Abstract
INTRODUCTION:
Primary sclerosing cholangitis (PSC) is a cholestatic liver disorder that is frequently associated with ulcerative colitis (UC). Patients with PSC and UC (PSC-UC) have a higher risk of colorectal neoplasia compared with patients with UC. The oncogenic properties of microRNA-346 (miR-346) have been recently reported. We investigated the expression of miR-346 and its 2 target genes, the receptor of vitamin D (VDR), and the tumor necrosis factor-α (TNF-α), which are known to modulate carcinogenesis.
METHODS:
Ascending and sigmoid colon biopsies were obtained from patients with PSC, PSC and UC (PSC-UC), UC, and healthy controls (n = 10 in each group). Expressions of VDR, TNF-α, 18S RNA, p27Kip1, miR-346, and reference microRNA, miR-191, were evaluated by real-time PCR using human TaqMan Gene Expression and TaqMan MicroRNA Assays. Functional studies with miR-346 mimic and inhibitor were conducted in HepG2 and Caco-2 cells. The effect of ursodeoxycholic acid on miR-346 expression was examined in Caco-2 cells.
RESULTS:
An increased expression of miR-346 in the ascending colon of PSC-UC was observed (P < 0.001 vs all groups). In patients with UC, an exceptionally low colonic expression of miRNA-346 was accompanied by the extensive upregulation of VDR and TNF-α genes. A functional in vitro analysis demonstrated that inhibition of miR-346 resulted in the upregulation of VDR and TNF-α, whereas the induction of miR-346 activity suppressed VDR, TNF-α, and p27Kip1.
DISCUSSION:
The upregulation of miRNA-346 in the colon of patients with PSC may be responsible for the disturbance of VDR and TNF-α signaling pathway, which could result in an inadequate suppression of neoplasia.
INTRODUCTION
Primary sclerosing cholangitis (PSC) is a chronic biliary disorder with a complex etiology, which is characterized by a progressive destruction of the biliary tract and consequently the liver through the mechanisms of autoimmunity and cholestasis. PSC mainly affects men and is commonly accompanied by inflammatory bowel disease (IBD), predominantly ulcerative colitis (UC) (1). Typically, patients with PSC exhibit an impaired hepatic excretion of secondary bile acids (BA), which were found to be positively associated with colonic carcinomas (2,3). Patients with PSC have an increased risk of developing primary bile duct cancer and colorectal cancer (CRC) (1,4). The risk of CRC development in patients with PSC with concurrent IBD was found to be 14% at 10 years and 31% at 20 years compared with a steady risk of 2.3% in patients without concurrent IBD (4), whereas in UC, the overall prevalence of CRC is 3.7% (5). Moreover, in most patients with IBD-PSC who developed CRC, tumors are located in the right-sided colon in contrast to patients with only IBD, in which the tumors more frequently occur in the left colon. This may suggest differences in the pathogenesis of CRC in these 2 groups of patients (6,7). CRC is not a uniform disease as it can be distinguished by a range of genomic and epigenomic modifications (8,9).
Recently, the mechanism of CRC tumorigenesis has been linked to the area of microRNAs (miRNAs) (10). MicroRNAs are a group of naturally occurring small noncoding RNA that have a length of 18–25 nucleotides, which are critical epigenomic regulators of gene expression and act either by translational repression or transcript degradation. Alterations in intracellular miRNAs were observed in numerous diseases, including carcinoma. MiRNAs may possess either tumor-suppressive or oncogenic activity, depending on target genes (11). Recently, miR-346 has been reported as an oncogenic miRNA (oncomiR) in numerous cancers, including prostate, lung, breast, and liver (12–14), but no data exist on its potential role in colorectal neoplasia.
MicroRNA-346 inhibits, among other target genes, the expression of the vitamin D receptor (VDR) via direct binding to a conserved target site within the 3′UTR of the VDR transcript (15). VDR is a nuclear receptor that mediates the biological activities of 1,25-dihydroxyvitamin D and is abundantly expressed in the epithelial cells of the gastrointestinal tract. Apart from the control of calcium homeostasis, it modulates the autocrine–paracrine regulation of cell proliferation and differentiation. The antiproliferative effects of VDR have been demonstrated in a wide variety of cancer cell lines. Several lines of evidence suggest that VDR activation, which induces the expression of cycle inhibitor p27(kip1), may be protective against cancer (16,17), and low levels of vitamin D have been associated both with cancer and altered immune responses (18–20). A reduction in epithelial VDR was suggested to affect the gut mucosal barrier and contributes to the development of IBD (21). Moreover, the role of vitamin D in immune-mediated diseases seems to be closely associated with bacterial metabolism and chronic dysbiosis may trigger VDR dysfunction (16). Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine and a key player in the pathogenesis of many inflammatory and autoimmune diseases. Recently, it was reported that miR-346 can indirectly modulate TNF-α expression either by the inhibition of Bruton tyrosine kinase (Btk) expression, which is required for TNF-α production, or by inducing tristetraprolin, which destabilizes TNF-α transcript (22).
