Abstract
BACKGROUND AND PURPOSE: Calcification in the coronary arteries has been correlated with significant vessel stenosis. The predictive value of calcification within the carotid siphon has not been characterized; however, stenosis in the carotid siphon is potentially important in determining management of patients with ipsilateral carotid bifurcation stenosis. The purpose of this study was to determine optimal parameters for assessing carotid siphon calcification on head CT scans and to compare the CT findings with angiographic results.
METHODS: We performed a retrospective review of patients referred for diagnostic carotid arteriography. Those patients who also had undergone a head CT study at our institution were selected. The CT scans and angiograms of 64 patients (128 vessels) were reviewed. Carotid siphon calcification on CT scans was characterized on brain and bone windows as mild, moderate, or severe. Comparison was then made with angiographic findings.
RESULTS: The sensitivity and specificity of CT for depicting greater than 50% angiographic stenosis in the carotid siphon were 86% and 98%, respectively, for bone windows and 100% and 0%, respectively, for brain windows. The positive predictive value (PPV) for a stenosis of greater than 50% as evidenced by severe calcification was 86% on bone windows and 11% on brain windows. The PPV for mild and moderate calcification on bone windows was 2.5% and 0%, respectively.
CONCLUSION: Severe CT calcification in the carotid siphon as characterized on bone windows correlates with a carotid siphon stenosis of greater than 50% as determined angiographically. Therefore, the identification of severe calcification offers a potential noninvasive method for identifying stenosis of the carotid siphon. This information may be essential in determining management and prognosis for patients with carotid bifurcation stenosis.
CT documentation of vascular calcification has gained widespread popularity as a screening technique for coronary artery disease. Most series report sensitivities of greater than 90% in detection of hemodynamically significant coronary arterial stenosis (1, 2). Calcification within the distal internal carotid artery (ICA) is a common finding on noncontrast cranial CT scans, yet the predictive value of such calcification for significant stenosis has not been characterized.
The preoperative imaging algorithm for patients being considered for carotid endarterectomy is in evolution, with many practitioners relying on carotid Doppler imaging alone in place of conventional angiography (3, 4). Extensive vascular calcification in the distal ICA on CT scans might suggest significant intracranial atherosclerotic disease. As this area is inaccessible to carotid Doppler imaging, siphon calcification might prompt the addition of conventional angiography, MR angiography, or CT angiography to the preoperative workup.
In this study, we compared the pattern and extent of carotid siphon calcification present on CT scans with the degree of ICA stenosis seen on conventional angiograms, with the expectation that this information will allow informed decisions regarding the significance of such calcifications as they relate to the need for further evaluation in the developing era of noninvasive carotid imaging.
Methods
The records of patients referred for diagnostic cerebral angiography between December 1995 and January 1997 at a tertiary care academic center were reviewed retrospectively. Selected for further review were those patients who had undergone a routine, noncontrast head CT study at our institution within 6 months of angiography. Patients with bilateral extracranial internal carotid occlusion as determined by conventional angiography were excluded. The CT scans and conventional angiograms of 64 patients (128 vessels) were reviewed independently by two investigators who were blinded to the results of the angiogram when reviewing the CT scans and to the results of the CT scans when reviewing the angiogram. The two investigators' readings correlated in 117 (91%) of 128 vessels on CT studies and in 123 (96%) of 128 vessels on angiographic evaluation. Differences in readings were resolved by consensus of the two readers on joint review. Two occluded vessels (1.5%) were excluded because they could not be assessed.
The presence of calcification in the carotid siphon, defined as that portion of the ICA between the petrous apex and anterior clinoid (5), was assessed on axial 5-mm-thick sections. Routine brain windows (level, 40 HU; window, 80 HU) and bone windows (level, 250 HU; window, 1500 HU) were viewed separately on a workstation. Calcification was characterized as absent; mild (thin, discontinuous); moderate (thin, continuous or thick, discontinuous); or severe (thick, continuous) (Figs 1 and 2).
fig 1.
