Abstract
Summary: We describe two cases of pituitary involvement by Wegener's granulomatosis. At initial presentation, or during subsequent disease “flares,” a pattern of pituitary abnormality was suggested. During periods of remission, we found the pituitary returned to a nearly normal appearance. Loss of the normal posterior pituitary T1 hyperintensity matched a clinical persistence of diabetes insipidus, suggesting there is permanent damage to this structure by the initial disease process.
Wegener's granulomatosis is a systemic disease histologically characterized by vasculitis, necrosis, and granulomas. The exact pathogenesis of the disease is not known, but it is widely believed that immunologic dysfunction plays a major role. While any organ may be affected, the upper respiratory tract, lungs, and kidneys are the primary sites of disease activity (1). Most frequently, the clinical manifestations of CNS disease are due to peripheral neuropathy resulting from vasculitis affecting the small vessels (1, 2). Active Wegener's granulomatosis resulting in intracranial abnormalities is uncommon (2). When the disease does extend intracranially, a number of neurologic complications have been reported, including cerebrovascular ischemic events, seizures, and cranial nerve palsies (1). Diabetes insipidus as a presenting symptom is rare (1, 3). In this article, we describe the findings in two cases of pituitary involvement by Wegener's granulomatosis that corroborate the findings reported in the literature. In addition, we evaluate the evolution of these lesions, clinically and radiologically, with respect to their response to treatment initially, during senescence, and with subsequent flaring of the disease.
Case Reports
Case 1
A 41-year-old woman with a history of nephrectomy in childhood was otherwise in excellent health until 10 months before admission, when she experienced fatigue, arthralgias, paranasal sinus congestion, galactorrhea, polydypsia, and polyuria. An MR examination performed at the referring institution revealed pituitary enlargement. Given the signs, symptoms, and imaging findings, pituitary adenoma was a consideration, and she was placed on vasopressin therapy. Seven months before admission, bilateral episcleritis, arthralgias, fatigue, sinus congestion, and an erythematous, papular rash, involving both lower extremities, developed. Results of a skin biopsy were consistent with leukocytoclastic vasculitis, she was found to have a positive antineutrophil cytoplasmic antibody in a cytoplasmic pattern (C-ANCA), and a nasal biopsy specimen revealed necrotizing vasculitis, all consistent with the diagnosis of Wegener's granulomatosis. She was placed on prednisone, 20 mg every other day, and referred to our institution.
Physical examination upon admission was remarkable for bilateral episcleritis, with possible early scleritis, and inflamed nasal mucosa. Significant laboratory values included prolactin, 39 ng/dL (normal, 2–16); and positive C-ANCA, 1:320. Levels of thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) were normal. During a 24-hour collection, her urine volume was over 10 L. An MR study of the pituitary showed abnormalities of the sellar region (Fig 1A–D).
fig 1.
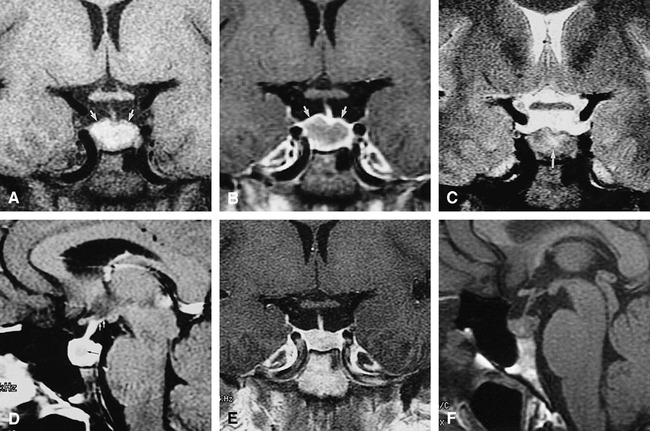
Case 1: MR findings in the sella turcica of 41-year-old woman with Wegener's granulomatosis.
A, Coronal T1-weighted (500/12/3) conventional spin-echo (CSE) image at initial presentation shows an enlarged pituitary gland (14 mm vertical height). The periphery of the pituitary is isointense with brain (arrows) whereas the remainder is hyperintense.
B, Coronal T1-weighted (500/12/3) CSE contrast-enhanced image shows enhancement of the periphery (arrows) of the pituitary gland whereas the remainder is relatively hypointense.
C, Coronal T2-weighted (2433/95/4) CSE image shows a subtle central area of hyperintensity (arrow) that is considerably smaller than the abnormality seen in A.