Given that patients with PSC have an increased risk of colorectal neoplasia in comparison to healthy subjects and patients with UC, we investigated the expression of miR-346 and its 2 target genes, including VDR and TNF-α, in human colonic biopsies of patients with PSC or with UC. Moreover, the effect of miR-346 on VDR, TNF-α, and cycle inhibitor p27kip1 expression was studied in vitro.
METHODS
Patient characteristics
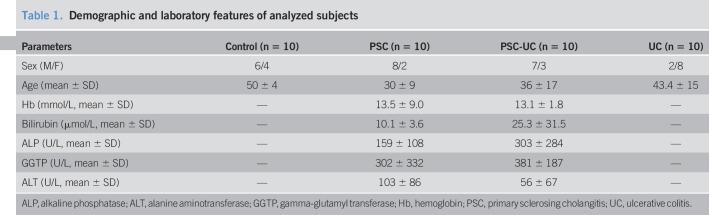
This study included 20 patients with PSC who underwent routine surveillance colonoscopies. Based on histopathological evaluation, they were divided into groups: the PSC group, who had never been diagnosed with concomitant IBD (n = 10), and the PSC-UC group showing the macroscopic features of UC on colonoscopy, which were confirmed with histology examination (n = 10). In addition, 10 patients with UC and 10 healthy controls who underwent colonoscopies for various indications and showed neither macroscopic nor microscopic abnormalities in their colons were included in the study (Table 1). All patients with PSC were treated with ursodeoxycholic acid (UDCA) (15 mg/kg body weight), and patients with PSC-UC additionally received 5ASA (2–3 g/daily). Three biopsy specimens per patients were obtained from both the ascending colon and the sigmoid colon. Each patient gave informed consent before participating in this study. The research protocol was approved by the Ethics Committee of Pomeranian Medical University and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Table 1.
Demographic and laboratory features of analyzed subjects
RNA and miRNA expression analysis
Total RNA was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany) following the manufacturer's recommendations. Expressions of specific genes were measured using human TaqMan Gene Expression Assays (TNF-α Hs00174128_m1 and p27Kip1 Hs00153277_m1) or TaqMan MicroRNA Assays (miR-346: 478046_mir). 18SRNA (Hs99999901_s1) and miR-191 (477952_mir) were used as control for data normalization. The fluorescence data were analyzed by the 7500 Fast Real-Time PCR System with 7500 Software v2.0.2 (Applied Biosystems, Foster City, CA), and relative amounts of transcripts were calculated using the 2−ΔΔCt formula.
Western blot analysis
After electrophoresis, the proteins were blotted into polyvinylidene difluoride membranes under semi-dry transfer conditions (Thermo Scientific, Rockford, IL). After blocking with 5% milk, the following antibodies were used: anti-VDR (1:200, sc-13133; Santa Cruz Biotechnology, Dallas, TX), anti-TNF-α (1:500, ab183896; Abcam, Cambridge, United Kingdom), and secondary antibodies (Code: 115-035-146; Code: 111-035-003, Jackson ImmunoResearch, West Grove, PA). Protein loading was normalized to anti-GAPDH (1:5,000, sc-365062; Santa Cruz). Bands were visualized by chemiluminescence detection (Chemiluminescent HRP Substrate, Millipore, MA) and quantified using the MicroChemi 2.0 System. Specific bands were quantified by densitometric analyses with GelQuant software (Jerusalem, Israel).
Immunohistochemistry
The paraffin sections of colon tissue after deparaffinization and antigen unmasking with citrate buffer were exposed to Normal Horse Serum to block unspecific binding (Vector Laboratories, Burlingame, CA). Anti-VDR (sc-13133, Santa Cruz) or anti-TNF-α (sc-52746, Santa Cruz) and biotinylated anti-mouse/anti-rabbit IgG (BA-1400, Vector Laboratories) were used as primary and secondary antibodies, respectively. Reactions were visualized using ABC Vectastain and DAB kits (Dako, Glostrup, Denmark). Tissue structures were visualized by Mayer hematoxylin staining (DAKO). The negative controls, in which the primary antibodies were omitted, were included in the study and uniformly demonstrated no reaction. Images were acquired with the ZEISS Axio Imager Z2 microscope.