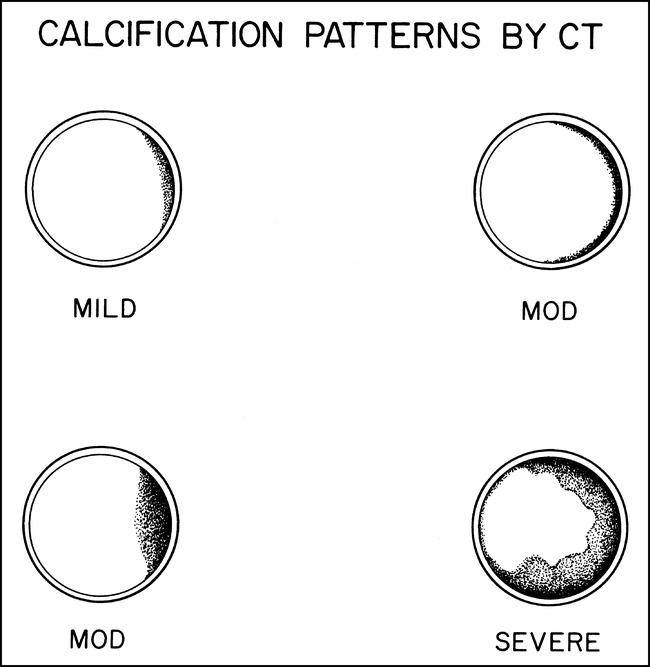
Schematic representation of CT calcification patterns. Calcification was classified as mild (thin, discontinuous); moderate (thin, continuous and thick, discontinuous); or severe (thick, continuous)
fig 2.
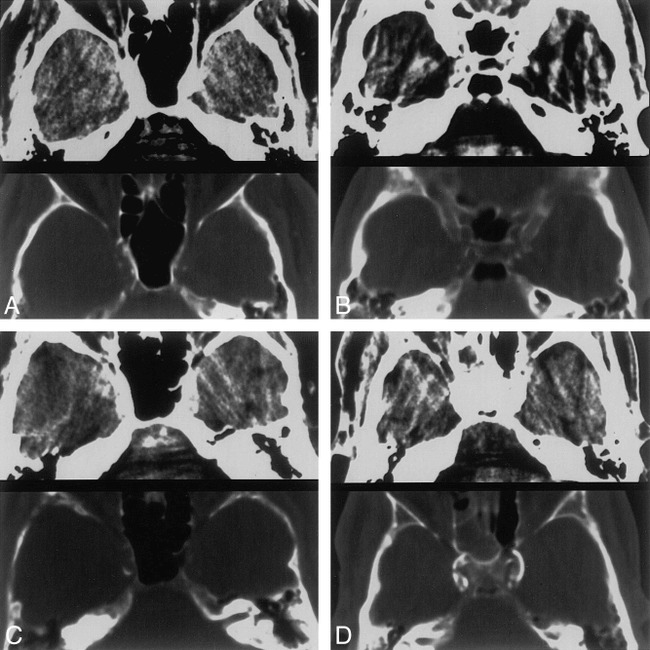
A–D, CT patterns of calcification. 5-mm-thick axial CT scans at the level of the carotid siphon. Brain (top) and bone (bottom) windows are shown in each image.
A, Thin, discontinuous calcification, bilaterally.
B, Thin, continuous calcification, right side.
C, Thick, discontinuous calcification, right side.
D, Thick, continuous calcification, bilaterally.
Conventional angiography was performed using selective common carotid artery injections of Omnipaque 300. Digital subtraction angiography was performed with at least two projections of the extracranial carotid circulation and at least two projections of the intracranial circulation. The filming rate was typically 2 frames per second. The degree of stenosis was assessed in the projection that best depicted the siphon and was measured in the projection in which the narrowing was greatest. The degree of stenosis was measured with precision calipers by dividing the minimum siphon diameter by the diameter of the normal vessel distal to the stenosis, excluding poststenotic dilatation. Angiographic findings in the carotid siphon were characterized as normal, as showing minimal irregularity of the vessel wall, as showing less than 50% diameter stenosis, or as showing greater than 50% diameter stenosis (Fig 3). Correlation between CT and angiography was then performed. The results were subjected to statistical analysis using a standard χ2-test.
fig 3.
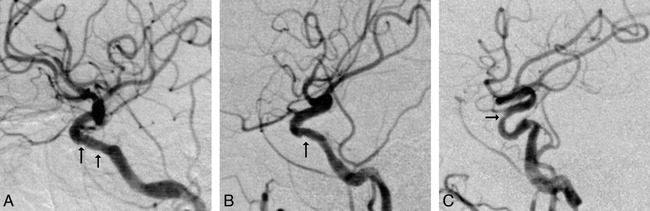
A–C, Angiographic stenoses. Digital subtraction angiograms of the intracranial circulation, lateral projections. Areas of interest are identified by the black arrows.
A, Mild irregularity of the carotid siphon.
B, Less than 50% diameter stenosis.
C, Greater than 50% diameter stenosis.