D, Sagittal T1-weighted (500/12/3) CSE contrast-enhanced image shows peripheral enhancement and a relatively hypointense center (black arrow) as well as minimal enhancement into hypothalamus (white arrows). The peripheral enhancing rim is broader in this image, which was obtained a few minutes after B. This was thought to be caused by the delayed enhancement by the more central tissues.
E, Coronal T1-weighted (400/9/4) CSE contrast-enhanced image after long-term immunosuppressive therapy shows the size of the pituitary gland has decreased to normal range (8 mm in height) with normal, uniform enhancement.
F, Sagittal T1-weighted (400/9/4) CSE image after immunosuppressive therapy shows persistent loss of normal posterior pituitary hyperintensity.
The patient was thought to have evidence of active Wegener's granulomatosis involving the pituitary gland, and she was begun on prednisone, 60 mg daily, and methotrexate, 15 mg weekly, which was increased by 2.5 mg per week to a maintenance dose of 20 mg weekly. She also received intranasal vasopressin, 0.1 mg twice a day. An MR examination 5 weeks later showed a return of normal pituitary size and a persistent loss of the normal posterior lobe T1 hyperintensity. Correspondingly, she had marked symptomatic improvement and was begun on a prednisone taper toward alternate-day therapy. After 4 months of treatment her serum prolactin remained elevated at 25 ng/mL.
Following her prednisone taper, she experienced worsening galactorrhea, polyuria, and polydipsia, accompanied by headaches. At this time, she was on 40 mg of prednisone every other day as well as 20 mg of methotrexate per week. An MR study revealed recurrent enlargement of the pituitary gland to 12 mm in vertical height.
Long-term therapy with immunosuppression resulted in overall improvement of the patient's symptoms, with intermittent galactorrhea from persistent low-level hyperprolactinemia and diabetes insipidus, requiring medical therapy. A final MR examination showed a normal-size gland as well as a return of normal pituitary signal characteristics, with the exception of persistent loss of the posterior lobe T1 hyperintensity (Fig 1E and F).
Case 2
This 18-year-old woman was in excellent health until 7 months before admission, when she experienced sinus congestion followed by progressive dyspnea, nasal deformity, polyuria, and polydipsia. Examination included a chest X-ray with findings of multiple nodules, cavitary lesions, and infiltrates. A nasal biopsy specimen revealed necrotizing granulomatous vasculitis, consistent with Wegener's granulomatosis, for which she was referred to our institution.
Physical examination upon admission was remarkable for an audible stridor, erythematous nasal mucosa with a septal perforation, and a saddlenose deformity. Laryngeal examination revealed subglottic stenosis, for which she underwent an emergent tracheostomy. A biopsy specimen of the subglottic tissue was positive for necrotizing granulomatous vasculitis. Significant laboratory features included an erythrocyte sedimentation rate of 108; prolactin, 76 ng/dL; and positive C-ANCA, 1:80. Levels of TSH, FSH, and LH were normal. During a 24-hour collection, her urine volume was over 8 L. A chest radiograph revealed multiple bilateral cavitary lesions. An MR image of the pituitary showed abnormalities in the sella region (Fig 2A–D).
fig 2.
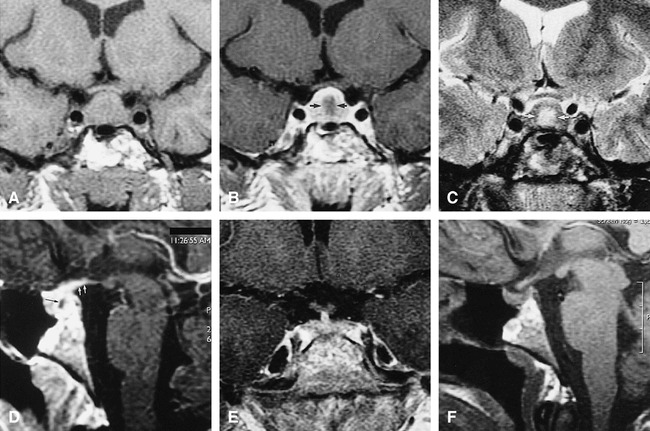
Case 2: MR findings in the sella turcica of 18-year-old woman with Wegener's granulomatosis.
A, Coronal T1-weighted (500/12/3) CSE image at initial presentation shows an enlarged pituitary gland (15 mm vertical height).
B, Coronal T1-weighted (500/12/3) CSE contrast-enhanced image shows enhancing periphery with relatively hypointense center (arrows).
C, Coronal T2-weighted (2433/95/4) CSE image with central hyperintensity (arrows) corresponding to the relatively hypointense region.