Cell culture and transfection
HepG2 cells, as miR-346 was demonstrated to be highly expressed in that cell line (14), and human epithelial colorectal adenocarcinoma cells, Caco-2 (American Type Culture Collection), were transfected with an miR-346 inhibitor (Ambion Anti-miR miRNA Inhibitor, hsa-miR-346; ID: AM10238; Thermo Fisher Scientific, Waltham, MA) or mimic (mirVana miRNA mimic, hsa-miR-346; ID: MC10238; Thermo Fisher Scientific) according to the producer's protocol using Lipofectamine RNAiMAX reagent (Invitrogen, Carlsbad, CA). Twenty-four hours after transfection, the cells were lysated, and RNA was isolated. Caco-2 cell lines were grown on 96-well plates and treated with different concentrations (50 or 150 µM) of UDCA (Sigma-Aldrich, UDCA ≥99%, ID: U5127-1G). Twenty-four hours after UDCA treatment, the cells were harvested for further molecular analysis.
Statistics
Results were statistically analyzed using the StatView Program (SAS Institute, Cary, NC). Comparisons between the groups were made using the nonparametric Mann–Whitney test. Correlations were assessed by nonparametric Spearman rank correlation coefficient tests. All in vitro experiments were performed for a total of 4 times, and comparisons between the groups were made using the Fisher Protected Least Significant Difference (PLSD) test. Data are presented either as mean ± SE or as median plus the interquartile range (Figures 1 and 2). A P value less than 0.05 was considered statistically significant.
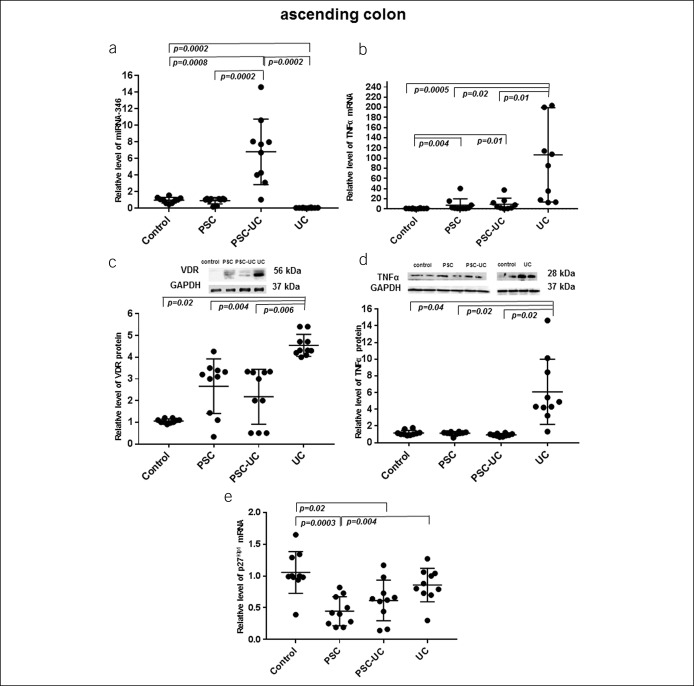
Figure 1.
Expression of miRNA-346, TNF-α, VDR, and p27 kip1 in the ascending colon. In patients with PSC-UC, the expression of miRNA-346 (a) was increased, whereas the levels of VDR protein (c) and TNF-α protein (d) were not changed in comparison to controls. The expression cell cycle inhibitor p27 kip1 (e) was substantially inhibited both in patients with PSC-UC and PSC. In contrast, in patients with UC, the miRNA-346 expression (a) was drastically suppressed, and it was accompanied by the enhanced expression of TNF-α mRNA (b), VDR protein (c), and TNF-α protein (d), whereas the expression cell cycle inhibitor p27 kip1 (e) was comparable to control values. MicroRNA-191 served as reference microRNA. Results are representative of n = 10 independent experiments per group. Data are presented as median plus IQR. IQR, interquartile range; VDR, receptor of vitamin D.
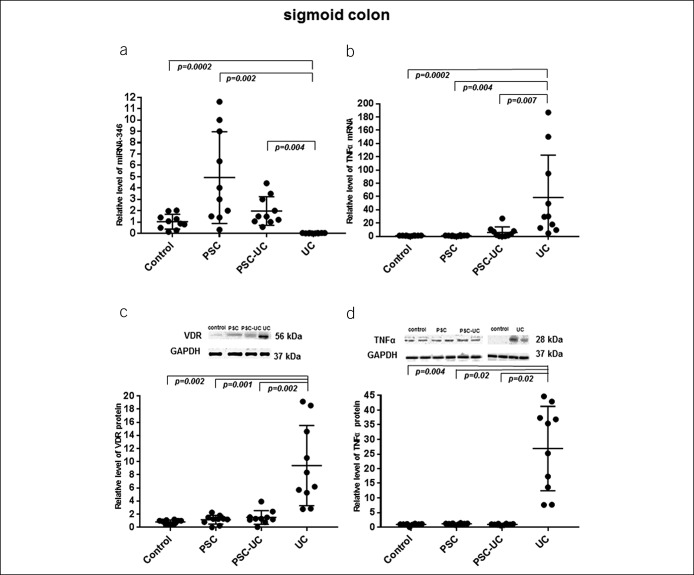
Figure 2.