Results
Patients ranged in age from 18 to 82 years (mean age, 53 years). Sixty-four (51%) of 126 vessels had calcification in the carotid siphon on CT bone windows. The patients with calcification ranged in age from 48 to 82 years, whereas those without calcification ranged from 18 to 66 years. The average age of patients with no calcification was 41 years; with mild calcification, 64 years; with moderate calcification, 71 years; and with severe calcification, 70 years.
CT Findings
Among the 64 vessels with calcification, bone windows characterized seven (11%) with severe (thick, continuous) calcification; 17 (27%) with moderate (thin, continuous, or thick, discontinuous) calcification; and 40 (63%) with mild (thin, discontinuous) calcification. Brain windows characterized all 64 vessels (100%) with severe calcification.
Angiographic Findings
Among all evaluated vessels, angiography characterized stenosis as a mild irregularity in 29 (23%) of the 126 vessels, as stenosis of less than 50% diameter in 23 (18%), and as stenosis of greater than 50% diameter in seven (6%).
Correlation of CT Calcification with Angiographic Findings
Sensitivity of CT for a greater than 50% angiographic stenosis using severe calcification as an inclusion criteria was 86% (6/7) for bone windows and 100% (7/7) for brain windows (P < .10). Specificity of CT for a greater than 50% stenosis using severe calcification was 98% (56/57) for bone windows and 0% (0/0) for brain windows (P < .01). The positive predictive value (PPV) of calcification on CT scans for a greater than 50% angiographic stenosis was 86% (6/7) for severe calcification seen on bone windows and 11% (7/64) for severe calcification seen on brain windows (P < .01). The PPV for a greater than 50% angiographic stenosis with mild and moderate calcification on bone windows was 2.5% (1/40) and 0% (0/17), respectively (P < .05). Sixty-two (49%) of the 126 vessels had no calcification on CT scans and none of these vessels had a greater than 50% angiographic stenosis.
Discussion
Our data indicate that the ability to predict angiographic stenosis using calcification seen on standard cranial CT scans is dependent both on the pattern of calcification and on the window/level settings used to view the CT scans. For instance, severe calcifications in the distal ICA evident on bone window settings had high (86%) PPV for greater than 50% stenosis. We conclude that the pattern of severe calcification on bone window settings may be predictive of severe stenosis.
Conversely, vessels with severe calcification on brain window settings had poor PPV for the presence of greater than 50% stenosis, regardless of the pattern of calcification. Vessels with severe calcification on brain window settings were noted to have stenosis in only 11% of cases. We conclude that siphon calcification seen on routine brain windows is nonspecific, regardless of pattern.
Our finding that bone windows are more predictive of stenosis than brain windows is in concert with the physical properties of CT. It is known that window and level settings are critical for the evaluation of the size of an object. The greater the difference in CT attenuation values between an object and the adjacent tissue, the more variation in size there will be on the display. Optimally, the CT level is set halfway between the object density and the adjacent tissue. Further, widening window settings deemphasizes partial volume effects when large variations in attenuation values exist between an object and adjacent structures. Optimal parameters are achieved for siphon calcification with bone window settings (6).
The findings in our study are in concordance with those reported for other anatomic sites. In the coronary circulation, findings of calcification by CT result in sensitivities of 91% to 94% for detection of significant stenosis (1, 2, 7). We similarly achieved a high sensitivity for detection of a greater than 50% stenosis. We emphasize, however, that it is the pattern of severe calcification that appears to be predictive of severe stenosis and that potentially offers a means of screening for stenosis. Such patterns of calcification have not been evaluated as to their predictive value in identifying stenosis in the carotid siphon. Our analysis marks the first imaging study to correlate the radiologic calcification pattern in the siphon with the degree of stenosis at that site.
The finding that certain patterns of calcification correlate with stenosis is in accord with the literature. Histologically, calcification is a common finding in the carotid siphon, as we have seen in our population (8–10). In addition, autopsy studies have shown that certain patterns of calcification are associated with stenosis. In particular, asymmetric calcification is associated with atherosclerosis (9). Our finding of a poor predictive value for mild and moderate calcification is also supported pathologically. Specifically, Gautier et al have noted that “by no means are siphon calcifications always associated with stenosis” (10).