D, Sagittal T1-weighted (500/12/3) CSE contrast-enhanced image shows enhancing periphery with relatively hypointense center (black arrow) as well as enhancement into hypothalamus (white arrows).
E, Coronal T1-weighted (400/9/4) CSE contrast-enhanced image after long-term immunosuppressive therapy shows size of pituitary gland has decreased to normal range (7 mm in height) with normal, uniform enhancement.
F, Sagittal T1-weighted (400/9/4) CSE image after immunosuppressive therapy shows persistent loss of normal posterior pituitary hyperintensity.
On the basis of these findings she was thought to have evidence of active Wegener's granulomatosis involving the pituitary and was begun on prednisone, 60 mg daily, as well as methotrexate, 15 mg weekly, which was increased by 2.5 mg per week to a maintenance dose of 20 mg weekly. Additionally, she received intranasal vasopressin, 0.1 mg twice a day. An MR study 6 weeks later showed a return of normal pituitary size and a persistent loss of the normal posterior lobe T1 hyperintensity. There was also marked improvement in her chest radiograph, and she was begun on a prednisone taper toward alternate-day therapy.
Three months later she began having severe headaches and fever. At that time her prednisone dose was 60 mg, alternating with 15 mg daily, and the remainder of her medications were unchanged. The prolactin level was further elevated to 82 ng/mL, with TSH, FSH, and LH levels remaining normal. A repeat MR study showed recurrent enlargement of the pituitary gland with its vertical height measuring 15 mm. Because of disease exacerbation, the prednisone dose was increased to 100 mg daily and the cytotoxic agent was changed from methotrexate to cyclophosphamide, 150 mg daily. On this regimen she experienced symptomatic improvement.
After long-term therapy, a final MR examination documented a return of the gland to normal size and signal characteristics, except for a persistent loss of the normal posterior pituitary lobe T1 hyperintensity (Fig 2E and F).
Discussion
Wegener's granulomatosis is a necrotizing granulomatous vasculitis that can involve virtually any organ (1). Capillaries as well as small and medium-sized arteries and veins are affected with histopathologic evidence of inflammatory infiltrates in the walls, leading to thrombosis and occlusion of the lumen (1). The presence of multinucleated cells within the inflammatory infiltrates is a prominent feature of this disorder, and granulomatous lesions with epithelioid histiocytes arranged around necrotic foci are common findings. The diagnosis of the disease is usually made from the demonstration of characteristic microscopic changes on a tissue biopsy specimen in a clinically compatible setting (1, 4). Autoantibodies reactive against the cytoplasm of human neutrophils (C-ANCA) are found in the serum of 85% of patients with Wegener's granulomatosis (4).
The nasal cavity and paranasal sinuses are the most common sites of disease activity, where involvement represents a significant feature of the disease. Pulmonary involvement is reported to occur in more than 80% of patients, with the subglottic region of the trachea affected in 16% (1). The kidneys also are a common site of involvement by Wegener's granulomatosis, causing glomerulonephritis in as many as 75% to 80% of patients (1). Untreated renal involvement by Wegener's granulomatosis ultimately progresses to renal failure. The reported prevalence of CNS involvement ranges from 15% to 54% (2, 5), and peripheral neuropathy consequent to small-vessel vasculitis is the most common clinical manifestation. Intracranial abnormalities are less common and are caused by three different mechanisms (5). The first, and most commonly implicated, is by direct extension of the granulomatous process from the sinuses, orbits, or mastoid air cells intracranially (2). Direct infiltration of the meninges, the cranial nerves, and, rarely, the brain surface by the granulomatous process may occur, with symptoms varying with the site of involvement. The second mechanism of CNS Wegener's granulomatosis is a vasculitis of the cerebral vessels, which produces ischemic and/or hemorrhagic infarctions (2). Finally, the third mechanism of CNS involvement is by direct formation of granulomas within the brain parenchyma proper remote from the primary site of the disease (2). This type may present clinically with seizures (6).
Involvement of the pituitary gland by Wegener's granulomatosis is an uncommon event. In earlier reports, a few cases with diabetes insipidus were described (1, 3), but MR findings of the underlying anatomic defect have rarely been reported (7, 8). Both our patients had diabetes insipidus, and one also experienced galactorrhea, although in both patients the prolactin levels were abnormally elevated. The pituitary gland showed similar MR characteristics in both patients. The gland was abnormally enlarged, and the normal hyperintensity of the posterior lobe was absent on the sagittal T1-weighted sequence. Abnormality of the anterior lobe in both patients included increased signal intensity on T2-weighted images. The first patient also had T1 hyperintensity within the anterior lobe, presumably due to hemorrhagic elements within granulomatous tissue. The contrast-enhanced studies also showed similar features in both cases, with central relative hypointense enhancement of the anterior lobe and preserved enhancement of the peripheral gland. In one case, the pituitary stalk was abnormally thickened, and in both cases abnormal contrast enhancement was found extending into the hypothalamus. The sella turcica was not enlarged and bone destruction was not present in either patient.