Expression of miRNA-346, TNF-α, and VDR in the sigmoid colon. In both patients with PSC and PSC-UC, the levels of miRNA-346 (a), TNF-α mRNA (b), VDR protein (c), and TNF-α protein (d) were comparable to the control values. In patients with UC, the miRNA-346 expression (a) was drastically suppressed, and it was accompanied by the enhanced expression of mRNA TNF-α (b), VDR protein (c), and TNF-α protein (d). MicroRNA-191 served as reference microRNA. Results are representative of n = 10 independent experiments per group. Data are presented as median plus IQR. IQR, interquartile range; PSC, primary sclerosing cholangitis; TNF-α, tumor necrosis factor-α; VDR, receptor of vitamin D.
RESULTS
In the ascending colon, the substantial upregulation of miR-346 expression was observed in patients with PSC-UC in comparison to all examined groups of patients (6.8 ± 3.0 in PSC-UC vs 1.1 ± 0.6 in controls, P = 0.0008; vs 0.8 ± 0.6 in PSC, P = 0.0002; and vs 0.003 ± 0.001 in UC, P = 0.0002; Figure 1a). In contrast, in patients with PSC without concurrent UC, the level of miRNA-346 was comparable to control values (Figure 1a), whereas in patients with UC alone, the miRNA-346 expression was hardly detectable (P = 0.0002 vs controls, Figure 1a). The very low expression of miRNA-346 in patients with UC was accompanied by an extensive increase in TNF-α mRNA and protein levels (85-fold vs controls, P = 0.0005, Figure 1b and 7-fold vs controls, P = 0.04, Figure 1d, respectively), with this substantial increase also evident in comparison to PSC and PSC-UC. In patients with PSC and PSC-UC, the level of TNF-α mRNA was increased in comparison to control values (10.2 ± 5.4 in PSC and 9.3 ± 3.9 in PSC-UC, P = 0.004 and P = 0.01, respectively; Figure 1b). Thus, the relative level of TNF-α mRNA in the ascending colon of patients with UC was 8.5-fold greater than that in PSC (P = 0.02) and 10-fold greater than that in PSC-UC (P = 0.01, Figure 1b). Furthermore, the level of TNF-α protein in the ascending colon of patients with UC was 8.3-fold higher than that in PSC (P = 0.02) and 8.3-fold greater than that in patients with PSC-UC (P = 0.02, Figure 1d). In addition, we demonstrated that in comparison to controls, the expression of p27Kip1 was low in the ascending colon of both patients with PSC and PSC-UC (0.4 ± 0.1 vs 1.1 ± 0.1 in controls, P = 0.0003; and 0.6 ± 0.1 vs 1.1 ± 0.1 in controls, P = 0.02, respectively; Figure 1e). In the sigmoid colon, the expression of miRNA-346 was enhanced in patients with PSC, but it did not reach statistical significance (7.6 ± 1.9 in PSC vs 1.0 ± 0.2 in controls; P = 0.09; Figure 2a). In contrast, in patients with UC alone, the miRNA-346 expression was very low (P = 0.0002 vs controls, Figure 2a), and it was associated with an extensive increase in both TNF-α mRNA (17-fold vs controls, P = 0.0002; 17-fold vs PSC, P = 0.004; and 8-fold vs PSC-UC, P = 0.007; Figure 2b) and TNF-α protein level (19-fold vs controls, P = 0.0004; 20-fold vs PSC P = 0.02 and 22-fold vs PSC-UC, P = 0.02; Figure 2d). TNF-α mRNA and protein levels were similar to the control group values in the PSC and PSC-UC groups (Figures 2b, d).
In terms of VDR protein levels, both in the ascending and sigmoid colons of patients with PSC and PSC-UC, there was a high variability between patients, and the overall mean values were similar to controls (Figures 1c and 2c). On the other hand, in patients with UC, the VDR protein expression was significantly increased in the ascending and sigmoid colons, not only in comparison to controls (4.7-fold, P = 0.02 and 9.5-fold, P = 0.002, respectively) but also when compared with PSC (2.5-fold, P = 0.004 and 9-fold, P = 0.001, respectively) and PSC-UC (2.5-fold, P = 0.006 and 6-fold, P = 0.002, respectively; Figures 1c and 2c).
A significant negative correlation was observed between VDR protein and miRNA-346 in patients with UC in both parts of the colon (sigmoid colon: Rho = −0.5; P = 0.04; and ascending colon: Rho = −0.8; P = 0.02). Furthermore, VDR protein levels were correlated positively with TNF-α mRNA in the sigmoid colon of all the patients (PSC: Rho = 0.8; P = 0.07; PSC + UC: Rho = 0.9; P = 0.05 and UC: Rho = 0.7; P = 0.01) and the ascending colon of patients with UC (Rho = 0.9; P = 0.02).