Clinically the role of a tandem siphon lesion (ie, occurring with an ipsilateral carotid bifurcation stenosis) as a source of increased stroke risk at carotid endarterectomy is in contention (11–14). Little definitive data exist on the stroke risk for siphon stenosis as a tandem lesion or for isolated carotid siphon stenosis of varying degrees. In one study, 22% of carotid thrombotic occlusions occurred in the siphon. However, in that study, four of six occlusions were seen with only moderate degrees of stenosis (15). Recently reported series have accepted 50% siphon stenosis as an intracranial tandem stenosis (12–14, 16, 17). In several of these studies the authors have stated that no significant differences in mortality or morbidity exist between those patients with isolated carotid bifurcation disease and those with tandem lesions. However, one of these studies had no perioperative morbidity and 2% mortality for isolated bifurcation disease, and 11% morbidity and 9% mortality for tandem lesions (13). Two other studies showed a trend toward increased recurrent symptoms or mortality without statistical significance (18, 19).
Other authors have reported that patients with tandem lesions are at higher risk for perioperative stroke and also for cardiac complications (12, 20). Nonetheless, no controlled, longitudinal study of outcomes with varying degrees of siphon stenosis has been performed. Debate continues regarding the preoperative significance of finding a tandem lesion (18, 19). Currently, however, as Masaryk et al have observed, “Evaluation of the distal carotid circulation is not only prudent but, in light of the NASCET patient selection guidelines, is the standard of care” (21). At present, such a recommendation requires conventional angiography as the standard of reference, since neither carotid Doppler imaging nor MR angiography has proved efficacious for evaluation of the intracranial carotid artery. However, some institutions now use noninvasive imaging in selecting patients for carotid endarterectomy (3, 4). Many of these patients undergo CT at some point in their diagnostic workup (22). CT criteria that accurately select patients who require additional evaluation of the carotid siphon would fulfill the goal both of assessing this area and of minimizing invasive testing. Our finding that severe calcification seen on bone window CT scans correlates with siphon stenosis provides evidence that patients with this finding should undergo evaluation of the intracranial circulation.
Several limitations of the CT study were noted during the review of this series. We found no established classification systems for CT calcification in the carotid siphon in the literature. Because pathologic studies suggest a possible association with asymmetric calcification and stenosis, we have incorporated this into our classification. We believe this represents a new radiologic approach that might be verified by future studies. In addition, averaging from surrounding structures presented a problem in several cases and potentially represents a source of error. We used a 5-mm section thickness for all patients in this study. The vessel diameter in the carotid siphon is typically on the order of 4 to 5 mm, and therefore partial volume averaging may also represent a source of error, particularly if a greater section thickness is used.
Several limitations were encountered at angiographic evaluation as well. We noted technical difficulties in measuring vessel diameters of small size (potentially increasing error) and in evaluating a siphon ipsilateral to a carotid bulb stenosis where underfilling occurred (potential overestimation of stenosis). Our measurements were made by comparing residual vessel lumen to expected vessel lumen distal to the site of stenosis. Studies at other anatomic sites have compared the stenosis to the distal vessel (23, 24). While no consensus exists for measurement at this site, we chose our method because it is accepted in the literature.
We selected a 50% diameter narrowing as indicative of severe stenosis because it is the figure used in the surgical and radiologic literature for the carotid siphon as described above. While 70% stenosis is considered significant at some sites, particularly the carotid bifurcation, no consensus exists for a significant stenosis of the carotid siphon (24). As this study represents a preliminary effort to determine whether any relationship is present, we attempted to achieve a high sensitivity while recognizing that specificity would necessarily be reduced. This was done by using a cutoff for severe stenosis (50%) that is at the low end of the range accepted as severe in the literature. Establishment of criteria for a significant degree of stenosis at this site is necessary to accurately assess the validity of the high PPV of severe calcification found in this study.
Conclusion
The intent of this study was to determine whether particular patterns of carotid siphon calcification correlated with angiographic stenosis. Severe CT calcification offers a potential method for identifying those patients with greater than 50% stenosis of the carotid siphon. Our findings show that the presence of extensive vascular calcification in the distal ICA on CT scans with bone windows suggests an intracranial stenosis. In an era when noninvasive imaging alone is used for preoperative assessment, this finding identifies a population of patients who might undergo further evaluation of the carotid siphon. As the natural history of varying degrees of both isolated and tandem siphon stenosis remains to be determined, future studies are necessary to address the outcome in these patients.
Footnotes
Address reprint requests to Richard J. Woodcock, Jr, MD, Box 170, Department of Radiology, Health Sciences Center, University of Virginia, Charlottesville, VA 22908.