The MR findings of our two patients, as well as in one previously reported case (7), exhibited a similar pattern. In another report, two patients with Wegener's involvement of the pituitary were described as having an intrasellar mass (8). Unfortunately, no detailed imaging descriptions were offered, and only a single T1-weighted image for each patient was shown. The MR abnormalities found in our two patients can occur in a variety of diseases that involve the pituitary. The most common abnormality to be considered in the differential diagnosis is a pituitary adenoma (9). Peripheral enhancement around a hypointense area, similar to that described in our patients, is a frequent finding in pituitary adenomas. Obliteration of the high signal of the posterior lobe does not usually take place with small adenomas, nor is diabetes insipidus a common clinical manifestation in adenomas of the anterior lobe. Enhancement of the hypothalamus is not a feature of adenomas, and was observed in all three patients for whom detailed MR descriptions are available (one in the literature, two described here).
Another abnormality of the anterior lobe to be considered is pituitary hyperplasia, which is seen fairly commonly in association with loss of feedback inhibition of primary hypothyroidism; however, this entity commonly presents as an enlarged gland that enhances homogeneously throughout its parenchyma (10). This is distinctly different from the findings in our two patients.
Lymphocytic hypophysitis has been reported as a cause of pituitary enlargement. This is an autoimmune disorder that commonly is found in women in the postpartum period and presents with clinical and laboratory findings of hypopituitarism (11, 12). The anterior lobe is the primary target in this entity, although on rare occasions diabetes insipidus has also been observed, indicative of posterior lobe involvement (11). On MR images, the enlarged pituitary gland exhibits homogeneous enhancement in contradistinction to the heterogenous pattern noted in both our patients with Wegener's granulomatosis before treatment (11, 12). A nonenhancing necrotic center is a rare finding in lymphocytic hypophysitis, representing an end-stage finding bearing the name necrotizing hypophysitis (13). In some cases there may be abnormal enhancement extending into the hypothalamus (12).
Granulomatous hypophysitis is another condition that presents with enlargement of the pituitary and can produce endocrine dysfunction (14). Tuberculosis, sarcoidosis, and Langerhans' cell histiocytosis (histiocytosis X) are included in this category, in which the diagnosis is usually made before the pituitary becomes involved (15–17). Similar to lymphocytic hypophysitis, these granulomatous processes display homogeneous enhancement throughout the parenchyma and lack the T1- and T2-weighted signal abnormalities noted in Wegener's granulomatosis (15, 17). Unlike lymphocytic hypophysitis, which primarily involves the adenohypophysis, granulomatous hypophysitis often presents with diabetes insipidus (14, 16). This is a reflection of the involvement of the stalk and hypothalamus, which occurs commonly in the latter.
A complete or partial remission of disease activity in Wegener's granulomatosis can be achieved with systemic immunosuppressive therapy (2, 4, 18, 19). During treatment of our patients, the pituitary size returned to normal within a short period of time, and the abnormal contrast enhancement of both the gland and hypothalamus resolved. The diabetes insipidus persisted in both cases, requiring continuation of vasopressin therapy and suggesting that the neurohypophysis was permanently damaged at an early stage of pituitary involvement, which is further supported by the finding that the normal T1 hyperintensity of the posterior lobe did not reappear. Finally, the clinical improvement obtained in these two patients was difficult to maintain, as in both cases the pituitary involvement recurred soon after the dose of immunosuppressive agent was decreased.
Conclusion
The MR findings in both our patients with Wegener's granulomatosis involving the pituitary gland were similar to the findings in a single case reported previously (7) and showed a “cycling” appearance (alternating between near-normal and abnormal) during clinically verified periods of senescence and recurrence. Although the imaging findings alone are not specific for this entity, correlation with clinical and endocrinologic abnormalities can lead to the correct diagnosis of Wegener's granulomatosis of the pituitary gland. Furthermore, the observed evolution of the pituitary lesions under treatment indicates that regression of the anatomic abnormalities may take place, but that administration of immunosuppressive agents is also critical to maintain control of the disease process. Finally, although there may be initial anatomic responses, manifested in our cases by the reduction in size of the pituitary gland to the normal range, reversal of the posterior lobe dysfunction may not be achieved.