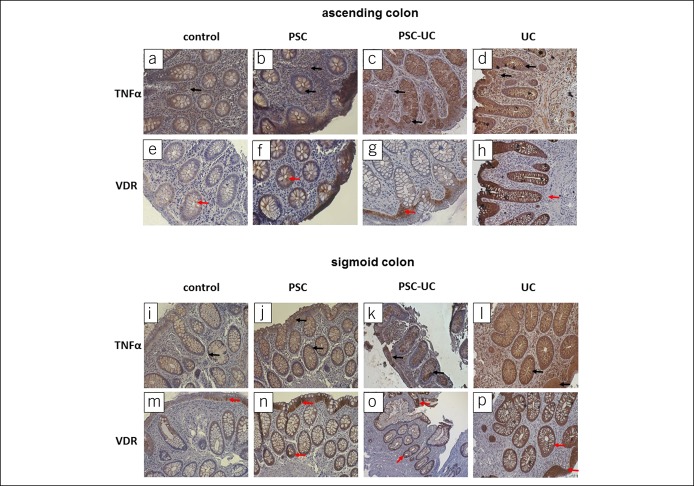
The localization of VDR and TNF-α protein in control, PSC, PSC-UC, and UC in human intestinal tissue is presented in Figure 3.
Figure 3.
Immunohistochemical localization of VDR and TNF-α proteins in human intestinal tissue. Representative immunostaining of colonic biopsies from controls, patients with PSC, patients with PSC-UC, and patients with UC with anti-TNF-α antibodies (a–d and I–l) and anti-VDR antibodies (e–h and m–p). Brown staining indicates either VDR protein (red arrows), which is located typically in the epithelial cells, or TNF-α protein (black arrows), which is depicted in expanded apical portions of goblet cells. Nuclei were visualized by hematoxylin. Original magnification was 200×. PSC, primary sclerosing cholangitis; TNF-α, tumor necrosis factor-α; VDR, receptor of vitamin D.
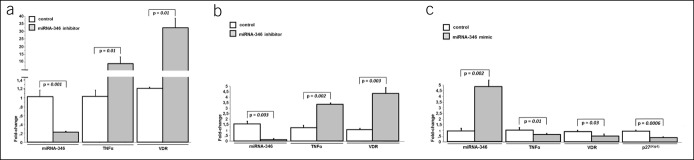
Finally, to find whether miRNA‐346 is directly involved in the regulation of TNF-α and VDR gene expression, we used antisense oligonucleotide molecules to block miR-346 activity. Transfection of the inhibitor targeting miR-346 into HepG2 cells effectively suppressed miR-346 (Figure 4a) and simultaneously induced TNF-α and VDR mRNA expression (6.6 ± 1.9 vs 1.0 ± 1.7 in controls, P = 0.01; and 32.7 ± 13.8 vs 1.4 ± 1.2 in controls, P = 0.01, respectively, Figure 4a). Similarly, transfection of the miR-346 inhibitor into Caco-2 cells effectively inhibited miR-346 expression (Figure 4b) and simultaneously enhanced the levels of TNF-α and VDR mRNA (3.3 ± 0.1 vs 1.2 ± 0.2 in controls, P = 0.002; and 4.4 ± 0.9 vs 1.1 ± 0.2 in controls, P = 0.003, respectively, Figure 4b). In contrast, transfection of a modified double-stranded RNA that mimics endogenous miR-346 into Caco-2 cells resulted in upregulation of that miRNA expression (4.5-fold increase, P = 0.002 vs controls; Figure 4c) and led to the downregulation of TNFα and VDR mRNA (0.7 ± 0.1 vs 1.1 ± 0.2 in controls, P = 0.01; and 0.5 ± 0.2 vs 0.9 ± 0.1 in controls, P = 0.03, respectively, Figure 4c). These data demonstrate that miR-346 is implicated in the negative regulation of TNFα and VDR synthesis. Given that miR-346 may promote cell cycle by inhibition of p27 Kip1 via suppression of sFRP4 (23), we additionally analyzed the level of p27 Kip1 in Caco-2 cells transfected with miR-346 mimics. Increased miR-346 expression contributed to the downregulation of p27 Kip1 (0.4 ± 0.1 vs 1.1 ± 0.2 in controls, P = 0.0006; Figure 4c).
Figure 4.
Effect of miR-346 inhibition or activation on TNF-α and VDR expression in cell lines. Caco-2 cells were untransfected (control) or transfected with miR-346 inhibitor (a, b) or miRNA-346 mimic (c). Twenty-four hours after transfection, levels of miR-346, TNF-α, and VDR transcript were quantified by qRT-PCR. Suppression of miR-346 by miR-346 antisense molecules was confirmed in both cell lines. Inhibition of miR-346 led to the strong upregulation of TNFα and VDR in both HepG2 (a) and Caco-2 (b) cell lines. Induction of miR-346 led to a significant downregulation of TNFα, VDR, and p27kip1 in Caco-2 cells (c). Each experiment was repeated 4 times with similar results. Data are presented as mean ± SE. TNF-α, tumor necrosis factor-α; VDR, receptor of vitamin D.