References
- 1.Fallavollita JA, Brody AS, Bunnell IL, et al. Fast computed tomography detection of coronary calcification in the diagnosis of coronary artery disease. Circulation 1994;89:285-290 [DOI] [PubMed] [Google Scholar]
- 2.Shemesh J, Apter S, Rozenman J, et al. Calcification of coronary arteries: detection and quantification with double-helix CT. Radiology 1995;197:779-783 [DOI] [PubMed] [Google Scholar]
- 3.Balas P, Pagratis N, Massouridou E. Is cerebral arteriography necessary for decision making in carotid endarterectomy? Int J Angiol 1991;10:213-216 [PubMed] [Google Scholar]
- 4.Gelabert HA, Moore WS, et al. Carotid endarterectomy without angiography. Surg Clin North Am 1990;70:213-223 [DOI] [PubMed] [Google Scholar]
- 5.Cotran R, Kumar V, Robins S. Robins Pathologic Basis of Disease.. 4th ed. Philadelphia: Saunders; 1989;1385-1431
- 6.Curry TS, Dowdey JE, Murry RC. Christenson's Physics of Diagnostic Radiology.. 4th ed. Baltimore: Williams & Wilkins; 1990;289-323
- 7.Bartel AG. The significance of coronary calcification detected by fluoroscopy. Circulation 1974;49:1247-1253 [DOI] [PubMed] [Google Scholar]
- 8.Mitchell JRA, Schwartz CJ. Arterial Disease. Oxford: Blackwell Scientific; 1965
- 9.Fisher CM. Atherosclerosis of the carotid and vertebral arteries. J Neuropathol 1965;24:455-456 [Google Scholar]
- 10.Barnett HJM, Mohr JP, Stein BM. Internal carotid artery disease. In: Gautier JC, Mohr JP, eds. Stroke: Pathophysiology, Diagnosis and Management. 2nd ed. New York: Churchill-Livingstone; 1992;292-295
- 11.Barnett HJ, Barnes RW. The uncertainties surrounding carotid endarterectomy. JAMA 1992;268:3120-3121 [PubMed] [Google Scholar]
- 12.Schuler JJ, Flanigan DP, Leonardo LT, et al. The effect of carotid siphon stenosis on stroke rate, death and relief of symptoms following elective carotid endarterectomy. Surgery 1982;92:1058-1067 [PubMed] [Google Scholar]
- 13.Marzewski DJ, Furlan AJ, St Louis P. Intracranial internal carotid artery stenosis: long-term prognosis. Stroke 1982;13:821-824 [DOI] [PubMed] [Google Scholar]
- 14.Rouleau PA, Huston J, Gilbertson J. Carotid artery tandem lesions: frequency of detection and consequences for endarterectomy. In: Book of Abstracts of the American Society of Neuroradiology 1998. Oak Brook, IL: American Society of Neuroradiology; 1998:338. [PMC free article] [PubMed] [Google Scholar]
- 15.Castaigne P. Internal carotid occlusion. Brain 1970;93:231. [DOI] [PubMed] [Google Scholar]
- 16.Day AL, Rhoton AL, Quisling RG. Resolving siphon stenosis following endarterectomy. Stroke 1980;11:278-281 [DOI] [PubMed] [Google Scholar]
- 17.Roederer GO, Langlois YE, Chan ARW. Is siphon disease important in predicting outcome of carotid endarterectomy? Arch Surg 1983;118:1177-1181 [DOI] [PubMed] [Google Scholar]
- 18.Mackey WC, O'Donnell TF, Callow AD. Carotid endarterectomy in patients with intracranial vascular disease: short-term risk and long-term outcome. J Vasc Surg 1989;10:432-438 [PubMed] [Google Scholar]
- 19.Mattos MA, van Bemmelen PS, Hodgson KJ. The influence of carotid siphon stenosis on short- and long-term outcome after carotid endarterectomy. J Vasc Surg 1993;17:902-911 [PubMed] [Google Scholar]
- 20.Goldstein LB, McCrory DC, Landsman PB, et al. Multicenter review of preoperative risk factors for carotid endarterectomy in patients with ipsilateral symptoms. Stroke 1994;25:1116-1121 [DOI] [PubMed] [Google Scholar]
- 21.Masaryk TJ, Obuchowski NA. Noninvasive carotid imaging: caveat emptor. Radiology 1993;186:326-331 [DOI] [PubMed] [Google Scholar]
- 22.Martin JD, Valentine RJ, Myers SI, et al. Is routine CT scanning necessary in the preoperative evaluation of patients undergoing carotid endarterectomy? J Vasc Surg 1991;14:267-270 [DOI] [PubMed] [Google Scholar]
- 23. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445-453 [DOI] [PubMed] [Google Scholar]
- 24.Fox AJ. How to measure carotid stenosis. Radiology 1993;316-318 [DOI] [PubMed]