Footnotes
Address reprint requests to N. J. Patronas, MD, National Institutes of Health, Clinical Center, Department of Radiology, Building 10, Bethesda, MD 20892.
References
- 1.Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992;116:488-498 [DOI] [PubMed] [Google Scholar]
- 2.Nishino H, Rubino FA, DeRemee RA, Swanson JW, Parisi JE. Neurological involvement in Wegener's granulomatosis: an analysis of 324 consecutive patients at the Mayo Clinic. Ann Neurol 1993;33:4-9 [DOI] [PubMed] [Google Scholar]
- 3.Rosete A, Cabral AR, Kraus A, Alarcon-Segovia D. Diabetes insipidus secondary to Wegener's granulomatosis: report and review of the literature. J Rheum 1991;18:761-765 [PubMed] [Google Scholar]
- 4.Sneller MC. Wegener's granulomatosis [clinical conference]. JAMA 1995;273:1288-1291 [PubMed] [Google Scholar]
- 5.Dwyer J, Janszen VD. Wegener's granulomatosis with intracerebral and nervous system involvement. J Otolaryngol 1981;10:476-480 [PubMed] [Google Scholar]
- 6.Miller KS, Miller JM. Wegener's granulomatosis presenting as a primary seizure disorder with brain lesions demonstrated by magnetic resonance imaging. Chest 1993;103:316-318 [DOI] [PubMed] [Google Scholar]
- 7.Czarnecki EJ, Spickler EM. MR demonstration of Wegener granulomatosis of the infundibulum, a cause of diabetes insipidus. AJNR Am J Neuroradiol 1995;16:968-970 [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts GA, Eren E, Sinclair H, et al. Two cases of Wegener's granulomatosis involving the pituitary. Clin Endocrinol (Oxf) 1995;42:323-328 [DOI] [PubMed] [Google Scholar]
- 9.Elster AD. Modern imaging of the pituitary. Radiology 1993;187:1-14 [DOI] [PubMed] [Google Scholar]
- 10.Dadachanji MC, Bharucha NE, Jhankaria BG. Pituitary hyperplasia mimicking pituitary tumor. Surg Neurol 1994;42:397-399 [DOI] [PubMed] [Google Scholar]
- 11.Koshiyama H, Sato H, Yorita S, et al. Lymphocytic hypophysitis presenting with diabetes insipidus: cases report and literature review. Endocr J 1994;41:93-97 [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi J, Meyers GS, Segall HD, Sharma OP, Hinton DR. Lymphocytic adenohypophysitis: contrast-enhanced MR imaging in five cases. Radiology 1995;195:30-34 [DOI] [PubMed] [Google Scholar]
- 13.Ahmed SR, Aiello DP, Page R, Hopper K, Towfighi J, Santen RJ. Necrotizing infundibulohypophysitis: a unique syndrome of diabetes insipidus and hypopituitarism. J Clin Endocrinol Metab 1993;76:1499-1504 [DOI] [PubMed] [Google Scholar]
- 14.Higuchi M, Arita N, Mori S, Satoh B, Mori H, Hayakawa T. Pituitary granuloma and chronic inflammation of hypophysis: clinical and immunohistochemical studies. Acta Neurochir (Wien) 1993;121:152-158 [DOI] [PubMed] [Google Scholar]
- 15.Pamir MINI, Zirh TA, Ozek MM, Sav A, Erzen C, Erbengi T. Magnetic resonance imaging in the diagnosis of idiopathic giant-cell granulomatous hypophysitis: a rare cause of hyperprolacinaemia. Neurochirurgia (Stuttgart) 1993;36:20-25 [DOI] [PubMed] [Google Scholar]
- 16.Ranjan A, Chandy MJ. Intrasellar tuberculoma. Br J Neurosurg 1994;8:179-185 [DOI] [PubMed] [Google Scholar]
- 17.Zouaoui A, Maillard J-C, Dormaont D, Chiras J, Marsault C. MR in neurosarcoidosis. J Neuroradiol 1992;19:271-284 [PubMed] [Google Scholar]
- 18.Sneller MC, Hoffman GS, Talar-Williams C, Kerr GS, Hallahan CW, Fauci AS. Analysis of forty-two Wegener's granulomatosis patients treated with methotrexate and prednisone. Arthritis Rheum 1995;38:608-613 [DOI] [PubMed] [Google Scholar]
- 19.Duna GF, Galperin C, Hoffman GS. Wegener's granulomatosis. Rheum Dis Clin North Am 1995;21:949-986 [PubMed] [Google Scholar]