In addition, we showed that UDCA did not affect the basal expression of miR-346 (1.0 ± 0.1 50 μM UDCA vs 1.0 ± 0.1 in controls, P = 0.8; and 1.1 ± 0.4 150 μM UDCA vs 1.0 ± 0.1 in controls, P = 0.8).
DISCUSSION
The 2 main findings of this study were (i) a divergent expression of miR-346 in the colonic tissues of patients with PSC in comparison to patients with UC alone and (ii) miR-346-dependent modulation of TNF-α and VDR expression in Caco-2 cell line.
Our study demonstrates the substantial increase in miR-346 expression in the intestinal mucosa of patients with PSC, but the localizations of these changes were different depending on the part of the examined colon. Hence, in patients with PSC-UC, miR-346 was upregulated in the ascending colon, whereas in patients with PSC without concurrent UC, the changes in miR-346 expression were observed in the sigmoid colon. In patients with PSC-UC, increased levels of miRNA-346 were seen in the part of colon with the highest concentrations of secondary BAs. It is known that the right proximal ascending colon is the predilection site for the development of colonic malignancies in patients with PSC-UC, and these patients tend to have more progressive tumors than patients with IBD without concomitant PSC (4). Of note, miRNA-346 was hardly detected in both the ascending and sigmoid colons of patients with UC. These results are in agreement with previous studies in which miRNA-346 expression in colonic biopsies was reported to be consistently downregulated in both quiescent and active UC in comparison to healthy controls (24,25).
The very low expression of miR-346 in both examined parts of the colonic tissue of patients with UC was associated with a very significant increase in TNF-α mRNA and protein levels. These data are consistent with previous reports, which showed independently either diminished expressions of miR-346 or increased levels of TNF-α in the colons of patients with UC (24–26). Based on the animal and human data, it was suggested that TNF-α promotes miR-346 expression (15). However, in our study, this was not the case as we did not observe the induction of miR-346 in the presence of a very high level of TNF-α transcript. In contrast, in the colonic tissues of patients with PSC, we demonstrated a negative association between miR-346 expression and TNF-α mRNA level. This observation can be supported by a report showing that miR-346 plays a crucial role in the control of the inflammatory responses as it acts as a negative regulator of TNF-α release (22). In activated macrophages and fibroblast-like synoviocytes isolated from the joint spaces of patients with rheumatoid arthritis, TNF-α secretion was substantially inhibited by miR-346 (22). Our observations from the functional studies in which the experimental inhibition of miR-346 in Caco-2 and HepG2 cells led to the enhanced expression of TNF-α are in line with these reports.
Our study reveals a distinct profile of TNF-α expression in patients with PSC vs UC. In the sigmoid colon of patients with PSC both with or without concomitant UC, the levels of TNF-α mRNA and protein were comparable to control values in contrast to patients with UC where the expression of TNF-α was drastically increased. In the ascending colon, the level of TNF-α mRNA was enhanced in all patients with PSC. However, this occurred to a significantly lesser extent (more than 8-fold less) than in the colonic mucosa of patients with UC. Moreover, the analyses of the TNF-α protein level showed that in contrast to patients with UC, the colonic expression of this cytokine in all patients with PSC was comparable to controls. The observed differences between mRNA and protein levels of TNF-α in patients with PSC and PSC-UC may be a result of epigenetic regulation of TNF-α gene expression by miR-346. TNF-α is a pleiotropic cytokine with dual roles in cancer biology as it has either pro- or anti-cancer activities (27). These paradoxical roles depend on its local tissue concentration (28). Hence, TNF-α is cytotoxic to tumor cells at high levels, whereas it fuels tumor-promoting inflammation and angiogenesis at low levels. The responses mediated by TNF-α are transduced via type 1 (TNFR-1) and type 2 receptors. TNFR-1 has a death membrane domain and mediates apoptosis, but apart from this, it triggers the transcription of NF-kB and c-Jun, which are involved in cell proliferation and growth (29). The association between chronic inflammation and the risk of colorectal cancer remains controversial. Several recent meta-analyses showed no evidence of the association between the circulation level of TNF-α and the risk of colorectal cancer (30,31). Moreover, anti-TNF-α therapies for the treatment of IBD, which aim to reduce the level of this cytokine, have been suggested to have some undesirable effects, such as the induction of malignancy (32).
