in humans.
TRANSLATIONAL IMPACT
✓ Mice to human studies indicate that REPIN 1 is an important genetic risk factor for the development of NAFLD in humans.
CONFLICTS OF INTEREST
Guarantor of the article: Nora Klöting, PhD.
Specific author contributions: N.K.: contributed to the initial concept, experimental data, data collection and interpretation of results, and manuscript writing. K.A., T.S.: contributed to protocol writing, submission, and manuscript review. A.D.: performed open abdominal surgery for Roux-en-Y bypass, sleeve gastrectomy, or cholecystectomy and took liver biopsies. C.W. performed hepatic scoring. C.B. performed genotyping. M.B., C.B., K.A., and M.S.: reviewed and contributed to the manuscript writing. All authors read and approved the final manuscript.
Financial support: This work was supported by the Deutsche Forschungsgemeinschaft (SFB1052/02, # 209933838, B04 [to NK] and AB 453/2–1 [to KA]) and supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1001 (to NK) and DZD:82DZD00601 (to N.K.).
Potential competing interests: None to report.
ACKNOWLEDGEMENT
The authors thank Susan Berthold and Daniela Kern for technical assistance.
REFERENCES
- 1.Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Stefan N, Fritsche A, Schick F, et al. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol 2016;4:789–98. [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Schork N, Chen CH, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology 2015;149:1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krüger J, Berger C, Weidle K, et al. Metabolic effects of genetic variation in the human REPIN1 gene. Int J Obes (Lond) 2019;43:821–31. [DOI] [PubMed] [Google Scholar]
- 5.Abshagen K, Mense L, Fischer F, et al. Repin1 deficiency in liver tissue alleviates NAFLD progression in mice. J Adv Res 2019;16:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruschke K, Illes M, Kern M, et al. Repin1 maybe involved in the regulation of cell size and glucose transport in adipocytes. Biochem Biophys Res Commun 2010;400:246–51. [DOI] [PubMed] [Google Scholar]
- 7.Krowczynska AM, Coutts M, Makrides S, et al. The mouse homologue of the human acidic ribosomal phosphoprotein PO: A highly conserved polypeptide that is under translational control. Nucleic Acids Res 1989;17:6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology 1994;20:15–20. [PubMed] [Google Scholar]
- 9.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289–93. [DOI] [PubMed] [Google Scholar]
- 10.Kleiner DE, Brunt EM, van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 11.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: Distinct clinicopathologic meanings. Hepatology 2011;53:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abshagen K, Degenhardt B, Liebig M, et al. Liver-specific Repin1 deficiency impairs transient hepatic steatosis in liver regeneration. Sci Rep 2018;8:16858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern M, Kosacka J, Hesselbarth N, et al. Liver-restricted Repin1 deficiency improves whole-body insulin sensitivity, alters lipid metabolism, and causes secondary changes in adipose tissue in mice. Diabetes 2014;63:3295–309. [DOI] [PubMed] [Google Scholar]
- 14.Luo RB, Suzuki T, Hooker JC, et al. How bariatric surgery affects liver volume and fat density in NAFLD patients. Surg Endosc 2018;32:1675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang A, Chen J, Le TA, et al. Cross-sectional and longitudinal evaluation of liver volume and total liver fat burden in adults with nonalcoholic steatohepatitis. Abdom Imaging 2015;40:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bora A, Alptekin C, Yavuz A, et al. Assessment of liver volume with computed tomography and comparison of findings with ultrasonography. Abdom Imaging 2014;39:1153–61. [DOI] [PubMed] [Google Scholar]