VDR signaling plays a critical role in maintaining the integrity of the mucosal epithelial barrier by suppressing the apoptosis of inflammation-induced cells (33,34). The data on mucosal VDR expression in patients with cholestasis are very limited. The VDR is known to be involved in the phenotypic features of PSC either by having an indirect impact on intestinal permeability or directly modulating of innate immunity. We previously demonstrated that VDR protein expression was decreased considerably in peripheral blood mononuclear cells and in the livers of patients with PSC, but its colonic expression has not been investigated (35). In this study, we found that the upregulation of miR-346 in the ascending colon of patients with PSC-UC and in the sigmoid colon of patients with PSC was associated with a reduction of VDR expression in comparison to the inflamed colon of patients with UC. Consequently, in the presence of pathological changes caused by cholangitis, the colon of patients with PSC is not efficiently protected by VDR-mediated defense due to miR-346-dependent inhibition of the VDR gene. In Caco-2 cells transfected with miR-346 mimcs, we observed a considerable suppression of both VDR and TNF-α mRNA. Our findings are in agreement with the previous reports demonstrating downregulation of VDR expression by miR-346 (15,36). Moreover, we noticed that upregulation of miR-346 activity in those cells was associated with decreased expression of p27kip, a tumor suppressor gene, which is known to be downregulated in several human cancers, including CRC (37–39). Thus, the increased miR-346 expression may be associated with the downregulation of p27kip in the ascending colon of patients with PSC and may contribute to the development and progression of colon cancer. To the best of our knowledge, the expression of both miR-346 and cell cycle inhibitor p27kip in the colon of patients with PSC has not been investigated before.
We observed that a barely detectable expression of miRNA-346 in both parts of the colon of patients with UC was associated with a substantial induction of VDR. Our in vitro functional studies in 2 cell lines (HepG2 and Caco-2) with single-stranded RNA molecule, which inhibits endogenous miRNA-346, confirmed the inhibitory effect of this microRNA on VDR and TNF-α transcript.
In summary, the present study provides novel insights into miRNA-346-dependent modulation of TNF-α and VDR expression. The evidence from this study implies that the substantial upregulation of miRNA-346 in the ascending colon of patients with PSC-UC may be accountable for the suppression of cellular protective responses modulated by VDR and TNF-α, which may lead to an inadequate suppression of neoplasia. In addition, the enormous reduction of miRNA-346 in both parts of the large intestine of patients with UC, which was accompanied by the extensive increase in VDR and TNF-α, supports this notion. Further investigation of the potential importance of colonic miRNA-346, including upstream factors, in pathophysiological mechanisms of CRC development in patients with PSC is necessary.
CONFLICTS OF INTEREST
Guarantor of the article: Agnieszka Kempinska-Podhorodecka, PhD.
Specific author contributions: A.K.-P.: study design, data collection, data analysis, and drafting of the manuscript. M.B.: performed immunohistochemistry. E.W., L.K., and K.G.: data collection. P.M and M.M.: critical review of the manuscript. All the authors reviewed the manuscript and approved the final draft submitted.
Financial support: This study was supported by the grant no. 2015/17/B/NZ5/02541 from National Science Centre in Poland.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ PSC increases the risk of colitis-associated CRC.
✓ miR-346 was reported as an oncogenic miRNA in numerous cancers.
✓ miR-346 modulates VDR and TNF-α gene, which are known to be protective against carcinogenesis.
WHAT IS NEW HERE
✓ Upregulation of colonic miRNA-346 in patients with PSC-UC in contrast to patients with UC alone.
✓ Complete suppression of miRNA-346 in UC was associated with the upregulation of VDR and TNF-α.
✓ The inhibitory effect of miRNA-346 on VDR and TNF-α transcript was confirmed in vitro.
TRANSLATIONAL IMPACT
✓ Inadequate colonic expression of VDR and TNF-α in PSC may lead to colorectal neoplasia.
✓ Inhibition of miRNA-346 is a potential target for pharmacological intervention in PSC.
REFERENCES
- 1.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet 2013;382:1587–99. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein H, Bernstein C, Payne CM, et al. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 2005;589:47–65. [DOI] [PubMed] [Google Scholar]
- 3.Bayerdorffer E, Mannes GA, Richter WO, et al. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology 1993;104:145–51. [DOI] [PubMed] [Google Scholar]
- 4.Claessen MM, Vleggaar FP, Tytgat KM, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol 2009;50:158–64. [DOI] [PubMed] [Google Scholar]
- 5.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claessen MM, Lutgens MW, van Buuren HR, et al. More right-sided IBD-associated colorectal cancer in patients with primary sclerosing cholangitis. Inflamm Bowel Dis 2009;15:1331–6. [DOI] [PubMed] [Google Scholar]
- 7.Marchesa P, Lashner BA, Lavery IC, et al. The risk of cancer and dysplasia among ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol 1997;92:1285–8. [PubMed] [Google Scholar]
- 8.Inamura K. Colorectal cancers: An update on their molecular pathology. Cancers (Basel) 2018;10:E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohammadi A, Mansoori B, Baradaran B. The role of microRNAs in colorectal cancer. Biomed Pharmacother 2016;84:705–13. [DOI] [PubMed] [Google Scholar]
- 11.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of MicroRNAs in cancer. Cancer Res 2016;76:3666–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F, Luo LJ, Zhang L, et al. MiR-346 promotes the biological function of breast cancer cells by targeting SRCIN1 and reduces chemosensitivity to docetaxel. Gene 2017;600:21–8. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Pan W, Lin X, et al. MicroRNA-346 functions as an oncogene in cutaneous squamous cell carcinoma. Tumour Biol 2016;37:2765–71. [DOI] [PubMed] [Google Scholar]
- 14.Yu Q, Yang X, Duan W, et al. miRNA-346 promotes proliferation, migration and invasion in liver cancer. Oncol Lett 2017;14:3255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Du J, Zhang Z, et al. MicroRNA-346 mediates tumor necrosis factor alpha-induced downregulation of gut epithelial vitamin D receptor in inflammatory bowel diseases. Inflamm Bowel Dis 2014;20:1910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del PR, Ferri C, Cominelli F. Vitamin D Axis in inflammatory bowel diseases: Role, current uses and future perspectives. Int J Mol Sci 2017;18:E2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Gocek E, Liu CG, et al. MicroRNAs181 regulate the expression of p27Kip1 in human myeloid leukemia cells induced to differentiate by 1,25-dihydroxyvitamin D3. Cell Cycle 2009;8:736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dankers W, Colin EM, van Hamburg JP, et al. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front Immunol 2016;7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. [DOI] [PubMed] [Google Scholar]
- 20.Haussler MR, Whitfield GK, Kaneko I, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int 2013;92:77–98. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Chen Y, Golan MA, et al. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest 2013;123:3983–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semaan N, Frenzel L, Alsaleh G, et al. miR-346 controls release of TNF-alpha protein and stability of its mRNA in rheumatoid arthritis via tristetraprolin stabilization. PLoS One 2011;6:e19827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma T, He C, Shi M, et al. MiR-346 promoted cell proliferation and cell cycle of human gastric cancer cells by suppressing sFRP4 expression. Int J Clin Exp Med 2016;9:1822–8. [Google Scholar]
- 24.Fisher K, Lin J. MicroRNA in inflammatory bowel disease: Translational research and clinical implication. World J Gastroenterol 2015;21:12274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasseu M, Treton X, Guichard C, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One 2010;5:e13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen T, Goll R, Cui G, et al. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol 2007;42:1312–20. [DOI] [PubMed] [Google Scholar]
- 27.Tse BW, Scott KF, Russell PJ. Paradoxical roles of tumour necrosis factor-alpha in prostate cancer biology. Prostate Cancer 2012;2012:128965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fajardo LF, Kwan HH, Kowalski J, et al. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol 1992;140:539–44. [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth MJ, Cretney E, Kershaw MH, et al. Cytokines in cancer immunity and immunotherapy. Immunol Rev 2004;202:275–93. [DOI] [PubMed] [Google Scholar]
- 30.Izano M, Wei EK, Tai C, et al. Chronic inflammation and risk of colorectal and other obesity-related cancers: The health, aging and body composition study. Int J Cancer 2016;138:1118–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Liu S, Zhou Y. Circulating levels of C-reactive protein, interleukin-6 and tumor necrosis factor-alpha and risk of colorectal adenomas: A meta-analysis. Oncotarget 2016;7:64371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melmed GY, Targan SR. Future biologic targets for IBD: Potentials and pitfalls. Nat Rev Gastroenterol Hepatol 2010;7:110–7. [DOI] [PubMed] [Google Scholar]
- 33.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 2008;294:G208–G216. [DOI] [PubMed] [Google Scholar]
- 34.Li YC, Chen Y, Du J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J Steroid Biochem Mol Biol 2015;148:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempinska-Podhorodecka A, Milkiewicz M, Wasik U, et al. Decreased expression of vitamin D receptor affects an immune response in primary biliary cholangitis via the VDR-miRNA155-SOCS1 pathway. Int J Mol Sci 2017;18:E289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Moreno JM, Herencia C, Montes de OA, et al. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J 2016;30:1367–76. [DOI] [PubMed] [Google Scholar]
- 37.Loda M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med 1997;3:231–234. [DOI] [PubMed] [Google Scholar]
- 38.Liu DF, Ferguson K, Cooper GS, et al. p27 cell-cycle inhibitor is inversely correlated with lymph node metastases in right-sided colon cancer. J Clin Lab Anal 1999;13:291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tenjo T, Toyoda M, Okuda J, et al. Prognostic significance of p27(kip1) protein expression and spontaneous apoptosis in patients with colorectal adenocarcinomas. Oncology 2000;58:45–51. [DOI] [PubMed] [Google Scholar]